- The First Affiliated Hospital of Zhejiang Chinese Medical University (Zhejiang Provincial Hospital of Chinese Medicine), Hangzhou, China
Background: Sarcopenia is a progressive, systemic skeletal muscle disorder. Resistance exercise and physical activity have been proven effective in its treatment, but consensus on pharmacological interventions has not yet been reached in clinical practice. β-Hydroxy-β-methylbutyrate (HMB) is a nutritional supplement that has demonstrated favorable effects on muscle protein turnover, potentially contributing to beneficial impacts on sarcopenia.
Aim: To assess the potential positive effects of HMB or HMB-containing supplements on individuals with sarcopenia, a systematic review and meta-analysis was conducted.
Methods: A systematic review and meta-analysis were conducted on randomized controlled trials (RCTs) examining the treatment of sarcopenia with HMB. Two assessors independently conducted screening, data extraction, and bias risk assessment. Outcome data were synthesized through a random-effects model in meta-analysis, using the mean difference (MD) as the effect measure.
Results: A meta-analysis was conducted on six studies. HMB or HMB-rich nutritional supplements showed a statistically significant difference in Hand Grip Strength (HGS) for sarcopenia patients [MD = 1.26, 95%CI (0.41, 2.21), p = 0.004], while there was no statistically significant difference in Gait Speed (GS) [MD = 0.04, 95%CI (−0.01, 0.08), p = 0.09], Fat Mass (FM) [MD = −0.18, 95%CI (−0.38, 0.01), p = 0.07], Fat-Free Mass (FFM) [MD = 0.09, 95%CI (−0.23, 0.42), p = 0.58], and Skeletal Muscle Index (SMI) [MD = 0.01, 95%CI (−0.00, 0.01), p = 0.13].
Conclusion: HMB or HMB-rich nutritional supplements are beneficial for muscle strength in sarcopenia patients. However, there is limited evidence demonstrating significant effects on both muscle strength and physical performance in sarcopenia individuals. HMB may be considered as a treatment option for sarcopenia patients.
Systematic review registration: CRD42024512119.
1 Introduction
Sarcopenia is clinically defined as a progressive, systemic musculoskeletal disorder (1). It is widely acknowledged that sarcopenia is more prevalent in the elderly population. In fact, the decline in muscle mass typically initiates around the age of 40 (2). Consequently, the adverse impacts of sarcopenia on quality of life, healthcare demands, incidence, and mortality may affect middle-aged and older individuals (3). Research suggests that this condition is commonly observed in both males and females, with an estimated prevalence of around 10% in individuals aged 65 and above (4). Another study indicates that sarcopenia affects 10–16% of the elderly population globally (5). With its increasing prevalence and the global trend of population aging, sarcopenia has become a focal point in contemporary clinical epidemiology (6). The diagnosis of sarcopenia comprises three aspects: muscle strength, muscle mass, and physical performance. Muscle strength is assessed through hand grip strength (HGS), typically measured using a dynamometer. Muscle mass encompasses measures such as fat mass (FM), fat-free mass (FFM), and skeletal muscle mass index (SMI), which can be assessed using dual-energy X-ray absorptiometry (DEXA), bioelectrical impedance analysis (BIA), ultrasound, magnetic resonance imaging (MRI), and computed tomography (CT), among others. Physical performance is often evaluated through gait speed (GS), which can be measured using an automated timer (6, 7). Currently, the preferred treatment for sarcopenia in clinical practice remains resistance exercise therapy (8), as there is still no evidence supporting pharmaceutical interventions for treating sarcopenia (9). While resistance exercise has demonstrated significant efficacy for sarcopenia patients, nutritional intervention is often the primary treatment for those unable to engage in physical activity (10).
β-Hydroxy-β-Methylbutyrate (HMB) is a nutritional supplement, a bioactive metabolite formed from the breakdown of the essential branched-chain amino acid leucine (11). HMB plays various roles in the human body, with its most crucial functions involving protein metabolism, insulin activity, and skeletal muscle hypertrophy (12). HMB can stimulate the mechanistic Target of Rapamycin (mTOR) signaling pathway, promoting protein synthesis, while inhibiting protein degradation through the attenuation of the proteasome pathway (12, 13). It also enhances muscle membrane integrity, making it a recognized key regulator of muscle protein synthesis metabolism in clinical settings (14, 15). Studies suggest that daily supplementation of HMB can increase muscle mass and reduce muscle damage in the elderly (16–18). Even for bedridden healthy elderly individuals without resistance training, HMB supplementation has shown positive effects in maintaining muscle mass (19), indicating its potential as an effective nutritional intervention for patients unable to engage in physical activity, such as those in critical conditions (20–22).
However, there is currently no high-quality clinical evidence supporting whether HMB has a beneficial therapeutic effect on sarcopenia patients. Assuming that HMB is beneficial for patients with sarcopenia. Therefore, we conducted a systematic review and meta-analysis to investigate whether the use of HMB alone or in protein supplements containing HMB has favorable impacts on sarcopenia patients.
2 Methods
2.1 Search strategy
This study strictly adheres to the guidelines outlined in the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) (23). Two researchers systematically conducted searches across four electronic databases—Medline/PubMed, Cochrane Library, Embase, and Web of Science—employing a comprehensive strategy that integrated both free terms and database-specific subject terms. This approach aimed to thoroughly retrieve relevant papers pertinent to this study. In cases of disagreement, a third researcher was responsible for facilitating discussions and reaching a consensus. The last search was conducted on October 24, 2023. The Medline/PubMed search example is as follows: “sarcopeni*”[Title/Abstract] OR “presarcopeni*”[Title/Abstract] OR “pre sarcopeni*”[Title/Abstract] OR “Sarcopenia”[MeSH Terms]) AND (“HMB”[Title/Abstract] OR “beta-hydroxy-beta-methylbutyrate”[Title/Abstract] OR “β-hydroxy-β-methylbutyrate”[Title/Abstract].
2.2 Study selection criteria
All retrieved papers are stored in EndNote X9. Two researchers individually conducted screening based on titles and abstracts to identify relevant literature for the study. In instances of disagreement between the two researchers, a third researcher facilitated discussions to reach a consensus. Inclusion criteria for the study were as follows: (1) Diagnosis of sarcopenia according to the European Working Group on Sarcopenia in Older People (EWGSOP), Asian Working Group on Sarcopenia (AWGS) (6, 24), consensus, or any other definition used by the authors of the original study; (2) Randomized controlled trial (RCT); (3) Use of HMB or HMB-rich preparations in the intervention group. Exclusion criteria were as follows: (1) Animal or in vitro studies; (2) Studies where data cannot be accurately extracted or where data is missing.
2.3 Date collection, and data extraction
Two researchers independently extracted data from the included study by reading the full text and entered the extracted data into a pre-designed data extraction form. In cases of discrepancies during the extraction process, a third researcher facilitated discussions to reach a consensus. The extracted study features included the authors, publication year, publication date, study location, research design, diagnostic criteria, basic characteristics and numbers of study subjects, interventions for the intervention and control groups, follow-up duration, and outcome data.
2.4 Quality assessment
Two researchers independently assessed the quality of the studies using the standards outlined in the Cochrane Handbook for Systematic Reviews of Interventions (25). These standards encompassed the following seven criteria: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome data, addressing incomplete outcome data, selective reporting, and other biases.
2.5 Statistical analysis
This study utilized the statistical software RevMan 5.3 for conducting meta-analyses. As all included outcome measures were continuous variables, the mean difference (MD) was chosen as the effect size, with each effect size accompanied by a point estimate and a 95% confidence interval (CI). For studies that provided data in terms of median and range, the data were transformed according to the appropriate formulas before being included in the pooled analysis (26, 27). Heterogeneity was assessed through a χ2 test (α = 0.1) and complemented by the I2 statistic. If the I2 value was less than 50%, a fixed-effects model was employed for the meta-analysis. If the I2 value was 50% or greater, indicating substantial statistical heterogeneity among the study results, further analysis of the sources of heterogeneity was conducted. After excluding apparent clinical and methodological heterogeneity, a random-effects model was then applied for the meta-analysis. Sensitivity analysis entails the stepwise exclusion of individual study results, followed by a reiteration of the meta-analysis, and an evaluation of the disparities between the revised outcomes and the initial combined results.
3 Results
3.1 Search result
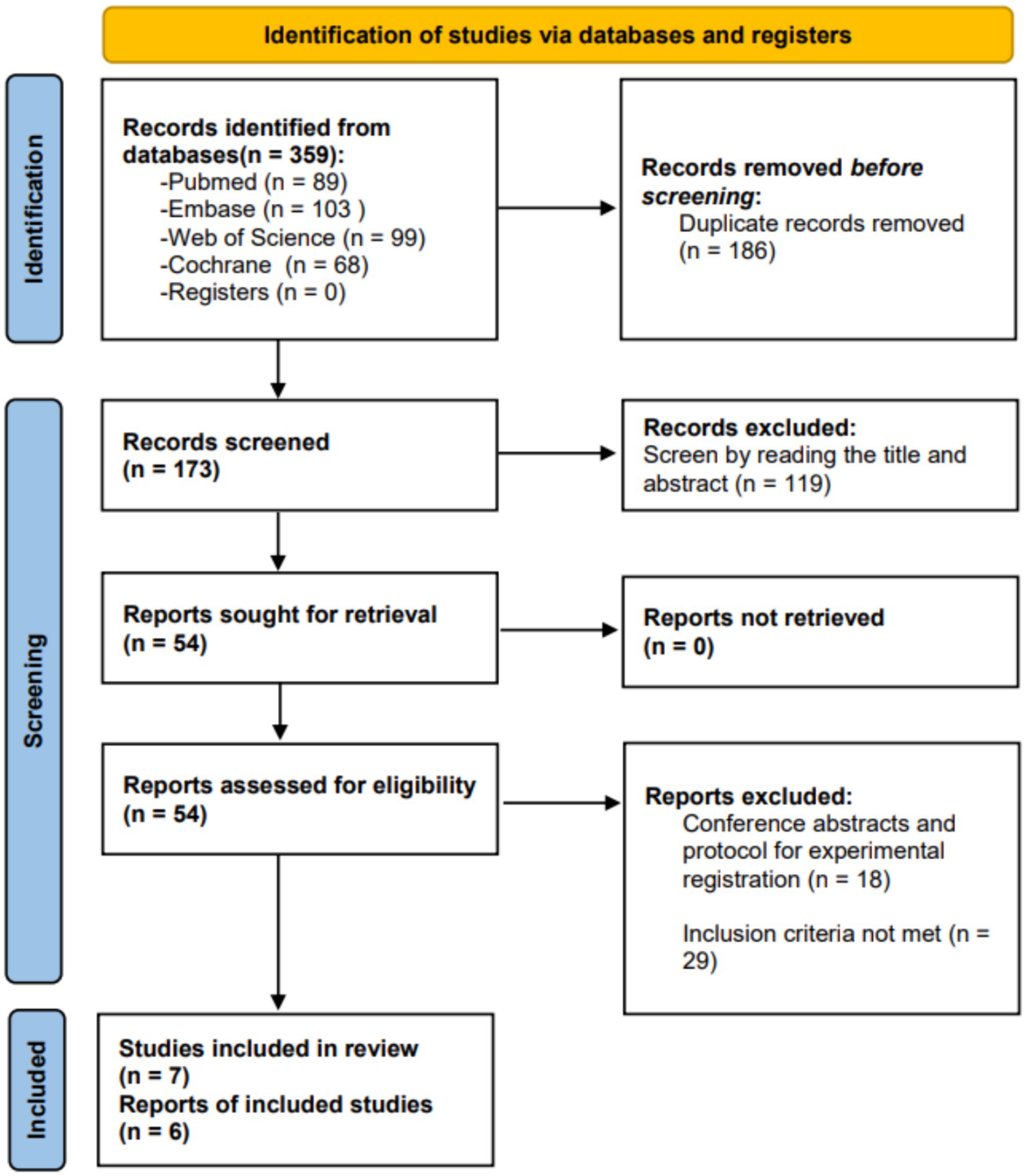
We retrieved a total of 359 studies. After removing duplicate studies, we identified 173 studies for title and abstract screening. Through the screening of title and abstract, we confirmed 54 studies and conducted a full-text review. Among them, 30 studies did not meet the inclusion criteria, and 18 studies were classified as conference abstracts or experimental registration plans. Finally, we included 7 studies in the systematic review. However, one study (28) had limited outcome indicators, allowing for quality assessment but preventing inclusion in the meta-analysis. Therefore, only 6 studies were included in the meta-analysis. Figure 1 illustrates the study selection process and search results.
3.2 Study characteristics
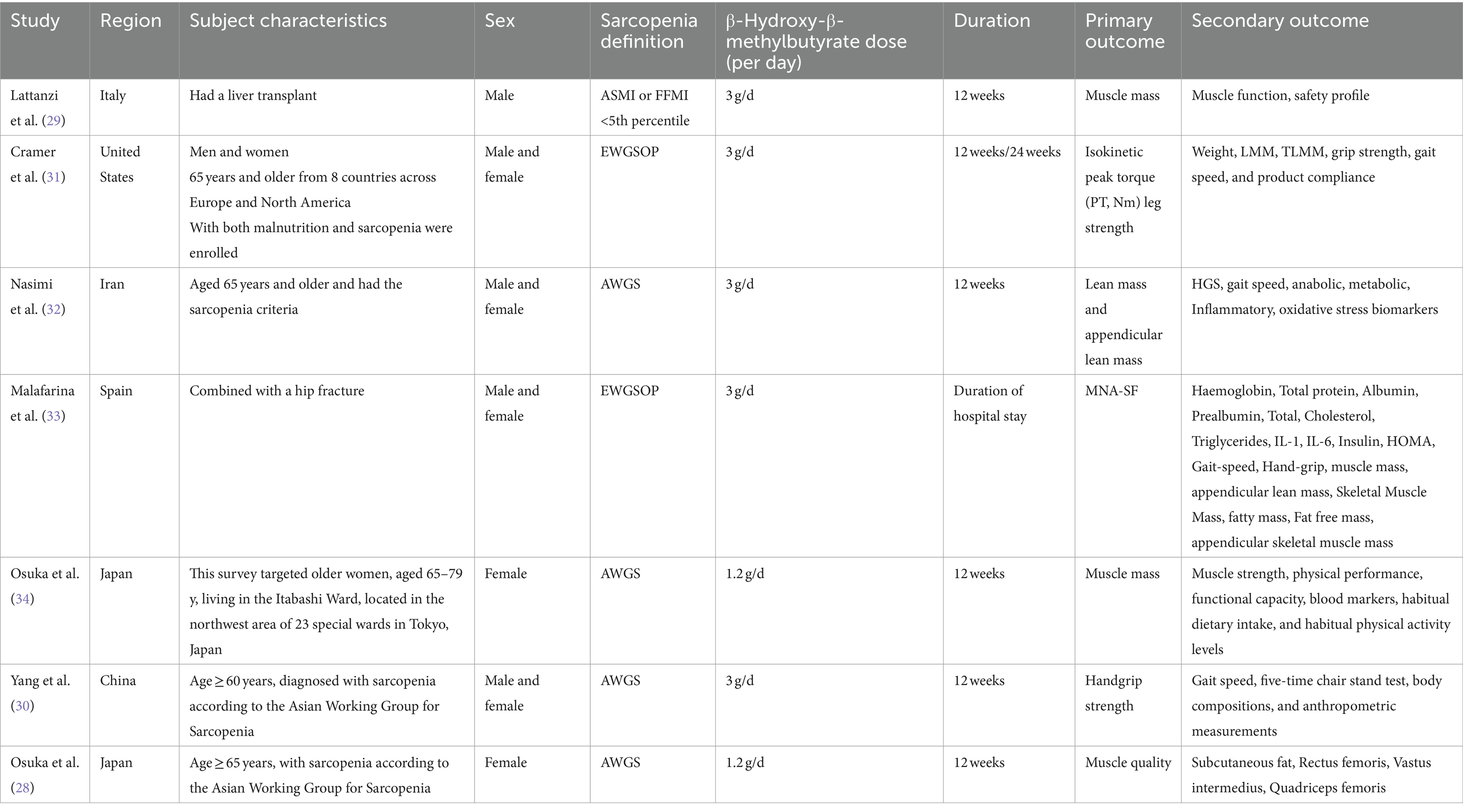
Seven studies met the inclusion criteria for quality assessment (28–34). All seven studies utilized established diagnostic criteria and included patients diagnosed with sarcopenia, such as the criteria from the EWGSOP and the AWGS. In one study (29), participants were post-liver transplant patients with concomitant sarcopenia, while another study (33) included sarcopenia patients with hip fractures. The HMB intake was 1.2 g/d in two studies (28, 34), and 3 g/d in the remaining five studies (29–33). The intervention duration in one study was only during the hospitalization period (33), while the nutrition intervention duration in the other six studies was 12 weeks. Detailed characteristics of the studies are presented in Table 1.
3.3 Quality assessment and publication bias
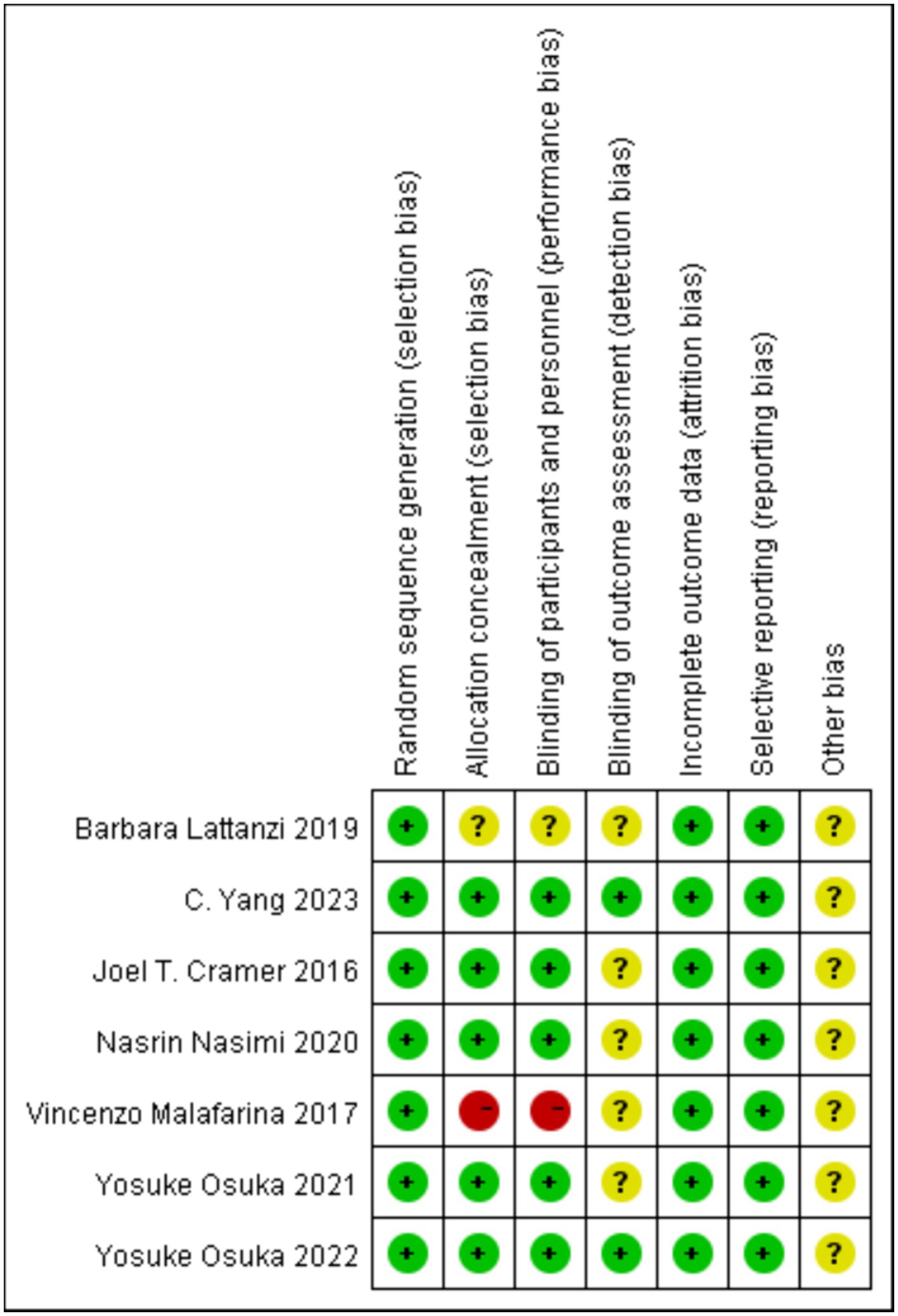
No study was considered at low risk across all items. All participants met the criteria for random allocation, but it remains unclear whether all trials adhered to all quality assessment criteria (Figure 2). As the literature included in this study is less than 10 articles, publication bias detection was not performed.
3.4 Study results
Regardless of the measurement approach used, our primary outcome measure is the patient’s HGS, and secondary outcome measures include GS, FM, FFM, and SMI. One study (34) included four treatment groups: exercise plus HMB, exercise plus placebo, education plus HMB, and education plus placebo. Therefore, the data from this study were divided into two groups for analysis. Another study (31) categorized sarcopenia patients into severe and mild–moderate groups, both undergoing intervention with HMB nutritional supplements. Consequently, the data from this study were also divided into two groups for analysis. The intervention period in one study extended from admission to discharge, in contrast to the remaining studies which uniformly had a 12-week intervention duration. Additionally, one study implemented a combined approach for interventions, while the others exclusively administered either HMB or HMB-rich nutritional supplements. In one study, the dosage of HMB was 1.2 g, while in the remaining studies, the dosage of HMB was 3 g. Three studies involved populations from Asia, and three studies involved populations from Europe. Two studies the patient combined with other diseases, and four studies the patient without other diseases. These variations may introduce heterogeneity into the analysis. Therefore, we conducted subgroup analyses based on intervention duration, intervention method, population, the dosage of HMB, and combined with other diseases for HGS, GS, FM, and FFM data during the analysis, aiming to reduce the heterogeneity in the article. The results of sensitivity analyses can be found in Supplementary materials.
3.4.1 HGS
A total of 6 studies, involving 667 patients, were included in the analysis. According to the random-effects model meta-analysis results, when the intervention duration was less than 12 weeks, there was no statistically significant difference in HGS between the HMB group and the control group [MD = 0.40, 95%CI (−2.99, 3.79), p = 0.82] (Figure 3). However, with an intervention duration of 12 weeks, a statistically significant difference in HGS emerged between the HMB group and the control group [MD = 1.31, 95%CI (0.43, 2.18), p = 0.003, I2 = 98%] (Figure 3). When the intervention involved the combination of HMB with other methods, there was a statistically significant difference in HGS between the HMB group and the control group [MD = 0.51, 95% CI (0.21, 0.81), p = 0.0008, I2 = 72%]. Similarly, when the intervention consisted solely of HMB, a statistically significant difference in HGS between the HMB group and the control group was also observed [MD = 1.78, 95% CI (0.31, 3.25), p = 0.02, I2 = 99%]. There was a significant difference in HGS between the HMB group and the control group when the population was from Asia [MD = 0.07, 95% CI (0.02, 0.13), p = 0.01, I2 = 99%]. However, when the population was from Europe, no statistically significant difference in HGS was observed between the HMB group and the control group [MD = −0.40, 95% CI (−0.17, 0.09), p = 0.05, I2 = 0%]. There was a significant difference in HGS between the HMB group and the control group when the dosage of HMB was less than 3 g [MD = 0.51, 95% CI (0.21, 0.81), p = 0.0008, I2 = 72%]. Similarly, a significant difference in HGS was observed between the HMB group and the control group when the dosage of HMB was 3 g [MD = 2.07, 95% CI (0.53, 3.60), p = 0.008, I2 = 99%]. There was a significant difference in HGS between the HMB group and the control group when the patient without other diseases [MD = 1.26, 95% CI (0.38, 2.13), p = 0.005, I2 = 99%]. However, when the patient combined with other diseases, no statistically significant difference in HGS was observed between the HMB group and the control group [MD = 1.79, 95% CI (−2.84, 6.43), p = 0.45, I2 = 31%] (Details can be found in Supplementary materials). Overall, the results indicated a statistically significant difference in HGS between the HMB group and the control group [MD = 1.26, 95%CI (0.41, 2.21), p = 0.004, I2 = 98%] (Figure 3).
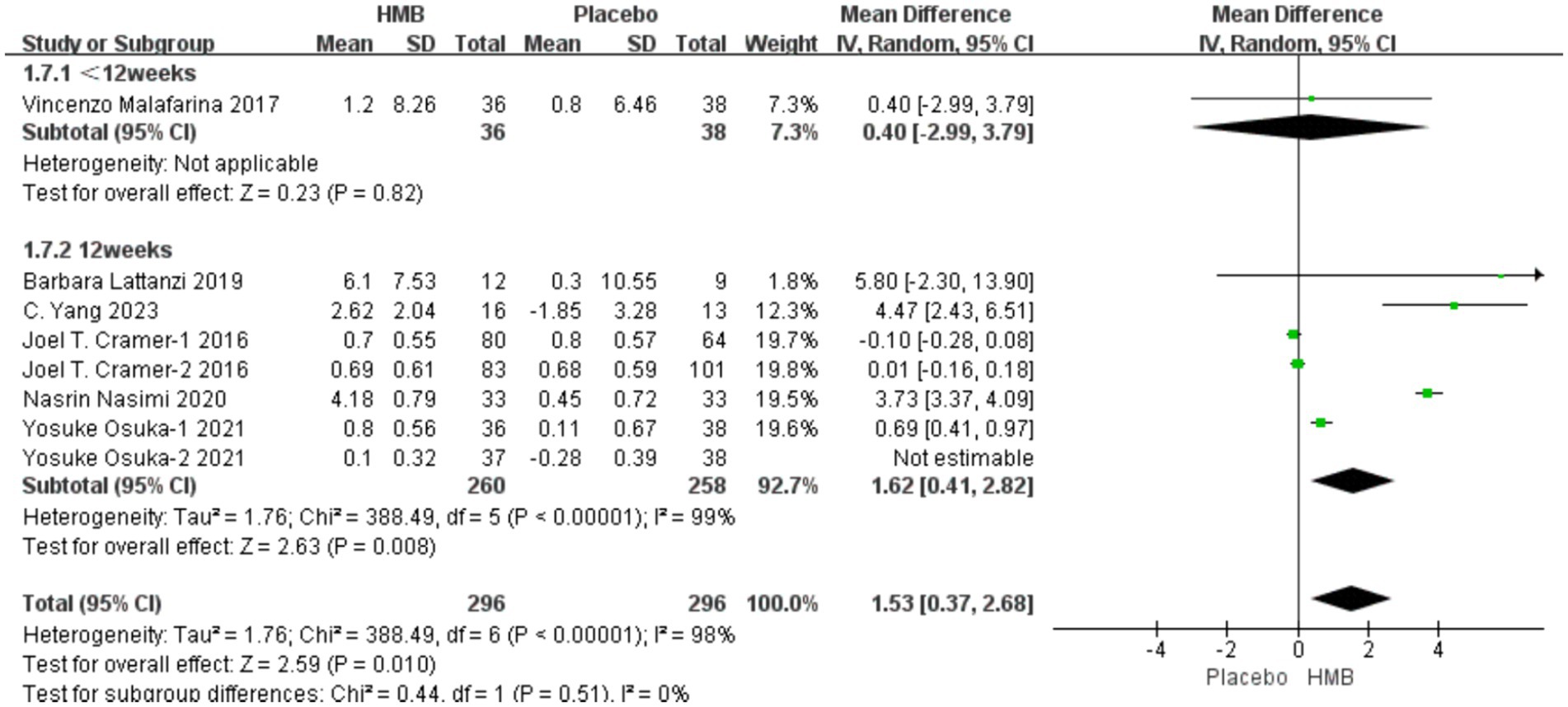
Figure 3. Forest plot for changes in hand grip strength. The horizontal lines represent 95% CI. The diamond data markers indicate the mean difference (MD) of HMB supplementation on hand grip strength.
3.4.2 GS
A total of 5 studies, including 646 patients, were included in the analysis. According to the random-effects model meta-analysis results, when the intervention duration was less than 12 weeks, there was no statistically significant difference in GS between the HMB group and the control group [MD = 0.00, 95%CI (−0.14, 0.14), p = 1.00] (Figure 4). With an intervention duration of 12 weeks, there was no statistically significant difference in GS between the HMB group and the control group [MD = 0.04, 95%CI (−0.00, 0.09), p = 0.08, I2 = 99%] (Figure 4). When the intervention involved the combination of HMB with other methods, there was no statistically significant difference in GS between the HMB group and the control group [MD = 0.06, 95% CI (−0.04, 0.16), p = 0.23, I2 = 100%]. Similarly, when the intervention consisted solely of HMB, there was no statistically significant difference in GS between the HMB group and the control group [MD = 0.03, 95% CI (−0.02, 0.07), p = 0.29, I2 = 99%]. There was a significant difference in GS between the HMB group and the control group when the population was from Asia [MD = 0.07, 95% CI (0.02, 0.13), p = 0.01, I2 = 99%]. However, when the population was from Europe, no statistically significant difference in GS between the HMB group and the control group was observed [MD = −0.40, 95%CI (−0.17, 0.09), p = 0.52, I2 = 0%]. However, the sensitivity analyses did not support this conclusion, and it is possible that there were false positives. There was no significant difference in GS between the HMB group and the control group when the dosage of HMB was less than 3 g [MD = 0.06, 95% CI (−0.04, 0.16), p = 0.23, I2 = 100%]. When the dosage of HMB was 3 g, there was no significant difference in GS between the HMB group and the control group [MD = 0.03, 95% CI (−0.02, 0.07), p = 0.29, I2 = 99%]. There was no significant difference in GS between the HMB group and the control group when the patient without other diseases [MD = 0.04, 95% CI (0.00, 0.09), p = 0.08, I2 = 99%]. When the patient combined with other diseases, no statistically significant difference in GS was observed between the HMB group and the control group [MD = 0.00, 95% CI (−0.14, 0.14), p = 1.00] (Details can be found in Supplementary materials). Overall, the results indicated a statistically non-significant difference in GS between the HMB group and the control group [MD = 0.04, 95%CI (−0.01, 0.08), p = 0.09, I2 = 99%] (Figure 4).
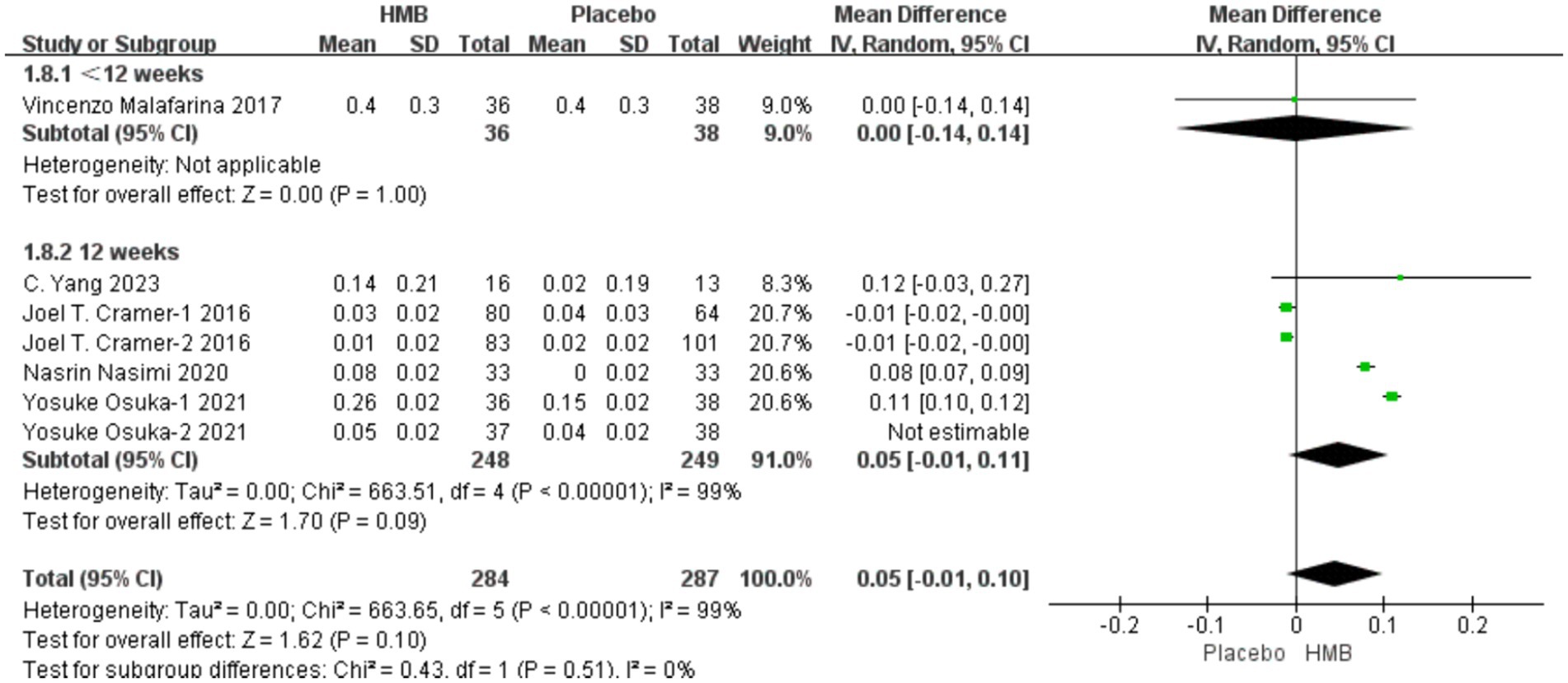
Figure 4. Forest plot for changes in gait speed. The horizontal lines represent 95% CI. The diamond data markers indicate the mean difference (MD) of HMB supplementation on gait speed.
3.4.3 FM
A total of 5 studies, including 638 patients, were included in the analysis. According to the random-effects model meta-analysis results, when the intervention duration was less than 12 weeks, there was no statistically significant difference in FM between the HMB group and the control group [MD = 3.09, 95%CI (−2.05, 8.23), p = 0.24] (Figure 5). With an intervention duration of 12 weeks, there was no statistically significant difference in FM between the HMB group and the control group [MD = −0.19, 95%CI (−0.39, 0.01), p = 0.06, I2 = 95%] (Figure 5). When the intervention involved the combination of HMB with other methods, there was no statistically significant difference in FM between the HMB group and the control group [MD = −0.28, 95%CI (−0.67, 0.11), p = 0.16, I2 = 98%]. Similarly, when the intervention consisted solely of HMB, there was no statistically significant difference in FM between the HMB group and the control group [MD = −0.12, 95%CI (−0.29, 0.04), p = 0.15, I2 = 76%]. For populations from Asia, there was a significant difference in FM between the HMB group and the control group [MD = 0.07, 95% CI (0.01, 0.13), p = 0.03, I2 = 99%]. However, for populations from Europe, there was no statistically significant difference in FM between the HMB group and the control group [MD = −0.40, 95%CI (−0.17, 0.09), p = 0.54, I2 = 0%]. There was no significant difference in FM between the HMB group and the control group when the dosage of HMB was less than 3 g [MD = −0.28, 95% CI (−0.67, 0.11), p = 0.16, I2 = 98%]. Similarly, when the dosage of HMB was 3 g, there was no significant difference in FM between the HMB group and the control group [MD = −0.12, 95% CI (−0.29, 0.04), p = 0.07, I2 = 76%]. There was no significant difference in FM between the HMB group and the control group when the patient without other diseases [MD = −0.19, 95% CI (−0.39, 0.1), p = 0.06]. When the patient combined with other diseases, no statistically significant difference in FM was observed between the HMB group and the control group [MD = 1.13, 95% CI (−1.68, 3.94), p = 0.37, I2 = 0%] (Details can be found in Supplementary materials). Overall, the results indicated no statistically significant difference in FM between the HMB group and the control group [MD = −0.18, 95%CI (−0.38, 0.01), p = 0.07, I2 = 94%] (Figure 5).
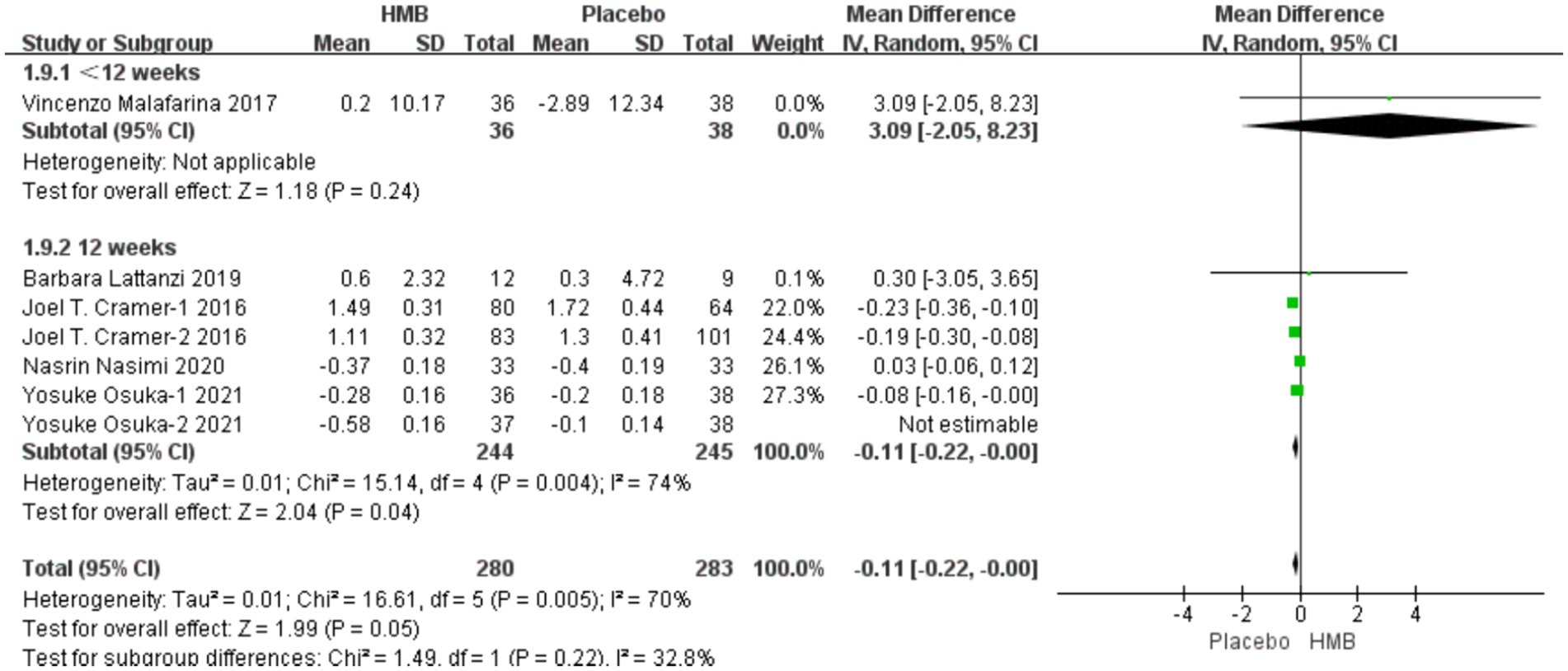
Figure 5. Forest plot for changes in fat mass. The horizontal lines represent 95% CI. The diamond data markers indicate the mean difference (MD) of HMB supplementation on fat mass.
3.4.4 FFM
A total of 4 studies, including 273 patients, were included in the analysis. According to the random-effects model meta-analysis results, when the intervention duration was less than 12 weeks, there was no statistically significant difference in FFM between the HMB group and the control group [MD = −2.70, 95%CI (−6.72, 1.32), p = 0.19] (Figure 6). With an intervention duration of 12 weeks, there was no statistically significant difference in FFM between the HMB group and the control group [MD = 0.11, 95%CI (−0.21, 0.43), p = 0.50, I2 = 96%] (Figure 6). When the intervention involved the combination of HMB with other methods, there was no statistically significant difference in FFM between the HMB group and the control group [MD = 0.11, 95%CI (−0.24, 0.46), p = 0.54, I2 = 99%]. Similarly, when the intervention consisted solely of HMB, there was no statistically significant difference in FFM between the HMB group and the control group [MD = 0.20, 95%CI (−0.59, 0.99), p = 0.62, I2 = 0%]. There was no significant difference in FFM between the HMB group and the control group in the Asian population [MD = 0.13, 95% CI (−0.20, 0.46), p = 0.45, I2 = 97%]. Similarly, in the European population, there was no statistically significant difference in FFM between the HMB group and the control group [MD = −0.76, 95%CI (−2.62, 1.09), p = 0.42, I2 = 15%]. There was no significant difference in FFM between the HMB group and the control group when the dosage of HMB was less than 3 g [MD = 0.11, 95% CI (−0.24, 0.46), p = 0.54, I2 = 99%]. Similarly, when the dosage of HMB was 3 g, there was no significant difference in FFM between the HMB group and the control group [MD = 0.20, 95% CI (−0.59, 0.99), p = 0.62, I2 = 0%] There was no significant difference in FFM between the HMB group and the control group when the patient without other diseases [MD = 0.13, 95% CI (−0.20, 0.46), p = 0.58, I2 = 94%]. When the patient combined with other diseases, no statistically significant difference in FFM was observed between the HMB group and the control group [MD = −0.76, 95% CI (−2.62, 1.09), p = 0.37, I2 = 15%] (Details can be found in Supplementary materials). Overall, the results indicated no statistically significant difference in FFM between the HMB group and the control group [MD = 0.09, 95%CI (−0.23, 0.42), p = 0.58, I2 = 94%] (Figure 6).
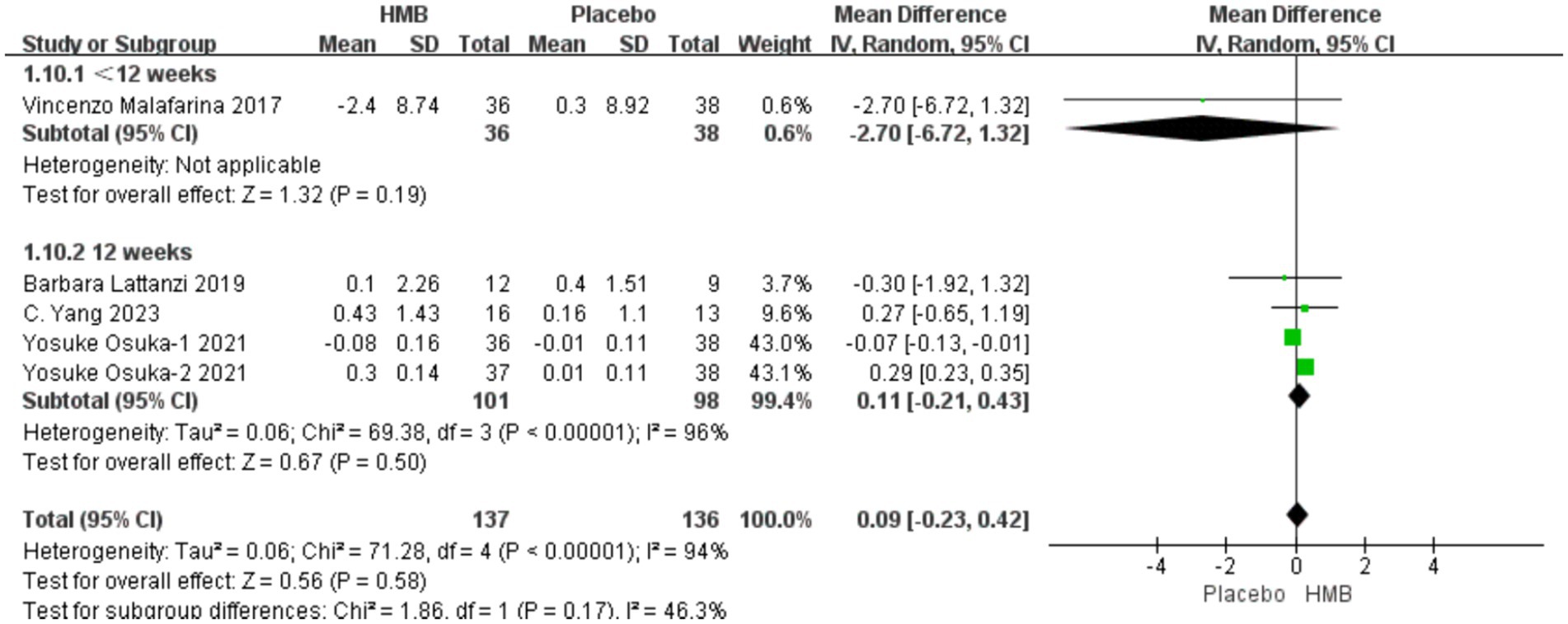
Figure 6. Forest plot for changes in fat free mass. The horizontal lines represent 95% CI. The diamond data markers indicate the mean difference (MD) of HMB supplementation on fat free mass.
3.4.5 SMI
A total of 3 studies, including 199 patients, were included in the analysis. According to the random-effects model meta-analysis results, there was no statistically significant difference in SMI between the HMB group and the control group [MD = 0.01, 95%CI (−0.00, 0.01), p = 0.13] (Figure 7). When the intervention involved the combination of HMB with other methods, there was no statistically significant difference in SMI between the HMB group and the control group [MD = 0.01, 95%CI (−0.00, 0.01), p = 0.54, I2 = 57%]. Similarly, when the intervention consisted solely of HMB, there was no statistically significant difference in SMI between the HMB group and the control group [MD = 0.01, 95%CI (−0.00, 0.01), p = 0.13, I2 = 3%]. There was no significant difference in SMI between the HMB group and the control group in populations from Asia [MD = 0.01, 95% CI (−0.00, 0.01), p = 0.12, I2 = 16%]. Similarly, in populations from Europe, there was no statistically significant difference in SMI between the HMB group and the control group [MD = −0.37, 95%CI (−1.24, 0.50), p = 0.13, I2 = 3%]. When the dosage of HMB was less than 3 g, there was no significant difference in SMI between the HMB group and the control group [MD = 0.01; 95% CI (−0.00, 0.01); p = 0.54; I2 = 57%]. When the dosage of HMB was 3 g, there was no significant difference in SMI between the HMB group and the control group [mean difference (MD) = 0.01; 95% confidence interval (CI) = (−0.00, 0.01); p = 0.13; I2 = 3%]. There was no significant difference in SMI between the HMB group and the control group when the patient without other diseases [MD = 0.01, 95% CI (−0.00, 0.01), p = 0.38, I2 = 3%]. When the patient combined with other diseases, no statistically significant difference in SMI was observed between the HMB group and the control group [MD = −0.37, 95% CI (−1.24, 0.50), p = 0.40] (Details can be found in Supplementary materials).
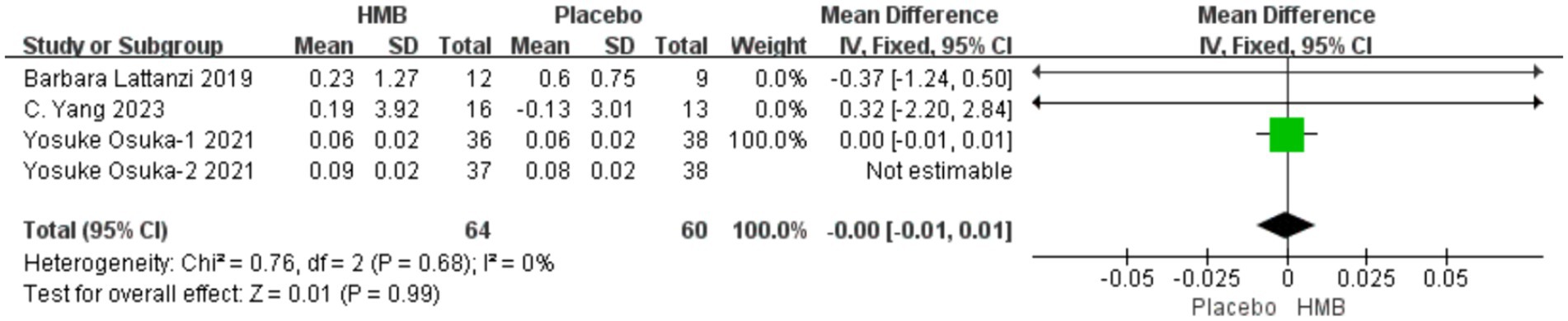
Figure 7. Forest plot for changes in skeletal muscle index. The horizontal lines represent 95% CI. The diamond data markers indicate the mean difference (MD) of HMB supplementation on skeletal muscle index.
4 Discussion
The results of this meta-analysis indicate a positive effect of HMB or HMB-rich nutritional supplements on HGS in individuals with sarcopenia. However, the present study did not demonstrate significant benefits for GS, FM, FFM, and SMI. Sarcopenia is diagnosed based on muscle strength, muscle mass, and physical performance (1, 35). Different measurement methods are employed for muscle strength, muscle mass, and physical performance. For instance, muscle strength can be assessed through HGS (36–38), muscle mass through SMI, FFM, and FM (39–42), and physical performance through GS (43, 44). Presently, the efficacy of HMB for individuals with sarcopenia remains inconclusive. To our knowledge, this study represents the first meta-analysis investigating the effects of supplementing with HMB or HMB-rich nutritional supplements on individuals diagnosed with sarcopenia.
The study by Bear et al. (11) indicates that HMB or supplements containing HMB have beneficial effects on muscle mass and strength in frail individuals. Wu et al.’s (45) research suggests that HMB can improve muscle loss in the elderly. Han et al.’s (46) study found that HMB may help mitigate muscle loss in patients after hip replacement surgery. Results from Martin-Cantero et al.’s (47) research suggest that HMB may effectively improve muscle mass in the elderly. A randomized controlled trial (48) indicates that supplementing with HMB, especially without combining exercise, can enhance muscle mass in the elderly. These studies consistently demonstrate the beneficial effects of HMB on the elderly or frail populations, which aligns with our study results. What sets our study apart is the inclusion of individuals with sarcopenia, analyzing the impact of HMB on muscle strength, muscle mass, and physical performance in this specific population—a distinction not found in other studies. The study by Courel-Ibáñez et al. (13) indicates that HMB does not lead to significant improvements in physical performance, muscle strength, and overall performance in the elderly. A difference from previous meta-analyses is that their intervention involved adding HMB to existing exercise routines, suggesting that physical activity might yield similar or even greater benefits compared to HMB alone (49–51). This is inconsistent with our study results, and the discrepancy may be attributed to differences in the study populations. Regrettably, for sarcopenia patients who face difficulties or are unable to engage in physical activity, sports do not appear to be their optimal choice. Our study results, for the first time, demonstrate that supplementing with HMB or HMB-rich supplements has a certain effect on muscle strength in sarcopenia patients. During the revision process of sarcopenia guidelines, there has been an increasing emphasis on muscle strength. It is recognized that muscle strength is more accurate than muscle mass in predicting adverse outcomes. HGS, as a representative measure of muscle strength, has garnered significant attention in many guidelines and is considered a key marker for evaluating and diagnosing sarcopenia (6, 7). In our study, patients showed improved grip strength after oral intake of HMB- or HMB-rich nutritional supplements. This suggests an enhancement in muscle strength among sarcopenia patients, which can contribute to building confidence and facilitating better rehabilitation. HMB may serve as an effective therapeutic approach for sarcopenia patients, particularly those unable to engage in physical exercise. HMB increases protein synthesis through the mTOR pathway, inhibits catabolic metabolic pathways to reduce protein breakdown, enhances satellite cell proliferation, and decreases the release of inflammatory factors, thereby promoting muscle tissue repair (52–54). Additionally, it increases mitochondrial synthesis and fatty acid oxidation to improve aerobic capacity (55–57). Research indicates (58, 59) a positive correlation between plasma HMB levels and muscle strength as well as muscle mass. Studies (60, 61) also demonstrate that oral HMB supplementation can alleviate the decline in muscle mass in the elderly. HMB may potentially serve as an effective therapeutic drug for treating sarcopenia in clinical settings, offering a promising solution for sarcopenia patients who are unable to engage in physical activity. However, the impact of HMB on sarcopenia patients remains a subject of debate, and further randomized controlled trials are needed to provide high-quality evidence.
The studies included in our analysis exhibit significant heterogeneity, and despite employing various methods to adjust the results, a high degree of heterogeneity persists. Despite our thorough investigation and the application of subgroup analyses and sensitivity analyses, unfortunately, the source of heterogeneity remains unidentified. This may be attributed to the lack of uniform diagnostic criteria for sarcopenia, as well as factors such as ethnicity, gender, nutritional supplements, intervention methods, and dosage. This inference is supported by the fact that the studies included participants from different countries, diagnosed based on specific regional standards. Additionally, there are variations in the gender distribution of study participants, with some studies including only male or female patients, and others including both genders. Furthermore, the nutritional supplement components used in each study vary, and there are differences in intervention methods and the dosage of supplemented HMB. Fortunately, the results of subgroup analyses and sensitivity analyses align with the main outcomes, leading us to believe that the impact of this heterogeneity on the primary outcomes is limited (Details can be found in Supplementary materials).
This meta-analysis has several limitations. Firstly, we included only six studies, as there is a scarcity of randomized controlled trials investigating the use of HMB or HMB-rich nutritional supplements as intervention measures for treating sarcopenia patients. To address this limitation, we incorporated various types of HMB supplements, different control group settings, and diverse sarcopenia patient populations, inevitably increasing the heterogeneity of this study. Additionally, the prevalence and diagnostic criteria for sarcopenia differ between men and women (62), underscoring the need to distinguish the impact of sarcopenia on males and females. However, among the six studies included, five recruited both male and female patients, and unfortunately, there was no differentiation between genders in the results section. Therefore, future research should continue to conduct large-scale, carefully designed randomized controlled trials to provide high-quality evidence.
5 Conclusion
Through this study, we have found that HMB or HMB-rich nutritional supplements are beneficial for muscle strength in sarcopenia patients. However, there is limited evidence demonstrating its efficacy on both muscle mass and physical performance in sarcopenia individuals. Furthermore, HMB may be a treatment option for sarcopenia patients, but larger-scale randomized controlled trials are still needed to confirm this conclusion.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
HS: Formal analysis, Writing – review & editing, Writing – original draft, Data curation. HZ: Data curation, Writing – review & editing. YG: Writing – review & editing, Data curation. SX: Writing – review & editing. WS: Writing – review & editing. XZ: Writing – review & editing. HL: Writing – review & editing. GC: Writing – review & editing. PT: Formal analysis, Investigation, Supervision, Writing – review & editing. JL: Writing – review & editing, Methodology, Supervision, Validation, Funding acquisition.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was supported by the National Nature Science Foundation of China (No. 82274549). Funding provider: JL.
Acknowledgments
Thanks to all staff involved in the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1348212/full#supplementary-material
References
1. Cruz-Jentoft, AJ, and Sayer, AA. Sarcopenia. Lancet. (2019) 393:2636–46. doi: 10.1016/S0140-6736(19)31138-9
2. Petermann-Rocha, F, Balntzi, V, Gray, SR, Lara, J, Ho, FK, Pell, JP, et al. Global prevalence of sarcopenia and severe sarcopenia: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. (2022) 13:86–99. doi: 10.1002/jcsm.12783
3. Beaudart, C, Demonceau, C, Reginster, JY, Locquet, M, Cesari, M, Cruz Jentoft, AJ, et al. Sarcopenia and health-related quality of life: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. (2023) 14:1228–43. doi: 10.1002/jcsm.13243
4. Komici, K, Dello Iacono, A, De Luca, A, Perrotta, F, Bencivenga, L, Rengo, G, et al. Adiponectin and sarcopenia: a systematic review with Meta-analysis. Front Endocrinol (Lausanne). (2021) 12:576619. doi: 10.3389/fendo.2021.576619
5. Yuan, S, and Larsson, SC. Epidemiology of sarcopenia: prevalence, risk factors, and consequences. Metabolism. (2023) 144:155533. doi: 10.1016/j.metabol.2023.155533
6. Cruz-Jentoft, AJ, Bahat, G, Bauer, J, Boirie, Y, Bruyere, O, Cederholm, T, et al. The extended group for, sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. (2019) 48:16–31. doi: 10.1093/ageing/afy169
7. Chen, LK, Woo, J, Assantachai, P, Auyeung, TW, Chou, MY, Iijima, K, et al. Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. (2020) 21:300.e2–7.e2. doi: 10.1016/j.jamda.2019.12.012
8. Shen, Y, Shi, Q, Nong, K, Li, S, Yue, J, Huang, J, et al. Exercise for sarcopenia in older people: a systematic review and network meta-analysis. J Cachexia Sarcopenia Muscle. (2023) 14:1199–211. doi: 10.1002/jcsm.13225
9. Papadopoulou, SK. Sarcopenia: a contemporary health problem among older adult populations. Nutrients. (2020) 12:1293. doi: 10.3390/nu12051293
10. Lee, SY, Lee, HJ, and Lim, JY. Effects of leucine-rich protein supplements in older adults with sarcopenia: a systematic review and meta-analysis of randomized controlled trials. Arch Gerontol Geriatr. (2022) 102:104758. doi: 10.1016/j.archger.2022.104758
11. Bear, DE, Langan, A, Dimidi, E, Wandrag, L, Harridge, SDR, Hart, N, et al. Beta-hydroxy-beta-methylbutyrate and its impact on skeletal muscle mass and physical function in clinical practice: a systematic review and meta-analysis. Am J Clin Nutr. (2019) 109:1119–32. doi: 10.1093/ajcn/nqy373
12. Kaczka, P, Michalczyk, MM, Jastrzab, R, Gawelczyk, M, and Kubicka, K. Mechanism of action and the effect of beta-hydroxy-beta-methylbutyrate (HMB) supplementation on different types of physical performance – a systematic review. J Hum Kinet. (2019) 68:211–22. doi: 10.2478/hukin-2019-0070
13. Courel-Ibanez, J, Vetrovsky, T, Dadova, K, Pallares, JG, and Steffl, M. Health benefits of beta-hydroxy-beta-methylbutyrate (HMB) supplementation in addition to physical exercise in older adults: a systematic review with Meta-analysis. Nutrients. (2019) 11:2082. doi: 10.3390/nu11092082
14. Prado, CM, Landi, F, Chew, STH, Atherton, PJ, Molinger, J, Ruck, T, et al. Advances in muscle health and nutrition: a toolkit for healthcare professionals. Clin Nutr. (2022) 41:2244–63. doi: 10.1016/j.clnu.2022.07.041
15. Kamei, Y, Hatazawa, Y, Uchitomi, R, Yoshimura, R, and Miura, S. Regulation of skeletal muscle function by amino acids. Nutrients. (2020) 12:261. doi: 10.3390/nu12010261
16. Saez de Asteasu, ML, Martinez-Velilla, N, Zambom-Ferraresi, F, Casas-Herrero, A, and Izquierdo, M. Role of physical exercise on cognitive function in healthy older adults: a systematic review of randomized clinical trials. Ageing Res Rev. (2017) 37:117–34. doi: 10.1016/j.arr.2017.05.007
17. Lazarus, NR, Izquierdo, M, Higginson, IJ, and Harridge, SDR. Exercise deficiency diseases of ageing: the primacy of exercise and muscle strengthening as first-line therapeutic agents to combat frailty. J Am Med Dir Assoc. (2018) 19:741–3. doi: 10.1016/j.jamda.2018.04.014
18. Travers, J, Romero-Ortuno, R, Bailey, J, and Cooney, MT. Delaying and reversing frailty: a systematic review of primary care interventions. Br J Gen Pract. (2019) 69:e61–9. doi: 10.3399/bjgp18X700241
19. Deutz, NEP, Pereira, SL, Hays, NP, Oliver, JS, Edens, NK, Evans, CM, et al. Effect of β-hydroxy-β-methylbutyrate (HMB) on lean body mass during 10 days of bed rest in older adults. Clin Nutr. (2013) 32:704–12. doi: 10.1016/j.clnu.2013.02.011
20. Viana, MV, Becce, F, Pantet, O, Schmidt, S, Bagnoud, G, Thaden, JJ, et al. Impact of beta-hydroxy-beta-methylbutyrate (HMB) on muscle loss and protein metabolism in critically ill patients: a RCT. Clin Nutr. (2021) 40:4878–87. doi: 10.1016/j.clnu.2021.07.018
21. Tanaka, K, Nakamura, S, and Narimatsu, H. Nutritional approach to Cancer Cachexia: a proposal for dietitians. Nutrients. (2022) 14:345. doi: 10.3390/nu14020345
22. Huang, L, Li, M, Deng, C, Qiu, J, Wang, K, Chang, M, et al. Potential therapeutic strategies for skeletal muscle atrophy. Antioxidants (Basel). (2022) 12:44. doi: 10.3390/antiox12010044
23. Shamseer, L, Moher, D, Clarke, M, Ghersi, D, Liberati, A, Petticrew, M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. (2015) 350:g7647. doi: 10.1136/bmj.g7647
24. Chen, LK, Liu, LK, Woo, J, Assantachai, P, Auyeung, TW, Bahyah, KS, et al. Sarcopenia in Asia: consensus report of the Asian working Group for Sarcopenia. J Am Med Dir Assoc. (2014) 15:95–101. doi: 10.1016/j.jamda.2013.11.025
25. Cumpston, M, Li, T, Page, MJ, Chandler, J, Welch, VA, Higgins, JP, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane handbook for systematic reviews of interventions. Cochrane Database Syst Rev. (2019) 10:ED000142. doi: 10.1002/14651858.ED000142
26. McGrath, S, Zhao, X, Steele, R, Thombs, BD, and Benedetti, AD.E.S.D. Collaboration. Estimating the sample mean and standard deviation from commonly reported quantiles in meta-analysis. Stat Methods Med Res. (2020) 29:2520–37. doi: 10.1177/0962280219889080
27. Cai, S, Zhou, J, and Pan, J. Estimating the sample mean and standard deviation from order statistics and sample size in meta-analysis. Stat Methods Med Res. (2021) 30:2701–19. doi: 10.1177/09622802211047348
28. Osuka, Y, Kojima, N, Nishihara, K, Sasai, H, Wakaba, K, Tanaka, K, et al. Beta-hydroxy-beta-methylbutyrate supplementation may not enhance additional effects of exercise on muscle quality in older women. Med Sci Sports Exerc. (2022) 54:543–50. doi: 10.1249/MSS.0000000000002836
29. Lattanzi, B, Giusto, M, Albanese, C, Mennini, G, D'Ambrosio, D, Farcomeni, A, et al. The effect of 12 weeks of beta-Hydroxy-beta-methyl-butyrate supplementation after liver transplantation: a pilot randomized controlled study. Nutrients. (2019) 11:2259. doi: 10.3390/nu11092259
30. Yang, C, Song, Y, Li, T, Chen, X, Zhou, J, Pan, Q, et al. Effects of beta-hydroxy-beta-methylbutyrate supplementation on older adults with sarcopenia: a randomized, double-blind, placebo-controlled study. J Nutr Health Aging. (2023) 27:329–39. doi: 10.1007/s12603-023-1911-1
31. Cramer, JT, Cruz-Jentoft, AJ, Landi, F, Hickson, M, Zamboni, M, Pereira, SL, et al. Impacts of high-protein Oral nutritional supplements among malnourished men and women with sarcopenia: a multicenter, randomized, double-blinded, controlled trial. J Am Med Dir Assoc. (2016) 17:1044–55. doi: 10.1016/j.jamda.2016.08.009
32. Nasimi, N, Sohrabi, Z, Dabbaghmanesh, MH, Eskandari, MH, Bedeltavana, A, Famouri, M, et al. A novel fortified dairy product and sarcopenia measures in Sarcopenic older adults: a double-blind randomized controlled trial. J Am Med Dir Assoc. (2021) 22:809–15. doi: 10.1016/j.jamda.2020.08.035
33. Malafarina, V, Uriz-Otano, F, Malafarina, C, Martinez, JA, and Zulet, MA. Effectiveness of nutritional supplementation on sarcopenia and recovery in hip fracture patients. A multi-centre randomized trial. Maturitas. (2017) 101:42–50. doi: 10.1016/j.maturitas.2017.04.010
34. Osuka, Y, Kojima, N, Sasai, H, Wakaba, K, Miyauchi, D, Tanaka, K, et al. Effects of exercise and/or beta-hydroxy-beta-methylbutyrate supplementation on muscle mass, muscle strength, and physical performance in older women with low muscle mass: a randomized, double-blind, placebo-controlled trial. Am J Clin Nutr. (2021) 114:1371–85. doi: 10.1093/ajcn/nqab176
35. Beaudart, C, McCloskey, E, Bruyere, O, Cesari, M, Rolland, Y, Rizzoli, R, et al. Sarcopenia in daily practice: assessment and management. BMC Geriatr. (2016) 16:170. doi: 10.1186/s12877-016-0349-4
36. Roberts, HC, Denison, HJ, Martin, HJ, Patel, HP, Syddall, H, Cooper, C, et al. A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach. Age Ageing. (2011) 40:423–9. doi: 10.1093/ageing/afr051
37. Celis-Morales, CA, Welsh, P, Lyall, DM, Steell, L, Petermann, F, Anderson, J, et al. Associations of grip strength with cardiovascular, respiratory, and cancer outcomes and all cause mortality: prospective cohort study of half a million UK biobank participants. BMJ. (2018) 361:k1651. doi: 10.1136/bmj.k1651
38. Ho, FKW, Celis-Morales, CA, Petermann-Rocha, F, Sillars, A, Welsh, P, Welsh, C, et al. The association of grip strength with health outcomes does not differ if grip strength is used in absolute or relative terms: a prospective cohort study. Age Ageing. (2019) 48:684–91. doi: 10.1093/ageing/afz068
39. Buckinx, F, Landi, F, Cesari, M, Fielding, RA, Visser, M, Engelke, K, et al. Pitfalls in the measurement of muscle mass: a need for a reference standard. J Cachexia Sarcopenia Muscle. (2018) 9:269–78. doi: 10.1002/jcsm.12268
40. Sbrignadello, S, Gobl, C, and Tura, A. Bioelectrical impedance analysis for the assessment of body composition in sarcopenia and type 2 diabetes. Nutrients. (2022) 14:1864. doi: 10.3390/nu14091864
41. Aleixo, GFP, Shachar, SS, Nyrop, KA, Muss, HB, Battaglini, CL, and Williams, GR. Bioelectrical impedance analysis for the assessment of sarcopenia in patients with cancer: a systematic review. Oncologist. (2020) 25:170–82. doi: 10.1634/theoncologist.2019-0600
42. Correa-de-Araujo, R, Harris-Love, MO, Miljkovic, I, Fragala, MS, Anthony, BW, and Manini, TM. The need for standardized assessment of muscle quality in skeletal muscle function deficit and other aging-related muscle dysfunctions: a symposium report. Front Physiol. (2017) 8:87. doi: 10.3389/fphys.2017.00087
43. Pavasini, R, Guralnik, J, Brown, JC, di Bari, M, Cesari, M, Landi, F, et al. Short physical performance battery and all-cause mortality: systematic review and meta-analysis. BMC Med. (2016) 14:215. doi: 10.1186/s12916-016-0763-7
44. Cubillos Arcila, DM, Dariva Machado, G, Martins, VF, Leotti, VB, Schule, R, Peyre-Tartaruga, LA, et al. Long-term progression of clinician-reported and gait performance outcomes in hereditary spastic paraplegias. Front Neurosci. (2023) 17:1226479. doi: 10.3389/fnins.2023.1226479
45. Wu, H, Xia, Y, Jiang, J, Du, H, Guo, X, Liu, X, et al. Effect of beta-hydroxy-beta-methylbutyrate supplementation on muscle loss in older adults: a systematic review and meta-analysis. Arch Gerontol Geriatr. (2015) 61:168–75. doi: 10.1016/j.archger.2015.06.020
46. Han, Z, Ji, NN, Ma, JX, Dong, Q, and Ma, XL. Effect of resistance training combined with Beta-Hydroxy-Beta-Methylbutyric acid supplements in elderly patients with sarcopenia after hip replacement. Orthop Surg. (2022) 14:704–13. doi: 10.1111/os.13208
47. Martin-Cantero, A, Reijnierse, EM, Gill, BMT, and Maier, AB. Factors influencing the efficacy of nutritional interventions on muscle mass in older adults: a systematic review and meta-analysis. Nutr Rev. (2021) 79:315–30. doi: 10.1093/nutrit/nuaa064
48. Stout, JR, Smith-Ryan, AE, Fukuda, DH, Kendall, KL, Moon, JR, Hoffman, JR, et al. Effect of calcium beta-hydroxy-beta-methylbutyrate (CaHMB) with and without resistance training in men and women 65+yrs: a randomized, double-blind pilot trial. Exp Gerontol. (2013) 48:1303–10. doi: 10.1016/j.exger.2013.08.007
49. Bandeira-Guimaraes, M, Blanco-Rambo, E, Vieira, AF, Saez de Asteasu, ML, Pinto, RS, Izquierdo, M, et al. Chronic effects of different intensities of power training on neuromuscular parameters in older people: a systematic review with meta-analysis. Sports Med Open. (2023) 9:98. doi: 10.1186/s40798-023-00646-9
50. Lopez, P, Pinto, RS, Radaelli, R, Rech, A, Grazioli, R, Izquierdo, M, et al. Benefits of resistance training in physically frail elderly: a systematic review. Aging Clin Exp Res. (2018) 30:889–99. doi: 10.1007/s40520-017-0863-z
51. Delaire, L, Courtay, A, Humblot, J, Aubertin-Leheudre, M, Mourey, F, Racine, AN, et al. Implementation and Core components of a multimodal program including exercise and nutrition in prevention and treatment of frailty in community-dwelling older adults: a narrative review. Nutrients. (2023) 15:4100. doi: 10.3390/nu15194100
52. Holecek, M. Beta-hydroxy-beta-methylbutyrate supplementation and skeletal muscle in healthy and muscle-wasting conditions. J Cachexia Sarcopenia Muscle. (2017) 8:529–41. doi: 10.1002/jcsm.12208
53. Gorji, AE, Ostaszewski, P, Urbanska, K, and Sadkowski, T. Does beta-hydroxy-beta-methylbutyrate Have any potential to support the treatment of Duchenne muscular dystrophy in humans and animals? Biomedicines. (2023) 11:2329. doi: 10.3390/biomedicines11082329
54. Tezze, C, Sandri, M, and Tessari, P. Anabolic resistance in the pathogenesis of sarcopenia in the elderly: role of nutrition and exercise in young and old people. Nutrients. (2023) 15:4073. doi: 10.3390/nu15184073
55. Wan, M, Zheng, C, Zheng, J, Duan, G, Yu, J, Zhang, P, et al. Different effects of dietary beta-hydroxy-beta-methylbutyrate on composition of fatty acid and free amino acid, and fatty metabolism in the different muscles of broilers. Poult Sci. (2023) 102:103001. doi: 10.1016/j.psj.2023.103001
56. Shefflette, A, Patel, N, and Caruso, J. Mitigating sarcopenia with diet and exercise. Int J Environ Res Public Health. (2023) 20:6652. doi: 10.3390/ijerph20176652
57. Liu, S, Zhang, L, and Li, S. Advances in nutritional supplementation for sarcopenia management. Front Nutr. (2023) 10:1189522. doi: 10.3389/fnut.2023.1189522
58. Matheson, EM, Nelson, JL, Baggs, GE, Luo, M, and Deutz, NE. Specialized oral nutritional supplement (ONS) improves handgrip strength in hospitalized, malnourished older patients with cardiovascular and pulmonary disease: a randomized clinical trial. Clin Nutr. (2021) 40:844–9. doi: 10.1016/j.clnu.2020.08.035
59. Engelen, M, and Deutz, NEP. Is beta-hydroxy beta-methylbutyrate an effective anabolic agent to improve outcome in older diseased populations? Curr Opin Clin Nutr Metab Care. (2018) 21:207–13. doi: 10.1097/MCO.0000000000000459
60. Sanz-Paris, A, Camprubi-Robles, M, Lopez-Pedrosa, JM, Pereira, SL, Rueda, R, Ballesteros-Pomar, MD, et al. Role of Oral nutritional supplements enriched with beta-hydroxy-beta-methylbutyrate in maintaining muscle function and improving clinical outcomes in various clinical settings. J Nutr Health Aging. (2018) 22:664–75. doi: 10.1007/s12603-018-0995-7
61. Cornejo-Pareja, I, Ramirez, M, Camprubi-Robles, M, Rueda, R, Vegas-Aguilar, IM, and Garcia-Almeida, JM. Effect on an Oral nutritional supplement with beta-hydroxy-beta-methylbutyrate and Vitamin D on Morphofunctional aspects, body composition, and phase angle in malnourished patients. Nutrients. (2021) 13:4355. doi: 10.3390/nu13124355
Keywords: β-hydroxy-β-methylbutyrate, HMB, sarcopenia, meta-analysis, muscle
Citation: Su H, Zhou H, Gong Y, Xiang S, Shao W, Zhao X, Ling H, Chen G, Tong P and Li J (2024) The effects of β-hydroxy-β-methylbutyrate or HMB-rich nutritional supplements on sarcopenia patients: a systematic review and meta-analysis. Front. Med. 11:1348212. doi: 10.3389/fmed.2024.1348212
Edited by:
Paolo Gallo, Campus Bio-Medico University Hospital, ItalyReviewed by:
Hubertus Axer, Jena University Hospital, GermanyEwa Tomaszewska, University of Life Sciences of Lublin, Poland
Elisa Julianti, Department of Food Science Universitas Sumatera Utara, Indonesia
Copyright © 2024 Su, Zhou, Gong, Xiang, Shao, Zhao, Ling, Chen, Tong and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peijian Tong, cGVpamlhbnRvbmd6anRjbUAxNjMuY29t; Ju Li, anVsaUB6Y211LmVkdS5jbg==
†These authors have contributed equally to this work
 Hai Su†
Hai Su† Haojing Zhou
Haojing Zhou Sicheng Xiang
Sicheng Xiang Weijie Shao
Weijie Shao Guoqian Chen
Guoqian Chen Peijian Tong
Peijian Tong