
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Med., 02 February 2024
Sec. Hematology
Volume 11 - 2024 | https://doi.org/10.3389/fmed.2024.1335969
This article is part of the Research TopicInfectious Diseases and Hematology: Diagnosis and ManagementView all 13 articles
A correction has been applied to this article in:
Refractory human cytomegalovirus infection without evidence of genetic resistance in the UL-54 and UL-97 genes in a pediatric hematopoietic stem cell transplant recipient: a case report
 Alejandra Pando-Caciano1*
Alejandra Pando-Caciano1* Ketty Adid Escudero-Ramirez1
Ketty Adid Escudero-Ramirez1 Jackeline Carol Rodríguez-Torres2
Jackeline Carol Rodríguez-Torres2 Holger Maita-Malpartida1,3
Holger Maita-Malpartida1,3Cytomegalovirus (CMV) infection is a common complication in patients undergoing hematopoietic stem cell transplantation (HSCT). Management of refractory CMV infections, especially in developing countries, can be challenging due to the limited availability of second and third-line antiviral drugs or alternative treatments. Here, we present a case of an 8 years-old patient diagnosed with acute myeloid leukemia. Eight months post-diagnosis, the patient underwent TCR-αβ+/CD19+-depleted haploidentical HSCT. Both the donor and recipient tested positive for anti-CMV IgG and negative for IgM antibodies. Before transplantation, the patient received CMV prophylaxis in the form of intravenous ganciclovir. Post-transplantation, the patient exhibited oscillating CMV viral loads and was diagnosed with a refractory infection. Treatment with ganciclovir, foscarnet, and cidofovir was unsuccessful. Sequencing of UL-54 and UL-97 genes was performed to rule out potential resistance to first-line treatment. Ten months after the HSCT, the child died from hypovolemic shock due to gastrointestinal bleeding. This is the first case reported in Peru and Latin America of a refractory CMV infection in a pediatric HSCT recipient without evidence of clinical symptoms and CMV genetic resistance. This case demonstrates the need for alternative treatments to manage refractory CMV infections, especially in haploidentical HSCT cases where drug resistance is frequent (~15%). Furthermore, this case highlights the importance of using highly sensitive genetic tools to detect mutations associated with virus resistance in a broader range of the viral genome.
Human herpesvirus 5 or human cytomegalovirus (CMV) is a member of the Herpesviridae family (1). CMV seroprevalence in Latin America (60%–90%) is significantly higher than in Europe or North America (2). The infection among immunocompetent individuals is usually asymptomatic, but among transplanted individuals, CMV can cause fatal diseases such as pneumonia, enteritis, cystitis, and encephalitis (3). Additionally, infection can cause graft failure due to the inhibition of the myelopoiesis (4).
Post-transplant CMV infection monitoring relies on weekly qPCR-based detection and quantification of blood viral load (5). This approach enables preemptive antiviral therapy to prevent end-stage organ disease (5). Approved antiviral agents for CMV infections include ganciclovir, valganciclovir, foscarnet, cidofovir, letermovir, and maribavir (6, 7).
One main challenge in managing CMV infection in transplant recipients is refractory infections, which may be related to genetic or non-genetic mechanisms (8). The significant incidence of resistant CMV, especially among haploidentical HSCT recipients (~15%) (9), makes the need for quick and accurate detection of resistant cases evident. Typically, the choice of therapeutic approaches is adjusted according to the specific resistance profile of the virus with the aim of achieving early remission of the infection. CMV drug resistance is evaluated by conventional PCR and sequencing of the UL-54 (phosphotransferase) and UL-97 (DNA polymerase) genes (10).
We report here the case of a high-risk pediatric patient with acute myeloid leukemia who developed a refractory CMV infection. Despite treatment with ganciclovir, valganciclovir, foscarnet, and cidofovir, the infection persisted. This is the first case reported in Peru and Latin America of refractory CMV infection in a pediatric HSCT recipient without evidence of clinical symptoms or genetic resistance.
This case highlights the need for highly sensitive genetic tests to characterize mutations associated with CMV resistance to drugs and for alternative treatments to manage refractory CMV infections in order to extend the survival chances of individuals undergoing HSCT.
We present the case of an 8 years-old male patient who underwent haploidentical hematopoietic stem cell transplantation (HSCT) for acute myeloid leukemia. The procedure was carried out on November 18th, 2019, at the Instituto Nacional de Salud del Niño San Borja in Lima, Peru. The patient received an allograft from his mother with a 3/6 HLA match after depleting CD19+ and TCRαβ+ cells.
The patient received chemotherapy according to the ALL-BFM 2009 protocol and achieved complete remission after four blocks of consolidation (11). The conditioning regimen involved the administration of fludarabine, antithymocyte globulin, cyclophosphamide, and total body irradiation. No prophylaxis for graft-versus-host disease (GvHD) was provided before transplantation. The histocompatibility study showed haploidentical compatibility with his mother at the HLA-B and HLA-DQ loci, resulting in the selection of the mother as the transplant donor.
The patient had no active CMV infection prior to transplantation. However, both the recipient and donor tested positive for anti-CMV IgG. Anti-CMV prophylaxis was started 16 days prior to transplantation, with the initial administration of ganciclovir at a dose of 5 mg/kg/12 h for 6 days, suspension of treatment for 7 days, and restart of ganciclovir treatment at a dose of 5 mg/kg/12 h for 3 days.
No immunosuppressive therapy was administered following transplantation. At day +14, immune reconstitution was assessed by absolute neutrophil count, which was greater than 0.5 × 109 cells/L (neutrophil count = 0.6 × 109 cells/L). Recovery of CD8+, CD19+, and NK cells was faster than CD4+ cells. CD4+ recovery occurred at 6 months post-HSCT, while CD8+, CD19+, and NK recovery occurred during the first trimester (Table 1). At day +35, the patient presented complete chimerism with >95% donor cells for all three cell lineages (T, B, and myeloid cells), confirming engraftment without GvHD.
The viral load of CMV was quantified by real-time PCR (targeting the 4 IE antigen gene) in peripheral blood (PB) samples. Reactivation of CMV infection was observed on day +32, with the presence of 688 DNA copies/mL. The kinetics of the viral load showed a pattern consistent with a CMV infection refractory to the administered drugs, observing an increase in viral load, accompanied by a fall and a new increase, throughout the post-HSCT period (see Figure 1).
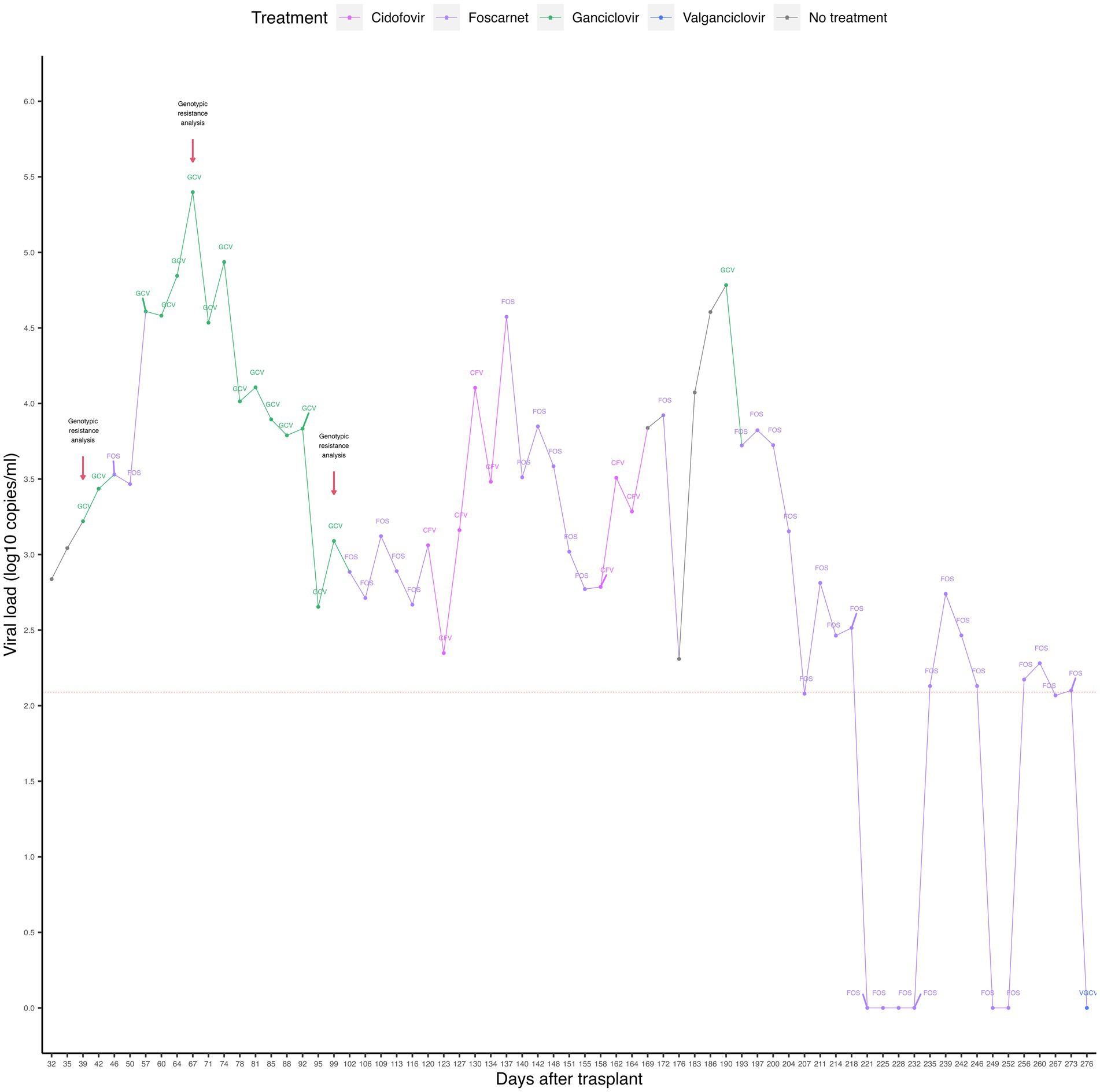
Figure 1. Timeline representing CMV viral loads and antiviral treatments of the case. The dashed red horizontal line represents the limit of detection of the qPCR assay. The red arrows indicate the time points of genotypic resistance analysis. GCV, ganciclovir; FOS, foscarnet; CFV, cidofovir; VGCV, valganciclovir.
Because of infection reactivation, preemptive treatment was started with intravenous ganciclovir (5 mg/kg/12 h) for 7 days, followed by foscarnet (180 mg/kg/day) for 10 days, ganciclovir (5 mg/kg/12 h) for 46 days, foscarnet (180 mg/kg/day) for 15 days, and cidofovir (5 mg/kg/week) for 4 weeks. The highest viral load (2.5 × 105 copies/mL) was recorded on day +67 during the application of the antiviral treatment and gradually decreased until it reached 223 copies/mL on day +123. An invasive pulmonary infection with small pseudo-nodules in the right lung was also recorded. No causative agents were identified, and the infection was managed with amphotericin B and voriconazole.
On day +127, an increase in viral load from 223 copies/mL to 1,452 copies/mL was observed, restarting treatment with foscarnet (180 mg/kg/day) for 18 days, cidofovir (5 mg/kg/week) for 2 weeks, ganciclovir (5 mg/kg/12 h) for 3 days and foscarnet (180 mg/kg/day) for 80 more days, until day +273. On the fourteenth day after the administration of foscarnet (day +207), the viral load fell below the detection limit (equivalent to 120 copies/mL). However, 4 days later (day +211), an increase in the viral load to 649 copies/ml was observed during the application of foscarnet. The viral load then became negative at day +221, remaining undetectable until day +232. Three days later, virus reactivation was observed with a viral load of 135 copies/mL (day +235), which increased to 549 copies/mL, then decreased to 292 copies/mL, 135 copies/mL, and finally became negative on day +249. The last reactivation was observed on day +256, with a viral load of 149 copies/mL. On day +275, the patient started treatment with oral valganciclovir (15–18 mg/kg/day) for 9 days. After the last reactivation, the viral load became undetectable on day +276, coinciding with the administration of valganciclovir.
Due to our suspicion of a refractory CMV infection, PB samples were analyzed by Sanger sequencing to identify mutations, deletions, insertions, or substitutions in the UL-54 and UL-97 genes known to confer genetic resistance to drugs. Nevertheless, genotypic analyses performed on days +39, +67, and +99 failed to detect modifications compatible with genetic drug resistance.
During the post-HSCT period, the patient showed coinfection with adenovirus and BK polyomavirus, detected by quantitative PCR in plasma and serum. These infections were detected on days +11 and +18 and successfully cleared at days +148 and +188, respectively.
On day +245 of HSCT, the patient was diagnosed with disease relapse by flow cytometry and PCR of bone marrow samples (7.49% of pathological myeloid blasts and 11.14% of AML1-ETO+ cells). The biopsy of the pterygoid mass confirmed infiltration of blasts in the right sphenoid maxillary sinus, which extended from the right pterygoid to invade the intracranial extra-axial space at the level of the temporal fossa and right choana. Intravenous cytarabine (30 mg every 24 h) was started for 8 days, and intrathecal cytarabine (30 mg every 24 h) for 3 days, with no effect on disease remission. Chimerism tests revealed mixed chimerism greater than 94%, with donor T, B, and myeloid cell populations greater than 95%, 94%, and 95%, respectively. The patient presented volume growth of the right side of the face, palpebral ptosis, and visual loss of the right eye due to infiltration of blasts. On day +276, the patient was discharged with parental consent and received palliative therapy for pain management with morphine administration.
On day +290, the patient was admitted to the emergency department due to hematemesis followed by fainting. Laboratory tests revealed severe anemia, acute kidney disease due to tumor lysis, leukocytosis with blastemia, and thrombocytopenia. The patient was discharged 2 days later with a poor short-term prognosis. On day +308, the patient was readmitted to the emergency department due to hematemesis. On physical examination, he presented poor general condition, with paleness, tachycardia, hypotension, fever, sunken eyes, multiple ecchymoses, increased volume of the left arm, and chronic malnutrition. Laboratory studies revealed leukocytosis due to the presence of blasts, thrombocytopenia, anemia, high levels of C-reactive protein (354.6 mg/L), urea (80.2 mg/dL), urea nitrogen (37.45 mg/dL), and procalcitonin (86.17 ng/mL). One day after readmission, the patient died of hypovolemic shock due to intestinal bleeding.
This is the first case reported in Peru and Latin America describing persistent CMV infection in a pediatric haploidentical HSCT recipient. CMV infection significantly increases morbidity in transplant patients and represents a major risk factor for survival (12). The incidence of CMV infection in individuals undergoing haploidentical HSCT is significantly higher than in those with matched sibling donors (MSD) or matched unrelated donors (MUD) (85.7%, 39.0%, 55.6%, respectively; p < 0.001) (13). Among haploidentical HSCT patients, CMV reactivation occurs in 55% of cases, leading to CMV disease in 5% (14). Receiving a haploidentical HSCT is considered a risk factor for CMV infection development (OR = 4.24, p = 0.007) (15).
Refractory infections in HSCT recipients are usually common (12%) (12). Refractory CMV infection is characterized by a viremia increase of more than 1-log10 after at least 2 weeks of antiviral treatment (8). It is important to note that some drugs used to treat the CMV infection in the present case could not be administered on time due to shortage at the institution.
The presence of refractory infection constitutes a significant risk for the survival of infected patients (3 years event-free survival: 53% in cases of resistant CMV vs. 87% in cases of susceptible CMV; p < 0.001) (12). In the present case, a persistent viral load was observed during the 9 months post-HSCT, with an increase of 2.5-log10 compared to the initial viral load of 688 copies/mL. The highest viral load was 2.5 × 105 copies/mL on day +67. These findings confirmed the presence of a refractory CMV infection, which is characterized by fluctuating viral loads.
Initial treatment for CMV infection among HSCT recipients consists of ganciclovir or valganciclovir for at least 2 weeks (3). In case of adverse events (neutropenia or thrombocytopenia) or intolerance, using foscarnet for 2–3 weeks or cidofovir for 2 weeks is recommended (3). Foscarnet administration as a first-line treatment has been suggested for adult patients presenting bone marrow function (16). In cases of mutations conferring high resistance to foscarnet and cidofovir, such as the C592G mutation in the UL97 gene, a switch to ganciclovir is recommended, expecting virus clearance within 14 days (16, 17).
In addition to conventional pharmacological approaches, CMV-specific T-cell therapy (CMV-TCT) has been suggested as a promising treatment option for CMV infections following HSCT. The therapy is suggested for cases of refractory CMV with progressive viral disease such as pneumonitis and colitis (18). Donor-derived CMV-TCT has been proven to be effective in treating CMV infections in pediatric patients undergoing HSCT, with an overall response rate of 89.5% (19). Combining CMV-TCT with foscarnet has demonstrated efficacy in resolving CMV-associated retinitis in a pediatric recipient of haploidentical TCRαβ+/CD19+ cell-depleted HSCT (20). Notably, this therapeutic strategy has not been reported for HSCT recipients in Peru. Challenges related to logistics and the cost of the T-cell separation process may pose significant obstacles to the future implementation of this therapy in developing countries.
Previous reports suggest combining viral load monitoring with genetic screening to detect mutations in the UL-97 and UL-54 genes for treatment adjustment based on the specific viral resistance profile (21). However, in the presented case, genetic resistance testing during the first 3 months post-HSCT did not reveal any known mutations associated with drug resistance. Contrarily, the highest viral load observed during the entire post-transplant period occurred at day +67 (2.5 × 105 copies/ml), approximately 2 months after the transplant.
Known drug resistance in CMV results primarily from mutations in the UL-97 and UL-54 genes. UL-97 mutations affect ganciclovir activation but do not impact susceptibility to foscarnet or cidofovir (22). UL-54 mutations confer resistance to all available drugs, and their combination with UL-97 mutations results in high-level resistance to multiple drugs (23). Additionally, in transplant recipients, rare mutations associated with letermovir resistance have also been reported in the UL-56 gene, which encodes a component of the viral terminase complex responsible for the cleavage and packaging of viral genome into nascent viral capsids (24–26). Among solid organ transplant recipients, mutations in the UL-97 and UL-54 genes account for 26% of drug resistance cases, whereas mutations in UL-56 account for only 3% of cases (27).
The case presented here is exceptional since there was no response to treatment with ganciclovir, foscarnet, and cidofovir despite the absence of mutations associated with CMV resistance. The last measurement of viral load before the patient’s discharge at day +276, suggested virus clearance by foscarnet, although no additional tests were performed to confirm a possible new reactivation. A similar case of an older adult with chronic myelomonocytic leukemia who underwent HSCT with an MUD donor was previously reported in 2022. The patient presented four episodes of CMV infection with no mutations on the UL-97 and UL-54 genes. Intriguingly, however, analysis of the UL-56 gene revealed an unknown mutation (R246C). This mutation was associated with a superior replicative capacity but did not necessarily lead to a reported increase drug resistance (27). The combination of enhanced replicative capacity and the absence of mutations in UL-97 and UL-54 genes may explain persistent viral loads in cases like the one presented here, suggesting the importance of including the analysis of the UL-56 gene in addition to UL-97 and UL-54 genes in similar cases.
On day +308, the patient died of gastrointestinal bleeding, possibly caused by CMV colitis, similar to a previous report (28). Since gastrointestinal biopsies were not performed, the diagnosis of a probable CMV infection of the gastrointestinal tract could not be verified. Upper and lower gastrointestinal tract infections are more frequent in transplant recipients with underlying hematological conditions than in transplant patients with solid tumors (p = 0.02) (29). Therefore, it is essential that healthcare institutions managing transplanted patients have access to tests permitting CMV detection and quantification in the gastrointestinal tract (30). In the case reported here, despite the availability of these laboratory tests, signs of gastrointestinal involvement in the patient did not manifest themselves in the final month before death. During this period, no further testing was performed to confirm probable CMV gastrointestinal disease, as the patient was in palliative care.
In transplant patients, the initial episode of DNAemia is usually asymptomatic (>80%). However, approximately 15% of these individuals have CMV disease when the first viremia is detected, which includes CMV syndrome, pneumonia, and gastrointestinal disease (31). Gastrointestinal bleeding in haploidentical HSCT recipients may occur from day +1 to +892 post-transplantation, with clinical presentations that include rectal bleeding (69%), hematemesis (20%) and melena (9%) (32). Timely treatment is crucial to increase the chances of survival in transplant patients, especially in HSCT patients, as mortality from CMV-induced gastrointestinal can be as high as 42% (29). Hence, monitoring the potential development of CMV disease in transplant patients with persistent viral loads is of critical importance.
Documented cases of HSCT recipients with refractory or resistant CMV infections are scarce worldwide and more typically involve adult solid organ transplants (26, 33–35). In Latin America, only one case of a heart transplant recipient with suspected genetic resistance has been reported, involving a 12 years-old pediatric patient initially treated with ganciclovir upon diagnosis of CMV-DNAemia. As viral loads persisted, foscarnet was administered, resulting in remission of the infection (36). Genetic resistance tests were not conducted, presumably due to the absence of this type of testing in the healthcare institution.
The case reported here highlights the importance of actively monitoring the development of CMV disease in patients with asymptomatic CMV DNAemia and considering alternative therapeutic options. It also highlights the need for more sensitive genetic tests, such as next-generation sequencing (NGS), since standard sequencing fails to detect relevant mutations in 9% of cases (37). The need for this approach is exemplified by the case of an 8 months-old who received an HSTC from an MSD. NSG detected the D588N mutation in the UL-54 gene, while standard sequencing failed to detect it. This mutation is clinically relevant due to its association with resistance to foscarnet, ganciclovir, and cidofovir (38).
In summary, this report described the case of a patient diagnosed with acute myeloid leukemia who underwent haploidentical HSCT and subsequently developed CMV infection refractory to first, second, and third-line treatment. The infection had an atypical asymptomatic presentation with no evidence of genetic resistance in the UL-97 and UL-54 genes. Although thrombocytopenia may have caused gastrointestinal bleeding, it cannot be ruled out that CMV colitis caused this episode.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
The study was approved by the Comité Institucional de Ética en Investigación, Instituto Nacional de Salud del Niño San Borja. Written informed consent was obtained from the minor(s)’ legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
AP-C: Conceptualization, Formal analysis, Methodology, Writing – original draft. KE-R: Investigation, Writing – original draft. JR-T: Supervision, Writing – review & editing. HM-M: Supervision, Writing – review & editing, Conceptualization, Funding acquisition.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was funded by PROCIENCIA—CONCYTEC within the framework of the call “Proyecto Investigación Básica, 2018-01” (Contract number 107-2018-FONDECYT). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
We would like to express our gratitude to the parents of the child whose case was reported for granting us permission to publish their child’s case. We would also like to thank K. Marshall McNagny for taking the time to review the manuscript and providing valuable suggestions that greatly improved it.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Gugliesi, F, Coscia, A, Griffante, G, Galitska, G, Pasquero, S, Albano, C, et al. Where do we stand after decades of studying human cytomegalovirus? Microorganisms. (2020) 8:685. doi: 10.3390/microorganisms8050685
2. Fowler, K, Mucha, J, Neumann, M, Lewandowski, W, Kaczanowska, M, Grys, M, et al. A systematic literature review of the global seroprevalence of cytomegalovirus: possible implications for treatment, screening, and vaccine development. BMC Public Health. (2022) 22:1659. doi: 10.1186/s12889-022-13971-7
3. Cho, S-Y, Lee, D-G, and Kim, H-J. Cytomegalovirus infections after hematopoietic stem cell transplantation: current status and future immunotherapy. Int J Mol Sci. (2019) 20:2666. doi: 10.3390/ijms20112666
4. Renzaho, A, Podlech, J, Kühnapfel, B, Blaum, F, Reddehase, MJ, and Lemmermann, NAW. Cytomegalovirus-associated inhibition of hematopoiesis is preventable by cytoimmunotherapy with antiviral CD8 T cells. Front Cell Infect Microbiol. (2020) 10:138. doi: 10.3389/fcimb.2020.00138
5. Jakharia, N, Howard, D, and Riedel, DJ. CMV infection in hematopoietic stem cell transplantation: prevention and treatment strategies. Curr Treat Options Infect Dis. (2021) 13:123–40. doi: 10.1007/s40506-021-00253-w
6. Panda, K, Parashar, D, and Viswanathan, R. An update on current antiviral strategies to combat human cytomegalovirus infection. Viruses. (2023) 15:1358. doi: 10.3390/v15061358
7. Yong, MK, Shigle, TL, Kim, Y-J, Carpenter, PA, Chemaly, RF, and Papanicolaou, GA. American Society for Transplantation and Cellular Therapy Series: #4—cytomegalovirus treatment and management of resistant or refractory infections after hematopoietic cell transplantation. Transplant Cell Ther. (2021) 27:957–67. doi: 10.1016/j.jtct.2021.09.010
8. Sassine, J, Khawaja, F, Shigle, TL, Handy, V, Foolad, F, Aitken, SL, et al. Refractory and resistant cytomegalovirus after hematopoietic cell transplant in the letermovir primary prophylaxis era. Clin Infect Dis. (2021) 73:1346–54. doi: 10.1093/cid/ciab298
9. Shmueli, E, Or, R, Shapira, MY, Resnick, IB, Caplan, O, Bdolah-Abram, T, et al. High rate of cytomegalovirus drug resistance among patients receiving preemptive antiviral treatment after haploidentical stem cell transplantation. J Infect Dis. (2014) 209:557–61. doi: 10.1093/infdis/jit475
10. Recio, V, González, I, and Tarragó, D. Cytomegalovirus drug resistance mutations in transplant recipients with suspected resistance. Virol J. (2023) 20:153. doi: 10.1186/s12985-023-02127-7
11. Instituto Nacional de Salud del Niño San Borja. Guía de Práctica Clínica de Leucemia Linfoblastica Aguda. Lima, Peru (2016). Available at: https://www.insnsb.gob.pe/guia-clinica-sub-unidad-de-atencion-integral-especializada-de-tph-2/. (Accessed September 4, 2023)
12. Szmit, Z, Frączkiewicz, J, Salamonowicz-Bodzioch, M, Król, A, Ussowicz, M, Mielcarek-Siedziuk, M, et al. The impact of high CMV viral load and refractory CMV infection on pediatric HSCT recipients with underlying non-malignant disorder. J Clin Med. (2022) 11:5187. doi: 10.3390/jcm11175187
13. Lin, C-H, Su, Y-J, Hsu, C-Y, Wang, P-N, and Teng, C-LJ. Haploidentical allogeneic hematopoietic stem cell transplantation increases the risk of cytomegalovirus infection in adult patients with acute leukemia. Transpl Infect Dis. (2019) 21:e13096. doi: 10.1111/tid.13096
14. Yeh, T-J, Yang, C-I, Huang, C-T, Wang, M-H, Chuang, T-M, Ke, Y-L, et al. Revisit of the association between cytomegalovirus infection and invasive fungal infection after allogeneic hematopoietic stem cell transplantation: a real-world analysis from a high CMV Seroprevalence area. J Fungi. (2022) 8:408. doi: 10.3390/jof8040408
15. Jaing, T-H, Chang, T-Y, Chen, S-H, Wen, Y-C, Yu, T-J, Lee, C-F, et al. Factors associated with cytomegalovirus infection in children undergoing allogeneic hematopoietic stem-cell transplantation. Medicine. (2019) 98:e14172. doi: 10.1097/MD.0000000000014172
16. Einsele, H, Ljungman, P, and Boeckh, M. How I treat CMV reactivation after allogeneic hematopoietic stem cell transplantation. Blood. (2020) 135:1619–29. doi: 10.1182/blood.2019000956
17. Springer, KL, Chou, S, Li, S, Giller, RH, Quinones, R, Shira, JE, et al. How evolution of mutations conferring drug resistance affects viral dynamics and clinical outcomes of cytomegalovirus-infected hematopoietic cell transplant recipients. J Clin Microbiol. (2005) 43:208–13. doi: 10.1128/JCM.43.1.208-213.2005
18. Kállay, K, Kassa, C, Réti, M, Karászi, É, Sinkó, J, Goda, V, et al. Early experience with CliniMACS prodigy CCS (IFN-gamma) system in selection of virus-specific T cells from third-party donors for pediatric patients with severe viral infections after hematopoietic stem cell transplantation. J Immunother. (2018) 41:158–63. doi: 10.1097/CJI.0000000000000197
19. Ruan, Y, Luo, T, Liu, Q, Liu, X, Chen, L, Wen, J, et al. Features of cytomegalovirus infection and evaluation of cytomegalovirus-specific T cells therapy in children’s patients following allogeneic hematopoietic stem cell transplantation: a retrospective single-center study. Front Cell Infect Microbiol. (2022) 12:1027341. doi: 10.3389/fcimb.2022.1027341
20. Seo, S, Smith, C, Fraser, C, Patheja, R, Shah, SP, Rehan, S, et al. Adoptive T-cell therapy for pediatric cytomegalovirus-associated retinitis. Blood Adv. (2019) 3:1774–7. doi: 10.1182/bloodadvances.2019000121
21. El Chaer, F, Shah, DP, and Chemaly, RF. How I treat resistant cytomegalovirus infection in hematopoietic cell transplantation recipients. Blood. (2016) 128:2624–36. doi: 10.1182/blood-2016-06-688432
22. Hakki, M, and Chou, S. The biology of cytomegalovirus drug resistance. Curr Opin Infect Dis. (2011) 24:605–11. doi: 10.1097/QCO.0b013e32834cfb58
23. Hantz, S, Garnier-Geoffroy, F, Mazeron, M-C, Garrigue, I, Merville, P, Mengelle, C, et al. Drug-resistant cytomegalovirus in transplant recipients: a French cohort study. J Antimicrob Chemother. (2010) 65:2628–40. doi: 10.1093/jac/dkq368
24. Kleiboeker, SB. Prevalence of cytomegalovirus antiviral drug resistance in transplant recipients. Antivir Res. (2023) 215:105623. doi: 10.1016/j.antiviral.2023.105623
25. Chou, S. Rapid in vitro evolution of human cytomegalovirus UL56 mutations that confer letermovir resistance. Antimicrob Agents Chemother. (2015) 59:6588–93. doi: 10.1128/AAC.01623-15
26. Cherrier, L, Nasar, A, Goodlet, KJ, Nailor, MD, Tokman, S, and Chou, S. Emergence of letermovir resistance in a lung transplant recipient with ganciclovir-resistant cytomegalovirus infection. Am J Transplant. (2018) 18:3060–4. doi: 10.1111/ajt.15135
27. Santos Bravo, M, Tilloy, V, Plault, N, Palomino, SS, Mosquera, MM, Navarro Gabriel, M, et al. Assessment of UL56 mutations before letermovir therapy in refractory cytomegalovirus transplant recipients. Microbiol Spectr. (2022) 10:e0019122. doi: 10.1128/spectrum.00191-22
28. Bhat, V, Joshi, A, Sarode, R, and Chavan, P. Cytomegalovirus infection in the bone marrow transplant patient. World J Transplant. (2015) 5:287–91. doi: 10.5500/wjt.v5.i4.287
29. Torres, HA, Kontoyiannis, DP, Bodey, GP, Adachi, JA, Luna, MA, Tarrand, JJ, et al. Gastrointestinal cytomegalovirus disease in patients with cancer: a two decade experience in a tertiary care cancer center. Eur J Cancer. (2005) 41:2268–79. doi: 10.1016/j.ejca.2005.07.011
30. Suárez-Lledó, M, Marcos, MÁ, Cuatrecasas, M, Bombi, JA, Fernández-Avilés, F, Magnano, L, et al. Quantitative PCR is faster, more objective, and more reliable than immunohistochemistry for the diagnosis of cytomegalovirus gastrointestinal disease in allogeneic stem cell transplantation. Biol Blood Marrow Transplant. (2019) 25:2281–6. doi: 10.1016/j.bbmt.2019.07.016
31. Lodding, IP, da Cunha Bang, C, Sørensen, SS, Gustafsson, F, Iversen, M, Kirkby, N, et al. Cytomegalovirus (CMV) disease despite weekly preemptive CMV strategy for recipients of solid organ and hematopoietic stem cell transplantation. Open Forum Infect Dis. (2018) 5:ofy080. doi: 10.1093/ofid/ofy080
32. Sun, X, Su, Y, Liu, X, Zhang, Y, He, Y, Han, W, et al. Overt gastrointestinal bleeding following haploidentical haematopoietic stem cell transplantation: incidence, outcomes and predictive models. Bone Marrow Transplant. (2021) 56:1341–51. doi: 10.1038/s41409-020-01187-5
33. Chon, WJ, Kadambi, PV, Xu, C, Becker, YT, Witkowski, P, Pursell, K, et al. Use of leflunomide in renal transplant recipients with ganciclovir-resistant/refractory cytomegalovirus infection: a case series from the University of Chicago. Case Rep Nephrol Dial. (2015) 5:96–105. doi: 10.1159/000381470
34. Paolucci, S, Campanini, G, Cassaniti, I, Tebaldi, A, Novazzi, F, Fratini, A, et al. Emergence of letermovir-resistant HCMV UL56 mutant during rescue treatment in a liver transplant recipient with ganciclovir-resistant infection HCMV: a case report. BMC Infect Dis. (2021) 21:994. doi: 10.1186/s12879-021-06694-4
35. Gómez Valbuena, I, Alioto, D, Serrano Garrote, O, and Ferrari Piquero, JM. Uso de leflunomida en infección de citomegalovirus resistente: a propósito de un caso. Farm Hosp. (2016) 40:52–4. doi: 10.7399/fh.2016.40.1.10161
36. Rojas-Contreras, C, De la Cruz-Ku, G, Vilcarromero, S, Villacaqui Ayllon, R, and Valcarcel-Valdivia, B. Reporte de caso de resistencia al ganciclovir en enfermedad por citomegalovirus postrasplante cardiaco: case report. Rev Peru Med Exp Salud Publica. (2018) 35:145–9. doi: 10.17843/rpmesp.2018.351.3562
37. López-Aladid, R, Guiu, A, Mosquera, MM, López-Medrano, F, Cofán, F, Linares, L, et al. Improvement in detecting cytomegalovirus drug resistance mutations in solid organ transplant recipients with suspected resistance using next generation sequencing. PLoS One. (2019) 14:e0219701. doi: 10.1371/journal.pone.0219701
Keywords: HSCT, cytomegalovirus infection, antiviral resistance, ganciclovir, foscarnet, cidofovir, case report
Citation: Pando-Caciano A, Escudero-Ramirez KA, Rodríguez-Torres JC and Maita-Malpartida H (2024) Refractory human cytomegalovirus infection without evidence of genetic resistance in the UL-54 and UL-97 genes in a pediatric hematopoietic stem cell transplant recipient: a case report. Front. Med. 11:1335969. doi: 10.3389/fmed.2024.1335969
Received: 09 November 2023; Accepted: 15 January 2024;
Published: 02 February 2024.
Edited by:
Pierpaolo Di Micco, Ospedale Santa Maria delle Grazie, ItalyReviewed by:
Nopporn Apiwattanakul, Mahidol University, ThailandCopyright © 2024 Pando-Caciano, Escudero-Ramirez, Rodríguez-Torres and Maita-Malpartida. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alejandra Pando-Caciano, YWxlamFuZHJhLnBhbmRvQHVwY2gucGU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.