
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 28 March 2024
Sec. Precision Medicine
Volume 11 - 2024 | https://doi.org/10.3389/fmed.2024.1332434
This article is part of the Research Topic Systems and Network Approaches to Precision Medicine and Healthcare View all 9 articles
 Carolyn G. Mazariego1*
Carolyn G. Mazariego1* Skye McKay1
Skye McKay1 Elijah Tyedmers1
Elijah Tyedmers1 Lauren Kelada2,3
Lauren Kelada2,3 Brittany C. McGill2,3
Brittany C. McGill2,3 Rebecca Daly2,3
Rebecca Daly2,3 Claire E. Wakefield2,3
Claire E. Wakefield2,3 David S. Ziegler3,4
David S. Ziegler3,4 Natalie Taylor1
Natalie Taylor1Objectives: Paediatric oncologists often encounter challenges when seeking compassionate access to off-label therapies for their patients. This study employed implementation science and co-design techniques to develop the ProCure medicines database, with the goal of streamlining the application process and addressing identified barriers in paediatric oncology.
Methods: This study utilised an exploratory qualitative research design. Seventeen healthcare providers, including oncologists, nurse consultants, and allied health professionals, participated in semi-structured interviews guided by the Consolidated Framework for Implementation Research (CFIR) and a visual process map aid. Deductive qualitative data analysis, according to the CFIR constructs, identified key barriers and facilitators. Collaborative design sessions engaged multidisciplinary teams to develop the ProCure beta version.
Results: Barriers to off-label therapy access included resource-intensive applications, time sensitive decision-making, and complex pharmaceutical information. Facilitators included Drug Access Navigators, Molecular Tumour Boards, and a multi-disciplinary approach. ProCure addressed end-user needs by centralising medicines information. Additional features suggested by healthcare providers included blood–brain-barrier penetrability data and successful application examples.
Conclusion: ProCure represents a promising solution to the challenges paediatric oncologists face in accessing off-label therapies. By centralising information, it simplifies the application process, aids decision-making, and promotes a collaborative approach to patient care. The potential of the database to stream and enhance off-label therapy access underscores its relevance in improving paediatric oncology practise. Further research and implementation efforts are warranted to assess ProCure’s real-world impact and refine its features based on user feedback.
Precision medicine has ushered in a new era in healthcare, particularly in the field of oncology. It has brought the promise of personalised treatment approaches, underpinned by the identification and validation of biomarkers intricately linked to therapeutic responses (1–3). Central to this paradigm shift is the practise of tumour profiling, a powerful tool that unveils the genetic intricacies indicate cancer specific to the individual, and can reveal potential targeted therapies (3). In this context, precision medicine trials have become a focal point of scientific inquiry and medical practise. These trials have the potential to identify ground-breaking treatment options that hold the promise of improved outcomes for patients. However, a significant challenge looms when these novel therapies, identified through precision medicine, are not approved for use in specific patient populations, most notably in paediatric oncology (4). While there have been significant improvements in treatments for childhood cancer, for an estimated 15% of children diagnosed with cancer, a cure is not possible. For example, Diffuse Midline Gliomas (DMG) represent the most aggressive of all cancers, with almost all children dying within 12 months; we therefore label cancers such as these as ‘hard-to-treat’ (5).
Australia has nine paediatric oncology centres, and it is estimated that ~750 children aged 0–14 years old are diagnosed with cancer each year (6). Given Australia’s vast geographical area, it is estimated that the mean travel distance from home to hospital is >100 km (7).
In Australia, the Therapeutic Goods Administration (TGA) is the regulatory authority responsible for the approval and monitoring of therapeutic goods, including targeted oncology drugs. For paediatric use, the TGA follows a risk-based approach, taking into consideration the specific needs of children (8). Manufacturers are required to provide data on the safety and efficacy of drugs in paediatric populations as part of the regulatory submission. Once approved, they are listed on the Australian Register of Therapeutic Goods (ARTG) for specific indications and populations with appropriate restrictions.
If there are no suitable treatment options available on the ARTG or through clinical trials, a clinician may decide to pursue off-label prescribing based on available evidence. Off-label prescribing means that the TGA has not approved the indication, route of administration or patient group. It does not mean that the TGA has rejected the indication (9). Commonly the TGA has not been asked to evaluate the indication in the context of paediatrics, perhaps due to a lack of robust safety and clinical evidence. For these reasons, prescribing off-label for paediatric patients is often unavoidable. Multiple patients receive off-label therapy at each hospital each year, however, there are no formally reported studies or data available as to how many off-label applications are processed or approved. Anecdotally, seeking off-label drug access is generally more common in children with hard-to-treat cancer.
Furthermore, the Therapeutic Goods Act 1989 (the Act) does not regulate clinical practise. ‘Off-label use’ is a clinical decision, made at the discretion of the treating clinician and does not require an exemption, approval or authorisation from the TGA in order to use a therapeutic good for an off-label use. This clinician is responsible for obtaining informed consent from their patient (which includes telling them if the use is off-label) and ensuring that the agent selected is the most appropriate treatment option.
The TGA may grant special consideration via the Special Access Scheme for compassionate use of imported drugs in situations where there are no satisfactory alternative treatments available in Australia, and the patient has a serious or life-threatening condition. This is generally considered on a case-by-case basis (10).
In contrast, the Food and Drug Administration (FDA) in the United States has specific regulations and guidelines for paediatric drug development. The Paediatric Research Equity Act (PREA) and the Best Pharmaceuticals for Children Act (BPCA) provide incentives for studying drugs in paediatric populations. Paediatric studies may be required for certain drugs, and paediatric labelling information is often included in drug approvals. The FDA also has mechanisms for expanded access or compassionate use, allowing seriously ill patients to access investigational drugs outside of clinical trials. This is regulated under the FDA’s expanded access programme (11).
In the European Union (EU), the European Medicines Agency (EMA) oversees the regulation of medicines. Paediatric requirements are outlined in the Paediatric Regulation, which aims to improve the development and availability of medicines for children. Like the FDA, the EMA encourages the inclusion of paediatric populations in clinical trials and has a system for paediatric investigation plans (PIPs). Compassionate use in the EU is governed by national regulations within each member state. The EMA provides guidance on compassionate use, and the decision to allow such use is made by the relevant national competent authority (12).
The regulatory frameworks in Australia, the FDA, and the EU all prioritise the safety and efficacy of drugs in paediatric populations. Paediatric investigation plans are a common theme, encouraging the inclusion of children in clinical trials. Compassionate use or expanded access programmes exist in all three regions, though specific details and processes may vary.
Regardless of varying practises, paediatric oncologists are at the frontline of dealing with access challenges, as they are the conduit between the patient and pharmaceutical companies, often having to submit lengthy applications to obtain off-label access. Clinicians grapple with a multitude of hurdles, ranging from navigating the regulatory intricacies and negotiating with pharmaceutical companies to meticulously adhering to stringent documentation requirements (4, 13, 14). The absence of readily available resources and comprehensive support exacerbates this already demanding process.
In response to these challenges we developed ProCure, a database which aims to streamline the application process for off-label medicines. ProCure offers a systematic approach designed to expedite the application process, enhance efficiency, promote consistency, and empower paediatric oncologists in their tireless efforts to secure the best possible care for their young patients.
To set the foundation for the design of the ProCure database, this research to explore paediatric healthcare professionals’ (PHCPs): (i) perceived role in identifying and accessing off-label medicines and apply implementation science methods to identify barriers and facilitators encountered, and (ii) PHCPs perceived acceptability of a resource like ProCure and elicit end-user needs to facilitate co-design of the resource.
• Can a database (ProCure) be built to streamline drug information that would be useful for paediatric oncologists when preparing off-label drug access applications?
• What features should ProCure include in order to facilitate ease of use to enable efficiency in the application process for off-label drug access?
This study did not take place in one specific setting (e.g., hospital); we recruited PHCPs from across Australian paediatric clinical sites. Any PHCP (e.g., oncologists, nurses, and pharmacists) who had direct involvement in the care of children or adolescents with hard-to-treat cancers within the previous 24 months, and self-reported experience applying for managing off-label cancer medicines were eligible to participate. In the first instance, known PHCPs were recruited via purposive sampling (i.e., the clinical investigator DZ emailed clinicians involved in Australian paediatric precision medicine trials) and via exponential snowball sampling, with consenting participants opting to provide contact details of other potentially eligible participants. Additional participants were recruited nationally, using study advertisements on social media (i.e., Twitter) and professional network platforms (e.g., Australian and New Zealand Children’s Haematology and Oncology Group). Online REDCap consent was required prior to the interview. This study was approved by the Sydney Children’s Hospital Network Ethics Committee. Informed written consent was obtained from each study participant (HREC Reference: 2021/ETH00583. SCHN HREC).
Individual semi-structured interviews aimed to explore PHCPs’ experiences with off-label therapy applications and access pathways, with a particular focus on challenges and barriers. Following completion of the online consent form the research team (CM, RD, SM, and BM) contacted participants to schedule a suitable interview time.
The first stage of the interview comprised of an implementation process mapping exercise; a visual representation of current practises associated with access to off-label therapeutics in the context of a paediatric oncology precision medicine trial. Implementation process mapping can help to create a visual representation to identify gaps in practise, or how new innovations that facilitate evidence-based practise can be integrated into current workflow (15–17). In order to conduct this process mapping exercise, we began with a draft process map of how the study team understood the steps needed to be taken by paediatric oncologists when attempting to obtain off-label therapies. The interviewer described this process map then asked participants if it was reflective of their experience. We asked participants to confirm or amend these current practises. With each interview, the process map was iterated to reflect amendments and new information (Figure 1). We then asked participants to reflect on the potential usefulness of a resource like ProCure for facilitating/improving current clinical practise.

Figure 1. Process map of current off-label access pathway (without ProCure resource support). MTB, Multi-disciplinary Tumour Board; PRISM, refers to the Australian precision medicine trial for children with hard-to-treat cancers; Pharma, Pharmaceutical company.
The second phase of our interview included additional questions guided by the Consolidated Framework for Implementation Research (CFIR) (18, 19). The CFIR to a determinant framework which can aid in systematically identifying factors influencing implementation of interventions at multiple levels within an organisational context. The CFIR comprises a taxonomy containing 31 constructs in five domains as follows: intervention characteristics, outer setting, inner setting, characteristics of the individual, and the process of implementation (18, 19). The CFIR domains and constructs served as a comprehensive framework to guide the interview process and ensure coverage of key potential barriers and enablers to ProCure use and implementation factors. Interviews continued until thematic saturation was reached (defined as three consecutive interviews with no new themes) (20). ProCure was further designed and iterated alongside interview data collection, and where possible, evolving iterations of the database were presented during interviews to obtain feedback.
This study utilised an exploratory qualitative research design, employing deductive data analysis (21) through the domains of the widely-used CFIR. Interviews were recorded and then transcribed verbatim using a third-party service. The qualitative interview data were analysed using a deductive thematic analysis approach by coding identified barriers and facilitators to the CFIR.
Thirty percent of interviews were double coded by SM and CM (SM is a trained genetic counsellor and CM is an implementation scientist) in NVivo version 12, with any discrepancies resolved through discussion until high intercoder agreement was reached. The coded data were transferred to an Excel spreadsheet and an iterative triangulation approach of developing a coding tree was employed to identify themes and patterns within the data, allowing for the identification of key barriers, end-user needs, and gaps that ProCure should aim to address. SM conducted the rest of the interview coding, and presented themes and extracted illustrative quotes back to the larger study team (CM, ET, and NT) for discussion and finalisation.
A web-designer (WebWorks) was contracted to help develop an engaging, user-friendly online interface for ProCure. Our implementation science team (CM, ET, SM, and NT) met with the web-designer fortnightly across a 9-month period to design ProCure. Three collaborative design sessions were organised, two with the project steering committee (which consists of researchers, paediatric oncologists, genetic counsellors, and two parents of children with hard-to-treat cancers). The project steering committee was presented the working draft iteration of ProCure and invited to provide informal qualitative feedback (either verbally during the meeting or via email to the study investigators post-meetings) during early/mid-development phases of ProCure. For the third collaborative design session, we arranged for ProCure to be presented to the multidisciplinary team within a precision medicine molecular tumour board (MTB) meeting that comprised of a pharmacist and seven paediatric oncologists. ProCure was presented to the MTB and informal feedback was collected to continue to guide the development of ProCure to be tailor-made for each of use by paediatric oncologists. This session occurred during the late development phase of ProCure (final 3 months). These three collaborative design sessions further facilitated the co-design process, allowing for immediate feedback, suggestions for improvements, and for MTB participants to contribute their expertise to the development of the resource. The feedback from these collaborative sessions was presented back to the web-designer for discussion/exploration of the feasibility of incorporating into ProCure. This manuscript presents the design process for the ProCure resource, however, the study team are also undertaking a formal evaluative Beta testing phase with the ProCure resource that will be reported separately to this design process.
A total of 17 PHCPs participated in the study, comprising nine oncologists and eight clinical nurse consultants/allied health professionals. The average amount of years of experience in delivering paediatric oncological care was 14.5 years (range < 1 year to 41 years, Table 1). This diverse group represented a range of professional roles within paediatric oncology, allowing for comprehensive ‘real world’ insights into the application process for off-label therapies. Interviews took place between June 2021 and May 2022.
The compassionate access application process was identified as resource-intensive, requiring substantial time and effort from PHCPs to identify, compile and submit the necessary documentation (Table 2, with supporting quotes and associated CFIR domains). Barriers included increased PHCP workload related to performing comprehensive literature reviews to support clinical treatment decision-making and identifying the correct pharmaceutical contacts and processes for off-label access applications. The application process was also considered relatively uncommon and unfamiliar to most PHCPs, which was further impeded by the inherent variability between pharmaceutical procedures and complexity of regulatory authority processes (e.g., hospital drug committees, ethics committees). The time-sensitive nature of decision-making regarding off-label therapies was also identified as a significant barrier, placing additional pressure on PHCPs to quickly gather and review relevant pharmaceutical information and to communicate this information to patients and their families.
Paediatric healthcare professionals highlighted the value of a Drug Access Navigator (DAN), who can play a pivotal role in assisting throughout the application process by providing guidance, coordinating documentation, and facilitating communication with regulatory authorities (Table 2). The establishment of a precision medicine Multi-disciplinary Tumour Board (MTB) was seen as another facilitator, enabling multidisciplinary discussions amongst experts to determine the appropriateness of off-label therapies based on molecular profiling and individual patient characteristics, and providing the opportunity to share experiential advice regarding off-label applications. Finally, the adoption of a collaborative, multi-disciplinary and consistent approach to care was emphasised as a facilitator, promoting shared decision-making and comprehensive support for patients.
A central aspect of ProCure, which aligns with PCHP needs, is the consolidation of information about medicines within an accessible and well-supported platform. By centralising relevant pharmaceutical information, ProCure was perceived to be a useful and valuable resource (acceptability outcome) that could alleviate the upstream burden on PHCPs in gathering and reviewing scattered data from various sources and potentially standardise drug access applications. One perceived challenge was the capability of maintaining up-to-date literature within a fast-evolving landscape, and some PHCPs expressed the intention to continue to perform their own literature reviews for applications. However, most PHCPs could not identify any major barriers to implementing ProCure into clinical practise.
Paediatric healthcare professionals provided valuable feedback and recommendations to enhance the functionality and acceptability of ProCure, for which high level inclusions and end-user needs are detailed in Table 3. One recurrent suggestion was the inclusion of blood–brain-barrier penetrability data, which would enable PHCPs to make informed decisions regarding the suitability of therapies for central nervous system tumours. PHCPs also expressed the need for examples of successful applications to serve as reference points for application building.
Based on the findings from the interviews, identified gaps, and the collaborative design sessions, a beta version of the ProCure database was developed (ProCure-Beta, July 2022; Figure 2).
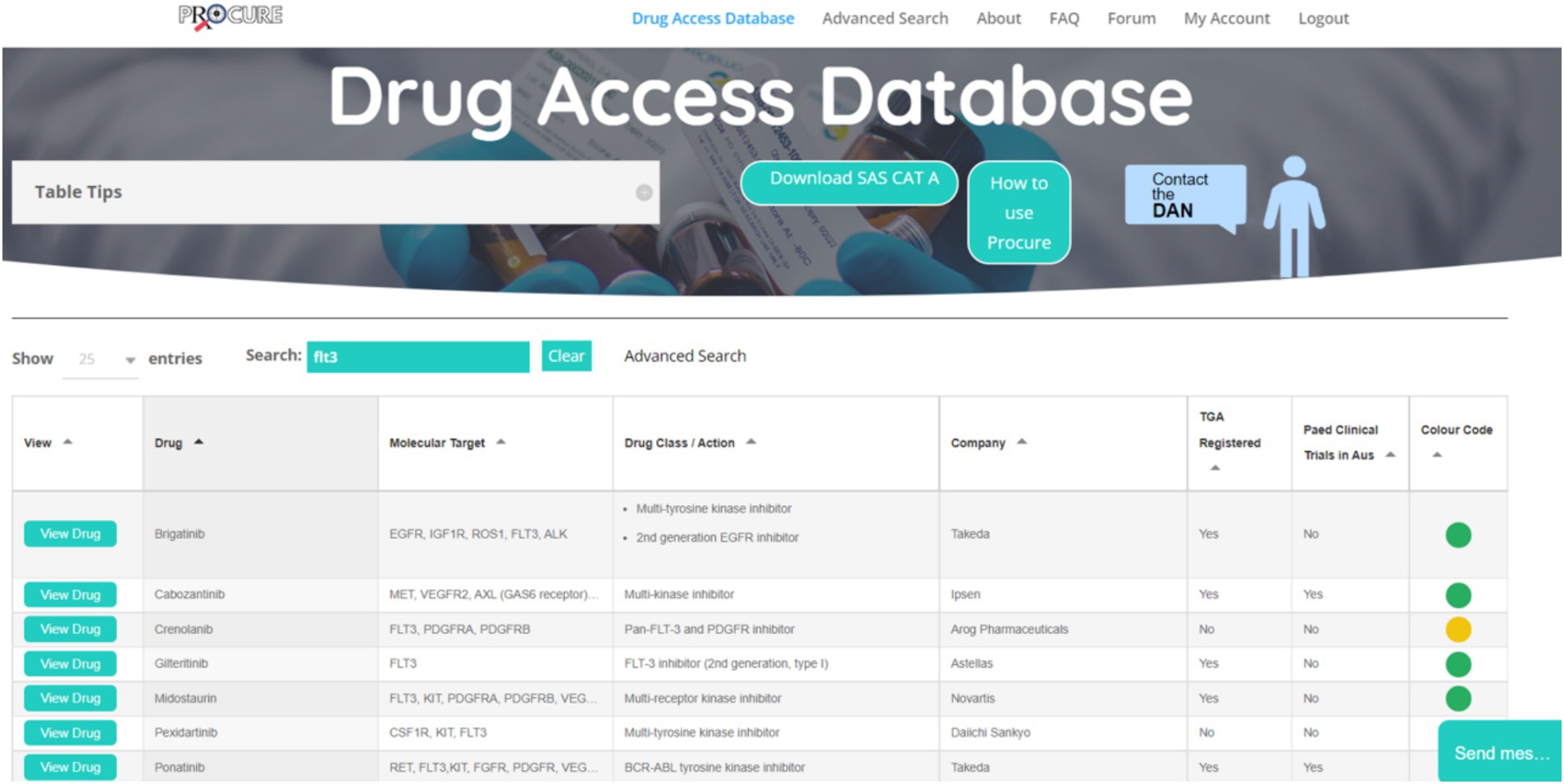
Figure 2. Screenshot of ProCure Beta. Cancer agents and molecular tumour markers can be searched for using the search bar at the top of the database. Searches return relevant results with drug profiles that can be expanded with the button ‘View Drug’. Colour coded dots refer to the level of drug accessibility in Australia; Green, Therapeutic Goods Administration (TGA) approved, established access programme for paediatric indications; a history of compassionate access for certain cancer types and/or molecular targets; known overseas supplier via special access schemes, clinical trial open in Australia; Yellow, Potentially accessible but not TGA approved, may have an access programme for restricted cancers/molecular targets, may have a clinical trial running but no longer recruiting in Australia and may only be recruiting overseas. Compassionate access is possible but less likely; and Red, Appears to be inaccessible to Australia at this time. No approval in any jurisdiction, no open clinical trials in Australia, no access programmes, may have clinical trials open abroad. FAQ, Frequently asked questions; SAS CAT A, Special access scheme—category A (download required when colour code of drug is red); DAN, Drug access navigator; and TGA, Therapeutic goods administration.
ProCure was designed to address the identified barriers, reinforce identified facilitators, and fulfil end-user needs. The study team identified the practical steps through which ProCure would ideally be used (Figure 3), which included: (1) Searching the ProCure resources for the relevant off-label therapy or tumour marker; (2) Consider the drug access options included in the presented search results; and (3) Gather supporting evidence and paperwork (included in the ProCure resource); and (4) Submit application to pharmaceutical contact/company.
This novel study explored PHCPs’ perspectives on their perceived roles in identifying and accessing off-label medicines by applying implementation science methods to identify barriers and facilitators of current processes. The novelty of this study is further strengthened by the end-user centric design of the ProCure database with the outcome of acceptability in mind.
The identified barriers and facilitators provide valuable insights into the challenges currently faced by PHCPs in accessing off-label therapies and the potential impact of ProCure in addressing these challenges. Barriers to off-label therapy access included resource-intensive applications, time sensitive decision-making, and complex pharmaceutical information. Facilitators included DANs, MTBs, and a multi-disciplinary approach. The resource-intensive nature of the current application process aligns with previous research on the complexities and time constraints associated with off-label therapy access (4, 13).
The incorporation of a DAN and the establishment of MTBs as facilitators align with the principles of collaborative and multidisciplinary care, emphasising the importance of interprofessional collaboration in optimising treatment decisions.
Our study employed an end-user centric approach in designing the ProCure resource. This approach increases the likelihood of ProCure aligning with end-user needs and workflow, enhancing its potential for successful implementation (22). However, we acknowledge that the field of precision medicine is rapidly evolving, and so to maintain relevance, ProCure must also evolve to continue to meet end-user needs. This study may not have captured the most recent needs as ProCure was designed in 2022. Ongoing updates and adjustments to ProCure will be required to retain effectiveness and efficiency. Updates to data contained in ProCure are currently facilitated by the DAN, however, for sustainability, we must consider reducing the manual updating aspects of the resource as much as possible, while still maintaining the rigour and relevance in the presented results of performed searches within the resource.
Finally, the use of implementation methodologies and tools to ‘design for implementation’ is a novel approach that this work has undertaken, as implementation outcomes are usually considered once an innovation has proved efficacy (23). The assessment of acceptability as a precursor to practical implementation and ‘real-world’ use of ProCure provided the benefit of being able to identify how to build ProCure to meet end-user needs. In eliciting these end-user needs and building ProCure to match, we established a strong foundation through which we can expect that latter stage implementation outcomes, such as uptake and adoption, would be further realised (19).
ProCure has the potential to streamline access to off-label therapies for paediatric cancer patients by assisting healthcare professionals in the application and procurement process. By simplifying administrative processes, ProCure can reduce the burden on healthcare professionals, freeing up valuable time and resources, allowing clinicians to focus more on patient care and less on navigating complex paperwork and regulatory hurdles.
Additionally, we envision that ProCure may have secondary effects in enhancing treatment decision making for clinicians. By centralising information about available therapies and molecular profiling, ProCure may empower healthcare professionals to make more informed and targeted treatment choices tailored to individuals. Finally, while ProCure has currently not been designed to serve this purpose, such a resource could potentially be used to not just facilitate off-label access to novel therapeutics, but also to track and monitor off-label use. This could detail data on quality outcomes of off-label therapy use and safety monitoring. These postulations will be explored during the next phase of research, effectiveness and implementation pilot testing.
At present there are no formal evaluations of success of applications to off-label access schemes, however a recent systematic review captured global off-label drug use in adult oncology patients, finding that off-label drug use in inpatients ranged from 18–41% (24). Amongst adult patients with cancer, 13–71% received a minimum of one off-label chemotherapy drug. However, a similar review for the paediatric context is not available, thus we can only speculate how these adult-based data points may be reflective of practises in paediatric care. Ideally, future research would be able to capture some of these data and follow up on instances where we see that access was obtained and how these results might influence policies/regulations within pharmaceutical companies and the ARTG at large.
Finally, ProCure was purpose-built to be iterated, meaning that as new end-user needs are identified they can be functionally added to the resource. Other studies have cited that clear communication between patients, families, and clinicians to manage patient and familial expectations of off-label treatments on a patient’s prognosis is often another challenge clinicians face (13, 25, 26). In future, we may consider a dedicated section of ProCure to provide training or information to clinicians on how best to approach these conversations.
Overall, the findings suggest that ProCure could address the key barriers faced by PHCPs in accessing off-label therapies for paediatric oncology patients. By meeting end-users’ needs for centralised and well-supported pharmaceutical information, ProCure has the potential to streamline the application process, facilitate clinician decision-making, and enhance patient care within the paediatric oncology community.
The datasets presented in this article are not readily available because the datasets generated and analysed for this study can be accessed through a separate application to the HREC. Requests to access the datasets should be directed to Yy5tYXphcmllZ29AdW5zdy5lZHUuYXU=.
The studies involving humans were approved by the Sydney Children’s Hospitals Network Human Research Ethics Committee (SCHN HREC). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
CM: Data curation, Formal analysis, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing. SM: Data curation, Formal analysis, Project administration, Writing – review & editing. ET: Writing – review & editing. LK: Data curation, Writing – review & editing. BM: Data curation, Writing – review & editing. RD: Data curation, Writing – review & editing. CW: Conceptualisation, Funding acquisition, Supervision, Writing – review & editing. DZ: Conceptualisation, Funding acquisition, Supervision, Writing – review & editing. NT: Conceptualisation, Funding acquisition, Methodology, Supervision, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. Funding for this research was supported by a Translational Programme Grant from the Cancer Institute NSW. RD was supported by Luminesce Alliance—Innovation for Children’s Health. Luminesce Alliance—Innovation for Children’s Health, was a not-for-profit cooperative joint venture between the Sydney Children’s Hospitals Network, the Children’s Medical Research Institute, and the Children’s Cancer Institute. It has been established with the support of the NSW Government to coordinate and integrate paediatric research. Luminesce Alliance is also affiliated with the University of Sydney and UNSW Sydney.
We would like to acknowledge all those involved in the ProCure Steering committee, with special thanks to the lived-experience consumers who sit on this committee.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Cahaney, C, Dhir, A, and Ghosh, T. Role of precision medicine in pediatric oncology. Pediatr Ann. (2022) 51:e8–e14. doi: 10.3928/19382359-20211209-01
2. Hadjadj, D, Deshmukh, S, and Jabado, N. Entering the era of precision medicine in pediatric oncology. Nat Med. (2020) 26:1684–5. doi: 10.1038/s41591-020-1119-6
3. Lee, J, Gillam, L, Visvanathan, K, Hansford, JR, and McCarthy, MC. Clinical utility of precision medicine in pediatric oncology: a systematic review. JCO Precis Oncol. (2021) 5:1088–102. doi: 10.1200/PO.20.00405
4. Moerdler, S, Zhang, L, Gerasimov, E, Zhu, C, Wolinsky, T, Roth, M, et al. Physician perspectives on compassionate use in pediatric oncology. Pediatr Blood Cancer. (2019) 66:e27545. doi: 10.1002/pbc.27545
5. Bartels, U, Hawkins, C, Vézina, G, Kun, L, Souweidane, M, and Bouffet, E. Proceedings of the diffuse intrinsic pontine glioma (DIPG) Toronto think tank: advancing basic and translational research and cooperation in DIPG. J Neuro-Oncol. (2011) 105:119–25. doi: 10.1007/s11060-011-0704-4
6. Cancer Australia (2022). Statistics for children’s cancers. Available at: https://childrenscancer.canceraustralia.gov.au/about-childrens-cancer/statistics-childrens-cancers
7. Daniel, G, Wakefield, CE, Ryan, B, Fleming, CA, Levett, N, and Cohn, RJ. Accommodation in pediatric oncology: parental experiences, preferences and unmet needs. Rural Remote Health. (2013) 13:2005. doi: 10.22605/RRH2005
8. Department of Health and Aged Care (2011). About the work of the TGA—a risk management approach: Australian Government. Available at: https://www.tga.gov.au/about-work-tga-risk-management-approach
9. Day, RO. Ongoing challenges of off-label prescribing. Aust Prescr. (2023) 46:86–9. doi: 10.18773/austprescr.2023.022
10. Therapeutic Goods Administration (2023). Special Access Scheme (SAS): Guidance for health practitioners accessing unapproved therapeutic goods. Available at: https://www.tga.gov.au/sites/default/files/2023-02/special-access-scheme-sas-guidance-health-practitioners-accessing-unapproved-therapeutic-goods.pdf
11. Food and Drug Administration (2016). Drug Research and Children. Available at: https://www.fda.gov/drugs/information-consumers-and-patients-drugs/drug-research-and-children
12. European Medicines Agency (2024). Needs for paediatric medicines. Available at: https://www.ema.europa.eu/en/human-regulatory-overview/research-and-development/paediatric-medicines-research-and-development/needs-paediatric-medicines
13. Daly, R, Hetherington, K, Hazell, E, Wadling, BR, Tyrrell, V, Tucker, KM, et al. Precision medicine is changing the roles of healthcare professionals, scientists, and research staff: learnings from a childhood Cancer precision medicine trial. J Personal Med. (2023) 13:1033. doi: 10.3390/jpm13071033
14. Wong, M, Mayoh, C, Lau, LMS, Khuong-Quang, D-A, Pinese, M, Kumar, A, et al. Whole genome, transcriptome and methylome profiling enhances actionable target discovery in high-risk pediatric cancer. Nat Med. (2020) 26:1742–53. doi: 10.1038/s41591-020-1072-4
15. Antonacci, G, Lennox, L, Barlow, J, Evans, L, and Reed, J. Process mapping in healthcare: a systematic review. BMC Health Serv Res. (2021) 21:342. doi: 10.1186/s12913-021-06254-1
16. Kononowech, J, Landis-Lewis, Z, Carpenter, J, Ersek, M, Hogikyan, R, Levy, C, et al. Visual process maps to support implementation efforts: a case example. Implement Sci Commun. (2020) 1:105. doi: 10.1186/s43058-020-00094-6
17. Morrow, A, Steinberg, J, Chan, P, Tiernan, G, Kennedy, E, Egoroff, N, et al. In person and virtual process mapping experiences to capture and explore variability in clinical practice: application to genetic referral pathways across seven Australian hospital networks. Transl Behav Med. (2023) 13:561–70. doi: 10.1093/tbm/ibad009
18. Damschroder, LJ, Aron, DC, Keith, RE, Kirsh, SR, Alexander, JA, and Lowery, JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. (2009) 4:50. doi: 10.1186/1748-5908-4-50
19. Damschroder, LJ, Reardon, CM, Opra Widerquist, MA, and Lowery, J. Conceptualizing outcomes for use with the consolidated framework for implementation research (CFIR): the CFIR outcomes addendum. Implement Sci. (2022) 17:7. doi: 10.1186/s13012-021-01181-5
20. Clarke, V, and Braun, V. Thematic analysis. J Posit Psychol. (2017) 12:297–8. doi: 10.1080/17439760.2016.1262613
21. Azungah, T. Qualitative research: deductive and inductive approaches to data analysis. Qual Res J. (2018) 18:383–400. doi: 10.1108/QRJ-D-18-00035
22. Slattery, P, Saeri, AK, and Bragge, P. Research co-design in health: a rapid overview of reviews. Health Res Policy Syst. (2020) 18:17. doi: 10.1186/s12961-020-0528-9
23. Lane-Fall, MB, Curran, GM, and Beidas, RS. Scoping implementation science for the beginner: locating yourself on the “subway line” of translational research. BMC Med Res Methodol. (2019) 19:133. doi: 10.1186/s12874-019-0783-z
24. Saiyed, MM, Ong, PS, and Chew, L. Off-label drug use in oncology: a systematic review of literature. J Clin Pharm Ther. (2017) 42:251–8. doi: 10.1111/jcpt.12507
25. Gereis, JM, Hetherington, K, Robertson, EG, Daly, R, Donoghoe, MW, Ziegler, DS, et al. Parents' and adolescents' perspectives and understanding of information about childhood cancer precision medicine. Cancer. (2023) 129:3645–55. doi: 10.1002/cncr.34914
26. McGill, BC, Wakefield, CE, Hetherington, K, Munro, LJ, Warby, M, Lau, L, et al. “Balancing expectations with actual realities”: conversations with clinicians and scientists in the first year of a high-risk childhood Cancer precision medicine trial. J Personal Med. (2020) 10:9. doi: 10.3390/jpm10010009
Keywords: paediatric oncology, precision medicine, novel therapies, co-design, implementation science, CFIR
Citation: Mazariego CG, McKay S, Tyedmers E, Kelada L, McGill BC, Daly R, Wakefield CE, Ziegler DS and Taylor N (2024) Co-design of a paediatric oncology medicines database (ProCure) to support complex care provision for children with a hard-to-treat cancer. Front. Med. 11:1332434. doi: 10.3389/fmed.2024.1332434
Received: 02 November 2023; Accepted: 05 March 2024;
Published: 28 March 2024.
Edited by:
Emma Kurnat-Thoma, Consultant, Bethesda, MD, United StatesReviewed by:
Michael Weaver, University of Florida, United StatesCopyright © 2024 Mazariego, McKay, Tyedmers, Kelada, McGill, Daly, Wakefield, Ziegler and Taylor. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Carolyn G. Mazariego, Yy5tYXphcmllZ29AdW5zdy5lZHUuYXU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.