
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 27 March 2024
Sec. Intensive Care Medicine and Anesthesiology
Volume 11 - 2024 | https://doi.org/10.3389/fmed.2024.1342752
This article is part of the Research Topic Insights in Intensive Care Medicine and Anesthesiology: 2023 View all 22 articles
 Sebastian Bratke1
Sebastian Bratke1 Sebastian Schmid1
Sebastian Schmid1 Bernhard Ulm1,2
Bernhard Ulm1,2 Bettina Jungwirth1
Bettina Jungwirth1 Manfred Blobner1,2
Manfred Blobner1,2 Laura Borgstedt2*
Laura Borgstedt2*Background: The prevalence of neurodegenerative diseases is increasing as is life expectancy with Alzheimer’s disease accounting for two-thirds of dementia cases globally. Whether general anesthesia and surgery worsen cognitive decline is still a matter of debate and most likely depending on the interplay of various influencing factors. In order to account for this complexity, Alzheimer’s disease animal models have been developed. The Tg2576 model of Alzheimer’s disease is a well-established mouse model exhibiting amyloidopathy and age-dependent sex-specific differences in Alzheimer’s disease symptomology. Yet, data on anesthesia in this mouse model is scarce and a systematic comparison of vital parameters during anesthesia with wild-type animals is missing. In order to investigate the safety of general anesthesia and changes in vital parameters during general anesthesia in Tg2576 mice, we did a secondary analysis of vital parameters collected during general anesthesia in aged Tg2576 mice.
Methods: After governmental approval (General Administration of the Free State of Bavaria, file number: 55.2-1-54-2532-149-11) 60 mice at 10-12 months of age were exposed to isoflurane (1.6 Vol%) for 120 min, data of 58 mice was analyzed. During general anesthesia, heart rate, respiratory rate, temperature, isoflurane concentration and fraction of inspired oxygen were monitored and collected. Data were analyzed using univariate and multivariate linear mixed regression models.
Results: During general anesthesia, heart rate decreased in a sex-specific manner. Respiratory rate decreased and body temperature increased dependent on genotype. However, the changes were limited and all vital parameters stayed within physiological limits.
Conclusion: Isoflurane anesthesia in the Tg2576 mouse model is safe and does not seem to influence experimental results by interacting with vital parameters. The present study provides information on appropriate anesthesia in order to advance research on anesthesia and AD and could contribute to improving laboratory animal welfare.
According to the World Health Organization (WHO) 55 million people worldwide are currently living with dementia, with Alzheimer’s disease (AD) being the underlying cause in 60–80% of all cases. This number of AD patients is projected to increase to 139 million people by 2050 accounting for 1.6 trillion USD in healthcare costs by 2050 (1, 2).
With an increase in life expectancy as well as medical progress, patients with a preexisting cognitive impairment or even a diagnosis of AD might require surgery and thus either general or regional anesthesia. When it comes to general anesthesia (GA), it is still unclear whether general anesthetics aggravate a preexisting cognitive dysfunction or accelerate cognitive decline (3, 4). Investigating the effect of general anesthesia on AD pathology in humans is impeded by confounders such as surgical trauma, comorbidities, preoperative fasting and post-operative complications (5).
To account for these limitations and to advance research on AD pathology, diagnosis and therapy, various AD mouse models have been developed (6). Mice have a complex nervous system with notable homologies to humans in anatomy (7) and function in terms of learning, memory and behavior (8). There are numerous transgenic, knock-in, injection, and neuroinflammation based AD mouse models, representing amyloidopathy, tauopathy or both (6). In our study, the Tg2576 model was used. It overexpresses the human amyloid precursor gene with the KM670/671NL (so called “Swedish mutation”) under a viral hamster prion promotor and was first described by Hsiao et al. (9). These animals show first symptoms of cognitive decline at the age of 10 months, with sex specific differences at the age of 12 months, along with amyloid plaques and microglial activation in the neocortex and hippocampus (10, 11).
The monitoring of vital parameters is a standard procedure and ensures adequate and safe anesthesia by detecting and thus preventing hypotension, subsequent cerebral hypoperfusion or hypoxia which can lead to an unfavorable cognitive outcome (12). Although the Tg2576 mouse model has been used for many years and has often been subjected to anesthesia in this context, the authors are not aware of any publications regarding the associated changes in vital parameters (13, 14). In some cases, very limited monitoring was carried out (15, 16). Furthermore, despite the frequent use of isoflurane for general anesthesia in Tg2576 mice, literature on the effects of 120 min of general anesthesia on this mouse model is scarce (15, 17). To our knowledge, a direct comparison between the vital signs of anesthetized wild-type animals and Tg2576 has not yet been carried out systematically. In a previously published study, our group investigated the influence of isoflurane anesthesia on neurocognition, behavior and amyloidopathy in 10 months old Tg2576 mice with respect to sex. Typical symptoms of early stage AD with corresponding histopathological alterations were found, whereas relevant sex-specific differences or an influence of isoflurane on AD symptomology and pathology could not be detected (18). We retrospectively assessed vital parameters during general anesthesia in order to address whether isoflurane affects heart rate, respiratory rate and body temperature dependent on transgenic status or sex. Data were obtained during isoflurane anesthesia in Tg2576 and wild type mice randomized to intervention during the above-mentioned study. Concerning laboratory animal welfare, another aim of this publication was to evaluate the safety and physiological changes of Tg2576 under general isoflurane anesthesia (19).
This study was carried out in strict accordance with the recommendations of the Federation of European Laboratory Animal Science Associations (FELASA). The following experimental procedures on animals were approved by the Governmental Animal Care Committee (Regierung von Oberbayern, Maximilianstr. 39, 80538 Munich, Germany, Chair: Dr. B. Wirrer, Registration number: 55.2-1-54-2532-67-2016, July 28th, 2016). All efforts were made to minimize suffering. Animal welfare was assessed daily.
We used the B6; SJL-Tg (APPSWE) 2576Kha mouse model of AD, also referred to as Tg2576. With the approval of Taconic (Taconic Europe, Lille Skensved, Denmark), male Tg2576 mice were crossed with female C57B6/SJL mice (The Jackson Laboratory, Bar Harbor, ME, USA) in a separate breeding facility. The genotype was confirmed by PCR, using DNA from tail tissues (Charles River Laboratories, Sulzfeld, Germany). Mice homozygous for the rd1-mutation were excluded from the analysis as these mice are blind. At least 14 days prior to anesthesia, cognitive and behavioral testing, mice were transferred to a test facility for acclimatization. Mice were housed under standard laboratory conditions (specific pathogen free environment, 12 h light/12 h dark cycle, 22°C, 60% humidity and free access to water and standard mouse chow) (11, 18).
Mice were randomly assigned to the experimental groups regarding isoflurane anesthesia or sham procedure using a computer-generated randomization list (18).
For induction of general anesthesia mice were placed in an acrylic glass chamber that had been pre-flushed with 4.5 Vol% isoflurane (Isofluran Baxter vet, Deerfield, IL, USA; Vaporizer: Draeger, Lübeck, Germany) and 50% oxygen. After loss of postural reflexes, mice were placed in sternal recumbency on a warming pad. General anesthesia was maintained for 120 min with 1.6 Vol% isoflurane (MAC 1.0) and a fraction of inspired oxygen of 50% (FiO2 0.5) administered via a nose chamber. Mice breathed spontaneously with an applied positive end-expiratory pressure of 5 mbar. Anesthetic depth was monitored every 15 min using the tail clamp test (20). Eye lubricant (Bepanthen®, Bayer Vital GmbH, 51368 Leverkusen, Germany) was applied and animals were individually covered with compresses (Vliwasoft®, Lohmann & Rauscher GmbH & Co. KG, 56567 Neuwied, Germany).
After 120 min mice were placed in the acrylic glass chamber again with 50% oxygen now without isoflurane until full recovery from anesthesia. Afterward the animals were weighed and placed in single cages. During general anesthesia respiratory rate, heart rate (both via subcutaneous electrocardiogram), gas concentrations, and rectal temperature were measured (Datex Ohmeda S/5 Anesthesia Monitor F-CM1-05 with MNESTPR Modul, Datex-Ohmeda GmbH, Duisburg, Germany).
All analyses were conducted with RStudio 2023.09.1 (RStudio, Boston, MA, USA) running R version 4.3.1 (R Foundation for Statistical Computing, Vienna, Austria).
Categorical values are presented as absolute and relative numbers, continuous variables with median and interquartile range (IQR). For group comparisons Mann-Whitey U-tests and Kruskal-Wallis tests with Bonferroni’s corrected post-hoc tests were used. To assess changes in vital parameters during the time of anesthesia in combination with the possibly influencing factors sex and genotype, linear mixed regression models were calculated. An alpha of 5% was seen as significant.
A total of 60 mice (median weight 28.1 g) underwent general anesthesia for 120 min (Table 1). Incomplete recordings in two mice due to a technical failure at the start of the experiments led to the exclusion of these animals from further analyses concerning general anesthesia. Therefore 58 animals in total with a median age of 10.5 months were analyzed, of which 27 (46.6%) were male. Genotype was equally distributed (Table 1).
The median heart rate was 466 (434–499) beats per minute (bpm) in all animals during general anesthesia, without significant sex- or genotype-specific differences in univariate analyses (Table 2). Anesthesia time [min], had a significant influence on heart rate [−0.26 (−0.38 to −0.13); p < 0.001, Table 3] as heart rate decreased over time in univariate analyses (Figures 1A, B).
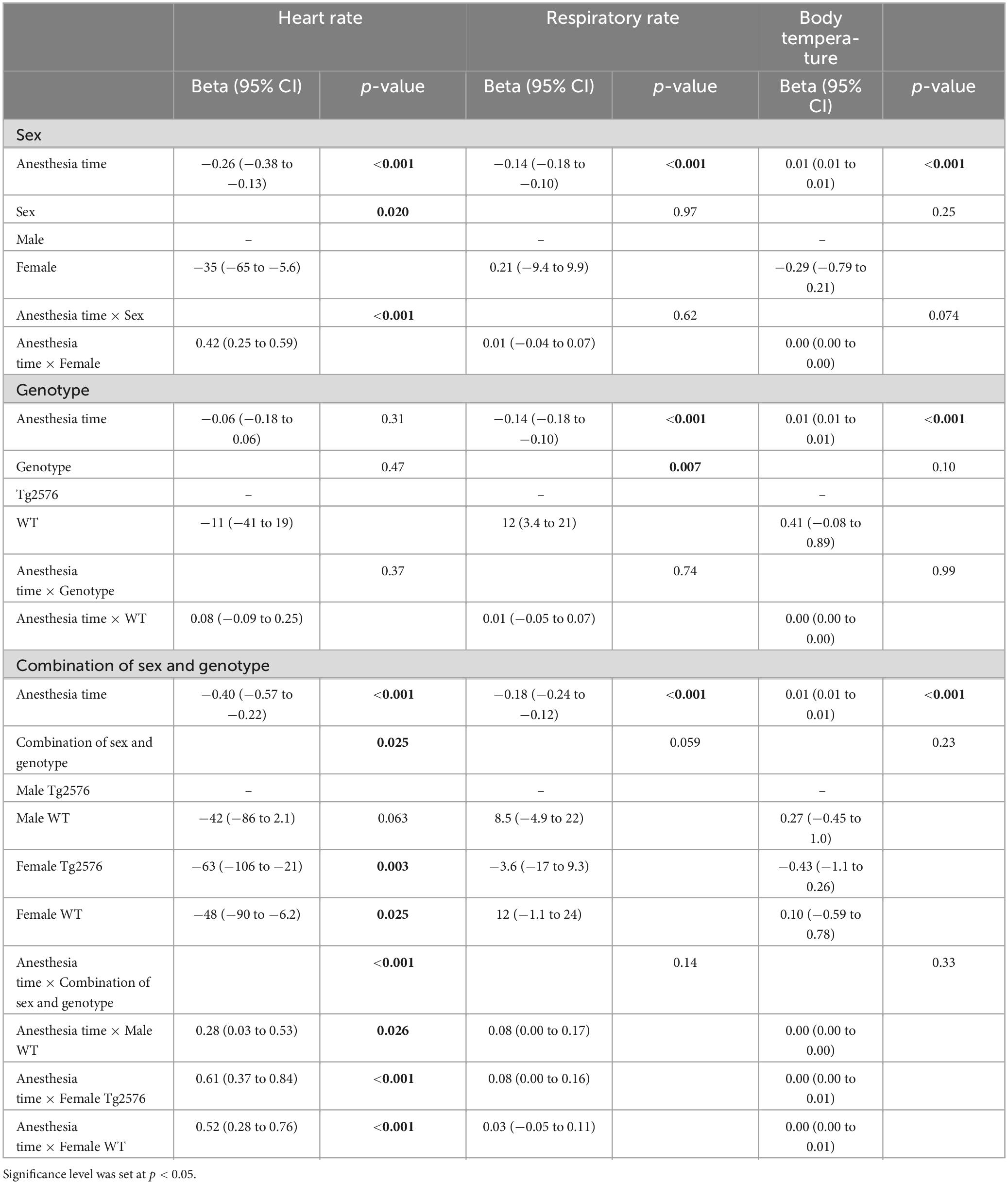
Table 3. Multivariate linear mixed regression models with variables time, sex, genotype and combination of sex and genotype for heart rate, respiratory rate and body temperature.
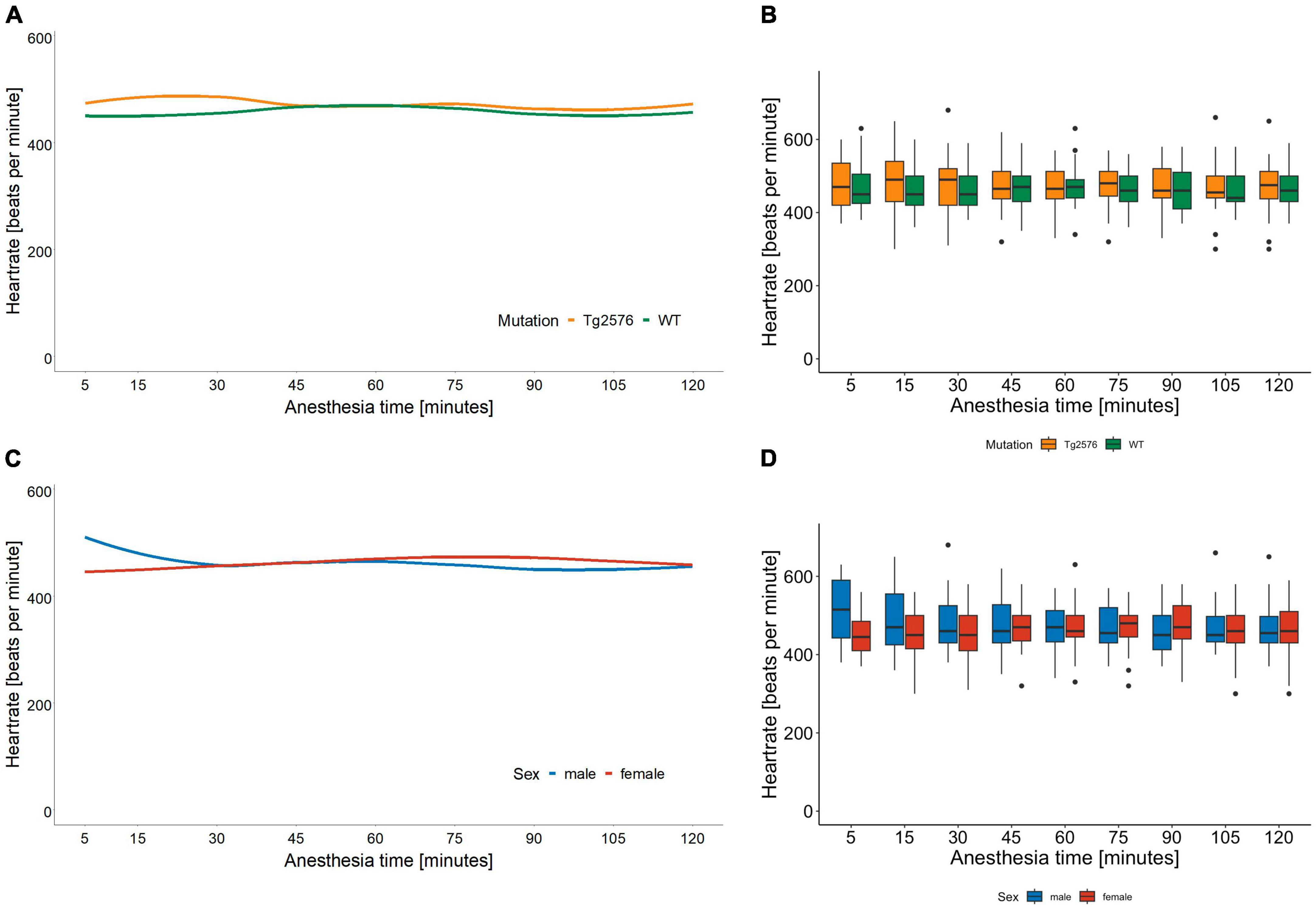
Figure 1. Heart rate in beats per minute and anesthesia time in minutes sorted by genotype (A) and corresponding boxplots over a 15 min interval (B); median (horizontal lines), interquartile range (box) and range (whiskers), dots represent outliers at least 1.5 times of the interquartile range (IQR). The heart rate of Tg2576 mice and WT mice did not show significant differences. Heart rate in beats per minute and anesthesia time in minutes sorted by sex (C) and corresponding boxplots over a 15 min interval (D); median (horizontal lines), interquartile range (box) and range (whiskers), dots represent outliers at least 1.5 times of the interquartile range (IQR). The heart rate of female animals was lower at the beginning of anesthesia and increased over the course of anesthesia. In male animals, the heart rate was higher at the beginning and decreased over time, with a tendency toward approximation in both sexes.
Overall, females had a significantly lower heart rate compared to males [−35 (−65 to −5.6) bpm; p = 0.020, Table 3] without a significant influence of genotype [−11 (−41 to 19); p = 0.47, Table 3 and Figures 1C, D].
There was a significant interaction between anesthesia time and sex with a positive value for females over time [0.42 (0.25 to 0.59); p < 0.001, Table 3]. The heart rate in female mice was lower at the beginning and increased over the course of anesthesia (Figures 1C, D).
The combination of sex and genotype showed a significantly negative influence of female sex in both Tg2576 [−63 (−106 to −21); p = 0.003] and WT [−48 (−90 to −6.2); p = 0.025] on heart rates (Table 3) compared to male Tg2576 as the heart rates of male Tg2576 remained above those of the other experimental groups throughout general anesthesia.
Anesthesia time had a significantly positive effect on the combination of sex and genotype in male WT [0.28 (0.03 to 0.53); p = 0.026], female Tg2576 [0.61 (0.37 to 0.84); p < 0.001] and female WT [0.52 (0.28 to 0.76); p < 0.001, Table 3].
Female Tg2576 had a significantly lower heart rate [−63 (−108 to −19); p = 0.005, Table 4] than male Tg2576.
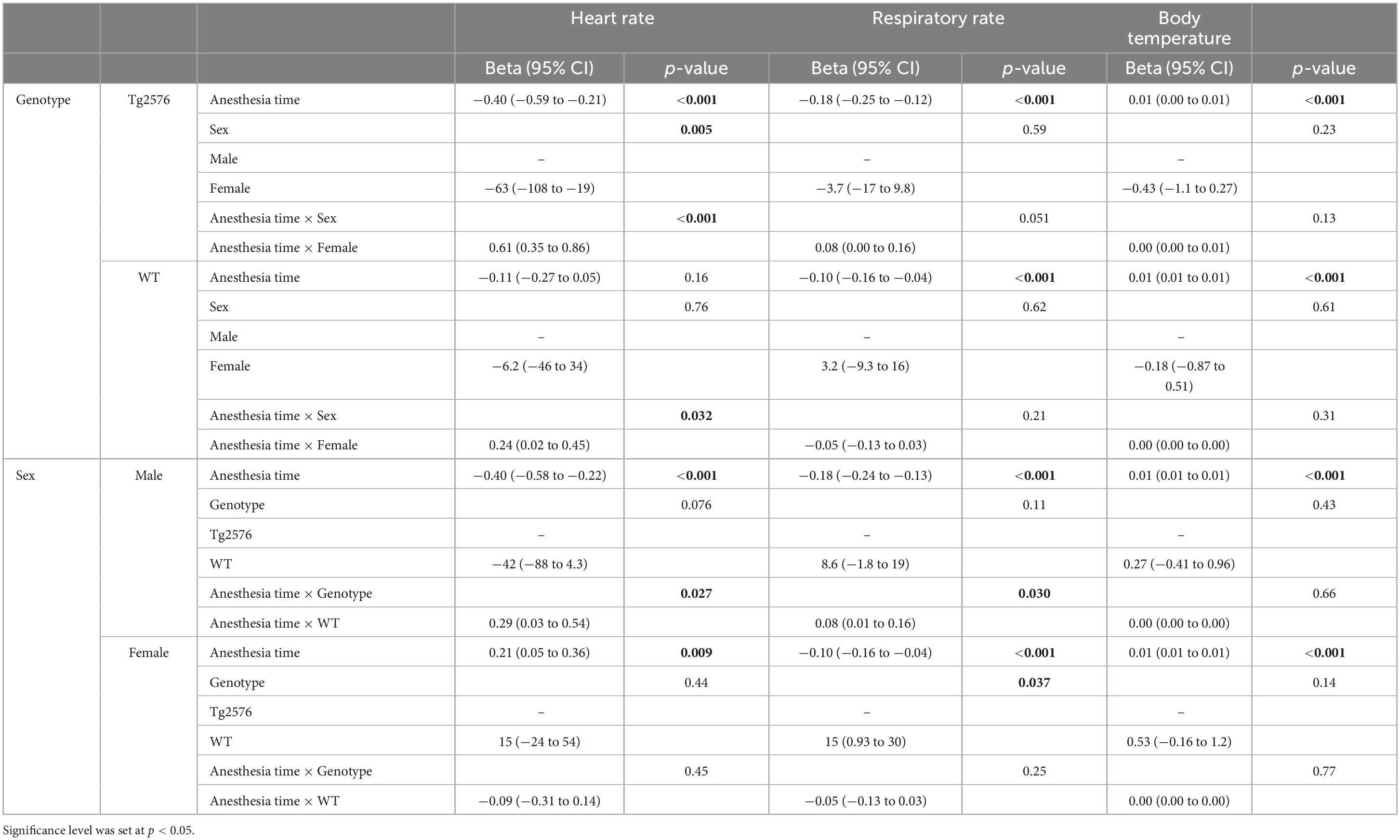
Table 4. Multivariate linear mixed regression model with variables time, sex, genotype and further stratification by experimental group for heart rate, respiratory rate and body temperature.
The combination of anesthesia time and female sex showed a significantly positive influence on heart rate in Tg2576 [0.61 (0.35 to 0.86); p < 0.001, Table 4] and WT [0.24 (0.02 to 0.45); p = 0.032, Table 4].
In male mice, WT genotype had a significantly positive influence on heart rate [0.29 (0.03 to 0.54); p = 0.027] when assessing the combination of anesthesia time and genotype (Table 4).
The median respiratory rate was 104 (93–115) breaths per minute in all animals during general anesthesia (Table 2).
Univariate comparisons showed a significant difference between genotype [Tg2576 96 (89–109) and WT 111 (100–121); p = 0.005, Table 2] and between the combination of sex and genotype [male Tg2576 94 (90–107), male WT 112 (103–120), female Tg2576 96 (87–112), female WT 111 (100–121); p = 0.039, Table 2] in median respiratory rates.
Overall, WT animals had a significantly higher respiratory rate throughout general anesthesia [12 (3.4 to 21); p = 0.007, Table 3 and Figures 2A, B].
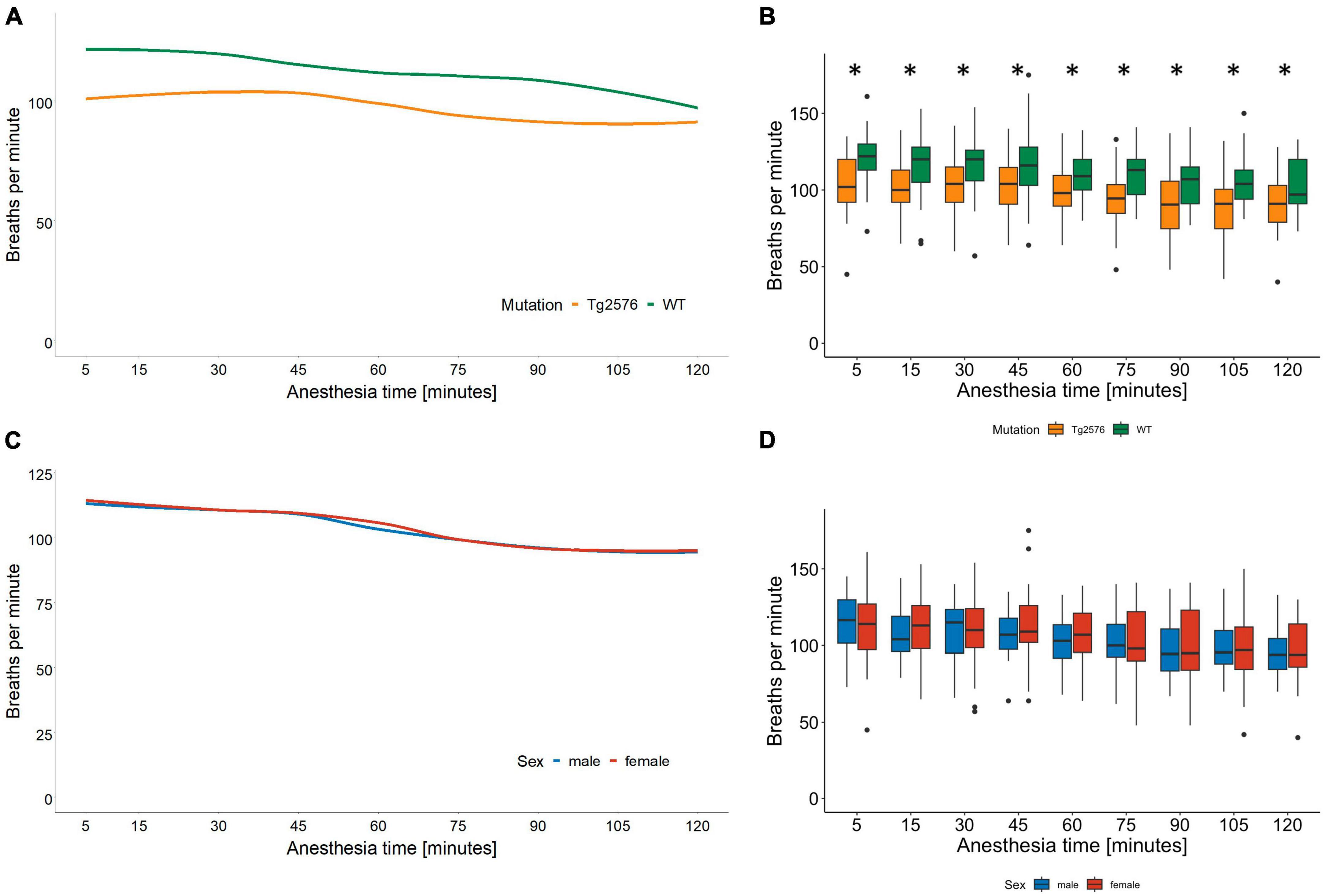
Figure 2. Respiratory rate in breaths per minute and anesthesia time in minutes sorted by genotype (A) and corresponding boxplots over a 15 min interval (B); median (horizontal lines), interquartile range (box) and range (whiskers), dots represent outliers at least 1.5 times of the interquartile range (IQR), *p = 0.007. The respiratory rate in Tg2576 mice was significantly lower compared to WT mice. Respiratory rate in breaths per minute and anesthesia time in minutes sorted by sex (C) and corresponding boxplots over a 15 min interval (D); median (horizontal lines), interquartile range (box) and range (whiskers), dots represent outliers at least 1.5 times of the interquartile range (IQR). The respiratory rate decreased in all animals over the course of isoflurane anesthesia, without a statistically significant difference between sex.
In male animals, WT genotype significantly positively influenced respiratory rate [0.08 (0.01 to 0.16); p = 0.030, Table 4].
The median body temperature in all animals was 36.8 (36.5–37.6)°C (Table 2) with a significant difference in genotype [Tg2576 36.7 (36.3–37.2)°C, WT 37.2 (36.8–38.1)°C; p = 0.022] in univariate comparisons (Table 2).
During anesthesia body temperature increased over time [0.001 (0.01 to 0.01); p < 0.001] without significant differences in regression models (Tables 3, 4 and Figures 3A–D).
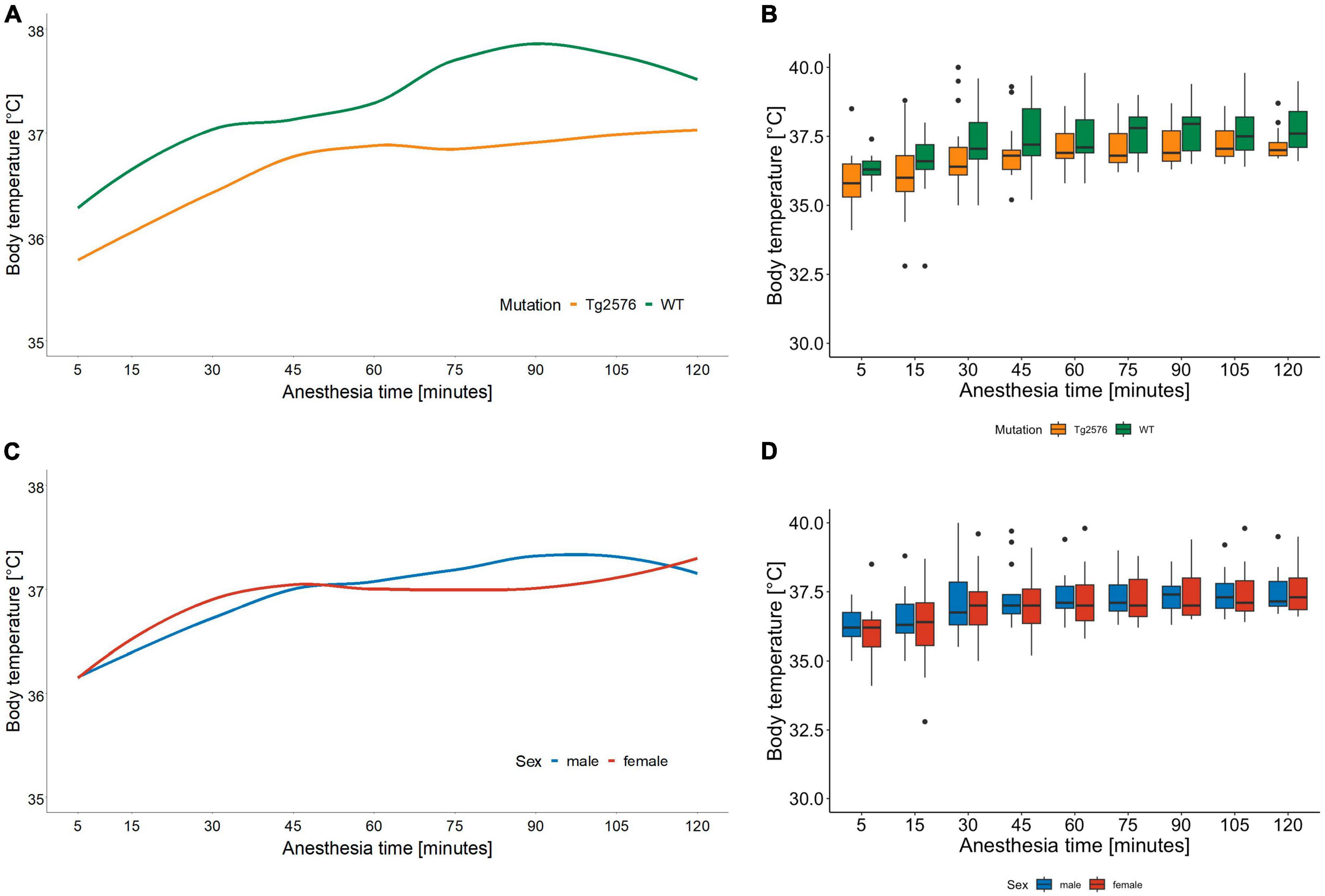
Figure 3. Body temperature in °C and anesthesia time in minutes sorted by genotype (A) and corresponding boxplots over a 15 min interval (B); median (horizontal lines), interquartile range (box) and range (whiskers), dots represent outliers at least 1.5 times of the interquartile range (IQR). Body temperature of Tg2576 and WT mice differ over the course of anesthesia. Throughout anesthesia body temperature increased in all animals, without a statistically significant difference between genotype. Body temperature in °C and anesthesia time in minutes sorted by sex (C) and corresponding boxplots over a 15 min interval (D); median (horizontal lines), interquartile range (box) and range (whiskers), dots represent outliers at least 1.5 times of the interquartile range (IQR). Body temperature increased in all animals over the course of anesthesia, without a statistically significant difference between sex.
Over the time of anesthesia, all vital parameters changed significantly, except for the model of heart rate and genotype [−0.06 (−0.18 to 0.06), p = 0.31, Table 3].
This study is a secondary analysis of data collected during general anesthesia in a mouse model of AD. Heart rate, respiratory rate and body temperature of both male and female 10–12 months old Tg2576 and wild type mice under isoflurane anesthesia were analyzed. We found time dependent as well as sex- and genotype-specific differences in vital parameters. After general anesthesia, mice underwent neurocognitive testing for 8 consecutive days. Results from the post-anesthesia part of the study have already been published (18). Data on anesthesia in Tg2576 mice over a period of 120 min are not very frequently encountered in the literature, as general anesthesia in animal experiments is often maintained for a shorter time period and published data on vital parameters is limited (13, 15, 17, 21). In view of this, these results can be considered a valuable contribution to the field of experimental AD research and provide a unique insight into the physiology of Tg2576 during general anesthesia.
Since its first description in 1996 (9), the Tg2576 animal model has been widely used in AD research to elucidate AD pathophysiology (10, 22–25), symptomology (26–28) and possible therapeutic strategies for this to date incurable neurodegenerative disease (29–33). Also, studies on the effect of general anesthesia on AD pathology have been done using this AD mouse model (17, 18, 21). Tg2576 animals have been very well described (34, 35) and are known to exhibit sex-specific differences similar to human AD pathology (11). In order to translate experimental evidence into the human organism, valid animal models are warranted. Although the Tg2576 animal model has already been well characterized in different aspects of AD research, literature on vital parameters during general anesthesia is limited. We therefore undertook a secondary analysis of heart rate, respiratory rate and body temperature in the Tg2576 mouse model taken during general anesthesia.
We found that the median heart rate did not differ significantly between animals in univariate analyses. However, in multivariate analyses female mice had a lower heart rate which increased over the course of anesthesia while the heart rate in male mice decreased, with a tendency toward approximation (Figures 1C, D). Heart rates in male Tg2576 and female mice approximated toward the end of general anesthesia (Figures 1A, B). This finding is in line with other studies on general anesthesia in laboratory animals, where heart rates in female (36) and animals of both sex (37, 38) increased over the course of isoflurane anesthesia. In (37) sex was not a significant factor for heart rate in C57BL/6J mice whereas our data showed a significant difference between male and female Tg2576 and WT mice. There is emergent evidence of a heart-brain-interaction in AD in terms of increased heart rate variability in males associated with better cognitive resilience (39) and susceptibility to AD pathology (40), so a sex-dependent effect on the heart rate based on the respective genotype in this AD mouse model cannot be excluded. Also, isoflurane is known to induce hypotension (41) and an increase in heart rate could be a compensatory mechanism. We did not measure blood pressure during anesthesia, which on the one hand can be viewed as a limitation of our study. On the other hand, it must be noted that the American Heart Association recommended avoiding anesthesia during blood pressure measurement due to the effect of anesthetics on cardiovascular function (42). We aimed to avoid invasive blood pressure measurement techniques in order to minimize distress for the animals and decided against a tail-cuff method since mice have complex thermoregulating processes involving fluctuating vasomotor tone of the tail, especially under general anesthesia (43). For the same reason we refrained from taking blood samples.
The respiratory rate decreased in all animals during isoflurane anesthesia (Figures 2C, D). Tg2576 mice had a lower respiratory rate than WT littermates without significant sex-specific differences (Figures 2A, B). Again, these observations reflect the current literature on isoflurane anesthesia in mice (36–38) in terms of a decrease in absolute numbers during anesthesia. It has to be noted that respiratory rates in our study ranged between 94 and 111 breaths per minute, whereas other publications stated respiratory rates of <80 per minute (37), <60 per minute (36), and <100 per minute (38). These differences in respiratory rates might be due to different isoflurane concentrations and subsequently differing anesthetic depths. In our study, 4.5 Vol% of isoflurane was administered for induction of general anesthesia, followed by 1.6 Vol% for anesthesia maintenance. Isoflurane concentration was reduced to 1.6 Vol% immediately after loss of righting reflex. This concentration was found to be sufficient by tail clamp test assessment while other authors used isoflurane concentrations of 1.5–2.1% (37), 2.8% (36), and 2% (38). Several aspects have to be considered when putting these data into perspective. The cited studies used C57BL/6J mice (36, 37) or ddY mice (38) at a significantly younger age [6 weeks (36), 8–20 weeks (37), and 7 weeks (38)] and applied isoflurane anesthesia for a shorter time [40 min (38), 50 min (36) and 60 min (37)] compared to the 120 min of anesthesia in our experiments. It is known that genetic variability influences susceptibility to isoflurane in mouse strains (44) and humans (45) and that anesthetic requirement decreases with age (46), which might account for the differences observed in our data. SpO2 was not measured in our study which is a limitation. However, other animal studies found SpO2 to be stable even with lower respiratory rates (37, 38), although it has to be noted that fractions of inspired oxygen as high as 100% were used.
Throughout the course of anesthesia body temperature increased in all animals while actively warmed using a warming pad (Figures 3C, D). Hypothermia is a known complication in general anesthesia with numerous deleterious effects on both human (47) and laboratory animal (48) physiology. Body temperature correlated positively with heart rate and respiratory rate in female C57BL/6J mice (48) during 30 min of isoflurane anesthesia which is again an anesthesia time shorter than in our study. Although animals were handled identically, we found that body temperature differed significantly between genotypes in univariate but not in multivariate analyses. Tg2576 had a median body temperature of 36.7°C compared to 37.2°C in WT (Figures 3A, B). This is reflective of AD pathology in humans where old age, disruption in thermoregulation and neurodegeneration seem to be interconnected (49).
In this secondary analysis of vital parameters collected during general anesthesia of Tg2576 mice and WT littermates we found marginal, yet significant differences in heart rate, respiratory rate and body temperature. Despite these differences, all vital parameters remained within physiological limits. Our findings indicate that isoflurane anesthesia in this AD mouse model is safe and does not seem to influence experimental results by interacting with vital parameters. The present study provides information on appropriate anesthesia in order to advance research on anesthesia and AD and could contribute to improving laboratory animal welfare.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The animal study was approved by the Regierung von Oberbayern, Maximilianstr. 39, 80538 Munich, Germany, Chair: Dr. B. Wirrer, Registration number: 55.2-1-54-2532-67-2016, July 28th, 2016. The study was conducted in accordance with the local legislation and institutional requirements.
SB: Formal analysis, Investigation, Validation, Writing – review and editing. SS: Conceptualization, Methodology, Supervision, Writing – review and editing. BU: Formal analysis, Methodology, Validation, Writing – review and editing, BJ: Conceptualization, Supervision, Writing – review and editing. MB: Methodology, Resources, Supervision, Writing – review and editing. LB: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Writing – original draft.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study received Institutional funding. We acknowledge support from Alzheimer Forschung Initiative e.V. for Open Access Publishing.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Long S, Benoist C, Weidner W. World Alzheimer report 2023: Reducing dementia risk: Never too early, never too late. London: Alzheimer’s Disease International (2023).
2. Velandia P, Miller-Petrie M, Chen C, Chakrabarti S, Chapin A, Hay S, et al. Global and regional spending on dementia care from 2000-2019 and expected future health spending scenarios from 2020-2050: An economic modelling exercise. EClinicalMedicine. (2022) 45:101337.
3. Sprung J, Warner D, Knopman D, Petersen R, Mielke M, Jack C Jr., et al. Exposure to surgery with general anaesthesia during adult life is not associated with increased brain amyloid deposition in older adults. Br J Anaesth. (2020) 124:594–602.
4. Patel D, Lunn A, Smith A, Lehmann D, Dorrington K. Cognitive decline in the elderly after surgery and anaesthesia: Results from the oxford project to investigate memory and ageing (OPTIMA) cohort. Anaesthesia. (2016) 71:1144–52.
5. Lee J, Choi G, Kang H, Baek C, Jung Y, Shin H, et al. Relationship between surgery under general anesthesia and the development of dementia: A systematic review and meta-analysis. Biomed Res Int. (2020) 2020:3234013.
6. Yokoyama M, Kobayashi H, Tatsumi L, Tomita T. Mouse models of Alzheimer’s disease. Front Mol Neurosci. (2022) 15:912995. doi: 10.3389/fnmol.2022.912995
7. Kluever V, Fornasiero E. Principles of brain aging: Status and challenges of modeling human molecular changes in mice. Ageing Res Rev. (2021) 72:101465.
8. Rosenthal N, Brown S. The mouse ascending: Perspectives for human-disease models. Nat Cell Biol. (2007) 9:993–9.
9. Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, et al. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. (1996) 274:99–102.
10. Frautschy S, Yang F, Irrizarry M, Hyman B, Saido T, Hsiao K, et al. Microglial response to amyloid plaques in APPsw transgenic mice. Am J Pathol. (1998) 152:307–17.
11. Schmid S, Rammes G, Blobner M, Kellermann K, Bratke S, Fendl D, et al. Cognitive decline in Tg2576 mice shows sex-specific differences and correlates with cerebral amyloid-beta. Behav Brain Res. (2019) 359:408–17.
12. Berger M, Schenning K, Brown C, Deiner S, Whittington R, Eckenhoff R, et al. Best practices for postoperative brain health: Recommendations from the fifth international perioperative neurotoxicity working group. Anesth Analg. (2018) 127:1406–13.
13. Jia Z, Geng L, Xie G, Chu Q, Zhang W. Sevoflurane impairs acquisition learning and memory function in transgenic mice model of Alzheimer’s disease by induction of hippocampal neuron apoptosis. Int J Clin Exp Med. (2015) 8:15490–7.
14. Chen R, Jiang Y, Hu J, Chen H, Li H, Meng X, et al. Hourly air pollutants and acute coronary syndrome onset in 1.29 million patients. Circulation. (2022) 145:1749–60.
15. Coleman R, Liang C, Patel R, Ali S, Mukherjee J. Brain and brown adipose tissue metabolism in transgenic Tg2576 mice models of Alzheimer disease assessed using (18)F-FDG PET imaging. Mol Imaging. (2017) 16:1536012117704557.
16. Xu W, Lopez-Guzman M, Schoen C, Fitzgerald S, Lauer S, Nixon R, et al. Spared piriform cortical single-unit odor processing and odor discrimination in the Tg2576 mouse model of Alzheimer’s disease. PLoS One. (2014) 9:e106431. doi: 10.1371/journal.pone.0106431
17. Bianchi S, Tran T, Liu C, Lin S, Li Y, Keller J, et al. Brain and behavior changes in 12-month-old Tg2576 and nontransgenic mice exposed to anesthetics. Neurobiol Aging. (2008) 29:1002–10.
18. Borgstedt L, Bratke S, Blobner M, Pötzl C, Ulm B, Jungwirth B, et al. Isoflurane has no effect on cognitive or behavioral performance in a mouse model of early-stage Alzheimer’s disease. Front Neurosci. (2022) 16:1033729. doi: 10.3389/fnins.2022.1033729
19. Gargiulo S, Greco A, Gramanzini M, Esposito S, Affuso A, Brunetti A, et al. Mice anesthesia, analgesia, and care, part I: Anesthetic considerations in preclinical research. ILAR J. (2012) 53:E55–69.
20. Deady J, Koblin D, Eger E II, Heavner J, D’Aoust B. Anesthetic potencies and the unitary theory of narcosis. Anesth Analg. (1981) 60:380–4.
21. Quiroga C, Chaparro R, Karlnoski R, Erasso D, Gordon M, Morgan D, et al. Effects of repetitive exposure to anesthetics and analgesics in the Tg2576 mouse Alzheimer’s model. Neurotox Res. (2014) 26:414–21.
22. Eeza M, Singer R, Höfling C, Matysik J, de Groot H, Roβner S, et al. Metabolic profiling of suprachiasmatic nucleus reveals multifaceted effects in an Alzheimer’s disease mouse model. J Alzheimers Dis. (2021) 81:797–808.
23. Khorani M, Bobe G, Matthews D, Magana A, Caruso M, Gray N, et al. The impact of the hAPP695SW transgene and associated amyloid-β accumulation on murine hippocampal biochemical pathways. J Alzheimers Dis. (2021) 85:1601–19.
24. Mehlhorn G, Hollborn M, Schliebs R. Induction of cytokines in glial cells surrounding cortical beta-amyloid plaques in transgenic Tg2576 mice with Alzheimer pathology. Int J Dev Neurosci. (2000) 18:423–31.
25. Pérez-González M, Badesso S, Lorenzo E, Guruceaga E, Pérez-Mediavilla A, García-Osta A, et al. Identifying the main functional pathways associated with cognitive resilience to Alzheimer’s disease. Int J Mol Sci. (2021) 22:9120.
26. Barnes P, Good M. Impaired Pavlovian cued fear conditioning in Tg2576 mice expressing a human mutant amyloid precursor protein gene. Behav Brain Res. (2005) 157:107–17.
27. Kosel F, Pelley J, Franklin T. Behavioural and psychological symptoms of dementia in mouse models of Alzheimer’s disease-related pathology. Neurosci Biobehav Rev. (2020) 112:634–47.
28. Ognibene E, Middei S, Daniele S, Adriani W, Ghirardi O, Caprioli A, et al. Aspects of spatial memory and behavioral disinhibition in Tg2576 transgenic mice as a model of Alzheimer’s disease. Behav Brain Res. (2005) 156:225–32.
29. Evans C, Miners J, Piva G, Willis C, Heard D, Kidd E, et al. ACE2 activation protects against cognitive decline and reduces amyloid pathology in the Tg2576 mouse model of Alzheimer’s disease. Acta Neuropathol. (2020) 139:485–502.
30. Latina V, Giacovazzo G, Cordella F, Balzamino B, Micera A, Varano M, et al. Systemic delivery of a specific antibody targeting the pathological N-terminal truncated tau peptide reduces retinal degeneration in a mouse model of Alzheimer’s disease. Acta Neuropathol Commun. (2021) 9:38.
31. Ma C, Hunt J, Kovalenko A, Liang H, Selenica M, Orr M, et al. Myeloid arginase 1 insufficiency exacerbates amyloid-β associated neurodegenerative pathways and glial signatures in a mouse model of Alzheimer’s disease: A targeted transcriptome analysis. Front Immunol. (2021) 12:628156. doi: 10.3389/fimmu.2021.628156
32. Schafer M, Alldred M, Lee S, Calhoun M, Petkova E, Mathews P, et al. Reduction of β-amyloid and γ-secretase by calorie restriction in female Tg2576 mice. Neurobiol Aging. (2015) 36:1293–302.
33. Wong L, Wong P, Ho P. Metabolic profiling of female Tg2576 mouse brains provides novel evidence supporting intranasal low-dose pioglitazone for long-term treatment at an early stage of Alzheimer’s disease. Biomedicines. (2020) 8:589.
34. Barnes P, Hale G, Good M. Intramaze and extramaze cue processing in adult APPSWE Tg2576 transgenic mice. Behav Neurosci. (2004) 118:1184–95.
35. Bilkei-Gorzo A. Genetic mouse models of brain ageing and Alzheimer’s disease. Pharmacol Ther. (2014) 142:244–57.
36. Cesarovic N, Nicholls F, Rettich A, Kronen P, Hassig M, Jirkof P, et al. Isoflurane and sevoflurane provide equally effective anaesthesia in laboratory mice. Lab Anim. (2010) 44:329–36.
37. David E, Pacharinsak C, Jampachaisri K, Hagan L, Marx J. Use of ketamine or xylazine to provide balanced anesthesia with isoflurane in C57BL/6J mice. J Am Assoc Lab Anim Sci. (2022) 61:457–67.
38. Tsukamoto A, Serizawa K, Sato R, Yamazaki J, Inomata T. Vital signs monitoring during injectable and inhalant anesthesia in mice. Exp Anim. (2015) 64:57–64.
39. Eissman J, Dumitrescu L, Mahoney E, Smith A, Mukherjee S, Lee M, et al. Sex differences in the genetic architecture of cognitive resilience to Alzheimer’s disease. Brain. (2022) 145:2541–54.
40. Molloy C, Choy E, Arechavala R, Buennagel D, Nolty A, Spezzaferri M, et al. Resting heart rate (variability) and cognition relationships reveal cognitively healthy individuals with pathological amyloid/tau ratio. Front Epidemiol. (2023) 3:1168847. doi: 10.3389/fepid.2023.1168847
41. Gaertner DH, Hankenson F, Batchelder M. Anesthesia and analgesia for laboratory rodents. 2nd ed. In: Fish R, Brown M, Danneman P, Karas A editors. Anesthesia and analgesia in laboratory animals. London: Academic Press, Elsevier (2008). p. 239–97.
42. Kurtz T, Griffin K, Bidani A, Davisson R, Hall J. Recommendations for blood pressure measurement in animals: Summary of an AHA scientific statement from the Council on high blood pressure research, professional and public education subcommittee. Arterioscler Thromb Vasc Biol. (2005) 25:478–9.
43. Bigiarelli K. Rodent thermoregulation: Considerations for tail-cuff blood pressure measurements. J Am Assoc Lab Anim Sci. (2022) 61:406–11.
44. Mogil J, Smith S, O’Reilly M, Plourde G. Influence of nociception and stress-induced antinociception on genetic variation in isoflurane anesthetic potency among mouse strains. Anesthesiology. (2005) 103:751–8.
45. Liem E, Lin C, Suleman M, Doufas A, Gregg R, Veauthier J, et al. Anesthetic requirement is increased in redheads. Anesthesiology. (2004) 101:279–83.
46. Nickalls R, Mapleson W. Age-related iso-MAC charts for isoflurane, sevoflurane and desflurane in man. Br J Anaesth. (2003) 91:170–4.
47. Sun Z, Honar H, Sessler D, Dalton J, Yang D, Panjasawatwong K, et al. Intraoperative core temperature patterns, transfusion requirement, and hospital duration in patients warmed with forced air. Anesthesiology. (2015) 122:276–85.
48. Caro A, Hankenson F, Marx J. Comparison of thermoregulatory devices used during anesthesia of C57BL/6 mice and correlations between body temperature and physiologic parameters. J Am Assoc Lab Anim Sci. (2013) 52:577–83.
Keywords: Alzheimer’s disease, Tg2576, isoflurane, general anesthesia, vital parameters, mouse model, transgenic mice
Citation: Bratke S, Schmid S, Ulm B, Jungwirth B, Blobner M and Borgstedt L (2024) Genotype- and sex-specific changes in vital parameters during isoflurane anesthesia in a mouse model of Alzheimer’s disease. Front. Med. 11:1342752. doi: 10.3389/fmed.2024.1342752
Received: 22 November 2023; Accepted: 20 February 2024;
Published: 27 March 2024.
Edited by:
Ata Murat Kaynar, University of Pittsburgh, United StatesReviewed by:
Ronald Balczon, University of South Alabama, United StatesCopyright © 2024 Bratke, Schmid, Ulm, Jungwirth, Blobner and Borgstedt. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Laura Borgstedt, bGF1cmEuYm9yZ3N0ZWR0QHR1bS5kZQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.