- 1Division of Pulmonary, Allergy, and Critical Care Medicine, Department of Internal Medicine, Dongguk University Gyeongju Hospital, Dongguk University College of Medicine, Gyeongju, Republic of Korea
- 2Division of Pulmonary, Allergy, and Critical Care Medicine, Department of Internal Medicine, Korea University Guro Hospital, Korea University College of Medicine, Seoul, Republic of Korea
- 3Department of Internal Medicine, Hanyang University Guri Hospital, Hanyang University College of Medicine, Seoul, Republic of Korea
- 4Division of Pulmonary Medicine and Allergy, Department of Internal Medicine, Hanyang Medical Center, Hanyang University College of Medicine, Seoul, Republic of Korea
Introduction: Obstructive sleep apnea (OSA) is frequently associated with airflow limitation (AFL). However, information on the prevalence of and factors associated with likely OSA in individuals with AFL in Korea is limited.
Methods: Data from the 2019 Korea National Health and Nutrition Examination Survey (KNHANES) were used, and 3,280 individuals (2,826 individuals without AFL and 454 individuals with AFL) were included. AFL was defined as forced expiratory volume in 1 s (FEV1)/forced vital capacity (FVC) < 0.7. A score ≥ 5 on the STOP-BANG questionnaire was used to identify individuals with likely OSA. The prevalence of likely OSA was compared between individuals with and without AFL. In addition, factors associated with likely OSA in individuals with AFL were evaluated using multivariable logistic regression analysis.
Results: Of 3,280 individuals, 13.8% had an AFL. The prevalence of likely OSA was significantly higher in individuals with AFL than in individuals without AFL (9.2% vs. 5.0%, p = 0.014). Among 454 individuals with AFL, obesity (adjusted odds ratio [aOR] = 14.78, 95% confidence interval [CI] = 4.20–52.02) was most strongly associated with likely OSA, followed by heavy alcohol consumption (aOR = 4.93, 95% CI = 1.91–12.70), hypertension (aOR = 4.92, 95% CI = 1.57–15.46), overweight (aOR = 4.71, 95% CI = 1.76–12.64), college graduate (aOR = 4.47, 95% CI = 1.10–18.22), and history of pulmonary tuberculosis (aOR = 3.40, 95% CI = 1.06–10.96).
Conclusion: In Korea, approximately 1 in 10 individuals with AFL had likely OSA. Overweight and obesity, heavy alcohol consumption, high educational level, hypertension, and history of pulmonary tuberculosis were associated with likely OSA in individuals with AFL.
Introduction
Obstructive sleep apnea (OSA) is a common sleep-related breathing disorder characterized by upper airway obstruction during sleep, leading to fragmented sleep and daytime sleepiness (1). OSA often coexists with respiratory diseases, such as bronchial asthma and chronic obstructive pulmonary disease (COPD), characterized by airflow limitation (AFL) (2, 3). In previous studies, up to 22% of patients with OSA had comorbid AFL (4).
When OSA and obstructive airway diseases coexist, sleep quality worsens, and quality of life decreases compared with either condition alone (5, 6). Patients with OSA and obstructive airway diseases also have more frequent cardiovascular diseases (7) and a higher risk of pulmonary hypertension (8), which may lead to a higher risk of hospitalization and mortality (9). Furthermore, OSA and obstructive airway diseases potentially form a vicious cycle. OSA triggers lower airway inflammation, while reduced lung volume from these airway diseases worsens OSA severity (10). Accordingly, to improve treatment outcomes, it is important to recognize the real-world prevalence of and factors associated with OSA in individuals with obstructive airway diseases. However, information regarding this subject in Korea is limited. Although this issue was evaluated in a previous study, they used regional cohort data (11), necessitating analysis using national representative information.
This study aimed to investigate the prevalence of and factors associated with likely OSA in individuals with AFL. For the current study, we used Korea National Health and Nutrition Survey (KNHANES) data, which includes spirometry results and questionnaires assessing likely OSA.
Materials and methods
Study population
We used the 2019 KNHANES, a cross-sectional nationwide survey that assesses the health and nutritional status of non-institutionalized Korean citizens. The KNHANES is designed, managed, and monitored by the Korea Disease Control and Prevention Agency (KDCA). The study participants were chosen based on a stratified multistage probability cluster sampling method. In brief, the KNHANES has collected data on demographics and personal habits, including age, sex, height, weight, and self-reported smoking history. It also includes spirometry results in participants aged ≥40 years. More detailed information regarding the KNHANES can be found in previous studies (12–16).
A total of 8,110 individuals were included in the 2019 KNHANES. After excluding 3,302 individuals aged ≤40 who did not perform spirometry, and 1,528 individuals with missing values in lung function measurements, 3,280 individuals were included in the prevalence analysis. In addition, 2,826 individuals without AFL were further excluded, leaving 454 individuals in the final analytic cohort for assessing factors associated with likely OSA in individuals with AFL (Figure 1).
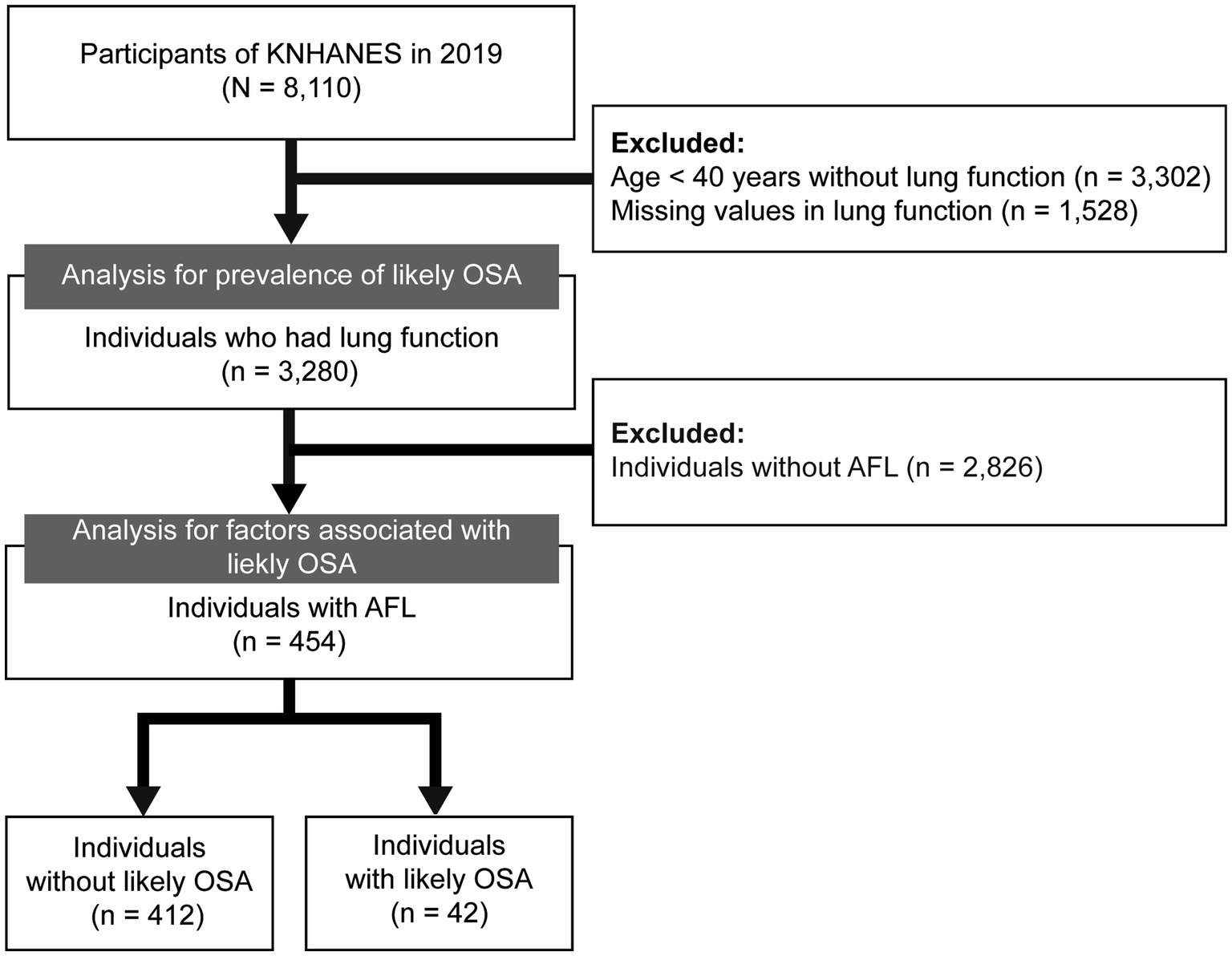
Figure 1. Flow chart of the study population. KNHANES, Korea National Health and Nutrition Examination Survey; OSA, obstructive sleep apnea; AFL, airflow limitation.
Definitions: AFL and likely OSA
Spirometry was assessed by well-trained technicians using a spirometer (Vyntus Spiro; Care Fusion, San Diego, CA, United States; or dry rolling seal spirometer Model 2,130; Sensormedics Corporation, Yorba Linda, CA, United States) following the American Thoracic Society (ATS)/European Respiratory Society (ERS) recommendations (17). Since KNHANES provides only pre-bronchodilator values, AFL was defined as the pre-bronchodilator value of forced expiratory volume in 1 s (FEV1)/forced vital capacity (FVC) < 0.7.
Likely OSA, suggesting a high risk for OSA, was assessed using a questionnaire on snoring, tiredness, observed apnea, blood pressure, body mass index (BMI), age, neck circumference, and gender (STOP-BANG). Likely OSA was defined as a STOP-BANG score ≥ 5, which is generally considered high risk. More detailed information on the STOP-BANG questionnaire was described in the previous studies (18–20).
Covariates
BMI was calculated as weight in kilograms divided by height in meters squared (kg/m2). Past smokers were defined as individuals with a history of smoking ≥5 cigarettes in their lifetime but did not currently smoke. Heavy drinking was defined as consuming >30 g/day of alcohol. Individuals who were single, separated, divorced, or widowed were considered unmarried. Income was categorized as low, intermediate, or high based on the tertiles of monthly personal income. Physical activity was determined using the Global Physical Activity Questionnaire based on intensity, frequency, and time. Depressive mood was defined as sadness for at least 2 weeks. Hypertension was defined as systolic blood pressure ≥ 140 mmHg, diastolic blood pressure ≥ 90 mmHg, use of antihypertensive drugs, or previous physician diagnosis. Diabetes mellitus was defined as fasting glucose level ≥ 126 mg/dL, use of insulin or antidiabetic drugs, or previous physician diagnosis. Dyslipidemia was defined as low-density lipoprotein cholesterol >130 mg/dL, high-density lipoprotein cholesterol <40 mg/dL, use of antidyslipidemic drugs, or previous physician diagnosis of dyslipidemia. Cardiovascular disease was defined as physician diagnosis of myocardial infarction or angina pectoralis. Educational level, respiratory symptoms (cough and sputum), sleep duration, stress, and other comorbidities (asthma, history of pulmonary tuberculosis) were assessed by self-reported questionnaires. Detailed definitions of comorbidities were described in our previous studies (21–28).
Statistical analysis
Data are expressed as weighted percentages with standard error (SE). The p-values for evaluating intergroup differences were calculated using Pearson’s chi-square test. We compared the prevalence of likely OSA in individuals with (n = 454) and without AFL (n = 2,826). The calculated power using post-hoc power analysis was 92.8% before adjusting for survey weights (29).
Multivariable logistic regression analysis was used to explore the factors associated with likely OSA in individuals with AFL. Two models were developed for multivariable analysis. Model 1 (excluding variables used to define OSA) included sociodemographic variables (educational level, income), habitual behaviors (smoking, heavy alcohol consumption), clinically significant variables (physical activity, respiratory symptoms, sleep duration), percentage of predicted FEV1 (FEV1%pred), and comorbidities (diabetes mellitus, dyslipidemia, cardiovascular disease, asthma, history of pulmonary tuberculosis). Model 2 included the variables from Model 1 and additional variables used to define OSA (age, BMI, and hypertension). Sex was not included due to the limited number of OSA cases in women. Weighting adjustments were implemented in all analyses and statistical significance was determined based on a two-sided p-value <0.05. All analyses were conducted in R version 4.3.1 (R Core Team 2023; R Foundation for Statistical Computing in Vienna, Austria).
Results
Prevalence of likely OSA in individuals with AFL
As shown in Figure 2, individuals with AFL were more likely to have likely OSA than were individuals without AFL (9.2 vs. 5.0%, p = 0.002). In subgroup analysis, the prevalence of likely OSA differed between the individuals with and without AFL, showing a pronounced gap in prevalence of likely OSA for younger individuals (9.7 vs. 2.8% for the 40–49 age group, p = 0.117), underweight individuals (8.5 vs. 0%, p = 0.014), and never-smokers (3.9 vs. 1.2%, p = 0.027). Detailed information on the prevalence of likely OSA based on subgroup (age, sex, BMI category, smoking status, and lung function) is shown in Figure 2.
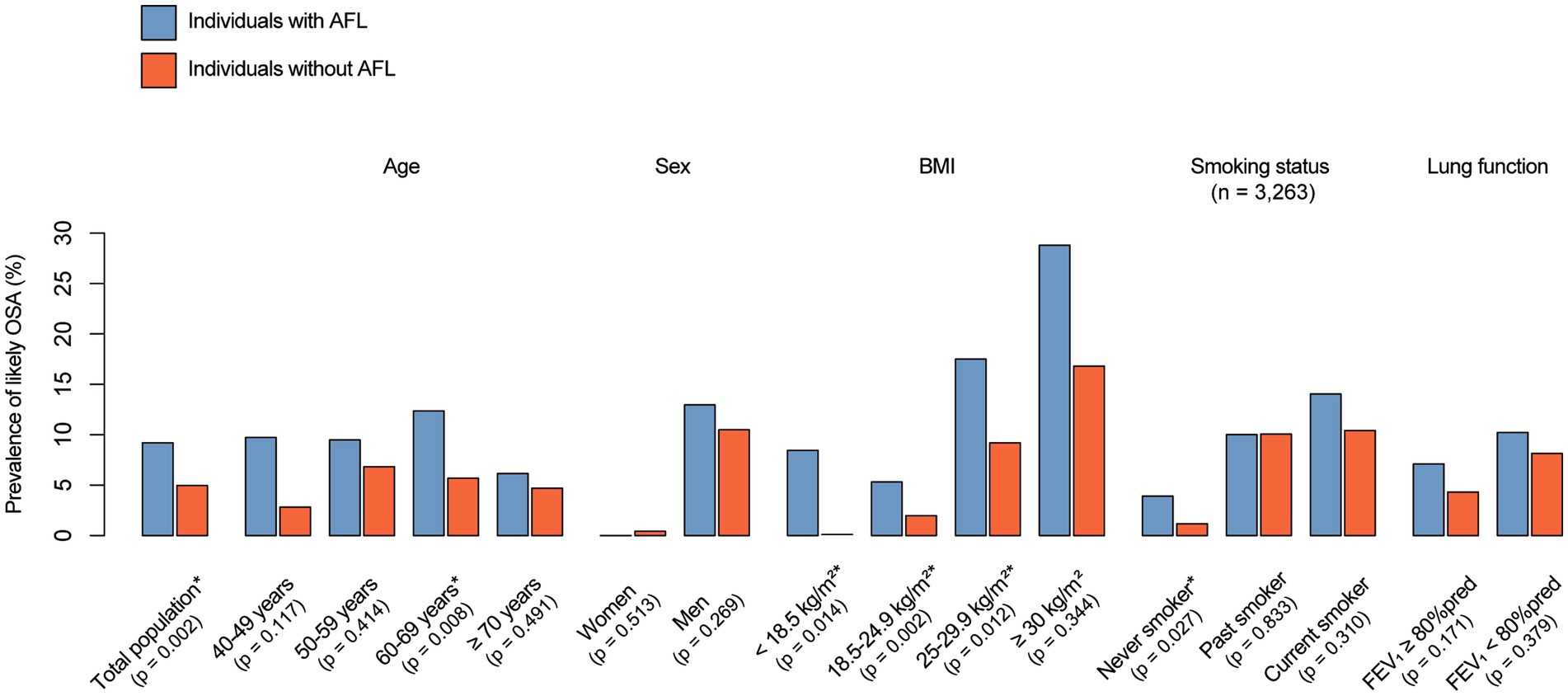
Figure 2. Prevalence of likely OSA and AFL. The p-value was calculated using a weight-adjusted chi-square test, with *indicating p < 0.05. OSA, obstructive sleep apnea; AFL, airflow limitation; BMI, body mass index.
Baseline characteristics
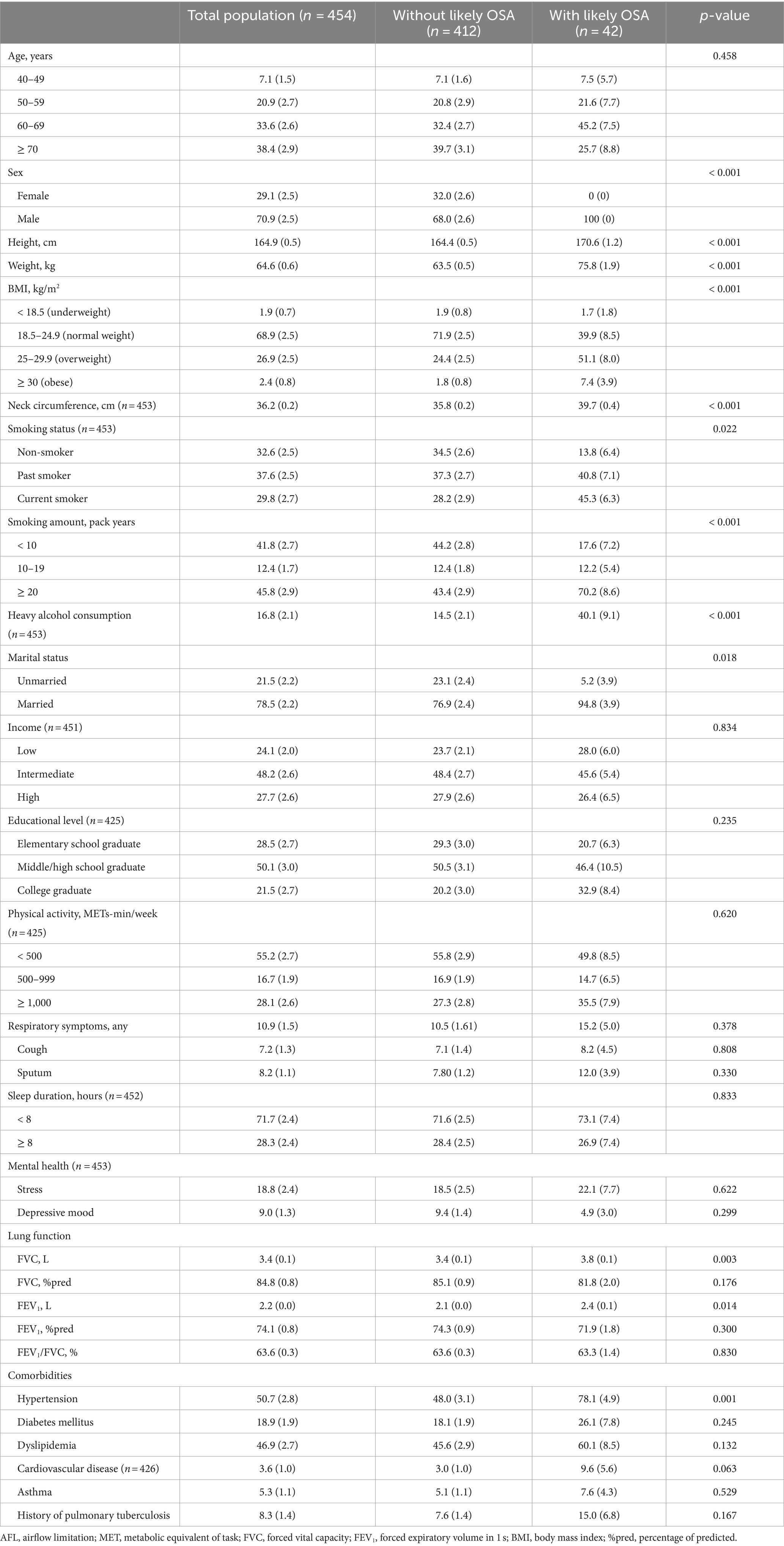
Table 1 shows the baseline characteristics of the study population. Among individuals with AFL, three-fourths were > 60 years of age, and two-thirds were male. Individuals with likely OSA were more likely to be male than those without likely OSA (100 vs. 68.0%, p < 0.001); however, age was similar between individuals with or without likely OSA. Individuals with likely OSA were taller (170.6 vs. 164.4 cm, p < 0.001), heavier (75.8 vs. 63.5 kg, p < 0.001), more likely to be overweight or obese (58.5 vs. 26.2%, p < 0.001), had a thicker neck circumference (39.7 vs. 35.8 cm, p < 0.001), had higher rate of a history of smoking (86.1 vs. 65.5%, p < 0.001), consumed more alcohol (heavy drinker, 40.1 vs. 14.5%, p < 0.001), and were more likely to be married (94.8 vs. 76.9%, p < 0.001) than individuals without likely OSA. There were no significant intergroup differences in socioeconomic status (income and education), physical activity, respiratory symptoms, sleep duration, and mental health (p > 0.05 for all variables).
Regarding lung function, individuals with likely OSA had higher FVC (3.8 vs. 3.4 L, p = 0.003) and FEV1 (2.4 vs. 2.1 L, p = 0.014) than individuals without likely OSA; however, FVC %pred, FEV1%pred, and FEV1/FVC ratio did not differ between individuals with and without likely OSA (p > 0.05 for all variables). Among comorbidities, individuals with likely OSA were more likely to have hypertension (78.1% vs. 48.0%, p = 0.001). However, there were no differences in other comorbidities (p > 0.05 for all variables).
Factors associated with likely OSA in individuals with AFL
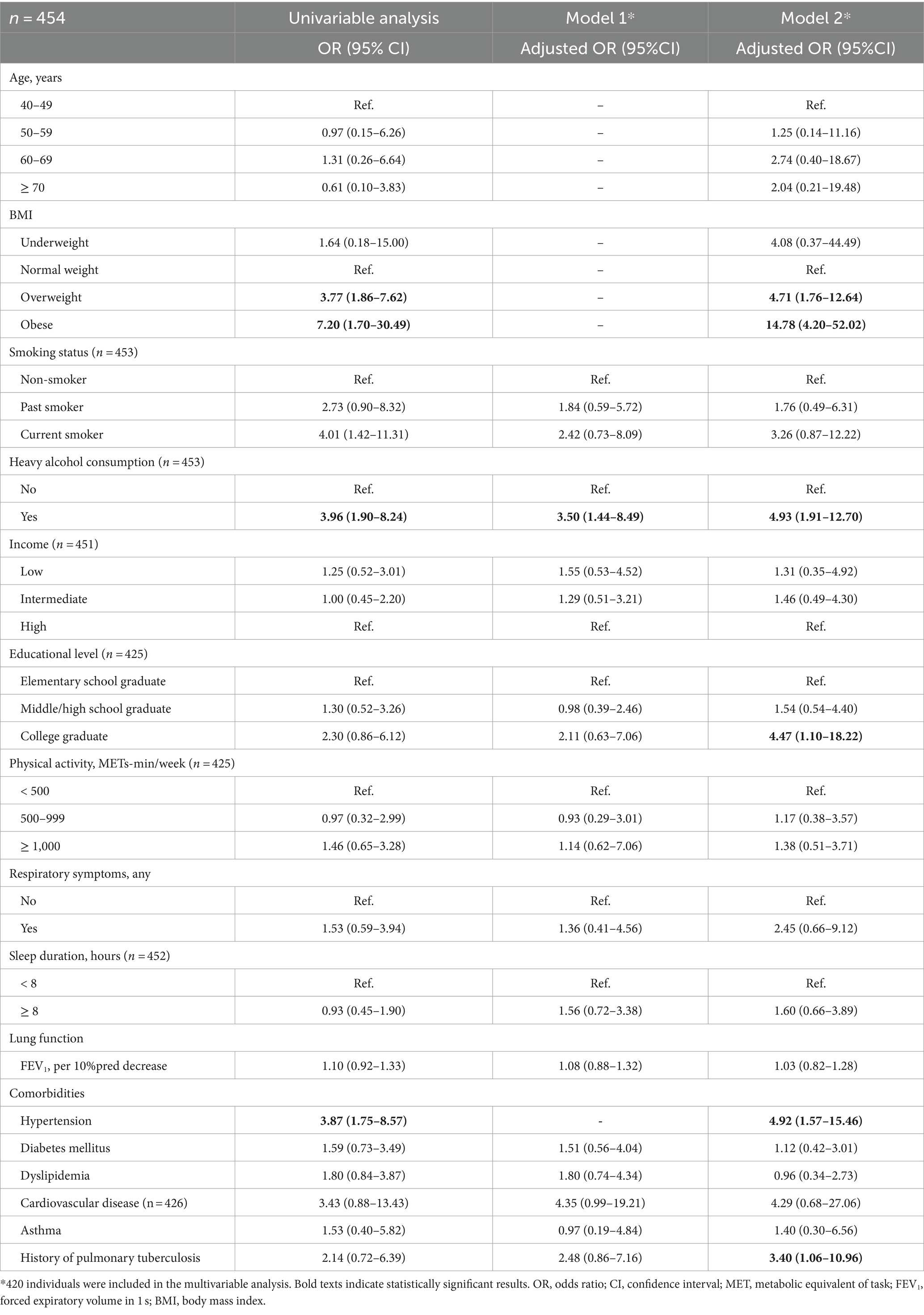
Table 2 shows the results of multivariable logistic regression analysis for factors associated with likely OSA among individuals with AFL. In model 1, only heavy alcohol consumption was associated with likely OSA (adjusted odds ratio [aOR] = 3.50, 95% confidence interval [CI] = 1.44–8.49). In Model 2, obesity was the factor most strongly associated with likely OSA (aOR = 14.78, 95% CI = 4.20–52.02). Other factors significantly associated with likely OSA were overweight (aOR = 4.71, 95% CI = 1.76–12.64), heavy alcohol consumption (aOR = 4.93, 95% CI = 1.91–12.70), college graduate (aOR = 4.47, 95% CI = 1.10–18.22), hypertension (aOR = 4.92, 95% CI = 1.57–15.46), and history of pulmonary tuberculosis (aOR = 3.40, 95% CI = 1.06–10.96).
Discussion
This nationwide, representative, cross-sectional study evaluated the prevalence of likely OSA and factors associated with likely OSA in individuals with AFL. Our study showed that approximately 1 in 10 individuals with AFL had likely OSA, which was significantly higher than in individuals without AFL. In addition, the prevalence gap of likely OSA between individuals with and without AFL was greater in younger and underweight individuals and never-smokers. Overweight or obesity, heavy alcohol consumption, high education, hypertension, and history of pulmonary tuberculosis were associated with increased odds of having likely OSA in individuals with AFL.
The prevalence of OSA in individuals with COPD varies depending on the definition of OSA and COPD. OSA is a common comorbidity in individuals with COPD, affecting approximately 60% of patients (30–32). In the present study, the prevalence of likely OSA in individuals with AFL was 9.2%, which was significantly higher than in individuals without AFL (5.0%). However, this rate was relatively lower than previously reported rates of comorbid OSA in individuals with COPD.
There are some possible explanations for this finding. First, not all individuals with AFL in this study can be classified as those with COPD based on traditional definition of COPD (21). Several other diseases and conditions can cause AFL. However, according to the recently revised GOLD definition for COPD, most individuals with AFL would be considered to have COPD (33). Moreover, there is an emerging concept advocating for more proactive strategies to diagnose COPD early, including the identification of pre-COPD. In this context, it seems acceptable that most individuals with AFL fall within the category of COPD. Second, the participants in our study were enrolled in a nationwide survey. Thus, a considerable proportion was more likely to be clinically stable, less symptomatic, and have better lung function than individuals from COPD cohorts (12, 34). The severity of COPD is associated with the risk of comorbid OSA (35). However, the strength of the present study is the use of a nationwide representative sample, and it was the first to outline the scheme on the overall burden of OSA in Koreans with AFL.
An important finding of our study is that individuals with AFL, particularly younger individuals, underweight individuals, and never-smokers, had a higher prevalence of likely OSA than individuals without AFL. This suggests that populations not typically at risk for OSA may have an increased risk if they have AFL. This observation has important implications, particularly for certain patients (i.e., pre-COPD, COPD in young people, individuals with sarcopenia, and never-smokers) who have recently attracted attention due to their potentially distinctive characteristics compared with the traditional COPD phenotype (36–38). Although the exact mechanisms are unclear, increasing evidence indicates that pathogenesis and clinical manifestations of COPD may differ in these patients compared to patients with classic smoking COPD (39). Our study suggests that comorbid OSA may be an additional potential explanation for underlying pathophysiology. Therefore, future studies on the role of OSA in these entities, such as pre-COPD, COPD in young people, sarcopenia, and never-smokers, are warranted.
We identified several factors associated with an increased risk of likely OSA in individuals with AFL. It is uncertain whether including variables used to define likely OSA, such as age, BMI, and hypertension, is necessary for the multivariable analysis. However, in the fully adjusted model that includes these variables, we identified additional factors, indicating the need to adjust these variables. Results from the fully adjusted model are similar to previous findings, showing that individuals who are overweight or obese, consume a large amount of alcohol, or have hypertension are more likely to have OSA (40–42). In addition, a history of pulmonary tuberculosis, which often results in respiratory sequelae, was associated with an increased risk of likely OSA in individuals with AFL. In alignment with the results from a previous study, lung function, as well as the quality of sleep, were worse in patients with tuberculosis compared with their non-tuberculosis family members (43). However, in contrast to previous studies, higher educational level was unexpectedly associated with an increased risk of likely OSA in the present study (44, 45), warranting further research.
It is gradually recognized that the coexistence of OSA in COPD has important clinical relevance (46). This recognition led to the emergence of the concept of COPD–OSA overlap syndrome, and several studies reported that this disease entity has been linked with a poor prognosis (7, 47). Reduced lung volume is associated with increased OSA severity, potentially due to increased upper airway collapsibility (1). Supporting this notion, patients with OSA who had reduced lung function exhibited a higher risk of death. Nevertheless, both diseases are frequently underdiagnosed (48, 49) despite available treatment options to reduce the burden of both COPD and OSA (50, 51). Therefore, it is imperative to consider strategies for early detection and effective management of both conditions.
There are some limitations in our study. First, we used the STOP-BANG questionnaire to define likely OSA rather than sleep polysomnography, which is the gold standard for diagnosing OSA (1). In addition, there has still been debate about the clinical utility of various questionnaires for screening OSA (52, 53). Nevertheless, the STOP-BANG questionnaire has been validated in several previous studies as a useful tool for identifying OSA (18, 54). Also, we expressed this condition as likely OSA, suggesting a high risk of OSA rather than a diagnosis of OSA. Second, as mentioned above, pre-bronchodilator lung function measurements allowed us to include individuals with AFL, which can mostly be classified as COPD. However, COPD is a heterogeneous condition, and our results may not represent specific COPD phenotypes or etiologies. Therefore, our findings should be generalized cautiously and interpreted from an epidemiological perspective. Third, the prevalence of OSA may be overestimated because individuals with COPD frequently exhibit symptoms similar to those found in OSA, particularly when OSA is assessed using a questionnaire. Fourth, we used only data from 1 year, which limited the number of study participants. Because KNHANES is an ongoing study, research using multiannual data will be possible in future studies. Fifth, we could not provide information when the lung function was measured. Since individuals with OSA had an evening-to-morning variability in lung function, our study results might have been influenced by this variability (55). Sixth, our results are based on the Korean population, and caution is needed when interpreting and applying the findings to populations in other countries.
In conclusion, approximately 1 in 10 individuals with AFL in Korea had likely OSA. Overweight and obesity, heavy alcohol consumption, high educational level, hypertension, and history of tuberculosis were associated with likely OSA in individuals with AFL.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found at: https://knhanes.kdca.go.kr/knhanes/main.do.
Ethics statement
The studies involving humans were approved by Institutional Review Board (IRB) of the Korea Disease Control and Prevention Agency (KDCPA) approved the protocol (IRB No. 2018-01-03-C-A). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
SHK: Data curation, Formal analysis, Visualization, Writing – original draft. JKS: Writing – original draft. JYC: Writing – original draft. J-YM: Writing – review & editing. HL: Supervision, Validation, Writing – review & editing. KHM: Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Jordan, AS, McSharry, DG, and Malhotra, A. Adult obstructive sleep apnoea. Lancet. (2014) 383:736–47. doi: 10.1016/S0140-6736(13)60734-5
2. Bonsignore, MR, Baiamonte, P, Mazzuca, E, Castrogiovanni, A, and Marrone, O. Obstructive sleep apnea and comorbidities: a dangerous liaison. Multidiscip Respir Med. (2019) 14:8. doi: 10.1186/s40248-019-0172-9
3. Bouloukaki, I, Fanaridis, M, Testelmans, D, Pataka, A, and Schiza, S. Overlaps between obstructive sleep apnoea and other respiratory diseases, including COPD, asthma and interstitial lung disease. Breathe (Sheff). (2022) 18:220073. doi: 10.1183/20734735.0073-2022
4. Sanders, MH, Newman, AB, Haggerty, CL, Redline, S, Lebowitz, M, Samet, J, et al. Sleep and sleep-disordered breathing in adults with predominantly mild obstructive airway disease. Am J Respir Crit Care Med. (2003) 167:7–14. doi: 10.1164/rccm.2203046
5. Ekici, A, Ekici, M, Kurtipek, E, Keles, H, Kara, T, Tunckol, M, et al. Association of asthma-related symptoms with snoring and apnea and effect on health-related quality of life. Chest. (2005) 128:3358–63. doi: 10.1378/chest.128.5.3358
6. Adler, D, Bailly, S, Benmerad, M, Joyeux-Faure, M, Jullian-Desayes, I, Soccal, PM, et al. Clinical presentation and comorbidities of obstructive sleep apnea-COPD overlap syndrome. PLoS One. (2020) 15:e0235331. doi: 10.1371/journal.pone.0235331
7. Shah, AJ, Quek, E, Alqahtani, JS, Hurst, JR, and Mandal, S. Cardiovascular outcomes in patients with COPD-OSA overlap syndrome: a systematic review and meta-analysis. Sleep Med Rev. (2022) 63:101627. doi: 10.1016/j.smrv.2022.101627
8. Shawon, MSR, Perret, JL, Senaratna, CV, Lodge, C, Hamilton, GS, and Dharmage, SC. Current evidence on prevalence and clinical outcomes of co-morbid obstructive sleep apnea and chronic obstructive pulmonary disease: a systematic review. Sleep Med Rev. (2017) 32:58–68. doi: 10.1016/j.smrv.2016.02.007
9. Akinnusi, M, El-Masri, AR, Lawson, Y, and El-Solh, AA. Association of overlap syndrome with incident atrial fibrillation. Intern Emerg Med. (2021) 16:633–42. doi: 10.1007/s11739-020-02469-y
10. Bikov, A, Losonczy, G, and Kunos, L. Role of lung volume and airway inflammation in obstructive sleep apnea. Respir Investig. (2017) 55:326–33. doi: 10.1016/j.resinv.2017.08.009
11. Choi, KM, Thomas, RJ, Kim, J, Lee, SK, Yoon, DW, and Shin, C. Overlap syndrome of COPD and OSA in Koreans. Medicine (Baltimore). (2017) 96:e7241. doi: 10.1097/MD.0000000000007241
12. Kim, SH, Lee, H, Kim, Y, Rhee, CK, Min, KH, Hwang, YI, et al. Recent prevalence of and factors associated with chronic obstructive pulmonary disease in a rapidly aging society: Korea National Health and nutrition examination survey 2015-2019. J Korean Med Sci. (2023) 38:e108. doi: 10.3346/jkms.2023.38.e108
13. Kim, SH, Lee, H, and Kim, Y. Health-related quality of life after pulmonary tuberculosis in South Korea: analysis from the Korea National Health and nutrition examination survey between 2010 and 2018. Health Qual Life Outcomes. (2021) 19:195. doi: 10.1186/s12955-021-01833-6
14. Kim, SH, Park, HY, Jung, H, Zo, S, Kim, S, Park, DW, et al. Trends and factors associated with influenza vaccination in subjects with asthma: analysis of the Korea National Health and nutrition examination survey between 2010 and 2019. Ther Adv Chronic Dis. (2022) 13:204062232211239. doi: 10.1177/20406223221123979
15. Lee, DH, Yang, B, Gu, S, Kim, EG, Kim, Y, Kang, HK, et al. Influenza vaccination trend and related factors among patients with diabetes in Korea: analysis using a nationwide database. Front Endocrinol (Lausanne). (2023) 14:1077846. doi: 10.3389/fendo.2023.1077846
16. Kim, SH, Gu, S, Kim, JA, Im, Y, Cho, JY, Kim, Y, et al. Association between Oral health and airflow limitation: analysis using a Nationwide survey in Korea. J Korean Med Sci. (2023) 38:e241. doi: 10.3346/jkms.2023.38.e241
17. Graham, BL, Steenbruggen, I, Miller, MR, Barjaktarevic, IZ, Cooper, BG, Hall, GL, et al. Standardization of spirometry 2019 update. An official American Thoracic Society and European Respiratory Society technical statement. Am J Respir Crit Care Med. (2019) 200:e70–88. doi: 10.1164/rccm.201908-1590ST
18. Chung, F, Subramanyam, R, Liao, P, Sasaki, E, Shapiro, C, and Sun, Y. High STOP-Bang score indicates a high probability of obstructive sleep apnoea. Br J Anaesth. (2012) 108:768–75. doi: 10.1093/bja/aes022
19. Nagappa, M, Liao, P, Wong, J, Auckley, D, Ramachandran, SK, Memtsoudis, S, et al. Validation of the STOP-Bang questionnaire as a screening tool for obstructive sleep apnea among different populations: a systematic review and Meta-analysis. PLoS One. (2015) 10:e0143697. doi: 10.1371/journal.pone.0143697
20. Chung, F, Abdullah, HR, and Liao, P. STOP-Bang questionnaire: a practical approach to screen for obstructive sleep apnea. Chest. (2016) 149:631–8. doi: 10.1378/chest.15-0903
21. Yang, B, Choi, H, Lim, JH, Park, HY, Kang, D, Cho, J, et al. The disease burden of bronchiectasis in comparison with chronic obstructive pulmonary disease: a national database study in Korea. Ann Transl Med. (2019) 7:770. doi: 10.21037/atm.2019.11.55
22. Yang, B, Jang, HJ, Chung, SJ, Yoo, SJ, Kim, T, Kim, SH, et al. Factors associated with bronchiectasis in Korea: a national database study. Ann Transl Med. (2020) 8:1350. doi: 10.21037/atm-20-4873
23. Chung, SJ, Kim, HI, Yang, B, Kim, T, Sim, YS, Kang, HK, et al. Impact of the severity of restrictive spirometric pattern on nutrition, physical activity, and quality of life: results from a nationally representative database. Sci Rep. (2020) 10:19672. doi: 10.1038/s41598-020-76777-w
24. Kang, N, Shin, SH, Gu, S, Kang, D, Cho, J, Jeong, HJ, et al. The impact of low forced vital capacity on behavior restrictions in a population with airflow obstruction. J Thorac Dis. (2019) 11:1316–24. doi: 10.21037/jtd.2019.03.77
25. Kim, Y, Lee, H, Park, Y, Chung, SJ, Yeo, Y, Park, TS, et al. Additive effect of obesity and dyslipidemia on wheezing in Korean adults: a Nationwide representative survey study. Allergy, Asthma Immunol Res. (2021) 13:808–16. doi: 10.4168/aair.2021.13.5.808
26. Lee, H, Shin, SH, Gu, S, Zhao, D, Kang, D, Joi, YR, et al. Racial differences in comorbidity profile among patients with chronic obstructive pulmonary disease. BMC Med. (2018) 16:178. doi: 10.1186/s12916-018-1159-7
27. Shin, SH, Park, J, Cho, J, Sin, DD, Lee, H, and Park, HY. Severity of airflow obstruction and work loss in a Nationwide population of working age. Sci Rep. (2018) 8:9674. doi: 10.1038/s41598-018-27999-6
28. Yang, B, Choi, H, Shin, SH, Kim, Y, Moon, JY, Park, HY, et al. Association of Ventilatory Disorders with Respiratory symptoms, physical activity, and quality of life in subjects with prior tuberculosis: a National Database Study in Korea. J Pers Med. (2021) 11:678. doi: 10.3390/jpm11070678
30. Machado, MC, Vollmer, WM, Togeiro, SM, Bilderback, AL, Oliveira, MV, Leitão, FS, et al. CPAP and survival in moderate-to-severe obstructive sleep apnoea syndrome and hypoxaemic COPD. Eur Respir J. (2010) 35:132–7. doi: 10.1183/09031936.00192008
31. Soler, X, Gaio, E, Powell, FL, Ramsdell, JW, Loredo, JS, Malhotra, A, et al. High prevalence of obstructive sleep apnea in patients with moderate to severe chronic obstructive pulmonary disease. Ann Am Thorac Soc. (2015) 12:1219–25. doi: 10.1513/AnnalsATS.201407-336OC
32. Turcani, P, Skrickova, J, Pavlik, T, Janousova, E, and Orban, M. The prevalence of obstructive sleep apnea in patients hospitalized for COPD exacerbation. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. (2015) 159:422–8. doi: 10.5507/bp.2014.002
33. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for Prevention, Diagnosis and Management of COPD. (2023). Available at: http://goldcopd.org/2023-gold-report-2. Updated 2023. Assessed October 04, 2023.
34. Lee, JY, Chon, GR, Rhee, CK, Kim, DK, Yoon, HK, Lee, JH, et al. Characteristics of patients with chronic obstructive pulmonary disease at the first visit to a pulmonary medical Center in Korea: the KOrea COpd subgroup study team cohort. J Korean Med Sci. (2016) 31:553–60. doi: 10.3346/jkms.2016.31.4.553
35. Owens, RL, Macrea, MM, and Teodorescu, M. The overlaps of asthma or COPD with OSA: a focused review. Respirology. (2017) 22:1073–83. doi: 10.1111/resp.13107
36. Kim, SH, Lee, H, Joo, H, Choi, H, Sim, YS, Rhee, CK, et al. Risk of rapid lung function decline in young adults with chronic obstructive pulmonary disease: a community-based prospective cohort study. J Korean Med Sci. (2023) 38:e3. doi: 10.3346/jkms.2023.38.e3
37. Park, HY, Kang, D, Shin, SH, Yoo, KH, Rhee, CK, Suh, GY, et al. Chronic obstructive pulmonary disease and lung cancer incidence in never smokers: a cohort study. Thorax. (2020) 75:506–9. doi: 10.1136/thoraxjnl-2019-213732
38. Benz, E, Trajanoska, K, Lahousse, L, Schoufour, JD, Terzikhan, N, De Roos, E, et al. Sarcopenia in COPD: a systematic review and meta-analysis. Eur Respir Rev. (2019) 28:190049. doi: 10.1183/16000617.0049-2019
39. Stolz, D, Mkorombindo, T, Schumann, DM, Agusti, A, Ash, SY, Bafadhel, M, et al. Towards the elimination of chronic obstructive pulmonary disease: a lancet commission. Lancet. (2022) 400:921–72. doi: 10.1016/S0140-6736(22)01273-9
40. Romero-Corral, A, Caples, SM, Lopez-Jimenez, F, and Somers, VK. Interactions between obesity and obstructive sleep apnea: implications for treatment. Chest. (2010) 137:711–9. doi: 10.1378/chest.09-0360
41. Simou, E, Britton, J, and Leonardi-Bee, J. Alcohol and the risk of sleep apnoea: a systematic review and meta-analysis. Sleep Med. (2018) 42:38–46. doi: 10.1016/j.sleep.2017.12.005
42. Konecny, T, Kara, T, and Somers, VK. Obstructive sleep apnea and hypertension: an update. Hypertension. (2014) 63:203–9. doi: 10.1161/HYPERTENSIONAHA.113.00613
43. Itagi, ABH, Dipankar, SP, Krishna Veni, D, and Yunus, GY. Evaluation of Spirometric measures and quality of sleep in tuberculosis patients and their non-tuberculosis family caregivers. Cureus. (2021) 13:e17788. doi: 10.7759/cureus.17788
44. Song, L, Li, H, Wang, J, Xie, J, Chen, G, Liang, T, et al. Educational attainment could be a protective factor against obstructive sleep apnea: a study based on Mendelian randomization. J Thorac Dis. (2022) 14:210–5. doi: 10.21037/jtd-21-945
45. Adams, RJ, Piantadosi, C, Appleton, SL, Hill, CL, Visvanathan, R, Wilson, DH, et al. Investigating obstructive sleep apnoea: will the health system have the capacity to cope? A population study. Aust Health Rev. (2012) 36:424–9. doi: 10.1071/AH11098
46. McNicholas, WT . COPD-OSA overlap syndrome: evolving evidence regarding epidemiology, clinical consequences, and management. Chest. (2017) 152:1318–26. doi: 10.1016/j.chest.2017.04.160
47. Wang, Y, Li, B, Li, P, Gong, T, Wu, M, Fu, J, et al. Severe obstructive sleep apnea in patients with chronic obstructive pulmonary disease is associated with an increased prevalence of mild cognitive impairment. Sleep Med. (2020) 75:522–30. doi: 10.1016/j.sleep.2020.05.002
48. Yoo, KH, Kim, YS, Sheen, SS, Park, JH, Hwang, YI, Kim, SH, et al. Prevalence of chronic obstructive pulmonary disease in Korea: the fourth Korean National Health and nutrition examination survey, 2008. Respirology. (2011) 16:659–65. doi: 10.1111/j.1440-1843.2011.01951.x
49. The Lancet Respiratory Medicine. Time to wake the giant of obstructive sleep apnoea. Lancet Respir Med. (2020) 8:1. doi: 10.1016/S2213-2600(19)30449-7
50. Sterling, KL, Pépin, JL, Linde-Zwirble, W, Chen, J, Benjafield, AV, Cistulli, PA, et al. Impact of positive airway pressure therapy adherence on outcomes in patients with obstructive sleep apnea and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. (2022) 206:197–205. doi: 10.1164/rccm.202109-2035OC
51. Marin, JM, Soriano, JB, Carrizo, SJ, Boldova, A, and Celli, BR. Outcomes in patients with chronic obstructive pulmonary disease and obstructive sleep apnea: the overlap syndrome. Am J Respir Crit Care Med. (2010) 182:325–31. doi: 10.1164/rccm.200912-1869OC
52. Jonas, DE, Amick, HR, Feltner, C, Weber, RP, Arvanitis, M, Stine, A, et al. Screening for obstructive sleep apnea in adults: evidence report and systematic review for the US preventive services task force. JAMA. (2017) 317:415–33. doi: 10.1001/jama.2016.19635
53. Kapur, VK, Auckley, DH, Chowdhuri, S, Kuhlmann, DC, Mehra, R, Ramar, K, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of sleep medicine clinical practice guideline. J Clin Sleep Med. (2017) 13:479–504. doi: 10.5664/jcsm.6506
54. Pivetta, B, Chen, L, Nagappa, M, Saripella, A, Waseem, R, Englesakis, M, et al. Use and performance of the STOP-Bang questionnaire for obstructive sleep apnea screening across geographic regions: a systematic review and Meta-analysis. JAMA Netw Open. (2021) 4:e211009. doi: 10.1001/jamanetworkopen.2021.1009
Keywords: sleep apnea, obstructive, pulmonary disease, chronic obstructive, epidemiology, prevalence, airflow obstruction
Citation: Kim SH, Sim JK, Choi JY, Moon J-Y, Lee H and Min KH (2024) Prevalence of and factors associated with likely obstructive sleep apnea in individuals with airflow limitation. Front. Med. 11:1343372. doi: 10.3389/fmed.2024.1343372
Edited by:
Bumhee Yang, Chungbuk National University Hospital, Republic of KoreaReviewed by:
András Bikov, The University of Manchester, United KingdomAkiko Sano, Kindai University, Japan
Sun-Hyung Kim, Chungbuk National University Hospital, Republic of Korea
Copyright © 2024 Kim, Sim, Choi, Moon, Lee and Min. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hyun Lee, bmFtdWhhbmF5ZXlvQG5hdmVyLmNvbQ==; Kyung Hoon Min, bWlua3l1bmdob29uQGtvcmVhLmFjLmty
†These authors have contributed equally to this work and share first authorship
 Sang Hyuk Kim
Sang Hyuk Kim Jae Kyeom Sim
Jae Kyeom Sim Jee Yea Choi
Jee Yea Choi Ji-Yong Moon
Ji-Yong Moon Hyun Lee
Hyun Lee Kyung Hoon Min
Kyung Hoon Min