
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 08 February 2024
Sec. Pulmonary Medicine
Volume 11 - 2024 | https://doi.org/10.3389/fmed.2024.1335469
 Michio Ozeki1,2*
Michio Ozeki1,2* Saori Endo1
Saori Endo1 Shiho Yasue1
Shiho Yasue1 Akifumi Nozawa1
Akifumi Nozawa1 Ryuta Asada2,3
Ryuta Asada2,3 Akiko M. Saito2
Akiko M. Saito2 Hiroya Hashimoto2,4
Hiroya Hashimoto2,4 Takumi Fujimura5
Takumi Fujimura5 Yohei Yamada5
Yohei Yamada5 Tatsuo Kuroda5
Tatsuo Kuroda5 Shigeru Ueno6
Shigeru Ueno6 Shoji Watanabe7
Shoji Watanabe7 Shunsuke Nosaka8
Shunsuke Nosaka8 Mikiko Miyasaka8
Mikiko Miyasaka8 Akihiro Umezawa9
Akihiro Umezawa9 Kentaro Matsuoka10
Kentaro Matsuoka10 Takanobu Maekawa11
Takanobu Maekawa11 Satoshi Hirakawa12
Satoshi Hirakawa12 Taizo Furukawa13
Taizo Furukawa13 Shigehisa Fumino13
Shigehisa Fumino13 Tatsuro Tajiri13,14
Tatsuro Tajiri13,14 Junkichi Takemoto14
Junkichi Takemoto14 Ryota Souzaki14
Ryota Souzaki14 Yoshiaki Kinoshita15
Yoshiaki Kinoshita15 Akihiro Fujino5,16
Akihiro Fujino5,16Introduction: Intractable lymphatic anomalies (LAs) include cystic lymphatic malformation (LM; macrocystic, microcystic, or mixed), generalized lymphatic anomaly, and Gorham–Stout disease. LAs can present with severe symptoms and poor prognosis. Thus, prospective studies for treatments are warranted. We conducted a prospective clinical trial of sirolimus for intractable LAs.
Methods: This was an open-label, single-arm, multicenter, prospective trial involving five institutions in Japan. All patients with LAs received oral sirolimus once daily, and the dose was adjusted to ensure that the trough concentration remained within 5–15 ng/mL. We prospectively assessed the drug response (response rate for radiological volumetric change in target lesion), performance state, change in respiratory function, visceral impairment (pleural effusion, ascites, bleeding, pain), laboratory examination data, quality of life (QOL), and safety at 12, 24, and 52 weeks of administration.
Results: Eleven patients with LAs (9 generalized lymphatic anomaly, 1 cystic LM, 1 Gorham–Stout disease) were treated with sirolimus, of whom 6 (54.5%; 95% confidence interval: 23.4–83.3%) demonstrated a partial response on radiological examination at 52 weeks of administration. No patients achieved a complete response. At 12 and 24 weeks of administration, 8 patients (72.7%) already showed a partial response. However, patients with stable disease showed minor or no reduction after 12 weeks. Adverse events, such as stomatitis, acneiform dermatitis, diarrhea, and fever, were common with sirolimus. Sirolimus was safe and tolerable.
Conclusion: Sirolimus can reduce the lymphatic tissue volume in LAs and may lead to improvements in clinical symptoms and QOL.
There are various types of lymphatic anomalies (LAs) that fall under the category of complex LAs (1). These include common lymphatic malformation (LM), generalized lymphatic anomaly (GLA), kaposiform lymphangiomatosis, Gorham–Stout disease (GSD), central conducting lymphatic anomaly, and others (2). These anomalies typically manifest during birth or early childhood, and rarely occur in adulthood. The clinical symptoms of LAs are influenced by factors such as their anatomical region, size, and degree of invasiveness. LAs can lead to severe conditions such as dyspnea, respiratory disorders, coagulation disorders, and bleeding. Challenging LA cases can be complicated to manage because of the location and high risk of recurrence. Conventional treatment options include surgical resection, sclerotherapy, and drainage; however, these methods have potential complications. Therefore, it is crucial to explore innovative pharmacological treatment strategies for effective management of these anomalies.
Recent genetic studies have revealed that LAs are caused by somatic activating mutations in genes such as PIK3CA, NRAS, CBL, KRAS, and ARAF (3). These disease-causing genes and their associated proteins are involved in signaling pathways, including the PI3K-AKT (protein kinase B) and RAS-mitogen-activated protein kinase (MAPK) pathways that have roles in lymphatic vessel development and growth in both normal tissue and LAs (3). Another study showed that rapamycin (sirolimus), a mammalian target of rapamycin (mTOR) inhibitor, can inhibit the excessive growth of lymphatic vessels in mouse models of LM driven by mutations of the PIK3CA gene (4). In clinical settings, sirolimus has emerged as a promising treatment for LAs and other vascular anomalies (5). Retrospective cohort studies demonstrated that 50–80% of LA patients treated with sirolimus experienced improvements in their clinical symptoms, such as pain, bleeding, and functional deficits, and a reduction in their lesion volume. However, it is important to note that most of these studies were retrospective (6).
We conducted a multicenter prospective study to investigate the impacts of sirolimus for intractable LAs. In this study, we measured the volume of LM lesions objectively and quantitatively using magnetic resonance imaging (MRI). Additionally, based on the natural history data and effectiveness data of sirolimus from clinical trials that used the same MRI assessment as ours, we statistically determined the sample size and prospectively evaluated the radiological effect of sirolimus. Herein, we report the results of this trial for sirolimus treatment of LAs.
We performed a clinical trial, designated Sirolimus for Intractable Lymphatic Anomalies (SILA), to assess the efficacy and safety of sirolimus in LAs. It was an open-label, single-arm, multicenter, prospective trial conducted at five institutions in Japan (Gifu University Hospital, Kyoto Prefectural University of Medicine, Kyushu University Hospital, National Center for Child Health and Development, and Keio University School of Medicine).
Patients who met the eligibility criteria and provided informed consent and assent were enrolled. Before enrollment, all patients were reviewed by a multi-institutional, multidisciplinary evaluation committee, which included individuals from pediatric surgery, pediatrics, and radiology departments. Clinical information, clinical photographs, and radiological findings were assessed. Although there are no globally established diagnostic criteria for cystic LM, GLA, or GSD, we used the diagnostic criteria used domestically in Japan for each disease (7, 8). The inclusion criteria were: >0.6 m2 of body surface area (BSA) and judged by the investigator to be able to take tablets; definite diagnosis of cystic LM, GLA, or GSD, and exclusion of other lymphatic diseases; presence of at least one target lesion (e.g., cystic LM, lymphedema) measurable on MRI; and presence of intractable and severe disorders and symptoms caused by the target disease (bleeding, chronic pain, recurrent cellulitis [>3 episodes/year], ulceration, visceral and/or bone involvement, potential effects on organ function including the eye, airway, and ear). The exclusion criteria were: Karnofsky performance status (PS) score ≤ 30 (≥10 years of age) or Lansky play-PS ≤30 (<10 years of age); dysfunction of the liver, renal, or cardiac systems; receipt of molecularly targeted drugs associated with the mTOR pathway, immunosuppressant drugs, or drugs that inhibit/induce CYP3A4 enzyme activity; active infections requiring systemic treatment; uncontrolled diabetes, hypertension, hyperlipidemia, chronic liver, kidney disease, or immunodeficiency conditions; and carrier status for hepatitis B and/or hepatitis C virus. Patients with contraindications to sirolimus and experience of therapies affecting assessment of the target lesions, including history of allergic reaction to sirolimus, receipt of surgery (resection, sclerotherapy, or endovascular treatment), or administration of therapeutic drugs (propranolol, Kampo medicines, interferon, octreotide, bisphosphonate, or denosumab), chemotherapy agents, or radiation therapy for the target lesion, were also excluded.
Initially, sirolimus (Rapalimus Tablet; 1 mg; Nobelpharma Co., Ltd., Tokyo, Japan) at 2 mg (BSA ≥1.0 m2) or 1 mg (BSA <1.0 m2) was administered orally once daily after meals or on an empty stomach. The dose was then adjusted based on the trough concentration of sirolimus measured during week 2 to reach a target concentration within 5 to 15 ng/mL (max dose: 4 mg). The trough concentrations were measured at 1 and 2 weeks, and every 4 weeks thereafter. Supportive therapies (antibiotics, blood transfusion, antipyretics, analgesics) and therapies that would not affect assessment of the LA lesions were allowed, while additional drugs that may have adverse effects in combination with sirolimus, drugs that inhibit/induce CYP3A4 enzyme activity, and therapies (surgery, sclerotherapy, and drugs) that may affect assessment of the target lesions were not allowed.
The primary outcome was the objective radiographic response rate to sirolimus treatment, defined as the population of patients who achieved a complete response (CR) or partial response (PR) based on the volume of the target lesion assessed on MRI at 52 weeks. For definitive evaluation, two central radiologists who were independent from the other investigators performed the assessment. On MRI with T2 fat-saturated sequence images of the lymphatic lesions or cysts, the area dimensions of the lesions were measured using the region of interest (ROI) tool in a Digital Imaging and Communications in Medicine (DICOM) viewer (OsiriX© v.9.0; Pixmeo, Bernex, Switzerland). Other pathologic lesions, such as inflammation, bleeding, and hematoma, were removed. The volume of the target lesion was calculated by multiplying the ROI areas by the slice width. The non-lymphatic tissues (bleeding, inflammation, and hematoma) were not examined. We classified the evaluation criteria as follows: CR, disappearance of all target lesions; PR, ≥20% decrease in volume of the target lesion; progressive disease (PD), ≥20% increase in volume of the target lesion; and stable disease (SD), insufficient shrinkage to qualify for PR and insufficient growth to qualify for PD.
The secondary outcomes were the radiological, clinical, and biological assessments of the efficacy of sirolimus as well as the safety. The radiological response rate (at 12 and 24 weeks), PS, change in respiratory function, visceral impairment (pleural effusion, ascites, bleeding, pain), laboratory examination data (complete blood count, liver, lipid profile, and blood coagulation parameters), and quality of life (QOL) were evaluated at 12, 24, and 52 weeks. The changes in vital signs and pharmacokinetic data every 4 weeks and the safety (adverse events) were examined during sirolimus treatment. PS, bleeding, pain, QOL, and adverse events were measured using the Karnofsky PS (≥10 years of age) or Lansky play-PS (<10 years of age), World Health Organization (WHO)-Bleeding Scale (9), visual analog scale for pain (10), PedsQL™ 4.0 Generic Core Scales (<25 years of age) (11) or Functional Assessment of Cancer Therapy-General (FACT-G) (≥25 years of age) (12), and the Common Terminology Criteria for Adverse Events V4.0 (13), respectively.
There is little available data on the natural history of intractable LAs. Therefore, we examined retrospective data from a Japanese national survey on intractable LAs (6). In 59 patients with intractable LAs (19 cystic LM, 18 GLA, and 22 GSD), only one patient (1.7%) showed radiological improvement for at least 1 year. Thus, the threshold response rate was 5%. The expected response rate was determined as 50% because data from past clinical trials showed radiological response rates of 52 and 50% (5, 14). Therefore, the sample size was calculated based on the probability that the lower limit of the exact 95% confidence interval (CI) for the primary outcome would be greater than the threshold of 5%. To maintain a statistical power of at least 90%, nine patients were needed. In consideration of drop-out, the target sample size was set at 10.
For the primary outcome, we used the full analysis set consisting of all enrolled patients and measured the response rate (proportion of patients evaluated as CR or PR) at 52 weeks and its exact 95% CI. If the lower limit of the 95% CI was >5%, efficacy of sirolimus would be concluded. For the secondary outcomes, the response rate and CI were calculated for pre-treatment versus 12, 24, and 52 weeks of treatment and compared by the Wilcoxon signed rank test if appropriate.
A total of 12 patients were enrolled in the study. Subsequently, 1 patient (case 9) was excluded because of an orthodontic appliance that may affect radiological assessment on MRI. Therefore, a total of 11 patients (9 GLA, 1 cystic LM, 1 GSD) were evaluated in the study. Of these, 2 patients did not complete the study because of discontinuation at the patient’s request and cessation of medication for >28 consecutive days during treatment, respectively (Figure 1).
The demographic characteristics of the patients treated with sirolimus (full analysis set) are shown in Table 1. All 11 patients had some complications (Table 1). The vital signs (blood pressure, heart rate, respiratory rate, body temperature, SpO2), electrocardiogram, and respiratory function tests of all patients before treatment were normal. Four patients (36.4%; cases 2, 3, 5, and 8) and 1 patient (9.1%; case 1) had pleural effusion and ascites. Cases 4 and 11 had lymphorrhea from the femoral skin. The target lesions for assessment comprised six subcutaneous lesions (cases 3, 6, 8, 10, 11, and 12), two retroperitoneal lesions (cases 1 and 4), two thoracic lesions (cases 2 and 5), and one cervical lesion (case 7). The mean drug-taking rate ± standard deviation was 97.3% ± 5.2%.
The detailed results for the individual cases are summarized in the Supplementary material. The radiological response rate at 52 weeks of administration was 54.5% (6/11; 95% CI: 23.4–83.3%; excluding case 3 who obtained PR but discontinued at day 287; Table 2). No patients achieved CR or PD. At 12 weeks of administration, 72.7% (8/11) already showed a PR and the volume of their lesions was further reduced at 24 weeks. However, in 7 PR patients (Figure 2, solid lines), the volume showed almost no change from 24 weeks to 52 weeks. In contrast, the SD patients (Figure 2, dotted lines) showed minor or no reduction after 12 weeks of treatment.
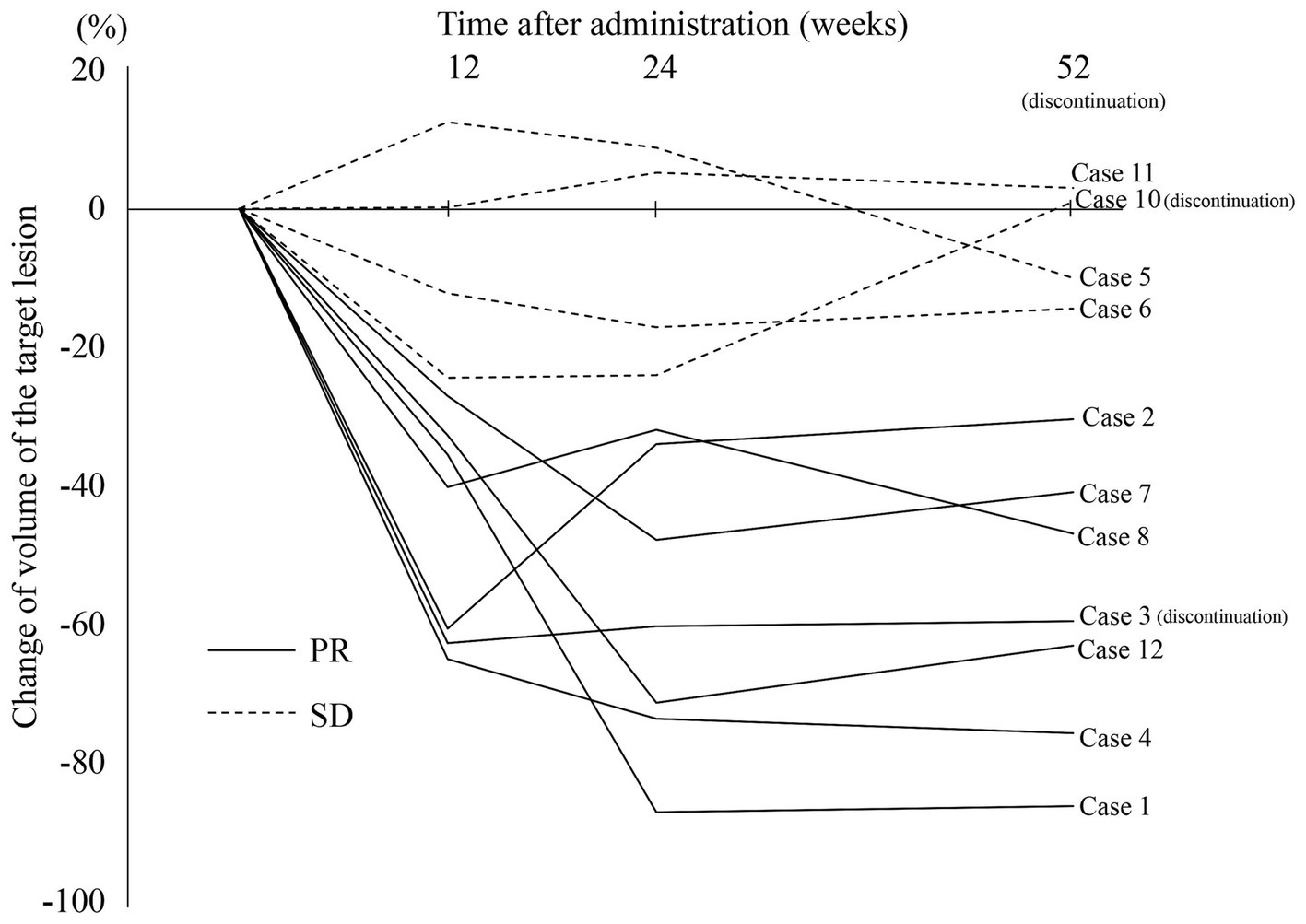
Figure 2. Changes in volume of the target lesions during sirolimus treatment. One patient (case 9) was excluded because of an orthodontic appliance that may affect radiological assessment on MRI. PR, partial response; SD, stable disease.
Before the start of sirolimus treatment, pleural effusion was identified in 36.4% of patients (4/11). This proportion declined to 18.2% (2/11) at 52 weeks of treatment or discontinuation. Bleeding in the skin, soft tissues, muscles, and bones was observed in 4 of 11 patients before the treatment (Grade 1 in 2 cases and Grade 2 in 2 cases according to the WHO-Bleeding Scale). This number decreased to just one case (Grade 2) at 52 weeks of treatment or discontinuation. There were no instances of bleeding in the oral and nasal cavities, gastrointestinal tract, body cavities, central nervous system, points of surgical invasion, or circulatory system. Regarding the pain scales, no significant differences were noted before and after treatment. For the QOL scores, no significant changes were seen in the overall scores of the PedsQL (5 patients aged <25 years) or FACT-G (2 patients aged ≥26 years) between the pre-treatment period and 52 weeks of treatment. Likewise, there were no significant changes for improvement in activities of daily living (ADL) based on the pre-treatment and post-treatment Karnofsky PS scores (8 cases) and Lansky play-PS scores (3 cases).
Among the subjects with abnormal blood coagulation parameters at baseline, the rates of normalization at 52 weeks of treatment or discontinuation were as follows: 0.0% (0/3) for platelet count, 57.1% (4/7) for fibrinogen, and 20.0% (2/10) for D-dimer. While not all parameters became normalized, certain improvements were noted. The mean fibrinogen level increased after treatment, exceeding the baseline value at all observation points. Conversely, the mean D-dimer level decreased below the baseline value at all observation points after treatment commencement. No significant differences were observed before and after treatment (Table 3).
We observed improvement of symptoms in three cases (cases 5, 6, and 11) that were assessed as SD (Supplementary material). In case 5, the pleural effusion observed before the start of treatment had disappeared by week 52 of treatment. The patient’s pre-treatment VAS score of 64 had improved to 23 at 52 weeks. In case 6, improvements were noted in the blood coagulation parameters: fibrinogen (74 mg/dL pre-treatment to 137 mg/dL at 52 weeks) and D-dimer (101.1 μg/mL pre-treatment to 53.4 μg/mL at 52 weeks). Although the volume change rate of the target lesion fell between −17.1 and − 12.2%, and the therapeutic effect did not reach PR, a discernible reduction was evident. In case 11, the pre-treatment VAS score of 76 improved to 24 at 52 weeks. Pre-treatment Grade 2 bleeding (involving the skin, soft tissue, muscle, bone) had resolved at 52 weeks. Before treatment, the patient had lymphatic leakage of 1–2 L/day, which progressively decreased up to 24 weeks until there was no leakage and the skin condition became softer.
The sirolimus trough levels were measured at 1 and 2 weeks, and then every 4 weeks thereafter. The target trough level was set between 5 to 15 ng/mL, and the dosage of sirolimus was adjusted accordingly. The sirolimus concentration at nearly all measurement points was within the target range (Figure 3). There were no notable differences in sirolimus concentrations between patients with PR and patients with SD. The sirolimus concentrations in all SD patients (cases 5, 6, 10, and 11) at 1 week and two SD patients (cases 6 and 11) at 2, 4, and 8 weeks were below the 5 ng/mL threshold. However, these low concentrations were not correlated with the effectiveness of sirolimus, and the concentrations in these patients varied throughout the rest of the treatment period.
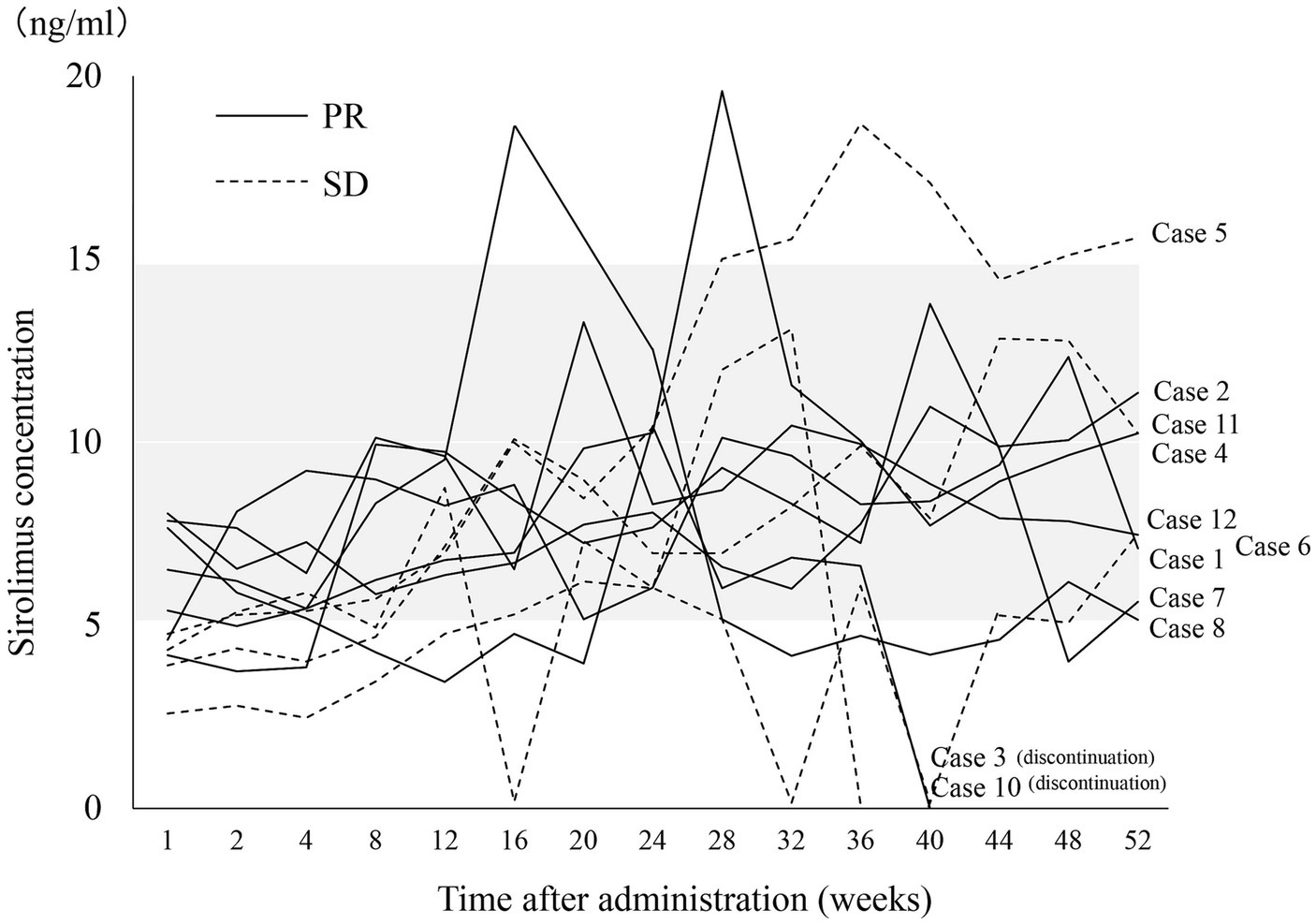
Figure 3. Changes in sirolimus concentrations during treatment. The gray band indicates the target trough sirolimus concentration range (5–15 ng/mL). One patient (case 9) was excluded because of an orthodontic appliance that may affect radiological assessment on MRI. PR, partial response; SD, stable disease.
Adverse events were observed in all 11 subjects, but none exceeded Grade 3 in severity. The Grade 3 adverse events were observed in 7 subjects (63.6%). There were no instances of fatal adverse events. Stomatitis was the most common adverse event, occurring in 9 patients (81.8%), followed by acneiform dermatitis in 8 (72.7%) and then diarrhea and fever in 6 subjects each (54.5%). Other observed events included upper respiratory infection in 4 patients (36.4%), and abdominal pain, pharyngitis, skin infection, and pain in 3 subjects each (27.3%). Eczema, bronchitis, pneumonia, influenza-like symptoms, dehydration, cough, arthropod bite, skin abrasion, muscle pain, headache, and heavy menstrual bleeding were observed in 2 subjects each (18.2%). All other adverse events were singular occurrences.
Intractable LAs are extremely rare and have limited experience details and treatment options, making these diseases exceptionally difficult to manage. Historically, treatment strategies have primarily consisted of surgical intervention, sclerotherapy, and symptomatic care. However, there is an urgent need for novel treatments to address the significant challenge of managing these diseases. The present study is the first multicenter clinical trial in Japan designed to prospectively investigate the therapeutic efficacy and safety of oral sirolimus for intractable LAs. Sirolimus was administered for a 1-year period, with blood level monitoring after each administration to maintain a therapeutic concentration between 5 and 15 ng/mL. MRI was employed to assess the target lesions, with evaluations for reduction in lesion size, clinical symptoms, bleeding, blood coagulation test data, QOL, ADL, and any adverse events. The findings demonstrated that sirolimus can significantly reduce the lesion size and may lead to improvements in symptoms associated with LAs.
Sirolimus is postulated to exert its therapeutic effects through the inhibition of mTOR-related proteins, resulting in suppression of both angiogenesis and lymphangiogenesis (4). Recent advances in genetic analyses have uncovered somatic mosaic mutations of the PIK3CA gene within the tissues of LA lesion sites, casting a spotlight on the correlations between these mutations and the pathogenesis of LAs (2). Current understanding posits that mouse models with PIK3CA gene mutations within lymphatic endothelial cells are prone to the development of purulent lesions (3). It is further hypothesized that the presence of PIK3CA gene mutations in LAs could result in a reduction in lesion size, coupled with a potential decrease in lymph leakage from the lesions, after sirolimus treatment. Although the anatomical location of lymphatic malformations is important when making treatment choices, in this study we included cases that presented with severe symptoms, regardless of being in anatomically risky locations, because sirolimus has the potential these symptoms as well.
In the present study, we refrained from conducting genetic analyses on the lesion sites, and thus no cases were officially confirmed to have PIK3CA gene mutations. Nevertheless, the notable reductions in lesion size and the resulting clinical improvements observed across a wide range of cases suggest a strong correlation between LAs and PIK3CA gene mutations. Alternatively, even in the absence of PIK3CA gene mutations, the suppressive effects on angiogenesis and lymphangiogenesis could be key mechanisms driving the observed clinical improvements. Biopsy or removal of the lesion site carries risks of severe bleeding, infection, and postoperative lymph leakage. In clinical practice, it is not easy to perform genetic analysis before administering sirolimus treatment. Additionally, the detection sensitivity for genetic mutations in lesions is not currently high, and the process is time-consuming and costly. To date and at the time of the study’s planning, there is no established evidence linking genetic mutations to the effectiveness of sirolimus treatment. These insights challenge the conventional belief on the necessity to confirm the presence or absence of genetic mutations prior to commencement of treatment. Further research is needed to understand the relationship between the genetic background of the patient and the treatment efficacy of sirolimus.
We have conducted a prospective study that assessed not only the reduction rate of the target lesions, but also the clinical symptoms, pleural effusion, ascites, blood and coagulation test data, pain scales, QOL, ADL, and drug kinetics during treatment. These consecutive and comprehensive data were valuable, and we confirmed the efficacy of sirolimus in reducing the size of LA lesions. We analyzed the MRI images of the lesion sites in all cases using a volumetric approach. Through application of the image analysis software OsiriX, we precisely detected and measured high-signal lymphatic cysts and edema that were visible on fat-suppressed T2-weighted images. Because lymphatic disease lesions have complex shapes, their measurement presents considerable challenges. However, OsiriX allowed us to establish ROIs based on MRI signal intensity variations, thereby facilitating area measurement. This makes OsiriX a valuable tool for evaluation of lymphatic diseases. In addition, we evaluated a variety of parameters during treatment. A global project that involves numerous doctors and patients, known as Outcome Measures for Vascular Malformations (OVAMA) (15), also promotes and is developing the use of various parameters such as symptoms, QOL, and patient satisfaction for treatment evaluation. Our trial, which was initiated before the OVAMA project, implemented similar evaluations as part of a prospective study. Even in cases where the lesion size was not reduced, we often observed improvements in other clinical symptoms. Consequently, it is vital that relevant judgments are based on an all-encompassing view, employing a variety of evaluation criteria and not solely relying on MRI images.
The optimal concentration and duration of sirolimus for vascular anomalies remain unknown. In our study, we adjusted the dosage to yield a target trough concentration of 5–15 ng/mL. Throughout the treatment, the fluctuations of sirolimus concentrations were largely confined within our target trough concentration range. However, the concentration levels in all SD patients (cases 5, 6, 10, and 11) were < 5 ng/mL after 1 week, and for two of these patients (cases 6 and 11), the levels remained below 5 ng/mL at 2, 4, and 8 weeks and the drug dosage was increased. Harbers et al. (16) pointed out the effectiveness of low target sirolimus levels (4–10 ng/mL) in 79.1% of patients with vascular anomalies. Despite these findings, low concentrations are not universally optimal for all cases. Karastaneva et al. (17) reported that the most remarkable volume reductions were detected within the first 4–6 months of therapy. In all of our PR patients, the tumors had already reduced by >20% at 12 weeks, and a similar trend persisted at 24 and 52 weeks. It is important to mention that the link between treatment responsiveness and blood concentration remains elusive. Nevertheless, it is plausible that an initially low blood concentration may lead to subpar treatment outcomes. High blood levels of sirolimus (>10 ng/mL) can lead to severe adverse events (18, 19), suggesting that if effective outcomes can be achieved at lower concentrations, there is no justification for enforcing higher levels. In our view, if the efficacy is not immediately apparent after treatment initiation, it is advisable to adjust the dosage to reach the range of 5–15 ng/mL and persist with the treatment for at least 6 months.
The adverse events observed were considered to be of a similar type and frequency to those reported in previous studies (5, 14, 16, 17, 19). Regarding our female patients, we found that 3 of 7 (42.9%) experienced menstrual disorders (1 irregular menstruation, 2 hypermenorrhea). These patients maintained regular menstrual cycles before administration of sirolimus, and their symptoms proved to be mild and reversible. Similarly, Triana et al. (20) reported that 7 of 74 women (9.4%) experienced menstrual alterations attributed to sirolimus treatment. All of these patients also maintained regular menstrual cycles before the treatment. One patient experienced a 4-month amenorrhea period after treatment initiation, which later resolved spontaneously, and the other six patients encountered hypermenorrhea. While most patients experienced mild menstrual alterations that did not necessitate dose reduction or withdrawal, one patient had to discontinue sirolimus due to hypermenorrhea, metrorrhagia, and hematuria. Following sirolimus withdrawal, five patients saw their regular menstrual cycles return. Using a rat model, Braun et al. (21) investigated the underlying mechanisms for sirolimus-associated ovarian toxicity. They discovered that sirolimus amplified signaling in rat ovarian follicles via the pro-proliferative PI3K pathway. We should take care for these potential adverse events.
Our study had some limitations. First, the open-label, single-arm design may have introduced bias in some outcome measures. Ideally, a randomized, placebo-controlled, double-blind clinical trial design should be used. However, these diseases are extremely rare and heterogeneous, and thus the trials are challenging. A nationwide survey by Japanese rare disease researchers in the 2010s showed that intractable cases in Japan included about 600 with a cystic lymphatic malformation and 100 with GLA/GSD (7, 8). These numbers highlight the limited number of potential participants in Japan. Enrolling suitable cases within the trial period was challenging given few patients could reach the trial sites and meet the inclusion criteria. Therefore, conducting a high evidence level trial for such rare diseases is difficult. Furthermore, evaluation methods for vascular anomalies have not been established, and it is difficult to assess the efficacy of sirolimus treatment.
Second, the number of participants was small. We designed the sample size to allow assessment of the radiological response rate as the primary outcome. There has been no previous clinical trial of sirolimus with a sample size determined statistically. However, the sample size was not necessarily appropriate for the secondary outcomes. Despite improvements in clinical symptoms, it is possible that there were no significant changes in almost all secondary outcomes such as QOL. Furthermore, the age group was limited to those who could take sirolimus tablets orally, so infants were not included. Separate trials may be necessary for infants.
Third, severity and radiological evaluation are not always correlated. Therefore, the reduction of lesions observed in our clinical trial does not necessarily indicate an improvement in clinical symptoms. However, it is evident that sirolimus significantly reduced the lesions compared with their natural history. Previous studies reported that the reduction of LM was associated with improvements in clinical symptoms and QOL (14). We evaluated secondary outcomes, such as other symptoms and QOL measures. Unfortunately, the number of cases was too small for a conclusive assessment of each item. Regardless, increasing the number of cases would enhance the probability of the data; thus, further investigation of higher numbers of cases is necessary in the future.
In Japan, sirolimus has been designated as an orphan drug. The results of this study have led to its regulatory approval in Japan. We reported the outcomes of the clinical trial based on the maximum information available and the scientific perspective at the time of planning the study.
In conclusion, our results show that sirolimus appears to be effective for decreasing the lesion size in intractable LAs. Adverse events of sirolimus were common, but were mostly mild and tolerable if monitored carefully. Further clinical trials are warranted to investigate the optimal concentration and duration of sirolimus treatment.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
The studies involving humans were approved by Gifu University Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
MO: Writing – original draft, Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Supervision. SE: Investigation, Methodology, Writing – review & editing. SY: Investigation, Methodology, Writing – review & editing. AN: Data curation, Investigation, Methodology, Writing – review & editing. RA: Conceptualization, Data curation, Funding acquisition, Methodology, Project administration, Writing – review & editing. AS: Data curation, Formal analysis, Methodology, Validation, Writing – review & editing. HH: Data curation, Formal analysis, Writing – review & editing. TkF: Investigation, Writing – review & editing. YY: Investigation, Writing – review & editing. TK: Investigation, Writing – review & editing. SU: Investigation, Methodology, Writing – review & editing. SW: Investigation, Methodology, Writing – review & editing. SN: Formal analysis, Investigation, Writing – review & editing. MM: Formal analysis, Investigation, Writing – review & editing. AU: Investigation, Methodology, Writing – review & editing. KM: Formal analysis, Investigation, Methodology, Writing – review & editing. TM: Conceptualization, Investigation, Methodology, Writing – review & editing. SH: Formal analysis, Investigation, Methodology, Writing – review & editing. TiF: Investigation, Methodology, Writing – review & editing. SF: Investigation, Writing – review & editing. TT: Investigation, Methodology, Writing – review & editing. JT: Investigation, Writing – review & editing. RS: Investigation, Writing – review & editing. YK: Investigation, Writing – review & editing. AF: Conceptualization, Investigation, Methodology, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The present study was supported in part by a Clinical Research-Clinical Trial Promotion Research Project (JP18lk0201055) and a Practical Research Project for Rare/Intractable Diseases (JP22ek0109515) from the Japan Agency for Medical Research and Development, AMED.
We thank Tomohiro Hori, Hidenori Ohnishi, and Asuka Ogawa from Gifu University, Motoi Kato, and Tadashi Iwanaka from Saitama Children’s Medical Center, Masataka Takahashi, Katsuhiro Ogawa, and Yoko Shioda from the National Center for Child Health and Development, and Kyoichi Deie and Miho Watanabe from The University of Tokyo for their helpful comments. We also thank Alison Sherwin and J. Ludovic Croxford, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
MO received research funding from Nobelpharma Co. Ltd. in Tokyo. Sirolimus tablets were supplied by Nobelpharma Co. Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1335469/full#supplementary-material
1. Trenor, CC, and Chaudry, G. Complex lymphatic anomalies. Semin Pediatr Surg. (2014) 23:186–90. doi: 10.1053/j.sempedsurg.2014.07.006
2. Ozeki, M, and Fukao, T. Generalized lymphatic anomaly and Gorham-stout disease: overview and recent insights. Adv Wound Care (new Rochelle). (2019) 8:230–45. doi: 10.1089/wound.2018.0850
3. Mäkinen, T, Boon, LM, Vikkula, M, and Alitalo, K. Lymphatic malformations: genetics, mechanisms and therapeutic strategies. Circ Res. (2021) 129:136–54. doi: 10.1161/CIRCRESAHA.121.318142
4. Boscolo, E, Coma, S, Luks, VL, Greene, AK, Klagsbrun, M, Warman, ML, et al. AKT hyper-phosphorylation associated with PI3K mutations in lymphatic endothelial cells from a patient with lymphatic malformation. Angiogenesis. (2015) 18:151–62. doi: 10.1007/s10456-014-9453-2
5. Adams, DM, Trenor, CC 3rd, Hammill, AM, Vinks, AA, Patel, MN, Chaudry, G, et al. Efficacy and safety of sirolimus in the treatment of complicated vascular anomalies. Pediatrics. (2016) 137:e20153257. doi: 10.1542/peds.2015-3257
6. Ozeki, M, Asada, R, Saito, AM, Hashimoto, H, Fujimura, T, Kuroda, T, et al. Efficacy and safety of sirolimus treatment for intractable lymphatic anomalies: a study protocol for an open-label, single-arm, multicenter, prospective study (SILA). Regen Ther. (2019) 10:84–91. doi: 10.1016/j.reth.2018.12.001
7. Japan Intractable. Diseases information center. (2024). Available at: https://www.nanbyou.or.jp/entry/4893 (Accessed Jan 8, 2024).
8. Japan Intractable. Diseases information center. (2024). Available at: https://www.nanbyou.or.jp/entry/4637 (Accessed Jan 8, 2024).
9. Fogarty, PF, Tarantino, MD, Brainsky, A, Signorovitch, J, and Grotzinger, KM. Selective validation of the WHO bleeding scale in patients with chronic immune thrombocytopenia. Curr Med Res Opin. (2012) 28:79–87. doi: 10.1185/03007995.2011.644849
10. McCormack, HM, Horne, DJ, and Sheather, S. Clinical applications of visual analogue scales: a critical review. Psychol Med. (1988) 18:1007–19. doi: 10.1017/S0033291700009934
11. Varni, JW, Seid, M, and Kurtin, P. PedsQL™ 4.0: reliability and validity of the pediatric quality of life inventory™ version 4.0 generic Core scales in healthy and patient populations. Med Care. (2001) 39:800–12. doi: 10.1097/00005650-200108000-00006
12. Fairclough, DL, and Cella, DF. Functional assessment of Cancer therapy (FACT-G): non-response to individual questions. Qual Life Res. (1996) 5:321–9. doi: 10.1007/BF00433916
13. National Cancer Institute. Common terminology criteria for adverse events (CTCAE) v4.0. (2023). Available at: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm#ctc_40 (Accessed January 3, 2023).
14. Ozeki, M, Nozawa, A, Yasue, S, Endo, S, Asada, R, Hashimoto, H, et al. The impact of sirolimus therapy on lesion size, clinical symptoms, and quality of life of patients with lymphatic anomalies. Orphanet J Rare Dis. (2019) 14:141. doi: 10.1186/s13023-019-1118-1
15. Horbach, SER, Van Der Horst, CMA, Blei, F, Van Der Vleuten, CJM, Frieden, IJ, Richter, GT, et al. Development of an international core outcome set for peripheral vascular malformations: the OVAMA project. Br J Dermatol. (2018) 178:473–81. doi: 10.1111/bjd.16029
16. Harbers, VEM, Rongen, GA, Van der Vleuten, CJM, Verhoeven, BH, De Laat, PC, Van der Horst, CMA, et al. Patients with congenital low-flow vascular malformation treated with low dose Sirolimus. Adv Ther. (2021) 38:3465–82. doi: 10.1007/s12325-021-01758-y
17. Karastaneva, A, Gasparella, P, Tschauner, S, Crazzolara, R, Kropshofer, G, Modl, M, et al. Indications and limitations of Sirolimus in the treatment of vascular anomalies-insights from a retrospective case series. Front Pediatr. (2022) 10:857436. doi: 10.3389/fped.2022.857436
18. Rial, MDC, Tedesco Silva, H, Pacheco-Silva, A, Cruz, J, Torres, R, Tortella, BJ, et al. Adverse events and discontinuation rates associated with Sirolimus treatment in adult renal transplant patients in Latin America vs non-Latin American countries. Transplant Proc. (2020) 52:767–74. doi: 10.1016/j.transproceed.2020.01.040
19. Rössler, J, Baselga, E, Davila, V, Celis, V, Diociaiuti, A, El Hachem, M, et al. Severe adverse events during sirolimus "off-label" therapy for vascular anomalies. Pediatr Blood Cancer. (2021) 68:e28936. doi: 10.1002/pbc.28936
20. Triana, P, and López-Gutierrez, JC. Menstrual disorders associated with sirolimus treatment. Pediatr Blood Cancer. (2021) 68:e28867. doi: 10.1002/pbc.28867
Keywords: lymphatic anomalies, lymphatic malformation, generalized lymphatic anomaly, Gorham–stout disease, mammalian target of rapamycin
Citation: Ozeki M, Endo S, Yasue S, Nozawa A, Asada R, Saito AM, Hashimoto H, Fujimura T, Yamada Y, Kuroda T, Ueno S, Watanabe S, Nosaka S, Miyasaka M, Umezawa A, Matsuoka K, Maekawa T, Hirakawa S, Furukawa T, Fumino S, Tajiri T, Takemoto J, Souzaki R, Kinoshita Y and Fujino A (2024) Sirolimus treatment for intractable lymphatic anomalies: an open-label, single-arm, multicenter, prospective trial. Front. Med. 11:1335469. doi: 10.3389/fmed.2024.1335469
Received: 09 November 2023; Accepted: 23 January 2024;
Published: 08 February 2024.
Edited by:
Bruno Guedes Baldi, University of São Paulo, BrazilReviewed by:
Juan Carlos Lopez Gutierrez, Hospital Infantil La Paz, SpainCopyright © 2024 Ozeki, Endo, Yasue, Nozawa, Asada, Saito, Hashimoto, Fujimura, Yamada, Kuroda, Ueno, Watanabe, Nosaka, Miyasaka, Umezawa, Matsuoka, Maekawa, Hirakawa, Furukawa, Fumino, Tajiri, Takemoto, Souzaki, Kinoshita and Fujino. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michio Ozeki, b3pla2kubWljaGlvLmo1QGYuZ2lmdS11LmFjLmpw
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.