
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Med. , 07 February 2024
Sec. Obstetrics and Gynecology
Volume 11 - 2024 | https://doi.org/10.3389/fmed.2024.1233962
Introduction: Recurrent reproductive failure (RRF) is a common pregnancy complication, imposing great physical, emotional and financial burden for the suffered couples. The leading cause of RRF is believed to be aneuploid embryo, which could be solved by preimplantation genetic testing for aneuploidy (PGT-A) in theory. With molecular genetic development, PGT-A based on comprehensive chromosomal screening (CCS) procedures and blastocyst biopsy is widely applied in clinical practice. However, its effects in RRF were not defined yet.
Methods: A systematic bibliographical search was conducted without temporal limits up to June, 2023. Studies about the effects of PGT-A based on CCS procedures and blastocyst biopsy in RRF were included.
Results: Twenty studies about the effects of PGT-A based on CCS procedures and blastocyst biopsy in RRF were included. It revealed that PGT-A could optimise the reproductive outcomes of RRF sufferers, especially in those with advanced age. However, in patients with multiple occurrences of pregnancy losses, the benefits of PGT-A were limited.
Discussion: More randomized controlled trials with large sample size are required to evaluate the benefits of PGT-A in RRF sufferers and identify which population would benefit the most.
Recurrent reproductive failure (RRF), a common pregnancy complication, mainly comprises recurrent pregnancy loss (RPL) and recurrent implantation failure (RIF) (1). RPL refers to ≥2 pregnancy losses or miscarriages (recurrent miscarriage, RM) before 20–24 weeks of gestation (2), affecting 1–2% of all couples (3), while RIF is defined as ≥3 failed embryo transfers with good-quality in in vitro fertilization (IVF) (4), affecting about 10% of couples undergoing IVF treatment (5). RRF had brought great physical and mental pressure to the suffered couples, which linked to increased risk of infertility and pregnancy loss (6).
Aneuploidy is a critical cause of RRF (7). In RPL, aneuploidy is identified in at least 55% of products of RPL sufferers’ conception (8), while the embryo is thought to be responsible for 30–50% of RIF (9). Therefore, euploid embryo transfer (ET) is speculated to optimize the reproductive outcomes of RRF. Fortunately, euploid embryos could be selected by preimplantation genetic testing for aneuploidy (PGT-A). Originally, PGT-A was achieved by fluorescence in situ hybridization procedure (10), which was highly limited since it assessed only nine out of 24 chromosomes simultaneously with low resolution (11). Multiple major professional societies recommended against its general use (12), as PGT-A based on the FISH procedure failed to improve reproductive outcomes in clinical practice (13, 14). With molecular genetic advances, comprehensive chromosomal screening (CCS) procedures and blastocyst biopsy were developed in PGT-A. The commonly used CCS procedure encompasses array comparative genomic hybridization (aCGH), quantitative real-time PCR (qRT-PCR) and next-generation sequencing (NGS), etc. (12). CCS procedures not only analyzed the number of all chromosomes, but also segmental abnormalities. As blastocysts could better tolerate the insults of biopsy with an increased accuracy rate, blastocyst biopsy was found to yield overall improved reproductive outcomes than cleaved embryo biopsy or polar biopsy (12, 15, 16). Therefore, PGT-A based on CCS procedure and blastocyst biopsy is widely used in clinical practice. However, no consensus has been reached about its effects in RRF sufferers. The review aims to investigate if PGT-A is beneficial for RRF sufferers, and identify suitable population by sub-group analysis.
Databases of PubMed, Embase, and Cochrane Central Register of Controlled Trials were searched with the following terms: (recurrent OR repeated OR habitual) AND (pregnancy loss OR spontaneous abortion OR miscarriage OR fetal wastage) AND (preimplantation genetic diagnosis OR preimplantation genetic screening OR euploid OR preimplantation genetic test) from inception to June, 2023. The inclusion criteria were as follows. Published in English in peer-reviewed journals; irrespective of study-design; studies focusing on the impact of PGT-A based on CCS procedures and blastocyst biopsy on the reproductive outcomes of RRF sufferers. Commentaries, letters, reviews, conference abstracts, and irrelevant studies were excluded. Studies in which PGT-A assay not based on CCS procedure and blastocyst biopsy were also excluded.
Following an initial search and duplicates removed, a total of 1,063 literatures were screened by two authors independently (XK and XL). An overview of and screening process is presented in Figure 1. The selected studies were comprehensively examined, and the relevant data were extracted according to our developed data extraction spreadsheet by authors (YM and YL). Information selected included author’s name, publication year and country of the study, study year, study aim, sample size, methodology, sample characteristics, and outcome measures. Any discrepancies would be resolved by discussion until consensus was reached. The primary outcomes of interest were live birth rate (LBR), defined as the percentage of couples achieving a live birth after 24 weeks’ gestation. Secondary outcomes of interest included implantation rate (IR), clinical pregnancy rate (CPR)/ongoing pregnancy rate (OPR), biochemical pregnancy loss (BPL) rate, and miscarriage rate (MR). This study was exempted from Institutional Review Board approval, as it was a systematic review. We adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (17).
Unfortunately, only two prospective studies were retrieved. The other 18 included studies were all retrospective. Meta-analysis was precluded owing to their great heterogeneity. Table 1 showed the basic clinical characteristics of the included studies. These studies were all published between 2014 and 2023, and most (n = 13) published between 2019 and 2023. The geographical spread of these studies was as follows: seven from China, five from the United States, three from India, two from Italy, and one from Japan, Greece, and Latvia, respectively. In the present review, the study population consisted of RRF sufferers using PGT-A, whereas the control population comprised of RRF sufferers not using PGT-A or those without RRF history using PGT-A. RPL/RIF PGT-A refers to RPL/RIF sufferers who underwent PGT-A. RPL/RIF NO PGT-A refers to RPL/RIF sufferers who did not undergo PGT-A. NO RPL/RIF PGT-A refers to patients without a history of RPL/RIF who underwent PGT-A. The reproductive outcomes were presented in Table 2.
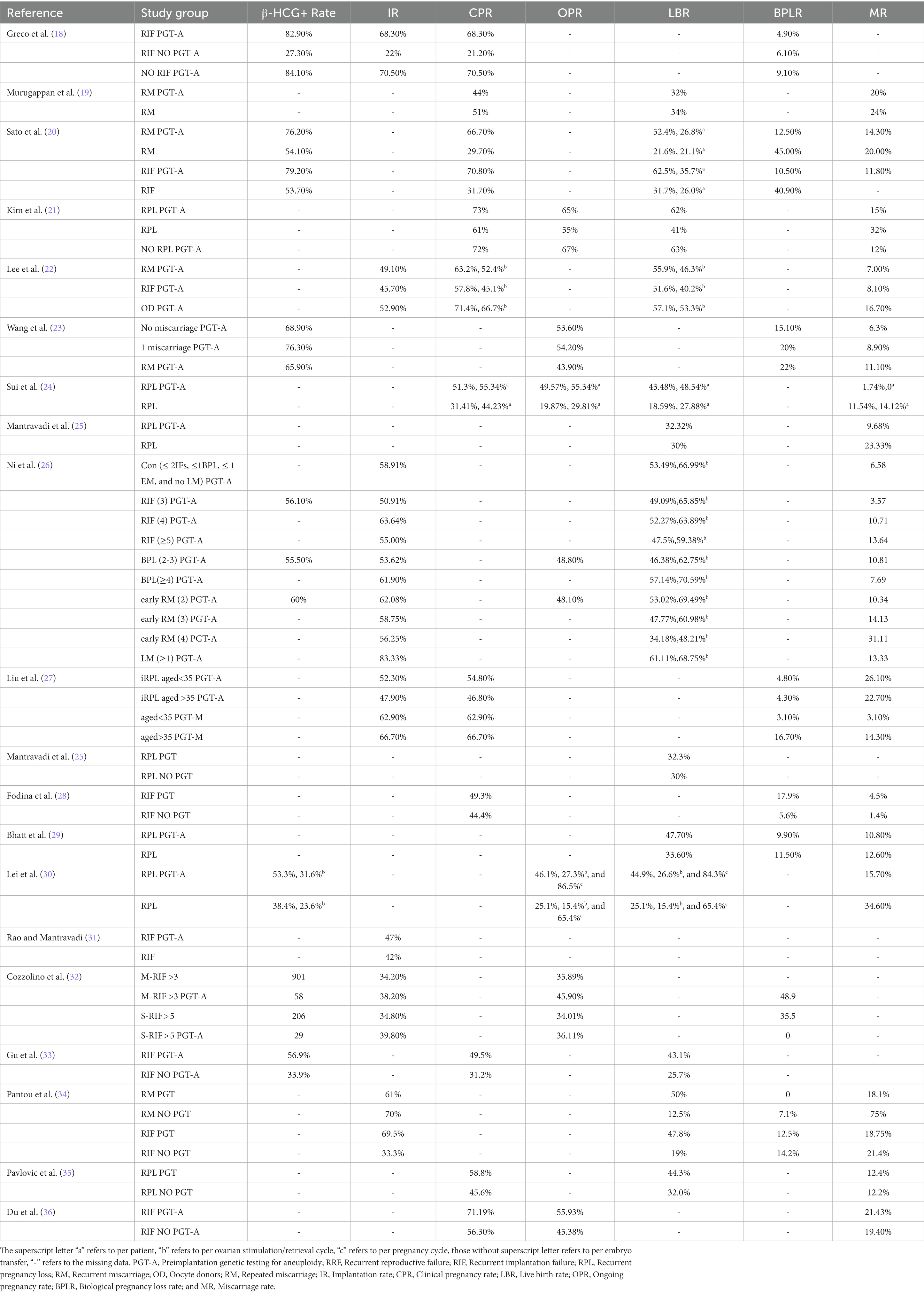
Table 2. The comparison of reproductive outcomes between the study group of patients with RRF using PGT-A and the control group.
Aneuploid embryos were commonly observed in the blastocysts from the IVF procedures. The euploidy rate was reported to be 56.4, 39.1, 42.8, and 25.5% in the excellent (≥3AA), good (3, 4, 5, 6 AB and BA), average (3, 4, 5, 6 BB, AC, and CA), and poor (≤3BB) blastocyst morphology groups, respectively (11). In RRF sufferers with blastocysts available for biopsy, the aneuploidy rate kept in line with maternal age. Sato et al. (20) reported that the aneuploidy rates were 43, 63, 69, and 91% in RPL sufferers and 56, 77, 77, and 94% in RIF sufferers according in the age groups of 35–36, 37-38, 39-40, and 41–42. Tong et al. (9) found that the aneuploidy rate in patients with RIF aged >38 years was significantly higher than that aged <38 years (68.9 vs. 39.9%, p < 0.001). Liu et al. (27) also reported that the aneuploidy rate in the idiopathic RPL group aged >35 years was higher than those aged <35 years (68.6 vs. 48.9%).
It should be noted that mosaic embryos were commonly observed according to the results of PGT-A based on CCS. Mosaicism is defined as the presence of ≥2 cell populations with different chromosomal constitutions within the same embryo (37), which may be contributed by many factors such as mitotic errors, amplification bias, contamination and the PGT-A provider, etc. (38). It was reported that the low-range mosaic embryos (<50%) showed a higher ongoing pregnancy rate and lower miscarriage rate, while a high-range mosaicism detection (>50%) was associated with whole chromosome aneuploidy in a significant proportion of cases. Therefore, cutoff 50% of mosaicism was recommended as a reference in clinical management (39). According to previous literature, the rate of mosaic embryo was 9.3 and 5.2% in RIF sufferers aged <38 and > 38 years (9), while 9.1 and 5.3% in RPL sufferers aged <35 aged group and RPL aged >35 aged group, respectively (27). This suggested that mosaicism rate did not always consistent with maternal age.
In 2019, Kim et al. (21) retrospectively reviewed the reproductive outcomes of RPL sufferers undergoing their first single embryo transfer (ET). Results found that RPL sufferers using PGT-A (n = 660) had significantly higher CPR (73 vs. 61%, p = 0.01), LBR (62 vs. 41%, p < 0.01), and reduced clinical pregnancy loss rate (15 vs. 32%, p < 0.01), compared with RPL sufferers not using PGT-A (n = 101). In the same year, Lei et al. (30) reported that RPL sufferers using PGT-A (n = 212) had acquired higher LBRs per cycle start (26.6 vs. 15.4%, p = 0.0004) and transfer (44.9 vs. 25.1%, p < 0.0001), and lower MR (15.7 vs. 34.6%, p = 0.0007) than those not using PGT-A (n = 294). In 2020, retrospective study of Mantravadi et al. (25) reported that the MR (9.68 vs. 23.33%, p = 0.0610) was lower in patients with idiopathic RPL using PGT-A (n = 82) than those not using PGT-A (n = 30), although the LBR and take home baby rates were not significantly different. Also in 2020, Sato et al. (20) made a multi-center prospective study which revealed that no significant differences were observed in the LBR and the MR in RPL sufferers given or not given PGT-A. However, PGT-A improved the LBR per embryo transfer in the RPL (52.4 vs. 21.6%, p = 0.028; PGT-A group vs. non-PGT-A group = 41 vs. 38). Additionally, PGT-A reduced BPLR in the RPL group (12.5 vs. 45.0%, p = 0.03). Similarly, Sui et al. (24) conducted a prospective randomized clinical trial (study group vs. control group = 104 vs. 103), results also revealed that PGT-A significantly increased the OPR (55.34 vs. 29.81%, p < 0.05), LBR (48.54 vs 27.88%, p < 0.05), and decreased the MR (0 vs 14.42%, p < 0.05) on a per-patient analysis in RPL sufferers. In 2021, Bhatt et al. (29) conducted a retrospective study which included IVF-FET cycles from 2010 to 2016 in the Society of Assisted Reproductive Technologies Clinical Outcomes Reporting System. The results revealed that PGT-A increased the LBR (48 vs. 34%, p < 0.001) and CPR (59 vs 47%, p < 0.001) and decreased the BPLR (9.9 vs 11.5%, p = 0.02) and MR (11 vs. 13%, p = 0.02) in RPL sufferers (PGT-A vs. non-PGT-A = 3,241 vs. 3,351). In 2022, Pantou et al.’s (34) retrospective study revealed that in the RM group, a significant decrease of early pregnancy loss rate (18.1 vs. 75%, p = 0.001) and significant increase in LBRs per transfer (50 vs. 12.5%, p = 0.002)/per patient (36 vs. 12.5%, p = 0.026) were observed in the PGT-A group compared with the non-PGT-A group (n = 25 vs. 40). In 2023, Pavlovic et al. (35) retrospectively compared the reproductive outcomes of idiopathic RPL sufferers between the PGT-A group and the non-PGT-A group. The use of PGT-A tested embryos resulted in significant increase in CPR (58.8 vs. 45.6%, p = 0.007) and LBR (44.3 vs. 32.0%, p = 0.011) compared to cycles using untested embryos. After adjusting for confounding factors, LBR remained significantly increased in the PGT-A cycles compared with the non-PGT-A cycles (OR = 2.26, 95%CI 1.19–4.31). However, the use of an euploid embryo does not significantly decrease MR (12.4 vs. 12.2%, p = 1.000).
For RIF, in Sato’s above mentioned study (20), the LBR per ET were significantly increased in RIF sufferers with PGT-A than those without PGT-A (62.5 vs. 31.7%, p = 0.016, PGT-A group vs. non-PGT-A group = 24 vs. 42). Additionally, BPLR was significantly reduced in the RIF PGT-A group compared with RIF NO PGT-A group (10.5 vs. 40.9%, p = 0.04). Fodina et al.’s (28) retrospective study reported that PGT-A group showed statistically significant higher chance in achieving both biochemical (17.9 vs. 5.6%, p = 0 0.01) and clinical pregnancy (49.3 vs. 44.4%, p = 0.049), as compared to those who did not undergo PGT-A (n = 72 vs. 22) in RIF sufferers. In the same year, Rao’s (31) retrospective study also reported that IR was higher in the PGT-A group (n = 54) than the control group (n = 189) (47 vs. 42%), although the difference was not significant. In 2022, Gu et al. (33) conducted a retrospective analysis which revealed that the positive serum human chorionic gonadotropin (56.9 vs. 33.9%, p < 0.01), clinical pregnancy (49.5 vs. 31.2%, p < 0.01), live birth (43.1 vs. 25.7%, p < 0.01), and fetal heart rates (50.0 vs. 29.8%, p < 0.01) per transfer were significantly higher in the RIF-PGT-A group (n = 209) than the RIF-non-PGT-A group (n = 257). In Pantou’s (34) above mentioned study, a significant increase in the IR (69.5 vs. 33.3%, p = 0.005) and the LBR per transfer (47.8 vs. 19%, p = 0.015) was observed between PGT-A and non-PGT-A group in RIF sufferers (n = 30 vs. 42). These studies demonstrated that PGT-A could optimize the reproductive outcomes in RRF than those not using PGT-A.
In 2019, Kim’s (21) above mentioned study revealed that the Cin patients with RPL CPR using PGT-A (n = 660) was comparable (73 vs. 72%, p = 0.01) to that of infertile patients using PGT-A without RPL history (n = 3,975), although the clinical pregnancy loss rate was higher (15 vs. 12%, p < 0.01). In the same year, Wang et al. (23) conducted a retrospective cohort study that enrolled patients who had their first IVF cycle with PGT-A. It revealed that the positive β-HCG (65.9 vs. 68.9%), ongoing pregnancy (43.9 vs. 53.6%), and total pregnancy loss rates (33.3 vs. 21.4%) did not significantly differ in patients with RPL (n = 41) compared with patients without a history of miscarriage (n = 183). Bhatt et al.’s (29) above mentioned study also revealed no difference was observed in the reproductive outcomes between patients with RPL using PGT-A and those with tubal factors. In 2020, Lee et al. (22) retrospectively compared the reproductive outcomes in patients who experienced RIF (n = 82), RM (n = 82), and oocyte donors (OD) (n = 45) using PGT-A. Results showed that the LBR were similar among patients RIF, RM, and OD groups (51.6 vs. 55.9 vs. 57.1%). These studies suggested that patients with RRF using PGT-A had comparable reproductive outcomes than patients without RRF history.
However, study of Murugappan et al. (19) concluded that PGT-A could not improve the reproductive outcomes of patients with RRF. This retrospective study, which included 112 RPL patients desired who preimplantation genetic screening and 188 patients who chose expectant management (without further examination and treatment), revealed that the rates of CPR, LBR, and MR were similar between the PGT-A and expectant management groups. Moreover, the median time to pregnancy was even longer in the PGT-A group than in the expectant management group (6.5 vs. 3.0 months). However, it should be noted that in this paper patients with expectant management were used as a control, while the control group usually refers to RRF sufferers who do not use PGT-A or patients without RRF history who underwent PGT-A in other papers (40).
In 2014, Greco et al. (18) conducted a retrospective study to investigate the effects of PGT-A in patients with RIF aged <36 years. The results revealed that the IR (68.3 vs. 22%, p = 0.001) and CPR (68.3 vs. 21.2%, p = 0.001) were significantly increased in the RIF PGT-A group (n = 43) than RIF NO PGT-A group (n = 33). On the other hand, the RIF PGT-A group had similar IR (68.3 vs. 70.5%, p = 1) and CPR (68.3 vs. 70.5%, p = 1), compared with NO RIF PGT-A group. Du et al. (36) also reported that the CPR was significantly increased in RIF PGT-A group (n = 59) compared with RIF NO PGT-A group (n = 119) (71.19 vs. 56.30%, p = 0.039) in RIF patients aged <38 years old. The OPR was also higher in the PGT-A group than the RIF without PGT-A group (55.93 vs. 45.38% p = 0.214), although the difference was not significant. These results indicated that PGT-A could optimize the reproductive outcomes in patents <38 years old.
Keiichi Kato (41) conducted a retrospective study which enrolled 32 patients who underwent PGT-A (18 in the RIF protocol and 14 in the RPL protocol) and 2,556 patients with IVF treatment at the same period for women aged 35–42 years in 2023. Results revealed that RPL patients with PGT-A had acquired increased LBR per ET (80.0 vs. 0, p = 0.005) and reduced MR (20.0 vs. 100.0%, p = 0.0098), compared with RPL sufferers without PGT-A. In the RIF sufferers, the PGT-A group also had better reproductive outcomes with higher LBR per ET [90.0 vs. 69.2% (p = 0.2313)], and lower MR (0 vs. 10.0%, p = 0.3297), although the difference was not significant. In 2021, Tong et al. (17) retrospectively compared the reproductive outcomes in RIF patients who underwent PGT-A between the younger (<38 years) and advanced (>38 years) age groups. Results revealed that there were no significant differences in the IR (39.1 vs. 51.0%), CPR (39.1 vs. 48.0%), and MR (4.3 vs. 7.8%) per ET between the two groups. In the same year, the above mentioned study conducted by Bhatt (30) revealed that in RPL sufferers, the adjusted odds ratio comparing IVF-FET with PGT-A vs. without PGT-A for live birth outcome was 1.31 (95% CI: 1.12, 1.52) for age < 35 years, 1.45 (95% CI: 1.21, 1.75) for ages 35–37 years, 1.89 (95% CI: 1.56, 2.29) for ages 38–40, 2.62 (95% CI: 1.94–3.53) for ages 41–42, and 3.80 (95% CI: 2.52, 5.72) for ages >42 years. These studies implied that PGT-A was beneficial in both young and patients with advanced age, particularly in patients with advanced age.
In 2020, Sui et al.’s (24) above mentioned study revealed that the benefits of PGT-A were limited in patients with >2 failed PGT-A cycles (who failed to achieve ongoing pregnancy). In the same year, another retrospective multi-center cohort study by Cozzolino et al. (32) also reported that PGT-A could significantly improve the IR and OPR in the moderate RIF group (>3 implantation failures). However, the IR and OPR were not different in the severe RIF group (>5 implantation failures). Similarly, Ni et al.’s (26) retrospective study concluded that compared with the control group (patients using PGT-A after one spontaneous abortion with abnormal genetic testing results in aborted villus tissues and women with ≤2 IFs and ≤ 1 BPL, n = 103), patients with ≥4 previous early miscarriages (n = 56) had a significantly increased early miscarriage rate (6.58 vs. 31.11%, p < 0.001) and a decreased live birth rate (53.49 vs. 34.18%, p = 0.007) after euploid transfer. These studies demonstrated that PGT-A’s effectiveness was limited in patients with multiple pregnancy losses.
Overall, our results indicated that PGT-A based on blastocyst biopsy and CCS procedures could optimize the reproductive outcomes of patients with RRF, which could be expected as the incidence of chromosomal abnormalities was higher in these patients. However, it should be noted that PGT-A cannot identify all possible genetic abnormalities or developmental defects. It could not guarantee successful pregnancy, which requires embryo with good quality and endometrial receptivity (42). As known, RPL and RIF are both complex and multifactorial condition. It is critical that RPL or RIF sufferers be properly evaluated to identify all possible causes and treated individually. Furthermore, they should be recommended to have prenatal diagnosis during the pregnancy period (27, 35). Their offspring should also have postnatal follow-up (25). In the future, the reproductive outcomes of RRF may be furtherly improved by artificial intelligence which could play a role in the following aspects: ultrasound monitoring of folliculogenesis, endometrial receptivity, embryo selection based on quality and viability, and prediction of post implantation embryo development, etc. (42).
In sub-analysis, we found that PGT-A was effective in RRF patients of any age, especially in patients with advanced aged. This made sense as the aneuploidy rate increased in patients with advanced maternal age in PGT-A cycles (16, 17, 20). However, it should be noticed that chances of women with advanced age getting blastocysts are less as their ovarian reserve is decreased. Deng et al. (43) reported that in patients with poor ovarian response, PGT-A cycles had less chance to reach embryo transfer compared with those not using PGT-A (13.7 vs. 70.6%, p < 0.001), and no difference were observed in the LBR per oocyte retrieval in cycles using or not using PGT-A (6.6 vs. 5.4%, p = 0.814). 31 PGT-A cycles were needed to avoid one clinical miscarriage. Therefore, PGT-A should be cautiously used for the population with advanced maternal age with poor ovarian response. Also, our results revealed that >3 instances of previous RRF or > 2 cycles of PGT-A cycles limited the benefits of PGT-A. This could be explained by the fact that no difference was observed in the prevalence of chromosomal abnormalities in couples with 2 and ≥ 3 pregnancy losses (44, 45), and PGT-A only selected euploidy embryos instead of changing the embryo pool (46). On the other hand, some other factors also affected the successful pregnancy rate other than an aneuploid embryo, such as thrombophilia, immunology, metabolic/endocrinological abnormalities, and anatomical abnormalities (47). Therefore, the benefits of PGT-A were not obvious in RRF with multiple pregnancy losses.
The primary limitation of this review was the paucity of high-quality studies which only included two prospective studies. Second, most studies did not show concomitant factors, such as AMH, previous times of pregnancy loss and other endocrine and immune disorders. As already known, AMH is an independent variable of increased aneuploidy embryo rate (27, 48). And previous occurrences of pregnancy loss and other endocrine and immune disorders were also closely associated with reproductive outcomes during the PGT-A cycles. Therefore, a direct comparison was challenging, because of the heterogeneity in patient cohorts of these studies.
Overall, PGT-A was beneficial for patients with RRF, especially in advanced aged patients. However, in patients with decreased ovarian reserve, the benefits of PGT-A may not be obvious as the probability of getting an euploid embryo was lower. In addition, PGT-A may have limited benefits for patients with multiple occurrences of pregnancy loss.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
YM drafted the manuscript and participated in data collection and analysis. YL participated in the design of the study and performed the statistical analysis. YC participated in its design and coordination. JZ, XK, and XL participated in data collection and analysis. FW participated in the design of the study and revised the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. al-Lamee, H, Ellison, A, Drury, J, Hill, CJ, Drakeley, AJ, Hapangama, DK, et al. Altered endometrial oestrogen-responsiveness and recurrent reproductive failure. Reprod Fertil. (2022) 3:30–8. doi: 10.1530/RAF-21-0093
2. Medicine PCotASfR . Evaluation and treatment of recurrent pregnancy loss: a committee opinion. Fertil Steril. (2012) 98:1103–11. doi: 10.1016/j.fertnstert.2012.06.048
3. The ESHRE Guideline Group on RPLBender Atik, R, Christiansen, OB, Elson, J, Kolte, AM, Lewis, S, et al. ESHRE guideline: recurrent pregnancy loss: an update in 2022. Hum Reprod Open. (2023) 2023:hoad002. doi: 10.1093/hropen/hoad002
4. Margalioth, EJ, Ben-Chetrit, A, Gal, M, and Eldar-Geva, T. Investigation and treatment of repeated implantation failure following IVF-ET. Hum Reprod. (2006) 21:3036–43. doi: 10.1093/humrep/del305
5. Busnelli, A, Reschini, M, Cardellicchio, L, Vegetti, W, Somigliana, E, and Vercellini, P. How common is real repeated implantation failure? An indirect estimate of the prevalence. Reprod Biomed Online. (2020) 40:91–7. doi: 10.1016/j.rbmo.2019.10.014
6. Kuroda, K, Horikawa, T, Moriyama, A, Ojiro, Y, Takamizawa, S, Watanabe, H, et al. Therapeutic efficacy of the optimization of thyroid function, thrombophilia, immunity and uterine milieu (OPTIMUM) treatment strategy on pregnancy outcomes after single euploid blastocyst transfer in advanced age women with recurrent reproductive failure. Reprod Med Biol. (2023) 22:e12554. doi: 10.1002/rmb2.12554
7. Martínez, MC, Méndez, C, Ferro, J, Nicolás, M, Serra, V, and Landeras, J. Cytogenetic analysis of early nonviable pregnancies after assisted reproduction treatment. Fertil Steril. (2010) 93:289–92. doi: 10.1016/j.fertnstert.2009.07.989
8. Papas, RS, and Kutteh, WH. Genetic testing for aneuploidy in patients who have had multiple miscarriages: a review of current literature. Appl Clin Genet. (2021) 14:321–9. doi: 10.2147/tacg.S320778
9. Tong, J, Niu, Y, Wan, A, and Zhang, T. Next-generation sequencing (NGS)-based preimplantation genetic testing for aneuploidy (PGT-A) of Trophectoderm biopsy for recurrent implantation failure (RIF) patients: a retrospective study. Reprod Sci. (2021) 28:1923–9. doi: 10.1007/s43032-021-00519-0
10. Handyside, AH, Kontogianni, EH, Hardy, K, and Winston, RM. Pregnancies from biopsied human preimplantation embryos sexed by Y-specific DNA amplification. Nature. (1990) 344:768–70. doi: 10.1038/344768a0
11. Capalbo, A, Rienzi, L, Cimadomo, D, Maggiulli, R, Elliott, T, Wright, G, et al. Correlation between standard blastocyst morphology, euploidy and implantation: an observational study in two centers involving 956 screened blastocysts. Hum Reprod. (2014) 29:1173–81. doi: 10.1093/humrep/deu033
12. Brezina, PR, Anchan, R, and Kearns, WG. Preimplantation genetic testing for aneuploidy: what technology should you use and what are the differences? J Assist Reprod Genet. (2016) 33:823–32. doi: 10.1007/s10815-016-0740-2
13. Mastenbroek, S, Twisk, M, van der Veen, F, and Repping, S. Preimplantation genetic screening: a systematic review and meta-analysis of RCTs. Hum Reprod Update. (2011) 17:454–66. doi: 10.1093/humupd/dmr003
14. Mastenbroek, S, Twisk, M, van Echten-Arends, J, Sikkema-Raddatz, B, Korevaar, JC, Verhoeve, HR, et al. In vitro fertilization with preimplantation genetic screening. N Engl J Med. (2007) 357:9–17. doi: 10.1056/NEJMoa067744
15. Sawarkar, S, and Munne, S. Genetic selection of the human embryos: from FISH to NGS, past and future. Reproductomics. (2018):227–42. doi: 10.1016/B978-0-12-812571-7.00014-9
16. Schoolcraft, WB, Fragouli, E, Stevens, J, Munne, S, Katz-Jaffe, MG, and Wells, D. Clinical application of comprehensive chromosomal screening at the blastocyst stage. Fertil Steril. (2010) 94:1700–6. doi: 10.1016/j.fertnstert.2009.10.015
17. Moher, D, Liberati, A, Tetzlaff, J, and Altman, DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. (2010) 8:336–41. doi: 10.1016/j.ijsu.2010.02.007
18. Greco, E, Bono, S, Ruberti, A, Lobascio, AM, Greco, P, Biricik, A, et al. Comparative genomic hybridization selection of blastocysts for repeated implantation failure treatment: a pilot study. Biomed Res Int. (2014) 2014:457913. doi: 10.1155/2014/457913
19. Murugappan, G, Shahine, LK, Perfetto, CO, Hickok, LR, and Lathi, RB. Intent to treat analysis of in vitro fertilization and preimplantation genetic screening versus expectant management in patients with recurrent pregnancy loss. Hum Reprod. (2016) 31:1668–74. doi: 10.1093/humrep/dew135
20. Sato, T, Sugiura-Ogasawara, M, Ozawa, F, Yamamoto, T, Kato, T, Kurahashi, H, et al. Preimplantation genetic testing for aneuploidy: a comparison of live birth rates in patients with recurrent pregnancy loss due to embryonic aneuploidy or recurrent implantation failure. Hum Reprod. (2020) 35:255. doi: 10.1093/humrep/dez289
21. Kim, JG, Murugappan, G, Lathi, RB, Kort, JD, Hanson, BM, Tiegs, AW, et al. Preimplantation genetic testing for aneuploidy (PGT-A) reduces miscarriage and improves live birth rates in recurrent pregnancy loss patients. Fertil Steril. (2019) 112:e401. doi: 10.1016/j.fertnstert.2019.07.1141
22. Lee, CI, Wu, CH, Pai, YP, Chang, YJ, Chen, CI, Lee, TH, et al. Performance of preimplantation genetic testing for aneuploidy in IVF cycles for patients with advanced maternal age, repeat implantation failure, and idiopathic recurrent miscarriage. Taiwan J Obstet Gynecol. (2019) 58:239–43. doi: 10.1016/j.tjog.2019.01.013
23. Wang, A, Kort, J, and Westphal, L. Miscarriage history association with euploid embryo transfer outcomes. Reprod Biomed Online. (2019) 39:617–23. doi: 10.1016/j.rbmo.2019.05.011
24. Sui, YL, Lei, CX, Ye, JF, Fu, J, Zhang, S, Li, L, et al. In vitro fertilization with single-nucleotide polymorphism microarray-based preimplantation genetic testing for aneuploidy significantly improves clinical outcomes in infertile women with recurrent pregnancy loss: a randomized controlled trial. Reprod Dev Med. (2020) 4:32–41. doi: 10.4103/2096-2924.281852
25. Mantravadi, K, Mathew, S, Soorve, S, Rao, DG, and Karunakaran, S. Does preimplantation genetic testing for aneuploidy optimise the reproductive outcomes in women with idiopathic recurrent pregnancy loss? Fertil Steril. (2020) 114:e437. doi: 10.1016/j.fertnstert.2020.08.1267
26. Ni, T, Wu, Q, Zhu, Y, Jiang, W, Zhang, Q, Li, Y, et al. Comprehensive analysis of the associations between previous pregnancy failures and blastocyst aneuploidy as well as pregnancy outcomes after PGT-A. J Assist Reprod Genet. (2020) 37:579–88. doi: 10.1007/s10815-020-01722-9
27. Liu, XY, Fan, Q, Wang, J, Li, R, Xu, Y, Guo, J, et al. Higher chromosomal abnormality rate in blastocysts from young patients with idiopathic recurrent pregnancy loss. Fertil Steril. (2020) 113:853–64. doi: 10.1016/j.fertnstert.2019.11.016
28. Fodina, V, Dudorova, A, and Erenpreiss, J. Evaluation of embryo aneuploidy (PGT-A) and endometrial receptivity (ERA) testing in patients with recurrent implantation failure in ICSI cycles. Gynecol Endocrinol. (2021) 37:17–20. doi: 10.1080/09513590.2021.2006466
29. Bhatt, SJ, Marchetto, NM, Roy, J, Morelli, SS, and McGovern, PG. Pregnancy outcomes following in vitro fertilization frozen embryo transfer (IVF-FET) with or without preimplantation genetic testing for aneuploidy (PGT-A) in women with recurrent pregnancy loss (RPL): a SART-CORS study. Hum Reprod. (2021) 36:2339–44. doi: 10.1093/humrep/deab117
30. Lei, CX, Ye, JF, Sui, YL, Zhang, YP, and Sun, XX. Retrospective cohort study of preimplantation genetic testing for aneuploidy with comprehensive chromosome screening versus nonpreimplantation genetic testing in normal karyotype, secondary infertility patients with recurrent pregnancy loss. Reprod Dev Med. (2019) 3:205–12. doi: 10.4103/2096-2924.274544
31. Rao, DG, and Mantravadi, K. Recurrent implantation failure—role of PGT and ERA to optimize reproductive outcomes? Fertil Steril. (2021) 116:e396–7. doi: 10.1016/j.fertnstert.2021.07.1061
32. Cozzolino, M, Diaz-Gimeno, P, Pellicer, A, and Garrido, N. Evaluation of the endometrial receptivity assay and the preimplantation genetic test for aneuploidy in overcoming recurrent implantation failure. J Assist Reprod Genet. (2020) 37:2989–97. doi: 10.1007/s10815-020-01948-7
33. Gu, R-H, Fu, J, Ge, N-D, Li, ZC, Huang, B, Xu, Y, et al. Preimplantation genetic testing for aneuploidy improves clinical outcomes in patients with repeated implantation failure. Reprod Dev Med. (2022) 7:12–9. doi: 10.1097/rd9.0000000000000043
34. Pantou, A, Mitrakos, A, Kokkali, G, Petroutsou, K, Tounta, G, Lazaros, L, et al. The impact of preimplantation genetic testing for aneuploidies (PGT-A) on clinical outcomes in high risk patients. J Assist Reprod Genet. (2022) 39:1341–9. doi: 10.1007/s10815-022-02461-9
35. Pavlovic, ZJ, Gallahan, S, Papri, S, New, EP, Sprague, R, Silva, C, et al. Analysis of pregnancy outcomes in patients with idiopathic recurrent pregnancy loss who utilized preimplantation genetic testing for aneuploidy. Fertil Steril. (2023) 120:e77–8. doi: 10.1016/j.fertnstert.2023.05.141
36. Du, Y, Guan, Y, Li, N, Shi, C, Zhang, Y, Ren, B, et al. Is it necessary for young patients with recurrent implantation failure to undergo preimplantation genetic testing for aneuploidy? Front Endocrinol. (2023) 14:1020055. doi: 10.3389/fendo.2023.1020055
37. Viotti, M . Preimplantation genetic testing for chromosomal abnormalities: aneuploidy, mosaicism, and structural rearrangements. Genes (Basel). (2020) 11:1–10. doi: 10.3390/genes11060602
38. Group PCotASfRMatGCP . Clinical management of mosaic results from preimplantation genetic testing for aneuploidy of blastocysts: a committee opinion. Fertil Steril. (2023) 120:973–82. doi: 10.1016/j.fertnstert.2023.08.969
39. ESHRE Working Group on Chromosomal Mosaicismde Rycke, M, Capalbo, A, Coonen, E, Coticchio, G, Fiorentino, F, et al. ESHRE survey results and good practice recommendations on managing chromosomal mosaicism. Hum Reprod Open. (2022) 2022:hoac044. doi: 10.1093/hropen/hoac044
40. Rienzi, L, Capalbo, A, Vajta, G, and Ubaldi, FM. PGS for recurrent pregnancy loss: still an open question. Hum Reprod. (2017) 32:476–7. doi: 10.1093/humrep/dew311
41. Kato, K, Kuroda, T, Yamadera-Egawa, R, Ezoe, K, Aoyama, N, Usami, A, et al. Preimplantation genetic testing for aneuploidy for recurrent pregnancy loss and recurrent implantation failure in minimal ovarian stimulation cycle for women aged 35-42 years: live birth rate, developmental follow-up of children, and embryo ranking. Reprod Sci. (2023) 30:974–83. doi: 10.1007/s43032-022-01073-z
42. Medenica, S, Zivanovic, D, Batkoska, L, Marinelli, S, Basile, G, Perino, A, et al. The future is coming: artificial intelligence in the treatment of infertility could improve assisted reproduction outcomes—the value of regulatory frameworks. Diagnostics. (2022) 12:2979–93. doi: 10.3390/diagnostics12122979
43. Deng, J, Hong, HY, Zhao, Q, Nadgauda, A, Ashrafian, S, Behr, B, et al. Preimplantation genetic testing for aneuploidy in poor ovarian responders with four or fewer oocytes retrieved. J Assist Reprod Genet. (2020) 37:1147–54. doi: 10.1007/s10815-020-01765-y
44. van Dijk, MM, Kolte, AM, Limpens, J, Kirk, E, Quenby, S, van Wely, M, et al. Recurrent pregnancy loss: diagnostic workup after two or three pregnancy losses? A systematic review of the literature and meta-analysis. Hum Reprod Update. (2020) 26:356–67. doi: 10.1093/humupd/dmz048
45. Ogasawara, M, Aoki, K, Okada, S, and Suzumori, K. Embryonic karyotype of abortuses in relation to the number of previous miscarriages. Fertil Steril. (2000) 73:300–4. doi: 10.1016/s0015-0282(99)00495-1
46. Kemper, JM, Wang, R, Rolnik, DL, and Mol, BW. Preimplantation genetic testing for aneuploidy: are we examining the correct outcomes? Hum Reprod. (2020) 35:2408–12. doi: 10.1093/humrep/deaa224
47. The ESHRE Guideline Group on RPLBender Atik, R, Christiansen, OB, Elson, J, Kolte, AM, Lewis, S, et al. ESHRE guideline: recurrent pregnancy loss. Hum Reprod Open. (2018) 2018:hoy004. doi: 10.1093/hropen/hoy004
48. La Marca, A, Minasi, MG, Sighinolfi, G, Greco, P, Argento, C, Grisendi, V, et al. Female age, serum antimüllerian hormone level, and number of oocytes affect the rate and number of euploid blastocysts in in vitro fertilization/intracytoplasmic sperm injection cycles. Fertil Steril. (2017) 108:777–783.e2. doi: 10.1016/j.fertnstert.2017.08.029
Keywords: preimplantation genetic testing for aneuploid, recurrent reproductive failure, recurrent implantation failure, recurrent pregnancy loss, comprehensive chromosomal screening, blastocyst biopsy, reproductive outcomes
Citation: Mei Y, Lin Y, Chen Y, Zheng J, Ke X, Liang X and Wang F (2024) Preimplantation genetic testing for aneuploidy optimizes reproductive outcomes in recurrent reproductive failure: a systematic review. Front. Med. 11:1233962. doi: 10.3389/fmed.2024.1233962
Received: 03 June 2023; Accepted: 26 January 2024;
Published: 07 February 2024.
Edited by:
Yuting Fan, Boston IVF, United StatesReviewed by:
Fallon Durant, Boston IVF, United StatesCopyright © 2024 Mei, Lin, Chen, Zheng, Ke, Liang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fang Wang, d2FuZ2ZjZDIwMjJAMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.