- 1Department of Internal Medicine and Nature-Based Therapies, Immanuel Hospital Berlin, Berlin, Germany
- 2Division of Oncology and Hematology, Department of Pediatrics, Charité Universitätsmedizin Berlin, Corporate member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Berlin, Germany
- 3Post Graduate Department of Kayacikitsa, J. S. Ayurveda College, Nadiad, India
- 4Head of Academic Advisory Board, European Academy of Ayurveda, Birstein, Germany
- 5Applied Social Psychology and Gender Research, CITEC, Bielefeld University, Bielefeld, Germany
- 6Institute of Social Medicine, Epidemiology, and Health Economics, Charité Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Berlin, Germany
Background: Maharishi Amrit Kalash (MAK) 4 and 5 are Ayurvedic herbal nutritional supplements that are believed to have beneficial effects on overall health and wellbeing. This study aimed to systematically review all available randomized controlled trials (RCTs) investigating the clinical effects and safety of MAK.
Methods: We included RCTs on therapy, health promotion, and prevention for patients and healthy volunteers of all ages. We systematically searched MEDLINE (via PubMed), EMBASE (via Ovid), the Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library), DHARA, Clinicaltrials.gov, the World Health Organization (WHO) International Clinical Trials Registry Platform, and Google Scholar from inception through 7 May 2023, with no time or language restrictions. The risk of bias was assessed using the Cochrane Risk of Bias Tool version 1. The protocol was registered with PROSPERO before conducting the review (CRD42023421655).
Results: Three RCTs with 418 study participants were included. Two studies were on breast cancer patients and one on healthy adults. The two studies on cancer evaluated the efficacy of MAK in reducing the side effects of chemotherapy in women with breast cancer. The study on healthy adults evaluated whether MAK has an effect on an age-related alertness task as an indicator of cognitive aging. Both studies on breast cancer patients found beneficial effects on performance status, anorexia, vomiting, and body weight. One study reported positive effects regarding stomatitis. Regarding visual alertness, results showed that individuals who received MAK improved in performance. None of the three included studies reported adverse events. The risk of bias was mixed. Due to the small number and heterogeneity of the RCTs, no meta-analysis could be performed.
Conclusion: There is evidence that MAK may have supportive effects in chemotherapeutic treatments for breast cancer patients and for healthy individuals regarding visual discrimination. However, it is difficult to verify treatment effects due to the small number of RCTs and the mixed risk of bias. Furthermore, none of the included studies recorded adverse events. Therefore, further high-quality studies are warranted to confirm the potential health benefits of MAK and to determine its optimal dosage and duration of use.
Systematic review registration: PROSPERO, CRD42023421655.
1 Introduction
Ayurveda is a traditional system of medicine originating in South Asia and has been practiced for more than 2000 years on the Indian subcontinent and elsewhere. It is based on the belief that a person’s physical, mental, and spiritual wellbeing is dependent on an individual balance between the body, mind, and soul. It is recognized by the World Health Organization (WHO) and is widely practiced today, including in the Western world (1–3). There are guidelines for the clinical evaluation of Ayurvedic interventions to ensure quality in this area of research (4). Ayurveda includes a wide range of medical practices, such as individualized treatments consisting of manual therapies, purification treatments (“Pancakarma”), nutritional therapy and herbs, lifestyle counseling, and yoga exercises (5). Maharishi Ayurveda is a contemporary revival that takes into account these traditional approaches in agreement with the classical texts (6). Since 2014, Ayurveda in India has been regulated by an independent ministry (Ministry of Ayurveda, Yoga, Naturopathy, Unani, Siddha, Sowa-Rigpa and Homoeopathy; abbreviated as AYUSH Ministry (7)).
Maharishi Amrit Kalash (MAK) 4 and 5 are Ayurvedic herbal preparations that are believed to have beneficial effects on overall health and wellbeing. These preparations are combinations of several herbs and minerals with rasayana (rejuvenative and immune boosting) effects and is said to be helpful in supporting the body’s natural defenses against disease. MAK 4 is prepared as a paste, whereas MAK 5 is administered as tablet. The ingredients of the two delivery forms differ from each other, a phytochemical standardization of the preparations is being sought (8), see Table 1. The preparations for MAK 4 and MAK 5 are based on the classic Ayurvedic formulation for Brahma Rasayana, as described in traditional texts and the Ayurvedic Pharmacopoeia of India (9, 10). The MAK preparation is complex, with a range of pharmacological activities on various organ systems. In terms of its preparation, it is comparable to the classic Ayurvedic formulation Chyavanprash with regard to numerous ingredients (e.g., Emblica officinalis) for which mechanisms have been discussed (11, 12). Studies have shown that Chyavanprash has immunostimulatory effects, enhancing the secretion of cytokines and stimulating macrophage and natural killer cell activity (13). Based on similarities in the preparations and the range of indications, similar mechanisms of action can also be assumed for MAK. Both Chyavanprash and the classic Ayurvedic recipe Brahma Rasayana, on which MAK is based, are classified as rasayana in Ayurveda, aimed at maintaining vigor, vitality, and delaying the aging process (14). These mechanisms might contribute to its therapeutic potential for various health conditions. In vitro effects for MAK 4 and 5 have been shown in different studies. Inaba et al. (15) evaluated the immunomodulatory effects of MAK 4 and MAK 5 in mice. MAK 4 increased the responsiveness of lymphocytes, and MAK 5 increased not only the responsiveness of lymphocytes but also macrophage function. In this study, it is also suggested that MAK 4 and 5 have mitogenic effects on lymphocytes. Sugiura et al. (16) found that MAK 4 and 5 were found to promote the phagocytic and digestive functions of macrophages in mice compared with control and also had a stimulatory effect on macrophages. Furthermore, Penza et al. (17) found that a MAK-supplemented diet inhibited liver carcinogenesis in urethane-treated mice. Several but fewer studies have also investigated the in vivo effects of MAK 4 and 5. Sundaram et al. (18) treated 10 hyperlipidemic patients receiving stable hypolipidemic therapy with MAK 4 and 5 for 18 weeks. Plasma lipoprotein, plasma lipid peroxide, and low-density lipoprotein oxidation studies were evaluated every 6 weeks. The results indicate that MAK 4 and MAK 5 may be useful in the prevention and treatment of atherosclerosis. Zanella et al. (19) put healthy people on diets with or without MAK and found that a MAK-enriched diet reduced oxidative stress parameters and increased antioxidant defenses in both short- and long-term treatment. Accordingly, there is quite some evidence that MAK may have positive effects on various health parameters. However, a systematic review of the available evidence is still lacking.
The preclinical evidence for MAK is already well-reviewed, but the necessary clinical evidence still largely lacking. To date, there has been no systematic review that assesses and compares the efficacy and safety of MAK for the prevention and treatment of various health conditions and in healthy individuals. The aim of this review is to summarize the existing randomized controlled trials (RCTs) on the efficacy and safety of MAK, to provide a comprehensive overview of the existing clinical evidence on MAK and to identify areas where further prospective clinical research is required.
2 Methods
This systematic review was conducted and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (20, 21). The protocol was registered with PROSPERO before conducting the review (CRD42023421655).
2.1 Eligibility assessment
Randomized controlled, randomized crossover, and cluster randomized trials were eligible. Studies of individuals of any age, sex, and origin were included. We included trials of therapeutic, health-promoting, or preventive use of MAK. Studies that compared MAK with (1) no specific intervention, (2) placebo, (3) other medicine treatment, or (4) other Ayurvedic preparations were eligible. Studies that examined MAK in combination with other procedures were included only if the concurrent intervention was comparable between all groups. There were no restrictions on the type of outcomes.
2.2 Search strategy and databases
MEDLINE (via PubMed), EMBASE (via Ovid), Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library), DHARA, Clinicaltrials.gov, the WHO International Clinical Trials Registry Platform were searched without time and language restrictions from inception through 7 May 2023. In order to include gray literature (e.g., reports, government documents, dissertations, theses, and conference abstracts), Google Scholar was also searched. The complete search strategy for PubMed is shown in Table 2. Search strategies for the other databases were identical in content except for the fact that we did not filter for RCTs for Google Scholar but included all hits for “Maharishi Amrit Kalash” for a maximum sensitive gray literature search.
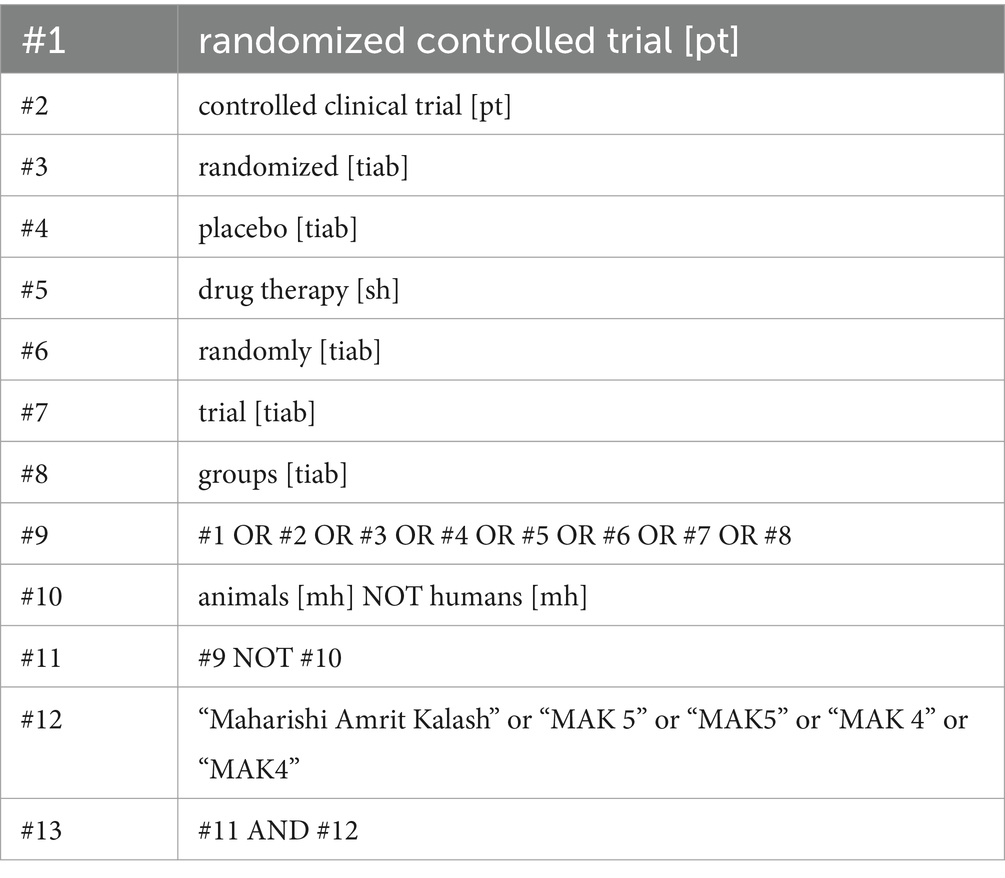
Table 2. Search strategy for MEDLINE (via PubMed) using the Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE: sensitivity-maximizing version (2008 revision); PubMed format (22).
2.3 Study selection and data extraction
Search results were checked for duplicates using the open-source software rayyan.ai. Two authors (AKK and RW) independently screened abstracts and full texts for eligibility using rayyan.ai. Disagreements were resolved in discussion with a third author (CK) until a consensus was reached. Study characteristics were extracted using a pre-developed data extraction form independently by two authors (AKK and RW). Data on publication type, design and funding, participants, intervention arms, dosage and pharmaceutical form, outcomes, and safety were extracted from the included full texts.
2.4 Risk of bias of individual studies
Two authors (AKK and RW) independently assessed the risk of selection bias, performance bias, detection bias, attrition bias, reporting bias, and other biases using the Cochrane Risk of Bias tool version 1 (23). Disagreements were resolved in discussion with a third author (CK) until a consensus was reached.
2.5 Data synthesis
If at least two studies assessing this specific outcome are available, meta-analyses were planned to be conducted using the Statistical Package for Social Sciences software (IBM SPSS Statistics for Windows, release 29.0; IBM Corporation, Armonk, NY) by a random effects model. Mean differences (MDs) between groups and their 95% confidence intervals (CIs) would have been calculated. The effects of MAK compared with different control interventions were planned to be analyzed separately. In case of data missing, attempts would have been made to obtain the missing data from the trial authors by email. Ultimately, pairwise meta-analyses could not be performed because of the small number of included studies.
2.6 Assessment of statistical heterogeneity
Statistical heterogeneity was planned to be evaluated using chi-square (χ2) statistics with a p-value of ≤0.10, indicating significant heterogeneity. The extent of heterogeneity was categorized using I2, with I2 > 25% representing moderate, I2 > 50% representing substantial, and I2 > 75% representing considerable heterogeneity (24). Ultimately, the assessment of statistical heterogeneity could not be performed because of the small number and poor reporting of included studies.
2.7 Subgroup analyses
The following subgroup analyses were planned a priori: If studies on both (1) MAK 4 and MAK 5 or (2) participants older and younger than 18 years were found, they would be considered separately. Due to the small number of included studies, none of the planned subgroup analyses could be performed.
3 Results
3.1 Literature search
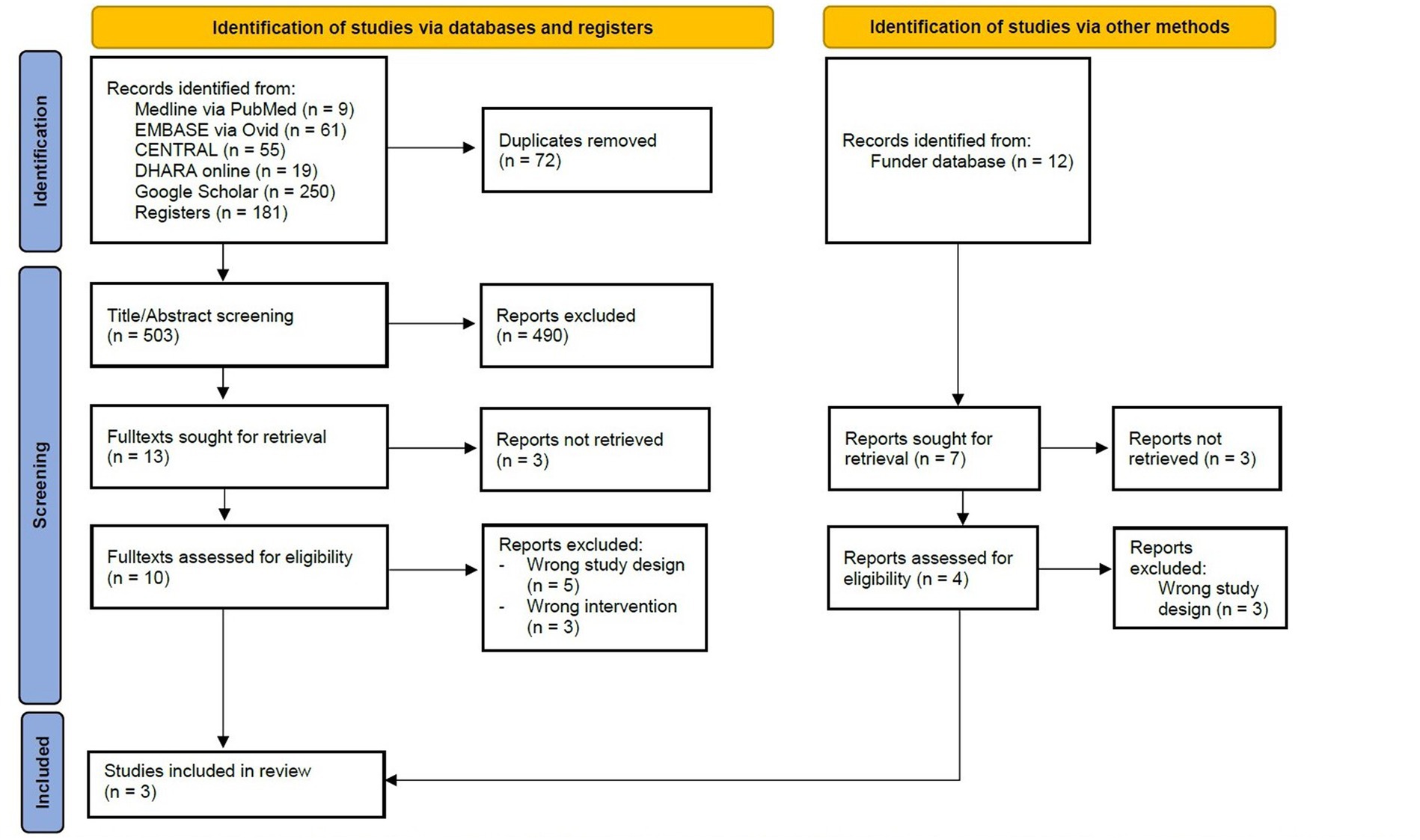
The literature search revealed 575 records; the identification of studies on other methods yielded 12 additional records (Figure 1). After excluding duplicates and irrelevant abstracts, 20 full texts were identified to be assessed for eligibility. For six of those studies, no full text could be retrieved at the time of analysis: Two study registries (25, 26), of which one has been published in the mean time (25) three conference abstracts (27–29), and one dissertation (30). Eight full texts were excluded because there were no RCT (18, 31–37), and three because it was the wrong intervention (37–39). Hence, three RCTs (N = 418) were included in the systematic review (40–42).
3.2 Study characteristics
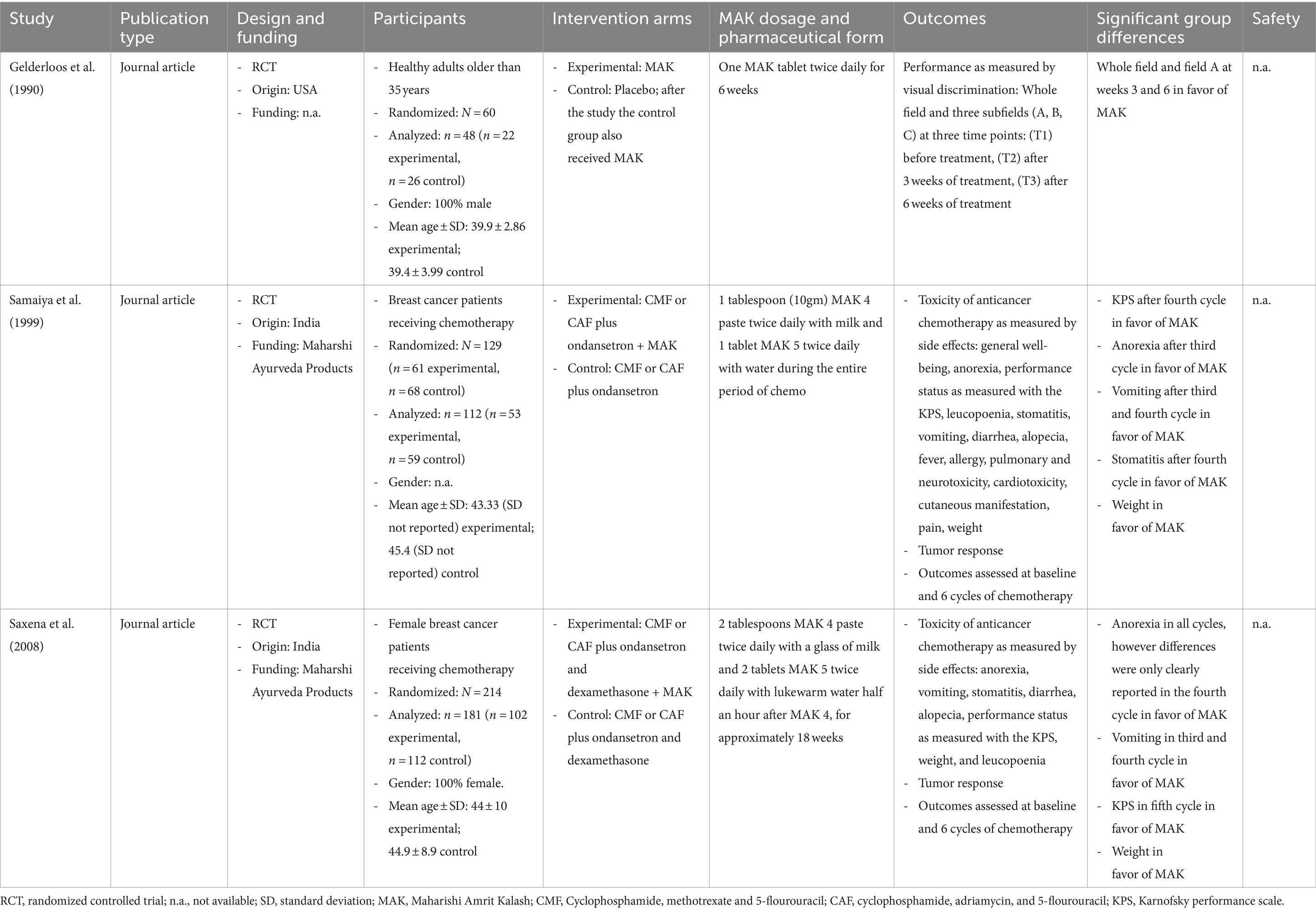
Study characteristics are presented in detail in Table 3. Two studies examined breast cancer patients (41, 42), and one focused on healthy adults (40). The two studies on cancer evaluated the efficacy of MAK in reducing the side effects of chemotherapy in women with breast cancer. The underlying hypothesis was that since MAK is rich in antioxidants, a reduction in the toxicity of chemotherapy can be achieved. The study on healthy adults evaluated whether MAK has an effect on an age-related alertness task. The underlying hypothesis was that MAK positively affects attentional capacity or alertness and thus can reverse the cognitive effects of aging. The studies were carried out in India (41, 42) and the USA (40). All studies were published between 1990 and 2008 and used a randomized study design. One study compared MAK to placebo (40), and the other two studies compared chemotherapy plus MAK to chemotherapy alone (41, 42). The sample size varied between n = 60 and n = 214 participants.
3.3 Study findings
3.3.1 MAK dosage and pharmaceutical form
Gelderloos et al. (40) administered one MAK tablet twice daily for 6 weeks. Samaiya et al. (41) administered 1 tablespoon (10gm) MAK 4 paste twice daily with milk and one tablet MAK 5 twice daily with water during the entire period of chemotherapy. Saxena et al. (42) administered 2 tablespoons MAK 4 paste twice daily with a glass of milk and two tablets MAK 5 twice daily with lukewarm water half an hour after MAK 4, for approximately 18 weeks.
3.3.2 MAK as a supplement to chemotherapy in breast cancer
Both studies on breast cancer patients (41, 42) assessed outcomes at baseline and at each of the 6 cycles of chemotherapy. Both studies found a positive effect of MAK on performance status as measured with the Karnofsky performance scale (43). Samaiya et al. (41) after the fourth chemotherapy cycle, and Saxena et al. (42) after the fifth cycle in favor of MAK. Saxena et al. (42) found positive effects of MAK in anorexia in all cycles; however, differences were only clearly reported in the fourth cycle in favor of MAK. Samaiya et al. (41) reported positive effects on anorexia after the third cycle in favor of MAK. Regarding vomiting, both studies found positive effects of MAK after the third and fourth cycles. Both studies found positive effects on body weight. Furthermore, Samaiya et al. (41) reported positive effects regarding stomatitis after the fourth cycle in favor of MAK.
3.3.3 MAK for reversing cognitive effects of aging
Gelderloos, Ahlstrom, Orme-Johnson, Robinson, Wallace, and Glaser (40) used a visual alertness task as an indicator of cognitive aging. Outcomes were assessed at three time points: before treatment with MAK, after 3 weeks of treatment, and after 6 weeks of treatment. Results showed that individuals who received MAK improved in performance on two of four measured fields at weeks 3 and 6.
3.3.4 Safety
None of the included studies recorded adverse events.
3.4 Assessment of the scope of unpublished data
During the literature search, 181 studies were identified via clinicaltrials.gov and the WHO International Clinical Trials Registry Platform. Of these, two were potentially suitable (25, 26). The study titled “Effects of Herbal Antioxidants on Cardiovascular Disease in Older Blacks” was updated as “completed” with a final update in 2010 (26). However, results were not filed and could not be identified during the literature search, so a final assessment was not possible. The study titled “Role of MAK, Ayurvedic herbal medicine on Breast Cancer” was registered in 2019, and results are not yet available (25). In addition, the literature search identified three conference abstracts (27–29) and one dissertation (30) that could potentially be considered. However, no full-text publication could be found or retrieved for these. No further information could be obtained by writing to the authors either. Thus, there is a restriction with respect to the conclusion due to the scope of unpublished data.
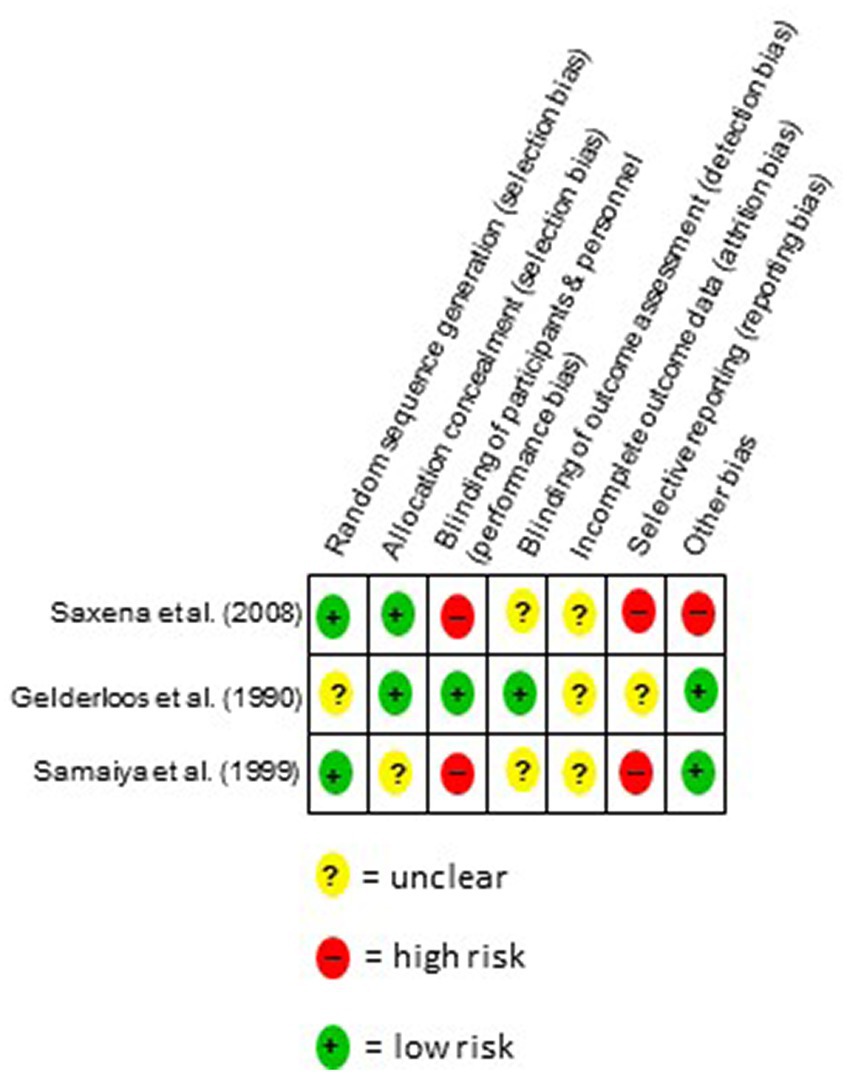
3.5 Risk of bias of individual studies
The risk of bias was highly variable across both studies and domains (Figure 2). The risk of selection bias was low for Saxena et al. (42) and mixed for Samaiya et al. (41) and Gelderloos, Ahlstrom, Orme-Johnson, Robinson, Wallace, and Glaser (40). In Saxena et al. (42) and Samaiya et al. (41), a high bias was observed with regard to the blindings of participants and personnel, while for Gelderloos et al. (40) blinding was rated as adequate. Detection bias was rated low for Gelderloos et al. (40) and unclear for Saxena et al. (42) and Samaiya et al. (41). Attrition bias was rated unclear for all studies. The risk of bias with regard to reporting bias in Saxena et al. (42) and Samaiya et al. (41) was rated as high, while for Gelderloos et al. (40) it was rated as unclear. Other bias were considered low in Gelderloss et al. (40) and Samaiya et al. (41) and high in Saxena et al. (42).
4 Discussion
4.1 Summary of evidence
This systematic review provides new evidence as it is the first systematic review on MAK. It is based on three RCTs that included 418 participants in total (40–42). The results suggest that MAK may alleviate the side effects of chemotherapy in breast cancer patients and may positively influence attentional capacity or alertness in healthy adults. The results from these few RCTs complement the findings from other clinical trials suggesting beneficial effects of MAK. A recent scoping review concluded that preclinical studies show promising results for the use of MAK as an anticancer and chemoprotective agent (25). Furthermore, the results of Zanella et al. (19), who placed healthy individuals on a diet with or without MAK, showed that a MAK-enriched diet decreased oxidative stress parameters and increased antioxidant defenses in both short- and long-term treatments (12). Research into the phytochemical aspects of the plants that form the basis for the production of MAK has also shown promising prospects for the treatment of oxidative stress and cancer (45). Experiments in the mouse model also show positive effects of MAK on cancer-associated parameters—although here an effect on tumor incidence but not on body weight was shown (15–17). This is in contrast to the results of the two RCTs (41, 42), which showed positive effects of MAK on body weight but not on tumor response. The effects on cognitive attention parameters found by Gelderloos et al. (40) are complemented by findings from Nidich, Morehead, Nidich, Sands, and Sharma (39). In this study, the non-verbal intelligence of students who received a Maharishi Student Rasayana Food Supplement over a longer period of time within an RCT was compared. The results show an increase in non-verbal intelligence in the MAK group compared to the placebo. However, further research is urgently needed to prove these effects with regard to the effect of MAK on the side effects of chemotherapy and cognitive aging processes. Traditional, complementary and integrative medicine offers a variety of approaches to alleviate symptoms associated with some of today’s most pressing medical conditions, such as cancer, pain, and bowel disease, through procedures such as lifestyle changes and manual medicine (46, 47). Together with other procedures from traditional and complementary medicine, MAK might be a promising therapeutic addition.
4.2 Strengths and limitations
To our knowledge, this is the first systematic review of the therapeutic, health-promoting, and preventive effects of MAK without time or language limitations using a broad search strategy. This included searching clinical trial registries as well as gray literature. The results of the review indicate that there is a paucity of high-quality RCTs on this topic. RCTs on Ayurveda for common medical conditions are mostly scarce (48). Only three RCTs could be included in the present review, which substantially limits the strength of the evidence. The included studies showed a mixed risk of bias. Furthermore, bias due to unpublished data cannot be ruled out. For example, three conference abstracts could not be retrieved as full texts and consequently could not be included in the review (27–29). Furthermore, one potentially eligible study (26), registered on clinicaltrials.gov and categorized as completed with results, could not be retrieved, including results as well. Furthermore, methodological limitations may apply as well. Within the two studies on breast cancer (41, 42), it is not apparent whether the statistical analysis corrected for multiple testing when testing for differences in each chemotherapy cycle. The presentation of the results and the description in the text cast serious doubt on this, which further calls into question the validity of the results. The lack of a priori registrations in public study registries, the lack of recording of adverse events, and the lack of mention of defined primary and secondary outcome parameters in all three included studies (40–42) also deserve critical mention. Most of the studies are also relatively old. More recent studies based on current quality guidelines are urgently needed. Finally, it does not become clear from the study on healthy adults which MAK preparation is used (40). It is assumed that it is MAK 5, but this is not explicitly mentioned.
4.3 Clinical implications
Based on the available results, it is too early to make specific clinical implications. As with all dietary supplements, caution is advised when using them in a clinical context due to potential interaction effects with medications. It is also very important in this context that doctors and patients talk openly about the potential use of such supplements.
4.4 Implications for future research
Given that Ayurveda is not only widely practiced in South Asia but has become increasingly popular on a global scale (1, 2), there is a great need for high-quality RCTs to improve the quality of evidence for the effects and safety of MAK. Future RCTs should adhere to the established Ayurveda research quality standards (4) as well as take into account the international quality standards for RCTs, such as the Consolidated Standards of Reporting Trials (49, 50). There is also an urgent need for a structured recording and reporting of adverse events.
4.5 Conclusion
MAK 4 and 5 exhibit potential health benefits in vivo, but limited clinical RCTs and a high risk of bias complicate confirming treatment effects. Consultation with the treating physician is necessary, especially when supplementing conventional oncological therapy. High-quality studies are required to confirm MAK’s health benefits and to establish optimal dosage and intake duration.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
AK: Conceptualization, Data curation, Formal analysis, Methodology, Project administration, Validation, Visualization, Writing – original draft. MP: Supervision, Writing – review & editing. SG: Supervision, Writing – review & editing. RW: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft. MJ: Supervision, Writing – review & editing. CK: Conceptualization, Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was funded by Maharishi Ayurveda Europe BV. The funder was not involved in design, conduct, analysis and publication of this review.
Acknowledgments
The authors would like to thank Maharishi Ayurveda Europe BV for funding this review. Particular thanks to Richa Shrivastava, who enabled this research project. The authors would like to thank Manfred Wischnewsky, Andreas Michalsen, and Gunda Loibl for their valuable input.
Conflict of interest
MP is salaried professor in J. S. Ayurveda College, Nadiad, India. He also treats patients with Ayurveda in the teaching hospital on a regular basis. SG is salaried professor in J. S. Ayurveda College, Nadiad, India. He treats patients with Ayurveda in the teaching hospital on a regular basis. He also receives honoraria for lecturing in courses on Ayurveda at European Academy for Ayurveda, Birstein, Germany. MJ receives consultancy fees from European Academy for Ayurveda, Birstein, Germany and for lecturing in courses on Ayurveda and Yoga at Sonne und Mond, Berlin, Germany. CK receives honoraria for lecturing in courses on Ayurveda and Yoga at European Academy for Ayurveda, Birstein, Germany and at Sonne und Mond, Berlin, Germany and receives consultancy fees from Bruno Zimmer, Oberthal, Germany for scientific advisory. He also treats patients with Ayurveda in a hospital department specialized on Integrative Medicine on a regular basis.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Chaturvedi, S, and Patwardhan, B. Building bridges for integrative medicine. Lancet Psychiatry. (2016) 3:705–6. doi: 10.1016/s2215-0366(16)30145-6
2. Morandi, A, and Nambi, ANN. An integrated view of health and well-being—Bridging Indian and Western knowledge. Dordrecht: Springer (2013). 39–57.
3. World Health Organization. (2022). WHO benchmarks for the training of Ayurveda. WHO Website. https://www.who.int/publications/i/item/9789240042711
4. AYUSH General guidelines for clinical evaluation of Ayurvedic interventions. https://www.ayush.gov.in/docs/clinical_evaluation.pdf (Accessed December 5, 2023).
5. Sharma, RK, and Agnivesa’s, Dash B.Caraka Samhita: Text with English translation & critical exposition based on Cakrapani Datta’s Ayurveda Dipika. Varanasi: Chowkhamba Sanskrit Series Office (2002).
6. Sharma, H, and Clark, CS. Ayurvedic healing: Contemporary maharishi Ayurveda medicine and science. Second ed Singing Dragon (2011).
7. Ministry of Ayurveda, Yoga, Naturopathy, Unani, Siddha, Sowa-Rigpa and Homoeopathy. Government of India Website. Available at: https://www.ayush.gov.in/ (Accessed December 01, 2023).
8. Kamath, CR, and Shah, B. Phytochemical screening and standardization of polyherbal formulation: maharishi Amrit Kalash 5. Int J Pharm Pharm Sci. (2014) 6:96–8.
9. Government of India, Ministry of Health and Family Welfare, Department of Indian System of Medicines and Homeopathy: The Ayurvedic Formulary of India, 2nd Edition, Part 1. Brahma Rasayana. New Delhi. (2003). 43.
10. Srikantha Murthy, KR. Vagbhata’s Astanga Hrdayam. Uttarasthana, Adhyaya 39: Chaukhamba Orientalia, Varanasi: Chaukhamba Orientalia (2000) 39:15–23.
11. Sharma, R, Martins, N, Kuca, K, Chaudhary, A, Kabra, A, Rao, MM, et al. Chyawanprash: a traditional Indian bioactive health supplement. Biomol Ther. (2019) 9:161. doi: 10.3390/biom9050161
12. Venkataraman, ND, Sundaram, RM, Somanathan, SS, Prabhu, TP, Rama, KP, and Priya, SJS. Chyavanprash: a multimodal approach to COVID-19 management. Int J Pathogen Res. (2021) 8:12–8. doi: 10.9734/ijpr/2021/v8i130193
13. Madaan, A, Kanjilal, S, Gupta, A, Sastry, JL, Verma, R, Singh, AT, et al. Evaluation of immunostimulatory activity of Chyawanprash using in vitro assays. Indian J Exp Biol. (2015) 53:158–63.
14. Parle, M, and Bansal, N. Traditional medicinal formulation, Chyawanprash— A review. Indian Journal of Traditional Knowledge, (2006). 5:484–488.
15. Inaba, R, Sugiura, H, and Iwata, H. Immunomodulatory effects of maharishi Amrit Kalash 4 and 5 in mice. Nihon Eiseigaku Zasshi. (1995) 50:901–5. doi: 10.1265/jjh.50.901
16. Sugiura, H, Inaba, R, Iwata, H, Nishida, H, and Tanaka, T. Modifying effects of maharishi amrit kalash 4 and 5 on phagocytic and digestive functions of macrophages in male icr mice. Environ Health Prev Med. (1998) 3:50–4. doi: 10.1007/bf02931239
17. Penza, M, Montani, C, Jeremic, M, et al. MAK-4 and -5 supplemented diet inhibits liver carcinogenesis in mice. BMC Complement Altern Med. (2007) 7:19. doi: 10.1186/1472-6882-7-19
18. Sundaram, V, Hanna, AN, Lubow, GP, Koneru, L, Falko, JM, and Sharma, HM. Inhibition of low-density lipoprotein oxidation by oral herbal mixtures maharishi Amrit Kalash-4 and maharishi Amrit Kalash-5 in hyperlipidemic patients. Am J Med Sci. (1997) 314:303–10. doi: 10.1097/00000441-199711000-00007
19. Zanella, I, Lorenzo, RD, and Lorenzo, DD. Effects of the dietary supplement MAK4 on oxidative stress parameters: a ¡°three-cases¡± report. Open Access Libr J. (2015) 2:68904:1. doi: 10.4236/oalib.1102150
20. Moher, D, Liberati, A, Tetzlaff, J, and Altman, DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. (2010) 8:336–41. doi: 10.1016/j.ijsu.2010.02.007
21. Page, MJ, McKenzie, JE, Bossuyt, PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
22. Higgins, JP, and Green, S. (Eds.) (2011). Cochrane handbook for systematic reviews of interventions. Version 5.1.0. Updated 2011. cochrane Website. https://handbook-5-1.cochrane.org/ (Accessed March 31, 2024).
23. Higgins, JP, Altman, DG, Gøtzsche, PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928
24. Higgins, JPT, Thompson, SG, Deeks, JJ, and Altman, DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
25. Vohra, R, Singh, R, and Shrivastava, RA. scoping review on ‘maharishi Amrit Kalash’, an ayurveda formulation for cancer prevention and management. J Ayurveda Integ Med. (2024) 15:100866. doi: 10.1016/j.jaim.2023.100866
26. ClinicalTrials.gov.Website. NCT00010725. Effects of herbal antioxidants on cardiovascular disease in older blacks. https://www.clinicaltrials.gov/study/NCT00010725 (Accessed March 31, 2024).
27. Dogra, J, and Bhargava, A. “Lipid peroxide in ischemic heart disease (IHD): inhibition by Maharishi Amrit Kalash (MAK-4 and MAK-5) herbal mixtures,” Federation of the American Societies for Experimental Biology Journal. 14.4 (2000). A121.
28. Glaser, JL, Roberts, DL, and Wallace, RK. Improvement in seasonal respiratory allergy with maharishi Amrit Kalash 5, an Ayurvedic herbal immunomodulator. Presented at: proceedings of the American Association of Ayurvedic Medicine. (1991).
29. Gurlee, PG, Gustavson, J, Keely, M, Wronskie-Bodier, C, and Glaser, JL. Clinical effect of maharishi Amrit Kalash 4 and 5 herbal preparations in the rehabilitation of late neurological deficits following head injury. Presented at: proceedings of the American Association of Ayurvedic Medicine. (1991).
30. Robinson, DK. Health promotion in the elderly: changes in endocrine function after three months of modern or traditional treatment. Dissertation. Fairfield: Maharishi University of Management (1999).
31. Arenander, A. The Cognitive & Behavioral Effects of the Transcendental Meditation Program & Maharishi Vedic Medicine on children with attention deficit hyperactivity disorder (ADHD). Brain Research Institute; (2000). 1–33. https://www.brainresearchinstitute.org/research/adhd/adhd_background.pdf (Accessed May 26, 2023).
32. Hicks, J. The use of nutrition and dietary supplements as complimentary Care in Children with Cancer. Cure Our Children. (2012) 12
33. Sharma, HM, Hanissian, S, Rattan, AK, Stern, SL, and Tejwani, GA. Effect of maharishi Amrit Kalash on brain opioid receptors and neuropeptides. J Res Educ Indian Med. (2015) 10:1.
34. Tomlinson, PF Jr. Superoxide scavenging, hydrogen peroxide deactivation, and benzo (a) pyrene chemoprotective activities of a maharishi Ayur-Veda food supplement. Maharishi Amrit Kalash: Maharishi University of Management (1994).
35. Hanna, AN, Sundaram, V, Falko, JM, Stephens, RE, and Sharma, HM. Effect of herbal mixtures MAK-4 and MAK-5 on susceptibility of human LDL to oxidation. Compl Med Int. (1996) 3:28–36.
36. Mamtani, R, and Mamtani, R.. (2005). Ayurveda and yoga in cardiovascular diseases. Cardiology in review, 13, 155–162.
37. Fields, JZ, Walton, KG, Schneider, RH, Nidich, S, Pomerantz, R, Suchdev, P, et al. Effect of a multimodality natural medicine program on carotid atherosclerosis in older subjects: a pilot trial of maharishi Vedic medicine. Am J Cardiol. (2002) 89:952–8. doi: 10.1016/s0002-9149(02)02245-2
38. Sharma, HM, Dillbeck, MC, and Dillbeck, SL. Implementation of the transcendental meditation program and maharishi Ayur-Veda to prevent alcohol and drug abuse among juveniles at risk. Alcohol Treat Q. (1994) 11:429–57. doi: 10.1300/J020v11n03_08PT-Review
39. Nidich, SI, Morehead, P, Nidich, RJ, Sands, D, and Sharma, H. The effect of the maharishi student Rasayana food supplement on non-verbal intelligence. Personal Individ Differ. (1993) 15:599–602. doi: 10.1016/0191-8869(93)90345-4
40. Gelderloos, P, Ahlstrom, HHB, Orme-Johnson, DW, Robinson, DK, Wallace, RK, and Glaser, JL. Influence of a maharishi Ayur-Vedic herbal preparation on age-related visual discrimination. Int J Psychosom. (1990) 37:25–9.
41. Samaiya, A, Srivastava, A, Taranikanli, V, et al. Reduction in toxicity of Cancer chemotherapy by maharishi Amrit Kalash (MAK) - an Ayurvedic herbal compound. Ann Nat Acad Med Sci. (1999) 35:109–19.
42. Saxena, A, Dixit, S, Aggarwal, S, Seenu, V, Prashad, R, Bhushan, S, et al. An ayurvedic herbal compound to reduce toxicity to cancer chemotherapy: a randomized controlled trial. Indian journal of medical and Paediatric. Oncology. (2008) 29:11–8. doi: 10.4103/0971-5851.51426
43. Karnofsky DA. Criteria of performance status (P. S.) In: WJ Williams, E Beutler, AJ Erslev, and RW Rundles, editors. Hematology. New York: McGraw-Hill (1972). 4.
45. Fatima, N, Baqri, SSR, Alsulimani, A, Fagoonee, S, Slama, P, Kesari, KK, et al. Phytochemicals from Indian Ethnomedicines: promising prospects for the Management of Oxidative Stress and Cancer. Antioxidants. (2021) 10, 1606. doi: 10.3390/antiox10101606
46. Martínez-Pozas, O, Sánchez-Romero, EA, Beltran-Alacreu, H, Arribas-Romano, A, Cuenca-Martínez, F, Villafañe, JH, et al. Effects of orthopedic manual therapy on pain sensitization in patients with chronic musculoskeletal pain: an umbrella review with Meta-Meta-analysis. Am J Phys Med Rehabil. (2023) 102:879–85. doi: 10.1097/phm.0000000000002239
47. Gonzalez-Alvarez, ME, Sanchez-Romero, EA, Turroni, S, Fernandez-Carnero, J, and Villafañe, JH. Correlation between the altered gut microbiome and lifestyle interventions in chronic widespread pain patients: A systematic review. Medicina. (2023) 59:256. doi: 10.3390/medicina59020256
48. Kessler, CS, Pinders, L, Michalsen, A, and Cramer, H. Ayurvedic interventions for osteoarthritis: a systematic review and meta-analysis. Rheumatol Int. (2015) 35:211–32. doi: 10.1007/s00296-014-3095-y
49. Schulz, KF, Altman, DG, and Moher, D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med. (2010) 152:726–32. doi: 10.7326/0003-4819-152-11-201006010-00232
Keywords: Ayurveda, systematic review, traditional Indian medicine, herbal medicine, maharishi Amrit Kalash
Citation: Koch AK, Patel M, Gupta S, Wullenkord R, Jeitler M and Kessler CS (2024) Efficacy and safety of the Ayurvedic herbal preparation Maharishi Amrit Kalash: a systematic review of randomized controlled trials. Front. Med. 11:1325037. doi: 10.3389/fmed.2024.1325037
Edited by:
Puja Khare, Council of Scientific and Industrial Research, IndiaReviewed by:
Eleuterio A. Sánchez Romero, European University of Madrid, SpainAmit Kar, Institute of Bio-Resources and Sustainable Development, India
Dhirajsingh Rajput, Central Council for Research in Ayurvedic Science, India
Copyright © 2024 Koch, Patel, Gupta, Wullenkord, Jeitler and Kessler. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anna K. Koch, YW5uYS1rYXRoYXJpbmEua29jaEBjaGFyaXRlLmRl
 Anna K. Koch
Anna K. Koch Manish Patel
Manish Patel Shivenarain Gupta3,4
Shivenarain Gupta3,4 Michael Jeitler
Michael Jeitler Christian S. Kessler
Christian S. Kessler