- 1Department of Rheumatology and Immunology, Beijing Anzhen Hospital, Capital Medical University, Beijing, China
- 2Department of the National Clinical Research Center for Mental Disorders and Beijing Key Laboratory of Mental Disorders, Beijing Anding Hospital and the Advanced Innovation Center for Human Brain Protection, Capital Medical University, Beijing, China
Objective: Depression is a common complication in Takayasu arteritis (TA). Disorders of the immune system play an important role in both diseases. This study aimed to clarify the feature of cytokines in TA patients with depression.
Methods: In this cross-sectional study, serum cytokines were tested in 40 TA patients and 11 healthy controls using the Bio-Plex Magpix System (Bio-Rad®). The state of depression was measured by the Zung Self-Rating Depression Scale (SDS) in TA patients. Logistic regression analysis was performed to find the risk factors of depression in patients with TA.
Results: TA patients with depression had higher ESR, hsCRP, NIH, and ITAS.A than patients without depression (16.00 [10.00, 58.50]mm/H vs. 7.50 [4.50, 17.75]mm/H, p = 0.013; 7.60 [2.32, 46.52]mg/L vs. 0.71 [0.32, 4.37]mg/L, p = 0.001; 2.00 [2.00, 3.00] vs. 1.00 [0.00, 2.00], p = 0.007; 7.00 [4.00, 9.50] vs. 1.50 [0.00, 5.75], p = 0.012, respectively). Additionally, the lower age of onset and levels of IL-4, IL-13, eotaxin, and IP-10 were observed in the depressed group compared with the non-depressed (23.50 [19.25, 32.50]pg./ml vs. 37.00 [23.25, 42.50]pg./ml, p = 0.017; 2.80 [2.17, 3.18]pg./ml vs. 3.51 [3.22, 4.66]pg./ml, p < 0.001; 0.66 [0.60, 1.12]pg./ml vs. 1.04 [0.82, 1.25]pg./ml, p = 0.008; 46.48 [37.06, 61.75]pg./ml vs. 69.14 [59.30, 92.80]pg./ml, p = 0.001; 184.50 [138.23, 257.25]pg./ml vs. 322.32 [241.98, 412.60]pg./ml, p = 0.005, respectively). The lower level of IL-4 and age of onset were the independent risk factors for depression in TA patients (OR [95% CI] 0.124 [0.018, 0.827], p = 0.031; 0.870 [0.765, 0.990], p = 0.035, respectively).
Conclusion: Our data suggested that lower cytokine levels, especially IL-4, might be involved in the development of TA patients with depression. Clinicians can probably use serum IL-4 level testing as a potential indicator of depression in TA.
Introduction
Takayasu arteritis (TA) is a chronic inflammatory vasculitis that affects the aorta and its major branches, predominantly occurring in young women in 80–90% of cases (1). Inflammation within the vasculature can result in segmental stenosis, occlusion, dilatation, and/or aneurysm formation (2). Existing evidence strongly indicates that cell-mediated immunity is a crucial factor in the pathogenic cascade leading to these lesions (3, 4). However, the quantitative and qualitative changes in cytokine production observed in TA remain incompletely elucidated in current studies.
Pathophysiological investigations into depression have elucidated a strong association between neuroimmune inflammatory pathways and the cytokine-mediated activation of cellular immunity (5). Individuals with major depression often manifest elevated levels of inflammatory mediators, including cytokines, chemokines, and acute-phase proteins in blood or cerebrospinal fluid (6). It is proposed that the onset and persistence of depression are substantially influenced by the secretion of peripheral pro-inflammatory cytokines and other factors. This activation initiates localized, low-grade, yet chronic inflammation within the central nervous system (6, 7). It is now recognized that the cytokines themselves are capable of triggering or inhibiting the release of other cytokines (8). Against the background of inflammation, cytokines act beyond the normal regulatory range. The abnormal phenomenon, in turn, leads to an increase in oxidative stress, thus maintaining, even enhancing chronic inflammation till depression happens (9).
TA is a disease caused by a dysregulation of the systemic immune system (10). In previous studies, a certain proportion of TA patients would have developed depressive co-morbidities (11). Though the mechanism of the disease is not fully defined, it is generally believed that the pathogenic process includes disruption of the cytokine network, which may underlie the pathophysiology of combined depression in TA (12). Previous studies have identified dysregulation of pro- and anti-inflammatory cytokines during inflammatory disease progression as an underlying factor in depression (13). However, the cytokine levels in depression TA patients were unclear. In our study, we detected the serum cytokines (27 types) in depression TA patients. The authors aimed to clarify the characteristics and risk factors of depression in TA patients and provided new evidence for the clinical manifestations and mechanisms of such co-morbidities in the context of systemic inflammatory states.
Materials and methods
Patients
A cross-sectional study was carried out to determine whether there is a relationship between cytokines and depression in TA patients. The authors consecutively enrolled 40 hospitalized patients with TA who attended Beijing Anzhen Hospital from March 2021 to September 2022, along with 11 healthy controls recruited from the general population. Cytokine profiles were compared with non-depressed healthy controls to examine the differences between these populations. Clinical and biological data were collected concurrently with the recruitment process of the patient registry.
Inclusion and exclusion criteria
TA patients who had been treated for more than 6 months were recruited, fulfilling the classification criteria of TA developed by the American College of Rheumatology (ACR)/EULAR in 2022 (14). The following were the exclusion criteria: (1) chronic or current infections, (2) tumors, (3) other autoimmune diseases, and (4) other psychiatric diseases or a history of psychiatric diseases. The authors also included healthy controls with matched age–sex ratios and no previous history of illness, which were evaluated by the SDS to confirm the absence of depressive symptoms.
Ethics statement
This study was conducted in accordance with the ethical principles Helsinki Declaration and was approved by the Ethics Committee of Beijing Anzhen Hospital (Approval Number: 2023183X).
Collection of clinical data and laboratory parameters
The authors collected the age of onset, gender, education level, and body mass index (BMI) as demographic variables. Clinical parameters consisted of NIH (15), ITAS.A, and ITAS2010 (16), which were defined to evaluate disease activity and Numano type (17). The authors also recorded in detail the typical clinical symptoms presented during the course of the disease, such as fever, neck pain, chest tightness/chest pain, bilateral pulse inequality, intermittent claudication, dizziness/headache, and abdominal pain (18). All patients were treated with medications, including glucocorticoids, conventional synthetic disease-modifying antirheumatic drugs (cDMARDs), and biologic disease-modifying antirheumatic drugs (btDMARDs). The authors documented the glucocorticoid dosage at the time of enrollment and converted it to prednisone equivalents. The dosages and methods of administration of DMARDs were methotrexate 15 mg Qw, martimecrolide 0.75 g bid, cyclophosphamide 100 mg Qod, hydroxychloroquine sulfate 0.2 g Qd, azathioprine 100 mg Qd, tocilizumab 8 mg/kg Qm, adalimumab 40 mg Q2w, and baricitinib 2 mg Qd. Laboratory parameters included white blood cell count (WBC), neutrophils (NEUT), hemoglobin (Hb), platelets (PLT), erythrocyte sedimentation rate (ESR), and high-sensitivity c-reactive protein (hsCRP), which were assessed at the same time as the estimation of psychological status by the laboratory medicine of Anzhen Hospital.
Evaluation of depression
Depressive symptoms were evaluated by using the Zung Self-Rating Depression Scale (SDS), a well-established and valid instrument for identifying depression in primary care patients (19). Participants with SDS scores equal to or exceeding 52 were categorized as experiencing depression, while those with SDS scores below 52 were classified as not having depression (20). The healthy controls were also screened for depressive tendencies through the SDS. None reported the presence of depressive symptoms.
Detection of cytokines
Venous blood (5 mL) was drawn into a vacuum blood collection tube on the day of assessing depressive symptoms. The clotted blood stood for 30 min before centrifugation at 2,000 × g for 10 min at room temperature. The serum was aliquoted and stored in an −80°C freezer for subsequent analysis.
Cytokine levels were determined by the Bio-Plex Pro Human Inflammation and Treg Cytokine Assays (United States) on the Bio-Plex Magpix System (Bio-Rad®). The panel analyzed 27cytokines, including IL-1β, IL-1rα, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12(p70), IL-13, IL-15, IL-17, eotaxin, FGF basic, G-CSF, GM-CSF, IFN-γ, IP-10, MCP-1, MIP-1α, MIP-1β, PDGF-BB, RANTES, TNF-α, and VEGF. The samples were processed according to the Bio-Plex Pro Human Inflammation Panel I Assay Quick Guide (Bio-Rad®, 10,044,282). The concentrations of the analytes in the test samples were determined by the standard curves of the different concentrations of cytokines.
Statistical analysis
The demographic and clinical data were expressed as median and IQR as the data results did not conform to normal distribution. The Wilcoxon test was used to determine the significance. The ANOVA test was used to analyze the relationship between the levels of each cytokine profile and the different groups. Spearman’s test was used to determine the correlation between SDS scores, ESR, hsCRP, and cytokines. Multivariate logistic regression analysis was performed to find out the risk factors of TA patients with depression. The analysis and visualization of all statistical tests were exhibited by R version 3.1.1 and SPSS.26.
Results
Sociodemographic characteristics and clinical differences in the depressed TA group versus the non-depressed group
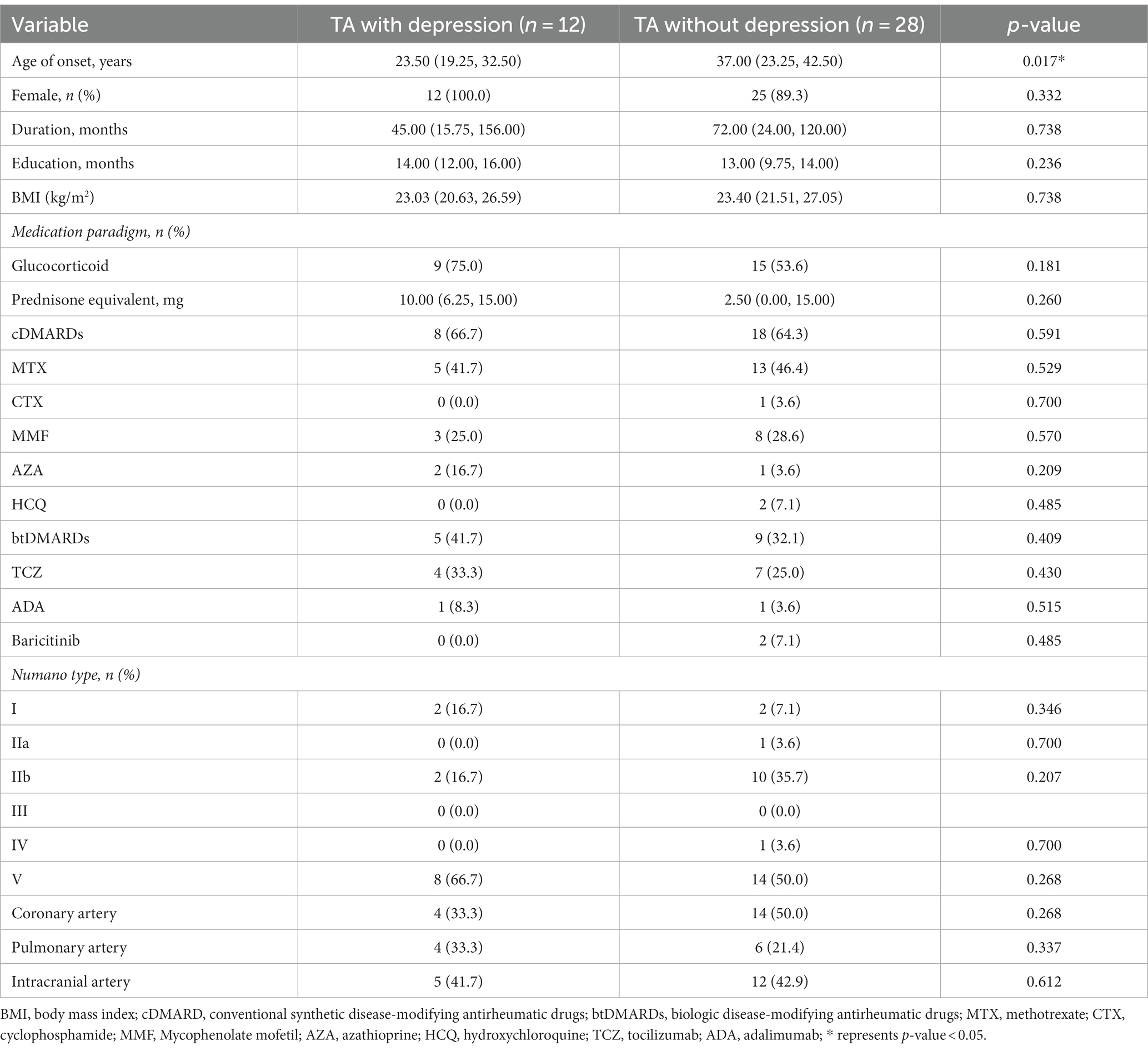
In our research, 40 TA patients who had received pharmacological treatment for more than 6 months were recruited. The sociodemographic and clinical data are shown in Table 1; 12 patients with SDS scores ≥52 were categorized as the depressed group and 28 patients with SDS scores lower than 52 were recognized as the non-depressed. The authors found that the age of onset in the depressed group was significantly lower than the non-depressed group in TA patients (23.50 [19.25, 32.50] vs. 37.00 [23.25, 42.50], p = 0.017). There were no significant differences between the depressed and non-depressed groups in terms of disease duration, percentage of females, duration of education, BMI, and medications. On the other hand, no differences were observed in Numano types, coronary, pulmonary, intracranial artery involvement, nor in the typical clinical symptoms in TA (Table 1).
Laboratory indicators for TA patients with or without depression
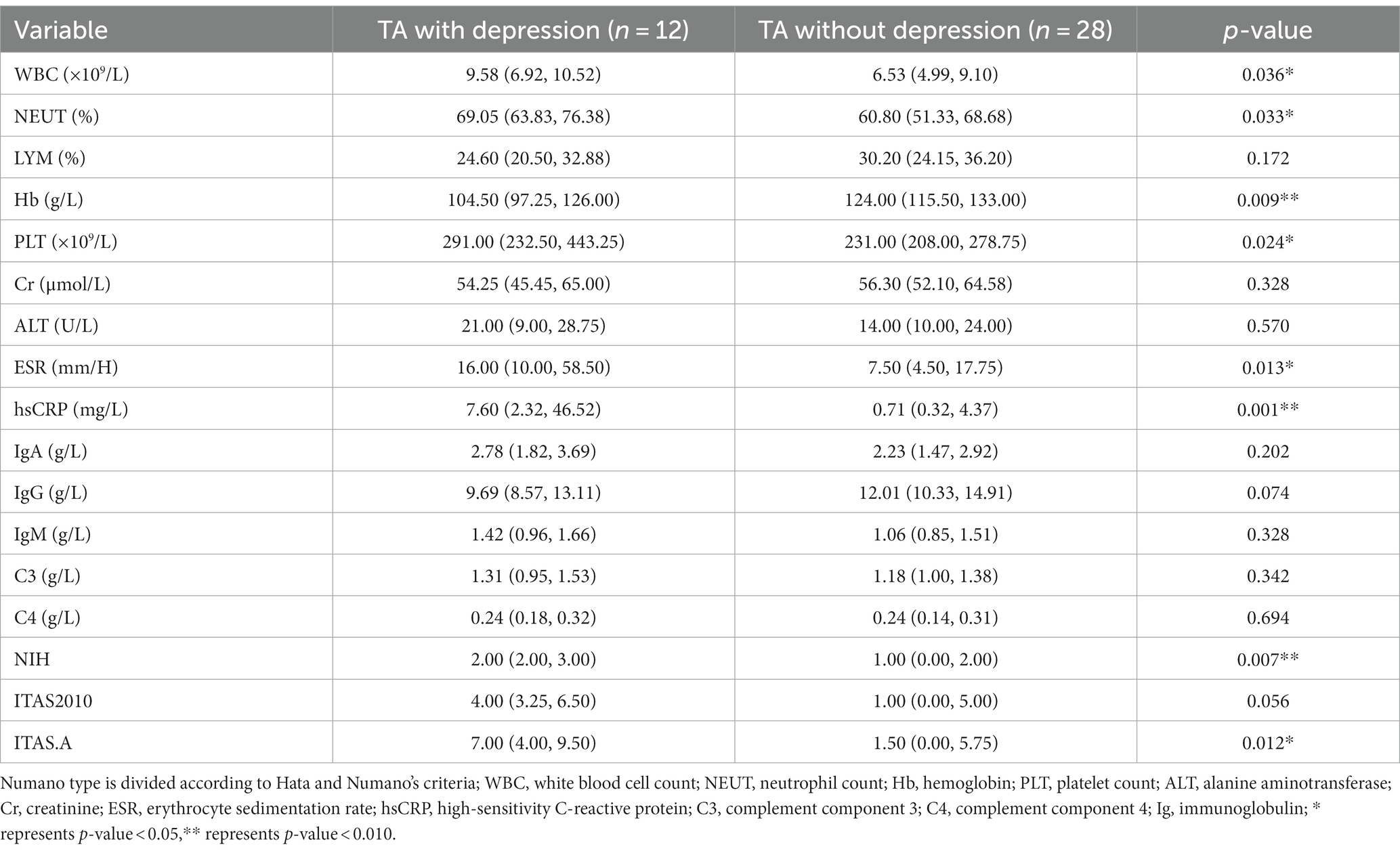
In the comparison of laboratory indicators, the authors found that the depressed group in TA had higher levels of WBC, NEUT, PLT, ESR, and hsCRP than the non-depressed group (9.58 [6.92, 10.52] ×109/L vs. 6.53 [4.99, 9.10]×109/L, p = 0.036; 69.05 [63.83, 76.38]% vs. 60.80 [51.33, 68.68]%, p = 0.033; 291.00 [232.50, 443.25]×109/L vs. 231.00 [208.00, 278.75]×109/L, p = 0.024; 16.00 [10.00, 58.50]mm/H vs. 7.50 [4.50, 17.75]mm/H, p = 0.013; 7.60 [2.32, 46.52]mg/L vs. 0.71 [0.32, 4.37]mg/L, p = 0.001), while the level of Hb was lower in the depressed group (104.50 [97.25, 126.00]g/L vs. 124.00 [115.50, 133.00]g/L, p = 0.009). The authors also compared the indexes of activity between the two groups. The levels of NIH and ITAS.A suggested significant differences (2.00 [2.00, 3.00] vs. 1.00 [0.00, 2.00], p = 0.007; 7.00 [4.00, 9.50] vs. 1.50 [0.00, 5.75], p = 0.012), while the level of ITAS2010 also emerged on a trend toward higher in the depressed group despite p > 0.05 (4.00 [3.25, 6.50] vs. 1.00 [0.00, 5.00], p = 0.056) (Table 2).
The differences in cytokine profiles between TA patients and healthy controls
As in previously known studies, many cytokines were elevated in TA patients compared to healthy controls, which was consistent with the inflammatory essence of the disease. The levels of IL-1rα, IL-2, IL-4, IL-6, IL-8, IL-12 (p70), eotaxin, FGF basic, G-CSF, IP-10, MCP-1, MIP-1α, and TNF-α were significantly higher in TA patients than the levels of 13 cytokines in the healthy controls (Table 3). No heterogeneity was found in the other 14 cytokines between TA patients and controls in our study. The authors further compared the cytokine profiles between depressed and non-depressed groups in TA patients. The levels of IL-4, IL-13, eotaxin, and IP-10 were significantly lower in the depressed group than in the non-depressed group (2.80 [2.17, 3.18]pg./ml vs. 3.51 [3.22, 4.66]pg./ml, p < 0.001; 0.66 [0.60, 1.12]pg./ml vs. 1.04 [0.82, 1.25]pg./ml, p = 0.008; 46.48 [37.06, 61.75]pg./ml vs. 69.14 [59.30, 92.80]pg./ml, p = 0.001; 184.50 [138.23, 257.25]pg./ml vs. 322.32 [241.98, 412.60]pg./ml, p = 0.005). However, there were no significant differences between the depressed TA group and healthy controls in the level of IL-4, IL-13, eotaxin, IP-10 (2.80 [2.17, 3.18]pg./ml vs. 2.25 [2.14, 2.64]pg./ml, p = 0.091; 0.66 [0.60, 1.12]pg./ml vs. 0.82 [0.82, 1.04]pg./ml, p = 0.487; 46.48 [37.06, 61.75]pg./ml vs. 50.45 [35.63, 53.05]pg./ml, p = 0.608; 184.50 [138.23, 257.25]pg./ml vs. 202.44 [163.94, 211.78]pg./ml, p = 0.976). In contrast, the levels of IL-4, IL-13, eotaxin, and IP-10 were significantly higher in the non-depressed TA group than in the healthy control group (3.51 [3.22, 4.66]pg./ml vs. 2.25 [2.14, 2.64]pg./ml, p < 0.001; 1.04 [0.82, 1.25]pg./ml vs. 0.82 [0.82, 1.04]pg./ml, p = 0.015; 69.14 [59.30, 92.80]pg./ml vs. 50.45 [35.63, 53.05]pg./ml, p < 0.001; 322.32 [241.98, 412.60]pg./ml vs. 202.44 [163.94, 211.78]pg./ml, p < 0.001) (Figure 1 and Table 3).
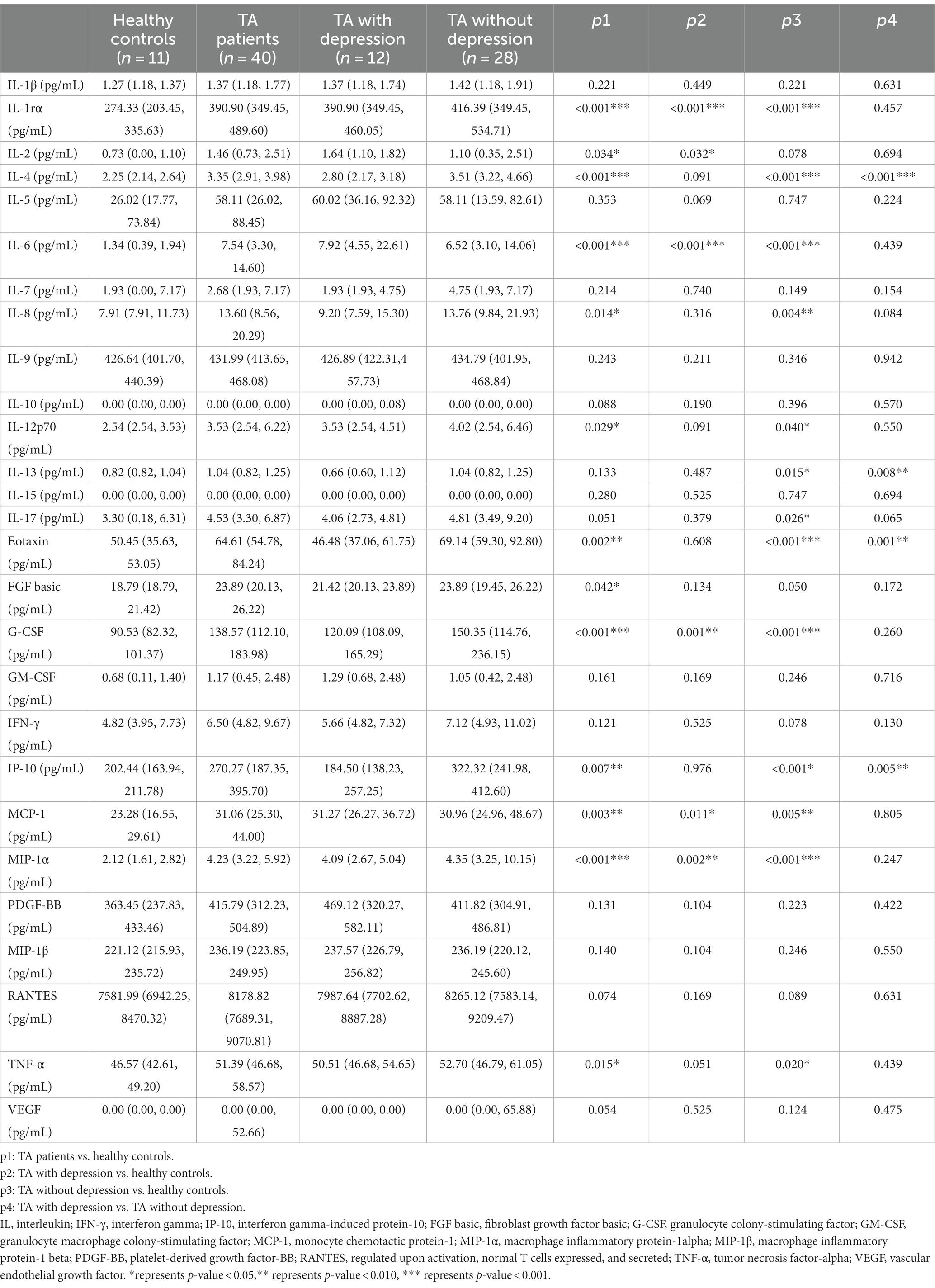
Table 3. Baseline levels of cytokines and chemokines in TA patients with depression, without depression, and healthy controls.
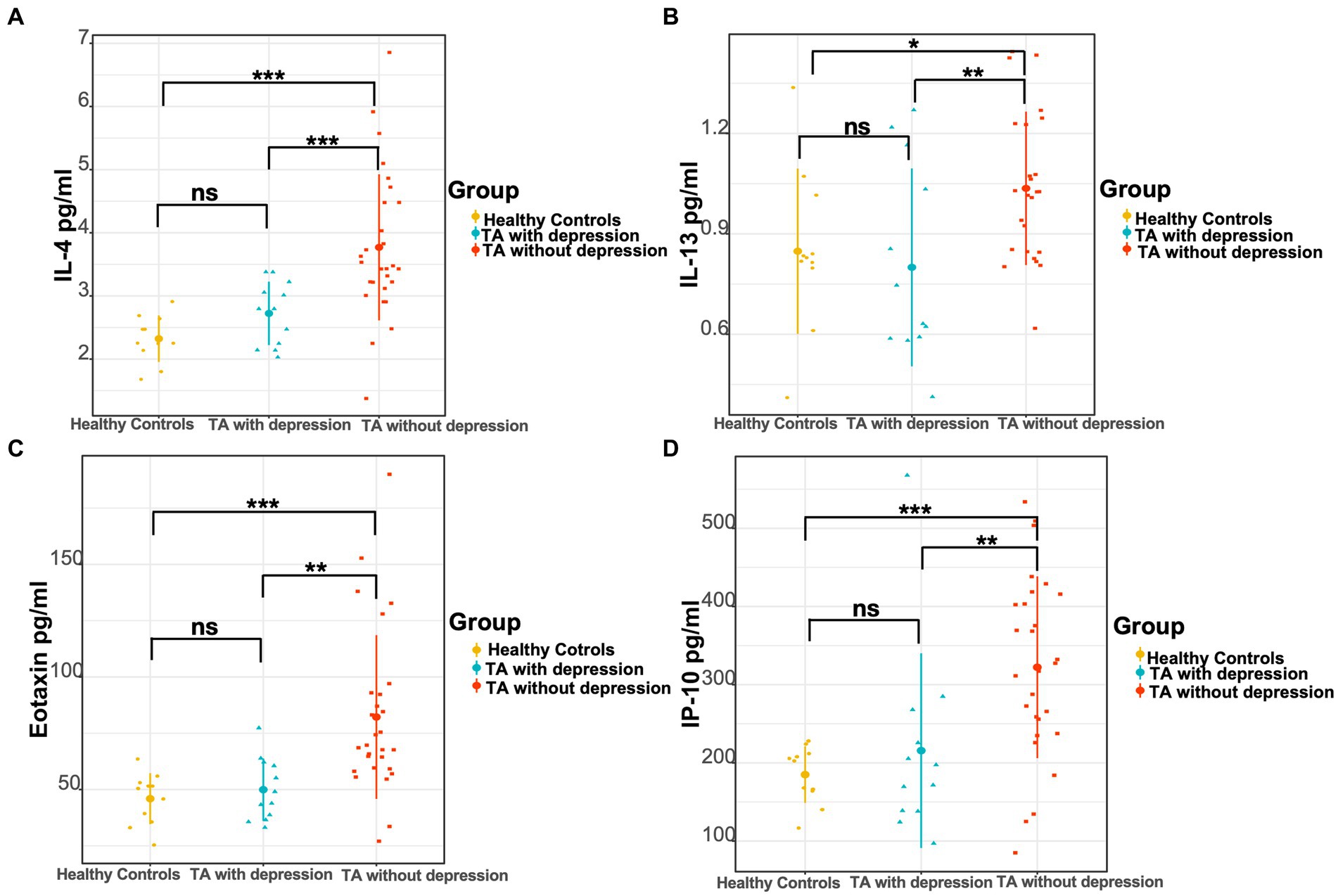
Figure 1. Comparison of three groups of cytokine levels. (A) The levels of IL-4, (B) IL-13, (C) eotaxin, and (D) IP-10 in the TA with depression, TA without depression, and healthy controls, respectively. *p < 0.05; **p < 0.01; ***p < 0.001.
Correlation between self-rating depression scale and cytokines, ESR, hsCRP, and disease activity
The authors used Spearman’s test as appropriate to assess the correlations between SDS and other significant variables in TA patients. The authors presented statistically correlated parameters in Figure 2 (p-value < 0.05) and the correlation coefficient r was shown in the form, while the blank indicated no statistical association. SDS was negatively correlated with the levels of IL-4 and IP-10 (r = −0.337, p = 0.033; −0.330, p = 0.038), while positively correlated with hsCRP, NIH, ITAS.A and ITAS2010 (r = 0.421, p = 0.007; r = 0.366, p = 0.020; r = 0.391, p = 0.013; r = 0.369, p = 0.019, respectively). In the meantime, the levels of IL-4, IL-13 and eotaxin were negatively associated with ESR (r = −0.416, p = 0.008; r = −0.459, p = 0.003; r = −0.518, p = 0.001) and hsCRP (r = −0.418, p = 0.007; r = −0.456, p = 0.003; r = −0.554, p < 0.001).
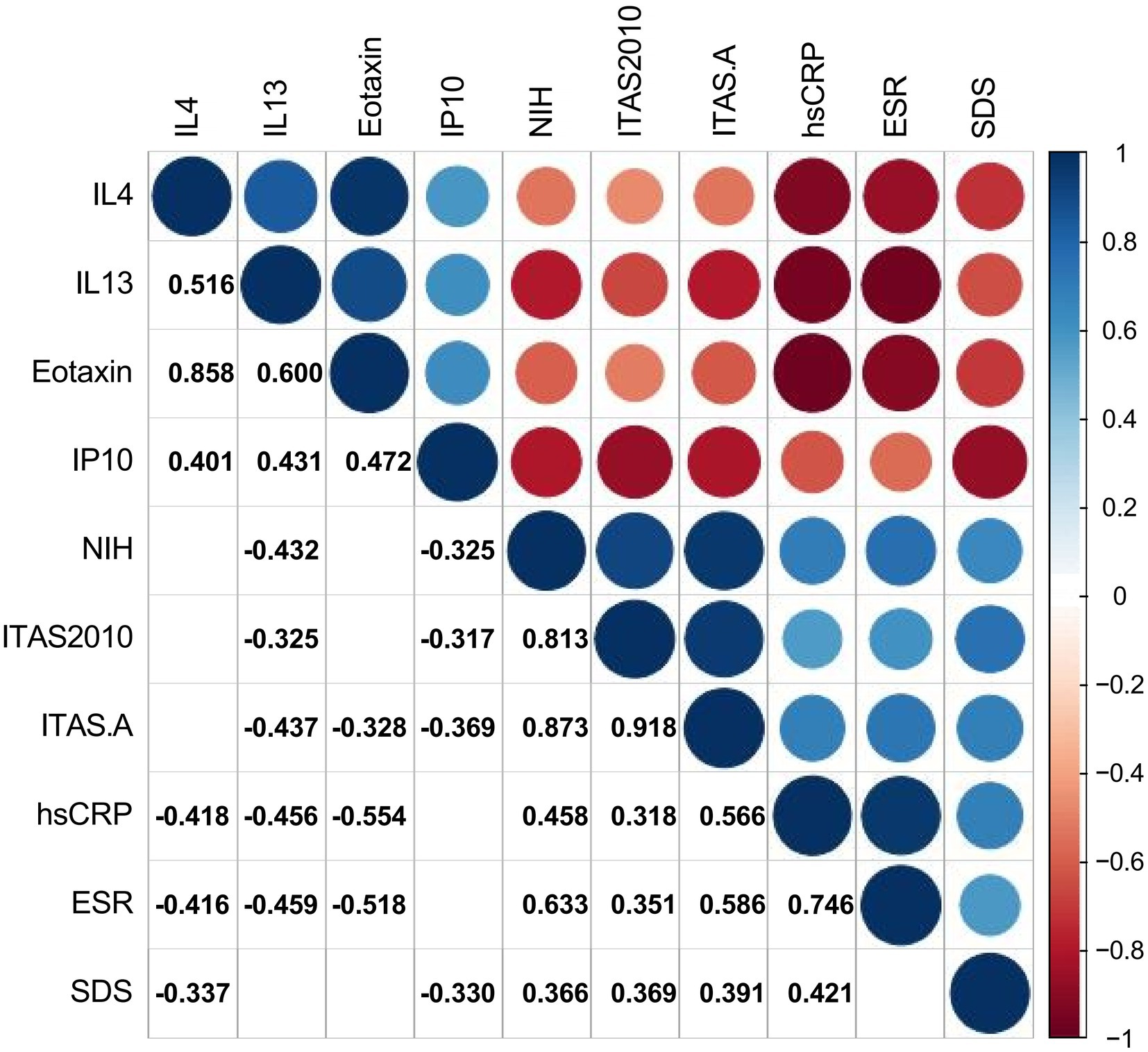
Figure 2. Correlations between cytokines, ESR, hsCRP, disease activity, and Self-Rating Depression Scale. The number and color in each box represented the correlation coefficient for two covariates corresponding to the box, of which blank indicated a non-statistically significant association.
Risk factors of depression in TA patients
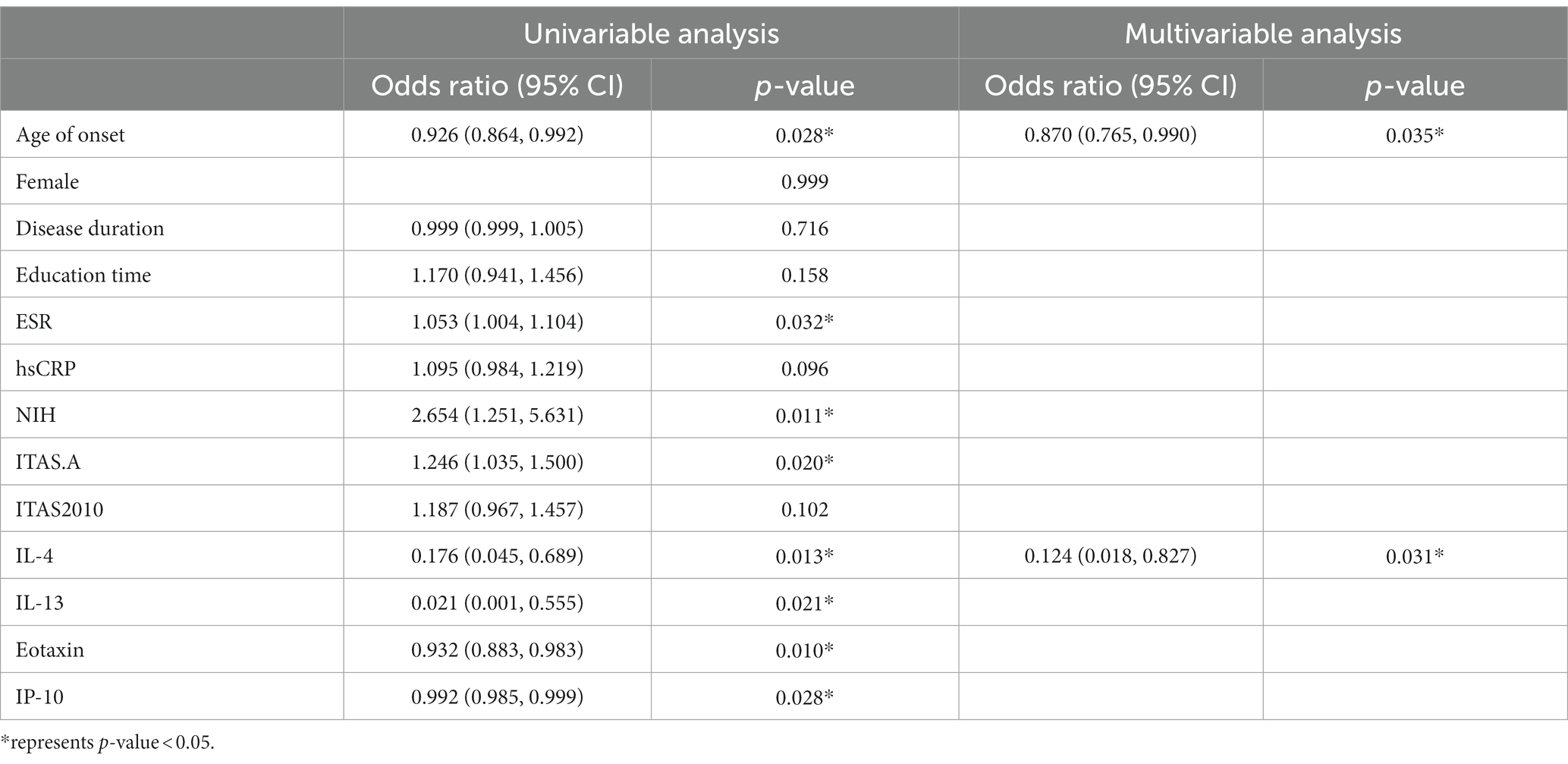
To identify independent predictors of depression in TA, the authors use multivariate logistic regression analysis to confirm the value of cytokines, which were significantly different in univariate analysis (p < 0.05) (Table 4). The univariate logistic regression analysis showed that age of onset and the levels of IL-4, IL-13, eotaxin, and IP-10 were protective in the depressed TA group (OR [95% CI] 0.926 [0.864, 0.992], p = 0.028; 0.176 [0.045, 0.689], p = 0.013; 0.021 [0.001, 0.555], p = 0.021; OR [95% CI] 0.932 [0.883, 0.983], p = 0.010; 0.992 [0.985, 0.999], p = 0.028, respectively), while ESR and NIH, ITAS.A as potential risk factors (OR [95% CI] 1.053 [1.004, 1.104], p = 0.032; 2.654 [1.251, 5.631], p = 0.011; OR [95% CI] 1.246 [1.035, 1.500], p = 0.020, respectively). In order to exclude confounding factors and build a multivariate regression model to better identify risk factors in the depressed group, the authors included traditional demographic risk factors, adjusted for gender, education, and disease duration. The results suggested that both the lower level of IL-4 and age of onset were risk factors for depression in TA patients (OR [95% CI] 0.124 [0.018, 0.827], p = 0.031; 0.870 [0.765, 0.990], p = 0.035, respectively).
Discussion
In this research, the authors recorded the levels of 27 cytokines, laboratory, and clinical data in patients with Takayasu arteritis (TA). Our study has reported the age of onset and IL-4 were risk factors for depressed TA patients and showed the depression group was more active in the disease for the first time. The authors found that depression was correlated with lower levels of IL-4, IL-13, eotaxin, and IP-10, suggesting the abnormal immune responses contributing to the evolution of depression in TA.
The authors found the depressed group was more active in TA patients. There were higher levels of ESR, hsCRP, NIH, ITAS.A, and ITAS2010 in TA with depression compared with non-depression. Previous studies have found depression and inflammation are intertwined, which depression facilitates inflammatory responses and inflammation promotes depression (21). Due to the inflammatory properties of autoimmune disease, many research studies devoted to studying the bidirectional inflammatory pathway between autoimmune diseases and depression (22). Imran et al. (23) identified a positive correlation between the severity of depression and the disease activity in rheumatoid arthritis (RA). Similar to other autoimmune disorders such as Behçet disease and rheumatoid arthritis (23, 24), the disease activity also showed a positive correlation with depression scores in TA (11, 25), which was consistent with our results.
Our study then compared the clinical characterizations between the depressed and non-depressed groups in TA patients. There was no heterogeneity in disease duration, BMI, educational time, and the female ratio. The younger age of onset was identified as an independent risk factor for depression in TA patients. It was shown that the younger age had well-known associations with depression (26). This can also be observed in autoimmune diseases. Previous research has shown that younger age in people under 40 years old had a significant interaction with RA in terms of depression risk (27, 28). In the existing life quality reports on TA, it was argued that age was negatively associated with emotional scores (29). However, studies on the relationship between age in TA patients with depression are lacking. Consistent with RA, the authors found that the younger age of onset showed a significantly positive correlation with depression in TA. This may be related to the fact that young patients are often actively involved in social work. The physical discomfort of TA combined with work stress can have a negative impact on the individual and trigger negative emotions.
The coexistence of immune-mediated inflammatory diseases and depression has long been recognized (7). Therefore, the authors focused on the analysis of the link between cytokines and depression in TA. In our study, the authors found the levels of IL-4, IL-13, eotaxin, and IP-10 were significantly lower in the depression of TA patients. Furthermore, the lower level of IL-4 proved to be an independent risk factor for depressed TA patients. IL-4 is an important anti-inflammatory factor produced by activated Th2 cells, thereby reducing the inflammatory response (30). Previous studies have proved that deficiencies in IL-4 can lead to an inability to recover in depressed mice caused by stress (31). It was shown that IL-4 reprogrammed the microglia, which activated the brain-derived neurotrophic factor signaling pathways to protect hippocampal nerves in depressed mice (32). These results indicated that IL-4 exerted neuroprotective function to fight depression. Current research found an elevated level of IL-4 in depressed populations and rats after exposure to chronic mild stress (33, 34) to suppress the inflammatory response. Recent findings suggested that IL-4 and IL-13 might play a significant role in the downregulation of inflammatory processes underlying RA pathology and beneficially modulate the course of the disease (35). In autoimmune disease, scholars have found that an increase in the ratio of Th1/Th2 cells could promote the production of depressive symptoms in collagen-induced arthritis mice (36). Hyperactive or hypoactive stress systems through modulation of the Th1/Th2 cytokine balance might be associated with abnormalities of the “systemic anti-inflammatory feedback,” contributing to the pathogenesis of depression and autoimmune diseases (37). This can explain that a decrease in IL-4 may indicate a lower proportion of Th2 cells, leading to an increase in the Th1/Th2 ratio, which can lead to TA patients with depression. It was shown that TA patients had higher serum levels of IL-4 and IL-13 than healthy controls. However, Gao et al. (38), in their investigation of Th2 cell characteristics in TA, identified there was not a significant difference in IL-4 between active and inactive groups. This aligns with our discovery that IL-4 levels did not show a correlation with NIH/ITAS.A/ITAS2010, which implies that the reduction of IL-4 in TA patients with depression might be specific to this condition.
The cytokine IL-13 is grouped together with IL-4 as anti-inflammatory cytokines, both of which are closely linked in the genome (39, 40). However, IL-13 was proven only one-tenth of the inhibiting effect of IL-4 in RA (41), without a direct correlation with depression. A cross-sectional study on depression in patients with breast cancer identified that the level of IL-13 was lower in the depressed group than in the non-depressed (13), a lack of further research to clarify the pathological mechanisms. Previous studies have found that IL-13 is significantly elevated in TA (38). Our result that the lower level of IL-13 was associated with depression might be related to the synergy of IL-4 reduction, with the need for further research to prove it. Eotaxin is a potent eosinophil chemoattractant cytokine (42). Its main receptor, the CC chemokine receptor 3 (43–45), is expressed on Th2 lymphocytes, implicated in the production of the Th2 cytokines (IL-4, IL-13) (46). Consequently, eotaxin has been proven to tend to a Th2 response (47). However, the current research studies remains contradictory about the role of eotaxin in depression (46). In previous studies, eotaxin was found to be higher in depressed patients than in healthy controls, but still remained elevated from baseline after 8 weeks of effective treatment (48). The determination of chemokines found no significant difference in eotaxin between TA and healthy controls (49). The authors confirmed that the lower level of eotaxin had a suggestive effect on TA patients with depression to a certain extent, which might be consistent with the declined IL-4. IP-10 is a Th1-related chemokine, secreted by T lymphocytes, NK cells, and monocytes (50) and demonstrated to be associated with multiple rheumatic immune disease activity (51). It was known that the HIV+ subjects with depression had increased levels of IP-10 compared to their non-depressed counterparts (52). Similar findings have not been identified in autoimmune diseases. The authors first found the declined level of IP-10 attributed to the depression in TA, the mechanism remaining a mystery.
Given the rarity of TA, our study is limited by a small sample size. Additionally, being a retrospective and cross-sectional study, it lacks a prospective plan and a follow-up cohort. In future, the authors will expand the number of patients and collect samples from patients with other systemic inflammatory diseases for more rigorous research.
Conclusion
This study presented that the serum levels of IL-4 were significantly decreased in TA patients with depression. It suggests that IL-4 may play an important part in the development of depression in TA patients. Clinicians need to be vigilant about the decline of IL-4 in order to predict the potential risk of depression in TA patients and respond positively.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Beijing Anzhen Hospital (Approval Number: 2023183X). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YZ: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft. SY: Data curation, Writing – original draft. AF: Investigation, Methodology, Writing – original draft. JD: Data curation, Writing – original draft. NG: Funding acquisition, Validation, Writing – review & editing. LP: Conceptualization, Funding acquisition, Writing – review & editing. TL: Conceptualization, Data curation, Funding acquisition, Validation, Writing – review & editing.
Glossary
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was sponsored by the National Natural Science Foundation of China (nos. 82270427, 81900448); Beijing Natural Science Foundation (nos. 7222043, 7232038).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Saadoun, D, Garrido, M, Comarmond, C, Desbois, AC, Domont, F, Savey, L, et al. Th1 and Th17 cytokines drive inflammation in Takayasu arteritis. Arthritis Rheumatol. (2015) 67:1353–60. doi: 10.1002/art.39037
2. Numano, F, Okawara, M, Inomata, H, and Kobayashi, Y. Takayasu's arteritis. Lancet. (2000) 356:1023–5. doi: 10.1016/s0140-6736(00)02701-x
3. Dai, X, Sun, Y, Ma, L, Hou, J, Wang, L, Gong, Y, et al. A novel molecular mechanism of vascular fibrosis in Takayasu arteritis: macrophage-derived GPNMB promoting adventitial fibroblast extracellular matrix production in the aorta. Transl Res. (2023) 255:128–39. doi: 10.1016/j.trsl.2022.12.004
4. Savioli, B, Abdulahad, WH, Brouwer, E, Kallenberg, CGM, and de Souza, AWS. Are cytokines and chemokines suitable biomarkers for Takayasu arteritis? Autoimmun Rev. (2017) 16:1071–8. doi: 10.1016/j.autrev.2017.07.023
5. Beurel, E, Toups, M, and Nemeroff, CB. The bidirectional relationship of depression and inflammation: double trouble. Neuron. (2020) 107:234–56. doi: 10.1016/j.neuron.2020.06.002
6. Mindt, S, Neumaier, M, Hoyer, C, Sartorius, A, and Kranaster, L. Cytokine-mediated cellular immune activation in electroconvulsive therapy: a CSF study in patients with treatment-resistant depression. World J Biol Psychiatry. (2020) 21:139–47. doi: 10.1080/15622975.2019.1618494
7. Nerurkar, L, Siebert, S, McInnes, IB, and Cavanagh, J. Rheumatoid arthritis and depression: an inflammatory perspective. Lancet Psychiatry. (2019) 6:164–73. doi: 10.1016/s2215-0366(18)30255-4
8. Opal, SM, and DePalo, VA. Anti-inflammatory cytokines. Chest. (2000) 117:1162–72. doi: 10.1378/chest.117.4.1162
9. Howard Tripp, N, Tarn, J, Natasari, A, Gillespie, C, Mitchell, S, Hackett, KL, et al. Fatigue in primary Sjögren's syndrome is associated with lower levels of proinflammatory cytokines. RMD Open. (2016) 2:e000282. doi: 10.1136/rmdopen-2016-000282
10. Wen, D, Feng, L, Du, X, Dong, JZ, and Ma, CS. Biomarkers in Takayasu arteritis. Int J Cardiol. (2023) 371:413–7. doi: 10.1016/j.ijcard.2022.08.058
11. Yilmaz, N, Can, M, Oner, FA, Kalfa, M, Emmungil, H, Karadag, O, et al. Impaired quality of life, disability and mental health in Takayasu's arteritis. Rheumatology (Oxford). (2013) 52:1898–04. doi: 10.1093/rheumatology/ket238
12. Leighton, SP, Nerurkar, L, Krishnadas, R, Johnman, C, Graham, GJ, and Cavanagh, J. Chemokines in depression in health and in inflammatory illness: a systematic review and meta-analysis. Mol Psychiatry. (2018) 23:48–58. doi: 10.1038/mp.2017.205
13. Ho, HY, Chin-Hung Chen, V, Tzang, BS, Hsieh, CC, Wang, WK, Weng, YP, et al. Circulating cytokines as predictors of depression in patients with breast cancer. J Psychiatr Res. (2021) 136:306–11. doi: 10.1016/j.jpsychires.2021.02.037
14. Grayson, PC, Ponte, C, Suppiah, R, Robson, JC, Gribbons, KB, Judge, A, et al. 2022 American College of Rheumatology/EULAR classification criteria for Takayasu arteritis. Ann Rheum Dis. (2022) 81:1654–60. doi: 10.1136/ard-2022-223482
15. Kerr, GS, Hallahan, CW, Giordano, J, Leavitt, RY, Fauci, AS, Rottem, M, et al. Takayasu arteritis. Ann Intern Med. (1994) 120:919–29. doi: 10.7326/0003-4819-120-11-199406010-00004
16. Misra, R, Danda, D, Rajappa, SM, Ghosh, A, Gupta, R, Mahendranath, KM, et al. Development and initial validation of the Indian Takayasu clinical activity score (ITAS2010). Rheumatology (Oxford). (2013) 52:1795–01. doi: 10.1093/rheumatology/ket128
17. Hata, A, Noda, M, Moriwaki, R, and Numano, F. Angiographic findings of Takayasu arteritis: new classification. Int J Cardiol. (1996) 54:S155–63. doi: 10.1016/s0167-5273(96)02813-6
18. Hellmich, B, Agueda, A, Monti, S, Buttgereit, F, de Boysson, H, Brouwer, E, et al. 2018 update of the EULAR recommendations for the management of large vessel vasculitis. Ann Rheum Dis. (2020) 79:19–30. doi: 10.1136/annrheumdis-2019-215672
19. Dunstan, DA, Scott, N, and Todd, AK. Screening for anxiety and depression: reassessing the utility of the Zung scales. BMC Psychiatry. (2017) 17:329. doi: 10.1186/s12888-017-1489-6
20. Zung, WW. A self-rating depression scale. Arch Gen Psychiatry. (1965) 12:63–70. doi: 10.1001/archpsyc.1965.01720310065008
21. Kiecolt-Glaser, JK, Derry, HM, and Fagundes, CP. Inflammation: depression fans the flames and feasts on the heat. Am J Psychiatry. (2015) 172:1075–91. doi: 10.1176/appi.ajp.2015.15020152
22. Matcham, F, Rayner, L, Steer, S, and Hotopf, M. The prevalence of depression in rheumatoid arthritis: a systematic review and meta-analysis. Rheumatology (Oxford). (2013) 52:2136–48. doi: 10.1093/rheumatology/ket169
23. Imran, MY, Saira Khan, EA, Ahmad, NM, Farman Raja, S, Saeed, MA, and Ijaz, HI. Depression in rheumatoid arthritis and its relation to disease activity. Pak J Med Sci. (2015) 31:393–7. doi: 10.12669/pjms.312.6589
24. Lee, J, Kim, SS, Jeong, HJ, Son, CN, Kim, JM, Cho, YW, et al. Association of sleep quality in Behcet disease with disease activity, depression, and quality of life in Korean population. Korean J Intern Med. (2017) 32:352–9. doi: 10.3904/kjim.2016.367
25. Erdal, S, Nalbantoğlu, B, Gür, MB, Yıldırım, M, Kılıçarslan, A, Kaymaz-Tahra, S, et al. HADS-depression score is a mediator for illness perception and daily life impairment in Takayasu's arteritis. Clin Rheumatol. (2021) 40:4109–16. doi: 10.1007/s10067-021-05719-2
26. Margaretten, M, Julian, L, Katz, P, and Yelin, E. Depression in patients with rheumatoid arthritis: description, causes and mechanisms. Int J Clin Rheumtol. (2011) 6:617–23. doi: 10.2217/ijr.11.6
27. Lu, MC, Guo, HR, Lin, MC, Livneh, H, Lai, NS, and Tsai, TY. Bidirectional associations between rheumatoid arthritis and depression: a nationwide longitudinal study. Sci Rep. (2016) 6:20647. doi: 10.1038/srep20647
28. Fifield, J, Reisine, S, Sheehan, TJ, and McQuillan, J. Gender, paid work, and symptoms of emotional distress in rheumatoid arthritis patients. Arthritis Rheum. (1996) 39:427–35. doi: 10.1002/art.1780390310
29. Alibaz-Oner, F, Sreih, AG, Merkel, PA, and Direskeneli, H. Patient-reported outcomes in Takayasu's arteritis. Presse Med. (2017) 46:e225–7. doi: 10.1016/j.lpm.2017.03.023
30. Woodward, EA, Prêle, CM, Nicholson, SE, Kolesnik, TB, and Hart, PH. The anti-inflammatory effects of interleukin-4 are not mediated by suppressor of cytokine signalling-1 (SOCS1). Immunology. (2010) 131:118–27. doi: 10.1111/j.1365-2567.2010.03281.x
31. Wachholz, S, Knorr, A, Mengert, L, Plümper, J, Sommer, R, Juckel, G, et al. Interleukin-4 is a participant in the regulation of depressive-like behavior. Behav Brain Res. (2017) 326:165–72. doi: 10.1016/j.bbr.2017.03.020
32. Zhang, J, Rong, P, Zhang, L, He, H, Zhou, T, Fan, Y, et al. IL4-driven microglia modulate stress resilience through BDNF-dependent neurogenesis. Sci Adv. (2021) 7. doi: 10.1126/sciadv.abb9888
33. Wang, YL, Han, QQ, Gong, WQ, Pan, DH, Wang, LZ, Hu, W, et al. Microglial activation mediates chronic mild stress-induced depressive-and anxiety-like behavior in adult rats. J Neuroinflamm. (2018) 15. doi: 10.1186/s12974-018-1054-3
34. Xie, XH, Lai, WT, Xu, SX, di Forti, M, Zhang, JY, Chen, MM, et al. Hyper-inflammation of astrocytes in patients of major depressive disorder: evidence from serum astrocyte-derived extracellular vesicles. Brain Behav Immun. (2023) 109:51–62. doi: 10.1016/j.bbi.2022.12.014
35. Iwaszko, M, Biały, S, and Bogunia-Kubik, K. Significance of interleukin (IL)-4 and IL-13 in inflammatory arthritis. Cell. (2021) 10. doi: 10.3390/cells10113000
36. Miossec, P, Briolay, J, Dechanet, J, Wijdenes, J, Martinez-Valdez, H, and Banchereau, J. Inhibition of the production of proinflammatory cytokines and immunoglobulins by interleukin-4 in an ex vivo model of rheumatoid synovitis. Arthritis Rheum. (1992) 35:874–83. doi: 10.1002/art.1780350805
37. Calcagni, E, and Elenkov, I. Stress system activity, innate and T helper cytokines, and susceptibility to immune-related diseases. Ann N Y Acad Sci. (2006) 1069:62–76. doi: 10.1196/annals.1351.006
38. Gao, N, Cui, W, Zhao, LM, Li, TT, Zhang, JH, and Pan, LL. Contribution of Th2-like Treg cells to the pathogenesis of Takayasu's arteritis. Clin Exp Rheumatol. (2020) 38:48–54.
39. de Waal Malefyt, R, Figdor, CG, Huijbens, R, et al. Effects of IL-13 on phenotype, cytokine production, and cytotoxic function of human monocytes. Comparison with IL-4 and modulation by IFN-gamma or IL-10. J Immunol. (1993) 151:6370–81.
40. Zurawski, G, and de Vries, JE. Interleukin 13, an interleukin 4-like cytokine that acts on monocytes and B cells, but not on T cells. Immunol Today. (1994) 15:19–26. doi: 10.1016/0167-5699(94)90021-3
41. Morita, Y, Yamamura, M, Kawashima, M, Aita, T, Harada, S, Okamoto, H, et al. Differential in vitro effects of IL-4, IL-10, and IL-13 on proinflammatory cytokine production and fibroblast proliferation in rheumatoid synovium. Rheumatol Int. (2001) 20:49–54. doi: 10.1007/s002960000074
42. Sirivichayakul, S, Kanchanatawan, B, Thika, S, Carvalho, AF, and Maes, M. Eotaxin, an endogenous cognitive deteriorating chemokine (ECDC), is a major contributor to cognitive decline in Normal people and to executive, memory, and sustained attention deficits, formal thought disorders, and psychopathology in schizophrenia patients. Neurotox Res. (2019) 35:122–38. doi: 10.1007/s12640-018-9937-8
43. Kitaura, M, Nakajima, T, Imai, T, Harada, S, Combadiere, C, and Tiffany, TL. Molecular cloning of human eotaxin, an eosinophil-selective CC chemokine, and identification of a specific eosinophil eotaxin receptor, CC chemokine receptor 3. J Biol Chem. (1996) 271:7725–30. doi: 10.1074/jbc.271.13.7725
44. Ponath, PD, Qin, S, Ringler, DJ, Clark-Lewis, I, Wang, J, Kassam, NJ, et al. Cloning of the human eosinophil chemoattractant, eotaxin. Expression, receptor binding, and functional properties suggest a mechanism for the selective recruitment of eosinophils. J Clin Invest. (1996) 97:604–12. doi: 10.1172/jci118456
45. Teixeira, MM, Wells, TN, Lukacs, NW, Proudfoot, AE, Kunkel, SL, Williams, TJ, et al. Chemokine-induced eosinophil recruitment. Evidence of a role for endogenous eotaxin in an in vivo allergy model in mouse skin. J Clin Invest. (1997) 100:1657–66. doi: 10.1172/jci119690
46. Teixeira, AL, Gama, CS, Rocha, NP, and Teixeira, MM. Revisiting the role of Eotaxin-1/CCL11 in psychiatric disorders. Front Psych. (2018) 9:241. doi: 10.3389/fpsyt.2018.00241
47. Gutierrez-Ramos, JC, Lloyd, C, and Gonzalo, JA. Eotaxin: from an eosinophilic chemokine to a major regulator of allergic reactions. Immunol Today. (1999) 20:500–4. doi: 10.1016/s0167-5699(99)01522-4
48. de la Peña, FR, Cruz-Fuentes, C, Palacios, L, Girón-Pérez, MI, Medina-Rivero, E, Ponce-Regalado, MD, et al. Serum levels of chemokines in adolescents with major depression treated with fluoxetine. World J Psych. (2020) 10:175–86. doi: 10.5498/wjp.v10.i8.175
49. Dong, H, Zhang, Y, Zou, Y, Chen, Y, Yue, J, Liu, H, et al. Elevated chemokines concentration is associated with disease activity in Takayasu arteritis. Cytokine. (2021) 143:155515. doi: 10.1016/j.cyto.2021.155515
50. Dufour, JH, Dziejman, M, Liu, MT, Leung, JH, Lane, TE, and Luster, AD. IFN-gamma-inducible protein 10 (IP-10; CXCL10)-deficient mice reveal a role for IP-10 in effector T cell generation and trafficking. J Immunol. (2002) 168:3195–04. doi: 10.4049/jimmunol.168.7.3195
51. van Hooij, A, Boeters, DM, Tjon Kon Fat, EM, van den Eeden, SJF, Corstjens, PL, et al. Longitudinal IP-10 serum levels are associated with the course of disease activity and remission in patients with rheumatoid arthritis. Clin Vaccine Immunol. (2017) 24. doi: 10.1128/CVI.00060-17
52. Rivera-Rivera, Y, García, Y, Toro, V, Cappas, N, López, P, Yamamura, Y, et al. Depression correlates with increased plasma levels of inflammatory cytokines and a dysregulated oxidant/antioxidant balance in HIV-1-infected subjects undergoing antiretroviral therapy. J Clin Cell Immunol. (2014) 5. doi: 10.4172/2155-9899.1000276
Keywords: Takayasu arteritis, mental psychology, depression, cytokines, disease activity
Citation: Zhang Y, Yang S, Fan A, Du J, Gao N, Pan L and Li T (2024) Decreased IL-4 is the risk factor of depression in patients with Takayasu arteritis. Front. Med. 11:1337206. doi: 10.3389/fmed.2024.1337206
Edited by:
Xiaoming Shu, China-Japan Friendship Hospital, ChinaCopyright © 2024 Zhang, Yang, Fan, Du, Gao, Pan and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lili Pan, bGlseXBhbnN4dW1Ac2luYS5jb20=; Taotao Li, bGl0YW90YW83ODlAc2luYS5jb20=
†These authors have contributed equally to this work
 Yaxin Zhang1
Yaxin Zhang1 Anyuyang Fan
Anyuyang Fan Na Gao
Na Gao Lili Pan
Lili Pan