- 1Global Evidence Synthesis Initiative (GESI), Division of Evidence Synthesis, School of Epidemiology and Public Health, Jawaharlal Nehru Medical College, Datta Meghe Institute of Higher Education and Research, Wardha, Maharashtra, India
- 2Division of Reproductive, Maternal and Child Health, Indian Council of Medical Research Headquarters, New Delhi, India
- 3Centre of One Health Research, Department of Medicine, Jawaharlal Nehru Medical College, Datta Meghe Institute of Higher Education and Research, Wardha, Maharashtra, India
- 4Global Consortium of Public Health Research, Jawaharlal Nehru Medical College, Datta Meghe Institute of Higher Education and Research, Wardha, Maharashtra, India
- 5i-Health Consortium, Division of Evidence Synthesis, School of Epidemiology and Public Health, Jawaharlal Nehru Medical College, Datta Meghe Institute of Higher Education and Research, Wardha, Maharashtra, India
- 6Department of Pharmacology, All India Institute of Medical Sciences (AIIMS), Jodhpur, Rajasthan, India
- 7Department of Epidemiology, Indian Institute of Public Health, Gandhinagar, Gujarat, India
- 8Faculty of Medicine and Health, Sydney Medical School, The University of Sydney Institute for Infectious Disease (Sydney ID), University of Syndey, Camperdown, NSW, Australia
- 9School of Human and Health Sciences, Global Health at the University of Huddersfield, Huddersfield, United Kingdom
- 10Stepping Stones, Jawaharlal Nehru Medical College, Datta Meghe Institute of Higher Education and Research, Wardha, Maharashtra, India
- 11South Asia Infant Feeding Research Network, Global Health Academy, Jawaharlal Nehru Medical College, Datta Meghe Institute of Higher Education and Research, Wardha, Maharashtra, India
Introduction: Anemia remains a prevalent global health issue with varying severity. Intravenous iron supplementation, particularly with ferric carboxymaltose (FCM), has appeared as a possible therapeutic intervention for individuals with moderate to severe anemia. The study aimed to assess the efficacy and safety of ferric carboxymaltose (FCM) in reducing anemia.
Methods: We searched electronic databases, registries, websites, e-libraries, reference lists of reviews, citations, etc. We included randomized control trials (RCTs), non-RCTs, and single-arm studies, while observational studies, case series, and case studies were excluded. Two reviewers independently screened the studies and extracted the data. We included studies of moderate-to-severely anemic Indians and excluded Indians with other comorbidities. We assessed the risk of bias and the overall quality of evidence (QoE) using GRADE GDT.
Result: We identified 255 studies and included 14 studies (11 RCT, one non-RCT, and two single-arm studies) with 1,972 participants for qualitative analysis and 10 studies in the meta-analysis. All the included studies detailed the use of FCM for anemia. The primary outcomes assessed in the included studies were anemia, hemoglobin, and adverse events. The outcomes assessed ranged from 2 weeks to 12 weeks. The risk of bias varied across different studies with different outcomes. FCM is consistent with a fewer number of adverse events as compared to other interventions and provides “moderate” to “very low” QoE.
Conclusion: A slow single infusion of 1 gram of FCM is well-tolerated, safe, and effective in treating iron deficiency anemia (IDA) and surpasses other interventions (Iron Sucrose Complex (ISC), Iron sucrose, and ferrous ascorbate) in elevating hemoglobin levels and replenishing iron stores.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=459363, CRD42023459363.
1 Introduction
Anemia is indicated by a deficiency in the number of red blood cells or below-average hemoglobin levels within these cells (1). This condition presents a noteworthy public health challenge, influencing not just individual well-being but also significantly impacting societal and economic advancement (2). As per WHO 2023 estimates, 42% of children under five and 40% of pregnant women are anemic globally (1). According to National Family Health Survey (NFHS-5) data organized in India (between 2019 and 2021), 57% of women and 25% of men in the age group of 15–49 years are anemic in India (3). Despite the availability of treatments and guidelines (Anemia Mukt Bharat), the slightest improvement is observed in the anemia status in India (4).
Causes of anemia can often be distinct but frequently coexist. The primary cause of anemia encompasses nutritional deficiencies, hemoglobinopathies, and infectious diseases (like malaria, tuberculosis, HIV, and parasitic infections). While it’s mostly presumed that around 50% of anemia cases stem from iron deficiency, this ratio might vary among different population groups and regions (5). Acknowledging the multifaceted nature of this ailment, rectifying anemia necessitates a comprehensive strategy (5). An integrated approach is crucial to combat it effectively, identifying and mitigating contributing factors.
Iron deficiency typically evolves gradually, often without evident symptoms or clinical manifestations. As iron reserves are gradually exhausted, iron availability to tissues diminishes, resulting in symptomatic anemia (6). This includes fatigue, weakness, dizziness and shortness of breath (6).
Although anemia can occur at any stage of life, pregnant women and young children are more inclined. The health effects of anemia include a high risk of maternal and child mortality, a negative impact on children’s cognitive development, physical development, physical performance, and increased susceptibility to infections in adults. Anemia during the antepartum period distinctly impacts both maternal and fetal well-being. It is intricately associated with more significant morbidity and risk of several challenges throughout pregnancy, such as greater susceptibility towards infection, increased need for blood transfusion during delivery, cardiovascular complications, intrauterine growth retardation, preterm delivery, and perinatal mortality and morbidity (7, 8). During the first trimester, IDA harms fetal growth more than during late pregnancy (6, 7, 9). Anemia during the post-partum period inflicts a significant disease burden at a vital phase of maternal–infant interaction and may result in developmental impairments in afflicted mothers’ newborns (6).
In regions where Iron Deficiency Anemia (IDA) is the predominant cause of anemia (especially in low-income contexts), supplementary iron is often administered through supplements to vulnerable groups. Strategies like fortifying food and diversifying diets to enhance iron consumption emerge as crucial and sustainable methods to combat IDA within the broader aspects. However, a comprehensive approach incorporating iron interventions alongside other strategies becomes imperative when anemia is not solely attributed to iron deficiency.
The primary approach to addressing it involves oral or intravenous (IV) iron supplementation, targeting the underlying cause of IDA, and restoring iron levels to normal. The initial method of choice is oral iron supplementation. However, challenges related to compliance and the potential for iron depletion undermine the efficacy of oral iron treatment (10). Oral interventions prove inadequate in cases of moderate to severe anemia, necessitating prompt elevation of hemoglobin levels and rapid iron store replenishment. Instead, expedited remedies like parenteral therapies become imperative (11). Notably, parenteral options, including intravenous iron preparations, facilitate swifter iron restoration compared to oral methods, and their tolerability during pregnancy is notable. Among the commonly utilized parenteral preparations, Iron Sucrose Complex (ISC) and iron dextran are dosed according to the level of iron deficiency. Nevertheless, it’s crucial to acknowledge that intravenous iron dextran formulations risk allergic reactions, whereas intravenous iron polymaltose mandates a lengthier infusion time.
Ferric carboxymaltose (FCM) is a third-generation intravenous dextran-free, intravenous iron formulation given in a single dose over a small duration, which overcomes the limitations of existing treatments and has a greater capacity for restoring iron (12). It minimizes the dose frequency but also has few drug-related side effects. Clinical findings have shown intravenous FCM’s efficacy is effective in spanning conditions like uterine bleeding, post-partum iron deficiency anemia, inflammatory bowel disease, and chronic kidney disease, irrespective of hemodialysis (13).
Ferric carboxymaltose is an innovative iron complex composed of a ferric hydroxide core, facilitating controlled iron delivery to reticuloendothelial cells and subsequently to iron-binding proteins like ferritin and transferring (13). This complex minimizes the risk of releasing excessive ionic iron into the serum. It is swiftly eliminated through the bloodstream and predominantly circulated to the bone marrow, liver, and spleen (13). This deliberate gradual release mechanism contributes to the low toxicity of FCM, establishing a substantial safety margin between standard and lethal doses. Furthermore, the FCM formulation’s neutral pH and physiological osmolarity permit the administration of elevated doses with favorable local tolerance. As long as the iron dosage is tailored to the patient’s needs, the likelihood of FCM-induced toxicity during clinical use remains relatively low. Additionally, FCM stands out for its absence of dextran ferumoxytol and iron isomaltose, minimizing the risk of dextran-induced anaphylactic reactions. Its exceptional safety profile, remarkably low immunogenicity, and often singular-dose regimen enhance its cost-effectiveness, particularly in most cases.
Though studies (8, 14–21) have demonstrated positive and encouraging effects of FCM in anemic individuals, there exists an urge to generate evidence for patients, practitioners and policymakers to determine the potential integration of intravenous FCM in the management of moderate to severely anemic individuals and, if data permits, to figure out the most appropriate drug dosage for this group of patients. Therefore, we plan to systematically review existing literature that reports intravenous FCM’s effectiveness in treating moderate-to-severe anemia. This systematic review aimed to assess the efficacy and safety of ferric carboxymaltose in reducing anemia.
2 Methods
The systematic review was conducted using a standard methodology suggested in the Cochrane Handbook of Systematic Reviews (22). The protocol of this systematic review was registered in Prospero. The registration number of the proposed protocol is https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42023459363. This systematic review was funded by Indian Council of Medical Research (ICMR), India.
2.1 Inclusion and exclusion criteria
2.1.1 Types of studies
Inclusion criteria encompassed Randomized Control Trials (RCTs), non-RCTs, and single-arm studies while observational studies, case series, and case studies were excluded. Full journal publication was mandatory for inclusion, though extended abstracts of otherwise unpublished clinical trials were accepted.
2.1.2 Types of participants
Studies based on moderate to severely anaemic Indians irrespective of age groups, gender, ethnicity, educational status, community or setting, other socio-demographic factors, type of anaemia (nutritional deficiencies, such as iron, folate, vitamins B12 and A; haemoglobinopathies; infectious diseases, such as malaria, tuberculosis, HIV and parasitic infections) were incorporated in this systematic review. Studies done in South-East Asia or at the global level were considered if they provided data separately for the Indian inhabitants. Studies confined to Indians with other comorbidities were excluded from the review.
2.1.3 Types of interventions
Studies in which intravenous ferric carboxymaltose injection was administered to moderately or severely anaemic Indians irrespective of dose, frequency and duration were incorporated in this systematic review.
2.1.4 Types of comparisons
The following comparisons were made in the review:
1. FCM versus placebo
2. FCM versus no treatment
3. FCM versus alternative experimental treatment modality (Iron sucrose or other);
4. FCM in combination with other treatments versus FCM treatment alone.
2.1.5 Types of outcomes
The following outcomes were considered in the review:
Primary outcomes:
1. Anaemia
2. Haemoglobin
3. Adverse events: Adverse reaction was considered if the patient experienced any reaction during infusion or after drug administration. It was assessed as a dichotomous outcome with a number of participants who reported adverse events.
Secondary outcomes:
1. Iron profile (such as serum iron, serum ferritin, transferrin saturation, Total Iron Binding Capacity).
Reporting of these outcome measures did not form part of the criteria for including studies in a review.
2.2 Search methods for identification of studies
The Cochrane Central Register of Controlled Trials (CENTRAL) (via the Cochrane Library), MEDLINE (via PubMed) Medical subject headings (MeSH) or equivalent and text-word terms were used in order to search bibliographic databases without language restrictions. We preferred studies published in English. Searches were tailored to individual databases. Furthermore, we searched the metaRegister of controlled trials (mRCT),1 clinicaltrials.gov,2 and the WHO International Clinical Trials Registry Platform (ICTRP)3 for ongoing trials. Moreover, we examined the reference lists of retrieved articles and conducted hand searches of abstracts from relevant conferences. To uncover additional literature pertinent to the review, we engaged field experts for insights into unpublished and ongoing trials.
2.2.1 CENTRAL search strategy
Search Name:ICMR_Ferric carboxymaltose for anemia
Last Saved:09/01/2023 13:29:47
Comment:
IDSearch
#1“ferric carboxymaltose”
#2“ferric carboxy-maltose”
#3“ferric carboxy-maltose”
#4“iron carboxymaltose”
#5“iron carboxy maltose”
#6“iron carboxy-maltose”
#7“ferric compounds”
#8“iron compounds”
#9“iron complex*”
#10“Iron polymaltose”
#11“polynuclear iron”
#12Ferinject*
#13Injectafer*
#14VIT-45
#15“VIT 45”
#16MeSH descriptor: [Ferric Compounds] explode all trees
#17#1 OR #2 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #8 OR #9 OR #10 OR #11 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16
#18anemi*
#19anaemi*
#20hemoglobin
#21hemoglobin
#22MeSH descriptor: [Anemia] explode all trees
#23MeSH descriptor: [Hemoglobins] explode all trees
#24#18 OR #19 OR #20 OR #21 OR #22 OR #23
#25India*
#26MeSH descriptor: [India] explode all trees
#27#23 OR #26
#28#17 AND #24 AND #27
2.3 Selection of studies
Two reviewers (SU and AA) independently screened the articles retrieved from the searches using the Rayyan online screening tool and determined eligibility by reading the abstract of each study. Subsequently, the review authors eliminated studies that failed to satisfy inclusion criteria and acquired full copies of the remaining studies. Two reviewers (SU and AA) read these studies independently to examine relevant studies, and a third author (MNK) was adjudicated in the event of disagreement. The studies were not anonymised before the assessment. A Preferred Reporting Items for Systematic Reviews and Meta-Analyzes (PRISMA) flow chart was incorporated in the review for a comprehensive overview (23). Notably, the studies included in this review were, irrespective of measured outcome data, reported in a “usable” way.
2.4 Data extraction and management
Three review authors (AA, SU and NW) independently extracted data utilizing standardized form, ensuring consistency. Details of the study, participants, intervention and outcomes were extracted and populated in the ‘Characteristics of Studies Table’. The multiple reports of the same study were amalgamated, thus treating each study as the primary unit of focus rather than individual reports.
2.5 Assessment of risk of bias in included studies
In each study, two authors (AG and DS) independently assessed the risk of bias, referencing the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions, with any disparities fixed by discussion. We completed a “Risk of Bias” table for each, including using the “Risk of bias 2” (RoB 2) (24) tool for RCTs and ROBINS-I tool (25) for non-RCTs.
2.6 Measures of treatment effect
We employed fixed-effect and random-effects models that gage the comprehensive effect’s direction, size, and consistency. Risk ratios (RR) along with 95% confidence intervals (CI) were calculated for dichotomous variables, while mean differences (MD) with 95% CI were used for continuous data when measurements were consistent across studies. Standardized mean differences (SMD) were applied for conceptually similar results with varied measurement scales. Comprehensive records of means and standard deviations were noted. Imputation was performed in cases lacking sufficient information for calculating standard deviations of changes.
When published data were missing, incomplete or inconsistent with RCT protocols, we pursued additional information from the original authors/manufacturers. We emailed authors to solicit the necessary details for studies presenting data discrepancies.
The clinical heterogeneity was assessed using the Chi2 test (p value <0.1 for statistical significance) and quantified with the I2 statistic. Heterogeneity was considered considerable if I2 exceeded 75%, substantial between 50 and 90%, moderate between 30 and 60%, and mild if below 40%. In cases of statistical heterogeneity (I2 ≥ 50%), we conducted prespecified subgroup analysis and employed a random-effects model to explore potential causes. Subgroup analyzes were performed based on ferric carboxymaltose administration duration.
In the case of 10 or more included studies, we intended to perform the funnel plot test to detect reporting bias. We also investigated potential and feasible sources of asymmetry in the funnel plot (Supplementary material).
We used the statistical package RevMan 5.4 for analysis, conducting a meta-analysis only when participants, interventions, comparisons, and outcomes were identical, ensuring a clinically meaningful and relevant answer.
As recommended by ‘The Cochrane Handbook’, chapter 4.6.6., and the ‘GRADE Handbook for grading the quality of evidence and strength of recommendations’ (26), we included a ‘Summary of findings’ (SoF) table. SoF tables were presented for comparisons of Hemoglobin, serum ferritin, and adverse events between FCM and alternative experimental treatments (ISC, iron sucrose, ferrous ascorbate). Utilizing the GRADE gdt system, two review authors assessed the overall evidence quality for each outcome and presented findings in the SoF tables. Decisions to downgrade study quality were substantiated through footnotes.
2.7 The grades of evidence as per the GRADE working group are
High quality
We are very confident that the true effect lies close to that of the estimate of the effect.
Moderate quality
We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low quality
Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect.
Very low quality
We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect (26).
3 Results
A total 255 records were retrieved from electronic sources. After removing the duplicates, the search yielded 213 records. We discarded 171 records in initial screening based on title and abstracts. We assessed full text of the remaining 42 articles for eligibility and excluded 28 articles based on wrong population (n = 23), wrong intervention (n = 2), and wrong design and outcome (n = 3). Finally, we included the remaining 14 studies in qualitative synthesis and ten in the meta-analysis. We presented the selection process as a PRISMA Flow diagram in Figure 1.
We found 14 studies (11 RCT, one non-RCT and two single-arm studies) which fulfilled our inclusion criteria (1,972 participants). All the studies were single-centric and carried out in different states of India such as New Delhi (15, 27), Cuttack (28), Kolkata (18), Jammu & Kashmir (19), Maharashtra (8), Central India (29), Gujarat (20), Rajasthan (14), North-eastern region (30), Haryana (16, 17), Karnataka (31), and one study (21) conducted in India, however, did not mention the place of research. The care settings reported were hospitals (Tertiary-care hospitals and sub-district hospitals), teaching Institutes and research centers.
All the studies included adults, with the mean age of the study participants ranging from 18 to 40 years. All the studies recruited people with moderate to severe anemia. Eight studies were conducted on pregnant women (8, 14–16, 18–21), five on post-partum women (17, 19, 28–30), two on females with menorrhagia (19, 27), and two studies on women of reproductive age groups (19, 31). None of the studies were conducted on men. All studies except two that failed to specify the type of anemia (16, 17) included participants with IDA.
All the included studies detailed the use of FCM for anemia. Eleven studies (8, 14, 15, 18–21, 27–30) compared FCM versus ISC; among them, one study compared FCM versus ISC as well as intramuscular (IM) Iron sorbitol (21), and one study compared FCM versus ISC as well as oral ferrous ascorbate (28). One study (31) only compared FCM versus ferrous ascorbate. Two studies (16, 17) were single-armed studies that assessed only the effect of FCM. All included studies except one study by Dakhale et al., 2021 (29) administered maximum single dose of 1 g diluted in either 100 mL (16, 17, 21, 29) or 200 mL (8, 15, 20, 27) or 250 mL (19, 28, 30, 31) of 0.9% normal saline solution as drip infusion for 15 min (16, 17, 21, 27, 28, 30, 31) or 30 min (8, 15, 20) or 45 min (19) at baseline. Dakhale et al. (29) administered 500 mg FCM diluted in 100 mL normal saline. In five studies (14, 15, 19, 27, 28), subsequent doses of FCM, if needed were administered not more than one infusion every week (14, 15, 19, 27, 28). Single doses were administered in three included studies (17, 21, 31).
In the comparison group, ISC was slowly infused intravenously as 200 mg (8, 14, 19–21, 29, 30) or 300 mg (15, 27, 28) in 100 mL (8, 20, 21, 29) or 200 mL (15, 19, 27, 30) or 300 mL (28) of 0.9% NS over 15–30 min (8, 15, 19–21, 28, 30) or two hours (27) or daily (21) or on alternate days (8, 14, 19, 28, 30) or twice weekly (15, 20, 27), or after 2 weeks (29).
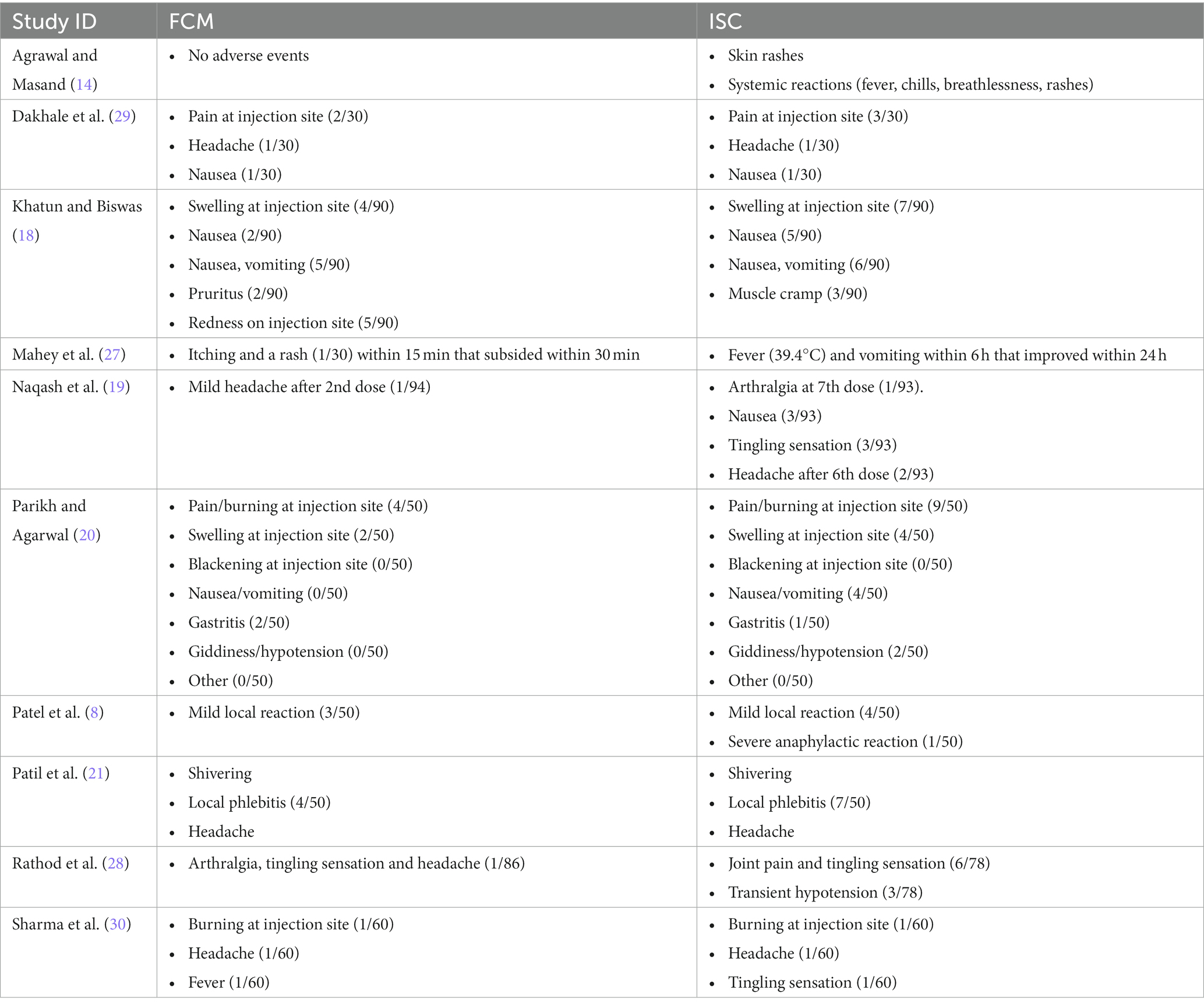
Hemoglobin was assessed in all the included studies. Except for the two included studies (27, 31), all other studies assessed the serum ferritin levels. Most of the studies except four studies (15, 17, 19, 27) reported adverse events. The outcomes assessed ranged between 2 weeks to 12 weeks. We have presented characteristics of included studies in Table 1.
3.1 Details of ongoing studies
Details of four ongoing studies can be found in Supplementary Table S1.
3.2 Risk of bias in included studies
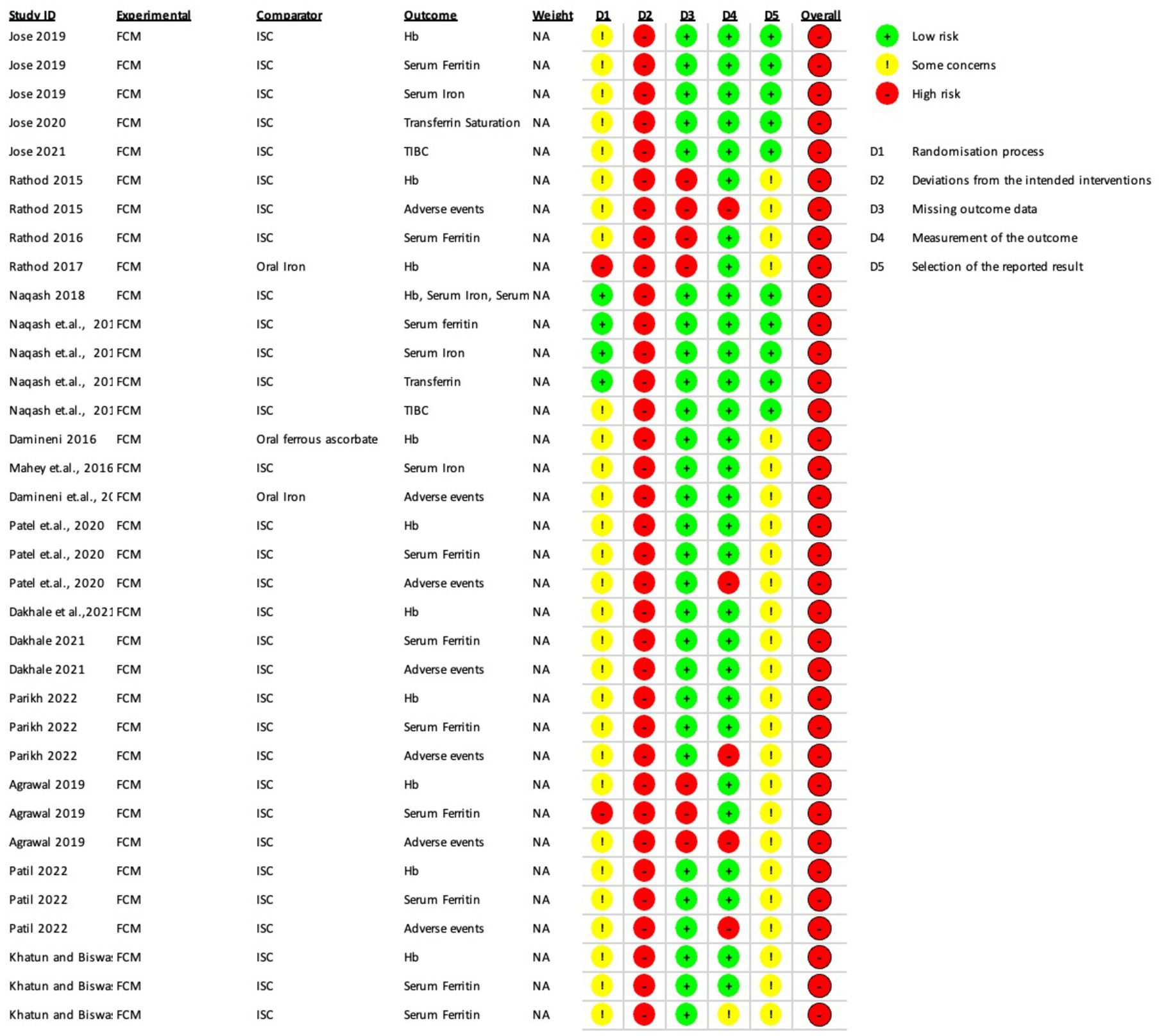
The Risk of Bias (ROB 2) assessment was conducted for several studies, and the results are summarized below and depicted in Figures 2, 3.
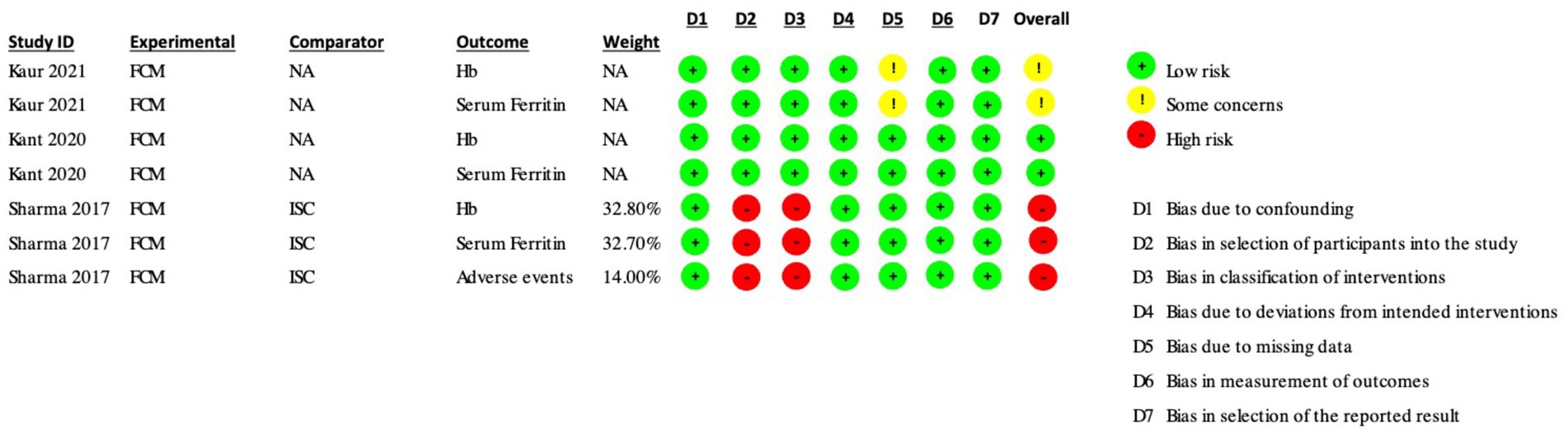
Figure 3. Risk of Bias assessments (ROBINS-I) in included non-randomized controlled trials and single-arm studies.
In the study by Jose et al. (15) several outcomes such as hemoglobin, serum ferritin, serum iron, transferrin saturation, and TIBC had some concerns overall.
In Rathod et al. (28) iron sucrose group, both hemoglobin and serum ferritin had high risk of bias overall due to deviations from intended intervention and missing outcome data. Adverse events also had a high risk of bias overall due to the outcome’s measurement, deviations from the intended intervention, and missing outcome data.
Naqash et al. (19) study had a high risk of bias in all outcomes, namely hemoglobin, serum ferritin, serum iron, transferrin saturation, and TIBC, due to deviations from intended interventions. TIBC also had some concerns due to randomization process.
In Damineni et al. (31) there was a high risk of bias in hemoglobin and adverse events outcomes due to the high risk of deviations from intended interventions and some concerns due to the randomization process and selection of reported results in the group where oral ferrous ascorbate was the comparator.
In Mahey et al. (27) there was a high risk of bias in hemoglobin, serum iron, and adverse events due to the high risk of deviations from intended interventions and some concerns due to the randomization process and selection of reported outcome.
In Patel et al. (8) hemoglobin, serum ferritin, and adverse events had an overall high risk of bias due to the high risk of deviations from intended interventions and some concerns due to the randomization process and selection of reported results. Adverse events also had a high risk in measuring the reported outcome.
Dakhale et al. (29) had an overall high risk of bias in hemoglobin, serum ferritin, and adverse events due to the high risk of deviations from intended interventions and some concerns due to the randomization process and selection of reported outcome.
Parikh and Agarwal et al. (20) also had an overall high risk of bias due to the high risk of deviations from intended interventions and some concerns due to the randomization process and selection of reported results. Adverse events also had a high risk in the measurement of reported results.
In Agrawal and Masand et al. (14) there was an overall high risk of bias in hemoglobin, serum ferritin, and adverse events outcomes due to deviations from intended intervention and missing outcome data along with some concerns in the selection of reported results. Hemoglobin also had some concerns in the randomization process, serum ferritin had a high risk in the randomization process, and adverse events had a high risk in the measurement of outcomes.
In Patil and Tehalia et al. (21) iron sucrose comparator group, both hemoglobin and serum ferritin had an overall high risk of bias due to deviations from intended interventions, some concerns due to the randomization process, and selection of reported results. Adverse events outcomes also had high risk in the measurement of the outcome. In the ISC group of Patil et al., hemoglobin had a high risk of bias due to the high risk of deviations from intended interventions and some concern in the randomization process and selection of reported results. In the oral iron group of Patil et al., both adverse events and serum ferritin had a high risk of bias due to the high risk of deviations from intended interventions and some concern in the randomization process and selection of reported outcomes.
In the ROBINS-I assessment, Kaur et al. (17) had only some concerns about the hemoglobin and serum ferritin outcomes due to some concerns of bias due to missing data. Kant et al. had a low risk of bias in both the hemoglobin and serum ferritin outcomes. Sharma et al. (30) had an overall high risk of bias in the hemoglobin, serum ferritin, and adverse events outcomes due to a high risk of bias in selecting participants for the study and bias in the classification of interventions.
To summarize, the studies reviewed in the ROB 2 tool had various levels of risk of bias in different outcomes, with some studies having a high risk of bias in multiple outcomes and others having only some concerns in one or two outcomes. The ROBINS-I tool assessed the risk of bias differently, with some studies having a low risk of bias in certain outcomes and others having a high risk of bias overall. Both tools provided a systematic approach to evaluating the risk of bias in studies, which was crucial for accurately evaluating their outcome.
3.3 Effects of interventions
3.3.1 Comparison 1: ferric carboxymaltose versus iron sucrose complex
3.3.1.1 Anemia
None of the included studies reported this outcome.
3.3.1.2 Hemoglobin
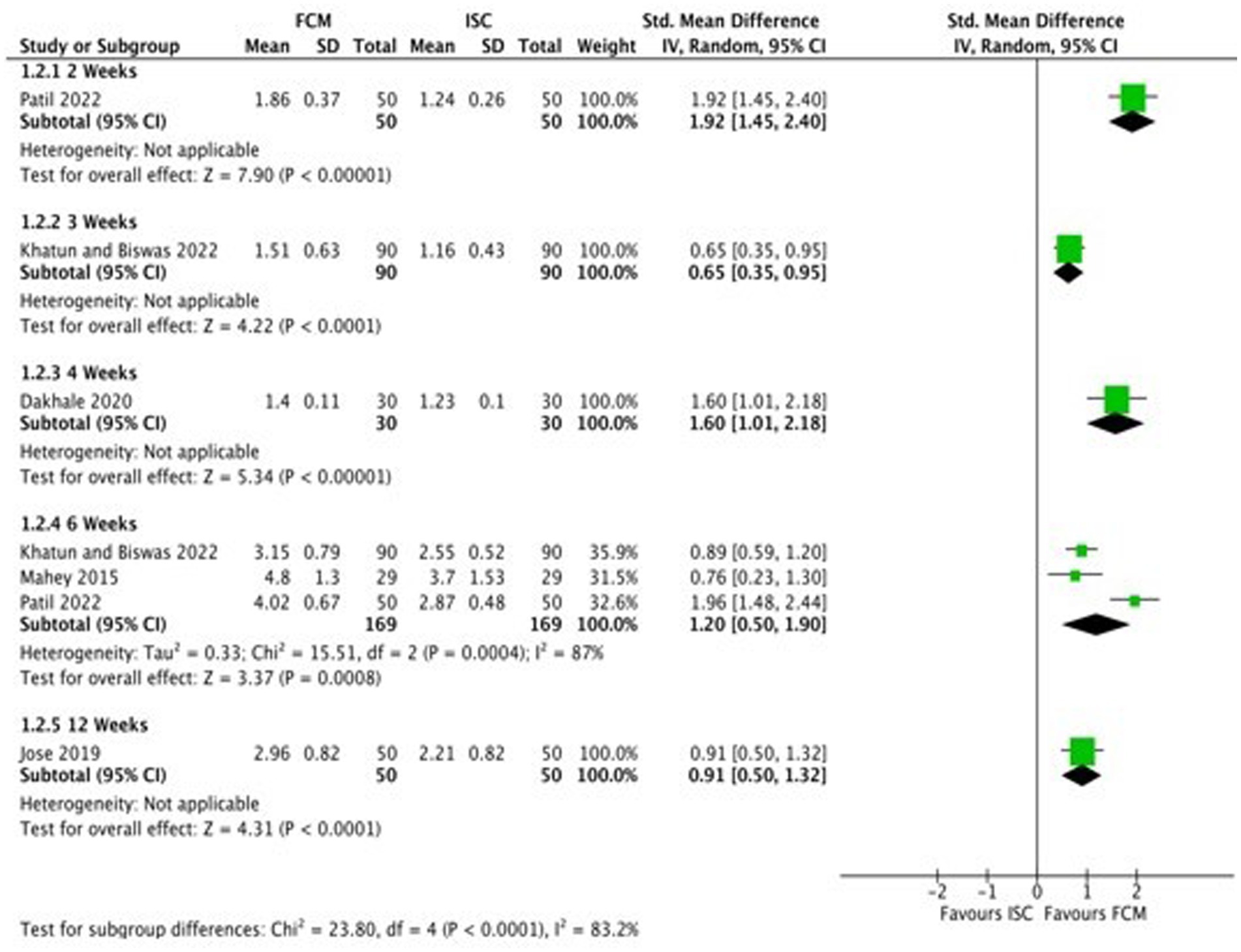
Six studies compared FCM with ISC on hemoglobin levels in moderate to severe anemic participants as post-scores, and four studies as change-scores. The outcomes were assessed at 2, 3, 4, 6 and 12 weeks. At 12 weeks, all studies showed significant improvements in Hb levels at post-scores and change-scores except for Mahey et al. (27) (Figure 4; Supplementary Figure S1).
3.3.1.3 Adverse events
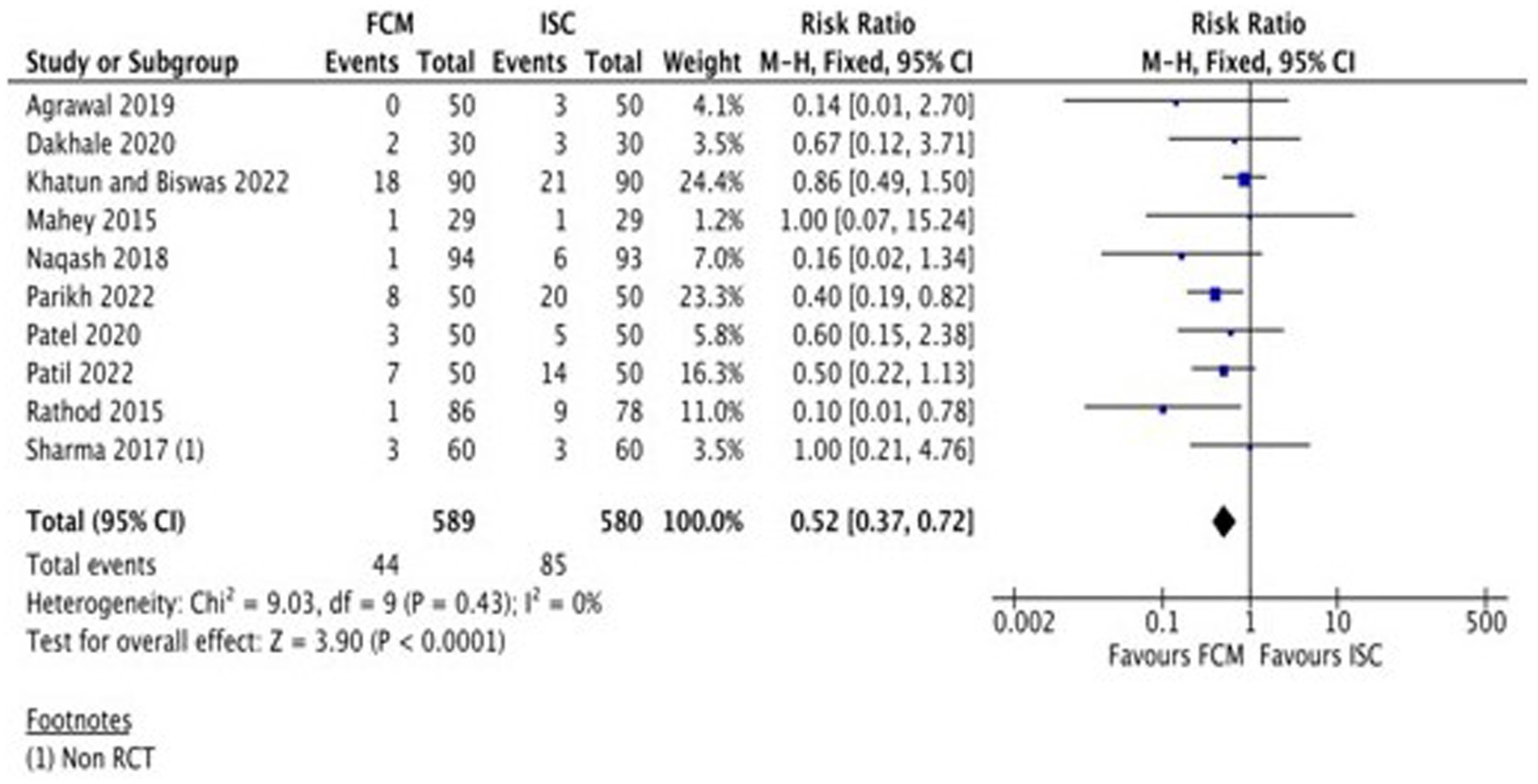
A total of eight studies (Table 2) assessed the adverse events of FCM and ISC when administered in moderate to severely anemic participants. All studies showed fewer adverse events with FCM as compared to ISC. The pooled analysis shows that the risk of adverse events in FCM group was 48% less than in ISC group (RR 0.52, 95% CI 0.37 to 0.72; participants = 1,169; studies = 10; I2 = 0%) (Figure 5; Supplementary Figure S2).
None of the studies reported any serious adverse drug reaction in FCM group requiring hospitalization.
3.3.1.4 Serum ferritin
A total of six studies (18–21, 28, 30) reported data on serum ferritin levels at baseline and at the end of the treatment. Studies reported serum ferritin levels at baseline and at different time points after 2, 3, 4, 6, and 12 weeks (18–21, 28, 30). Three studies reported change scores of serum ferritin levels (18, 21, 29). Subgroup analysis was undertaken according to the different time points. All the included studies demonstrate that the serum ferritin levels in the FCM group were significantly higher than in the ISC group. As the heterogeneity amongst the studies was substantial (I2 = 98.3% for post-scores and I2 = 92.9% for change-scores), and as some studies (18, 21, 28) reported serum ferritin levels at different time points, we did not pool the findings of the studies (Figure 6; Supplementary Figure S3).
3.3.1.5 Serum iron
Only two studies (19, 27) reported data on serum iron levels before treatment and after treatment at 4 weeks, 6 weeks, and at 12 weeks. One study by Mahey et al. (27) reported data at 6, and 12 weeks and Naqash et al. (19) at 4 weeks. Subgroup analysis was undertaken according to the different time points. All the included studies demonstrate that the serum ferritin levels in the FCM group were higher than in the ISC group. As the heterogeneity amongst the studies was substantial (I2 = 99.2%), and as one study (27) reported serum ferritin levels at different time points, we did not pool the findings of the studies (Supplementary Figure S4).
3.3.1.6 Total iron binding capacity (TIBC)
Only two studies (15, 19) reported data on TIBC. One study by Jose et al. (15) reported data at 3, 6, and 12 weeks and Naqash et al. (15), at 4 weeks. Subgroup analysis was undertaken according to the different time points. All the included studies demonstrates that the serum iron levels in the FCM group were lower than in the ISC group in two studies at 3 weeks and at 4 weeks (19) but not at 6 and 12 weeks (15). As the heterogeneity amongst the studies was substantial (I2 = 99.2%), and as one study (15) reported serum ferritin levels at different time points, we did not pool the findings of the studies (Supplementary Figure S5).
3.3.2 Comparison 2: ferric carboxymaltose versus iron sorbitol
3.3.2.1 Anemia
None of the included studies reported this outcome.
3.3.2.2 Hemoglobin
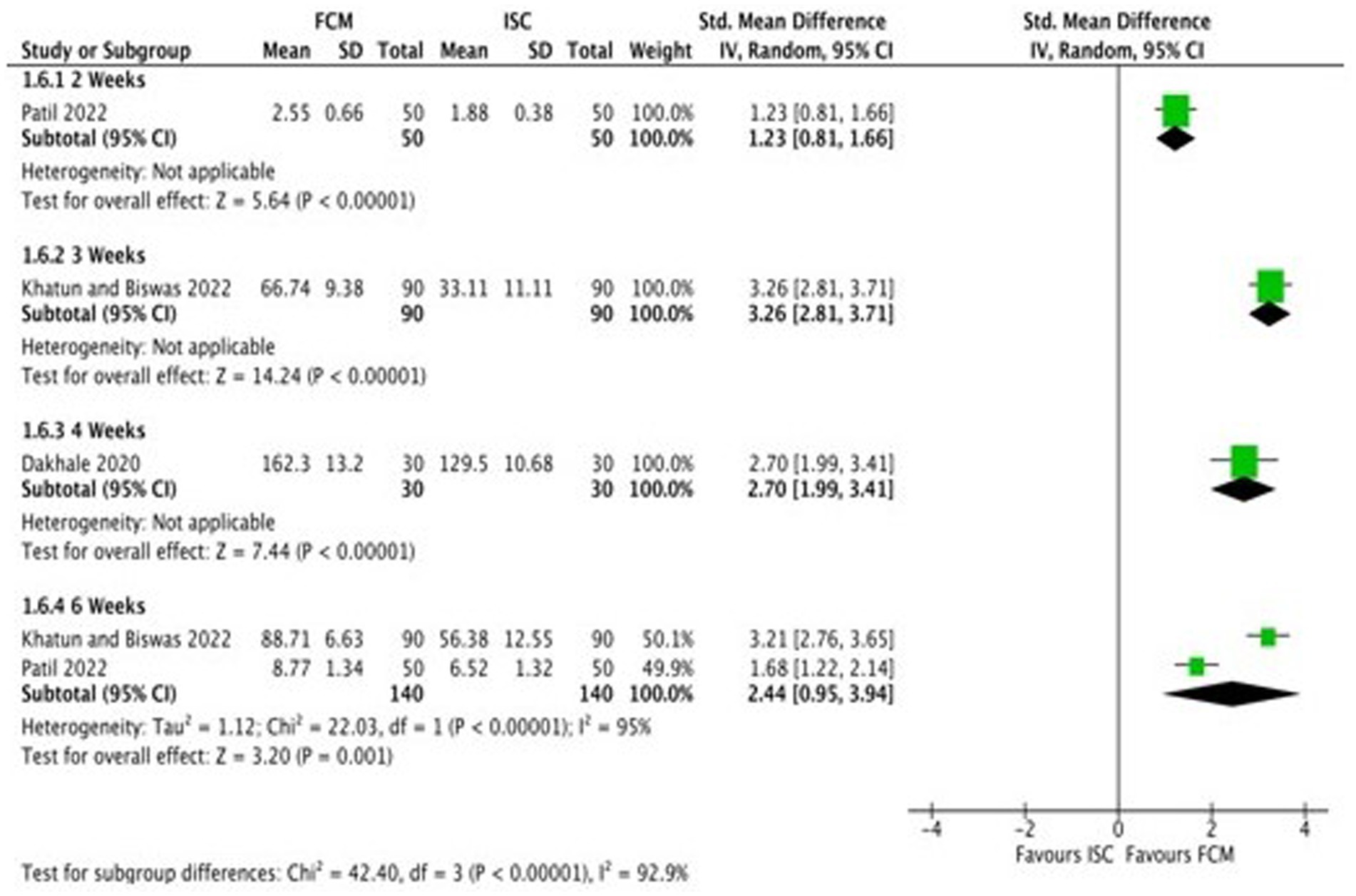
Only one study (21) compared FCM with intramuscular injection of iron sorbitol on hemoglobin levels in anemic participants as post-scores and two studies as change-scores. The outcomes were assessed at 2 and 6 weeks. The study showed significant improvements in Hb levels at post-scores as well as change-scores (Figure 7; Supplementary Figure S6).
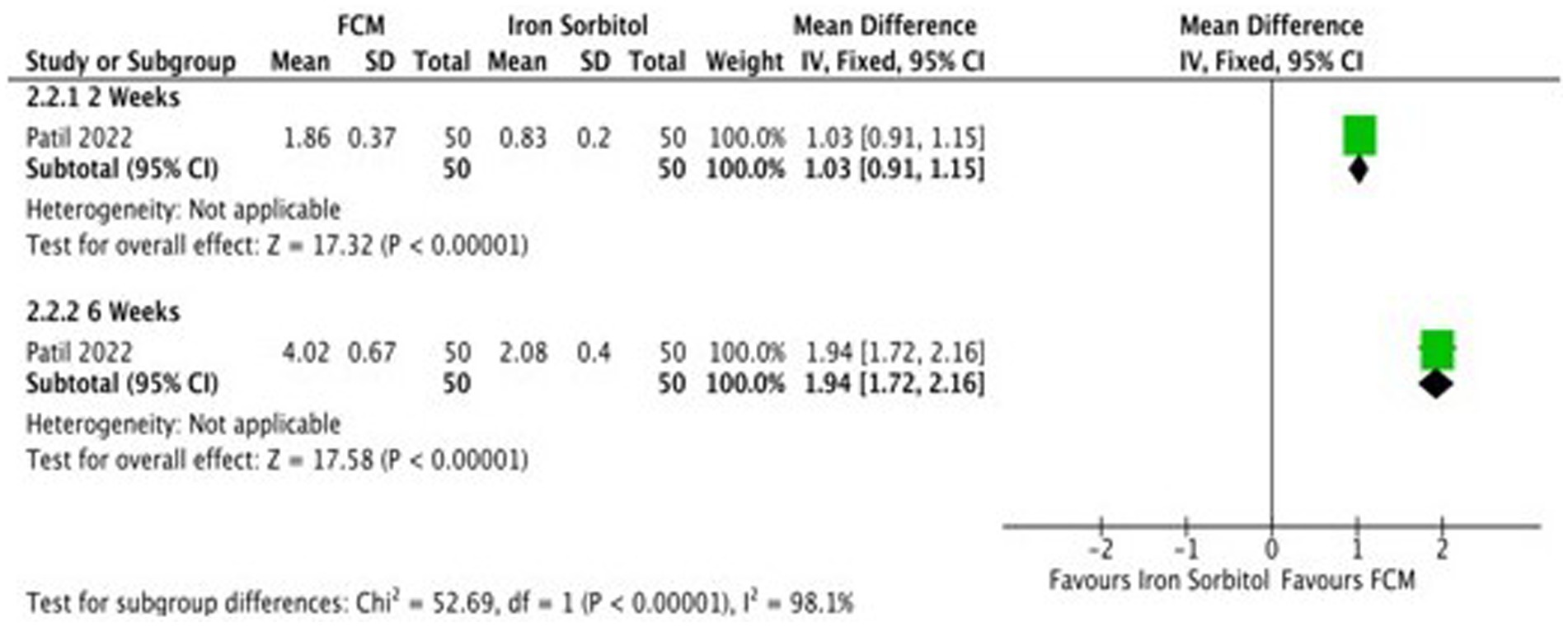
Figure 7. Forest plot of comparison: FCM verses alternative experimental treatment modality, outcome: hemoglobin (change-scores).
3.3.2.3 Adverse events
Only one study (21) assessed the adverse events of FCM and iron sorbitol when administered to anemic participants. The study showed fewer adverse events with FCM as compared to iron sorbitol. The pooled analysis shows that the risk of adverse events in FCM group was 78% less than that in iron sorbitol (RR 0.22, 95% CI 0.11 to 0.45; participants = 100; studies = 1).
3.3.2.4 Serum ferritin
Only one study (21) detailed the data on serum ferritin levels at baseline and at the end of the treatment and changed scores at baseline level and at 2 weeks, and 6 weeks. Subgroup analysis was undertaken according to the different time points. The study demonstrates that the serum ferritin levels in the FCM group were significantly higher as compared to iron sorbitol. The serum ferritin levels were higher at 6 weeks as compared to 2 weeks (Figure 8; Supplementary Figure S7).
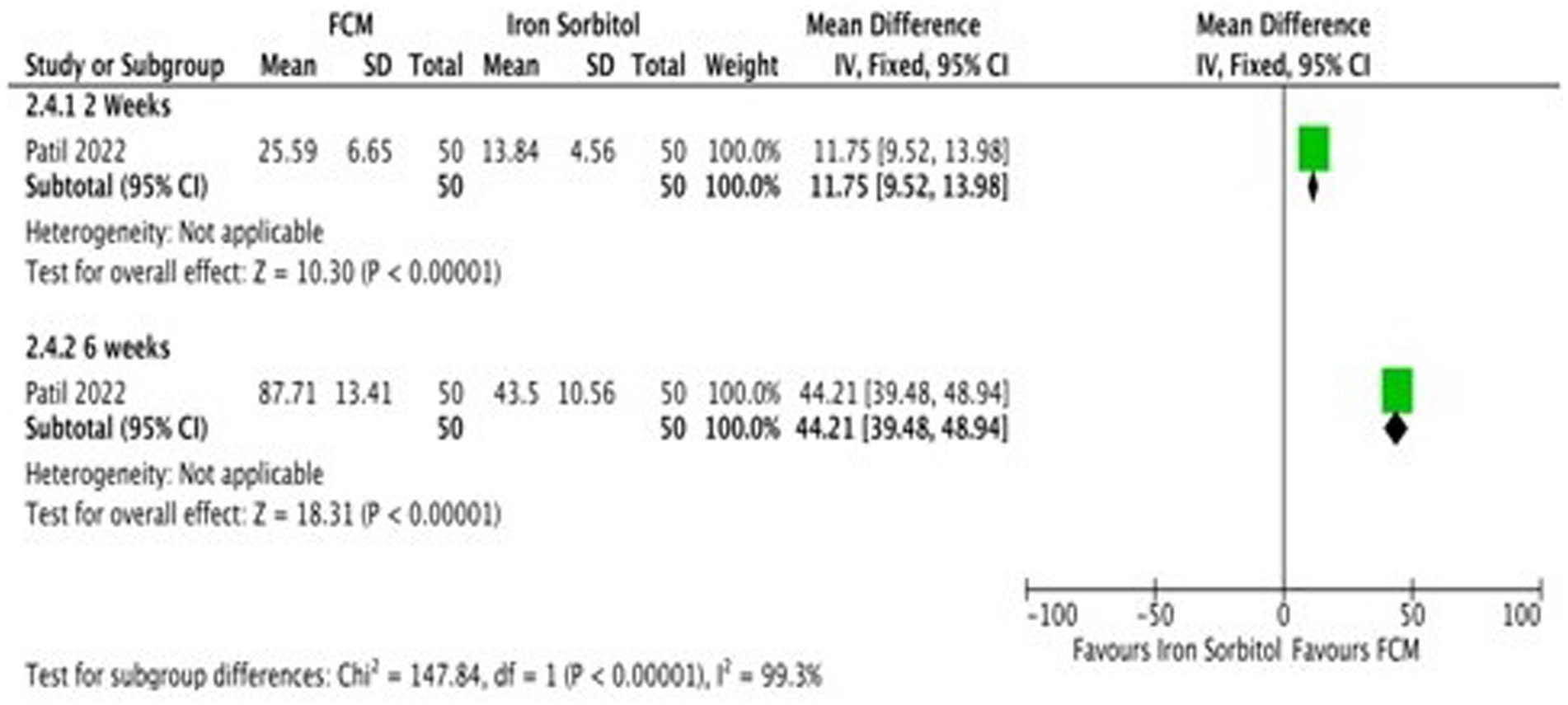
Figure 8. Forest plot of comparison: FCM verses alternative experimental treatment modality, outcome: serum ferritin (change scores).
3.3.2.5 TIBC
None of the included studies reported this outcome.
3.3.3 Comparison 3: ferric carboxymaltose versus oral iron
3.3.3.1 Anemia
None of the included studies reported this outcome.
3.3.3.2 Hemoglobin
Only two studies (28, 31) compared FCM with oral iron (ferrous ascorbate) on hemoglobin levels in anemic participants as post-scores as well as change-scores. In one study (31) outcomes were assessed at 1 week and 4 weeks, and in the other study (28), the outcomes was assessed at 2 weeks and 6 weeks. Both the studies showed significant improvements in Hb levels at post-scores as well as change-scores in FCM group as compared to oral iron (Figure 9; Supplementary Figure S8).
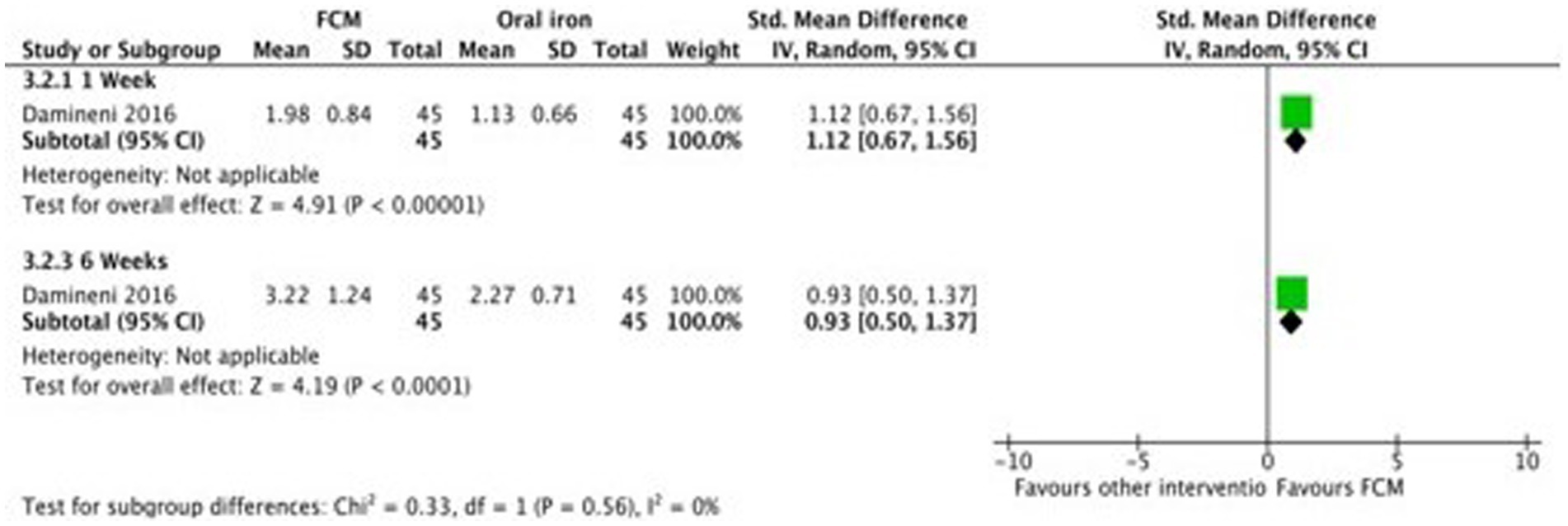
Figure 9. Forest plot of comparison: FCM verses alternative experimental treatment modality, outcome: hemoglobin (change-scores).
3.3.3.3 Adverse events
Only two studies (28, 31) assessed the adverse events of FCM and oral iron (ferrous ascorbate) when administered in anemic participants. The study showed fewer adverse events with FCM as compared to oral iron. The pooled analysis shows that the risk of adverse events in FCM group was 98% less than in oral iron (RR 0.02, 95% CI 0.01 to 0.12; participants = 246; studies = 2) (Figure 10).
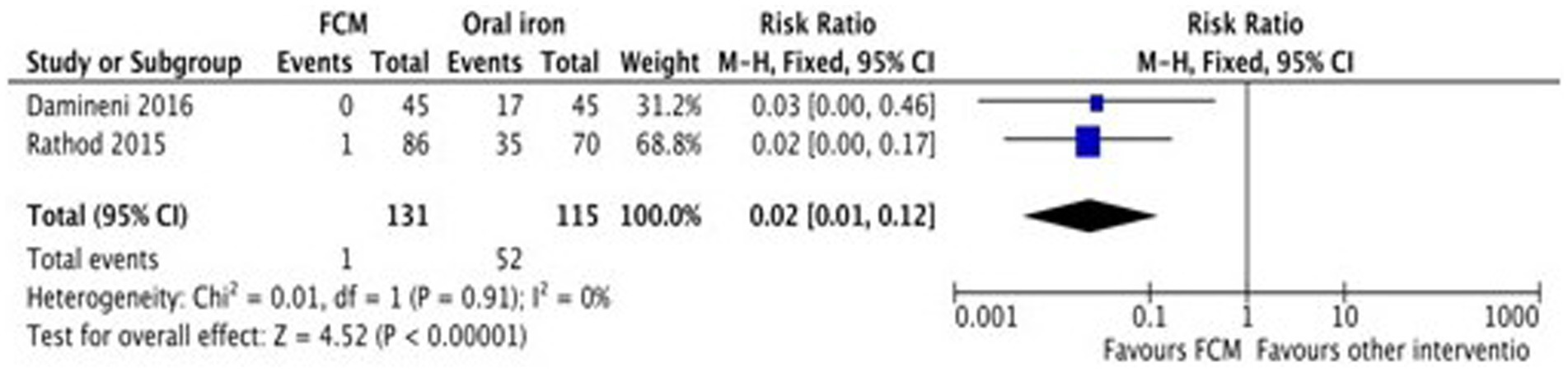
Figure 10. Forest plot of comparison: FCM verses alternative experimental treatment modality, outcome: adverse events.
3.3.3.4 Serum ferritin
Only one study (28) reported data on serum ferritin levels at baseline and at the end of the treatment at 2 weeks, and 6 weeks. The study demonstrates that the serum ferritin levels in the FCM group were significantly higher as compared to oral iron. The serum ferritin levels were lesser at 6 weeks as compared to 2 weeks (Supplementary Figure S9).
3.3.3.5 TIBC
None of the included studies reported this outcome.
3.3.4 Comparison 4: ferric carboxymaltose in combination with other treatments versus ferric carboxymaltose treatment alone
None of the included studies assessed this comparison.
3.4 Quality of evidence
3.4.1 GRADE assessments for FCM compared to ISC for anemia in Indians
The assessment of hemoglobin quality using Tables 3, 4 revealed ‘low quality’ at 2, 4, and 6 weeks, “moderate quality” at 3 weeks, and “very low quality” at 12 weeks. This was attributed to a high risk of bias and limited participant numbers in the analysis. The quality of evidence for serum ferritin was “low quality” at 2, 4, and 12 weeks, and “very low quality” at 6 weeks due to factors like high risk of bias, presence of heterogeneity, and a few number of participants in the analysis. The quality of evidence for adverse events was assessed as ‘moderate quality’ due to the presence of high risk of bias.
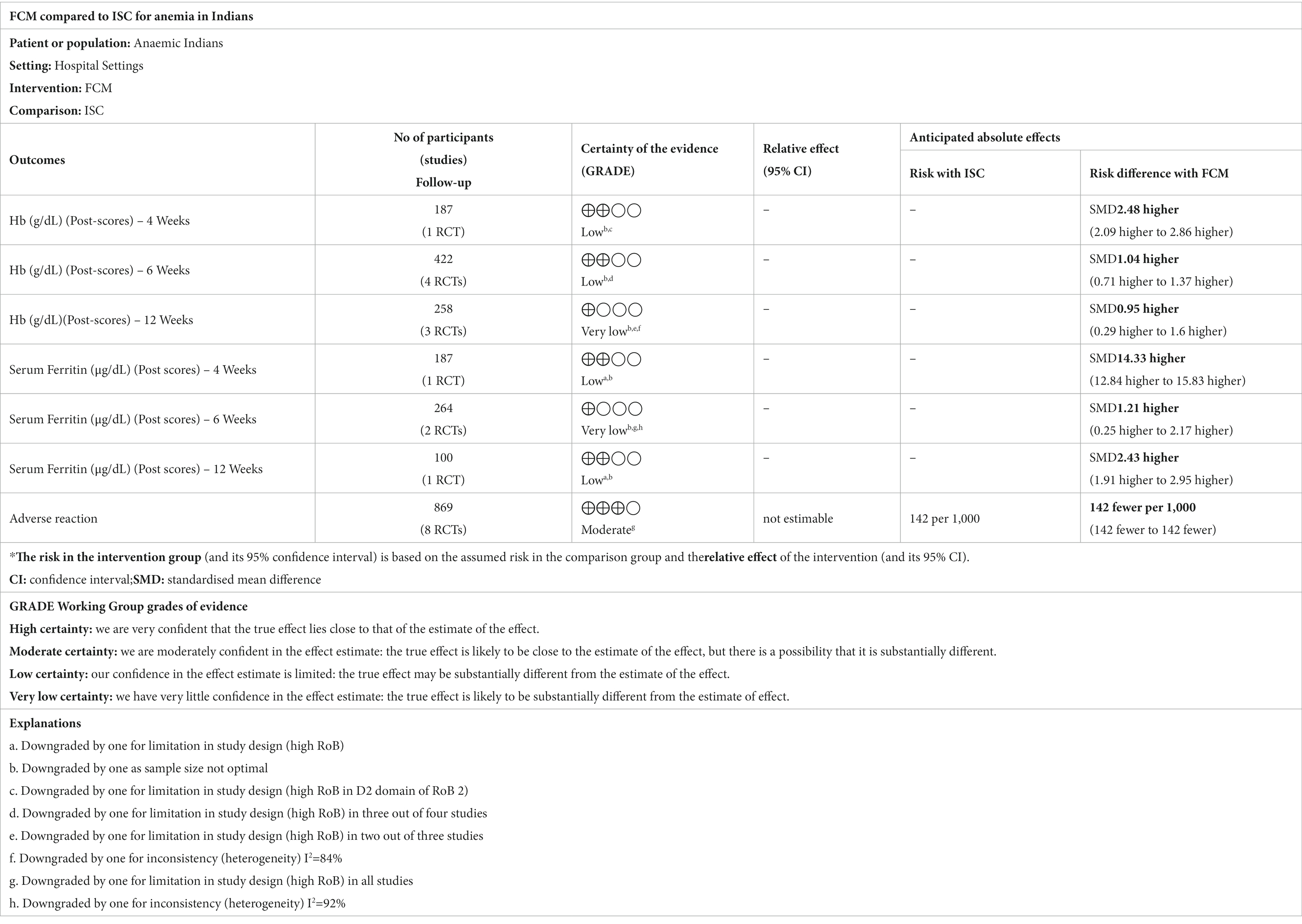
Table 3. GRADE assessments for ferric carboxymaltose compared to iron sucrose complex (ISC) for anemia in Indians.
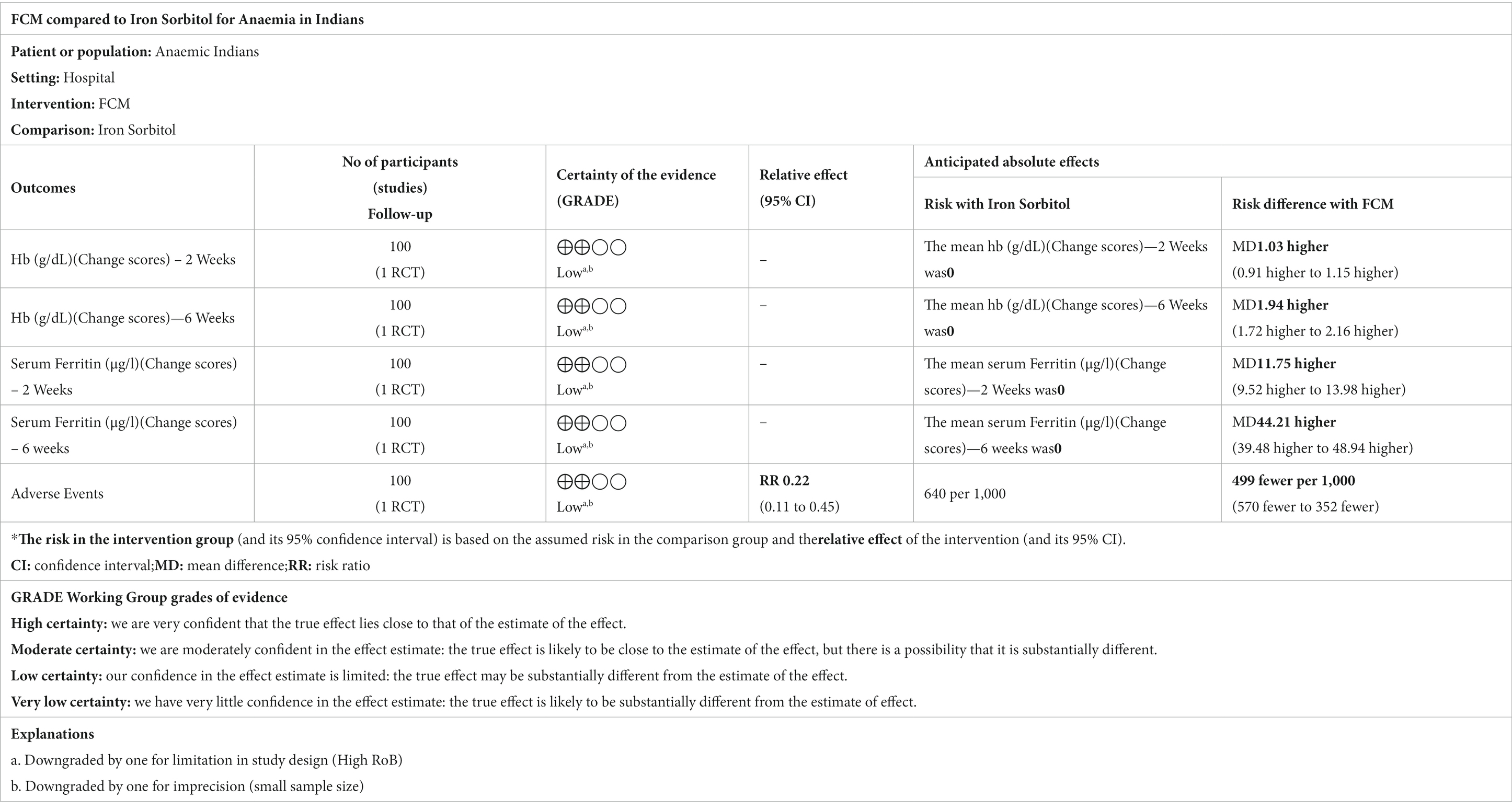
Table 4. GRADE assessments for ferric carboxymaltose compared to iron sorbitol for anemia in Indians.
When using the “RoB 2” tool, we found that all included studies had high risk of bias in at least one of the six domains. Most of the included studies did not describe the method of randomization and allocation concealment that may pose a serious selection bias (D1: Randomization process). The majority of the studies were open-label, and it was uncertain if blinding was successful in blinded studies, raising the potential of performance bias (D2: Deviation from intended intervention). Clinical outcome measures, such as hemoglobin, serum ferritin not affected by the subjectivity of the participants, were highly subjected to selection and performance bias whereas adverse events were highly subjected to selection (D1: Randomization process), performance (D2: Deviation from intended intervention), and detection bias (D4: Measurement of the outcome).
The inconsistency was only high for two outcomes (Hemoglobin and serum ferritin, Comparison 1 and 2) owing to a considerable level of heterogeneity, which was addressed through subgroup analyzes. The evidence in this review did not have issues regarding indirectness. Imprecision was an issue owing to small sample sizes, which lowered our confidence in the effects by one level. Except for two studies (15, 19), we did not find the protocol available. Hence, the risk of reporting bias had some concern. There were insufficient trials included in the meta-analyzes to utilize a funnel plot and assess the possible risk of publication bias.
4 Discussion
Our findings indicate that FCM can serve as a viable option for women with IDA, addressing not only the correction of hemoglobin deficiency but also the replenishment of iron stores. Other treatment of IDA, such as Iron sucrose complex, Iron sorbitol, or ferrous ascorbate, showed an increase in the hemoglobin level; however, the increment was significantly higher in the participants treated with FCM as compared to ISC infusion or iron sorbitol infusion, or oral iron. Serum ferritin level was also increased in the other treatment modalities but was higher in participants treated with FCM. FCM was well tolerated in patients with IDA, and most drug-related adverse events considered mild to moderate in severity.
The convenient dosing with a lesser total number of required doses decreased the reduces the frequency of hospital visits and, in turn, resulted in satisfactory compliance (18), higher patient satisfaction (28), higher acceptability, better general well-being (28), better HRQOL (19), minimum requirement of hospital resources and increase in acceptability as compared to patients treated with other treatment modalities (19).
4.1 Overall completeness and applicability of evidence
Evidence regarding FCM for anemia in India is limited, with data available only from small sample-sized RCTs that limits us from reaching reliable conclusions regarding the effects of FCM. These studies are also limited in their generalisability, as all the studies included women between 18 to 40 years of age.
The results of this systematic review can only be interpreted in consideration of the following factors.
• None of the studies assessed the comparison between FCM in combination with other treatments versus FCM alone.
• Only two studies assessed serum iron and TIBC in FCM and ISC group.
• Only one study assessed hemoglobin and adverse events in FCM verses iron Sorbitol
• Two ongoing studies (Table 2) will substantially increase the amount of available data to analyze.
4.2 Potential biases in the review process
Our review followed the principles outlined in Cochrane’s Handbook of Systematic Reviews (32). We executed a thorough search and searched data sources (including multiple databases, and clinical trial registries) that necessitate the inclusion of all published studies concerning FCM formulations. Although language limitations were taken into account, our focus remained on studies published in languages we anticipated. The evaluation of each study’s relevance was carried out meticulously, with the screening process executed by independent reviewers in duplicate. For robustness, data extraction, encompassing assessments of risk of bias (RoB) as well as GRADE assessments, were undertaken in duplicate by two independent reviewers. This dual approach served to guarantee the precision and accuracy of data extraction and reporting.
The present study was based on comprehensive bibliographical search that encompassed the inclusion of all published clinical trials addressing various intravenous formulations.
Due to a lack of details in the methodology and results section of the included studies, we had to pool some incompatible data. Some data were provided graphically in the published reports. The absence of crucial information like standard deviations hindered the execution of specific analyzes.
4.3 Agreements and disagreements with other studies or reviews
The findings of this systematic review confirm the results from already published systematic reviews on studies from other countries. In all the available systematic reviews (Table 5), among the different iron formulations available for the treatment of IDA, FCM was found to be superior when compared with other iron regimens in terms of improving hemoglobin levels and serum ferritin levels in different populations with iron deficiency anemia and indicated a high safety profile.
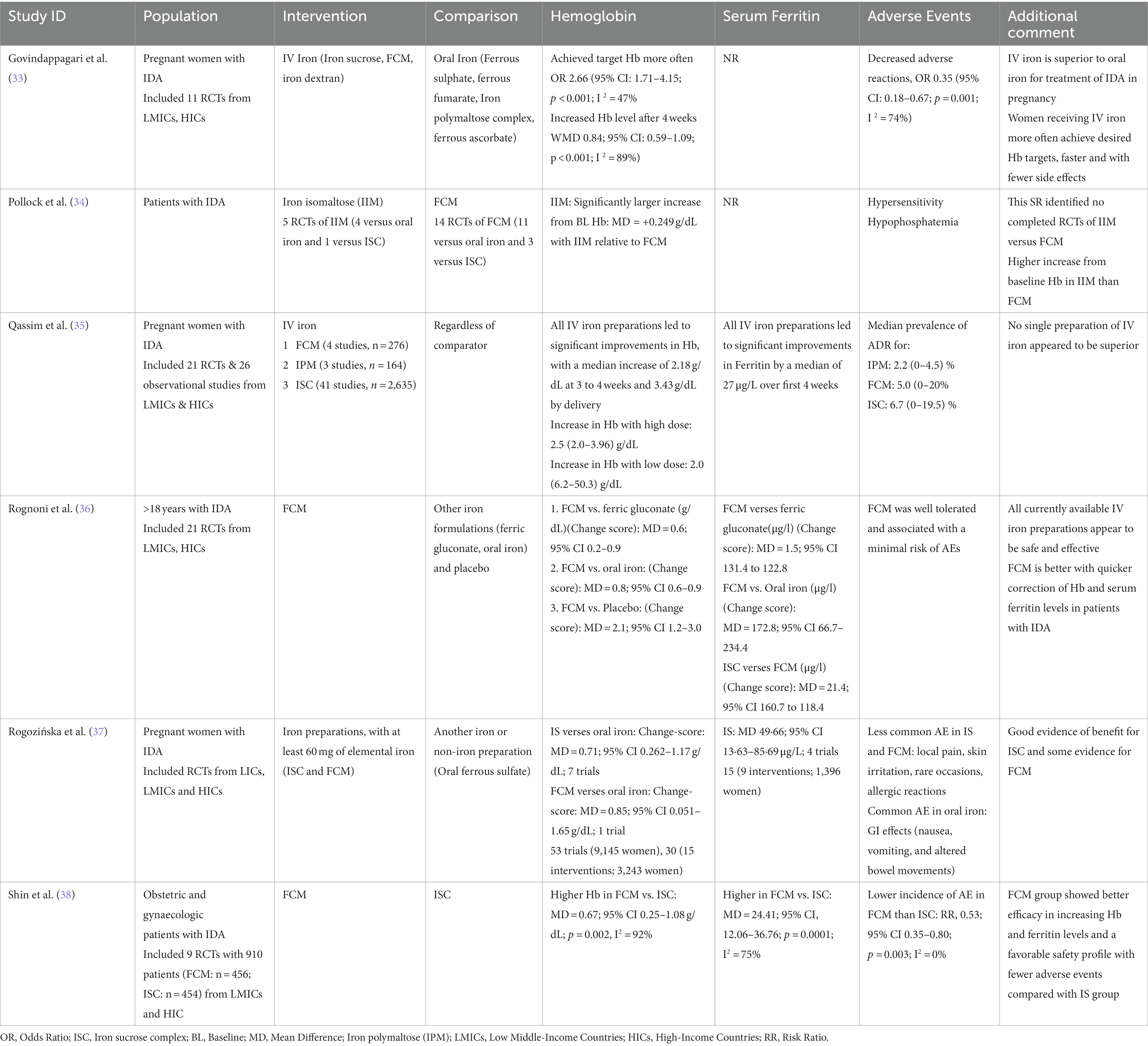
Table 5. Findings of other systematic reviews for all countries on effect of FCM for treatment of anemia.
5 Conclusion
The evidence from this SR does not support a robust clinical efficacy conclusion. In summary, this review indicates that a gradual single 1 g FCM infusion is both safe and effective for treating IDA in women. FCM demonstrates superior elevation of hemoglobin levels and restoration of iron stores compared to other interventions (ISC, Iron sucrose, and ferrous ascorbate), with minimal adverse events. However, the evidence is of ‘low’ to ‘very low’ quality, primarily based on ten studies with a high risk of bias and insufficient participant numbers for conclusive results. Further research is likely to influence these findings. FCM consistently shows fewer adverse events than other interventions, with evidence ranging from ‘moderate’ to ‘very low’ quality. The outcomes indicate FCM’s well-tolerated, safe, and effective role as an alternative to other interventions for IDA in women.
FCM offers the advantage of swiftly addressing IDA in some patients within just 2 weeks, requiring a single dose without the need for repeated administrations—thus offering a more convenient treatment approach. This approach also offers benefits such as administering a substantial dose per session, minimizing the number of required doses, reducing hospital visits, lowering transportation costs, needing less infusion-related equipment, and alleviating patient discomfort linked to multiple needle insertions.
However, despite these advantages, the body of evidence in this SR falls short of supporting a definitive conclusion regarding clinical efficacy.
5.1 Implications for research
Information from adequately powered, multicentric, methodologically rigorous RCTs to compare the efficacy and safety of FCM over other alternative treatment modalities for the treatment of anemia are necessitated. Cost-effectiveness analyzes are also necessitated.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
MK: Supervision, Writing – review & editing, Conceptualization, Funding acquisition, Formal analysis, Investigation, Methodology, Software, Validation, Writing – original draft. AS: Conceptualization, Formal analysis, Investigation, Resources, Validation, Supervision, Writing – review & editing. SG: Conceptualization, Validation, Visualization, Writing – review & editing. SU: Data curation, Formal analysis, Methodology, Software, Writing – original draft, Writing – review & editing. NW: Data curation, Formal analysis, Methodology, Writing – original draft. AA: Data curation, Formal analysis, Methodology, Software, Writing – original draft, Writing – review & editing. DS: Formal analysis, Methodology, Supervision, Validation, Visualization, Writing – review & editing. SS: Formal analysis, Supervision, Visualization, Writing – review & editing. PS: Formal analysis, Methodology, Supervision, Validation, Writing – review & editing. AG: Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – review & editing. ZQ: Formal analysis, Methodology, Project administration, Resources, Supervision, Visualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This systematic review was funded by the Indian Council of Medical Research (ICMR), Department of Health Research, Ministry of Health and Family Welfare, Government of India, New Delhi, India. Grant Number: 5/7/594/1/-RHN.
Acknowledgments
We gratefully acknowledge the Research and Development department of Datta Meghe Institute of Higher Education and Research for their administrative support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1340158/full#supplementary-material
Footnotes
References
1. World Health Organization . Anemia. Available at: https://www.who.int/health-topics/anemia#tab=tab_1
2. Adolescent Division (2013). Ministry of Health and Family Welfare, Government of India. Guidelines for control of iron deficiency anemia. Available at: https://www.nhm.gov.in/images/pdf/programmes/child-health/guidelines/Control-of-Iron-Deficiency-Anemia.pdf
3. International Institute for Population Sciences (IIPS) and ICF (2022). National Family Health Survey (NFHS – 5), 2019–21. Available at: https://dhsprogram.com/pubs/pdf/FR375/FR375.pdf
4. Trivedi, P , Chitra, S , Natarajan, S , Amin, V , Sud, S , Vyas, P, et al. Ferric Carboxymaltose in the Management of Iron Deficiency Anemia in pregnancy: a subgroup analysis of a Multicenter real-world study involving 1191 pregnant women. Obstet Gynecol Int. (2022) 2022:1–7. doi: 10.1155/2022/5759740
5. WHO . Worldwide prevalence of anemia 1993–2005: WHO global database on anemia. Geneva, Switzerland: WHO (2008).
6. Abu-Ouf, NM , and Jan, MM . The impact of maternal iron deficiency and iron deficiency anemia on child’s health. Saudi Med J. (2015) 36:146–9. doi: 10.15537/smj.2015.2.10289
7. Gautam, CS , Saha, L , Sekhri, K , and Saha, PK . Iron deficiency in pregnancy and the rationality of iron supplements prescribed during pregnancy. Medscape J Med. (2008) 10:283.
8. Patel, AR , Patel, VS , and Patel, PR . A comparative study of ferric carboxymaltose and iron sucrose as a parenteral iron treatment in iron deficiency anemia during pregnancy. Int J Reprod Contracept Obstet Gynecol. (2020) 9:2437. doi: 10.18203/2320-1770.ijrcog20202325
9. Allen, LH . Anemia and iron deficiency: effects on pregnancy outcome. Am J Clin Nutr. (2000) 71:1280S–4S. doi: 10.1093/ajcn/71.5.1280s
10. Vanobberghen, F , Lweno, O , Kuemmerle, A , Mwebi, KD , Asilia, P , Issa, A, et al. Efficacy and safety of intravenous ferric carboxymaltose compared with oral iron for the treatment of iron deficiency anemia in women after childbirth in Tanzania: a parallel-group, open-label, randomized controlled phase 3 trial. Lancet Glob Health. (2021) 9:e189–98. doi: 10.1016/S2214-109X(20)30448-4
11. Charmila, A , Natarajan, S , Chitra, TV , Pawar, N , Kinjawadekar, S , Firke, Y, et al. Efficacy and safety of ferric Carboxymaltose in the Management of Iron Deficiency Anemia: a multi-Center real-world study from India. J Blood Med. (2022) 13:303–13. doi: 10.2147/JBM.S361210
12. Froessler, B , Collingwood, J , Hodyl, NA , and Dekker, G . Intravenous ferric carboxymaltose for anemia in pregnancy. BMC Pregnancy Childbirth. (2014) 14:115. doi: 10.1186/1471-2393-14-115
13. Lyseng-Williamson, KA , and Keating, GM . Ferric Carboxymaltose: a review of its use in iron-deficiency anemia. Drugs. (2009) 69:739–56. doi: 10.2165/00003495-200969060-00007
14. Agrawal, D , and Masand, DL . A study for efficacy and safety of ferric carboxymaltose versus iron sucrose in iron deficiency anemia among pregnant women in tertiary care hospital. Int J Reprod Contracept Obstet Gynecol. (2019) 8:2280. doi: 10.18203/2320-1770.ijrcog20192418
15. Jose, A , Mahey, R , Sharma, JB , Bhatla, N , Saxena, R , Kalaivani, M, et al. Comparison of ferric Carboxymaltose and iron sucrose complex for treatment of iron deficiency anemia in pregnancy-randomized controlled trial. BMC Pregnancy Childbirth. (2019) 19:54. doi: 10.1186/s12884-019-2200-3
16. Kant, S , Haldar, P , Malhotra, S , Kaur, R , Rath, R , and Jacob, OM . Intravenous ferric carboxymaltose rapidly increases hemoglobin and serum ferritin among pregnant females with moderate-to-severe anemia: a single-arm, open-label trial. Natl Med J India. (2020) 33:324–8. doi: 10.4103/0970-258X.321145
17. Kaur, R , Kant, S , Haldar, P , Ahamed, F , Singh, A , Dwarakanathan, V, et al. Single dose of intravenous ferric Carboxymaltose prevents Anemia for 6 months among moderately or severely Anemic postpartum women: a case study from India. Curr Dev Nutr. (2021) 5:nzab078. doi: 10.1093/cdn/nzab078
18. Khatun, F , and Biswas, C . Comparative study of intravenous iron sucrose versus intravenous ferric carboxymaltose in the management of iron deficiency anemia in pregnancy. Int J Reprod Contracept Obstet Gynecol. (2022) 11:505. doi: 10.18203/2320-1770.ijrcog20220179
19. Naqash, A , Ara, R , and Bader, GN . Effectiveness and safety of ferric carboxymaltose compared to iron sucrose in women with iron deficiency anemia: phase IV clinical trials. BMC Womens Health. (2018) 18:6. doi: 10.1186/s12905-017-0506-8
20. Parikh, A , and Agarwal, S . Intravenous ferric carboxymaltose versus iron sucrose in iron deficiency anemia of pregnancy. IJOGR. (2022) 9:10–4. doi: 10.18231/j.ijogr.2022.003
21. Patil, KA , and Tehalia, MK . Comparative efficacy and safety of ferric carboxymaltose, iron sucrose and iron sorbitol in treatment of iron deficiency anemia in Indian pregnant women. Int J Reprod Contracept Obstet Gynecol. (2022) 11:2692. doi: 10.18203/2320-1770.ijrcog20222464
22. Higgins, J , Thomas, J , Chandler, J , Cumpston, M , Li, T , Page, M, et al. Cochrane handbook for systematic reviews of interventions. 2nd ed. Chichester, UK: John Wiley & Sons (2019).
23. Page, MJ , Moher, D , Bossuyt, PM , Boutron, I , Hoffmann, TC , Mulrow, CD, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. (2021):n160:372. doi: 10.1136/bmj.n160
24. Sterne, JAC , Savović, J , Page, MJ , Elbers, RG , Blencowe, NS , Boutron, I, et al. RoB 2: a revised tool for assessing risk of bias in randomized trials. BMJ. (2019):l4898. doi: 10.1136/bmj.l4898
25. Sterne, JA , Hernán, MA , Reeves, BC , Savović, J , Berkman, ND , Viswanathan, M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomized studies of interventions. BMJ. (2016) 355:i4919. doi: 10.1136/bmj.i4919
26. Schünemann, H , Brożek, J , Guyatt, G , and Oxman, A (2013). GRADE handbook for grading quality of evidence and strength of recommendations [internet]. The GRADE working group. Available at: https://gdt.gradepro.org/app/handbook/handbook.html
27. Mahey, R , Kriplani, A , Mogili, KD , Bhatla, N , Kachhawa, G , and Saxena, R . Randomized controlled trial comparing ferric carboxymaltose and iron sucrose for treatment of iron deficiency anemia due to abnormal uterine bleeding. Int J Gynaecol Obstet. (2016) 133:43–8. doi: 10.1016/j.ijgo.2015.09.007
28. Rathod, S , Samal, SK , Mahapatra, PC , and Samal, S . Ferric carboxymaltose: a revolution in the treatment of postpartum anemia in Indian women. Int J Appl Basic Med Res. (2015) 5:25–30. doi: 10.4103/2229-516X.149230
29. Dakhale, GN , Kalikar, MV , Fuke, RP , Parmarthi, AS , and Chokhandre, MK . Comparative study of efficacy and safety of parenteral iron sucrose versus ferric carboxymaltose in treatment of postpartum iron deficiency anemia. Int J Reprod Contracept Obstet Gynecol. (2021) 11:100. doi: 10.18203/2320-1770.ijrcog20215083
30. Sharma, N , Thiek, JL , Natung, T , and Ahanthem, SS . Comparative study of efficacy and safety of ferric Carboxymaltose versus iron sucrose in post-partum anemia. J Obstet Gynecol India. (2017) 67:253–7. doi: 10.1007/s13224-017-0971-x
31. Damineni, SC , and Thunga, S . IV ferric Carboxymaltose vs Oral iron in the treatment of post-partum iron deficiency anemia. J Clin Diagn Res. (2016) 10:QC08–10. doi: 10.7860/JCDR/2016/19375.8937
32. Higgins, JPT , Thomas, J , Chandler, J , Cumpston, M , Li, T , Page, MJ, et al. (2022). Cochrane handbook for systematic reviews of interventions version 6.3 (updated February 2022) [internet]. Cochrane. Available at: www.training.cochrane.org/handbook
33. Govindappagari, S , and Burwick, RM . Treatment of iron deficiency Anemia in pregnancy with intravenous versus Oral iron: systematic review and meta-analysis. Am J Perinatol. (2019) 36:366–76. doi: 10.1055/s-0038-1668555
34. Pollock, RF , and Muduma, G . A systematic literature review and indirect comparison of iron isomaltoside and ferric carboxymaltose in iron deficiency anemia after failure or intolerance of oral iron treatment. Expert Rev Hematol. (2019) 12:129–36. doi: 10.1080/17474086.2019.1575202
35. Qassim, A , Mol, BW , Grivell, RM , and Grzeskowiak, LE . Safety and efficacy of intravenous iron polymaltose, iron sucrose and ferric carboxymaltose in pregnancy: a systematic review. Aust N Z J Obstet Gynaecol. (2018) 58:22–39. doi: 10.1111/ajo.12695
36. Rognoni, C , Venturini, S , Meregaglia, M , Marmifero, M , and Tarricone, R . Efficacy and safety of ferric Carboxymaltose and other formulations in iron-deficient patients: a systematic review and network meta-analysis of randomized controlled trials. Clin Drug Investig. (2016) 36:177–94. doi: 10.1007/s40261-015-0361-z
37. Rogozińska, E , Daru, J , Nicolaides, M , Amezcua-Prieto, C , Robinson, S , Wang, R, et al. shin. Lancet Haematol. (2021) 8:e503–12. doi: 10.1016/S2352-3026(21)00137-X
38. Shin, HW , Go, DY , Lee, SW , Choi, YJ , Ko, EJ , You, HS, et al. Comparative efficacy and safety of intravenous ferric carboxymaltose and iron sucrose for iron deficiency anemia in obstetric and gynecologic patients: a systematic review and meta-analysis. Medicine (Baltimore). (2021) 100:e24571. doi: 10.1097/MD.0000000000024571
Keywords: ferric carboxymaltose, iron deficiency anemia, intravenous iron supplementation, moderate to severe anemia, hemoglobin
Citation: Khatib MN, Sinha AP, Gaidhane S, Upadhyay S, Waghmare N, Anil A, Saxena D, Sawleshwarkar S, Simkhada PP, Gaidhane A and Quazi ZS (2024) Effect of IV ferric carboxy maltose for moderate/severe anemia: a systematic review and meta-analysis. Front. Med. 11:1340158. doi: 10.3389/fmed.2024.1340158
Edited by:
Ahmet Emre Eskazan, Istanbul University-Cerrahpasa, TürkiyeReviewed by:
Pinar Yalcin Bahat, University of Health Sciences, TürkiyeZixing Zhong, Zhejiang Provincial People's Hospital, China
Copyright © 2024 Khatib, Sinha, Gaidhane, Upadhyay, Waghmare, Anil, Saxena, Sawleshwarkar, Simkhada, Gaidhane and Quazi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zahiruddin Syed Quazi, emFoaXJxdWF6aUBnbWFpbC5jb20=
 Mahalaqua Nazli Khatib
Mahalaqua Nazli Khatib Anju Pradhan Sinha
Anju Pradhan Sinha Shilpa Gaidhane
Shilpa Gaidhane Shilpa Upadhyay
Shilpa Upadhyay Nikita Waghmare
Nikita Waghmare Abhishek Anil
Abhishek Anil Deepak Saxena
Deepak Saxena Shailendra Sawleshwarkar
Shailendra Sawleshwarkar Padam Prasad Simkhada
Padam Prasad Simkhada Abhay Gaidhane
Abhay Gaidhane Zahiruddin Syed Quazi
Zahiruddin Syed Quazi