- 1Department of Breast Surgery, Longhua Hospital Shanghai University of Traditional Chinese Medicine, Shanghai, China
- 2Graduate School, Shanghai University of Traditional Chinese Medicine, Shanghai, China
Granulomatous lobular mastitis (GLM) is a rare inflammatory breast disease with unknown etiology, characterized by non-caseous granulomatous inflammation of the lobules, which infiltrate lymphocytes, neutrophils, plasma cells, monocytes, and eosinophils may accompany. GLM is often misdiagnosed as breast cancer due to the lack of specificity in clinical and imaging examinations, and therefore histopathology is the main basis for confirming the diagnosis. This review provides an overview of the pathological features of granulomatous lobular mastitis and cystic neutrophil granulomatous mastitis (CNGM, a pathologic subtype of GLM). As well as pathologic manifestations of other breast diseases that need to be differentiated from granulomatous lobular mastitis such as breast tuberculosis, lymphocytic mastopathy/diabetic mastopathy, IgG4-related sclerosing mastitis (IgG4-RSM), nodular disease, Wegener’s granulomatosis, and plasma cell mastitis. Besides, discusses GLM and CNGM, GLM and breast cancer, emphasizing that their relationship deserves further in-depth exploration. The pathogenesis of GLM has not yet been clearly articulated and needs to be further explored, pathology enables direct observation of the microscopic manifestations of the disease and contributes to further investigation of the pathogenesis.
1 Introduction
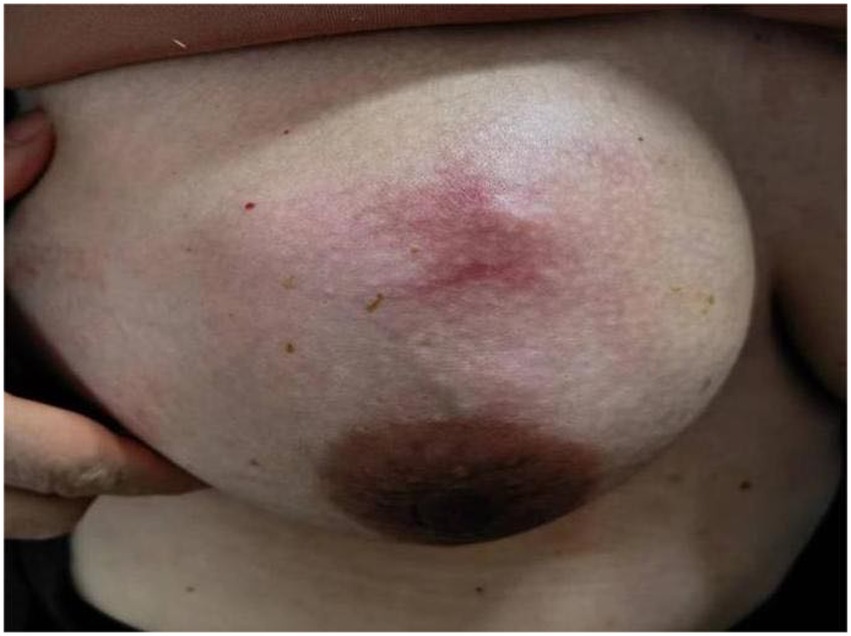
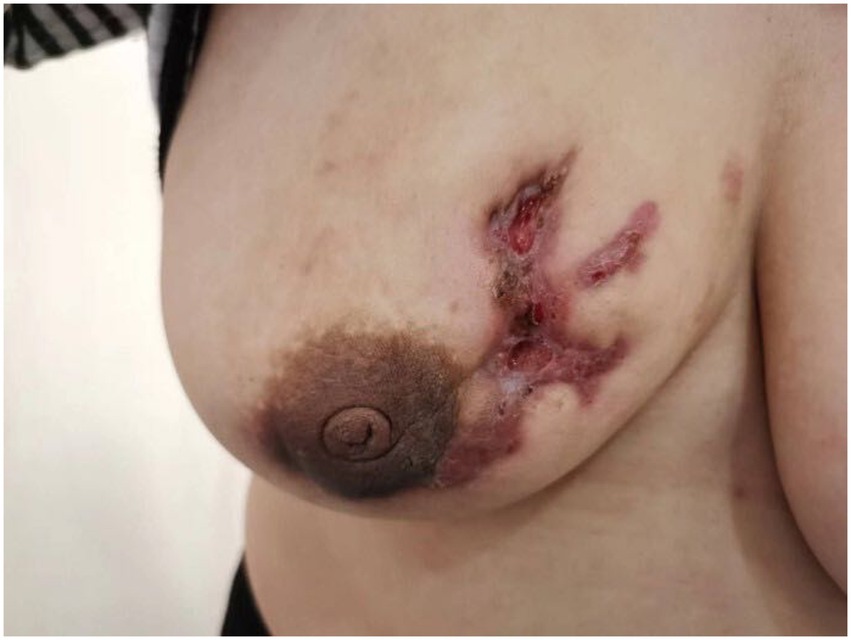
Granulomatous lobular mastitis is a chronic benign inflammatory disease of the breast, predominantly observed in non-lactating women of reproductive age with a history of gestation and lactation (1, 2). More cases were reported in Asia and Mediterranean countries such as China and Turkey (3, 4). The etiology of granulomatous mastitis is still unclear and is currently thought to be related to immunity, bacterial infections, and hyperprolactinemia, besides, labor and lactation, trauma, oral contraceptive pill (OCP) and psychotropic use, alpha1-antitrypsin (AAT) deficiency, and type 2 diabetes mellitus are also thought to be associated with GLM, It may be due to physical or chemical stimuli increased ductal permeability and delayed-type hypersensitivity caused by spillage of secretions such as milk from the ductal lumen (2, 5, 6). GLM presents a unilateral (or occasionally bilateral) painful lump in the breast (Figure 1). Initially, the skin may be red or unchanged in color, but the lump gradually becomes septic, involving the skin and forming a deep sinus tract or ulcerated surface (Figure 2). Some patients may have sunken nipples and swollen axillary lymph nodes or have extramammary symptoms such as fever, joint pain, and nodular erythema of the lower limbs. Common sequelae include scarring, retraction of skin and nipple, and even shrinkage of the entire breast, which affects the quality of life in young women (7). The imaging presentation of granulomatous lobular mastitis is nonspecific. On ultrasound, it showed multiple irregular hypoechoic masses, tubular connections, angular margins, hyperechoic rim, internal vascularity, and fistulae, tiny flowing spots of light when an abscess forms (Figures 3–5) (8–10), could accompanied by skin thickening, subcutaneous edema, and reactive hyperplasia of axillary lymph nodes (11). MRI specifically shows peripherally enhancing fluid or solid masses (Figure 6) (12). Current treatment focuses on observation, application of antibiotics, steroids, immunosuppressants, surgery (13–16), and traditional Chinese medicine (17, 18), lack of standardized treatment protocols. Recurrence rates vary between treatments (5, 19, 20). PRL levels, overweight, FSH/LH, and Corynebacterial infection had an association with GLM recurrence (21, 22).
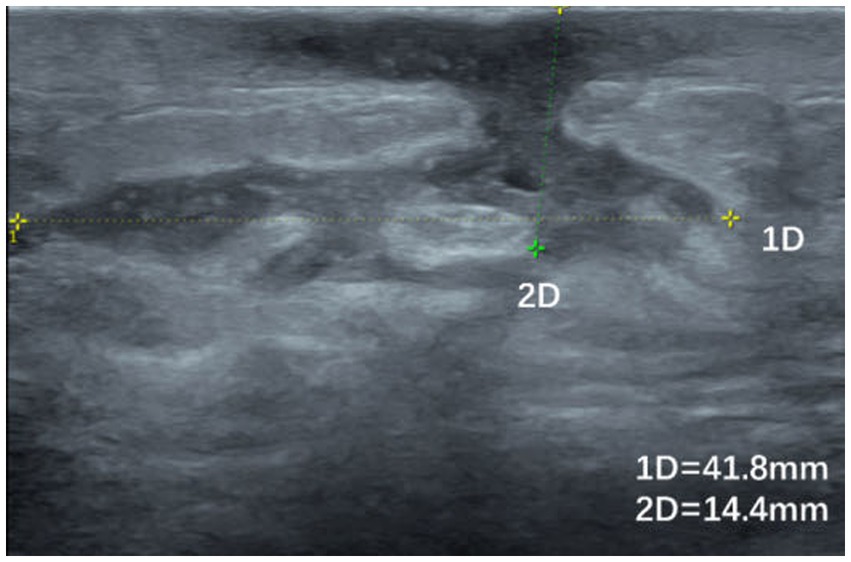
Figure 3. Ultrasound image of a patient with GLM with hypoechoic areas leading to the skin and sinus tract formation.
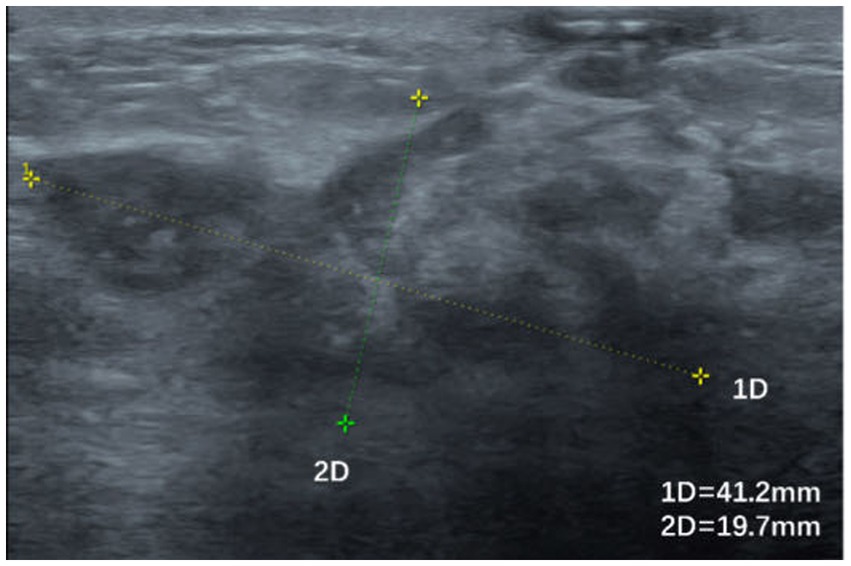
Figure 4. Irregularly shaped hypoechoic area with poorly defined borders and inhomogeneous internal echoes.
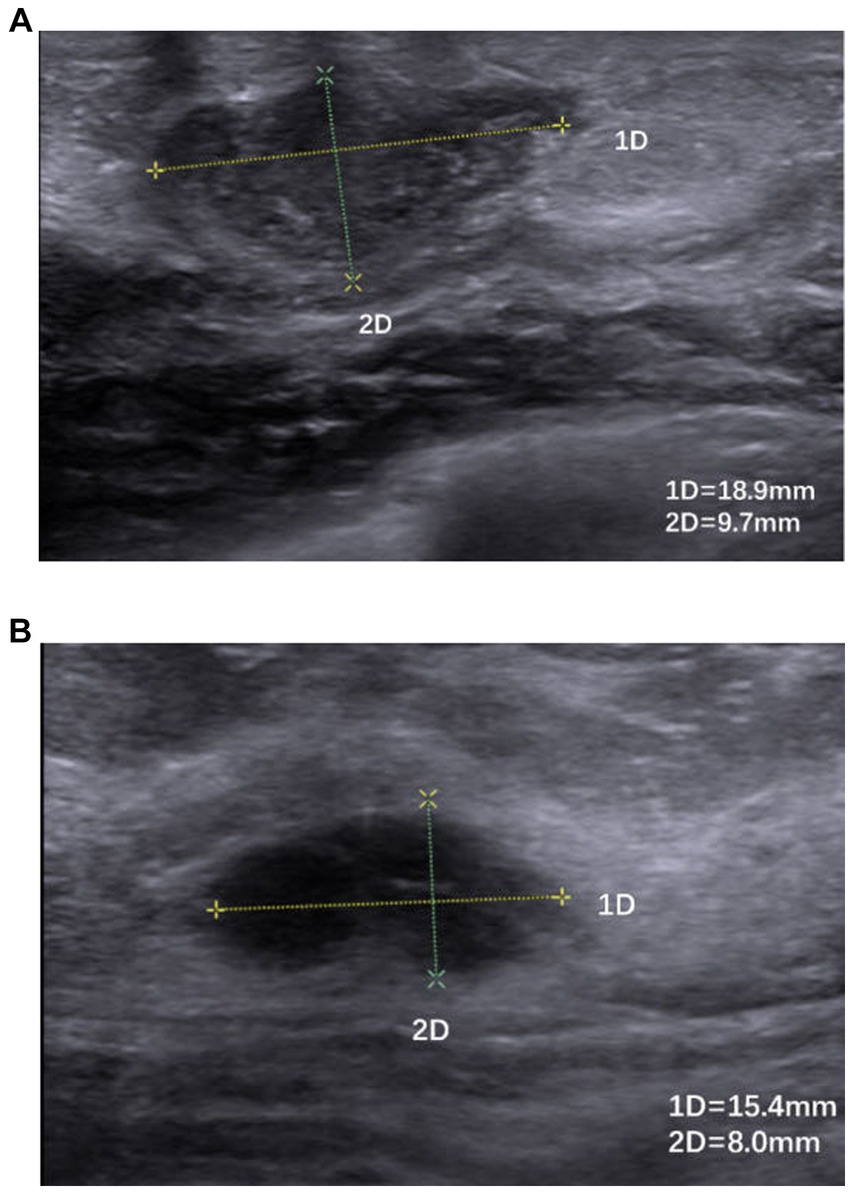
Figure 5. (A,B) Hypoechoic nodule with poorly defined borders, irregular morphology, and inhomogeneous internal echoes.
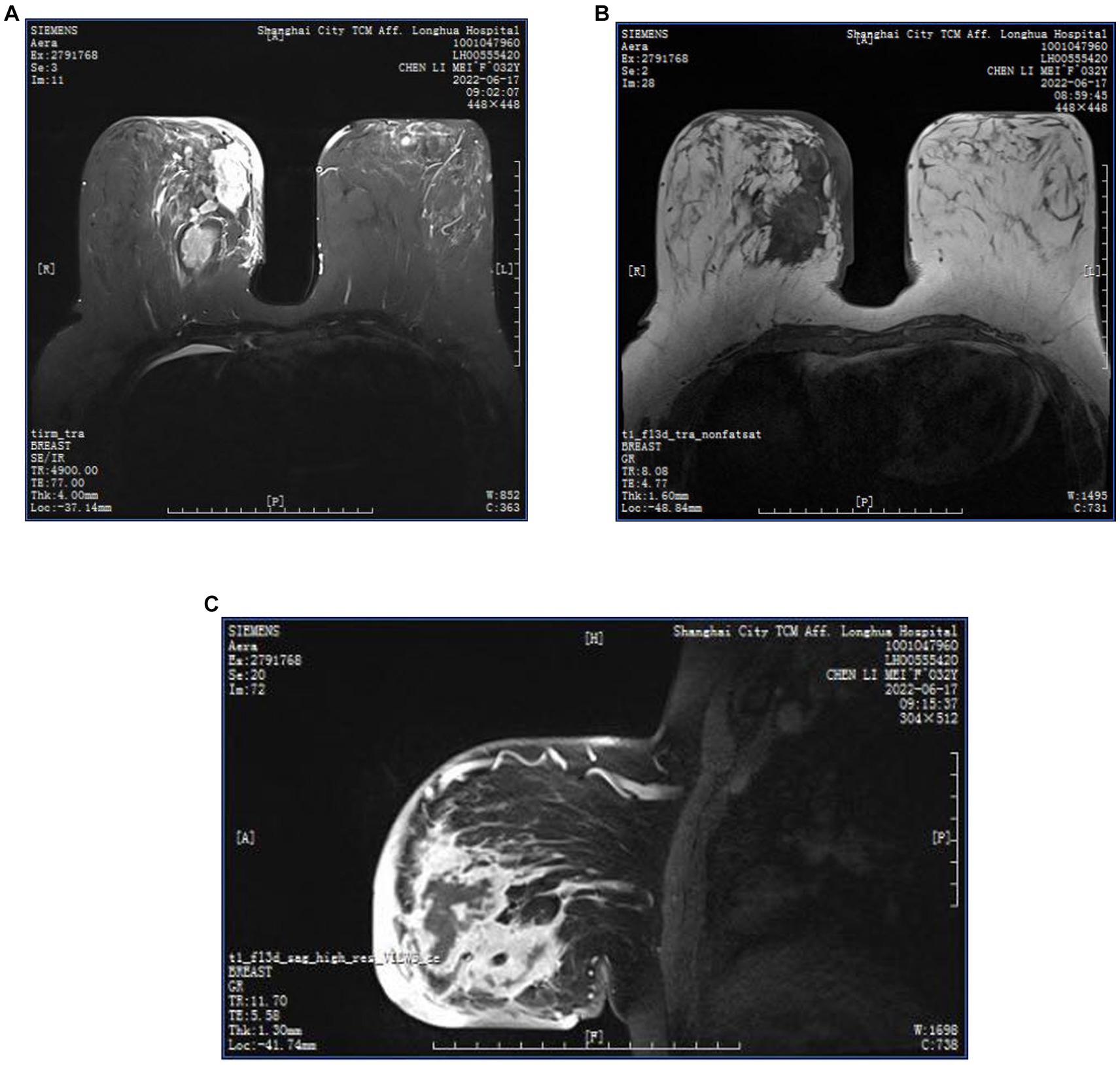
Figure 6. (A–C) MRI images of a GLM patient, multiple regionally distributed non-mass enhancing foci in the outer upper and inner upper quadrant of the left breast, inner upper and inner lower quadrant of the right breast, T1WI iso-slightly high signal, T2WI high signal, oedema around the foci, clusters of ring enhancement, thickening, and enhancement of the skin of the right inner breast, subcutaneous oedema. Multiple axillary lymph nodes on both sides, partially enlarged, with visible lymphatic gates.
The fact that the diagnosis is almost based on pathology, the difficulty in treatment, prolonged disease duration, and the high recurrence rate make GLM a refractory benign disease. It is crucial to diagnose this disease correctly as it can lead to different treatment options, especially since it is difficult to differentiate GLM from breast cancer. Pathology as the gold standard for diagnosis, is the most intuitive and convenient way to access the microscopic manifestations of the disease. This narrative review provides an overview of the pathologic manifestations of GLM and common differential diagnoses, discussing the relationship between GLM and CNGM, and the relationship between GLM and breast cancer, exploring the pathogenesis of GLM in terms of bacterial infections and inflammation-cancer theories.
2 Methods of obtaining pathologic tissue
The three main methods of obtaining pathologic tissue in breast disease are fine needle aspiration, core needle biopsy, and surgery. Fine needle aspiration has an irreplaceable role in determining the benignity and malignancy of the disease and in obtaining bacterial culture material because of its convenience and low invasiveness (23), However, the amount of pathological tissue extracted is not sufficient and the presence of granulomas may be missed, or it may not be sufficient to determine whether the inflammation is mainly centered on the lobules of the breast (24). The core needle, although more tissue can be obtained than fine needle aspiration, is not sufficiently diagnostic (inflammation) (25) or difficult to distinguish from tuberculosis mastitis (TB) (26). In view of its convenience, histopathology obtained by core needle biopsy or surgery is clinically recommended as a diagnostic source.
3 Histopathological manifestations of GLM
3.1 Gross examination
The lesions seen in general are mostly ill-defined, tough or hard, a few are soft and hard, a few are brittle, irregularly solid or cystic in section, grayish white or grayish red or grayish yellow, with dark red or yellow corn-like nodules, scattered with multiple pus cavities/pitting foci of necrosis of varying sizes, and some describe the cystic area as decaying (27). The cystic areas contain grayish, grayish-yellow, or grayish-brown secretions, with sinus tract formation visible on the cut surface (25, 28–32). It has been described as a decaying cystic area containing gray, grayish-yellow, or gray-brown secretions, with sinus tract formation on the cut surface.
3.2 Microscopic presentation
3.2.1 Typical microscopic presentation of GLM
Microscopically, GLM is a non-caseating granulomatous inflammation of the ductal units of the lobules/terminal duct lobular unit, which may involve multiple lobules and may be associated with microabscesses (33, 34). The background is also infiltrated with inflammatory cells, mainly lymphocytes, neutrophils, plasma cells, monocytes, and eosinophils (Figure 7).
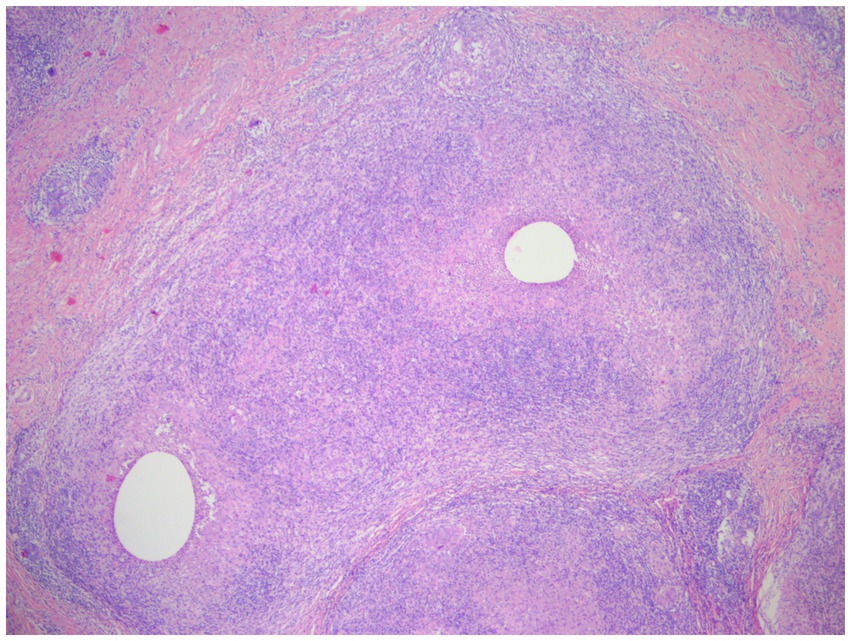
Figure 7. Two lesions with non-caseating necrotizing granulomatous lesions are partially fused, each with a vesicle visible in the center, and the normal tissue structure is disappearing. Visible epithelioid cells, and occasionally giant cells, acute and chronic inflammatory cell infiltration (lymphocytes, plasma cells, neutrophils) (H&E, ×40 magnification).
The lobular focus of granulomas can sometimes be masked and therefore makes the differential diagnosis difficult, mainly because (i) granulomatous tissue gradually replaces some or even all alveolar tissue, and lobular lesions are obscured and can fuse to form large lesions (35, 36). (ii) In the advanced stages, abscesses form, areas of necrosis expand, septic inflammation predominates and lobular structures fuse and disappear. (iii) Or small granulomas may form between lobules in the interstitium around the lesion, with a large number of microabscesses obscuring the granulomas, or even partially fused lesions with no visible granulomas (32). Tariq et al. pointed out that in the early stages of GLM, when suppuration is mild, the lobular structures are preserved in their entirety in the sections and are easily identifiable, which better defines classic GLM, but in the abscess phase the lobules gradually fuse, and in the late ulcerative stage the lobules fuse into a sheet, and in the more advanced stage the ducts are heavily necrotic and the lobular structures are eliminated, making them difficult to identify, with a small number of fibroblasts and neovascularisation visible, granulation tissue formation, and the formation of resorbing cystic vacuoles more easily seen than in the first two stages, with septic inflammation is predominant, creating a distinctive appearance of CNGM (28, 35, 37). Tariq et al. prefer that CNGM is a later stage of GLM rather than a subtype of GLM as most people believe. Associated with the close association of CNGM with Corynebacterium, this seems to point to the etiology of GLM as a bacterial infection.
Microabscesses are composed of neutrophils surrounded by epithelioid cells and monocytes and can be found in the lobular and ducts, in a few cases the entire epithelial lining of the duct is disrupted and replaced by inflammatory cells (38, 39). When larger abscesses or multiple small abscesses are formed, the lesions are more extensive and the inflammatory cell infiltration is more pronounced, involving the fat and skin, with skin breakdown (32, 35).
Multinucleated giant cells are predominantly Langhans-type giant cells with horseshoe-shaped nuclei, but a few foreign body giant cells can also be seen, and both can be present at the same time (23, 40). Ail DA believes that foreign body giant cells are more common in GLM and differentiates it from tuberculosis mastitis with a high number of Langhans-type giant cells (41). Ail DA suggests that foreign body giant cells are more common in GLM, and thus differentiates it from tuberculosis mastitis with more Langhans type giant cells. No phagocytosis in multinucleated giant cells (30). The presence of multinucleated giant cells in the ducts is sometimes associated with penetration (38). Eosinophilic infiltration is usually rare and variable in number (42, 43). Lacambra et al. found large numbers of eosinophil infiltrates, presumably related to the flow of protein secretions into the lobular stroma (44). Plasma cells are uncommon, accounting for essentially no more than 35%, and may be associated with mild to moderate lymphocytic vasculitis (40, 45, 46). Lymphocytic infiltration is common (47), and normal breast lobules also contain myeloid and lymphoid cells. In the study of Tse et al. (40), lymphocytes accounted for the majority of inflammatory cells (≥65%), with a lower proportion of neutrophils mostly accounting for less than 35%, and a large plasma cell infiltrate has also been seen (44, 48). In granulomatous lesions, neutrophil aggregation bands have more peripheral CD3+ lymphocytes than CD20+ lymphocytes (49). Anousha et al. suggest that granulomas, lobular central inflammation, and neutrophil infiltration are the pathological triad of GLM (50). This may be related to the different clinical staging of the obtained GLM tissue, with Yu et al. noting that the microscopic neutrophil count varies from the mass stage to the abscess stage to the post-ulcerative stage, and can vary from rare to numerous neutrophil infiltrates (28). Few researchers have described the pathological features of GLM according to clinical stage, so it is often difficult to detect microscopic patterns of GLM in terms of the way in which the various cell types are arranged and the changes in their proportions.
3.2.2 Typical GLM accompaniment and merging
GLM is often associated with mammary duct ectasia (MDE), with inflammation in or around the ducts, but the inflammatory response is usually unremarkable. In the study by Ling Chen et al., the percentage of GLM merged with PDM reached 5.3%, they hypothesized that this was due to the involvement of the ducts by lesions in the lobules (49). The ducts are often lined with secretions, inflammatory exudates, exfoliated epithelium, and foamy histiocyte collections, with hyperplastic degeneration and focal exfoliation of the ductal epithelium, and fibrous thickening of the ductal wall, surrounded by a distinct lymphoplasmacytic infiltrate. The fusion lesions are mainly between GLM lesions, whereas fusion lesions between mammary duct ectasia and GLM are rare (51). There may also be subacute or chronic inflammation of the interstitium, combined fibromatous nodules/fibroadenomas, combined intraductal papillomas, combined fibrocystic changes/cystic hyperplasia, combined sclerosing adenopathy, cholesterol crystals and calcification (25, 43, 46). Metaplasia apocrine may be present (52). There may be mild fibrosis of the interlobular stroma surrounding the lesion (30). There may be mild fibrosis of the interlobular stroma surrounding the lesion. Fat necrosis may be seen (42, 50). Squamous epithelial metaplasia may occur in both lobules and ducts (23).
3.2.3 Atypical microscopic presentation of GLM
Naik et al. suggest that one of the new features of CNGM is basophilic fibrous material surrounded by inflammatory cells and giant cells (38). This feature has not been described by others and its significance is unknown. Other uncommon features are caseous necrosis, marked eosinophil infiltration, non-granulomatous inflammation, and foamy histiocyte infiltration, granulation tissue formation (48, 50).
3.2.4 GLM atypically presents as a combination of other diseases
Sometimes GLM is not isolated, Choi SH showed an example of an initial right breast lesion diagnosed as GLM and a secondary lesion in the opposite breast diagnosed as tuberculosis mastitis 5 months later, where the patient had a positive TBC-PCR (polymerase chain reaction for tuberculosis) and the mass completely disappeared after 12 months of anti-tuberculosis treatment (53). GLM can also be combined with atypical manifestations or/and malignancy, Çalış H reported a case of GM combined with breast cancer (54). Özşen M reported 2 cases of ductal carcinoma in situ associated with lesions detected in patients with recurrent GLM (52). Sometimes breast cancer can also cause inflammation and look like GLM (50, 54). This tells us to be careful in identifying GLM even when pathological tissue is obtained, and that as much breast tissue as possible will be better for a clear diagnosis, which is why we do not recommend fine needle aspiration.
4 Histopathological manifestations of CNGM
4.1 CNGM microscopic presentation
CNGM is now considered a specific subtype of GLM with distinctive histopathological features, possibly associated with Corynebacterium, microscopically appears as a purulent lipid granuloma, surrounded by a central vacuole (cystic space), usually thought to be formed by lipolysis, with the size of several merged adipocytes (200–800 μm), surrounded by neutrophils, the thickness of the edge of which can be thin or thick (Figure 8). The number of neutrophils varies and the thickness of the rim surrounding the vesicle can be thin or thick, thus forming a microabscess in the granuloma, which is then surrounded by histiocytes, variable numbers of lymphocytes, plasma cells and Langhans giant cells, forming a definite granuloma (37, 55, 56). Sometimes granulomas can be poorly formed (57). However, not all granulomas have cystic vesicles, and neutrophilic inflammation and microabscesses can be seen outside the granuloma, and more often the granulomas fuse with each other to obscure the lobule-centered distribution characteristic of the granuloma (55). There are also cases where there are microorganisms in the lipid vacuoles surrounded by neutrophils but no granulomas. Gram-positive bacilli (GPB) are sometimes seen in the vesicles (Figure 9), apparently rod-shaped, and some of the bacilli are internally beaded or dendritic, arranged in a fenestrated pattern, forming wedge-shaped features (36, 45, 47). The microcystic vacuoles may sometimes be in clusters (38). Although GPBs are not always visible in the vesicles, they are confined to the interior of the lipid vacuoles in identifiable cases (24). Immunohistochemistry is increasingly used in the diagnosis and differential diagnosis of difficult breast lesions and is also the main means of molecular typing of breast cancer and screening of precise therapeutic markers, but in GLM and CNGM, in addition to identifying IgG4-related sclerosing mastitis (IgG4-RSM), its specific role is still unclear and lacks clinical significance (Figures 10–13).
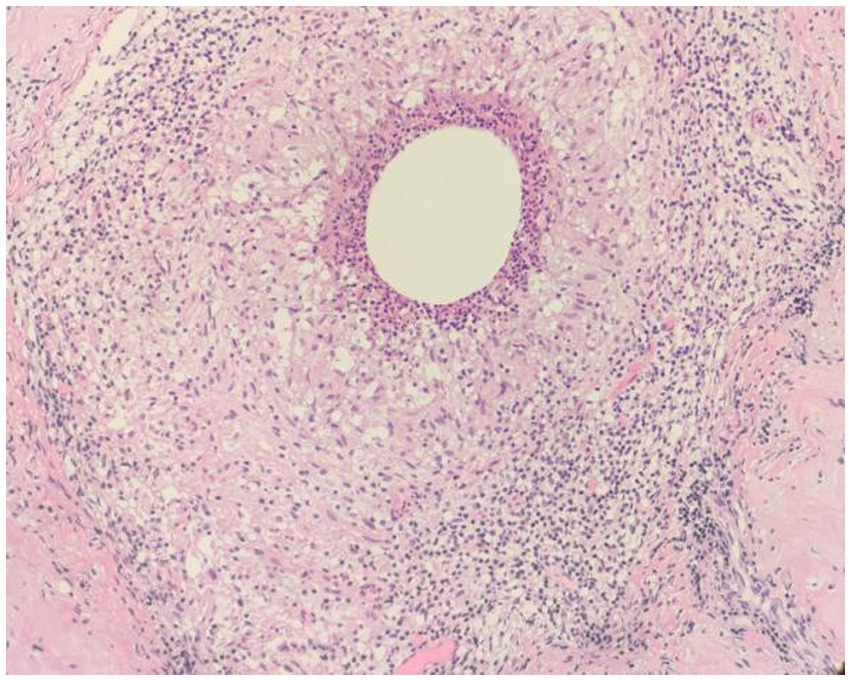
Figure 8. Examples of CNGM, granuloma formation from epithelioid cells, with a central vesicle surrounded by neutrophil accumulation. Infiltration of peripheral lymphocytes, plasma cells, neutrophils and a few eosinophils (H&E, ×200 magnification).
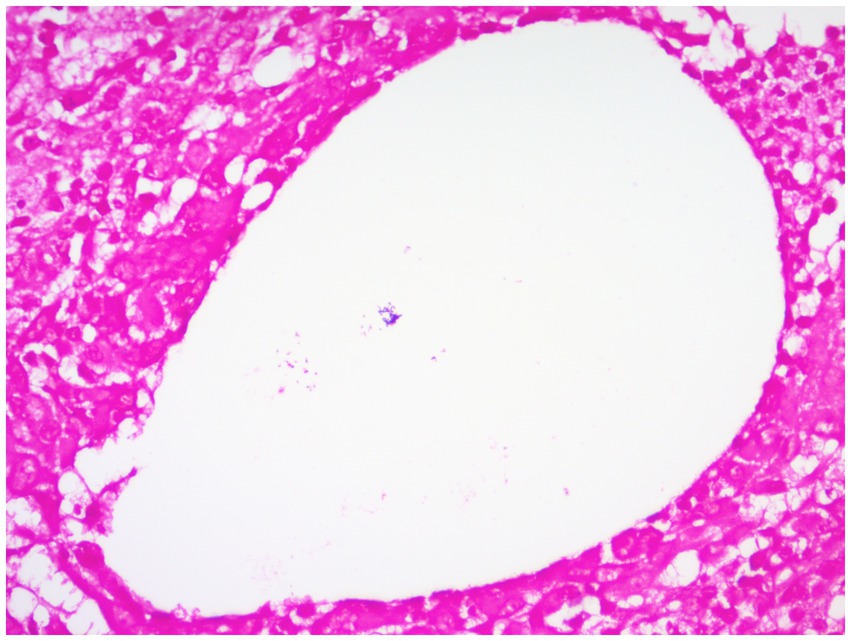
Figure 9. Gram-positive coryneform bacteria arranged in clusters stained purple can be seen in blank lipid vesicles (Gram stain, ×400 magnification).
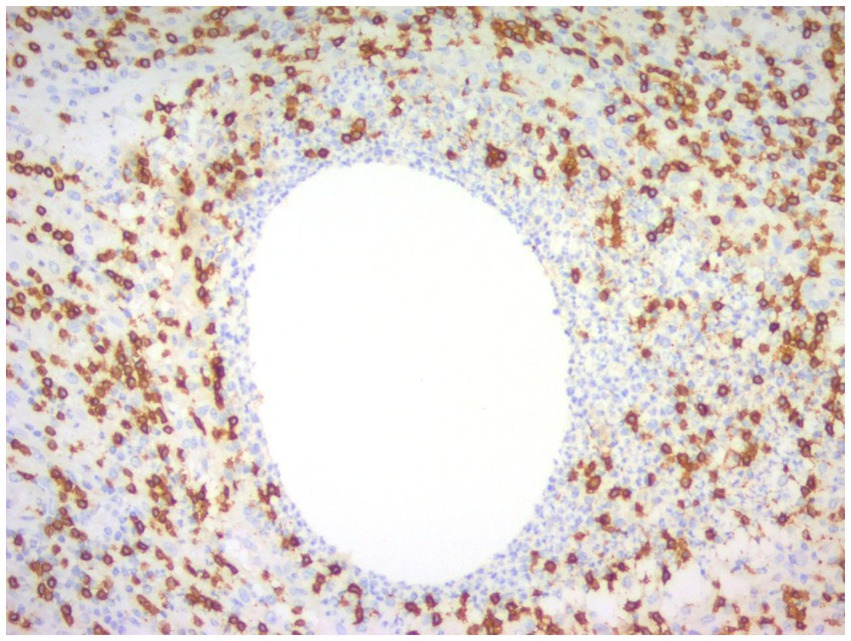
Figure 10. Immunohistochemical picture of CNGM with CD3-positive T lymphocytes stained brownish yellow and distributed at the periphery of the neutrophil aggregation band (En Vision, ×200 magnification).

Figure 11. Immunohistochemical picture of CNGM, CD20-positive B lymphocytes were stained brownish yellow and distributed in the periphery of the neutrophil aggregation band, and their location was more peripheral compared to CD3-positive T lymphocytes (En Vision, ×200 magnification).
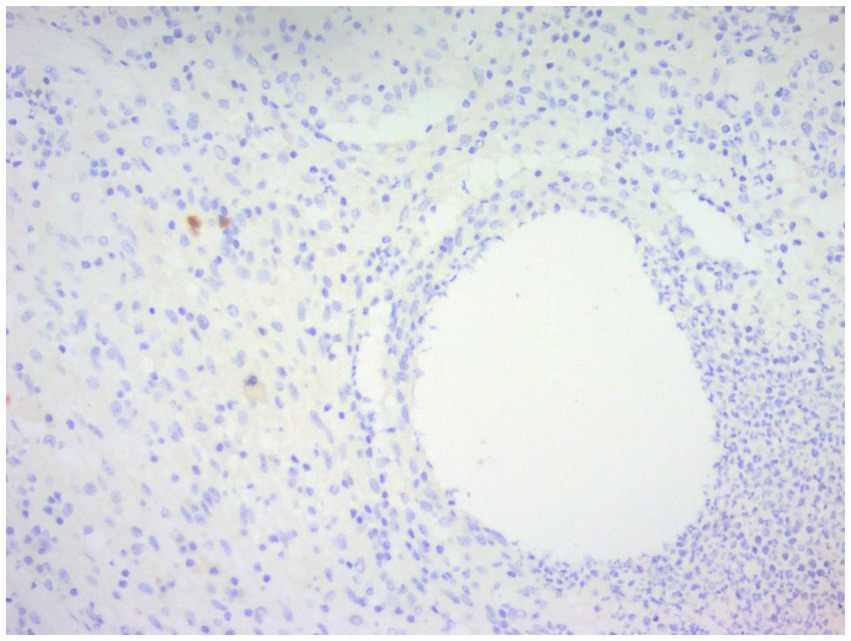
Figure 12. Immunohistochemical staining of CNGM, a few IgG4-positive plasma cells stained brownish-yellow can be seen around the vesicle (En Vision, ×200 magnification).
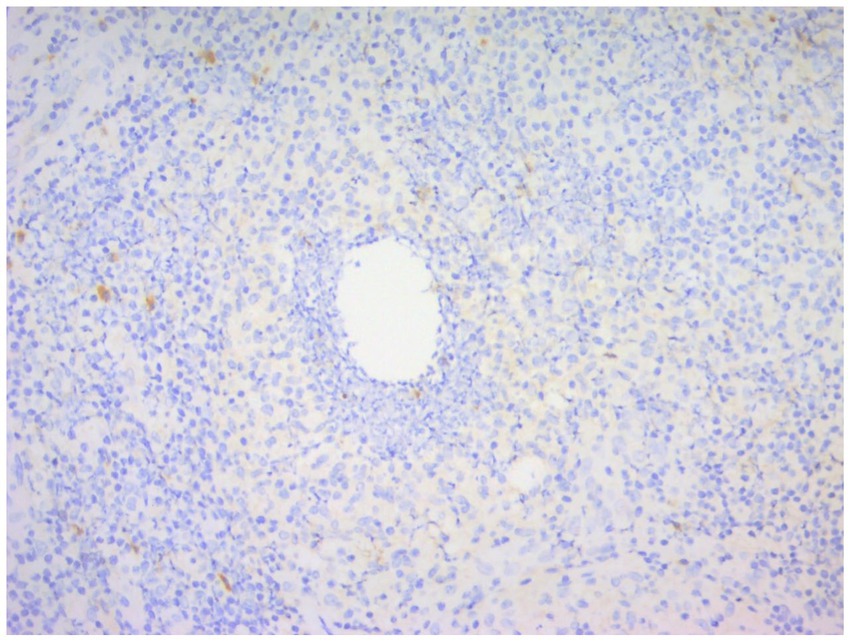
Figure 13. Immunohistochemical staining of CNGM, a few IgG-positive plasma cells stained brownish-yellow can be seen around the vesicles (En Vision, ×200 magnification).
4.2 Corynebacterium and CNGM
Gram-positive bacilli in lipid vacuoles are currently dominated by Corynebacterium, with the most common isolate being C. kroppenstedtii, followed by C. amycolatum and C. tuberculostearicum (50, 58). Other pathogens such as P. oleovorans, human gammaherpesvirus 4, A. baumannii, T. thermophilus are likely to be closely related to GLM (48). Staphylococcus epidermidis, Staphylococcus aureus, Pseudomonas aeruginosa etc. have also been cultured (59). There are also cases of mixed infections of Corynebacterium with other bacteria, or Corynebacterium is not the predominant pathogen (60, 61). The abundance of Corynebacterium in GLM patients showed inter-individual variability (62). The pus samples are more diverse than their skin samples (63) and are more abundant than in tissue samples. In a study by Wen Chen et al., the samples from 34.1% GLM patients had a Corynebacterium abundance of >1% (1.08–80.8%), with 53.3% displaying an abundance of >10% (64). Pathogen discovery will help guide clinical treatment. Corynebacterium was first isolated in 1998 from a human sputum sample (65). Lipophilic antibiotics may be more effective in treating Corynebacterium-associated breast infections, such as rifampicin, clarithromycin, and methotrexate-sulfamethoxazole (66). Other sensitive drugs such as vancomycin and gentamicin may also be used as adjunctive therapy (67, 68).
Positive bacterial cultures for Corynebacterium are most often seen in the abscess and refractory types (the two types are not statistically different) (35, 37). Patients with CNGM are younger, have larger masses, are more likely to be painful, febrile or with high neutrophils, form sinus tracts, and are more likely to recurrence (24, 69, 70). In the study by Tan QT et al. the risk of recurrence 2.64 times higher in patients with Corynebacterium infection (71). This may confirm the role of Corynebacterium in GLM in promoting abscess formation.
In fact, CNGM may be underdiagnosed because (i) Gram staining is usually limited to one or a few sections of formalin-fixed paraffin-embedded. (ii) Not all vesicles contain GPB. (iii) Corynebacterium are difficult to culture, whether the microbiological finding should be part of the diagnostic criteria remains debatable (72).
Based on nanopore sequencing and bacterial culture, Xin-Qian Li et al. find that the bacteria positive rate and vacuoles positive cases in the early stage (only hard mass) was significantly higher than that in late stage (medium/soft mass, with skin inflammation, abscess, fistulas or ulcers), the detection rate of bacteria in the early stage of GLM was over 80% and the dominant bacteria were Corynebacterium species (64%). Thus they speculate that C. kroppenstedtii may be initially present in the breast tissue at the early stage (73). Tariq H considers the early identification of Corynebacterium in deep breast tissue, causing peripheral granulomas and purulent inflammation, to be strong evidence of Corynebacterium pathogenicity (37).
However, the idea of Corynebacterium as the cause of GLM is still being questioned due to the negative microbiological cultures, the poor response to antibiotics, improvement of symptoms with cortisol or immunosuppressive drugs, the concomitant erythema nodosum of the lower limbs in some patients, and the self-limiting nature of GLM, which tends to be an autoimmune disease. And it remains controversial whether CNGM, as a pathological subtype of GLM, belongs to two different diseases from non-CNGM GLM, the relationship between GLM and CNGM needs further clarification.
Additionally, the classification of the period attributed to the patient’s disease in future research needs to be further refined, cause not all patients clinically undergo the process of abscess formation, and all patients with abscesses do not present it at the same period, in addition to the type of mass, the duration of the presence of the mass type, and the impact of treatment methods needs to be taken into account.
5 Techniques used in the diagnostic process of GLM, CNGM
5.1 Differential diagnosis of GLM from other diseases by staining
As an exclusionary disease, GLM is not associated with infectious diseases, other immune diseases, etc. Wu JM believes that once malignancy is excluded, infectious factors causing granulomatous mastitis should be considered and all cases should be subjected to special staining and microbiological cultures to exclude bacterial, fungal, parasitic and other sources of infection; especially in areas where tuberculosis is endemic, breast tuberculosis should be kept in doubt (72). In addition to the usual Gram stains the main ones are silver hexamine and Schiff periodate stains to exclude fungal infections, antacid stains (Wade-Fite, Ziehl-Neelsen), PCR tests, molecular tests for Mycobacterium tuberculosis to exclude Mycobacterium tuberculosis/Nontuberculosis mycobacteria infection (36, 57, 74, 75).
5.2 Methods for detecting Corynebacterium
Corynebacterium is generally detected by Gram staining, but its staining sensitivity is low in paraffin sections, and in a study by Tariq H et al., the Gram staining positivity of paraffin sections of Corynebacterium was significantly lower than that of PCR (17.9% vs. 68.7%) (76). Sangoi AR et al. found that cutting Gram-stained tissue sections of CNGM cases to a thickness of 6 μm instead of the traditional 4 μm increased the detection rate of Gram-positive bacilli (77). However, even with PCR testing, as only one section is selected per case, sampling may be falsely negative, and there is attrition of lesion tissue during processing, and dewaxing and DNA extraction may result in loss of cystic space contents, so Tariq H strongly recommends obtaining fresh biopsies for microbiological studies in suspected GLM cases, not only to improve PCR detection rates, but also to allow for culture. This would not only improve PCR detection but also allow antimicrobial susceptibility testing of positive cases, which would hopefully improve clinical outcomes with targeted antibiotics (76). Zhu Yongze et al. concluded that as long as a single Corynebacterium is isolated from a sterile site specimen in culture, it should be treated as pathogenic, even if no Gram-positive bacilli are seen in the original smear and only a certain number of leukocytes are present (58). The culture of Corynebacterium is now mostly done with blood plate, blood plate +1% Tween 80, extended incubation time and retention of tissue around the pus can increase the positive culture rate of Corynebacterium (59). The rate of positive culture of Corynebacterium can be increased by prolonging the culture time and leaving the tissue around the pus.
The more advanced methods for the identification of pathogenic organisms in CNGM include 16SrRNA gene sequencing, matrix-assisted laser-resolved ionization time-of-flight mass spectrometry (MALDI-TOF MS), rpoB gene sequence amplification by PCR, Sanger sequencing techniques (37, 38, 62, 67).
6 Differential diagnosis of GLM
6.1 Breast cancer
As the patient’s presentation is similar to breast cancer both clinically and on imaging, breast cancer becomes the primary diagnosis to be differentiated. The use of core needle biopsy or surgery to obtain pathological tissue is recommended. As the incidence of GLM has been increasing in recent years, cases of GLM in combination with breast cancer have gradually emerged. The diagnosis of breast cancer could be delayed because GLM has a long course and varied clinical presentation, its inflammatory features could have masked the presence of breast cancer until the clinical and imaging manifestations continued to suggest the risk of breast cancer or a new lump was found, which was then taken seriously by the clinician, lead to a new round of pathological biopsy (78–80).
Inflammation has now been progressively shown to be an important factor in tumor progression, chronic inflammatory responses promote cell division and repair, creating an environment that stimulates cancer growth and progression (81). Mammary ductal epithelial cells of GLM and plasma cell mastitis (PCM) showed injury and apoptosis, and MAC (C5b-9n) was mainly located on their cell membrane (82). Compared to healthy tissues, IGM tissues have elevated levels of both immune system and cancer-related proteins (83).
Currently, some large samples have shown that patients with mastitis have an increased risk of developing breast cancer and that mastitis can be considered a risk factor for breast cancer (84, 85). Further, respectively, observational research is needed on the relationship between lactational mastitis, GLM, and breast cancer, and specific mechanisms need to be explored.
6.2 Breast tuberculosis
There is a lot of histological overlap between GLM and tuberculosis, with granulomas and giant cells present, and it is particularly easy to misdiagnose on the basis of cytologic features alone, especially with a small number of fine needle punctures. Unlike the granulomas of GLM, which are centered on the lobules of the breast, the granulomas of TB distribution are irregular. The granulomas of GLM can be septic and necrotic, but TB is more prone to necrosis, especially the characteristic caseous necrosis (26). Ail DA et al. noted that Langhans giant cells are common in tuberculosis of the breast, whereas foreign body giant cells are common in GLM (41). However, most scholars have observed that Langhans cells are in fact not a minority in GLM (23, 40, 55). The predominance of neutrophils in the inflammatory infiltrate also contributes to the diagnosis of GLM (86). neutrophils and vesicles in TB are uncommon. Positive bacterial culture and Z-N staining is the gold standard for the diagnosis of TB, but the sensitivity is low and can be found by PCR for Mycobacterium tuberculosis (26, 41).
6.3 Lymphocytic mastopathy/diabetic mastopathy
Usually the patient has diabetes mellitus (especially type I) or other autoimmune deficiency, and the mass is firm and irregular, with a white, homogeneous solid section, and microscopically shows a lymphocytic infiltrate of mainly B lymphocytes in the lobules, periductal and perivascular areas (87–89). Lobular inflammation is more common in women, with widespread ductal inflammation, atrophy of the ducts and thickening of the basal lamina, and vasculitis involving mainly small and medium-sized vessels. It is associated with marked interlobular fibrosis, scar vitrification, epithelial fibroblasts, and lymphatic nodule formation with or without germinal centers (34, 90). Tomaszewski JE et al. suggest that epithelioid fibroblasts are unique to diabetic patient (91).
6.4 IgG4-related sclerosing mastitis (IgG4-RSM)
IgG4-related sclerosing mastitis is characterized by the formation of dense lymphoplasmacytic infiltrates, lymphoid follicle formation, occlusive phlebitis, extensive sclerosis, or fibrosis with at least a localized stellate distribution, with marked stromal sclerosis and loss of breast lobules (47, 72, 87). Immunohistochemical staining reveals large numbers of IgG4+ plasma cells and elevated IgG4 serum concentrations, but Goulabchand R et al. argue that in fact increased numbers of IgG4+ plasma cells are only part of the classical histological presentation and are not necessary for the diagnosis, nor does the diagnosis of GLM require consideration of the number of IgG4+ plasma cells or the IgG4:IgG ratio or the presence of other histological features of IgG4 -RSM (47, 92). Ogura K et al. have classified GLM into IgG4-associated and non-IgG4-associated subtypes (92). Interestingly Kong C et al. found that nipple invagination is a necessary basis for differentiating between IgG4-related and non-IgG4-related GLM, suggesting that the pathogenesis and immune mechanisms of the two diseases may be different and that the required treatment may be different (93).
6.5 Lupus mastitis
Lupus mastitis is characterized by lymphocytic lobular lipofuscinosis with plasma cell and hyaline fat necrosis (34). Lymphocytic infiltration may be nodular, diffuse, periductal and/or perifollicular and germinal center, and lymphocytic vasculitis is also common, involving mostly small and medium-sized vessels. Immunohistochemistry shows a mixed population of T and B lymphocytes, mainly CD3+ and CD4+ T cells mixed with CD20-positive B cells and polyclonal plasma cells (94).
6.6 Nodular disease and Wegener’s granulomatosis
When granuloma is present in conjunction with lymphocytic vasculitis, it needs to be differentiated from Wegener’s disease and nodular disease. Both nodular disease and Wegener’s granulomatosis affect mainly small and medium-sized vessels, the lesions are not lobularly centered, and both have necrotizing vasculitis and thrombosis, with a few cases of necrosis. Wegener’s disease is characterized by necrotizing vasculitis and granulomatous inflammation, mainly affecting the upper and lower respiratory tract and the kidneys (47). Nodular disease results in well-defined epithelioid nodules with microscopic lymphocytic infiltration in the vessel wall, but without neutrophil infiltration, and rarely vasculitis and fatty necrosis (25). Breast nodular disease is often secondary to generalized nodular disease, invading the dermis, with microscopic clusters of epithelioid cells of variable size, rarely extending into the subcutaneous tissue, and without neutrophil infiltration (39).
6.7 Plasma cell mastitis/periductal mastitis (PDM)/mammary duct ectasia (MDE)
Plasma cell mastitis is a late stage in the development of ductal dilatation of the breast or accompanies it, but is not an inevitable part of it. It mainly invades the large ducts behind the areola (especially within the 2 cm ring of the areola) (95). The affected ducts are highly dilated and the duct lumen contains secretions, exfoliated epithelium and foamy histiocytes. The duct wall is fibrotic and thickened, with atrophy of the epithelial cells of the duct wall. The duct is surrounded by a large infiltrate of diffuse lymphocytic plasma cells and other inflammatory cells, with a predominance of plasma cell infiltration. The ductal or lobular structures are frequently obscured or distorted (29, 34, 96). Necrosis may occur in the later stages and the masses are often interspersed with lipid-like (pimple-like) material after they have broken down (25). The mass is often interspersed with lipid-like material after rupture. Although GLM and ductal dilatation are pathologically distinct, they can occur together, and PDM can also present with granulomas and chronic purulent inflammation (75). The two can be difficult to distinguish when GLM is associated with dilated ducts and high plasma cell infiltration. The fusion lesions are mainly between GLM lesions, but fusion between ductal dilatation and GLM is rare (51). Cholesterol crystals and calcifications may also be present in MDE and are not statistically different from GLM (46).
6.8 Summary and outlook
Granulomatous lobular mastitis is a non-caseating granulomatous inflammatory disease occurring in the lobules of the breast. Granulomas are composed of epithelioid cells and multinucleated giant cells with an infiltration of inflammatory cells such as lymphocytes, neutrophils, plasma cells, and eosinophils, and as their features can be masked by focal fusion and abscess formation, as much breast tissue as possible should be selected to aid in the diagnosis. CNGM in the center of a granuloma containing neutrophils surrounded by lipid vesicles with Gram-positive bacilli visible in the vesicles is currently considered a subtype of GLM and its association with Corynebacterium is a current hot topic of research. However, recent studies have also tended to suggest that CNGM is a later stage of the GLM, a view that still needs to be confirmed. Our next step will be to conduct clinical studies based on this. We believe that the significance of pathology, in addition to diagnostic and differential diagnosis, can also contribute to the understanding of the disease by explaining its pathogenesis at the cytological level. Granulomatous lobular mastitis has been reported for the first time since 1972, and its incidence has gradually increased in recent years. Although there are many hypothese, the pathogenesis of granulomatous mastitis has not yet been fully established. In addition, GLM not only needs to be differentiated from breast cancer, but may also be combined with breast cancer and is a risk factor for breast cancer, which requires clinicians to spend effort on the diagnostic step of the disease. The relationship between inflammatory diseases of the breast, such as granulomatous lobular mastitis, and breast cancer needs to be further investigated. At present, animal studies of granulomatous mastitis have a high failure rate due to modeling difficulties, and are mostly clinical studies. In the future, there is a need not only for prospective clinical trials incorporating more samples, but also for collaborative imaging, testing and pathology contracts.
Author contributions
LC: Writing – original draft. CS: Writing – review & editing. JG: Writing – review & editing. XZ: Writing – review & editing. SL: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by Shanghai Municipal Health Commission (202240160) and Shanghai Hospital Development Center (SHDC2020CR1050B).
Acknowledgments
We thank Yunyuan Li for providing pathological pictures of granulomatous lobular mastitis and cystic neutrophilic granulomatous mastitis. We thank the patients who were involved in the case presentation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Al-Khaffaf, B, Knox, F, and Bundred, NJ. Idiopathic granulomatous mastitis: a 25-year experience. J Am Coll Surg. (2008) 206:269–73. doi: 10.1016/j.jamcollsurg.2007.07.041
2. Barreto, DS, Sedgwick, EL, Nagi, CS, and Benveniste, AP. Granulomatous mastitis: etiology, imaging, pathology, treatment, and clinical findings. Breast Cancer Res Treat. (2018) 171:527–34. doi: 10.1007/s10549-018-4870-3
3. Bhattarai, P, Srinivasan, A, Valenzuela, CD, Sulzbach, C, Wallack, MK, and Mariadason, JG. Idiopathic granulomatous mastitis: experience at a New York hospital. Ann R Coll Surg Engl. (2022) 104:543–7. doi: 10.1308/rcsann.2021.0239
4. Altintoprak, F, Kivilcim, T, and Ozkan, OV. Aetiology of idiopathic granulomatous mastitis. World J Clin Cases. (2014) 2:852–8. doi: 10.12998/wjcc.v2.i12.852
5. Fattahi, AS, Amini, G, Sajedi, F, and Mehrad-Majd, H. Factors affecting recurrence of idiopathic granulomatous mastitis: a systematic review. Breast J. (2023) 2023:9947797. doi: 10.1155/2023/9947797
6. Tang, X. Progress in the etiology of granulomatous mastitis. China Mater Child Health Care. (2018) 33:1672–5. doi: 10.7620/zgfybj.j.issn.1001-4411.2018.07.77
7. Pereira, FA, Mudgil, AV, Macias, ES, and Karsif, K. Idiopathic granulomatous lobular mastitis. Int J Dermatol. (2012) 51:142–51. doi: 10.1111/j.1365-4632.2011.05168.x
8. Yildiz, S, Aralasmak, A, Kadioglu, H, Toprak, H, Yetis, H, Gucin, Z, et al. Radiologic findings of idiopathic granulomatous mastitis. Med Ultrasonic. (2015) 17:39–44. doi: 10.11152/mu.2013.2066.171.rfm
9. Fazzio, RT, Shah, SS, Sandhu, NP, and Glazebrook, KN. Idiopathic granulomatous mastitis: imaging update and review. Insights Imaging. (2016) 7:531–9. doi: 10.1007/s13244-016-0499-0
10. Lin, Y, Lin, Y, Chang, J, Zhou, R. Analysis of ultrasonic features in 123 cases of granulomatous lobular mastitis. Chinese J New Clin Med (2021) 14:1112–6. doi: 10.3969/j.ion.1674-3806.2021.11.11
11. Alper, F, Abbasguliyev, H, Özmen, S, Yalçin, A, Yılmaz Çankaya, B, and Akçay, MN. Clinical, histopathological, imaging, and treatment perspectives of inflammatory granulomatous mastitis: review of the literature. Eurasian J Med. (2022) 54:172–8. doi: 10.5152/eurasianjmed.2022.22306
12. Gautier, N, Lalonde, L, Tran-Thanh, D, El Khoury, M, David, J, Labelle, M, et al. Chronic granulomatous mastitis: imaging, pathology and management. Eur J Radiol. (2013) 82:e165–75. doi: 10.1016/j.ejrad.2012.11.010
13. Karabacak, H, and Balas, Ş. Evaluation of early results of triamcinolone acetonide treatment in the treatment of idiopathic granulomatous mastitis introduction. Eur Rev Med Pharmacol Sci. (2023) 27:9801–8. doi: 10.26355/eurrev_202310_34155
14. Moldoveanu, D, Lee, C, and Hesley, G. Framework and guide for intralesional steroid injections in idiopathic granulomatous mastitis. Eur J Radiol. (2023) 168:111118. doi: 10.1016/j.ejrad.2023.111118
15. Yildirim, E, Kayadibi, Y, Bektas, S, Ucar, N, Oymak, A, Er, AM, et al. Comparison of the efficiency of systemic therapy and intralesional steroid administration in the treatment of idiopathic granulomatous mastitis. The novel treatment for granulomatous mastitis. Ann Ital Chir. (2021) 92:234–41.
16. Kaya, MN, Tekgöz, E, Çolak, S, Kılıç, Ö, Çınar, M, and Yılmaz, S. Drug-free remission is an achievable target with immunosuppressive treatment in idiopathic granulomatous mastitis. Ir J Med Sci. (2023) 192:2815–9. doi: 10.1007/s11845-023-03338-1
17. Li, ZY, Sun, XM, Li, JW, Liu, XF, Sun, ZY, Chen, HH, et al. Treatment of bilateral granulomatous lobular mastitis during lactation with traditional Chinese medicine: a case report. World J Clin Cases. (2021) 9:8249–59. doi: 10.12998/wjcc.v9.i27.8249
18. Zuo, X, Shi, X, Gao, X, Lai, R, Liu, P, and Zhao, Z. A retrospective study on 221 patients with granulomatous lobular mastitis treated by a combination of traditional Chinese medicine and western medicine. Ann Ital Chir. (2021) 92:135–41.
19. Martinez-Ramos, D, Simon-Monterde, L, Suelves-Piqueres, C, Queralt-Martin, R, Granel-Villach, L, Laguna-Sastre, JM, et al. Idiopathic granulomatous mastitis: a systematic review of 3060 patients. Breast J. (2019) 25:1245–50. doi: 10.1111/tbj.13446
20. Llancari, PA, Ortiz, A, Becerra, J, Muñoz, R, Valeriano, C, and Novoa, RH. Treatment and management experience of idiopathic granulomatous mastitis in a low-income country. Rev Bras Ginecol Obstet. (2023) 45:319–24. doi: 10.1055/s-0043-1770089
21. Huang, Y, and Wu, H. A retrospective analysis of recurrence risk factors for granulomatous lobular mastitis in 130 patients: more attention should be paied to prolactin level. Ann Palliat Med. (2021) 10:2824–31. doi: 10.21037/apm-20-1972
22. Tsai, MJ, Huang, WC, Wang, JT, Wang, MY, Lee, YH, Lin, SW, et al. Factors associated with treatment duration and recurrence rate of complicated mastitis. J Microbiol Immunol Infect. (2020) 53:875–81. doi: 10.1016/j.jmii.2020.03.028
23. Benson, JR, and Dumitru, D. Idiopathic granulomatous mastitis: presentation, investigation and management. Future Oncol. (2016) 12:1381–94. doi: 10.2217/fon-2015-0038
24. Taylor, GB, Paviour, SD, Musaad, S, Jones, WO, and Holland, DJ. A clinicopathological review of 34 cases of inflammatory breast disease showing an association between corynebacteria infection and granulomatous mastitis. Pathology. (2003) 35:109–19.
25. Min, S, Lang, R, Fu, X, and Fu, L. Analysis on the clinicopathological characteristics of granulomatous mastitis in 89 cases. Chin J Breast Di. (2013) 7:15–9. doi: 10.3877/cma.j.issn.1674-0807.2013.03.004
26. Liu, QL, and Lei, ME. Clinicopathological features of granulomatous lobular mastitis easily misdiagnosed as breast tuberculosis (with analysis of 31 cases). Compendium of papers from the 2019 National Tuberculosis Academic Conference of the Chinese Medical Association Branch of Tuberculosis. Suzhou, Jiangsu Province, China (2019). 497–501.
27. Niu, D, Xu, M, Yang, J, Yao, F, and Xia, Q. Granulomatous lobular mastitis: A clinicopathologic analysis of 21 cases. Modern Oncol. (2022) 30:626–9. doi: 10.3969/j.issn.1672-4992.2022.04.014
28. Yu, M, Dong, Y, Zhu, C, Xu, F, and Miao, X. Clinicopathological features and the change of CD4 +/CD8 + ratio in granulomatous lobular mastitis with different stages. J Diag Pathol. (2022) 29:485–90. doi: 10.3969/j.issn.1007-8096.2022.06.002
29. Sun, L. Differentiation and diagnosis of plasmacytic mastitis and granulomatous mastitis. J Mod Med Health. (2017) 33:2129–31. doi: 10.3969/j.issn.1009-5519.2017.14.016
30. Liao, QH, An, HM, Guo, DC, and Zhang, LF. Clinicopathological analysis of 33 cases of granulomatous lobular mastitis. J Clin Exp Pathol. (2014) 30:794–5. doi: 10.13315/j.cnki.cjcep.2014.07.024
31. Soltany, A, Hraib, M, Alkhayer, M, Ibraheem, B, and Alshehabi, Z. Clinicopathological features of idiopathic granulomatous mastitis: a retrospective study & educational lessons from Syria. Ann Med Surg. (2022) 77:103587. doi: 10.1016/j.amsu.2022.103587
32. Cheng, J, Du, YT, and Ding, HY. Granulomatous lobular mastitis: a clinicopathologic study of 68 cases. Chin J Patho. (2010) 39:678–80. doi: 10.3760/cma.j.issn.0529-5807.2010.10.00
33. Yu, H, Wang, Q, Yang, J, Lian, Z, Zhang, A, Gao, H, et al. Clinicopathological characteristics of granulomatous mastitis and its comprehensive treatment. Chin J Breast Dis. (2013) 7:20–3. doi: 10.3877/cma.j.issn.1674-0807.2013.03.005
34. Cheng, L, Reddy, V, Solmos, G, Watkins, L, Cimbaluk, D, Bitterman, P, et al. Mastitis, a radiographic, clinical, and histopathologic review. Breast J. (2015) 21:403–9. doi: 10.1111/tbj.12430
35. Yu, H, Wang, Q, He, Z, Yang, J, Gao, H, and Han, N. Clinicopathological characteristics and classified treatment of 218 patients with granulomatous mastitis. Chin J Breast Dis. (2018) 12:84–92. doi: 10.3877/cma.j.issn.1674-0807.2018.02.005
36. Xiao, F, Shen, J, Wang, J, Lin, Y, Zheng, Q, and Qu, J. Clinicopathological analysis of 141 cases of granulomatous lobular mastitis. Chinese For Med Res. (2021) 19:84–6. doi: 10.14033/j.cnki.cfmr.2021.05.030
37. Tariq, H, and Nazarullah, AN. The emerging role of corynebacteria in the pathogenesis of granulomatous lobular mastitis. Arch Breast Cancer. (2022) 9:249–53. doi: 10.32768/abc.202293SI250-254
38. Naik, MA, Korlimarla, A, Shetty, ST, Fernandes, AM, and Pai, SA. Cystic neutrophilic granulomatous mastitis: a clinicopathological study with 16s rRNA sequencing for the detection of corynebacteria in formalin-fixed paraffin-embedded tissue. Int J Surg Pathol. (2020) 28:371–81. doi: 10.1177/1066896919896021
39. Yip, CH, Jayaram, G, and Swain, M. The value of cytology in granulomatous mastitis: a report of 16 cases from Malaysia. Aust N Z J Surg. (2000) 70:103–5. doi: 10.1046/j.1440-1622.2000.01764.x
40. Tse, GM, Poon, CS, Ramachandram, K, Ma, TK, Pang, LM, Law, BK, et al. Granulomatous mastitis: a clinicopathological review of 26 cases. Pathology. (2004) 36:254–7. doi: 10.1080/00313020410001692602
41. Ail, DA, Bhayekar, P, Joshi, A, Pandya, N, Nasare, A, Lengare, P, et al. Clinical and cytological spectrum of granulomatous mastitis and utility of FNAC in picking up tubercular mastitis: an eight-year study. J Clin Diagn Res. (2017) 11:EC45–9. doi: 10.7860/JCDR/2017/25635.9591
42. Ren, X, Huang, Y, Wu, L, Qiu, L, and Zhao, Z. Clinicopathological and cytological analysis of granulomatous lobular mastitis. Zhejiang Clin Med. (2004) 6:905–6. doi: 10.3969/j.issn.1008-7664.2004.10.060
43. Feng, J, and Yu, Y. Granulomatous lobular mastitis: a clinicopathological study of 24 cases. J Diagn Pathol. (2002) 2:21–23+74.
44. Lacambra, M, Thai, TA, Lam, CC, Yu, AM, Pham, HT, Tran, PV, et al. Granulomatous mastitis: the histological differentials. J Clin Pathol. (2011) 64:405–11. doi: 10.1136/jcp.2011.089565
45. Helal, TE, Shash, LS, Saad El-Din, SA, and Saber, SM. Idiopathic granulomatous mastitis: cytologic and histologic study of 65 Egyptian patients. Acta Cytol. (2016) 60:438–44. doi: 10.1159/000448800
46. Jiang, L, Li, X, Sun, B, Ma, T, Kong, X, and Yang, Q. Clinicopathological features of granulomatous lobular mastitis and mammary duct ectasia. Oncol Lett. (2020) 19:840–8. doi: 10.3892/ol.2019.11156
47. Goulabchand, R, Hafidi, A, Van de Perre, P, Millet, I, Maria, ATJ, Morel, J, et al. Mastitis in autoimmune diseases: review of the literature, diagnostic pathway, and pathophysiological key players. J Clin Med. (2020) 9:958. doi: 10.3390/jcm9040958
48. Bi, J, Li, Z, Lin, X, Li, F, Xu, H, Yu, X, et al. Etiology of granulomatous lobular mastitis based on metagenomic next-generation sequencing. Int J Infect Dis. (2021) 113:243–50. doi: 10.1016/j.ijid.2021.10.019
49. Chen, L, Zhang, X, Wang, Y, Zhao, Q, and Ding, H. Granulomatous lobular mastitis: a clinicopathological analysis of 300 cases. Chin J Pathol. (2019) 48:231–6. doi: 10.3760/cma.j.issn.0529-5807.2019.03.012
50. Anousha, K, Azizi, M, Soleimani, V, Rezvani, A, Azmoudeh Ardalan, F, and Jahanbin, B. Idiopathic granulomatous mastitis: diagnosis and histopathologic features. Arch Breast Cancer. (2022) 9:272–8. doi: 10.32768/abc.202293SI272-278
51. Cheng, J, Ding, H, Du, Y, Wu, X, and Dai, X. Granulomatous lobular mastitis, mammary duct ectasia and erythema nodosum: a clinicopathologic study of 4 cases with review of literature. J Diagn Pathol. (2014) 21:488–490+494. doi: 10.3969/j.issn.1007-8096.2014.08.008
52. Özşen, M, Tolunay, Ş, and Gökgöz, MŞ. Granulomatous lobular mastitis: clinicopathologic presentation of 90 cases. Recurrence in uterine tumors with ovarian sex-cord tumor resemblance: a case report and systematic review. Turk Patoloji Derg. (2018) 34:215–9. doi: 10.5146/tjpath.2018.01431
53. Choi, SH, Jang, KS, and Chung, MS. Bilateral granulomatous mastitis with a different etiology. Cancer Biomark. (2015) 15:333–8. doi: 10.3233/CBM-140447
54. Çalış, H, and Kilitçi, A. Granulomatous mastitis concurrence with breast cancer. Eur J Breast Health. (2018) 14:58–60. doi: 10.5152/ejbh.2017.3804
55. D'Alfonso, TM, Moo, TA, Arleo, EK, Cheng, E, Antonio, LB, and Hoda, SA. Cystic neutrophilic granulomatous mastitis: further characterization of a distinctive histopathologic entity not always demonstrably attributable to corynebacterium infection. Am J Surg Pathol. (2015) 39:1440–7. doi: 10.1097/PAS.0000000000000479
56. Gautham, I, Radford, DM, Kovacs, CS, Calhoun, BC, Procop, GW, Shepardson, LB, et al. Cystic neutrophilic granulomatous mastitis: the Cleveland Clinic experience with diagnosis and management. Breast J. (2019) 25:80–5. doi: 10.1111/tbj.13160
57. Johnstone, KJ, Robson, J, Cherian, SG, Wan Sai Cheong, J, Kerr, K, and Bligh, JF. Cystic neutrophilic granulomatous mastitis associated with corynebacterium including Corynebacterium kroppenstedtii. Pathology. (2017) 49:405–12. doi: 10.1016/j.pathol.2017.01.006
58. Zhu, Y, Li, X, Wu, Q, and Chen, L. Analysis of species and antibiotic sensitivity of Corynebacterium in female breast abscess. Chin J Health Lab Tec. (2022) 32:2061–3.
59. Liu, X, Tong, L, Luo, Q, Xu, B, Lai, M, and Wang, L. Bacteriological analysis of granulomatous lobular mastitis. Guangdong Med. (2016) 37:2454–6. doi: 10.13820/j.cnki.gdyx.2016.16.023
60. Wang, Y, LeGolvan, M, Chapin, K, and Mainiero, M. Cystic neutrophilic granulomatous mastitis with corynebacterium and staphylococcus mimicking breast carcinoma. Clin Case Rep. (2018) 6:2208–10. doi: 10.1002/ccr3.1801
61. Li, Y, Chen, L, Zhang, C, Wang, Y, Hu, J, Zhou, M, et al. Clinicopathologic features and pathogens of granulomatous lobular mastitis. Breast Car. (2023) 18:130–40. doi: 10.1159/000529391
62. Yu, HJ, Deng, H, Ma, J, Huang, SJ, Yang, JM, Huang, YF, et al. Clinical metagenomic analysis of bacterial communities in breast abscesses of granulomatous mastitis. Int J Infect Dis. (2016) 53:30–3. doi: 10.1016/j.ijid.2016.10.015
63. Ong, SS, Xu, J, Sim, CK, Khng, AJ, Ho, PJ, Kwan, PKW, et al. Profiling microbial communities in idiopathic granulomatous mastitis. Int J Mol Sci. (2023) 24:1042. doi: 10.3390/ijms24021042
64. Chen, W, Zhang, D, Zeng, Y, Cui, J, Yu, J, Wang, J, et al. Clinical characteristics and microbiota analysis of 44 patients with granulomatous mastitis. Front Microbiol. (2023) 14:1175206. doi: 10.3389/fmicb.2023.1175206
65. Collins, MD, Falsen, E, Akervall, E, Sjöden, B, and Alvarez, A. Corynebacterium kroppenstedtii sp. nov., a novel corynebacterium that does not contain mycolic acids. Int J Syst Bacteriol. (1998) 48:1449–54. doi: 10.1099/00207713-48-4-1449
66. Dobinson, HC, Anderson, TP, Chambers, ST, Doogue, MP, Seaward, L, and Werno, AM. Antimicrobial treatment options for granulomatous mastitis caused by corynebacterium species. J Clin Microbiol. (2015) 53:2895–9. doi: 10.1128/JCM.00760-15
67. Tang, L, Liu, C, Ji, C, Diao, F, Lai, W, and Mu, X. Distribution and drug sensitivity analysis of pathogenic bacteria in granulomatous mastitis. Chinese J Antibiotics. (2019) 44:736–40. doi: 10.13461/j.cnki.cja.006677
68. Ye, H, Xing, F, Lu, Q, Lin, Z, Li, C, Liu, J, et al. Bacteriological and pathological analysis of non-lactation mastitis associated with Corynebacterium kroppenstedtii infection. J Trop Med. (2022) 22:1227–47. doi: 10.3969/j.issn.1672-3619.2022.09.011
69. Liang, Y, Zhan, H, Krishnamurti, U, Harigopal, M, and Sun, T. Further characterization of clinicopathologic features of cystic neutrophilic granulomatous mastitis. Am J Clin Pathol. (2022) 158:488–93. doi: 10.1093/ajcp/aqac074
70. Li, S, Huang, Q, Song, P, Han, X, Liu, Z, Zhou, L, et al. Clinical characteristics and therapeutic strategy of granulomatous mastitis accompanied by Corynebacterium kroppenstedtii: a retrospective cohort study. BMC Womens Health. (2023) 23:388. doi: 10.1186/s12905-023-02509-7
71. Tan, QT, Tay, SP, Gudi, MA, Nadkarni, NV, Lim, SH, and Chuwa, EWL. Granulomatous mastitis and factors associated with recurrence: an 11-year single-centre study of 113 patients in Singapore. World J Surg. (2019) 43:1737–45. doi: 10.1007/s00268-019-05014-x
72. Wu, JM, and Turashvili, G. Cystic neutrophilic granulomatous mastitis: an update. J Clin Pathol. (2020) 73:445–53. doi: 10.1136/jclinpath-2019-206180
73. Li, XQ, Yuan, JP, Fu, AS, Wu, HL, Liu, R, Liu, TG, et al. New insights of Corynebacterium kroppenstedtii in granulomatous lobular mastitis based on nanopore sequencing. J Invest Surg. (2022) 35:639–46. doi: 10.1080/08941939.2021.1921082
74. Lin, Y, Zhang, H, Qu, L, Zeng, Y, and Wu, J. Clinicopathological features and etiological analysis of 106 cases of granulomatous lobular mastitis. J Clin Exp Pathol. (2017) 33:1013–5. doi: 10.13315/j.cnki.cjcep.2017.09.016
75. Liu, Q, and Lei, M. Diagnosis and differential diagnosis of granulomatous lobular mastitis. Med Recap. (2020) 26:1994–8. doi: 10.3969/j.issn.1006-2084.2020.10.023
76. Tariq, H, Menon, PD, Fan, H, Vadlamudi, KV, Pandeswara, SL, Nazarullah, AN, et al. Detection of Corynebacterium kroppenstedtii in granulomatous lobular mastitis using real-time polymerase chain reaction and sanger sequencing on formalin-fixed, paraffin-embedded tissues. Arch Pathol Lab Med. (2022) 146:749–54. doi: 10.5858/arpa.2021-0061-OA
77. Sangoi, AR. "Thick section" gram stain yields improved detection of organisms in tissue sections of cystic neutrophilic granulomatous mastitis. Am J Clin Pathol. (2020) 153:593–7. doi: 10.1093/ajcp/aqz200
78. Mazlan, L, Suhaimi, SN, Jasmin, SJ, Latar, NH, Adzman, S, and Muhammad, R. Breast carcinoma occurring from chronic granulomatous mastitis. Malays J Med Sci. (2012) 19:82–5.
79. Zangouri, V, Niazkar, HR, Nasrollahi, H, Homapour, F, Ranjbar, A, and Seyyedi, MS. Benign or premalignant? Idiopathic granulomatous mastitis later diagnosed as ductal carcinoma breast cancer: case report and review of literature. Clin Case Rep. (2022) 10:e6323. doi: 10.1002/ccr3.6323
80. Xie, S, Yu, H, Gao, H, Lian, Z, and Wang, Q. Breast cancer coexisting with granulomatous mastitis in a young woman. Br J Hosp Med. (2022) 83:1–3. doi: 10.12968/hmed.2021.0426
81. Jha, NK, Arfin, S, Jha, SK, Kar, R, Dey, A, Gundamaraju, R, et al. Re-establishing the comprehension of phytomedicine and nanomedicine in inflammation-mediated cancer signaling. Semin Cancer Biol. (2022) 86:1086–104. doi: 10.1016/j.semcancer.2022.02.022
82. Zhang, HJ, Ding, PP, Zhang, XS, Wang, XC, Sun, DW, Bu, QA, et al. MAC mediates mammary duct epithelial cell injury in plasma cell mastitis and granulomatous mastitis. Int Immunopharmacol. (2022) 113:109303. doi: 10.1016/j.intimp.2022.109420
83. Albayrak, MGB, Simsek, T, Kasap, M, Akpinar, G, Canturk, NZ, and Guler, SA. Tissue proteome analysis revealed an association between cancer, immune system response, and the idiopathic granulomatous mastitis. Med Oncol. (2022) 39:238. doi: 10.1007/s12032-022-01845-2
84. Peters, F, Kiesslich, A, and Pahnke, V. Coincidence of nonpuerperal mastitis and noninflammatory breast cancer. Eur J Obstet Gynecol Reprod Biol. (2002) 105:59–63. doi: 10.1016/S0301-2115(02)00109-4
85. Chang, CM, Lin, MC, and Yin, WY. Risk of breast cancer in women with non-lactational mastitis. Sci Rep. (2019) 9:15587. doi: 10.1038/s41598-019-52046-3
86. Sripathi, S, Ayachit, A, Bala, A, Kadavigere, R, and Kumar, S. Idiopathic granulomatous mastitis: a diagnostic dilemma for the breast radiologist. Insights Imaging. (2016) 7:523–9. doi: 10.1007/s13244-016-0497-2
87. Cheuk, W, Chan, AC, Lam, WL, Chow, SM, Crowley, P, Lloydd, R, et al. IgG4-related sclerosing mastitis: description of a new member of the IgG4-related sclerosing diseases. Am J Surg Pathol. (2009) 33:1058–64. doi: 10.1097/PAS.0b013e3181998cbe
88. Ely, KA, Tse, G, Simpson, JF, Clarfeld, R, and Page, DL. Diabetic mastopathy: a clinicopathologic review. Am J Clin Pathol. (2000) 113:541–5. doi: 10.1309/K5HM-9603-PQ5T-KY3F
89. Valdez, R, Thorson, J, Finn, WG, Schnitzer, B, and Kleer, CG. Lymphocytic mastitis and diabetic mastopathy: a molecular, immunophenotypic, and clinicopathologic evaluation of 11 cases. Mod Pathol. (2003) 16:223–8. doi: 10.1097/01.MP.0000056627.21128.74
90. Kirby, RX, Mitchell, DI, Williams, NP, Cornwall, DA, and Cawich, SO. Diabetic mastopathy: an uncommon complication of diabetes mellitus. Case Rep Surg. (2013) 2013:198502. doi: 10.1155/2013/198502
91. Tomaszewski, JE, Brooks, JS, Hicks, D, and Livolsi, VA. Diabetic mastopathy: a distinctive clinicopathologic entity. Hum Pathol. (1992) 23:780–6. doi: 10.1016/0046-8177(92)90348-7
92. Ogura, K, Matsumoto, T, Aoki, Y, Kitabatake, T, Fujisawa, M, and Kojima, K. IgG4-related tumour-forming mastitis with histological appearances of granulomatous lobular mastitis: comparison with other types of tumour-forming mastitis. Histopathology. (2010) 57:39–45. doi: 10.1111/j.1365-2559.2010.03581.x
93. Kong, C, Zhang, C, Wu, Y, Zeng, Z, Yu, H, Zeng, J, et al. The expression and meaning of CD68, CD163, CD57, and IgG4 in granulomatous lobular mastitis. Gland Surg. (2020) 9:936–49. doi: 10.21037/gs-20-419
94. Kinonen, C, Gattuso, P, and Reddy, VB. Lupus mastitis: an uncommon complication of systemic or discoid lupus. Am J Surg Pathol. (2010) 34:901–6. doi: 10.1097/PAS.0b013e3181da00fb
95. Yang, J, Wang, Q, Zhang, A, Xu, J, Gao, H, Li, W, et al. Identification and treatment of periductal mastitis and granulomatous mastitis. Chinese J Breast Dis. (2011) 5:306–12. doi: 10.3969/j.issn.1674-0807.2011.03.006
Keywords: granulomatous lobular mastitis (GLM), cystic neutrophil granulomatous mastitis (CNGM), pathology, Corynebacterium, differential diagnosis
Citation: Cui L, Sun C, Guo J, Zhang X and Liu S (2024) Pathological manifestations of granulomatous lobular mastitis. Front. Med. 11:1326587. doi: 10.3389/fmed.2024.1326587
Edited by:
Luigi Tornillo, University of Basel, SwitzerlandReviewed by:
Lovenish Bains, University of Delhi, IndiaNguyen Minh Duc, Pham Ngoc Thach University of Medicine, Vietnam
Copyright © 2024 Cui, Sun, Guo, Zhang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chenping Sun, c2NwdGNtQDEyNi5jb20=
†ORCID: Leyin Cui, https://orcid.org/0000-0001-5439-9289
Xuliu Zhang, https://orcid.org/0009-0001-7808-8984
 Leyin Cui
Leyin Cui Chenping Sun1*
Chenping Sun1*