
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Med. , 08 July 2024
Sec. Infectious Diseases: Pathogenesis and Therapy
Volume 11 - 2024 | https://doi.org/10.3389/fmed.2024.1322700
 Jiaji Ling1,2
Jiaji Ling1,2 Liting Liang1,2
Liting Liang1,2 Xingxin Liu1,2
Xingxin Liu1,2 Wenjing Wu1,2
Wenjing Wu1,2 Ziyi Yan1,2
Ziyi Yan1,2 Wei Zhou1,2
Wei Zhou1,2 Yongmei Jiang1,2
Yongmei Jiang1,2 Linghan Kuang1,2*
Linghan Kuang1,2*Fusarium solani, as an opportunistic pathogen, can infect individuals with immunosuppression, neutropenia, hematopoietic stem cell transplantation (HSCT), or other high-risk factors, leading to invasive or localized infections. Particularly in patients following allogeneic HSCT, Fusarium solani is more likely to cause invasive or disseminated infections. This study focuses on a pediatric patient who underwent HSCT for severe aplastic anemia. Although initial blood cultures were negative, an abnormality was detected in the 1,3-β-D-glucan test (G test) post-transplantation. To determine the causative agent, blood samples were subjected to metagenomic next-generation sequencing (mNGS) and blood cultures simultaneously. Surprisingly, the results of matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) and mNGS differed slightly, with mNGS identifying Nectria haematonectria, while MALDI-TOF MS based on culture showed Fusarium solani. To clarify the results, Sanger sequencing was performed for further detection, and the results were consistent with those of MALDI-TOF MS. Since the accuracy of Sanger sequencing is higher than that of mNGS, the diagnosis was revised to invasive Fusarium solani infection. With advancements in technology, various detection methods for invasive fungi have been developed in recent years, such as mNGS, which has high sensitivity. While traditional methods may be time-consuming, they are important due to their high specificity. Therefore, in clinical practice, it is essential to utilize both traditional and novel detection methods in a complementary manner to enhance the diagnosis of invasive fungal infections.
Fusarium is a common saprophytic fungus in soil and is a conditional pathogen capable of causing invasive or localized infections (18). As an opportunistic pathogen, Fusarium has been increasingly reported to cause invasive or localized infections in humans in recent years, primarily reported in the United States, France, Italy, and others. However, there are limited relevant reports in China (22). In patients with normal immune function, Fusarium often causes superficial or localized lesions, such as onychomycosis and keratitis (12). A favorable prognosis can be achieved after active antifungal treatment in most cases (9). However, in patients undergoing organ transplantation, hematological malignancies, or allogeneic HSCT, Fusarium can easily cause invasive or even disseminated infections, typically affecting the lungs, blood vessels, and sinuses, as well as manifest as cranial-cerebral infections (20).
As an opportunistic pathogen, Fusarium infection is not as common as Aspergillus infection. Some Fusarium species are opportunistic pathogens that mainly cause local infections in individuals with normal immune systems (10). However, Fusarium can lead to invasive infections in patients with malignant hematological diseases, aplastic anemia, organ transplantation, or those undergoing chemotherapy (18, 20). Disseminated Fusarium disease almost only occurs in immunocompromised individuals, especially in cancer patients with neutropenia caused by cytotoxic drug therapy or bone marrow transplantation (13, 21). Granulocytes and macrophages play an important role in the immune defense against Fusarium (33). Fusarium can cause disseminated infections in patients with severely compromised immunity. In some research centers, Fusarium is the second most common pathogen after Aspergillus infection in high-risk populations such as leukemia patients, solid organ transplant recipients, and allogeneic bone marrow or stem cell transplant recipients (1). The prognosis of disseminated Fusarium disease, which can be life-threatening, is largely influenced by immune status (13, 21, 30).
Fusarium mainly works by producing Fusarium toxins; some of them produce A-type and B-type trichothecene mycotoxins (25). The A-type trichothecene includes various toxins such as T-2 toxin and HT-2 toxin. Of these, T-2 toxin is the most important toxin in this group because it inhibits eukaryotic protein synthesis and is highly toxic to white blood cells, often leading to immune suppression. B-type trichothecene mycotoxins include nivalenol, deoxynivalenol, 3-/15 acetyl nivalenol, and Fusarenon-X. Scientific research has shown that these toxins affect the immune system of experimental animals, leading to their increased susceptibility to various pathogens (7, 16).
In the past, invasive fungal infections were mainly diagnosed through host factors, clinical features, microbiological examination, and histopathology. With the rapid progress of society, novel technologies for diagnosing invasive fungal infections have emerged, such as polymerase chain reaction-based methods and metagenomic next-generation sequencing. In this study, we diagnosed an invasive Fusarium solani infection with the collaboration of traditional microbial detection methods and metagenomic next-generation sequencing in a pediatric patient.
A 3-year-old girl was diagnosed with severe aplastic anemia in another hospital 9 months ago. After admission to the Pediatric Hematology Department of our hospital, HSCT was scheduled according to an individualized treatment plan. Before transplantation, a chemotherapy pretreatment regimen was administered according to standard protocol. Cefoperazone sodium and tazobactam sodium were used to prevent bacterial infections, micafungin was used to prevent fungal infections, ganciclovir was used to prevent viral infections, and the combination of propranolol and rituximab was used to prevent graft-versus-host disease (GVHD). Twelve days after admission, the patient underwent HLA 7/12-compatible haploid allogeneic HSCT and HLA 5/10-compatible non-hematopoietic stem cell transplantation. Hematologic blood pressure increased during infusion, and no other abnormalities were observed.
Seventeen days after HSCT, the patient showed GVHD-related reactions, which gradually worsened. At the same time, liver function-related enzymes increased, and treatment was performed using plasma exchange. Then, the child developed intermittent fevers. Considering that the infection was worsening and C-reactive protein (CRP) levels were continuously elevated, imipenem and cilastatin sodium were administered to fight bacterial infections, and micafungin was continued to prevent fungal infections.
Forty-nine days after HSCT, the patient had a repeated fever with a thermal peak of 39.2°C, accompanied by increased CRP. The G test was positive at 150 pg/mL (normal value <60 pg/mL), the galactomannan test (GM test) was negative, and the blood culture was negative. A chest computed tomography (CT) examination showed multiple nodules and patchy blurred shadows in both lungs. The lower lobes of both lungs were characterized by lamellar consolidation with obvious interstitial changes (Figure 1A). Thus, voriconazole was then added to control the fungal infection.
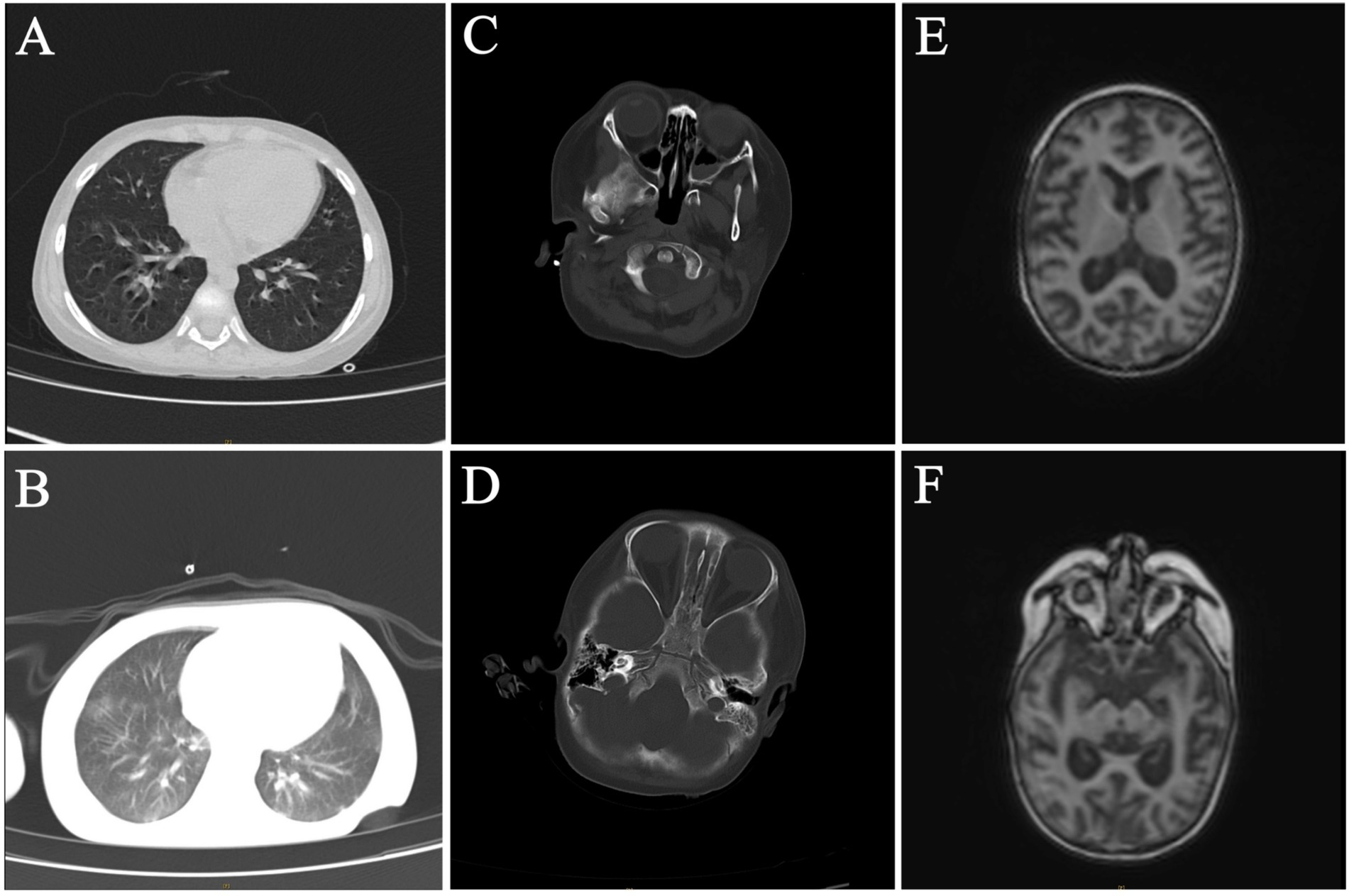
Figure 1. Radiological examination of the patient. (A,B) Chest CT on 49 days after HSCT and 81 days after HSCT. (C–F) Craniocerebral enhanced MRI and brain CT on 49 days after HSCT and 81 days after HSCT.
The same treatment regimen was continued after the initial HSCT, with consecutive negative blood culture results observed during this period. Eighty-one days after HSCT, the child developed convulsions, respiratory failure with central mechanism, and electrolyte disturbance. Compared to the previous chest CT results, the lesions and interstitial changes in the lungs were aggravated (Figure 1B). Compared to 49 days after HSCT, the craniocerebral MRI with contrast and brain CT showed abnormal signals in the cortex and subcortical regions of both cerebral hemispheres, as well as in the anterior and posterior corners of both cerebral ventricles, notably in the bilateral frontal lobe and the right temporal lobe. A nodular abnormal signal was noted in the right frontal white matter area, the cerebral sulci and fissures were widened and deepened, and the bilateral lateral ventricles were widened, indicating brain atrophy changes (Figures 1C–E).
The patient’s inflammatory markers were significantly elevated (CRP 370 mg/L, PCT 1.29 ng/mL), the G test result was positive (218 pg/mL), the GM test was negative, and the blood culture remained negative. Therefore, the antifungal treatment regimen was adjusted to the combination of micafungin and amphotericin B. The patient’s condition was complex and unstable, posing a threat to life at any time. Ninety-four days after HSCT, the patient was transferred to the PICU, and the G test turned negative after treatment. However, the patient was in a coma state, and blood tests indicated significantly reduced levels of red blood cells, white blood cells, and platelets, which led to diffuse intravascular coagulation abnormalities. Continuous infusion of blood products was used to support treatment.
One hundred and sixteen days after HSCT, the G test was positive again (254 pg/mL), but the GM test was still negative. The child developed herpes lesions around her lip and body, containing bloody substances, which gradually ruptured and formed necrotic scabs. In order to identify the cause as soon as possible, the medical team performed mNGS of the pathogen in addition to the routine double-vial blood cultures. Two days later, the mNGS revealed a rare fungus, Nectria haematonectria. Thus, according to the guidelines, the team used voriconazole combined with micafungin antifungal therapy and abandoned the use of vancomycin and ceftazidime.
The blood cultures showed a positive result after 4 days of culture, and oval fungal spores were observed under a microscope after Gram staining (Figure 2A), which supported our decision to initiate antifungal treatment. They were inoculated on Sabouraud agar (SDA) after 96 h of culture. The colony morphology on SDA is shown in Figure 2B; the colony exhibited dense, smooth, white mycelium, and the reverse color was reddish in the center and yellow in peripheral areas. The microstructure of the colony was observed by a KOH fungal smear test, Gram staining, lactic acid phenol cotton blue staining, and fungal fluorescent staining. Microscopic examination revealed septate, hyaline hyphae that were 4–5 microns in diameter, branching at acute angles, with numerous microconidia and sparse macroconidia. The microconidia were hyaline, unicellular to bicellular, and cylindrical to oval and formed long lateral phialides. The macroconidia were fusiform, cylindrical, moderately curved, and three or four septate (Figures 2C–F).
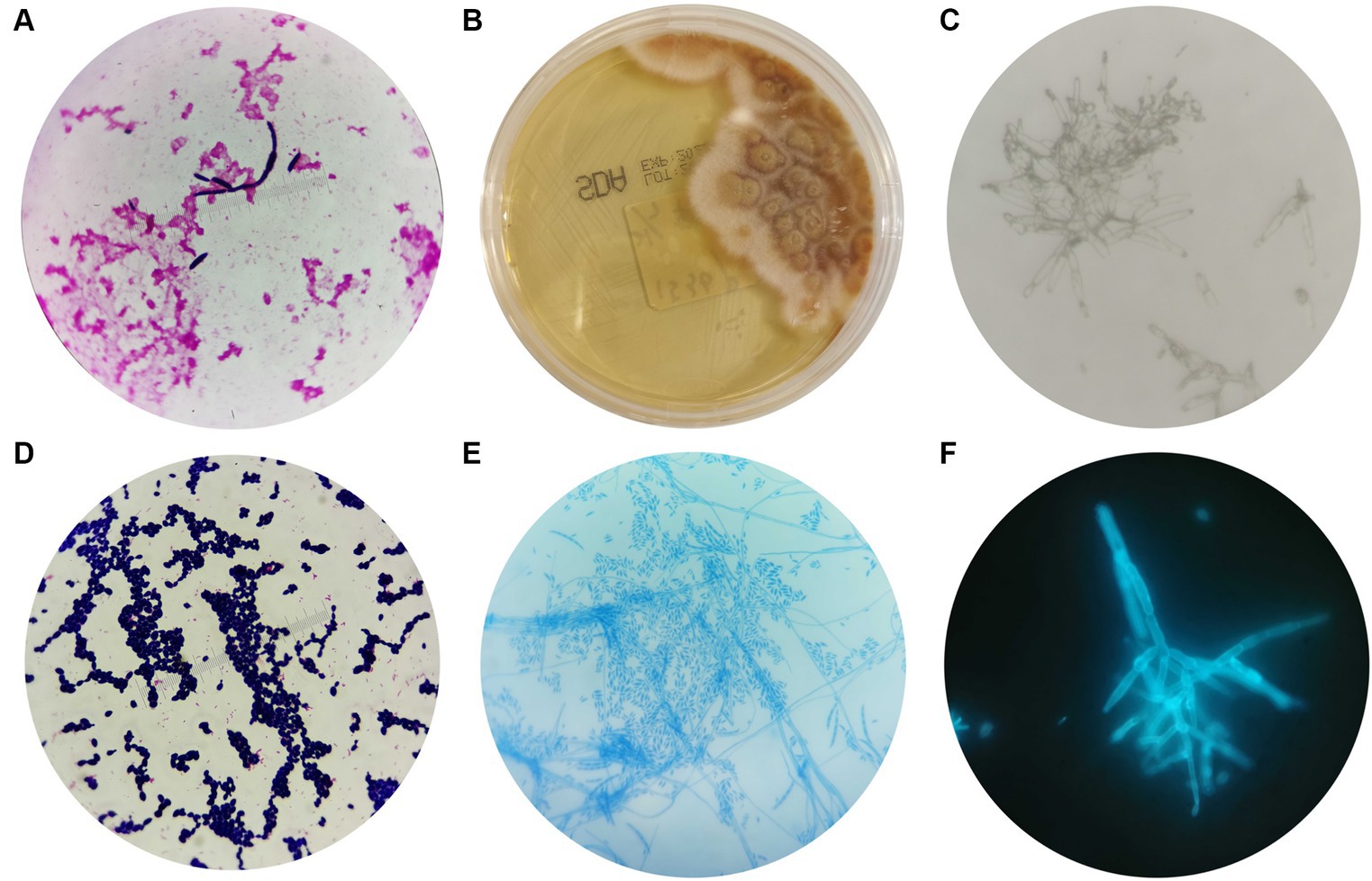
Figure 2. Morphological characteristics of Fusarium solani. (A) After 4 days of blood cultivation, oval fungal spores could be observed by microscope after Gram staining (magnification 10 × 100). (B) The morphology of Fusarium solani on SDA after 96 h of culture. (C–F) The morphologies of Fusarium solani were observed using a KOH fungal smear test (magnification 10 × 100), Gram staining (magnification 10 × 100), lactic acid phenol cotton blue staining (magnification 10 × 40), and fungal fluorescent staining (magnification 10 × 100).
In addition, the colony was identified as Fusarium solani with 99.9% confidence using MALDI-TOF MS. However, the results did not match those obtained from mNGS. Therefore, Sanger sequencing was adopted to confirm the identity of the fungus. Prior to sequencing, genomic DNA was extracted, and agarose gel electrophoresis was performed. The Sanger sequencing results confirmed the presence of Fusarium solani, which was consistent with the MALDI-TOF MS findings.
To explore the minimal inhibitory concentrations (MICs) of Fusarium solani, an antifungal susceptibility test was performed using TDR-YEAST, and the values are summarized in Table 1. MIC values 1 and 2 represent the MICs of Fusarium solani isolated from aerobic and anaerobic culture bottles, respectively.
Combined with multiple pieces of evidence, the patient developed recurrent fever after HSCT, indicative of a weakened immune system. Pulmonary CT scans indicated a fungal infection, with the G test showing positive results multiple times and the GM test remaining negative (Figure 3). Tests for respiratory pathogens, Epstein–Barr virus (EBV), and cytomegalovirus (CMV) were all negative. Although there were slight discrepancies between the results of MALDI-TOF MS and mNGS, Sanger sequencing was conducted for further detection and yielded consistent results with MALDI-TOF MS. Due to the higher accuracy of Sanger sequencing compared to mNGS, the diagnosis was revised to an invasive Fusarium solani infection. Following guidelines and MICs for Fusarium solani, we adjusted the anti-infective treatment regimen to include a combination of voriconazole and amphotericin B liposomes for the treatment of invasive Fusarium solani infection. We continued to monitor the antifungal efficacy of the treatment. Unfortunately, after more than 5 months of antifungal treatment, the child’s condition continued to deteriorate, and eventually, her parents had to face the difficult decision of discontinuing the treatment.
Although the efficacy of invasive Fusarium solani infection was not satisfactory in this study, experience can be accumulated for the management of similar cases in the future. In this study, after HSCT, successive blood cultures were negative in the early stage, but there was an abnormality in the G test. To identify the causative pathogen, blood samples were simultaneously sent for mNGS and blood culture. Interestingly, the results of MALDI-TOF MS and mNGS were different. The mNGS result was Nectria haematonectria, while culture-based MALDI-TOF MS showed Fusarium solani. To clarify the results, Sanger sequencing was performed for further detection and the results displayed the same with MALDI-TOF MS. Since the accuracy of Sanger sequencing is higher than mNGS, the diagnosis was revised to invasive Fusarium solani infection.
The main manifestations of invasive Fusarium solani infection are fever, skin appearance, and other related changes in invasive organs, but none of them are specific (31). Once infected, the skin lesions typically present as multiple erythematous papules and painful nodular lesions with central necrosis, which may appear as gangrenous purpura and can involve all parts of the body, especially the extremities. In addition, the lesions have an evolutionary stage, 75% of patients have skin lesions, and approximately 10% of patients have concentric rings. In this case, papular changes emerged at the beginning of admission, and there were cutaneous lesions of the trunk due to Fusarium solani infection. As we all know, invasive Fusarium solani infections have a poor prognosis, and patients are often accompanied by neutropenia and immunocompromised. An analysis of adult case data from the United States and Canada showed that the incidence of invasive Fusarium infection after HSCT was 35.4% (11). Maged et al. (17) calculated that the mortality rate of disseminated Fusarium infection reached 50%. A recent study of children with invasive Fusarium infection showed a 90-day survival rate of 77%, which is significantly higher than that of adults, but the primary disease distribution is different (2).
In this case, the results of MALDI-TOF MS and mNGS were slightly different. The mNGS result was Nectria haematonectria, while culture-based MALDI-TOF MS showed Fusarium solani. To find out the truth, Sanger sequencing was performed for further detection and the results displayed the same with MALDI-TOF MS. In order to clarify the results, many relevant studies have been read, and we have summarized the following reasons: first, Sanger sequencing has a longer read length and higher accuracy than mNGS, second, Fusarium is a sexual fungus of Nectria, so it is difficult to distinguish them using mNGS, and third, the amount of fungi in the original specimen was too small, and the background signal was too high. In addition, due to the thick fungal wall, it is difficult to break the wall to extract genomic DNA. Last but not least, improper storage of specimens led to the degradation of nucleic acids during mNGS sequencing.
The incidence of invasive fungal infection is on the rise with a high fatality rate (28). In order to improve the detection rate of invasive fungal infections and seize the opportunity for early treatment, many novel technologies and methods have been developed in recent years (3–6, 8, 14, 15, 19, 23, 26, 27, 29, 32, 34, 35). These novel methods are listed in Table 2 along with traditional invasive fungal detection methods.
The treatment of disseminated Fusarium infection is very difficult, and the mortality rate is extremely high (24). According to the 2021 global guideline for the diagnosis and management of rare mold infections, voriconazole or a lipid formulation of amphotericin B was strongly recommended for the primary treatment of invasive fusariosis. Amphotericin B deoxycholate should not be used if other active antifungal agents are available. For other agents, a conditional recommendation is indicated. Combination therapy is frequently used in the primary treatment of invasive fusariosis because of the severity of the disease, difficulties in achieving voriconazole trough concentrations within the targeted range, and because minimum inhibitory concentrations for azoles and polyenes are often high.
Primary combination therapy, with the potential for early step-down to monotherapy later (once minimum inhibitory concentrations of azoles and polyenes are available), is an approach strongly recommended (9). In this case, despite the patient continuing to use micafungin to prevent fungal infection, they developed a Fusarium solani bloodstream infection, indicating resistance to echinocandin drugs. The selection of antifungal drugs should be based on the adjustment plan of antifungal susceptibility testing conducted in vitro. Additionally, elevated neutrophils are an important prognostic factor for this disease. The patient has had extremely low neutrophil levels since admission, with a minimum absolute neutrophil count (ANC) of 0.00 × 109/L, which is also a significant factor for poor prognosis.
In recent years, the incidence rate of disseminated Fusarium disease in patients with hematologic malignancies has increased, and the mortality rate is extremely high. Attention should be paid to the diagnosis of the disease to distinguish it from other infections and to determine the most appropriate antifungal treatment program as soon as possible. As a case study has shown, early intervention using mNGS can be beneficial, especially when conventional treatments have failed; however, it is important to carefully evaluate the accuracy of the identification results. Additionally, a combination of traditional and modern detection methods should be utilized to enhance the diagnosis of invasive fungal infections.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
The studies involving humans were approved by Medical Ethics Committee, West China Second University Hospital, Sichuan University. The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from primarily isolated as part of your previous study for which ethical approval was obtained. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
JL: Conceptualization, Writing – review & editing, Methodology, Writing – original draft, Data curation, Validation. LL: Writing – review & editing, Formal analysis, Software. XL: Validation, Writing – review & editing. WW: Funding acquisition, Writing – review & editing. ZY: Funding acquisition, Writing – review & editing. WZ: Data curation, Writing – review & editing. YJ: Project administration, Resources, Writing – review & editing. LK: Funding acquisition, Resources, Supervision, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the National Science Foundation of China (No. 82300015) and the Sichuan Science and Technology Program (No. 2024NSFSC1942 to XL, No. 2023NSFSC1699 to LK, and No. 2022NSFSC1420 to WW).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Al-Hatmi, AMS, Bonifaz, A, Ranque, S, Sybren de Hoog, G, Verweij, PE, and Meis, JF. Current antifungal treatment of fusariosis. Int J Antimicrob Agents. (2018) 51:326–32. doi: 10.1016/j.ijantimicag.2017.06.017
2. Benish, M, Elitzur, S, Arad-Cohen, N, Barg, AA, Ben-Harosh, M, Bielorai, B, et al. Invasive fusariosis in pediatric hematology/oncology and stem cell transplant patients: a report from the Israeli Society of Pediatric Hematology-Oncology. J Fungi. (2022) 8:387. doi: 10.3390/jof8040387
3. Bhatnagar, I, Mahato, K, Ealla, KKR, Asthana, A, and Chandra, P. Chitosan stabilized gold nanoparticle mediated self-assembled gliP nanobiosensor for diagnosis of invasive Aspergillosis. Int J Biol Macromol. (2018) 110:449–56. doi: 10.1016/j.ijbiomac.2017.12.084
4. Dabrowski, M, Sharma, PS, Iskierko, Z, Noworyta, K, Cieplak, M, Lisowski, W, et al. Early diagnosis of fungal infections using piezomicrogravimetric and electric chemosensors based on polymers molecularly imprinted with d-arabitol. Biosens Bioelectron. (2016) 79:627–35. doi: 10.1016/j.bios.2015.12.088
5. Dina, NE, Gherman, AMR, Chis, V, Sarbu, C, Wieser, A, Bauer, D, et al. Characterization of clinically relevant fungi via SERS fingerprinting assisted by novel chemometric models. Anal Chem. (2018) 90:2484–92. doi: 10.1021/acs.analchem.7b03124
6. Filkins, LM, Bryson, AL, Miller, SA, and Mitchell, SL. Navigating clinical utilization of direct-from-specimen metagenomic pathogen detection: clinical applications, limitations, and testing recommendations. Clin Chem. (2020) 66:1381–95. doi: 10.1093/clinchem/hvaa183
7. Góral, T, Wiśniewska, H, Ochodzki, P, Nielsen, L, Walentyn-Góral, D, and Stępień, Ł. Relationship between Fusarium head blight, kernel damage, concentration of Fusarium biomass, and Fusarium toxins in grain of winter wheat inoculated with Fusarium culmorum. Toxins. (2018) 11:2. doi: 10.3390/toxins11010002
8. Han, D, Li, R, Shi, J, Tan, P, Zhang, R, and Li, J. Liquid biopsy for infectious diseases: a focus on microbial cell-free DNA sequencing. Theranostics. (2020) 10:5501–13. doi: 10.7150/thno.45554
9. Hoenigl, M, Salmanton-Garcia, J, Walsh, TJ, Nucci, M, Neoh, CF, Jenks, JD, et al. Global guideline for the diagnosis and management of rare mould infections: an initiative of the European Confederation of Medical Mycology in cooperation with the International Society for Human and Animal Mycology and the American Society for Microbiology. Lancet Infect Dis. (2021) 21:e246–57. doi: 10.1016/S1473-3099(20)30784-2
11. Horn, DL, Freifeld, AG, Schuster, MG, Azie, NE, Franks, B, and Kauffman, CA. Treatment and outcomes of invasive fusariosis: review of 65 cases from the PATH Alliance(®) registry. Mycoses. (2014) 57:652–8. doi: 10.1111/myc.12212
12. Kaur, R, and Maheshwari, M. Hyperkeratotic warty skin lesion of foot caused by Fusarium oxysporum. Indian J Dermatol. (2013) 58:159. doi: 10.4103/0019-5154.108082
13. Khwaja, A, Bjorkholm, M, Gale, RE, Levine, RL, Jordan, CT, Ehninger, G, et al. Acute myeloid leukaemia. Nat Rev Dis Primers. (2016) 2:16010. doi: 10.1038/nrdp.2016.10
14. Kwasny, D, Tehrani, SE, Almeida, C, Schjodt, I, Dimaki, M, and Svendsen, WE. Direct detection of Candida albicans with a membrane based electrochemical impedance spectroscopy sensor. Sensors. (2018) 18:2214. doi: 10.3390/s18072214
15. Mery, A, Sendid, B, Francois, N, Cornu, M, Poissy, J, Guerardel, Y, et al. Application of mass spectrometry technology to early diagnosis of invasive fungal infections. J Clin Microbiol. (2016) 54:2786–97. doi: 10.1128/JCM.01655-16
16. Meyer, JC, Birr, T, Hennies, I, Wessels, D, and Schwarz, K. Reduction of deoxynivalenol, T-2 and HT-2 toxins and associated Fusarium species during commercial and laboratory de-hulling of milling oats. Food Addit Contam A. (2022) 39:1163–83. doi: 10.1080/19440049.2022.2059576
17. Muhammed, M, Anagnostou, T, Desalermos, A, Kourkoumpetis, TK, Carneiro, HA, Glavis-Bloom, J, et al. Fusarium infection: report of 26 cases and review of 97 cases from the literature. Medicine. (2013) 92:305–16. doi: 10.1097/MD.0000000000000008
18. Nucci, M, and Anaissie, E. Fusarium infections in immunocompromised patients. Clin Microbiol Rev. (2007) 20:695–704. doi: 10.1128/CMR.00014-07
19. Obrucova, H, Kotaskova, I, Tihelkova, R, Hola, V, Ruzicka, F, and Freiberger, T. Fluorescent capillary electrophoresis is superior to culture in detecting Candida species from samples of urinary catheters and ureteral stents with mono- or polyfungal biofilm growth. J Clin Microbiol. (2019) 57:e01861. doi: 10.1128/JCM.01861-18
20. Pana, ZD, Roilides, E, Warris, A, Groll, AH, and Zaoutis, T. Epidemiology of invasive fungal disease in children. J Pediatric Infect Dis Soc. (2017) 6:S3–S11. doi: 10.1093/jpids/pix046
21. Pizzo, PA . Management of Patients with fever and neutropenia through the arc of time: a narrative review. Ann Intern Med. (2019) 170:389–97. doi: 10.7326/M18-3192
22. Poole, GJ, Smiley, RW, Walker, C, Huggins, D, Rupp, R, Abatzoglou, J, et al. Effect of climate on the distribution of Fusarium spp. causing crown rot of wheat in the Pacific Northwest of the United States. Phytopathology. (2013) 103:1130–40. doi: 10.1094/PHYTO-07-12-0181-R
23. Ribes, A, Aznar, E, Santiago-Felipe, S, Xifre-Perez, E, Tormo-Mas, MA, Peman, J, et al. Selective and sensitive probe based in oligonucleotide-capped nanoporous alumina for the rapid screening of infection produced by Candida albicans. ACS Sensors. (2019) 4:1291–8. doi: 10.1021/acssensors.9b00169
24. Salmanton-Garcia, J, Hoenigl, M, Gangneux, JP, Segal, E, Alastruey-Izquierdo, A, Arikan Akdagli, S, et al. The current state of laboratory mycology and access to antifungal treatment in Europe: a European Confederation of Medical Mycology survey. Lancet Microbe. (2023) 4:e47–56. doi: 10.1016/S2666-5247(22)00261-0
25. Schollenberger, M, Drochner, W, and Muller, HM. Fusarium toxins of the scirpentriol subgroup: a review. Mycopathologia. (2007) 164:101–18. doi: 10.1007/s11046-007-9036-5
26. Serin, I, and Dogu, MH. Serum Aspergillus galactomannan lateral flow assay for the diagnosis of invasive aspergillosis: a single-centre study. Mycoses. (2021) 64:678–83. doi: 10.1111/myc.13265
27. Shi, X, Zhang, X, Yao, Q, and He, F. A novel method for the rapid detection of microbes in blood using pleurocidin antimicrobial peptide functionalized piezoelectric sensor. J Microbiol Methods. (2017) 133:69–75. doi: 10.1016/j.mimet.2016.12.005
28. Supatharawanich, S, Narkbunnam, N, Vathana, N, Takpradit, C, Phuakpet, K, Pongtanakul, B, et al. Invasive fungal diseases in children with acute leukemia and severe aplastic anemia. Mediterr J Hematol Infect Dis. (2021) 13:e2021039. doi: 10.4084/MJHID.2021.039
29. Swetha, PDP, Nikitha, A, Shenoy, MM, Shim, YB, and Prasad, KS. Ni/Ni(OH)2-rGO nanocomposites sensor for the detection of long forgotten mycotoxin, xanthomegnin. Talanta. (2023) 253:123953. doi: 10.1016/j.talanta.2022.123953
30. Thornton, CR . Detection of the ‘Big Five’ mold killers of humans: Aspergillus, Fusarium, Lomentospora, Scedosporium and Mucormycetes. Adv Appl Microbiol. (2020) 110:1–61. doi: 10.1016/bs.aambs.2019.10.003
31. Tortorano, AM, Richardson, M, Roilides, E, van Diepeningen, A, Caira, M, Munoz, P, et al. ESCMID and ECMM joint guidelines on diagnosis and management of hyalohyphomycosis: Fusarium spp., Scedosporium spp. and others. Clin Microbiol Infect. (2014) 20:27–46. doi: 10.1111/1469-0691.12465
32. Tsai, SM, Goshia, T, Chen, YC, Kagiri, A, Sibal, A, Chiu, MH, et al. High-throughput label-free microcontact printing graphene-based biosensor for valley fever. Colloids Surf B. (2018) 170:219–23. doi: 10.1016/j.colsurfb.2018.06.011
33. Tupaki-Sreepurna, A, and Kindo, AJ. Fusarium: the versatile pathogen. Indian J Med Microbiol. (2018) 36:8–17. doi: 10.4103/ijmm.IJMM_16_24
34. Wang, J, Meng, S, Lin, K, Yi, X, Sun, Y, Xu, X, et al. Leveraging single-cell Raman spectroscopy and single-cell sorting for the detection and identification of yeast infections. Anal Chim Acta. (2023) 1239:340658. doi: 10.1016/j.aca.2022.340658
Keywords: Fusarium solani , invasive fungal infections, detection, MALDI-TOF MS, Sanger sequencing, mNGS
Citation: Ling J, Liang L, Liu X, Wu W, Yan Z, Zhou W, Jiang Y and Kuang L (2024) Invasive Fusarium solani infection diagnosed by traditional microbial detection methods and metagenomic next-generation sequencing in a pediatric patient: a case report and literature review. Front. Med. 11:1322700. doi: 10.3389/fmed.2024.1322700
Received: 16 October 2023; Accepted: 19 June 2024;
Published: 08 July 2024.
Edited by:
Ying Zhao, Peking Union Medical College Hospital (CAMS), ChinaReviewed by:
Min Wang, Second Affiliated Hospital of Soochow University, ChinaCopyright © 2024 Ling, Liang, Liu, Wu, Yan, Zhou, Jiang and Kuang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Linghan Kuang, a3VhbmdsaW5naGFuQDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.