- 1Department of Orthopedic, Hebei PetroChina Central Hospital, Langfang, China
- 2Hospital of Stomatology Hebei Medical University, Shijiazhuang, China
- 3Department of Endoscopy, Shijiazhuang Traditional Chinese Medicine Hospital, Shijiazhuang, China
Objective: High-density lipoprotein cholesterol (HDL-C) has been reported to be associated with pain symptoms of various diseases, and its anti-inflammatory and antioxidant mediation is related to the pathogenesis of chronic pain. This study aims to evaluate the relationship between HDL-C levels and chronic pain in American adults.
Methods: This cross-sectional study used data from American adults aged 20 and above during the 2003–2004 National Health and Nutrition Examination Survey (NHANES) cycle. Participants were divided into 4 groups based on HDL-C quartiles. We used chi-square tests and Student’s t-tests or Mann-Whitney U tests to analyze categorical variables and continuous variables to compare differences between groups. Multivariate logistic regression analysis was used to study the association between HDL-C levels and the risk of chronic pain. Likelihood ratio tests were used to assess interactions between subgroups, and sensitivity analyses were conducted.
Results: Our final analysis included 4,688 participants, of which 733 (16.4%) had chronic pain. In the multivariate logistic regression model adjusted for covariates, there was a negative correlation between HDL-C levels and chronic pain. Specifically, for every 20 unit increase in HDL-C, the risk of chronic pain decreased by 26%. Compared with the lowest HDL-C quartile (< 43 mg/dL), the highest HDL-C quartile (≥ 64 mg/dL) was associated with a 24% reduction in the risk of chronic pain. No interaction factors affecting the relationship between HDL-C and chronic pain were found in the subgroup analysis.
Conclusion: This study demonstrates a negative association between HDL-C levels and chronic pain in US adults, providing insights into the pathogenesis of chronic pain and potential improvements in chronic pain management strategies.
1 Introduction
Chronic pain, characterized as pain that lingers beyond the typical healing time frame (usually 3 months), poses a significant burden on individuals and economies (1). Research indicates that over 30% of people worldwide are affected by chronic pain (2). Individuals with chronic pain are more likely to experience anxiety, depression, activity limitations, opioid dependence, and reduced quality of life (3–5). Currently, the mechanisms underlying chronic pain remain challenging to identify, resulting in suboptimal management strategies that focus solely on symptoms or diseases (2). Therefore, to develop better prevention and management strategies, more research into risk factors for chronic pain is needed (6).
Chronic pain is typically viewed as a symptom rather than a disease. A variety of conditions, such as cardiovascular disease, diabetes, arthritis, and disk disease, may present with symptoms of chronic pain (7). Lipids are crucially involved in the pathophysiological processes of chronic pain. High-density lipoprotein cholesterol (HDL-C) is a molecule that is prevalent in or bound to HDL (8). HDL-C is considered beneficial for human health, and an increasing body of evidence suggests that low levels of HDL-C are associated with the aforementioned conditions and their concomitant symptoms of chronic pain, such as chest pain associated with acute coronary disease (9), neuropathic pain linked to diabetic peripheral neuropathy in individuals with type 2 diabetes (10), pain from osteoarthritis (11), and back pain associated with disc degeneration (12). HDL-C mediates anti-inflammatory and antioxidant effects (13). Low levels of HDL-C can lead to endothelial dysfunction, oxidative stress (10), and abnormal cytokine production, all of which are implicated in the pathogenesis of chronic pain (2). Therefore, we hypothesize that HDL-C may be associated with chronic pain.
Although several cohort studies in other countries have presented conflicting findings (14–17), there have been no reports on the independent association between HDL-C levels and chronic pain in American adults. As a result, the objective of this study is to explore this relationship utilizing data derived from the 2003–2004 National Health and Nutrition Examination Survey (NHANES).
2 Materials and methods
2.1 Study population
The study was conducted using NHANES data from 2003 to 2004. The NHANES project, a national survey spearheaded by the National Center for Health Statistics (NCHS) under the Centers for Disease Control and Prevention (CDC), is designed to evaluate the health and nutritional conditions of non-institutionalized US civilians. Utilizing a stratified multi-stage probability sampling approach, this survey has been in operation since 1999. It gathers nationally representative data through interviews covering demographic, dietary, socioeconomic, and health-related issues, as well as physical examinations and laboratory tests. The survey is conducted every 2 years. The NHANES database includes three consecutive cycles of chronic pain data (1999–2000, 2001–2002, and 2003–2004). The 1999–2002 HDL cholesterol measurements showed unsatisfactory deviations (> 4%) from the laboratory quality control (Solomon Park Research Laboratory, Kirkland, Washington), which is determined by the CDC. Therefore, NHANES data from 2003–2004 was chosen as the bias of the HDL-C method was acceptable (<4%).
2.2 Chronic pain
The NHANES database only conducted the Miscellaneous Pain section of the questionnaire survey for adults over the age of 20. Chronic pain was defined using the variables MPQ100 (pain duration exceeding 24 h in the last month) and MPQ110 (pain duration). Participants experiencing pain issues for a duration of 3 months or more were categorized into the chronic pain group (18). On the other hand, participants who indicated no pain issues in the previous month, as well as those with pain issues lasting less than 3 months, were grouped into the non-chronic pain category.
2.3 HDL-cholesterol
HDL-cholesterol was measured using the direct immunoassay method. Blood samples were processed, stored, and shipped to Johns Hopkins Hospital in Baltimore, Maryland for analysis. The NHANES Quality Assurance and Quality Control protocol adheres to the Clinical Laboratory Improvement Amendments Act of 1988. The NHANES Laboratory/Medical Technologist Procedure Manual provides detailed instructions on specimen collection, processing, quality control, and quality assurance.
2.4 Other covariates
Several potential covariates extracted from the NHANES were assessed, including age, sex, body mass index (BMI), marital status (living alone and married or living with a partner), alcohol consumption (average number of drinks per occasion with alcohol in the past year), smoking status (19), poverty income ratio (PIR), education level (did not graduate from high school, graduated from high school, and college education or above), race (Mexican American, other Hispanic non-Hispanic white non-Hispanic black, and other races), physical activity, triglyceride (TG), total cholesterol (TC), cotinine, blood lead, arthritis, cancer or malignancy, osteoporosis, hypertension, diabetes, and coronary heart disease. Physical activity was self-reported and categorized into four groups: mainly sit, walk around, light load, and heavy load. Hypertension and diabetes mellitus were determined based on medication use, self-reported physician diagnosis, and relevant testing indicators including blood pressure measurements, fasting blood glucose levels, and hemoglobin A1c levels (20, 21).
2.5 Statistical analysis
Data analyses were conducted using the statistical software packages R and Free Statistics software version 1.7.1 (22). Descriptive statistics were employed to summarize all data, encapsulating both the frequencies (percentages) and the mean ± standard deviation. The Chi-square test was used to evaluate differences between groups for categorical variables, while the Student’s t-test or the Mann-Whitney U-test was used for continuous variables, depending on which was most suitable. Multivariate logistic regression was conducted to explore the association between HDL-C levels and the risk of chronic pain. Four models were used for the multivariate logistic regression analysis, adjusting for various sociodemographic and clinical covariates. Model 1 was calibrated with age, sex, PIR, education level, marital status, and race. Model 2 incorporated all covariates from Model 1, with the addition of smoking status, alcohol consumption, physical activity, body mass index, coronary heart disease, arthritis, cancer or malignancy, osteoporosis, diabetes, and hypertension. Model 3 was an extension of Model 2, further adjusted for total cholesterol, triglyceride, cotinine, and blood lead. The likelihood ratio test was conducted on the interaction between subgroups, and sensitivity analysis was performed after excluding participants with missing data.
3 Results
3.1 Participants characteristics
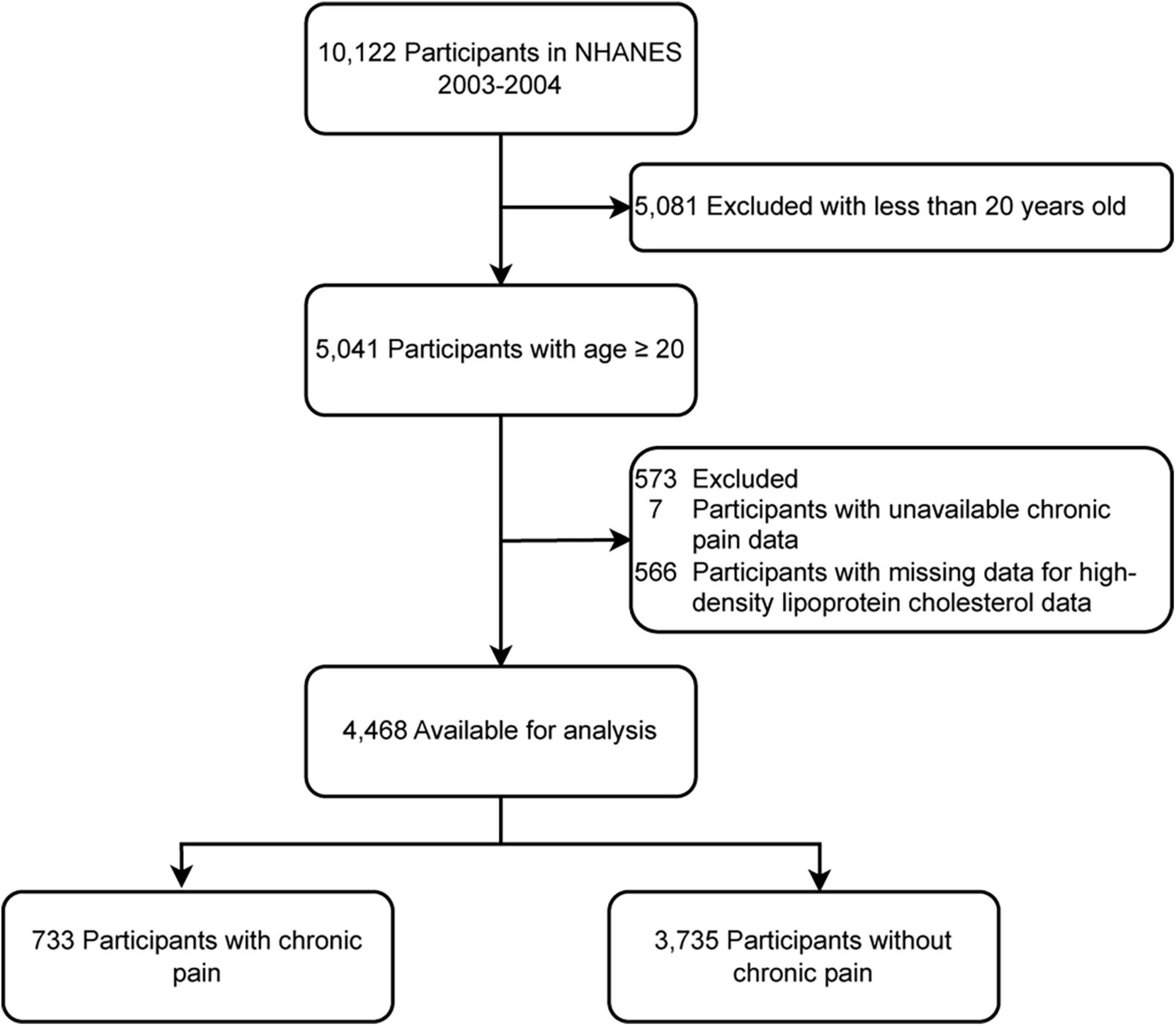
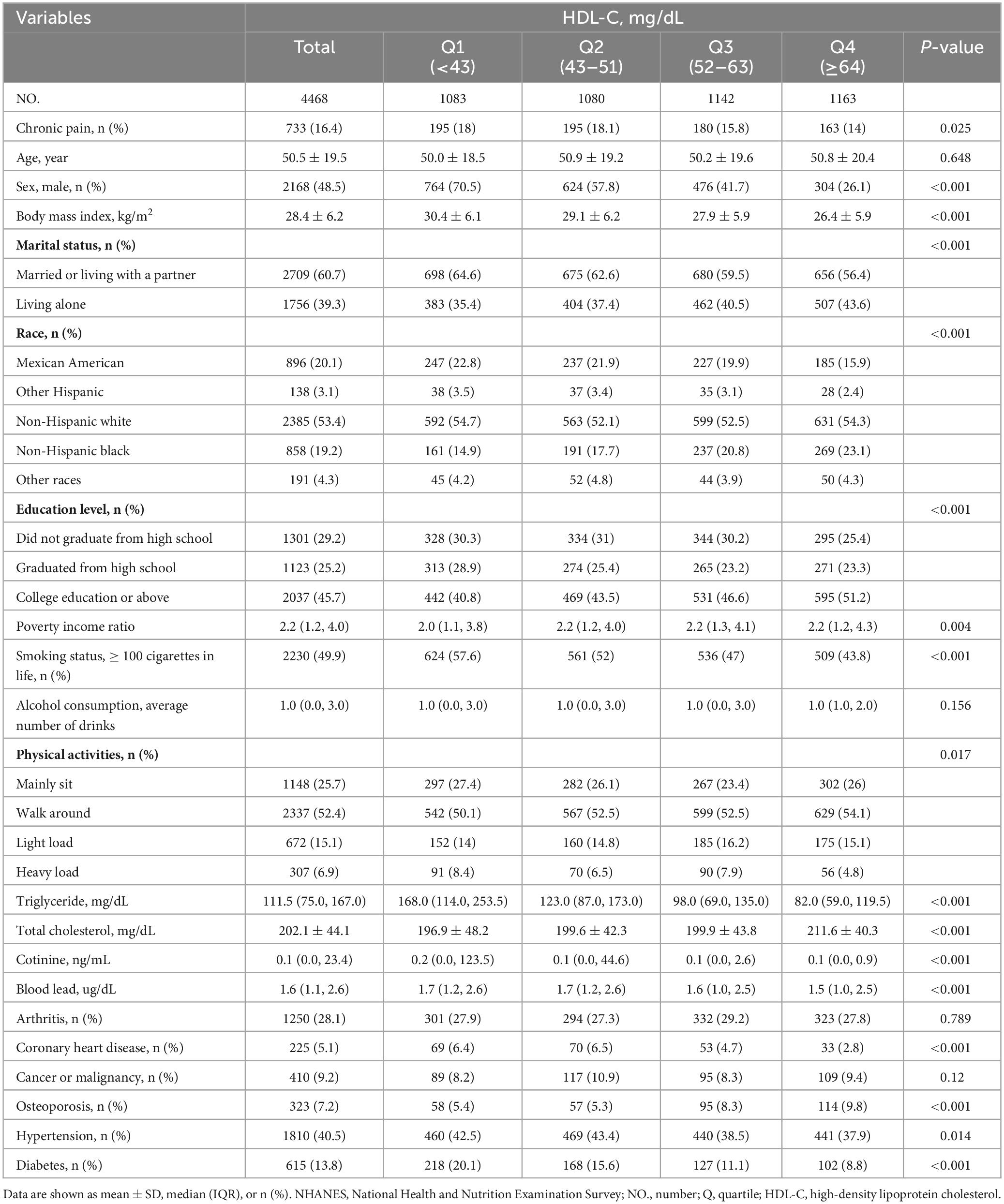
A total of 10,122 potential participants were identified, and 4,468 adults (≥ 20 years) were included in the study. The flowchart illustrating the exclusion criteria is presented in Figure 1. The baseline characteristics of patients according to HDL-C level categories are shown in Table 1. Table 1 shows that individuals with higher levels of HDL-C were more likely to have a lower risk of chronic pain.
3.2 Relationship between HDL-C and chronic pain
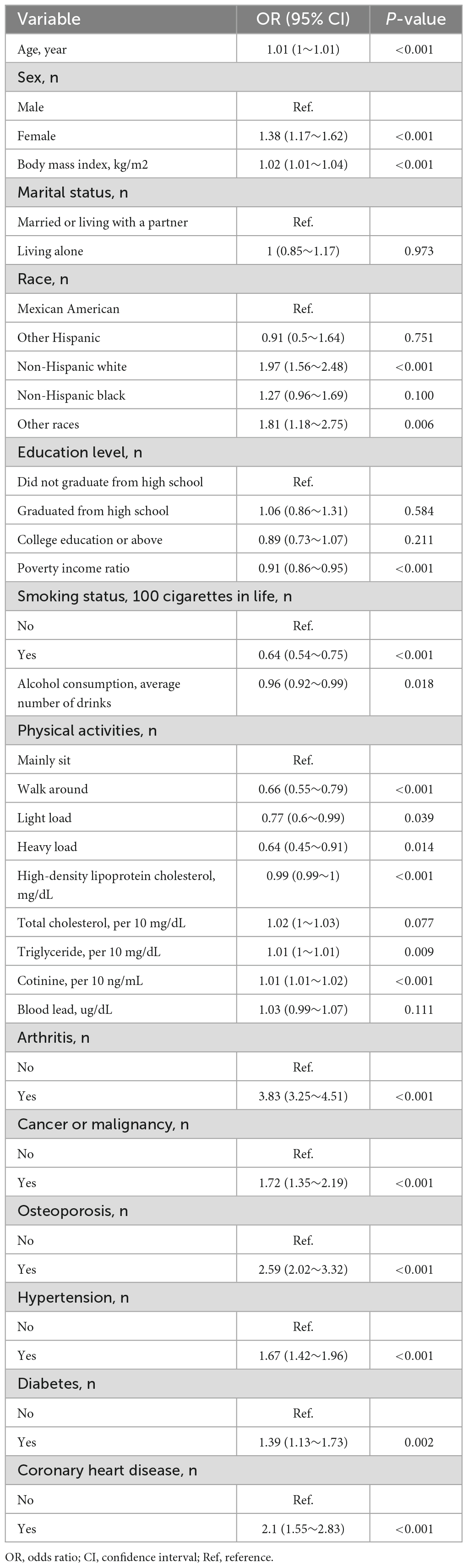
Univariate analysis revealed that age, sex, physical activity, PIR, BMI, smoking status, alcohol consumption, triglyceride levels, cotinine levels, arthritis, cancer or malignancy, coronary heart disease, osteoporosis, hypertension, and diabetes were associated with chronic pain (Table 2).
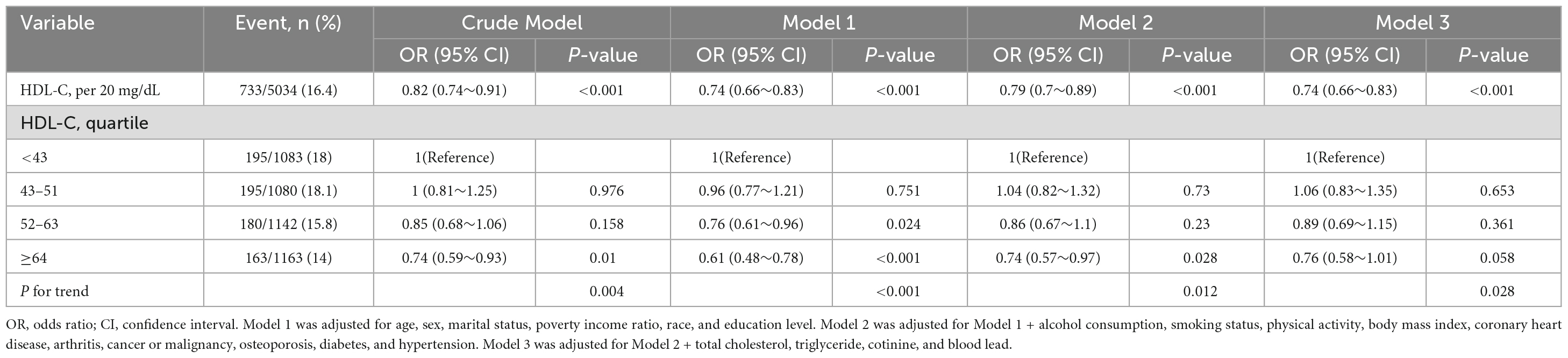
Table 3 presents the association between HDL-C and chronic pain. In Model 1, adjusting for age, sex, marital status, PIR, race, and education level, HDL-C was negatively related to chronic pain (HDL-C per 20 unit, OR: 0.74 [0.66–0.83], P < 0.001). This association remained stable even after adjusting for additional potential covariates in Model 2–3 [HDL-C per 20 mg/L; Model 2: OR = 0.79 (0.7–0.89), P < 0.001; Model 3: OR = 0.74 (0.66–0.83)]. Additionally, in the fully adjusted model, compared with individuals in the lowest HDL-C level (mg/dL) group (Q1, <43), the odds ratios (ORs) for chronic pain in the Q2 (43-51), Q3 (52–63), and Q4 (≥64) groups were 1.06 (95% CI: 0.83–1.35), 0.89 (95% CI: 0.69–1.15), and 0.76 (95% CI: 0.58–1.01), respectively. Compared with the Q1 group, there was no significant change in the risk of chronic pain in the Q2 group, while in the Q3–4 groups, the risk of chronic pain was reduced by 11% and 24%, respectively.
3.3 Sensitivity analysis
Figure 2 shows that no significant interactions were observed after stratification by sex, age (< 65 and ≥65 years), marital status, education level, physical activity, arthritis, coronary heart disease, cancer or malignancy, osteoporosis, hypertension, and diabetes. Due to multiple testing, the P-value (0.048) of the interaction in the arthritis subgroup may not be statistically significant. After excluding individuals with missing data (leaving 3,205 participants), the relationship between HDL-C and chronic pain remained robust in the sensitivity analysis (Table 4).
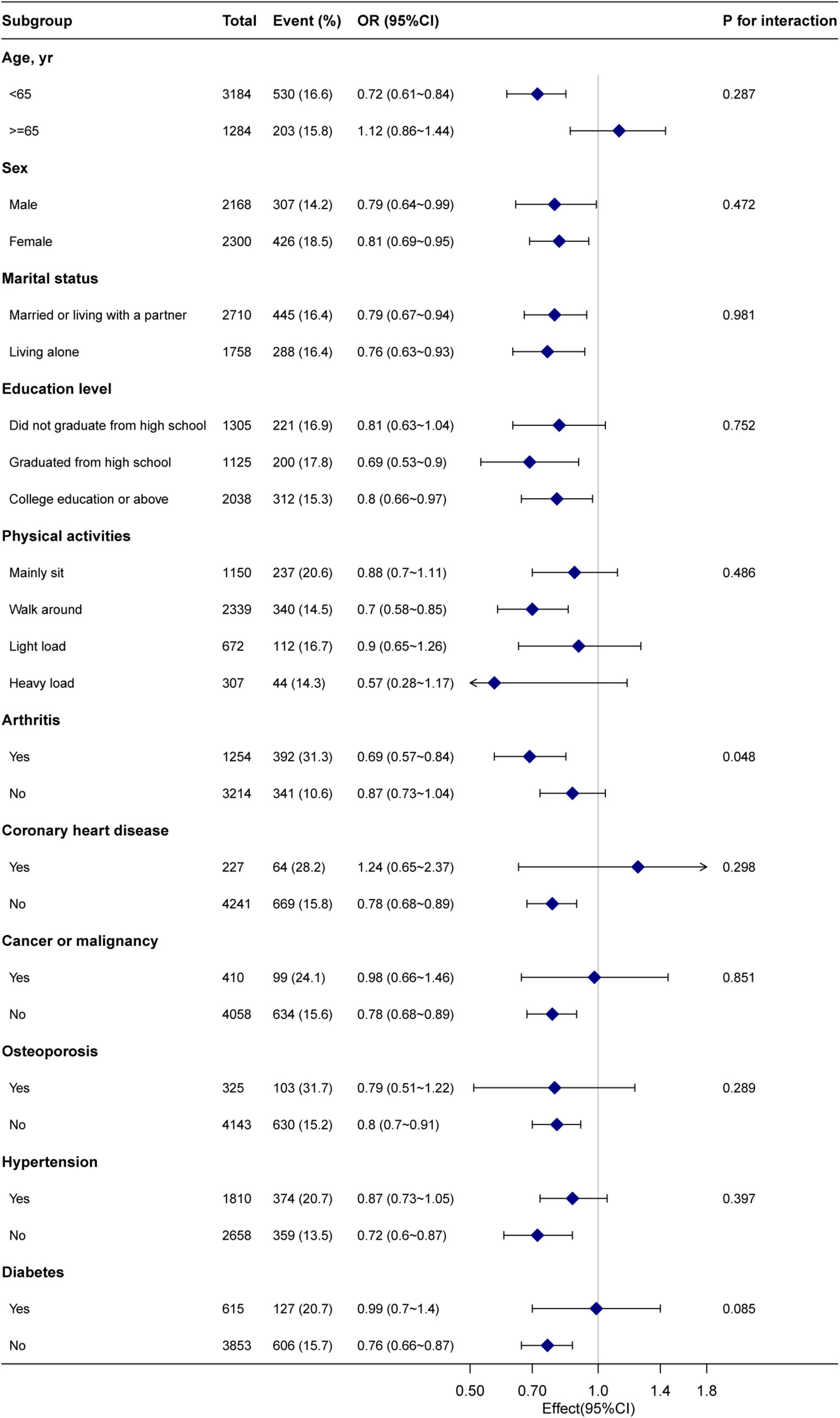
Figure 2. Subgroup analysis of the high-density lipoprotein cholesterol and chronic pain. Each stratification factor was adjusted for age, sex, body mass index, marital status, poverty income ratio, race, alcohol consumption, smoking status, education level, physical activity, coronary heart disease, arthritis, cancer or malignancy, osteoporosis, hypertension, diabetes, triglyceride, total cholesterol, cotinine, and blood lead.
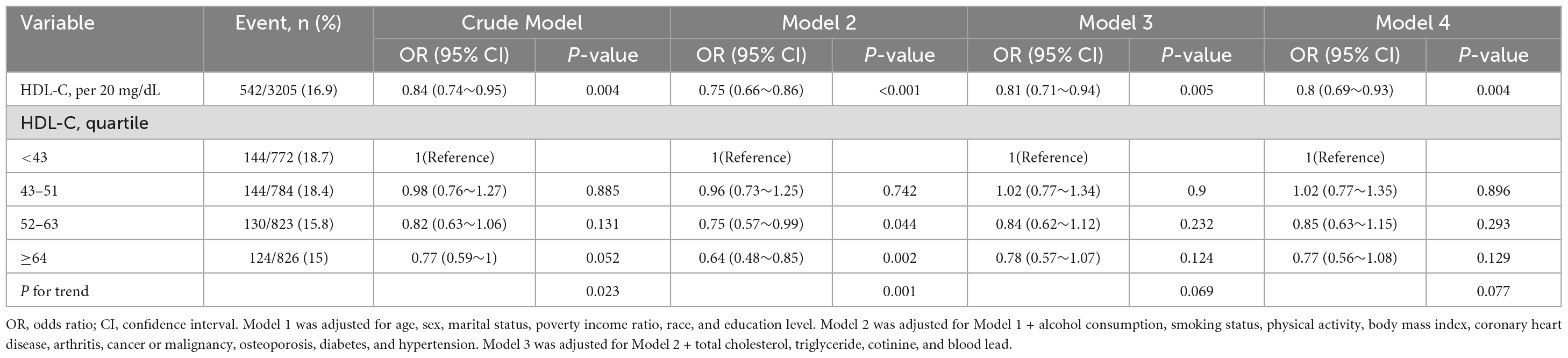
Table 4. Association of high-density lipoprotein cholesterol with chronic pain (3,205 participants with complete data).
4 Discussion
This study utilized a nationally representative database from NHANES to investigate the relationship between HDL-C and chronic pain in US adults. The findings showed that as HDL-C increased, the incidence of chronic pain decreased. Specifically, for every 20 units increase in HDL-C level, the risk of chronic pain is reduced by 26%. The relationship between HDL-C and chronic pain remained consistent in both subgroups and sensitivity analyses.
Few studies have explored the relationship between chronic pain and HDL-C. A cohort study with a sample size of 13,328 from the Scottish Family Health Study found that after adjustment by age and sex, HDL-C levels were associated with chronic pain [OR, 0.69 (0.63–0.76)]. Our findings are similar to this study, but our analysis also adjusts for additional conditions that may contribute to chronic pain, such as arthritis. Other studies examining specific types of chronic pain and HDL-C levels have yielded different conclusions. For example, studies on women with fibromyalgia (14) and shoulder and neck pain (23) found no association with HDL-C, while studies on chronic low back pain showed a negative correlation with HDL-C (17, 23). These discrepancies may be attributed to the different pathogenic mechanisms underlying different types of chronic pain.
Chronic pain can arise from nociceptive stimuli (caused by tissue injury), neuropathic stimuli (caused by nerve injury), or nociplastic stimuli (caused by a sensitized nervous system). It can also result from a combination of these factors (2). Currently, there is no established mechanism to explain the relationship between chronic pain and HDL-C, but we have proposed several possible mechanisms. Firstly, injurious pain may occur due to tissue damage or degenerative changes, such as those seen in inflammatory diseases like primary osteoarthritis and traumatic arthritis (2). Inflammatory responses have been associated with decreased levels of HDL-C (24, 25). Secondly, neuropathic pain can be caused by diseases or injuries that affect the nervous system, such as nerve compression, metabolism issues, or ischemia (2). For instance, in diabetes, low HDL-C levels may contribute to endothelial dysfunction, oxidative stress, and abnormal cytokine production (26), which are linked to the development of painful diabetic neuropathy (27, 28).
Given the high prevalence and significant impact of chronic pain, it is essential to understand its risk factors. This study contributes to the existing literature by examining the correlation between HDL-C and chronic pain. Although HDL-C is not currently a target for drug treatment (29), lifestyle changes such as physical activity, weight loss, alcohol consumption, smoking cessation, and adopting a Mediterranean diet have been shown to increase HDL-C levels (30). However, considering the limited mobility and physical or functional impairment often experienced by patients with chronic pain, lifestyle changes to increase HDL-C levels should be individualized.
The strength of this study lies in its ability to provide new evidence on the relationship between HDL-C levels and chronic pain. The findings are particularly compelling due to the extensive use of a large, representative US cohort, rigorous quality control measures, and the application of multiple statistical methods that consistently produced stable results.
However, the present study is subject to certain limitations. Firstly, given the observational nature of this study, a causal relationship between HDL-C and chronic pain cannot be conclusively established. Secondly, the findings of this research are based solely on data obtained from the NHANES database, which represents American adults. Consequently, additional investigations are necessary to ascertain the generalizability of these results to other populations. Lastly, the absence of repeated measurements of HDL-C data may compromise the accuracy of its long-term levels and its association with chronic pain.
5 Conclusion
There is a negative relationship between HDL-C and chronic pain, with individuals having low HDL-C levels being at a higher risk of chronic pain. Furthermore, this study posits that HDL-C potentially influences the development of chronic pain and could enhance the efficacy of chronic pain management approaches.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by The National Center for Health Statistics Research Ethics Review Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
HW: Writing–review and editing, Supervision, Conceptualization. PM: Data curation, Writing–original draft, Methodology, Formal analysis. HD: Data curation, Writing–original draft, Methodology, Formal analysis. SC: Writing–original draft, Formal analysis, Data curation. XG: Writing–original draft, Formal analysis, Data curation. XC: Writing–original draft, Formal analysis, Data curation. YL: Writing–original draft, Formal analysis, Data curation. GF: Writing–review and editing, Supervision, Project administration, Conceptualization.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Raja S, Carr D, Cohen M, Finnerup N, Flor H, Gibson S, et al. The revised international association for the study of pain definition of pain: Concepts, challenges, and compromises. Pain. (2020) 161:1976–82. doi: 10.1097/j.pain.0000000000001939
2. Cohen S, Vase L, Hooten W. Chronic pain: An update on burden, best practices, and new advances. Lancet. (2021) 397:2082–97. doi: 10.1016/S0140-6736(21)00393-7
3. Gureje O, Von Korff M, Simon G, Gater R. Persistent pain and well-being: A world health organization study in primary care. JAMA. (1998) 280:147–51. doi: 10.1001/jama.280.2.147
4. Smith B, Elliott A, Chambers W, Smith W, Hannaford P, Penny K. The impact of chronic pain in the community. Fam Pract. (2001) 18:292–9. doi: 10.1093/fampra/18.3.292
5. Dueñas M, Ojeda B, Salazar A, Mico J, Failde I. A review of chronic pain impact on patients, their social environment and the health care system. J Pain Res. (2016) 9:457–67. doi: 10.2147/JPR.S105892
6. Wang W, Lu X, Li Q, Chen D, Zeng W. The relationship between blood lead level and chronic pain in us adults: A nationwide cross-sectional study. Pain Ther. (2023) 12:1195–208. doi: 10.1007/s40122-023-00535-9
7. Argoff C, Bhullar R, Galluzzi K. Specific conditions causing persistent pain in older adults. In: G Cordts, P Christo editors. Effective treatments for pain in the older patient. New York, NY: Springer New York (2019). p. 71–107.
8. Chiesa S, Charakida M. High-density lipoprotein function and dysfunction in health and disease. Cardiovasc Drugs Ther. (2019) 33:207–19. doi: 10.1007/s10557-018-06846-w
9. Cordero A, Moreno-Arribas J, Bertomeu-González V, Agudo P, Miralles B, Masiá M, et al. Low levels of high-density lipoproteins cholesterol are independently associated with acute coronary heart disease in patients hospitalized for chest pain. Rev Esp Cardiol. (2012) 65:319–25. doi: 10.1016/j.rec.2011.09.003
10. Pai Y, Lin C, Lee I, Chang M. Prevalence and biochemical risk factors of diabetic peripheral neuropathy with or without neuropathic pain in Taiwanese adults with type 2 diabetes mellitus. Diabetes Metab Syndr. (2018) 12:111–6. doi: 10.1016/j.dsx.2017.09.013
11. Li H, George D, Jaarsma R, Mao X. Metabolic syndrome and components exacerbate osteoarthritis symptoms of pain, depression and reduced knee function. Ann Transl Med. (2016) 4:133. doi: 10.21037/atm.2016.03.48
12. Yoshimoto T, Ochiai H, Shirasawa T, Nagahama S, Kobayashi M, Minoura A, et al. Association between serum lipids and low back pain among a middle-aged Japanese population: A large-scale cross-sectional study. Lipids Health Dis. (2018) 17:266. doi: 10.1186/s12944-018-0907-1
13. Iqbal F, Baker W, Khan M, Thukuntla S, McKinney K, Abate N, et al. Current and future therapies for addressing the effects of inflammation on HDL cholesterol metabolism. Br J Pharmacol. (2017) 174:3986–4006. doi: 10.1111/bph.13743
14. Loevinger B, Muller D, Alonso C, Coe C. Metabolic syndrome in women with chronic pain. Metabolism. (2007) 56:87–93. doi: 10.1016/j.metabol.2006.09.001
15. Goodson N, Smith B, Hocking L, McGilchrist M, Dominiczak A, Morris A, et al. Cardiovascular risk factors associated with the metabolic syndrome are more prevalent in people reporting chronic pain: Results from a cross-sectional general population study. Pain. (2013) 154:1595–602. doi: 10.1016/j.pain.2013.04.043
16. Heuch I, Heuch I, Hagen K, Zwart J. Do abnormal serum lipid levels increase the risk of chronic low back pain? The nord-trøndelag health study. PLoS One. (2014) 9:e108227. doi: 10.1371/journal.pone.0108227
17. Heuch I, Heuch I, Hagen K, Zwart J. Associations between serum lipid levels and chronic low back pain. Epidemiology. (2010) 21:837–41. doi: 10.1097/EDE.0b013e3181f20808
18. Treede R, Rief W, Barke A, Aziz Q, Bennett M, Benoliel R, et al. A classification of chronic pain for icd-11. Pain. (2015) 156:1003–7. doi: 10.1097/j.pain.0000000000000160
19. Hollingshead N, Vrany E, Stewart J, Hirsh A. Differences in Mexican Americans’ prevalence of chronic pain and co-occurring analgesic medication and substance use relative to non-Hispanic white and black Americans: Results from NHANES 1999-2004. Pain Med. (2016) 17:1001–9. doi: 10.1093/pm/pnv003
20. Zheng Y, Wang J, Wang Y, Xu K, Chen X. The hidden dangers of plant-based diets affecting bone health: A cross-sectional study with U.S. National health and nutrition examination survey (NHANES) data from 2005–2018. Nutrients. (2023) 15:1794. doi: 10.3390/nu15071794
21. Association A. 2. Classification and diagnosis of diabetes: Standards of medical care in diabetes-2020. Diabetes Care. (2020) 43(Suppl. 1):S14–31. doi: 10.2337/dc20-S002
22. Ruan Z, Lu T, Chen Y, Yuan M, Yu H, Liu R, et al. Association between psoriasis and nonalcoholic fatty liver disease among outpatient us adults. JAMA Dermatol. (2022) 158:745–53. doi: 10.1001/jamadermatol.2022.1609
23. Djade C, Diorio C, Laurin D, Dionne C. An exploratory identification of biological markers of chronic musculoskeletal pain in the low back, neck, and shoulders. PLoS One. (2022) 17:e0266999. doi: 10.1371/journal.pone.0266999
24. Esteve E, Ricart W, Fernández-Real J. Dyslipidemia and inflammation: An evolutionary conserved mechanism. Clin Nutr. (2005) 24:16–31. doi: 10.1016/j.clnu.2004.08.004
25. Zuliani G, Volpato S, Blè A, Bandinelli S, Corsi A, Lauretani F, et al. High interleukin-6 plasma levels are associated with low HDL-C levels in community-dwelling older adults: The InChianti study. Atherosclerosis. (2007) 192:384–90. doi: 10.1016/j.atherosclerosis.2006.05.024
26. Morton J, Zoungas S, Li Q, Patel A, Chalmers J, Woodward M, et al. Low HDL cholesterol and the risk of diabetic nephropathy and retinopathy: Results of the advance study. Diabetes Care. (2012) 35:2201–6. doi: 10.2337/dc12-0306
27. Doupis J, Lyons T, Wu S, Gnardellis C, Dinh T, Veves A. Microvascular reactivity and inflammatory cytokines in painful and painless peripheral diabetic neuropathy. J Clin Endocrinol Metab. (2009) 94:2157–63. doi: 10.1210/jc.2008-2385
28. Sandireddy R, Yerra V, Areti A, Komirishetty P, Kumar A. Neuroinflammation and oxidative stress in diabetic neuropathy: Futuristic strategies based on these targets. Int J Endocrinol. (2014) 2014:674987. doi: 10.1155/2014/674987
29. März W, Kleber M, Scharnagl H, Speer T, Zewinger S, Ritsch A, et al. HDL cholesterol: Reappraisal of its clinical relevance. Clin Res Cardiol. (2017) 106:663–75. doi: 10.1007/s00392-017-1106-1
Keywords: chronic pain, HDL-C, negative correlation, NHANES, cross-sectional study
Citation: Mi P, Dong H, Chen S, Gao X, Cao X, Liu Y, Wang H and Fan G (2024) Association between HDL-C and chronic pain: data from the NHANES database 2003–2004. Front. Med. 11:1340037. doi: 10.3389/fmed.2024.1340037
Received: 17 November 2023; Accepted: 27 February 2024;
Published: 11 March 2024.
Edited by:
Marcin Siwek, Jagiellonian University Medical College, PolandCopyright © 2024 Mi, Dong, Chen, Gao, Cao, Liu, Wang and Fan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huijie Wang, d2FuZ19odWlqaWVAb3V0bG9vay5jb20=; Guofeng Fan, MjQwMzA4MTE4MUBxcS5jb20=
†These authors have contributed equally to this work and share first authorship
 Panpan Mi1†
Panpan Mi1† Haoran Dong
Haoran Dong Huijie Wang
Huijie Wang