- 1Department of Gynecology and Obstetrics, Erlangen University Hospital, Friedrich Alexander University of Erlangen-Nuremberg, Comprehensive Cancer Center Erlangen-EMN, Erlangen, Germany
- 2First Department of Pathology, Medical School, National and Kapodistrian University of Athens, Athens, Greece
Endometriosis constitutes the most common cause of chronic pelvic pain in female patients and is associated with infertility. Although there is no known cause for the disease, it is a heritable condition that is determined by numerous genetic, epigenetic, and environmental aspects. Peroxisome proliferator-activated receptors (PPARs) represent nuclear receptor proteins that control gene expression. By using the MEDLINE and LIVIVO databases we conducted a literature review in order to look into the role of PPARs in the endometriosis pathophysiology and succeeded in revealing 36 pertinent publications between 2001 and 2022. In regards to PPAR expression in endometriosis, PPARγ seems to represent the most studied PPAR isoform in endometriosis and to influence various pathways involved in the disease onset and progression. It's interesting to note that diverse treatment agents targeting the PPAR system have been identified as innovative, effective therapeutic alternatives in the context of endometriosis treatment. In conclusion, PPARs appear to contribute an important role in both endometriosis pathophysiology and therapy.
1 Introduction
Endometrial tissue growing outside of the womb in females of reproductive age is known as endometriosis, a chronic disease (1–3). Depending on the localization of the different endometriotic lesions, various clinical characteristics of endometriosis exist (4, 5). More precisely, patients with endometriosis typically report dyspareunia, chronic pelvic pain, alongside with dysmenorrhea, vaginal bleeding and/or infertility (6). Affected patients may continue to be asymptomatic. Should endometriotic lesions invade the urinary tract, dys-/hematuria can occur, while dys-/hematochezia represent additional symptoms upon intestinal involvement (7). Initial diagnosis of endometriosis is mostly based on transvaginal ultrasound findings, in addition to a thorough physical examination and patient history (8, 9). The uterus is typically not engorged, but the existence of ovarian cysts or nodules in the rectovaginal septum and/or the bladder necessitates additional examinations (10). The best confirmatory test currently available is considered to be laparoscopy because it can detect endometriotic adhesions and implants (11). Most asymptomatic women should receive expectant management (12). When there are no complications and moderate pelvic pain, the use of analgesics in combination with continuous hormonal contraceptives is appropriate for treating symptomatic endometriosis (13). Gonadotropin-Releasing Hormone (GnRH) agonists or oral estrogen-progestin contraceptives may need to be administered if symptoms are severe (14, 15). Laparoscopic excision and thermal destruction of endometrial lesions are the most widely spread surgical treatments when pharmacological therapy fails to work or the disease course is complicated (16). Both retrograde menstruation and coelomic metaplasia are the two most common pathogenetic hypotheses, but the exact pathophysiology of endometriosis development and progression are still not fully explored (17, 18). The hematogenous/lymphatic spread theory, the embryogenetic theory, and the stem cell recruitment theory are additional pertinent hypotheses (17).
Nuclear receptors which get activated by fatty acids are known as peroxisome proliferator-activated receptors (PPARs) (19). The three PPAR isoforms PPARα, PPARβ/δ, and PPARγ, each of which has distinct metabolic regulatory functions, tissue distribution, and ligand-binding characteristics (20, 21), are all present in the body. PPARs may form heterodimers with the retinoid X receptors (RXRs) and bind to particular DNA response elements within promoter regions hence effectively inducing or repressing the expression of their target genes (22). Given the larger PPAR ligand binding cavity's size, diverse natural and synthetic ligands may attach, causing the replacement of co-repressors from co-activators and thereby enhancing the roles of PPARs (23, 24). Only a few of the processes that PPARs control include fatty acid disposition and metabolism, various cellular biology processes, energy homeostasis, cell differentiation and immunity mechanisms (25, 26). More specifically, PPARα co-determines fatty acid metabolism and is highly expressed in several organs such as the heart or the skeletal muscles, liver, intestinal tract, kidneys, and brown adipose tissue. Fatty acid oxidation is regulated by PPARβ/δ, which is widely expressed and also regulates blood sugar and cholesterol levels. When it comes to lipoprotein metabolism lipid biosynthesis, adipogenesis, and insulin sensitivity, PPARγ is most highly expressed in adipose cells (23, 27).
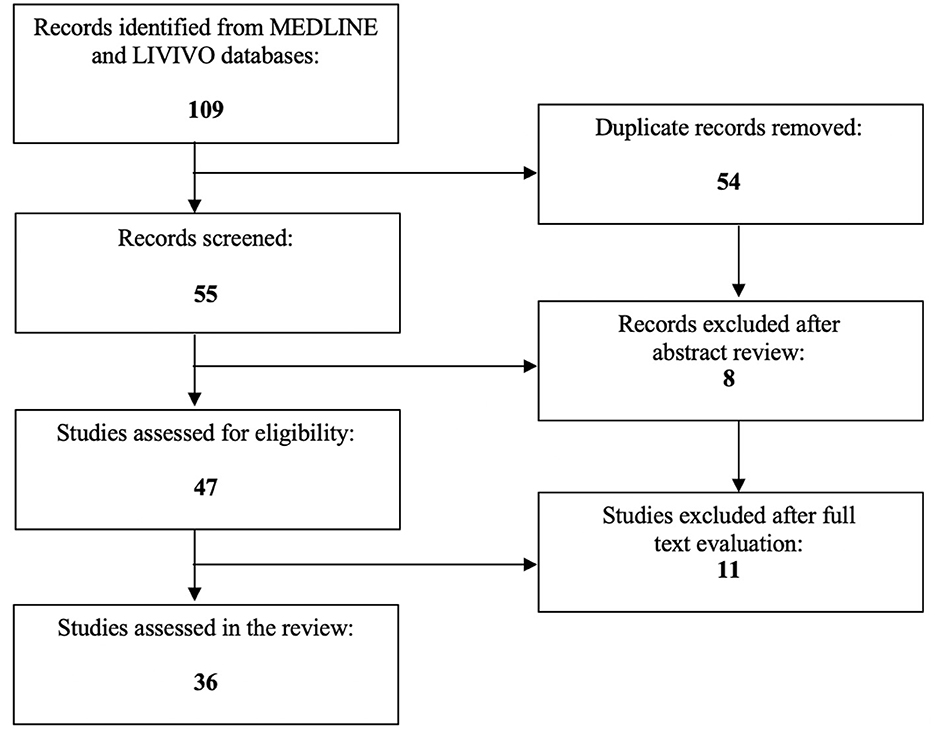
To date, PPARs have been proclaimed to directly or indirectly co-determine numerous procedures associated with cancer and/or gynecological health conditions (28–31). The focus of the present literature review is to investigate the exact role of PPARs in endometriosis. The MEDLINE and LIVIVO databases were used for the conduction of the literature search. In order to be eligible for inclusion, manuscripts needed to embody original research articles and scientific abstracts written in the English language that clearly reported on the role of PPARs in this gynecologic disease. Studies on the involvement of PPARs in uterine fibroids or endometrial cancer had to be excluded. The search terms “peroxisome proliferator-activated receptor,” “PPAR,” and “endometriosis” were employed. After the exclusion of duplicates a total of 55 articles published between 2001 and 2023 were identified. In the initial selection process, a total of eight works had to be discarded after abstract review. After detailed analysis of the full texts of the remaining 47 publications, a total of 36 relevant studies published between 2001 and 2022, that met the inclusion criteria, could be selected for the literature review. Figure 1 depicts the aforementioned selection process.
2 The role of genetics-epigenetics in endometriosis
According to the genetic-epigenetic (G-E) theory, endometriosis doesn't actually start until a number of cumulative G-E cellular changes have taken place. Predisposed women with more inherited incidents are hence at higher risk, while endometriosis could develop from any pluripotent cell other than the endometrium. This theory also explains why “endometrium-like cells” can harbor significant G-E differences. In this context, endometriosis cannot begin to develop until a number of cumulative G-E incidents have occurred (32). Errors can occur during cell division, and the risk is likely to rise in the presence of dioxin, oxidative stress, or ionizing radiation. The endometrium has a higher risk because it is the tissue in our body that grows the fastest. In addition, retrograde menstruation and microbiota, alongside the oxidative stress of the peritoneal cavity, are specifically associated with endometriosis (33, 34). In endometriosis, the frequent cancer-driver mutations brought on by genomic instability are examples of DNA mutations (35–37). Epigenetic incidents in particular have not yet been recorded. Nonetheless, one challenge is that epigenetic reorganizations can be too complex or even irreversible. Therefore, despite an eventual similarity in histology, the G-E theory proposes that the endometriotic cell has experienced irreversible G and/or E changes, permanently separating endometriosis from the endometrium (32). The possibility that these endometriotic cells could cause reversible metaplasia in the surrounding cells, which would cause them to histologically resemble endometrium, is currently more speculative. This is strongly implied, though not formally proven, when it is remembered that recurrences rise even after resections with safety margins. According to the G-E theory, endometriosis can develop from any type of poorly differentiated cell such as stem and bone marrow cells. Nonetheless, there is a higher chance of development from endometrium or embryological rests that have already progressed in that way (32). Each endometriosis lesion has a unique number and type of G-E incidents because they are clonal and have different molecular biology, which explains the various levels of progesterone resistance or aromatase activity (38, 39). This variation also provides an explanation why some lesions are not painful or do not cause distant discomfort (40) and why different patients may respond differently to hormonal therapy (41, 42). The specific arrangement of G-E incidents may also account for the emergence of superficial, cystic, or deep endometriosis in lesions. It is also crucial to note that the numerous endometriosis-related changes in the endometrium, immunology, infertility, and pregnancy are not always caused by endometriosis but can instead be attributed to an inherited propensity (32). Deep endometriosis in women not taking estrogens can occasionally start and progress years after menopause, which is also explained by the G-E pathogenesis (32).
3 The role of PPARs in the pathogenesis of endometriosis
Chen et al. performed a Gene Ontology and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis and stated that the PPAR signaling pathway is associated with the proliferation, metastasis, and autophagy of endometrial cells (43). Moreover, Hornung et al. evaluated the role of PPARs in macrophage attraction into the peritoneal cavity of women with endometriotic lesions. Using immunocytochemistry, the researchers localized PPARα and PPARγ within the nuclei of peritoneal macrophages and pro-monocytic, human histiocytic lymphoma U937 cells. Migration of U937 cells was increased by the PPARα agonist WY14643 and restrained by rosiglitazone. Peritoneal fluid from patients with endometriosis activated U937 cells which had been transiently transfected with a PPARα reporter, whereas it did not successfully activate PPARγ constructs. Notably, U937 cells, which had been transiently transfected with a PPAR reporter, showed endometriosis stage-dependent up-regulation upon peritoneal fluid treatment from women with endometriosis. On the contrary, PPAR response element transactivation was down-regulated after treatment with peritoneal fluid from healthy controls (44).
3.1 The role of PPARα in the pathogenesis of endometriosis
Two studies have, so far, examined the role of PPARα in endometriosis.
Peng et al. (45) demonstrated that high local estrogen level derived from ectopic endometrial stromal cells promote expression of prion, which serves as a critical mediator in augmenting cholesterol accumulation and estrogen production through negative regulation of the PPARα pathway, thus promoting endometriosis progression. Furthermore, Pergaliotis et al. examined mice with surgically-induced endometriosis and reported that PPARα-deficient mice cannot properly induce angiogenesis to sustain as many endometriotic crypts in the implantation site, with the amount of inflammation in the implantation site hence being significantly higher compared to control animal. However, in the control group, fibroblastic activity was found to be significantly up-regulated (46).
3.2 The therapeutic effects of PPARα-targeting agents in endometriosis
Chen et al. generated a first model of endometriosis in rats and a second model of endometriosis using the human ectopic endometrial stromal cells (HEcESCs) derived from the lesion tissues of endometriosis patients. Following the administration of resveratrol in the rat model, significant efficacy was observed in the context of lesion size attenuation and aberrant lipid profile rectification. Lipidomic analysis revealed, on the one hand, notable sphingolipid increases and, on the other hand, decreases of both glycerolipids and most phospholipids. Upon resveratrol admission, both proliferation capacity and invasiveness parameters diminished, whereas the early apoptosis proportion augmented for HEcESCs. The activation of PPARα was also described as a potential factor of recovery from endometriosis in both models (47). Massimi et al. (48) treated 12Z endometriotic epithelial cells with aspirin and other non-aspirin Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) which induced PPARα expression.
3.3 The role of PPARγ in the pathogenesis of endometriosis
The most studied PPAR in endometriosis is undoubtedly PPARγ.
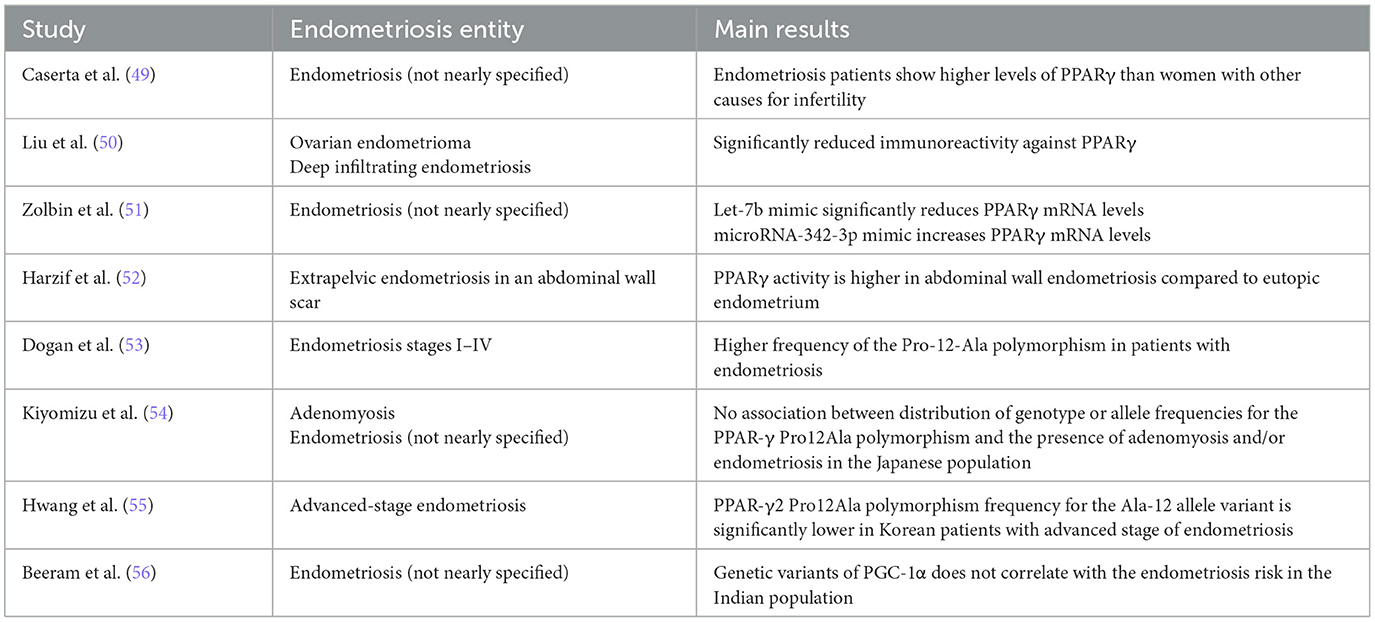
Caserta et al. (49) suggested that patients with endometriosis show higher PPARγ levels than women with other causes of infertility. Additionally, Liu et al. found that both ovarian endometriomas and deep infiltrating endometriosis had significantly reduced immunoreactivity against PPARγ. In particular, PPARγ staining levels were significantly lower in the deep infiltrating endometriosis patient group. Of note, the PPARγ staining levels was negatively associated with the fibrotic extent (50). Besides, Zolbin et al. performed a gene expression analysis and showed that endometriosis may alter the expression of PPARγ. More precisely, the let-7b mimic significantly reduced the messenger RiboNucleic Acid (mRNA) levels of PPARγ, whereas microRNA-342-3p mimic increased its expression levels (51). Harzif et al. presented the case of a 34-year-old patient with extrapelvic endometriosis in an abdominal wall scar. A sample of the abdominal endometriosis and uterine endometrium underwent Reverse Transcriptase quantitative Polymerase Chain Reaction (RT-qPCR) examination of PPARγ mRNA expression. PCR examination results revealed that PPARγ activity was higher in the abdominal wall endometriosis compared to the eutopic endometrium (52).
Dogan et al. investigated the correlation of the PPAR-γ2 Proline to Alanine at 12th amino acid (Pro12Ala) polymorphism with endometriosis in a case–control study incorporating 55 control women without endometriosis as opposed to 51 women with endometriosis stages I–IV. The study group observed a higher frequency of the Pro-12-Ala polymorphism in women with endometriosis compared with the control cases. Interestingly, the frequency was even greater in patients with one or more recurrences of endometriosis, thus implying that the 12-Pro allele might exert protective effects in terms of implantation and growth of ectopic endometrial fragments, whereas the 12-Ala allele could potentially facilitate endometrioid development, progression, and recurrence (53). Moreover, Kiyomizu et al. proved that there is no association between distribution of genotype or allele frequencies for the PPAR-γ Pro12Ala polymorphism and the presence of adenomyosis and/or endometriosis in the Japanese population. Nonetheless, the PPAR-γ 161CC genotype and 161C allele frequencies seem to show significant overexpression in women with adenomyosis and/or endometriosis (54). In a similar context, Hwang et al. performed a case–control study in a collective of 427 controls and 446 patients in the Korean population. The distribution of the PPAR-γ2 Pro12Ala polymorphism differed between the advanced-stage endometriosis group and the control group, while the frequency for the Ala-12 allele variant was significantly higher in the control group than in patients with advanced endometriosis stages (55). Last but not least, Beeram et al. reported that genetic variants of the PPARγ coactivator 1α (PGC-1α) do not seem to correlate with the risk of endometriosis in the Indian population, with GTT of PGC-1α gene constituting the most common haplotype in Indian women (56).
Table 1 summarizes the role of PPARγ in the pathogenesis of endometriosis.
3.4 The PPARγ-associated therapeutic effects of antihypertensive, antidiabetic and cholesterol-regulating agents in endometriosis
Glitazones represent synthetic derivatives of thiazolidinedione, and are designated as potent oral anti-diabetic agents (57). A Turkish study group transplanted endometrial tissue fragments onto the inner surface of the abdominal wall in 28 rats and, consecutively, orally administered rosiglitazone to this group. Surprisingly enough, the PPARγ agonist succeeded in significantly reducing the height, width, length, and spherical volumes, of endometriosis in the studied rat model (58, 59). Furthermore, Zhang et al. (60) applied rosiglitazone to surgically-induced endometriosis rats which enhanced the expression of PPARγ and impacted the development and progression of endometriosis by inhibition of angiogenesis as well as apoptosis induction. In 2002, Pritts et al. (61) described in their first relevant publication that rosiglitazone and 15 deoxy-Δ12,14 prostaglandin J2 decreases endometrial stromal cell transcription and translation of Regulated upon Activation Normal T-cell Expressed and Secreted (RANTES) in vitro. One year later, the same study group concluded that the addition of PPARγ ligands to endometriotic stromal cells inhibits RANTES promoter activity through a specific PPARγ response element (62). Moreover, Olivares et al. applied celecoxib in combination with rosiglitazone to a surgically induced endometriosis mouse model and outlined a statistically significant reduction in the mean number of lesions, the implant volume, or the vascularization level. Notably, the down-regulation of cell proliferation within the implants was statistically significant, while apoptosis showed a statistically significant boost (63). In 2010, Sharma et al. treated endometrial-endometriotic stromal cells isolated from ectopic endometrium with atorvastatin and observed a distinct expression increase of PPARγ (64). A year later, the same study group examined the therapeutic effect of rosiglitazone and 15d-PGJ2 on endometrial-endometriotic stromal cells and again reached the conclusion that both agents might support the expression of PPARγ (65).
Ohama et al. treated endometriotic stromal cells with pioglitazone and reported a significant reduction of the Tumor Necrosis Factor (TNF)-a-induced Interleukin-8 production, alongside a suppression of the growth of endometriotic stromal cells, as well as a reduction of the concentration of p65 (66). In addition, Kim et al. (67) treated infertile patients with stage III or IV endometriosis undergoing in vitro fertilization, alongside controlled ovarian stimulation with GnRH agonist, with pioglitazone and noticed both a significantly higher embryo implantation rate and significantly lower serum RANTES levels after pioglitazone treatment. In 2009, McKinnon et al. published their first original research article on the association of PPARγ and endometriosis. In peritoneal lesions, the pain reported by endometriosis patients increased as PPARγ expression augmented. Nonetheless, the extent of PPARγ expression did not correlate with the stage assigned to the patient (68). Three years later, the same study group treated endometrial stromal cells with ciglitazone and pioglitazone which attenuated a dose-dependent interleukin-6 and interleukin-8 release (69).
Wu et al. cultured immortalized endometrial and endometriotic cell lines with both the histone deacetylase inhibitor trichostatin A and ciglitazone and noted that trichostatin A up-regulates PPARγ expression in a dose-dependent way, while ciglitazone inhibits proliferation of endometriotic cells (70). Besides, Kavoussi et al. treated the endometrial epithelial cell line EM42 and the mesothelial cell line LP9 with the PPARγ agonist ciglitazone. Application of 40 mM ciglitazone to the EM42 cells decreased EM42 attachment to LP9 cells by 27%, while treatment of both EM42 and LP9 cells with 40 mM ciglitazone decreased EM42 attachment to LP9 by 37%. Administration of 40 mM ciglitazone to EM42 cells also decreased attachment to hyaluronic acid by 66%. Nevertheless, invasion of EM42 cells through the LP9 monolayer was not decreased by ciglitazone (71). In 2004, Lebovic et al. published their first original research article on the effects of ciglitazone in a rat model of endometriosis and reported that treatment with this thiazolidinedione might significantly decrease both the mean explant wet weight and the size of ectopic uterine tissues. Notably, the ciglitazone-treated rats experienced marked epithelial regression as well (72). Three years later, the same study group tested the effects of rosiglitazone in a baboon model of established endometriosis. The surface area of endometriotic lesions was statistically significantly higher in rosiglitazone non-treated baboons, with the rosiglitazone-treated baboons treated having a greater negative relative change in terms of peritoneal endometriotic lesion surface area (73). In 2010, the researchers conducted a prospective, randomized, placebo-controlled study in a baboon model to determine if, this time, pioglitazone could impede endometriosis development. Not only the surface area but also the volume of endometriotic lesions were significantly higher in the non-pioglitazone-treated baboons, with the overall number of endometriotic lesions being significantly greater (74). Three years later, Lebovic et al. revealed that ciglitazone succeeds in inhibiting the growth of the endometriotic epithelial cells 12Z up to 35% and of the endometriotic stromal cells 22B up to 70% via transformed cell cycle control and intrinsic apoptosis, diminishing the PGE2 receptor (EP) 2 and EP4 mRNA expression in 12Z and 22B cells, and down-regulating the expression and function of P450 aromatase mRNA and protein and estrone production in 12Z and 22B cells via EP2 and EP4 in a stromal-epithelial cell-specific way (75).
Wang et al. established a rat model of endometriosis by trans-implanting endometrial fragments to the peritoneal wall and then administered resveratrol to the rats. Intense staining of PPARγ expression was induced by high dose resveratrol treatment in glandular epithelial cells in the lesion tissues of model rats, hence inducing PPARγ activation in ectopic focus of endometriosis-induced rats (76).
In 2014, Nenicu et al. (77) proved that the angiotensin II type 1 receptor blocker telmisartan up-regulates PPARγ in peritoneal endometriosis-like lesions, hence resulting in a lower immune cell content, a reduced density of CD31-positive microvessels and a smaller number of Ki67-positive proliferating cells. Three years later, the researchers highlighted that co-treatment of peritoneal endometriotic lesions with telmisartan and the Cyclooxygenase (COX)-2 inhibitor parecoxib synergistically promotes the stromal and glandular expression of PPARγ (78).
Yang et al. established endometriosis rats with estradiol valerate and autologous transplantation and administered different doses of the Chinese traditional medicine QIU through oral gavage for 4 weeks, which promoted autophagy and inhibited angiogenesis by regulating the PPARγ/Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling pathway (79).
Figure 2 depicts all identified PPARγ-modulating treatment agents that provenly play a significant role in the therapy of endometriosis.
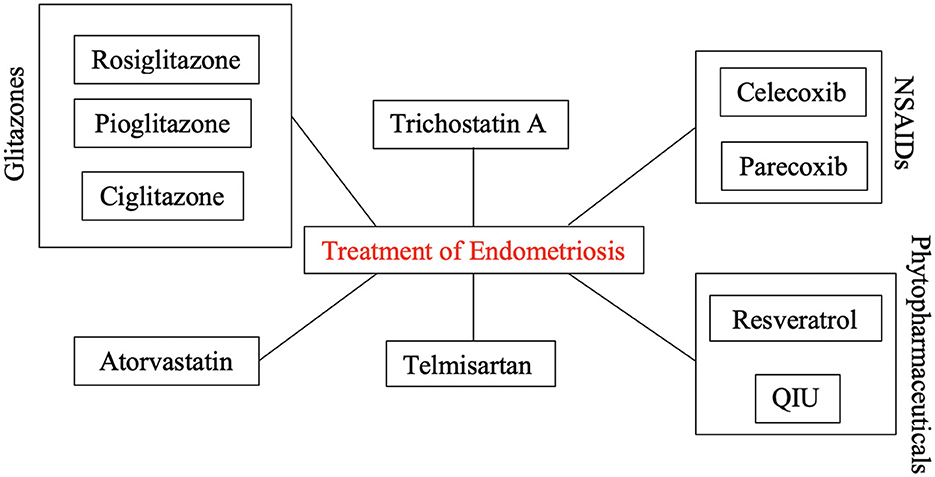
Figure 2. PPARγ-modulating treatment agents for the therapy of endometriosis. Selected well-established drug classes (analgesics; antidiabetics; etc.) seem to show promising therapeutic effects by up- or downregulating the expression of PPARγ in endometriotic cells.
4 Discussion
It has been shown that all PPAR isoforms are expressed in the uteri of various mammal species (80). However, the physiological status of each person or species may be the cause of the variation in their expression patterns (80). PPARs appear to be involved in the regulation of numerous uterine secretory functions essential for the implantation of the embryo during pregnancy or the lysis of the corpus luteum during the estrous cycle (81–83) given their differential expression during different reproductive conditions. Their interactions with prostaglandins, steroids, and cytokines may act as a conduit for their effects at the endometrial level (80). Additionally, alterations in the PPAR expression profile appear to have an effect on signal transduction during inflammatory processes that occur in the endometrium in cases of cystic endometrial hyperplasia and pyometra, as well as to cause hormonal disturbances (84). It is interesting to note that PPAR agonists have been shown to be correlated with estradiol-induced hyperplasia and proliferation in mouse uterus (85). So far, only few drugs such as hormonal therapy with progesterone or GnRH-modulators have received FDA approval for the treatment of endometriosis (86). Up to the present time, no review article has, to our knowledge, been published focusing on the role of PPARs in endometriosis. As such, the current article constitutes an up-to-date review comprehensive of the literature about the effects of the PPAR isoforms PPARα and PPARγ in endometriosis establishment and progression, alongside their potential as eventual anti-endometriotic treatment targets (Figure 3).
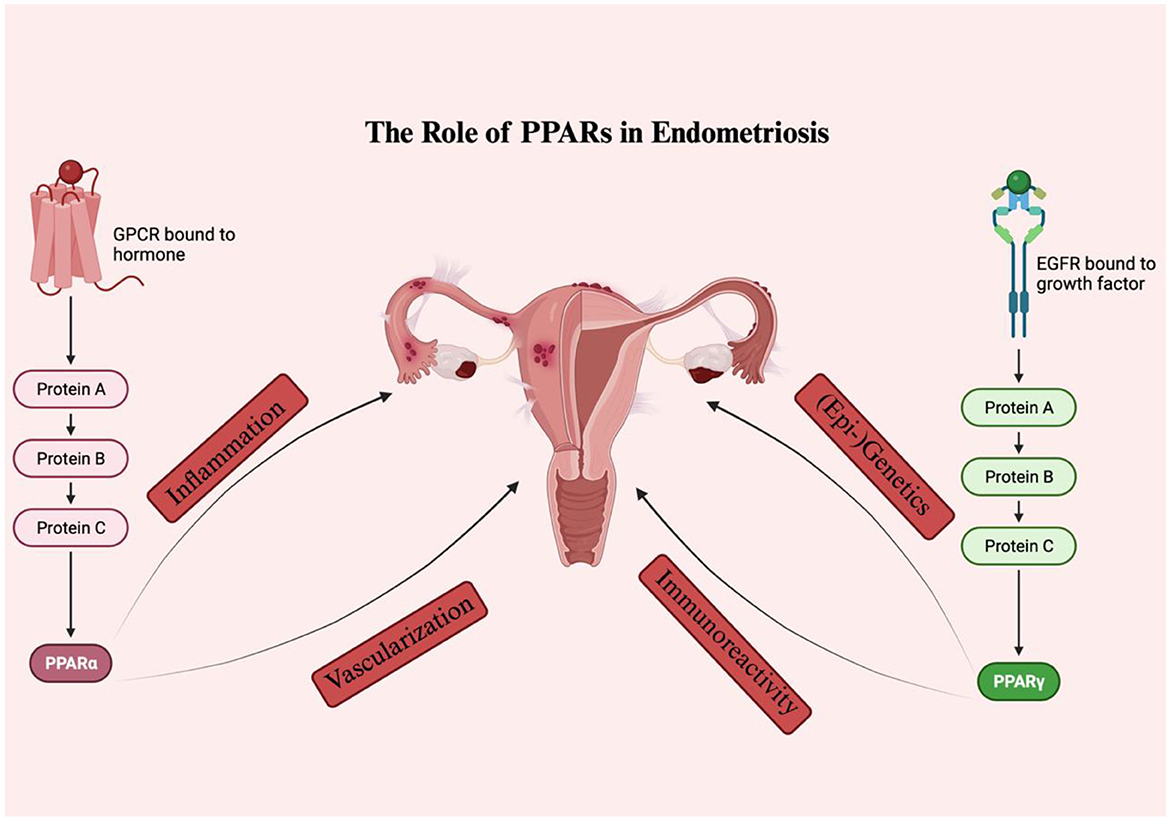
Figure 3. The role of PPARS in endometriosis. PPARα and PPARγ seem to influence various pathways involved in the disease onset and progression, including inflammation, vascularization, immunoreactivity, as well as (epi-) genetics.
Only two study groups have, so far, investigated the role of PPARα in endometriosis. Based on the study results, negative regulation of the PPARα pathway seems to promote endometriosis progression and to hinder adequate vascularization which directly correlates with the level of inflammation at the endometriotic implantation site. In the context of PPARα therapeutic targeting, the administration of resveratrol in endometriosis seemingly leads to the activation of PPARα which might potentially contribute to the recovery from this disease. Aspirin and other NSAIDs also succeed in inducing the PPARα expression.
PPARγ undoubtedly represents the most studied PPARγ isoform in endometriosis. Patients with endometriosis tend to have altered levels of PPARγ, with both ovarian endometriomas and deep infiltrating endometriosis showing significantly reduced immunoreactivity against PPARγ, but PPARγ activity being higher in abdominal wall endometriosis than in the eutopic endometrium. Interestingly enough, the exact location and extent of the endometriosis seems to have an influence on the PPARγ expression pattern. Additionally, the PPAR-γ2 Pro12Ala polymorphisms seem to differentially correlate with the risk of endometriosis in different ethnic populations. For instance, no association between distribution of genotype or allele frequencies for the PPAR-γ Pro12Ala polymorphism and the presence of adenomyosis and/or endometriosis could be noticed in the Japanese population. In the Korean population, on the other hand, the distribution of the PPAR-γ2 Pro12Ala polymorphism differs between advanced-stage endometriosis patients and healthy women. Significantly, genetic variants of the PGC-1α do not seem to correlate with the endometriosis risk in Indian female patients.
In the context of PPARγ therapeutic targeting, glitazones such as rosiglitazone, pioglitazone, and ciglitazone, seem to embody potent treatment agents which successfully suppress inflammation in endometriosis, decrease both the mean explant wet weight and the size of ectopic uterine tissues and even endorse fertility. Resveratrol treatment induces PPARγ activation in ectopic focus of endometriosis, while telmisartan up-regulates PPARγ in peritoneal endometriosis-like lesions with consecutive anti-inflammatory and anti-proliferative effects. Impressively, the Chinese traditional medicine QIU seems to effectively induce autophagy and block angiogenesis via the PPARγ/NF-κB signaling pathway.
Unfortunately, no study has been found that has, up to this point, included a sizable number of tissue samples, which would have provided more reliable and objective results. Furthermore, no PPAR agonist was given to patients with endometriosis as part of randomized controlled trials in order to assess the clinical applicability/efficacy of these drugs and identify potential unknown toxic effects. Future research should therefore address the aforementioned drawbacks and, ideally, examine the function of PPARs in early and advanced stage endometriosis separately. Most importantly, the efficacy of these novel agents on specific symptoms of endometriosis, namely infertility or chronic pelvic pain, should be thoroughly tested, given that these are two very different outcomes and therapeutic targets for various drugs.
The nonsystematic methodology used in the study selection is one of the review's limitations. In spite of the fact that systematic literature reviews adhere to strict rules and standards and represent the most accurate method for locating pertinent research articles, this methodology necessitates a precise research question by excluding broader topics like the function of PPARs in endometriosis. The publication bias (as data from statistically insignificant studies are less likely to be published), and the associated evidence selection bias, are also drawbacks of the present study.
5 Conclusions
In conclusion, the present literature review emphasizes the critical role of PPARs in the onset and progression of endometriosis and emphasizes the therapeutic potential of PPAR agonists against endometriotic cells. In order to come to unbiased and repeatable results and fully delineate the role of PPARs in endometriosis pathogenesis and therapy, additional systematic research is still needed in this field including knockout or knockdown study models and implementation of up- or down-regulators.
Author contributions
IP: Conceptualization, Methodology, Writing—original draft. ST: Writing—review & editing. MB: Writing—review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
2. Kalaitzopoulos DR, Lempesis IG, Samartzis N, Kolovos G, Dedes I, Daniilidis A, et al. Leptin concentrations in endometriosis: a systematic review and meta-analysis. J Reprod Immunol. (2021) 146:103338. doi: 10.1016/j.jri.2021.103338
3. Salmeri N, Vigano P, Cavoretto P, Marci R, Candiani M. The kisspeptin system in and beyond reproduction: exploring intricate pathways and potential links between endometriosis and polycystic ovary syndrome. Rev Endocr Metab Disord. (2023). doi: 10.1007/s11154-023-09826-0
4. Parasar P, Ozcan P, Terry KL. Endometriosis: epidemiology, diagnosis and clinical management. Curr Obstet Gynecol Rep. (2017) 6:34–41. doi: 10.1007/s13669-017-0187-1
5. Burghaus S, Klingsiek P, Fasching PA, Engel A, Haberle L, Strissel PL, et al. Risk factors for endometriosis in a german case-control study. Geburtshilfe Frauenheilkd. (2011) 71:1073–9. doi: 10.1055/s-0031-1280436
6. Maddern J, Grundy L, Castro J, Brierley SM. Pain in endometriosis. Front Cell Neurosci. (2020) 14:590823. doi: 10.3389/fncel.2020.590823
7. Nicolaus K, Reckenbeil L, Brauer D, Sczesny R, Diebolder H, Runnebaum IB. Cycle-related diarrhea and dysmenorrhea are independent predictors of peritoneal endometriosis, cycle-related dyschezia is an independent predictor of rectal involvement. Geburtshilfe Frauenheilkd. (2020) 80:307–15. doi: 10.1055/a-1033-9588
8. Doroftei B, Maftei R, Ilie OD, Simionescu G, Anton E, Armeanu T, et al. Transvaginal ultrasound as a first-line approach in deep endometriosis: a pictorial essay. Diagnostics. (2021) 11:444. doi: 10.3390/diagnostics11030444
9. Burghaus S, Schafer SD, Beckmann MW, Brandes I, Brunahl C, Chvatal R, et al. Diagnosis and treatment of endometriosis. guideline of the DGGG, SGGG and OEGGG (S2k Level, AWMF Registry Number 015/045, August 2020). Geburtshilfe Frauenheilkd. (2021) 81:422–46. doi: 10.1055/a-1380-3693
10. Scioscia M, Virgilio BA, Lagana AS, Bernardini T, Fattizzi N, Neri M, et al. Differential diagnosis of endometriosis by ultrasound: a rising challenge. Diagnostics. (2020) 10:848. doi: 10.3390/diagnostics10100848
11. Mettler L, Schollmeyer T, Lehmann-Willenbrock E, Schuppler U, Schmutzler A, Shukla D, et al. Accuracy of laparoscopic diagnosis of endometriosis. JSLS. (2003) 7:15–8.
12. Keyhan S, Hughes C, Price T, Muasher S. An update on surgical versus expectant management of ovarian endometriomas in infertile women. Biomed Res Int. (2015) 2015:204792. doi: 10.1155/2015/204792
13. Brown J, Crawford TJ, Datta S, Prentice A. Oral contraceptives for pain associated with endometriosis. Cochrane Database Syst Rev. (2018) 5:CD001019. doi: 10.1002/14651858.CD001019.pub3
14. Weisberg E, Fraser IS. Contraception and endometriosis: challenges, efficacy, and therapeutic importance. Open Access J Contracept. (2015) 6:105–15. doi: 10.2147/OAJC.S56400
15. Kupker W, Felberbaum RE, Krapp M, Schill T, Malik E, Diedrich K. Use of GnRH antagonists in the treatment of endometriosis. Reprod Biomed Online. (2002) 5:12–6. doi: 10.1016/S1472-6483(10)61590-8
16. Bafort C, Beebeejaun Y, Tomassetti C, Bosteels J, Duffy JM. Laparoscopic surgery for endometriosis. Cochrane Database Syst Rev. (2020) 10:CD011031. doi: 10.1002/14651858.CD011031.pub3
17. Signorile PG, Viceconte R, Baldi A. New insights in pathogenesis of endometriosis. Front Med. (2022) 9:879015. doi: 10.3389/fmed.2022.879015
18. Psilopatis I, Vrettou K, Fleckenstein FN, Theocharis S. The impact of histone modifications in endometriosis highlights new therapeutic opportunities. Cells. (2023) 12:1227. doi: 10.3390/cells12091227
19. Nuclear Receptors Nomenclature Committee. A unified nomenclature system for the nuclear receptor superfamily. Cell. (1999) 97:161–3. doi: 10.1016/S0092-8674(00)80726-6
20. Han L, Shen WJ, Bittner S, Kraemer FB, Azhar S. PPARs: regulators of metabolism and as therapeutic targets in cardiovascular disease. Part II: PPAR-beta/delta and PPAR-gamma. Future Cardiol. (2017) 13:279–96. doi: 10.2217/fca-2017-0019
21. Wagner KD, Wagner N. Peroxisome proliferator-activated receptor beta/delta (PPARbeta/delta) acts as regulator of metabolism linked to multiple cellular functions. Pharmacol Ther. (2010) 125:423–35. doi: 10.1016/j.pharmthera.2009.12.001
22. Berger J, Moller DE. The mechanisms of action of PPARs. Annu Rev Med. (2002) 53:409–35. doi: 10.1146/annurev.med.53.082901.104018
23. Grygiel-Gorniak B. Peroxisome proliferator-activated receptors and their ligands: nutritional and clinical implications–a review. Nutr J. (2014) 13:17. doi: 10.1186/1475-2891-13-17
24. Psilopatis I, Vrettou K, Fleckenstein FN, Theocharis S. The role of peroxisome proliferator-activated receptors in preeclampsia. Cells. (2023) 12:647. doi: 10.3390/cells12040647
25. Brunmeir R, Xu F. Functional regulation of PPARs through post-translational modifications. Int J Mol Sci. (2018) 19:1738. doi: 10.3390/ijms19061738
26. Bensinger SJ, Tontonoz P. Integration of metabolism and inflammation by lipid-activated nuclear receptors. Nature. (2008) 454:470–7. doi: 10.1038/nature07202
27. Christofides A, Konstantinidou E, Jani C, Boussiotis VA. The role of peroxisome proliferator-activated receptors (PPAR) in immune responses. Metabolism. (2021) 114:154338. doi: 10.1016/j.metabol.2020.154338
28. Yuvaraj S, Kumar BRP. Peroxisome proliferator-activated receptor-gamma As a novel and promising target for treating cancer via regulation of inflammation: a brief review. Mini Rev Med Chem. (2022) 22:3–14. doi: 10.2174/1389557521666210422112740
29. Muzio G, Barrera G, Pizzimenti S. Peroxisome proliferator-activated receptors (PPARs) and oxidative stress in physiological conditions and in cancer. Antioxidants. (2021) 10:1734. doi: 10.3390/antiox10111734
30. Psilopatis I, Vrettou K, Nousiopoulou E, Palamaris K, Theocharis S. The role of peroxisome proliferator-activated receptors in polycystic ovary syndrome. J Clin Med. (2023) 12:2912. doi: 10.3390/jcm12082912
31. Psilopatis I, Vrettou K, Troungos C, Theocharis S. The role of peroxisome proliferator-activated receptors in endometrial cancer. Int J Mol Sci. (2023) 24:9190. doi: 10.3390/ijms24119190
32. Amro B, Ramirez Aristondo ME, Alsuwaidi S, Almaamari B, Hakim Z, Tahlak M, et al. New understanding of diagnosis, treatment and prevention of endometriosis. Int J Environ Res Public Health. (2022) 19:6725. doi: 10.3390/ijerph19116725
33. Koninckx PR, Ussia A, Tahlak M, Adamyan L, Wattiez A, Martin DC, et al. Infection as a potential cofactor in the genetic-epigenetic pathophysiology of endometriosis: a systematic review. Facts Views Vis Obgyn. (2019) 11:209–16.
34. Leonardi M, Hicks C, El-Assaad F, El-Omar E, Condous G. Endometriosis and the microbiome: a systematic review. BJOG. (2020) 127:239–49. doi: 10.1111/1471-0528.15916
35. Koppolu A, Maksym RB, Paskal W, Machnicki M, Rak B, Pepek M, et al. Epithelial cells of deep infiltrating endometriosis harbor mutations in cancer driver genes. Cells. (2021) 10:749. doi: 10.3390/cells10040749
36. Guo SW. Cancer driver mutations in endometriosis: variations on the major theme of fibrogenesis. Reprod Med Biol. (2018) 17:369–97. doi: 10.1002/rmb2.12221
37. Murakami K, Kanto A, Sakai K, Miyagawa C, Takaya H, Nakai H, et al. Frequent PIK3CA mutations in eutopic endometrium of patients with ovarian clear cell carcinoma. Mod Pathol. (2021) 34:2071–9. doi: 10.1038/s41379-021-00861-3
38. Bulun SE, Monsivais D, Kakinuma T, Furukawa Y, Bernardi L, Pavone ME, et al. Molecular biology of endometriosis: from aromatase to genomic abnormalities. Semin Reprod Med. (2015) 33:220–4. doi: 10.1055/s-0035-1554053
39. Bulun SE, Yilmaz BD, Sison C, Miyazaki K, Bernardi L, Liu S, et al. Endometriosis. Endocr Rev. (2019) 40:1048–79. doi: 10.1210/er.2018-00242
40. Koninckx PR, Ussia A, Mashiach R, Vilos G, Martin DC. Endometriosis can cause pain at a distance. J Obstet Gynaecol Can. (2021) 43:1035–6. doi: 10.1016/j.jogc.2021.06.002
41. Koninckx PR, Ussia A, Adamyan L, Wattiez A, Gomel V, Martin DC. Heterogeneity of endometriosis lesions requires individualisation of diagnosis and treatment and a different approach to research and evidence based medicine. Facts Views Vis Obgyn. (2019) 11:57–61.
42. Becker CM, Gattrell WT, Gude K, Singh SS. Reevaluating response and failure of medical treatment of endometriosis: a systematic review. Fertil Steril. (2017) 108:125–36. doi: 10.1016/j.fertnstert.2017.05.004
43. Chen D, Zhou L, Qiao H, Wang Y, Xiao Y, Fang L, et al. Comparative proteomics identify HSP90A, STIP1 and TAGLN-2 in serum extracellular vesicles as potential circulating biomarkers for human adenomyosis. Exp Ther Med. (2022) 23:374. doi: 10.3892/etm.2022.11301
44. Hornung D, Waite LL, Ricke EA, Bentzien F, Wallwiener D, Taylor RN. Nuclear peroxisome proliferator-activated receptors alpha and gamma have opposing effects on monocyte chemotaxis in endometriosis. J Clin Endocrinol Metab. (2001) 86:3108–14. doi: 10.1210/jcem.86.7.7615
45. Peng HY, Lei ST, Hou SH, Weng LC, Yuan Q, Li MQ, et al. PrP(C) promotes endometriosis progression by reprogramming cholesterol metabolism and estrogen biosynthesis of endometrial stromal cells through PPARalpha pathway. Int J Biol Sci. (2022) 18:1755–72. doi: 10.7150/ijbs.68015
46. Pergialiotis V, Zarkadoulas N, Goula K, Frountzas M, Antoniadou F, Dimitroulis D, et al. Angiogenic and inflammatory alterations of endometriotic lesions in a transgenic animal experimental model with loss of expression of PPAR-alpha receptors. Cureus. (2022) 14:e30290. doi: 10.7759/cureus.30290
47. Chen Z, Wang C, Lin C, Zhang L, Zheng H, Zhou Y, et al. Lipidomic alterations and PPARalpha activation induced by resveratrol lead to reduction in lesion size in endometriosis models. Oxid Med Cell Longev. (2021) 2021:9979953. doi: 10.1155/2021/9979953
48. Massimi I, Pulcinelli FM, Piscitelli VP, Alemanno L, Maltese T, Guarino ML, et al. Non-steroidal anti-inflammatory drugs increase MRP4 expression in an endometriotic epithelial cell line in a PPARa dependent manner. Eur Rev Med Pharmacol Sci. (2018) 22:8487–96.
49. Caserta D, Bordi G, Ciardo F, Marci R, La Rocca C, Tait S, et al. The influence of endocrine disruptors in a selected population of infertile women. Gynecol Endocrinol. (2013) 29:444–7. doi: 10.3109/09513590.2012.758702
50. Liu X, Zhang Q, Guo SW. Histological and immunohistochemical characterization of the similarity and difference between ovarian endometriomas and deep infiltrating endometriosis. Reprod Sci. (2018) 25:329–40. doi: 10.1177/1933719117718275
51. Zolbin MM, Mamillapalli R, Nematian SE, Goetz TG, Taylor HS. Adipocyte alterations in endometriosis: reduced numbers of stem cells and microRNA induced alterations in adipocyte metabolic gene expression. Reprod Biol Endocrinol. (2019) 17:36. doi: 10.1186/s12958-019-0480-0
52. Harzif AK, Silvia M, Mariana A, Olivia L, Lovita BT, Wiweko B. Extrapelvic endometriosis in abdominal wall scar and PPAR gamma expression: a case report. Int J Surg Case Rep. (2018) 53:66–9. doi: 10.1016/j.ijscr.2018.10.026
53. Dogan S, Machicao F, Wallwiener D, Haering HU, Diedrich K, Hornung D. Association of peroxisome proliferator-activated receptor gamma 2 Pro-12-Ala polymorphism with endometriosis. Fertil Steril. (2004) 81:1411–3. doi: 10.1016/j.fertnstert.2003.10.026
54. Kiyomizu M, Kitawaki J, Obayashi H, Ohta M, Koshiba H, Ishihara H, et al. Association of two polymorphisms in the peroxisome proliferator-activated receptor-gamma gene with adenomyosis, endometriosis, and leiomyomata in Japanese women. J Soc Gynecol Investig. (2006) 13:372–7. doi: 10.1016/j.jsgi.2006.03.005
55. Hwang KR, Choi YM, Kim JM, Lee GH, Kim JJ, Chae SJ, et al. Association of peroxisome proliferator-activated receptor-gamma 2 Pro12Ala polymorphism with advanced-stage endometriosis. Am J Reprod Immunol. (2010) 64:333–8. doi: 10.1111/j.1600-0897.2010.00882.x
56. Beeram H, Siddamalla S, Tumu VR, Kv V, Vidala A, Deenadayal M, et al. Genetic variants of VDR and PGC-1alpha are not associated with the risk of endometriosis in indian women. DNA Cell Biol. (2022) 41:987–95. doi: 10.1089/dna.2022.0350
57. Stumvoll M, Haring HU. Glitazones: clinical effects and molecular mechanisms. Ann Med. (2002) 34:217–24. doi: 10.1080/713782132
58. Aytan H, Caliskan AC, Demirturk F, Aytan P, Koseoglu DR. Peroxisome proliferator-activated receptor-gamma agonist rosiglitazone reduces the size of experimental endometriosis in the rat model. Aust N Z J Obstet Gynaecol. (2007) 47:321–5. doi: 10.1111/j.1479-828X.2007.00744.x
59. Demirturk F, Aytan H, Caliskan AC, Aytan P, Koseoglu DR. Effect of peroxisome proliferator-activated receptor-gamma agonist rosiglitazone on the induction of endometriosis in an experimental rat model. J Soc Gynecol Investig. (2006) 13:58–62. doi: 10.1016/j.jsgi.2005.10.002
60. Zhang S, Zhuang L, Liu Q, Yu X, Min Q, Chen M, et al. Rosiglitazone affects the progression of surgically-induced endometriosis in a rat model. Mol Med Rep. (2021) 23:35. doi: 10.3892/mmr.2020.11673
61. Pritts EA, Zhao D, Ricke E, Waite L, Taylor RN. PPAR-gamma decreases endometrial stromal cell transcription and translation of RANTES in vitro. J Clin Endocrinol Metab. (2002) 87:1841–4. doi: 10.1210/jcem.87.4.8409
62. Pritts EA, Zhao D, Sohn SH, Chao VA, Waite LL, Taylor RN. Peroxisome proliferator-activated receptor-gamma ligand inhibition of RANTES production by human endometriotic stromal cells is mediated through an upstream promoter element. Fertil Steril. (2003) 80:415–20. doi: 10.1016/S0015-0282(03)00600-9
63. Olivares C, Ricci A, Bilotas M, Baranao RI, Meresman G. The inhibitory effect of celecoxib and rosiglitazone on experimental endometriosis. Fertil Steril. (2011) 96:428–33. doi: 10.1016/j.fertnstert.2011.05.063
64. Sharma I, Dhawan V, Mahajan N, Saha SC, Dhaliwal LK. In vitro effects of atorvastatin on lipopolysaccharide-induced gene expression in endometriotic stromal cells. Fertil Steril. (2010) 94:1639–46.e1. doi: 10.1016/j.fertnstert.2009.10.003
65. Sharma I, Dhawan V, Saha SC, Dhaliwal LK. In vitro effects of peroxisome proliferator-activated receptor-gamma ligands on gene expression in lipopolysaccharide-induced endometrial and endometriotic stromal cells. Fertil Steril. (2011) 95:829–31.e1–5. doi: 10.1016/j.fertnstert.2010.09.008
66. Ohama Y, Harada T, Iwabe T, Taniguchi F, Takenaka Y, Terakawa N. Peroxisome proliferator-activated receptor-gamma ligand reduced tumor necrosis factor-alpha-induced interleukin-8 production and growth in endometriotic stromal cells. Fertil Steril. (2008) 89:311–7. doi: 10.1016/j.fertnstert.2007.03.061
67. Kim CH, Lee YJ, Kim JB, Lee KH, Kwon SK, Ahn JW, et al. Effect of pioglitazone on production of regulated upon activation normal T-cell expressed and secreted (RANTES) and IVF outcomes in infertile women with endometriosis. Dev Reprod. (2013) 17:207–13. doi: 10.12717/DR.2013.17.3.207
68. McKinnon B, Bersinger NA, Huber AW, Kuhn A, Mueller MD. PPAR-gamma expression in peritoneal endometriotic lesions correlates with pain experienced by patients. Fertil Steril. (2010) 93:293–6. doi: 10.1016/j.fertnstert.2009.07.980
69. McKinnon B, Bersinger NA, Mueller MD. Peroxisome proliferating activating receptor gamma-independent attenuation of interleukin 6 and interleukin 8 secretion from primary endometrial stromal cells by thiazolidinediones. Fertil Steril. (2012) 97:657–64. doi: 10.1016/j.fertnstert.2011.12.001
70. Wu Y, Guo SW. Peroxisome proliferator-activated receptor-gamma and retinoid X receptor agonists synergistically suppress proliferation of immortalized endometrial stromal cells. Fertil Steril. (2009) 91:2142–7. doi: 10.1016/j.fertnstert.2008.04.012
71. Kavoussi SK, Witz CA, Binkley PA, Nair AS, Lebovic DI. Peroxisome-proliferator activator receptor-gamma activation decreases attachment of endometrial cells to peritoneal mesothelial cells in an in vitro model of the early endometriotic lesion. Mol Hum Reprod. (2009) 15:687–92. doi: 10.1093/molehr/gap061
72. Lebovic DI, Kir M, Casey CL. Peroxisome proliferator-activated receptor-gamma induces regression of endometrial explants in a rat model of endometriosis. Fertil Steril. (2004) 82(Suppl 3):1008–13. doi: 10.1016/j.fertnstert.2004.02.148
73. Lebovic DI, Mwenda JM, Chai DC, Mueller MD, Santi A, Fisseha S, et al. PPAR-gamma receptor ligand induces regression of endometrial explants in baboons: a prospective, randomized, placebo- and drug-controlled study. Fertil Steril. (2007) 88:1108–19. doi: 10.1016/j.fertnstert.2006.12.072
74. Lebovic DI, Mwenda JM, Chai DC, Santi A, Xu X, D'Hooghe T. Peroxisome proliferator-activated receptor-(gamma) receptor ligand partially prevents the development of endometrial explants in baboons: a prospective, randomized, placebo-controlled study. Endocrinology. (2010) 151:1846–52. doi: 10.1210/en.2009-1076
75. Lebovic DI, Kavoussi SK, Lee J, Banu SK, Arosh JA. PPARgamma activation inhibits growth and survival of human endometriotic cells by suppressing estrogen biosynthesis and PGE2 signaling. Endocrinology. (2013) 154:4803–13. doi: 10.1210/en.2013-1168
76. Wang C, Chen Z, Zhao X, Lin C, Hong S, Lou Y, et al. Transcriptome-based analysis reveals therapeutic effects of resveratrol on endometriosis in arat model. Drug Des Devel Ther. (2021) 15:4141–55. doi: 10.2147/DDDT.S323790
77. Nenicu A, Korbel C, Gu Y, Menger MD, Laschke MW. Combined blockade of angiotensin II type 1 receptor and activation of peroxisome proliferator-activated receptor-gamma by telmisartan effectively inhibits vascularization and growth of murine endometriosis-like lesions. Hum Reprod. (2014) 29:1011–24. doi: 10.1093/humrep/deu035
78. Nenicu A, Gu Y, Korbel C, Menger MD, Laschke MW. Combination therapy with telmisartan and parecoxib induces regression of endometriotic lesions. Br J Pharmacol. (2017) 174:2623–35. doi: 10.1111/bph.13874
79. Yang HD, Zhu QF Li H, Jiang XL, Xu XQ, Guo Y. Effect of neiyi prescription of QIU on autophagy and angiogenic ability of endometriosis via the PPARgamma/NF-kappaB signaling pathway. Arch Gynecol Obstet. (2022) 306:533–45. doi: 10.1007/s00404-022-06537-w
80. Bogacka I, Kurzynska A, Bogacki M, Chojnowska K. Peroxisome proliferator-activated receptors in the regulation of female reproductive functions. Folia Histochem Cytobiol. (2015) 53:189–200. doi: 10.5603/fhc.a2015.0023
81. MacLaren LA, Guzeloglu A, Michel F, Thatcher WW. Peroxisome proliferator-activated receptor (PPAR) expression in cultured bovine endometrial cells and response to omega-3 fatty acid, growth hormone and agonist stimulation in relation to series 2 prostaglandin production. Domest Anim Endocrinol. (2006) 30:155–69. doi: 10.1016/j.domaniend.2005.07.003
82. Cammas L, Reinaud P, Bordas N, Dubois O, Germain G, Charpigny G. Developmental regulation of prostacyclin synthase and prostacyclin receptors in the ovine uterus and conceptus during the peri-implantation period. Reproduction. (2006) 131:917–27. doi: 10.1530/rep.1.00799
83. Nishimura K, Yamauchi N, Chowdhury VS, Torii M, Hattori MA, Kaneto M. Expression of peroxisome proliferator-activated receptor isoforms in the rat uterus during early pregnancy. Cell Tissue Res. (2011) 345:275–84. doi: 10.1007/s00441-011-1208-4
84. Socha BM, Socha P, Szostek-Mioduchowska AZ, Nowak T, Skarzynski DJ. Peroxisome proliferator-activated receptor expression in the canine endometrium with cystic endometrial hyperplasia-pyometra complex. Reprod Domest Anim. (2022) 57:771–83. doi: 10.1111/rda.14121
85. Gunin AG, Bitter AD, Demakov AB, Vasilieva EN, Suslonova NV. Effects of peroxisome proliferator activated receptors-alpha and -gamma agonists on estradiol-induced proliferation and hyperplasia formation in the mouse uterus. J Endocrinol. (2004) 182:229–39. doi: 10.1677/joe.0.1820229
Keywords: endometriosis, peroxisome proliferator-activated receptors, epigenetics, inflammation, vascularization
Citation: Psilopatis I, Theocharis S and Beckmann MW (2024) The role of peroxisome proliferator-activated receptors in endometriosis. Front. Med. 11:1329406. doi: 10.3389/fmed.2024.1329406
Received: 28 October 2023; Accepted: 01 April 2024;
Published: 16 April 2024.
Edited by:
Xin Zhang, Jiangmen Central Hospital, ChinaReviewed by:
Noemi Salmeri, San Raffaele Scientific Institute (IRCCS), ItalyRahul Basu, University of Texas at San Antonio, United States
Copyright © 2024 Psilopatis, Theocharis and Beckmann. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Matthias W. Beckmann, bWF0dGhpYXMuYmVja21hbm5AdWstZXJsYW5nZW4uZGU=
 Iason Psilopatis
Iason Psilopatis Stamatios Theocharis
Stamatios Theocharis Matthias W. Beckmann
Matthias W. Beckmann