- 1Department of Public Health and Community Medicine, Institute of Medicine, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden
- 2The Centre for Disaster Medicine, University of Gothenburg, Gothenburg, Sweden
Background: Sulfur mustard (SM) exposure causes acute and chronic respiratory diseases. The extent of small airway dysfunction (SAD) in individuals exposed to SM is unclear. This study evaluated and compared SAD in SM-exposed and SM-unexposed participants using noninvasive lung function tests assessing small airway function.
Methods: This retrospective cohort study involved SM-exposed (n = 15, mean age: 53 ± 8 years) and SM-unexposed (n = 15, mean age: 53 ± 7 years) Kurdish-Swedish individuals in Sweden. Small airway resistance and reactance were assessed using impulse oscillometry (IOS). Nitrogen (N2) multiple breath washout (MBW) was employed to assess lung ventilation heterogeneity. The gas-exchanging capacity of the lungs was assessed using the diffusing capacity of the lungs for the carbon monoxide (DLCO) test. Lung function outcomes were reported as absolute values and z-scores. Group comparisons were performed using the Mann–Whitney U test.
Results: No statistically significant differences in age, height, or body mass index were observed between the two groups. IOS showed significantly increased small airway resistance, while N2MBW exhibited significantly increased global and acinar ventilation heterogeneity in SM-exposed individuals compared to that in unexposed individuals. SAD was identified in 14 of 15 SM-exposed individuals, defined as at least one abnormal IOS difference between resistance at 5 and 20 Hz (R5-R20) and/or area of reactance (AX) or N2MBW lung’s acinar zone (Sacin), and DLCO adjusted to the alveolar volume (DLCO/VA) outcome. Of these 14 individuals, only 5 demonstrated concordant findings across the IOS and N2MBW tests.
Conclusion: Exposure to SM was positively associated with long-term impairment of respiratory tract function in the small airways in the majority of the previously SM-exposed individuals in the present study. Furthermore, both IOS and N2MBW should be employed to detect SAD in SM-exposed survivors as they provide complementary information. Identifying and characterizing the remaining pathology of the small airways in survivors of SM exposure is a first step toward improved treatment and follow-up.
1 Introduction
Sulfur mustard (SM) is a blistering agent with alkylation capacity and lipophilic ability to form a highly reactive intermediate cyclic sulfonium ion, which can bind to many biological molecules (1–3). Details of its underlying pathological and biochemical mechanisms of action are unclear; hence, no antidotes or specific medications have been identified that treat or delay respiratory symptom progression (4).
The short and long-term effects of SM exposure primarily affect the eyes, skin, and lungs (5, 6). Over 80% of SM-exposed survivors reportedly develop respiratory symptoms, which are identified as the leading cause of mortality in survivors post-exposure (7–9).
One significant long-term consequence of SM exposure is bronchiolitis obliterans (10, 11). Other long-term effects include varying severe symptoms, such as hypersensitivity reactions, chronic dyspnea, recurrent pneumonia, chronic bronchitis, and chronic obstructive pulmonary disease (COPD) (12–15). Imaging and clinical investigations have revealed various pulmonary injuries, such as bronchiectasis, atelectasis, mosaic parenchymal attenuation, irregular and dilated central airways, bronchial wall thickening, interlobular septal wall thickening, pulmonary fibrosis, cryptogenic organizing pneumonia, emphysema, tracheomalacia, airways stenosis, and cancer, in both large and small airways (12–17).
The pulmonary pathological manifestations of exposure to SM may deteriorate over time, with individuals exposed to SM who were neither evacuated nor hospitalized after the exposure, being at risk of developing respiratory complications several decades later (9). Therefore, it is crucial to monitor individuals at high risk for any potential respiratory problems arising from SM exposure and employ alternative non-traditional monitoring methods to identify early-stage respiratory issues. While spirometry tests are the gold standard for measuring lung function, they have demonstrated a mixed picture of asthma, chronic obstructive pulmonary disease, and even normal results in SM-exposed survivors (18–21). Owing to the limited resources, conducting high-quality spirometry in many participants proved challenging; therefore, it was excluded from the study protocol in this article. Moreover, spirometry tests register modifications in airways in symptomatic patients and may not detect early-stage respiratory issues; further, they are not sensitive enough to measure the function of the small airways (airways with an internal diameter <2 mm) until significant clinical symptoms occur (22–24). Small airway dysfunction (SAD) is a major pathological aspect of lung illness, including bronchiolitis obliterans in SM-exposed survivors (25) and in patients with asthma and COPD (13, 26).
Assessing SAD can be challenging as no noninvasive gold standard is currently available (22). Spirometry, although most commonly employed, lacks consensus on the best parameter or criteria for identifying SAD. Forced expiratory flow at 25% and 75% of the pulmonary volume (FEF 25–75) is the most widely used parameter. However, its reproducibility is limited by its dependence on the forced expiratory volume in 1 s (FEV1)/forced vital capacity (FVC) and lack of adjustment for lung volume (27–29). Thus, the spirometry test is considered less sensitive in detecting modifications in small airways in the early stage; however, it may detect mild lung injury (23, 30, 31). Despite normal spirometry test results, biopsy findings indicated obliterative bronchiolitis in the small airways of the lungs in 50% of the patients 20 years after exposure to SM (10). However, spirometry tests for identifying SAD is questionable, warranting further research (32, 33). Chest high-resolution computed tomography (HRCT) is another noninvasive method that has been used for studying and evaluating abnormal changes in the morphology of small airways; however, its repeated use is limited owing to radiation risks (34). Nevertheless, follow-up assessments should be conducted in SM-exposed patients with SAD to determine the effectiveness of therapeutic interventions.
Hence, alternative lung function methods are warranted to non-invasively identify early SAD in patients, allowing timely follow up and early interventions in case of clinical respiratory manifestations. Previous studies have suggested that impulse oscillometry (IOS) and nitrogen (N2) multiple breath washout (MBW) may exhibit greater sensitivity in identifying early-stage SAD than spirometry (23, 30, 35). IOS and N2MBW are noninvasive physiological lung tests that measure different aspects of small airway function. IOS measures the mechanical properties of the lung (i.e., resistance and reactance) (36), while N2MBW provides information about ventilation distribution within the lung as a whole (lung clearance index, LCI) and more distally by using the indices of ventilation heterogeneity in the conducting (Scond) and acinar airways (Sacin), which are indicators of the rate of nitrogen washout (24, 37). Moreover, the gas exchange capacity in the lung across the alveolar-capillary interface can be assessed using the diffusing capacity of the lungs for the carbon monoxide (DLCO) test (38). The DLCO was employed to examine whether exposure to SM could impact this region of the lung in long-term. SAD has not been thoroughly studied in individuals exposed to SM with other non-traditional, noninvasive pulmonary function tests (PFTs). This study aimed to compare the outcomes of different noninvasive PFTs, e.g., IOS, N2MBW, and DLCO, to assess small airways, including alveolar function in SM-exposed survivors. We further compared these results with those of individuals who were not exposed to SM from the same ethnic group.
2 Materials and methods
This study evaluated data from a retrospective cohort study of participants exposed to SM from 1987 to 1988 in the Kurdistan Regions of Iraq and Iran. Participants recruited from Kurdish-Swedish survivors resettled in Sweden (exposed) were compared with SM-unexposed first-generation Kurdish-Swedish citizens (unexposed), ensuring that the sociodemographic characteristics were as similar as possible in both groups. Participants were recruited using posters about the study, social media announcements, and word of mouth.
The inclusion criteria for the SM-exposed group were as follows: (i) persons originally from the provenance of SM-attacked areas in the Kurdistan regions of Iraq and Iran who survived the chemical attacks of 1987 and 1988, (ii) persons with physical symptoms that developed at the time of SM exposure (signs of SM exposure), and (iii) persons aged 34–80 years. The inclusion criteria for the SM-unexposed group were as follows: (i) persons originally from Kurdistan, (ii) persons with no history of SM exposure, and (iii) persons aged 34–80 years. According to the study protocol, each exposed participant was matched with one unexposed participant. Data from 30 participants (exposed vs. unexposed: IOS, 15 vs. 15; N2MBW, 15 vs. 14; and DLCO, 13 vs. 14) were collected and included in the current study.
All the participants provided written informed consent prior to inclusion in the study. Data from five unexposed participants and one SM-exposed participant were collected between October and November 2019, and the remaining data were collected between March and June 2022. Data collection ceased during the coronavirus disease pandemic. The Regional Ethical Review Board of Gothenburg, Sweden, approved this study (2017-12-07599-17).
2.1 Exposure assessment
During the Iran-Iraq War in the 1980s, the Kurdish cities of Halabja and Sardasht were heavily attacked using chemical warfare agents, such as SM (39–41). There is no objective confirmation of SM exposure owing to a lack of evidence-based laboratory tests to verify exposure to SM after so many years. However, the exposure history was determined based on the anamnesis of the exposed participants, who originally came from chemical warfare agent-attacked regions in Kurdistan in Iraq and Iran. It is worth noting that survivors of the Halabja chemical attack faced challenges in receiving initial medical care due to limited support and delayed access to aid by the Iraqi regime. Despite some individuals receiving assistance in Iran, not all could escape or receive urgent treatment, resulting in a lack of medical documentation and verification of their exposure. Primary exposure was defined as direct contact with or inhalation of SM owing to an explosion of nearby bombs. Secondary exposure involved indirect contact with contaminated bodies or objects following the chemical attacks.
2.2 Pulmonary function measurements
Before the lung investigations, SM-exposed participants completed a questionnaire concerning the possible dose, duration, and route of SM exposure, initial signs and symptoms, current physical symptoms, medication, and other demographic variables. The IOS and N2MBW tests were conducted before the DLCO test to minimize the effect of forced expiration on airway tone; no bronchodilators were used during the tests. No coherence was used for quality control of IOS.
Impulse oscillometry was performed using the Jaeger Master Screen system (CareFusion, Germany) according to the current guidelines (42). A loudspeaker generated sound waves ranging from 5 to 35 Hz, which were transferred into the respiratory system via a suitable mouthpiece. Participants sat upright on a chair fitted with a nose clip and were asked to breathe normally through a mouthpiece for 30 s. To reduce the effect of “shunt-impedance,” the participant was asked to support their cheeks with their hands during the recordings. Mean values from three artifact-free recordings were reported. Results were acceptable if the coefficient of variation of at least 2 sets of data was less than 10%. The reported IOS variables included: (i) resistance at 5 Hz (R5) as a measure of airway resistance from the mouth to the distal point in the airway tree where the soundwave faded out, (ii) resistance at 20 Hz (R20) as a measure of central airway resistance, (iii) the difference between resistance at 5 and 20 Hz (R5-R20) as a measure of small airway resistance (43), (iv) area of reactance (AX), (v) reactance at 5 Hz (X5), both reflecting the elastance (stiffness) of the small airways, and (vi) resonant frequency (fres) reflecting the frequency at which total reactance is zero (44). All the IOS outcomes were reported in kPa/L/s (kPa/L for AX) and in z-scores (except for fres) based on the findings in a locally collected healthy control cohort (24), fres was measured in Hz (44).
Nitrogen multiple breath washout was measured using an ExhalyzerD device (EcoMedics, Switzerland) with the software Spiroware 3.3.1. Resident N2 was washed out from the lung by breathing 100% oxygen (O2). ExhalyzerD was validated (45) according to the current guidelines for inert gas washout tests. The study’s recordings were also conducted according to these guidelines; however, two artifact-free registrations deemed sufficient instead of three (46). Participants were instructed to wear a nose clip, sit straight with both feet flat on the floor, and breathe normally into a mouthpiece in a relaxed position. When a stable tidal breathing pattern was attained, the washout phase was initiated by breathing 100% oxygen. It was completed when the exhaled end-tidal N2 concentration was below 1/40th (2.5%) of its initial concentration. The reported outcomes included LCI 2.5%, Scond, and Sacin. LCI 2.5%, which reflects global ventilation heterogeneity, is calculated by dividing the cumulative expired volume during the washout by functional residual capacity (FRC) obtained from the same washout curve. Thus, LCI 2.5% is an index of how often the lung volume (FRC) needs to be “turned over” to wash out resident N2 below 2.5% of the starting concentration. Scond and Sacin are derived from a so-called concentration-normalized phase III slope (SnIII) analysis. These indices provide information about the location within the airway tree where ventilation heterogeneity is present. Scond reflects ventilation heterogeneity in the conducting airways, while Sacin reflects ventilation heterogeneity close to or at the entrance to the acinar airway zone (37). The z-scores were calculated using two locally collected healthy control cohorts (24).
Diffusing capacity of the lungs for the carbon monoxide was measured using the single-breath technique with a Jaeger Master Screen system (CareFusion, Germany). The test procedure was performed according to the guidelines for the test (47), and the outcomes were adjusted based on the participant’s current hemoglobin level. The test procedure involved the following steps: (i) Seating the participant in a chair-fitted with a nose clip and instructing them to breathe calmly at a normal rate. (ii) Having the participant perform maximum exhalation before a rapid and full inspiration to total lung capacity. (iii) Finally, instructing the participant to hold their breath at total lung capacity for approximately 10 s, followed by exhalation. During full inspiration, the participants inhaled medical lung test gas (ICOMAS, Linde Healthcare, Sweden) containing 0.3% carbon monoxide, 0.3% methane, 0.3% acetylene, and 20.9% O2 balanced in N2. The reported outcomes were alveolar volume (VA), DLCO, and their ratio (DLCO/VA). The z-scores were calculated based on the Global Lung Function Initiative reference equations for DLCO (48).
2.3 Statistical analysis
All the continuous demographic variables are presented as mean (standard deviation, SD) and median (min; max), while categorical variables are reported using frequency. Lung function outcomes are expressed as absolute values and z-scores. Limits of normality were defined as a z-score of <−1.96 or >1.96. The differences between the exposed and unexposed groups were determined based on the median (min–max) and z-score. Group comparisons were performed using the Mann–Whitney U test owing to not normally distributed data and small sample size. The significance level was set at P < 0.05. All the statistical analyses were performed using SPSS, version 28 (IBM Corp., Armonk, NY, USA).
3 Results
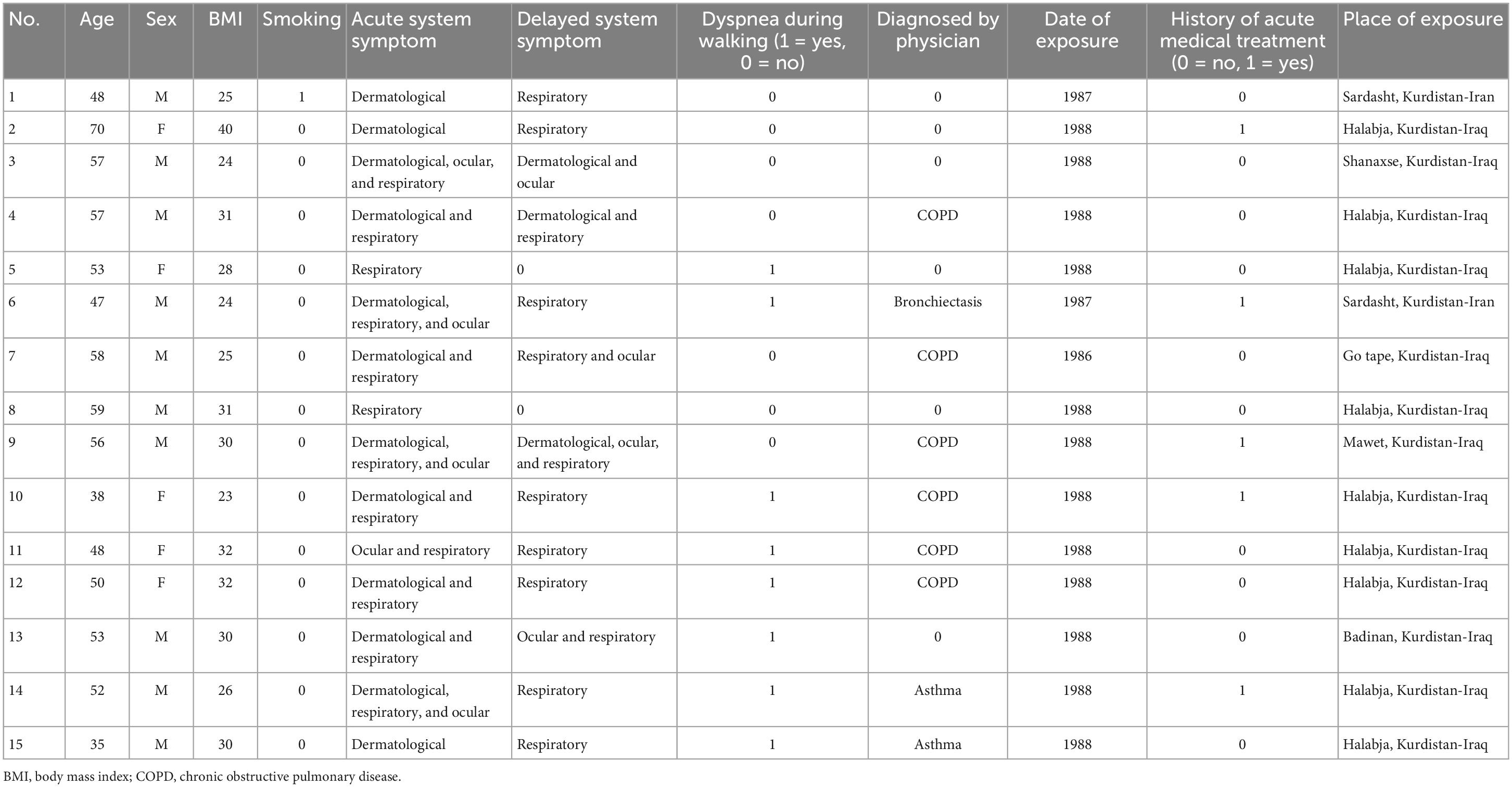
The participants’ characteristics are summarized in Table 1. No statistically significant differences were observed in the age, height, or body mass index between the two groups. All the participants, except one in the exposed group, reported no current or previous smoking history. Table 2 demonstrates the exposed participants’ characteristics, e.g., physical symptoms following exposure, of which 12 participants reported respiratory symptoms in the acute phase, 5 participants reported receiving early treatment following SM exposure, 12 experienced delayed respiratory symptoms, 6 participants had physician-diagnosed COPD, 2 were diagnosed for asthma and 1 for bronchiectasis, and 8 participants reported experiencing dyspnea upon walking on smooth ground (Table 2). None of the participants in the current study was wearing the oxygen.
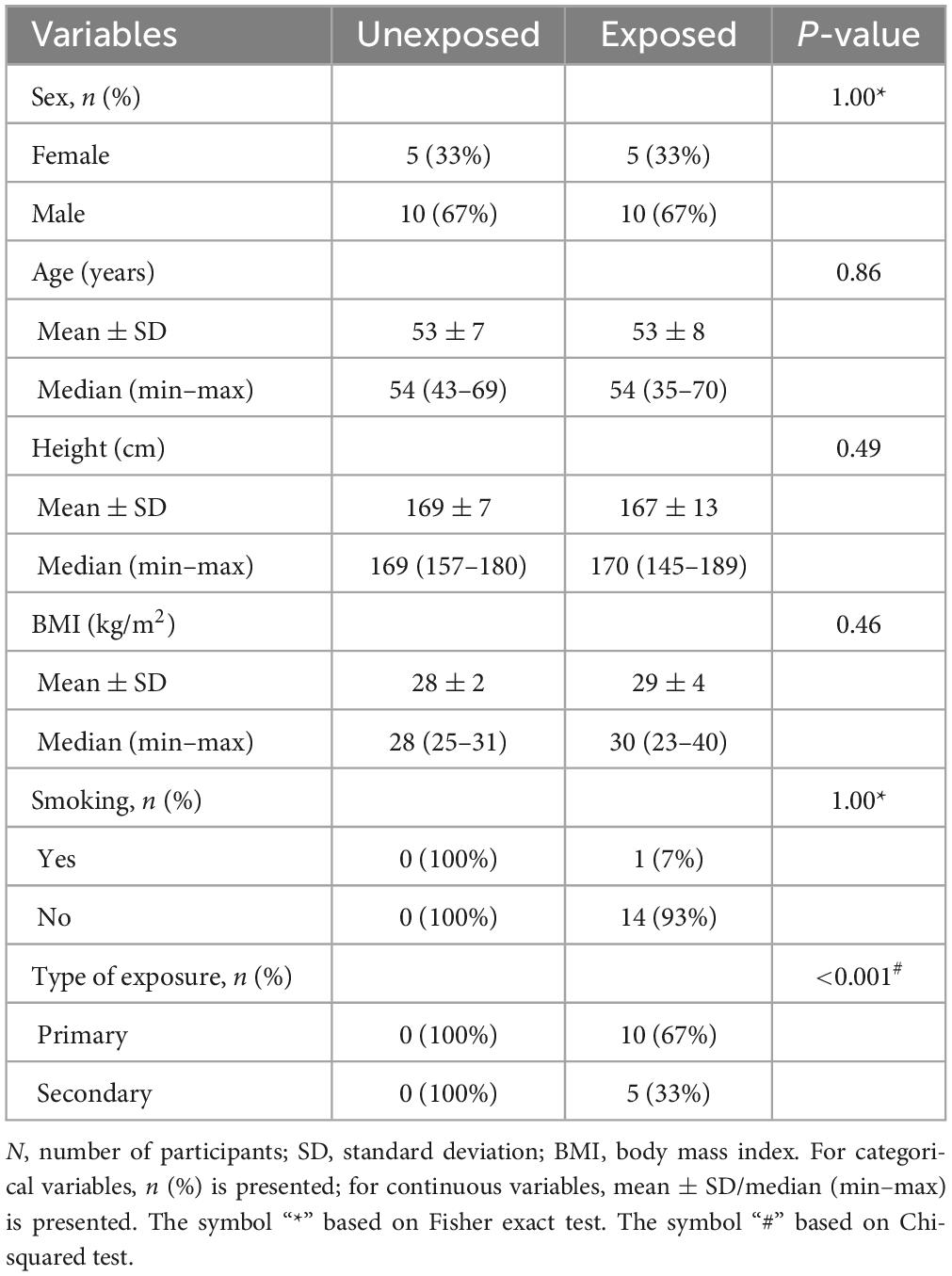
Table 1. Characteristics of the study participants; exposed vs. unexposed in IOS (n = 15 vs. 15), N2MBW (n = 15 vs. 14), and DLCO (n = 13 vs. 14).
3.1 Pulmonary function measurement outcomes
The results of the variables derived from the IOS measurements provided insights into the respiratory function of both the exposed and unexposed groups, suggesting impaired function in the small airways for the exposed group. The exposed group showed significantly higher resistance in the peripheral small airways for measured value (R5-R20, P = 0.023) in Figure 1. On the other hand, Figures 2–4 depict the reactance of the respiratory system wherein the exposed group tended to have higher stiffness in the small airways (AX, P = 0.056), as the exposed group illustrated more negative reactance values (X5, P = 0.202) and higher resonant frequency (fres, P = 0.057) than the unexposed group. Nevertheless, the differences were not statistically significant.

Figure 1. Outcomes of impulse oscillometry (IOS) variables demonstrating the respiratory resistance system total airway resistance (R5), central airways (R20), and peripheral small airways (R5-R20) in 15 exposed vs. 15 unexposed participants. P-value is based on independent samples Mann–Whitney U test. Box plots of IOS measurements: the boxes represent the 25th–75th percentiles with medians, and the top and bottom tails represent the highest/lowest scores without outliers. X shows the mean marker, and the circle shows the inner point.
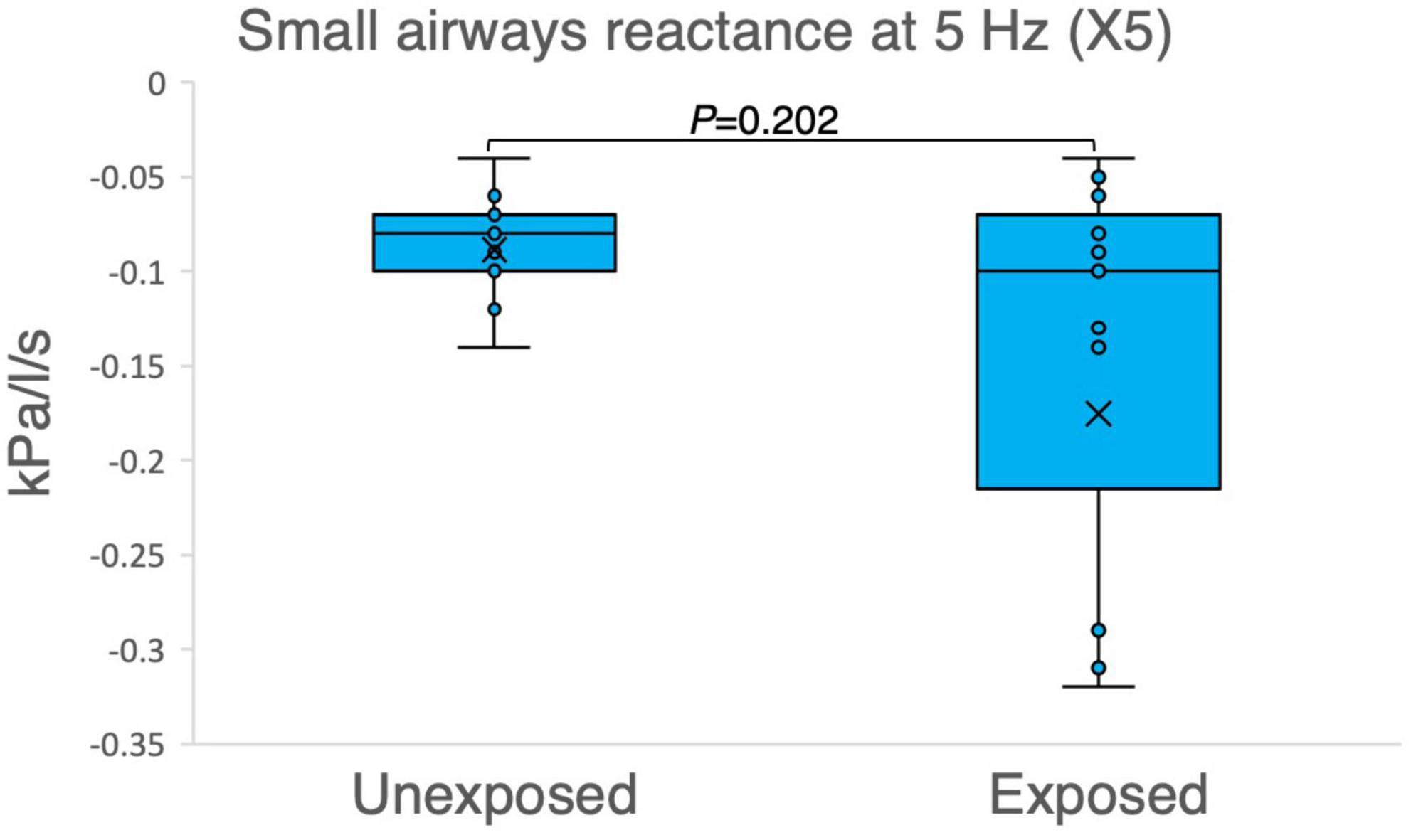
Figure 2. Outcomes of impulse oscillometry X5 (reactance at 5 Hz) in 15 sulfur mustard (SM)-exposed vs. 15 unexposed participants. P-value is based on independent-samples Mann–Whitney U test, used for between-group comparisons. Significance was set at P < 0.05.
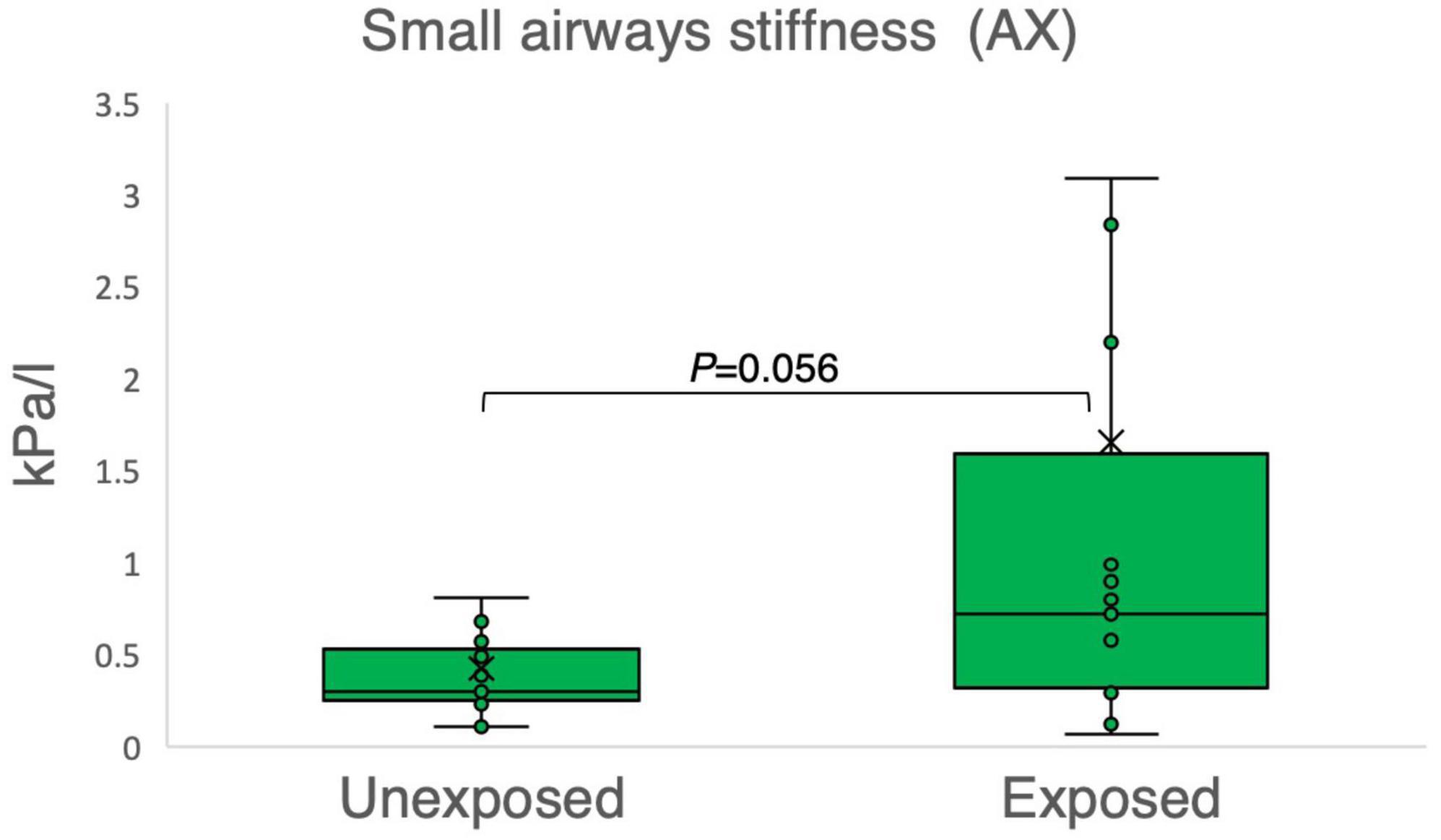
Figure 3. Outcomes of impulse oscillometry for the area of reactance (AX) in 15 sulfur mustard (SM)-exposed vs. 15 unexposed participants. P-value is based on independent-samples Mann–Whitney U test, used for between-group comparisons. Significance was set at P < 0.05.
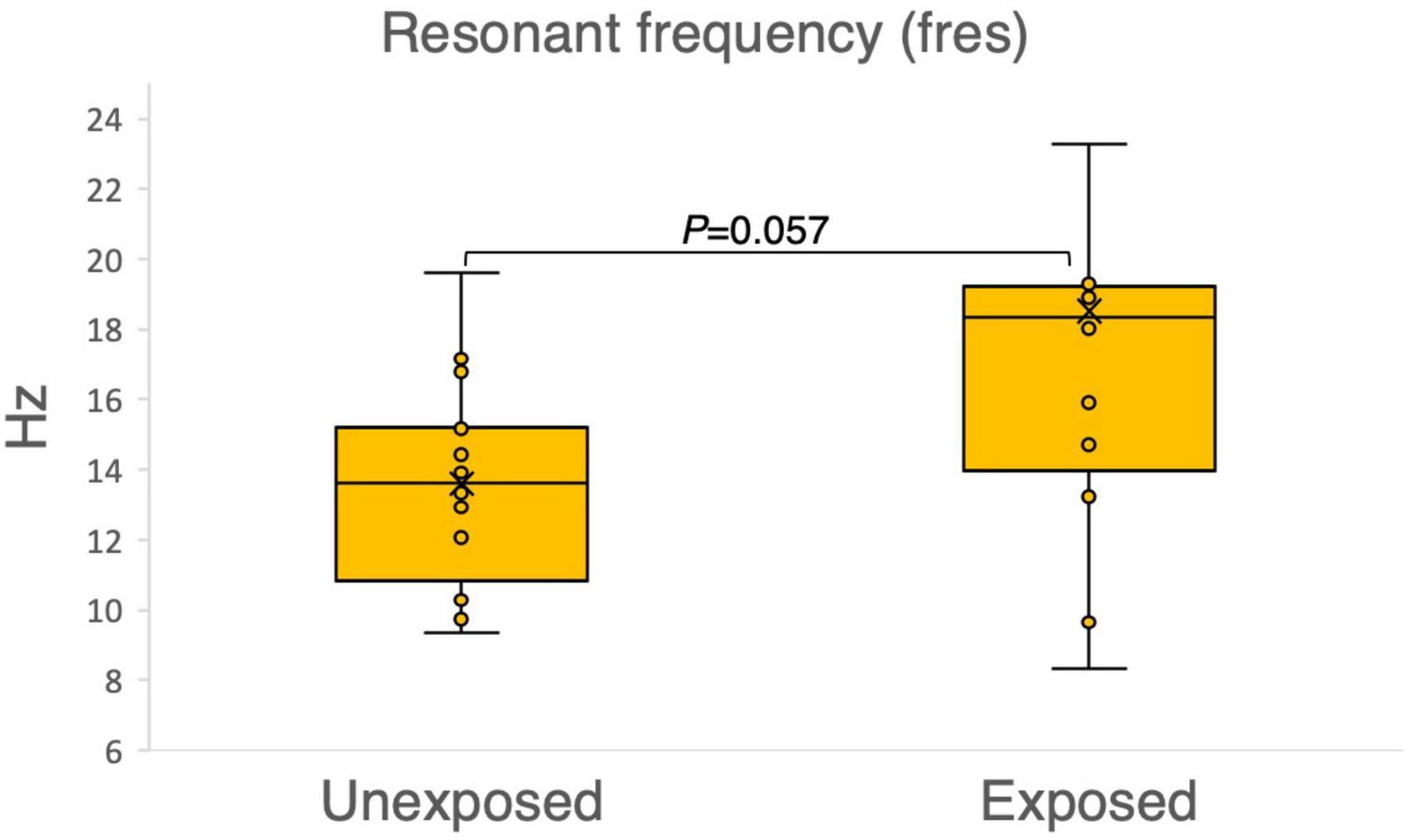
Figure 4. Outcomes of the impulse oscillometry resonant frequency (fres) in 15 sulfur mustard (SM)-exposed vs. 15 unexposed participants. P-value is based on independent-samples Mann–Whitney U test, used for between-group comparisons. Significance was set at P < 0.05.
Figure 5 illustrates the number of individuals with abnormalities in variables related to small airways for different techniques used in the study. The results revealed that among the exposed group, 10 of 15 individuals had abnormal values for IOS (AX and R5-R20), compared to 4 out of 15 individuals in the unexposed. Moreover, 8 out of 15 individuals in the exposed group and 3 out of 14 in the unexposed group had abnormal values for N2MBW (Sacin). Lastly, the study found that 3 out of 13 individual and 2 out of 14 individuals had abnormal values for DLCO in exposed and unexposed group, respectively.
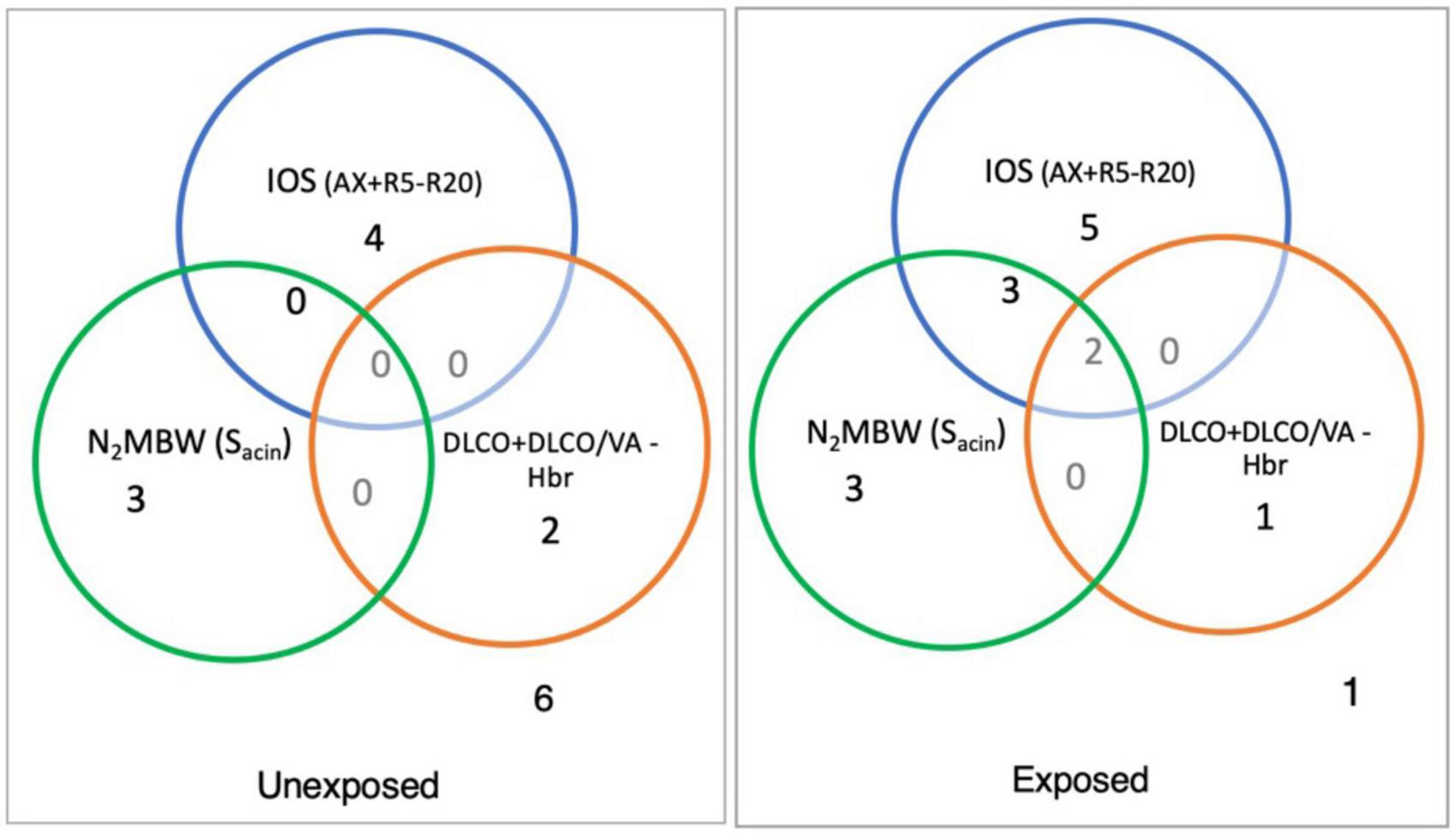
Figure 5. The number in each circle shows the number of abnormal values in the sulfur mustard (SM)-exposed and unexposed groups with different outcomes for specific variables for peripheral airways measurement with impulse oscillometry [R5-R20 and area of reactance (AX)), and nitrogen multiple breath washout method ventilation heterogeneity in the acinar airway zone (Sacin) and the ratio of the diffusing capacity of the lung for carbon monoxide (DLCO) to the alveolar volume (DLCO/VA) separately or in combination (exposed vs. unexposed: IOS, 15 vs. 15; N2MBW, 15 vs. 14; and DLCO, 13 vs. 14).
Based on the study findings, the prevalence of abnormalities varied greatly among participants when using different methods, such as IOS, N2MBW, and DLCO. The IOS method detected the highest number of abnormalities, while N2MBW showed a mix of abnormal results, and DLCO identified a lower number of abnormalities. Table 3 presents the details of the abnormalities found in the study population based on z-scores for various variables. Interestingly, the exposed individuals showed significantly more abnormalities than unexposed individuals in the IOS method variables R5 (6:0), R20 (3:0), and particularly in R5-R20 (8:0) and AX (10:4), which are specific to small airways.
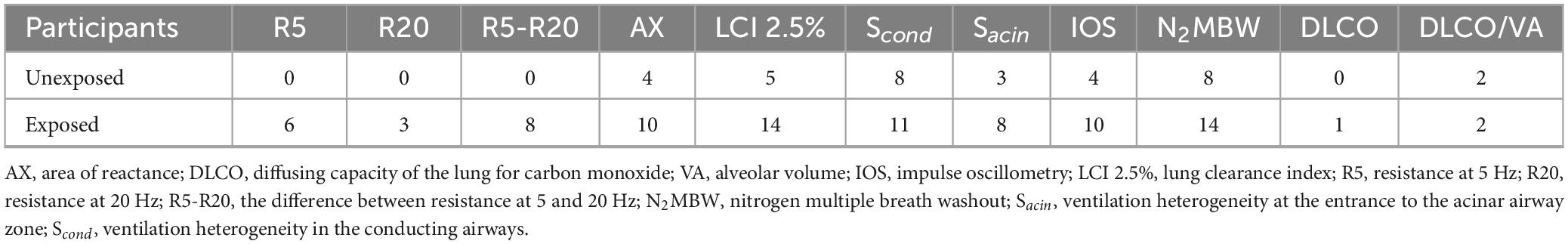
Table 3. Abnormal values for variables based on z-score in the SM-exposed vs. unexposed groups; exposed vs. unexposed in IOS (n = 15 vs. 15), N2MBW (n = 15 vs. 14), and DLCO (n = 13 vs. 14).
Similarly, using the N2MBW method, abnormalities were detected in the levels of LCI 2.5% (14:5), Scond (11:8), and Sacin (8:3). Although Sacin represents the distal part of the small airways and exhibited noticeable differences, fewer differences were observed in Scond, which represents the central airways. Additionally, minor differences between the groups regarding DLCO (3:2) were noted.
The results of the N2MBW measurements revealed a more significant degree of global airway ventilation heterogeneity and a trend toward higher acinar ventilation heterogeneity in the exposed group than in the unexposed group (Figures 6, 7). The exposed group exhibited significant variation, with a few individuals displaying substantial deviations. Eight and three participants of the exposed and unexposed groups, respectively, had abnormal Sacin values (Figure 5). The overlap was even more pronounced for LCI, with 14 exposed participants and 4 unexposed participants surpassing the reference level (Table 3). However, LCI 2.5% and Sacin values of the exposed group differed significantly from those of the unexposed group (Table 3). In addition, the variation of Scond within both the exposed and unexposed groups was not significant, with the presence of 11 and 8 individuals with Scond abnormalities in the exposed and unexposed group, respectively (Table 3). Furthermore, no significant difference was observed in Scond between the two groups (Figure 7).
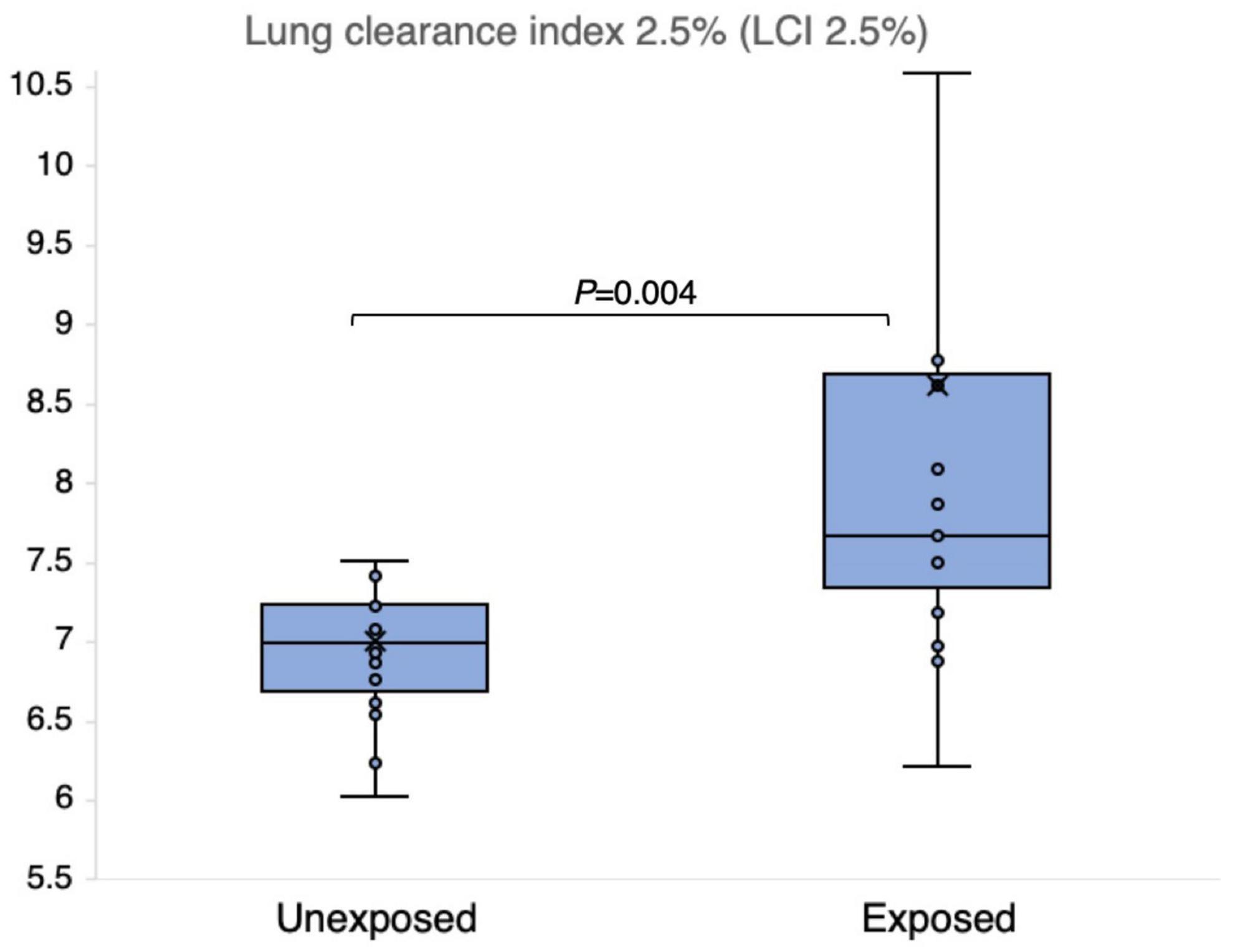
Figure 6. Outcomes of nitrogen multiple breath washout lung compliance index 2.5% (LCI 2.5%) in 15 sulfur mustard (SM)-exposed and 14 unexposed participants. P-value is based on independent-samples Mann–Whitney U test, used for between-group comparisons. Significance was set at P < 0.05.
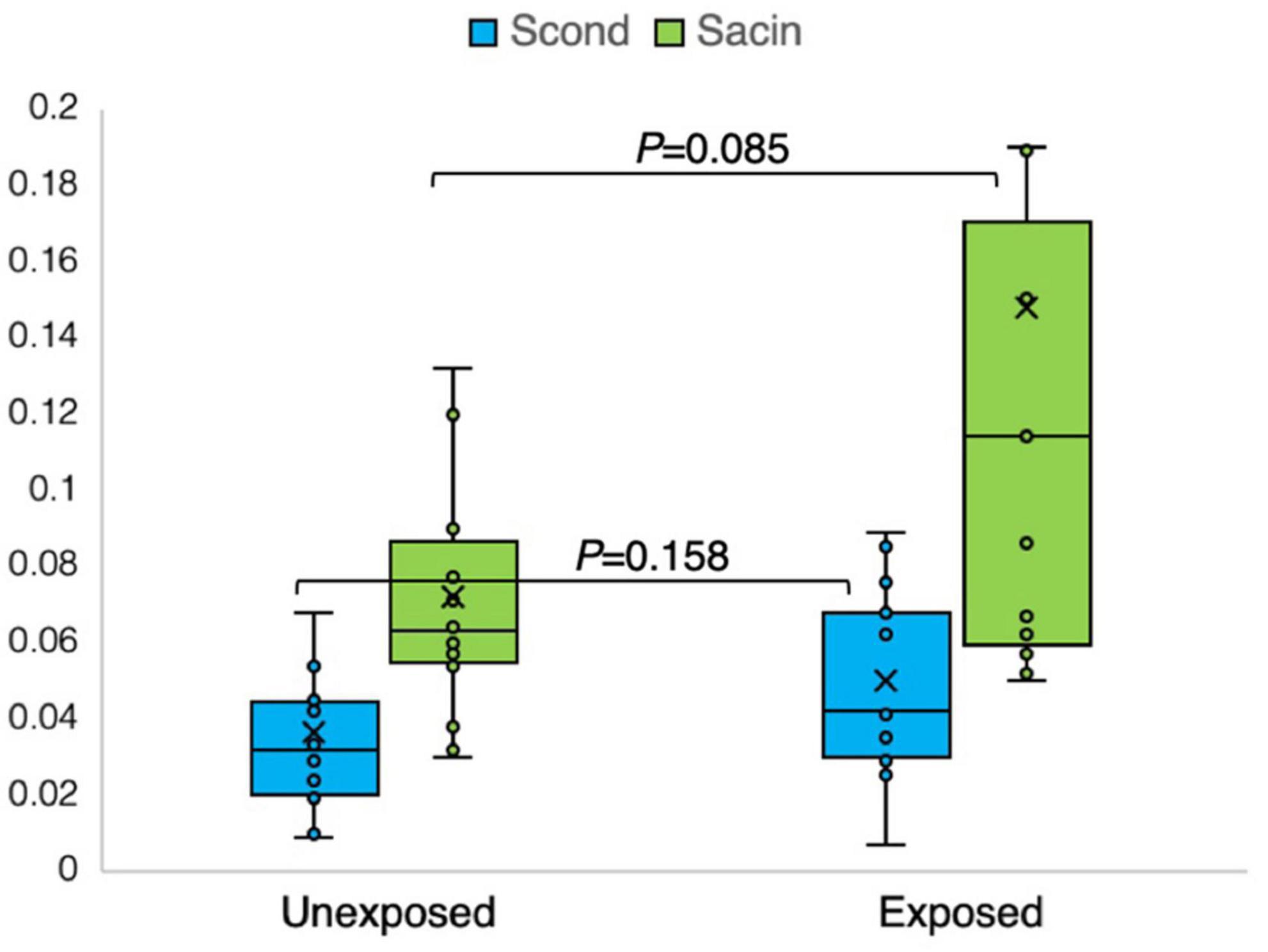
Figure 7. Outcomes of nitrogen multiple breath washout in the conducting (Scond) and acinar airway zone (Sacin) in 15 sulfur mustard (SM)-exposed and 14 unexposed participants. P-value is based on independent-samples Mann–Whitney U test, used for between-group comparisons. Significance was set at P < 0.05.
The results obtained from the analysis of the DLCO-derived variables demonstrated no significant impact on the studied groups; however, a few individuals demonstrated noteworthy abnormalities for the z-scores (Table 3). Figure 8 clearly illustrates no significant differences in the absolute measured values and z-scores of the DLCO/VA between the exposed and unexposed groups.
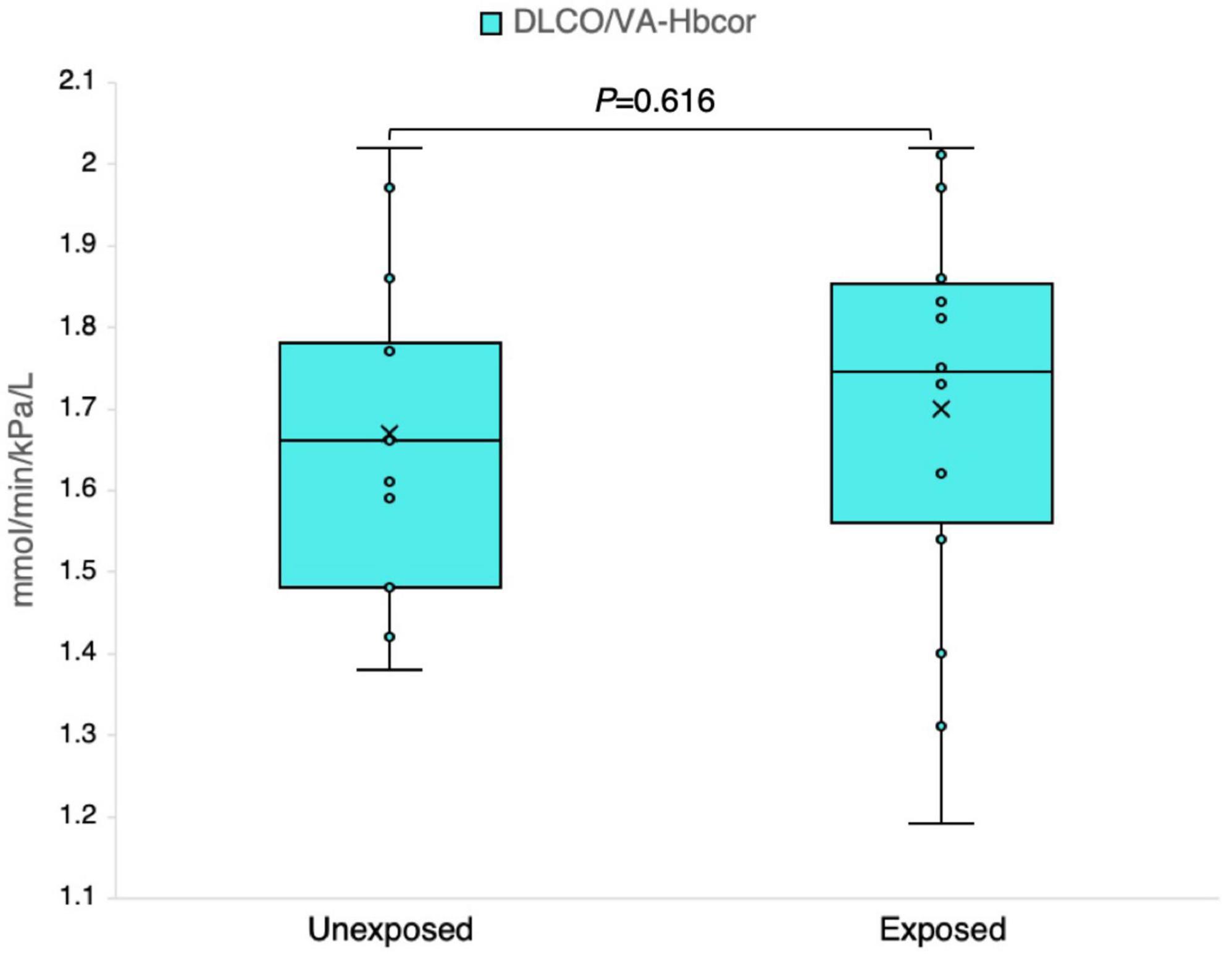
Figure 8. Outcomes of the ratio of the diffusing capacity of the lung for carbon monoxide (DLCO) to the alveolar volume (DLCO/VA) adjusted for the hemoglobin level in 13 sulfur mustard (SM)-exposed and 14 unexposed individuals. P-value is based on independent-samples Mann–Whitney U test, used for between-group comparisons. Significance was set at P < 0.05.
Based on the findings from the calculation of z-scores, more significant differences were observed between the exposed and unexposed groups—the analysis of the data indicated that the exposed group had lower respiratory function than the unexposed group. Table 4 provides a detailed summary of the z-scores for IOS, N2MBW, and DLCO. The results showed that the exposed group had significantly impaired results for R5-R20 (P = 0.029), LCI 2.5% (P = 0.002), and Sacin (P = 0.033) when compared to the unexposed group. Additionally, a noticeable tendency toward higher values for R5 (P = 0.059), AX (P = 0.051), and X5 (P = 0.054) was observed in the exposed group.
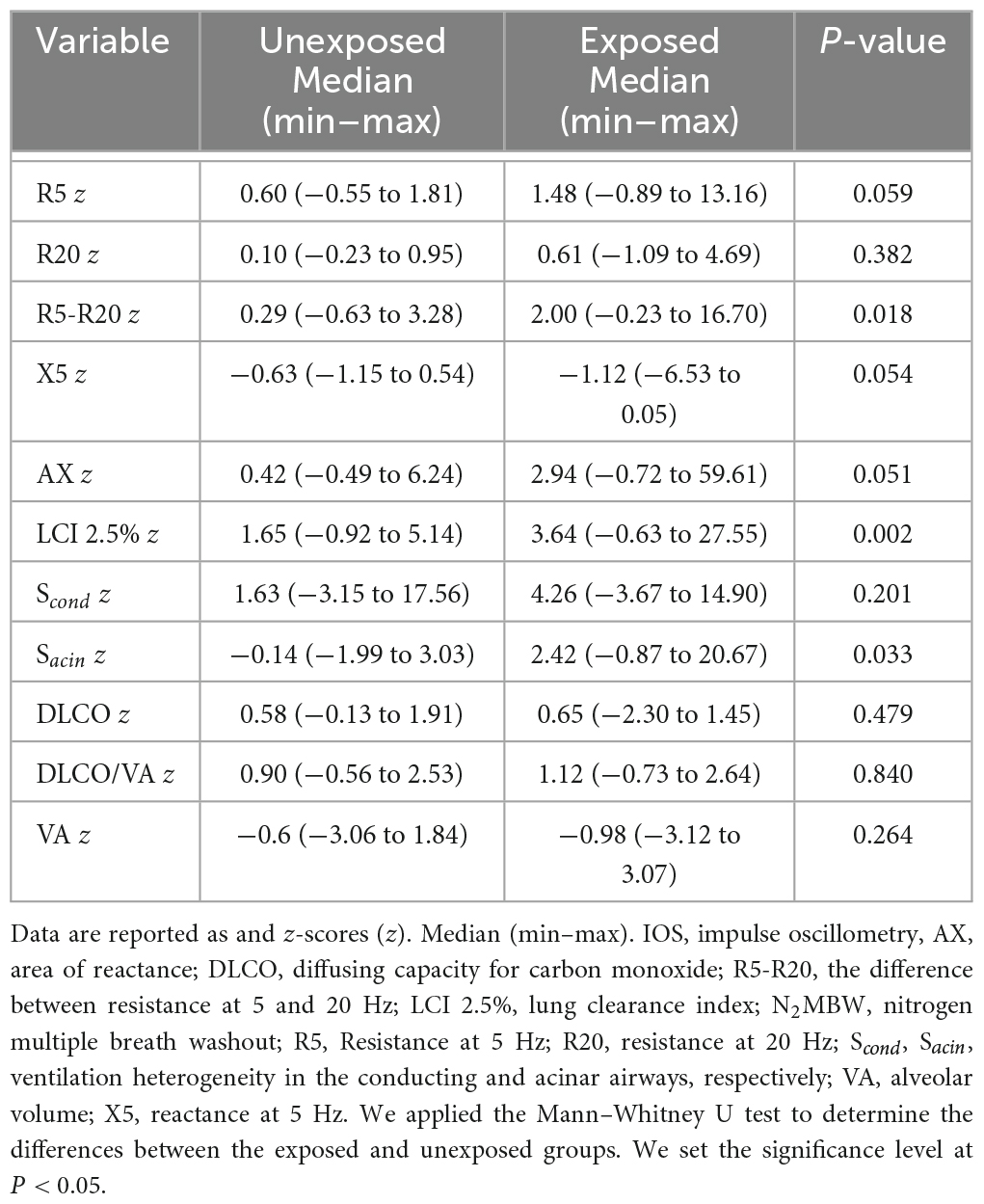
Table 4. Lung function tests z-scores for exposed vs. unexposed in IOS (n = 15 vs. 15), N2MBW (n = 15 vs. 14), and DLCO (n = 13 vs. 14).
After analyzing the scatter plots, no statistically significant relationships were observed between the different measures of lung function with IOS, N2MBW, and DLCO (Figure 9). For instance, a very weak negative correlation was found between Sacin and R5-R20 (P = 0.68). Similarly, the association between Sacin and DLCO/VA had a P-value of 0.81, which was not statistically significant. Another weak negative correlation was observed between Sacin and AX, with a P-value of 0.82. However, this correlation was not statistically significant. Lastly, R5-R20 and DLCO/VA had a moderate positive correlation, with a P-value of 0.11. Nevertheless, this correlation was not statistically significant.
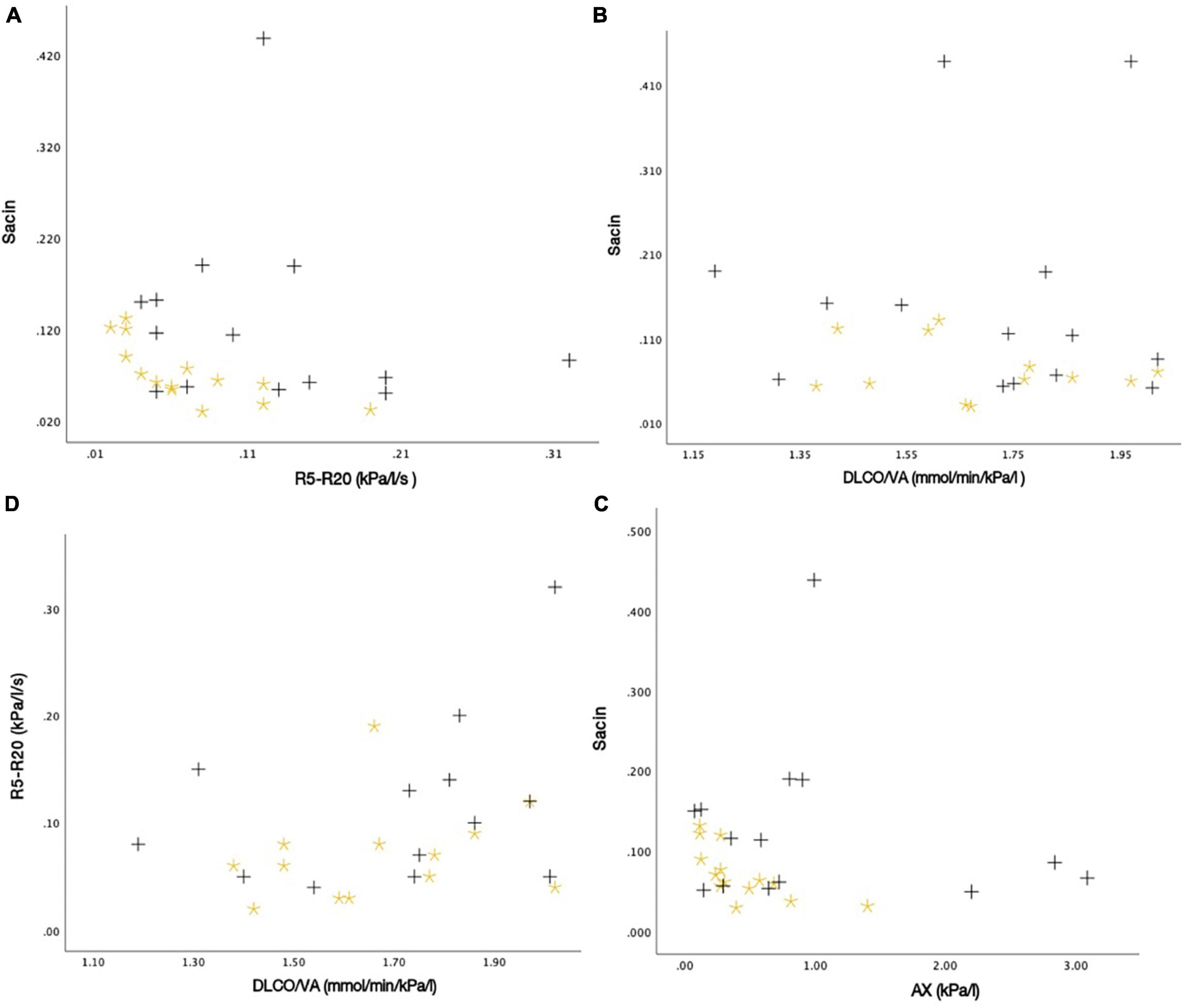
Figure 9. The associations between different measures of lung function as scatter plots: (A) Sacin and R5-R20 (r = –0.082, P-value = 0.678), (B) Sacin and DLCO/VA (r = –0.023, P-value = 0.810), (C) Sacin and AX (r = –0.045, P-value = 0.820), and (D) R5-R20 and DLCO/VA (r = 0.324, P-value = 0.106). Black plus = SM-exposed participant. Yellow star = Unexposed participant.
4 Discussion
Individuals exposed to SM can experience serious, long-lasting respiratory problems that gradually worsen over time, potentially leading to disabilities. This study is among the first to investigate the small airway function in SM-exposed individuals using non-traditional noninvasive PFTs, e.g., ISO and N2MBW, compared to unexposed control individuals. The findings revealed a positive association between exposure to SM and SAD. The exposed group had significantly higher respiratory resistance, stiffness, and ventilation heterogeneity in the small airways than the unexposed group.
Impulse oscillometry and N2MBW showed small airway dysfunction in the SM-exposed group, with statistically significant differences in the absolute measured values and abnormal z-scores for variables related to peripheral small airways compared to none in the unexposed group. In addition, the method was validated on individual levels; 8 of the 15 exposed participants who reported dyspnea upon walking had abnormal results for Sacin, LCI 2.5%, R5-R20, and AX.
Impulse oscillometry devices revealed that 8 of the 15 exposed participants had abnormal values for R5-R20, suggesting higher respiratory resistance in small airways, and 10 had abnormal values for AX, revealing stiffness in small airway. This contrasts with the unexposed group, where only four participants had abnormal values for AX. In addition, five participants in the exposed group had abnormal values for R5 indicators of total airway resistance, and two had abnormal values for R20 predicting central airway resistance compared to none in the unexposed group, indicating that the exposed group had more impaired respiratory function in large and central part of airways in addition to small airways. These findings are consistent with those of other studies, indicating that exposure to SM can cause significant damage to the central airways, as detected through traditional techniques such as spirometry and HRCT (11, 12, 49).
Concerning the N2MBW test, the variation within the exposed group was large, with a few individuals showing large deviations, possibly due to different exposure levels and susceptibility; Eight participants in the exposed group and three participants in the unexposed group had abnormal Sacin values (Figure 5). For LCI 2.5%, the overlap was bigger: 14 exposed and 5 unexposed participants had values above the reference level (z-scores of +1.96) (Table 3). However, we cannot exclude the possibility that other factors may also have played a role; for instance, the individual in the unexposed group also exhibited abnormal values.
Among the IOS variables, R5-R20 is presumably more specific for peripheral airway resistance; however, it does not reflect the pathology of the smallest airways. AX measures the reactance in the small airways, that is, the elastance or stiffness of the airways. In comparison, Sacin detects ventilation heterogeneity in the very distal/acinar airways. Consequently, these parameters are considered complementary for the identification of SAD. The current study found that all these measures may be influenced; however, they may show different patterns in different individuals, suggesting that exposure to SM may have different long-term consequences on small airways, possibly reflecting different SM exposure situations and different inherent susceptibilities. However, other factors may have also influenced the results, as a long time had passed since the SM exposure.
The results of DLCO in this study are consistent with those of a previous study that included 23 SM-exposed patients with abnormal spirometry, HRCT, and bronchoscopy results but normal DLCO when assessed almost 14 years after SM exposure (17). The large alveolar surface and large reserve capacity for gas diffusion at rest might explain this. Thus, DLCO is not considered a useful method, especially to assess SAD in SM-exposed individuals.
The abnormal values for R20 and R5, LCI 2.5%, and Scond suggest that lung function impairment may extend in other areas of the lung beyond the small airways. This finding aligns with that of other studies, which demonstrated bronchiectasis, atelectasis, dilated central airways, bronchial wall thickening, and interlobular septal wall thickening in individuals exposed to SM (11, 12, 49).
With IOS and N2MBW peripheral small airways related variables, at least one abnormal value was revealed for 93% of the exposed participants compared to 53% in the unexposed group (Figure 5); these individuals with abnormal values in the exposed group had clinical manifestation in terms of dyspnea upon walking on smooth ground, but not in the unexposed one. The reasons for the abnormal values in the unexposed participants may vary. The inclusion criteria for the control group did not consider other potential sources of exposure, such as environmental pollutants, second-hand smoking, occupational hazards, or other diseases that could potentially impact lung function. Additionally, the use of local Swedish references (24) in the study may have narrowed the reference range, resulting in abnormal values for both the control and exposed groups. Moreover, the prevalence of undiagnosed asthma and COPD in the population is worth noting (50, 51). However, one unexposed participant with abnormal Sacin was later diagnosed with lung disease by their family physician. It is worth noting that the unexposed participants with abnormal z-scores did not report respiratory clinical symptoms contrary to the exposed participants. The existence of abnormal values in unexposed control in our article is consistent with the results of a previous study that found both pulmonary symptoms and abnormal spirometry findings in the unexposed control group compared to SM-exposed individuals (52).
The current study’s results, suggesting SAD, align with those of other studies conducted on SM-exposed participants 10–15 years following SM exposure. Previous studies reported proinflammatory and inflammatory mediators in the bronchoalveolar lavage fluid, such as neutrophils, indicating injury to the bronchiole tree and a high incidence of bronchiole obliterans in SM-exposed survivors (11, 16). Exposure to SM may induce chronic inflammation in the airways by the accumulation of inflammatory cells that release proinflammatory substances, such as cyclooxygenase-2 and 12-lipoxygenase, and reduce the production of protective substances, including surfactants (18, 53–55). These pathological processes may successively deteriorate and result in small airway scarring that is undetectable by conventional imaging and spirometry tests in the early stages.
Furthermore, the results of the present study are significant as they indicate that certain SM-exposed participants had abnormal indices linked to small airways, even though they showed no clinical symptoms. This finding is consistent with that of a previous study, which identified signs of air trapping on HRCT in asymptomatic individuals exposed to SM (26). These findings suggest that the effects of SM exposure on lung function may not be immediately apparent, emphasizing the need to monitor for any potential respiratory issues that could develop in high-risk individuals who were exposed to SM (9).
Fres, another indicator in IOS, is the frequency at which the airways and lung tissues vibrate smoothly during breathing at the normal interval reference (7–12 Hz) reported in a previous study (42). With a score range (8–42) and a mean value (18.5), we noticed a considerable difference (P = 0.057) in the fres values between the exposed and unexposed groups in our study population (as shown in Figure 4). This indicates impacted airways among the exposed group. Unfortunately, we could not calculate the z-score for fres as we could not access a reference.
Previous studies have revealed a mixed picture of the lung status in SM-exposed participants, which may be attributed to the diagnostic techniques used (56). In a study of 15 Iranian SM-exposed survivors with respiratory symptoms, 13 had normal spirometry tests, 1 had mild restriction, and 1 had an obstructive pattern 20 years post-exposure. Histological biopsy analysis revealed that 50% of these patients had either bronchiolitis obliterans or signs indicative of its presence (10). This is in line with the results of our study, where 53% of the SM-exposed group had heterogeneity in Sacin and higher resistance in small airways (R5-R20) and 66% showed stiffness in small airways (AX). Hence, IOS and N2MBW as small airway-sensitive tests in SM-exposed lung investigations might help clarify the structural and parenchymal pulmonary pathological changes. The current study, which revealed the presence of SAD in SM-exposed participants, might provide a plausible explanation for the gradual manifestation of clinical respiratory symptoms in SM-exposed survivors (9). Small airways are the quiet zone of the airways; thus, SAD can often remain undetected by spirometry until the manifestation of significant clinical symptoms (10, 23). Furthermore, the presence of SAD may be important when assessing the responses to cortisone and bronchodilator inhalation in SM-exposed patients. Traditional inhalers may not adequately reach damaged peripheral airways owing to the powder’s particle size (57, 58).
The SAD verified by biopsy in SM-exposed individuals despite normal spirometry and HRCT results (27, 59) highlights the limitations of HRCT and spirometry in detecting the existence of SAD in the early stages among SM-exposed patients. While expiratory CT air trapping is a reliable indicator of SAD, visually detecting diffuse air trapping can be challenging (59). Spirometry tests also require a good technique and the patient to maneuver. Moreover, it registers SAD only after obstruction of a significant portion of the small airways (27, 28). Thus, early detection of SAD is crucial for controlling damage to small airways (26). Noninvasive methods, such as IOS and N2MBW, are easy to perform, independent of patient effort, register modifications in small airways long before spirometry (60), and lack radiation risk as in HRCT with repeated application. Moreover, IOS could prove useful particularly in SM-exposed patients who are unable to undergo spirometry tests. Additionally, these methods may be useful in providing complementary information to spirometry tests and HRCT for monitoring SAD and evaluating therapeutic intervention in SM-exposed patients (61).
4.1 Limitations and strengths
This study, while providing valuable insights into SAD in individuals exposed to SM, has certain limitations. Firstly, its generalizability to larger populations is limited owing to the small sample size; moreover, a selection bias could exist as participants with residual respiratory symptoms might have been more inclined to participate. Our findings illustrate the importance of including measurements of SAD in individuals with residual respiratory symptoms after SM exposure. Secondly, the study’s observational nature makes it difficult to comment on the causality between SM exposure and the outcomes. A long time had elapsed since the exposure, and many other exposures may also have affected the respiratory system. Moreover, we have limited data on the extent of exposure, which varied in the SM-exposed group.
Another limitation might be the lack of spirometry tests and imaging data. The initial study protocol included spirometry; however, most participants could not undergo repeated spirometry tests with adequate quality, leading to the exclusion of spirometry tests from the 2022 study protocol. Overall, this could be attributed to a combination of participant-related factors (lack of motivation or language barrier), and technical issues (limited resources). Comparing the results of physiological lung function tests with imaging would be highly interesting; however, this was impossible owing to limited resources.
Despite these limitations, the strength of this study is that it is one of the first to investigate SAD in SM-exposed participants vs. an unexposed control group and compare different non-traditional noninvasive lung physiological diagnostic techniques. IOS and N2MBW are noninvasive PFTs that can detect SAD in survivors long after exposure. The absolute measured values and z-scores were highly consistent in evaluating abnormal values, which strengthened the outcomes of this study. The existence of abnormal values among unexposed controls indicate that some of controls may have been sick too, thus the significance of the findings by this study may have been underestimated.
In summary, no notable correlations were observed between the small airways indicators, IOS, N2MBW, and DLCO. Of the three, IOS is the most precise, N2MBW is sensitive but has some limitations in the specificity, and DLCO exhibits low sensitivity and specificity (Table 3). Ultimately, the IOS and N2MBW are highly valuable in detecting SAD in the early stage among SM-exposed individuals, providing additional information to that obtained using traditional methods for monitoring SAD, and evaluating therapeutic interventions for patients exposed to SM.
However, neither IOS nor N2MBW is the gold standard for evaluating small airways. Nevertheless, further assessment of SAD with IOS and N2MBW in a larger population of SM-exposed participants is warranted.
5 Conclusion
Exposure to SM was positively associated with long-term impairment of respiratory tract function in the small airways in 14 of 15 SM-exposed participants, of whom 8 individuals reported dyspnea during walking and 6 of 15 in the unexposed group with no reported respiratory clinical symptoms in the present study. Whether they are at risk of further deterioration and lung disease is unknown; however, exploring this in a large SM-exposed population would be of great interest. DLCO measurements suggest that SM is unlikely to significantly affect lung gas exchange at the alveolar-capillary interface in the long-term. Furthermore, both IOS and N2MBW should be employed to detect SAD in SM-exposed survivors as they provide complementary information. Identifying and characterizing the remaining pathology of the small airways in survivors of SM exposure is the first step toward improved treatment and follow-up.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Regional Ethical Review Board of Gothenburg, Sweden (2017-12-07599-17). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
FM and A-CO conceived and designed the study. SK and FM prepared the materials and collected the data. FM conducted the statistical analysis in close collaboration with SK, which was then validated by YL. FM, AC-O, SK, and BD interpreted the data. FM wrote the initial draft of the manuscript. All authors reviewed and commented on various versions of the manuscript, read, and ultimately approved the final manuscript.
Funding
FM received funding for this study through a doctoral study grant (DOS) as part of their PhD thesis. The grant (no. 272597) was awarded by Region Västra Götaland, Närhälsan Research, and Development Primary Health Care, Gothenburg, Sweden. However, the funder did not play a role in the study’s design, data collection, analysis, interpretation, or manuscript writing.
Acknowledgments
We would like to extend our gratitude to all the participants who were involved in this study. Additionally, we would like to express our deep appreciation to the staff at the Laboratory of the Occupation and Environmental for their valuable assistance in the data collection process.
Conflict of interest
A-CO has invented a method to examine small airways, the PExA method, and is a shareholder and board member of PExA AB. However, results from that method are not presented in the current manuscript. She is also a scientific adviser for a pharmacological study carried out by Chiesi AB.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Somani S, Babu S. Toxicodynamics of sulfur mustard. Int J Clin Pharmacol Ther Toxicol. (1989) 27:419–35.
2. Beigi Harchegani A, Mirnam Niha M, Sohrabiyan M, Ghatrehsamani M, Tahmasbpour E, Shahriary A. Cellular and molecular mechanisms of sulfur mustard toxicity on spermatozoa and male fertility. Toxicol Res. (2018) 7:1029–35. doi: 10.1039/c8tx00062j
3. Cheng X, Liu C, Yang Y, Liang L, Chen B, Yu H, et al. Advances in sulfur mustard-induced DNA adducts: Characterization and detection. Toxicol Lett. (2021) 344:46–57.
4. Weinberger B, Malaviya R, Sunil V, Venosa A, Heck D, Laskin J, et al. Mustard vesicant-induced lung injury: Advances in therapy. Toxicol Appl Pharmacol. (2016) 305:1–11. doi: 10.1016/j.taap.2016.05.01
5. Ghanei M, Naderi M, Kosar A, Harandi A, Hopkinson N, Poursaleh Z. Long-term pulmonary complications of chemical warfare agent exposure in Iraqi Kurdish civilians. Inhal Toxicol. (2010) 22:719–24. doi: 10.3109/08958371003686016
6. Shahriary A, Ghanei M, Rahmani H. The systemic nature of mustard lung: Comparison with COPD patients. Interdiscip Toxicol. (2017) 10:114–27. doi: 10.1515/intox-2017-0018
7. Graham J, Schoneboom B. Historical perspective on effects and treatment of sulfur mustard injuries. Chem Biol Interact. (2013) 206:512–22. doi: 10.1016/j.cbi.2013.06.013
8. Ghabili K, Agutter P, Ghanei M, Ansarin K, Panahi Y, Shoja M. Sulfur mustard toxicity: History, chemistry, pharmacokinetics, and pharmacodynamics. Crit Rev Toxicol. (2011) 41:384–403. doi: 10.3109/10408444.2010.541224
9. Amini H, Solaymani-Dodaran M, Mousavi B, Alam Beladi S, Soroush M, Abolghasemi J, et al. Long-term health outcomes among survivors exposed to sulfur mustard in Iran. JAMA Netw Open. (2020) 3:e2028894. doi: 10.1001/jamanetworkopen.2020.28894
10. Ghanei M, Tazelaar H, Chilosi M, Harandi A, Peyman M, Akbari H, et al. An international collaborative pathologic study of surgical lung biopsies from mustard gas-exposed patients. Respir Med. (2008) 102:825–30. doi: 10.1016/j.rmed.2008.01.016
11. Abtahi H, Peiman S, Foroumandi M, Safavi E. Long term follow-up of sulfur mustard related bronchiolitis obliterans treatment. Acta Med Iran. (2016) 54:605–9.
12. Hefazi M, Attaran D, Mahmoudi M, Balali-Mood M. Late respiratory complications of mustard gas poisoning in Iranian veterans. Inhal Toxicol. (2005) 17:587–92. doi: 10.1080/0895837059100059113
13. Balali-Mood M, Hefazi M. Comparison of early and late toxic effects of sulfur mustard in Iranian veterans. Basic Clin Pharmacol Toxicol. (2006) 99:273–82. doi: 10.1111/j.1742-7843.2006.pto_429.x
14. White C, Rancourt R, Veress L. Sulfur mustard inhalation: Mechanisms of injury, alteration of coagulation, and fibrinolytic therapy. Ann NY Acad Sci. (2016) 1378:87–95. doi: 10.1111/nyas.13130
15. Rowell M, Kehe K, Balszuweit F, Thiermann H. The chronic effects of sulfur mustard exposure. Toxicology. (2009) 263:9–11. doi: 10.1016/j.tox.2009.05.015
16. Beheshti J, Mark E, Akbaei H, Aslani J, Ghanei M. Mustard lung secrets: Long term clinicopathological study following mustard gas exposure. Pathol Res Pract. (2006) 202:739–44. doi: 10.1016/j.prp.2006.04.008
17. Kavousi S, Akbarialiabad H, Mehrabani D, Mohamadian A, Ghahramani A, Shirkhoda A, et al. The predictive association between radiological findings and lung cancer development in patients exposed to sulfur mustard gas: 4 decades follow up of 719 victims. BMC Pulm Med. (2022) 22:481. doi: 10.1186/s12890-022-02282-7
18. Tahmasbpour E, Ghanei M, Khor A, Panahi Y. Altered expression of cyclooxygenase-2, 12-lipoxygenase, inducible nitric oxide synthase-2 and surfactant protein D in lungs of patients with pulmonary injury caused by sulfur mustard. Drug Chem Toxicol. (2019) 42:257–63. doi: 10.1080/01480545.2018.144247419
19. Khazdair M, Boskabady M, Ghorani V. Respiratory effects of sulfur mustard exposure, similarities and differences with asthma and COPD. Inhal Toxicol. (2015) 27:731–44. doi: 10.3109/08958378.2015.1114056
20. Emad A, Emad Y. Relationship between eosinophilia and levels of chemokines (CCL5 and CCL11) and IL-5 in bronchoalveolar lavage fluid of patients with mustard gas-induced pulmonary fibrosis. J Clin Immunol. (2007) 27:605–12. doi: 10.1007/s10875-007-9114-y
21. Karacsonyi C, Shanmugam N, Kagan E. A clinically relevant in vitro model for evaluating the effects of aerosolized vesicants. Toxicol Lett. (2009) 185:38–44. doi: 10.1016/j.toxlet.2008.11.01522
22. Postma D, Brightling C, Baldi S, Van den Berge M, Fabbri L, Gagnatelli A, et al. Exploring the relevance and extent of small airways dysfunction in asthma (ATLANTIS): Baseline data from a prospective cohort study. Lancet Respir Med. (2019) 7:402–16. doi: 10.1016/s2213-2600(19)30049-923
23. Lu L, Peng J, Zhao N, Wu F, Tian H, Yang H, et al. Discordant spirometry and impulse oscillometry assessments in the diagnosis of small airway dysfunction. Front Physiol. (2022) 13:892448. doi: 10.3389/fphys.2022.892448
24. Kjellberg S, Houltz B, Zetterström O, Robinson P, Gustafsson P. Prevalence of peripheral airway dysfunction in adult asthmatics. Observ Stud Am Thorac Soc. (2016) A1740.
25. Ghanei M, Moqadam F, Mohammad M, Aslani J. Tracheobronchomalacia and air trapping after mustard gas exposure. Am J Respir Crit Care Med. (2006) 173:304–9. doi: 10.1164/rccm.200502-247OC
26. Baradaran Mahdavi M, Rafati M, Ghanei M, Arabfard M. Computer-assisted evaluation of small airway disease in CT scans of Iran-Iraq war victims of chemical warfare by a locally developed software: Comparison between different quantitative methods. BMC Med Imaging. (2023) 23:165. doi: 10.1186/s12880-023-01114-227
27. McNulty W, Usmani O. Techniques of assessing small airways dysfunction. Eur Clin Respir J. (2014) 1:25898. doi: 10.3402/ecrj.v1.25898
28. Burgel P, Bergeron A, de Blic J, Bonniaud P, Bourdin A, Chanez P, et al. Small airways diseases, excluding asthma and COPD: An overview. Eur Respir Rev. (2013) 22:131–47. doi: 10.1183/09059180.00001313
29. Xiao D, Wang C. Small airway abnormalities as a marker of early lung injury: Challenges ahead. Lancet Glob Health. (2023) 11:e8–9. doi: 10.1016/S2214-109X(22)00480-6
30. Li L, Yan T, Yang J, Li Y, Fu L, Lan L, et al. Impulse oscillometry for detection of small airway dysfunction in subjects with chronic respiratory symptoms and preserved pulmonary function. Respir Res. (2021) 22:68. doi: 10.1186/s12931-021-01662-7
31. Morris Z, Coz A, Starosta D. An isolated reduction of the FEV3/FVC ratio is an indicator of mild lung injury. Chest. (2013) 144:1117–23. doi: 10.1378/chest.12-2816
32. Knox-Brown B, Mulhern O, Feary J, Amaral A. Spirometry parameters used to define small airways obstruction in population-based studies: Systematic review. Respir Res. (2022) 23:67. doi: 10.1186/s12931-022-01990-2
33. Sorkness R, Bleecker E, Busse W, Calhoun W, Castro M, Chung K, et al. Lung function in adults with stable but severe asthma: Air trapping and incomplete reversal of obstruction with bronchodilation. J App Physiol. (2008) 104:394–403. doi: 10.1152/japplphysiol.00329.2007
34. Shao Y, Tsai K, Kim S, Wu Y, Demissie K. Exposure to tomographic scans and cancer risks. JNCI Cancer Spectr. (2020) 4:kz072. doi: 10.1093/jncics/pkz072
35. Stanojevic S, Bowerman C, Robinson P. Multiple breath washout: Measuring early manifestations of lung pathology. Breathe. (2021) 17:210016. doi: 10.1183/20734735.0016-2021
36. Porojan-Suppini N, Fira-Mladinescu O, Marc M, Tudorache E, Oancea C. Lung function assessment by impulse oscillometry in adults. Ther Clin Risk Manag. (2020) 16:1139–50. doi: 10.2147/tcrm.S275920
37. Robinson P, Goldman M, Gustafsson P. Inert gas washout: Theoretical background and clinical utility in respiratory disease. Respiration. (2009) 78:339–55. doi: 10.1159/000225373
38. Balasubramanian A, MacIntyre N, Henderson R, Jensen R, Kinney G, Stringer W, et al. Diffusing capacity of carbon monoxide in assessment of COPD. Chest. (2019) 156:1111–9. doi: 10.1016/j.chest.2019.06.035
39. Dworkin J, Prescott M, Jamal R, Hardawan S, Abdullah A, Galea S. The long-term psychosocial impact of a surprise chemical weapons attack on civilians in Halabja, Iraqi Kurdistan. J Nerv Ment Dis. (2008) 196:772–5. doi: 10.1097/NMD.0b013e3181878b69
40. Moradi F, Moradi F, Li Y, Olin A, Daka B. The impact of sulfur mustard on quality of life and mental health in Kurdish survivors in Sweden, thirty years after exposure. Health Qual Life Outcomes. (2022) 20:165. doi: 10.1186/s12955-022-02081-y
41. Hashemian F, Khoshnood K, Desai M, Falahati F, Kasl S, Southwick S. Anxiety, depression, and posttraumatic stress in Iranian survivors of chemical warfare. JAMA. (2006) 296:560–6. doi: 10.1001/jama.296.5.560
42. Hoang T, Sikdar S, Xu C, Lee M, Cardwell J, Forno E, et al. Epigenome-wide association study of DNA methylation and adult asthma in the agricultural lung health study. Eur Respir J. (2020) 56:2000217. doi: 10.1183/13993003.00217-2020
43. Foy B, Soares M, Bordas R, Richardson M, Bell A, Singapuri A, et al. Lung computational models and the role of the small airways in asthma. Am J Respir Crit Care Med. (2019) 200:982–91. doi: 10.1164/rccm.201812-2322OC
44. Desiraju K, Agrawal A. Impulse oscillometry: The state-of-art for lung function testing. Lung India. (2016) 33:410–6. doi: 10.4103/0970-2113.184875
45. Singer F, Houltz B, Latzin P, Robinson P, Gustafsson P. A realistic validation study of a new nitrogen multiple-breath washout system. PLoS One. (2012) 7:e36083. doi: 10.1371/journal.pone.0036083
46. Robinson P, Latzin P, Verbanck S, Hall G, Horsley A, Gappa M, et al. Consensus statement for inert gas washout measurement using multiple– and single- breath tests. Eur Respir J. (2013) 41:507–22. doi: 10.1183/09031936.00069712
47. King G, Bates J, Berger K, Calverley P, de Melo P, Dellacà R, et al. Technical standards for respiratory oscillometry. Eur Respir J. (2020) 55:1900753. doi: 10.1183/13993003.00753-2019
48. Stanojevic S, Graham B, Cooper B, Thompson B, Carter K, Francis R, et al. Official ERS technical standards: Global lung function initiative reference values for the carbon monoxide transfer factor for Caucasians. Eur Respir J. (2017) 50:1700010. doi: 10.1183/13993003.00010-2017
49. Ghanei M, Adibi I, Farhat F, Aslani J. Late respiratory effects of sulfur mustard: How is the early symptoms severity involved? Chron Respir Dis. (2008) 5:95–100. doi: 10.1177/1479972307087191
50. Bruzzese J, Kingston S, Falletta K, Bruzelius E, Poghosyan L. Individual and neighborhood factors associated with undiagnosed asthma in a large cohort of urban adolescents. J Urban Health. (2019) 96:252–61. doi: 10.1007/s11524-018-00340-2
51. Ho T, Cusack R, Chaudhary N, Satia I, Kurmi O. Under– and over-diagnosis of COPD: A global perspective. Breathe (Sheff). (2019) 15:24–35. doi: 10.1183/20734735.0346-2018
52. Pourfarzam S, Ghazanfari T, Merasizadeh J, Ghanei M, Azimi G, Araghizadeh H, et al. Long-term pulmonary complications in sulfur mustard victims of Sardasht, Iran. Toxin Rev. (2009) 28:8–13. doi: 10.1080/15569540802689220
53. Suryadevara V, Huang L, Kim S, Cheresh P, Shaaya M, Bandela M, et al. Role of phospholipase D in bleomycin-induced mitochondrial reactive oxygen species generation, mitochondrial DNA damage, and pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. (2019) 317:L175–87. doi: 10.1152/ajplung.00320.2018
54. Swendsen C, Skita V, Thrall R. Alterations in surfactant neutral lipid composition during the development of bleomycin-induced pulmonary fibrosis. Biochim Biophys Acta. (1996) 1301:90–6. doi: 10.1016/0005-2760(96)00023-9
55. Tahmasbpour E, Ghanei M, Qazvini A, Vahedi E, Panahi Y. Gene expression profile of oxidative stress and antioxidant defense in lung tissue of patients exposed to sulfur mustard. Mutat Res Genet Toxicol Environ Mutagen. (2016) 800-801:12–21. doi: 10.1016/j.mrgentox.2016.03.006
56. Tang F, Loke W. Sulfur mustard and respiratory diseases. Crit Rev Toxicol. (2012) 42:688–702. doi: 10.3109/10408444.2012.698405
57. Chrystyn H, Price D. Not all asthma inhalers are the same: Factors to consider when prescribing an inhaler. Prim Care Respir J. (2009) 18:243–9. doi: 10.4104/pcrj.2009.00029
58. de Boer A, Gjaltema D, Hagedoorn P, Frijlink H. Can ‘extrafine’ dry powder aerosols improve lung deposition? Eur J Pharm Biopharm. (2015) 96:143–51. doi: 10.1016/j.ejpb.2015.07.016
59. Hofmanninger J, Prayer F, Pan J, Röhrich S, Prosch H, Langs G. Automatic lung segmentation in routine imaging is primarily a data diversity problem, not a methodology problem. Eur Radiol Exp. (2020) 4:1–13. doi: 10.1186/s41747-020-00173-2
60. Kitahara Y, Shirai T, Watanabe H, Sugiyama S, Okawa K, Shiratori K, et al. Changes in oscillometry parameters in reversibility testing as predictors for cough variant asthma. J Allergy Clin Immunol. (2022) 149:AB190.
Keywords: small airways, impulse oscillometry, multiple breath washout, pulmonary disease, sulfur mustard, Halabja
Citation: Moradi F, Kjellberg S, Li Y, Daka B and Olin A-C (2024) Respiratory function after 30+ years following sulfur mustard exposure in survivors in Sweden. Front. Med. 11:1251500. doi: 10.3389/fmed.2024.1251500
Received: 01 July 2023; Accepted: 14 February 2024;
Published: 04 March 2024.
Edited by:
Dirk Steinritz, Ludwig Maximilian University of Munich, GermanyReviewed by:
Sermet Sezigen, University of Health Sciences, TürkiyeLivia A. Veress, University of Colorado Anschutz Medical Campus, United States
Copyright © 2024 Moradi, Kjellberg, Li, Daka and Olin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Faraidoun Moradi, ZmFyYWlkb3VuLm1vcmFkaUBnbWFpbC5jb20=; bW9yYWRpLmZhcmFpZG91bkBndS5zZQ==
 Faraidoun Moradi
Faraidoun Moradi Sanna Kjellberg1
Sanna Kjellberg1 Bledar Daka
Bledar Daka Anna-Carin Olin
Anna-Carin Olin