- 1The Second School of Clinical Medicine, Guangzhou University of Chinese Medicine, Guangzhou, Guangdong, China
- 2Clinical Medical College of Acupuncture Moxibustion and Rehabilitation, Guangzhou University of Chinese Medicine, Guangzhou, Guangdong, China
- 3Shenzhen Bao'an Chinese Medicine Hospital, The Seventh Clinical Medical School of Guangzhou University of Chinese Medicine, Shenzhen, Guangdong, China
- 4The Brain Cognition and Brain Disease Institute (BCBDI), Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences (CAS), Shenzhen-Hong Kong Institute of Brain Science-Shenzhen Fundamental Research Institutions, Shenzhen, Guangdong, China
- 5University of Chinese Academy of Sciences, Beijing, China
Objective: The objective of this study is to evaluate the methodological quality of Tuina clinical practice guidelines (CPGs).
Methods: Computer searches of China National Knowledge Infrastructure (CNKI), Chinese Technical Periodicals (VIP), Wanfang Data Knowledge Service Platform, PubMed, Cochrane Library, Embase, and other databases were conducted to search for published guidelines on Tuina, with a search time frame from database creation to March 2021. Four evaluators independently used the Appraisal of Guidelines for Research and Evaluation II instrument to evaluate the quality of the included guidelines.
Results: A total of eight guidelines related to Tuina were included in this study. The quality of reporting was low in all included guidelines. The highest quality report had a total score of 404 and was rated as “highly recommended.” The worst guideline had a final score of 241 and was rated as “not recommended.” Overall, 25% of the included guidelines were recommended for clinical use, 37.5% were recommended after revision, and 37.5% were not recommended.
Conclusion: The number of existing Tuina clinical practice guidelines is limited. The methodological quality is low, far from the internationally accepted clinical practice guideline development and reporting norms. In the future, reporting specifications of guidelines and the methodology of guideline development, including the rigor of the guideline development process, the clarity, application, and independence of reporting, should be emphasized in the development of the Tuina guidelines. These initiatives could improve the quality and applicability of clinical practice guidelines to guide and standardize the clinical practice of Tuina.
Advantages and limitations of this study
- To our knowledge, no studies have assessed the quality of Tuina clinical practice guidelines using the AGREE II checklist.
- The included guidelines were measured using the AGREE II instrument, which allows for the evaluation of guideline development methodology.
- A total of eight relevant guidelines were included in this study, which involved targeting different populations.
- The study showed inadequate reporting quality in some areas.
- The study suggests that AGREE II may be somewhat challenging in helping determine the specificity of recommendations related to traditional Chinese medicine or traditional Chinese medicine techniques.
1. Introduction
Tuina is a non-pharmacological therapy (1). At present, many studies have demonstrated that Tuina is effective in treating neck pain, shoulder pain, low back pain, and other pain caused by spinal cord disease (2–5). According to the 2016 guidelines for orthopedic therapy for non-specific low back pain formulated by the American Orthopedic Association (6), Tuina is recommended as an important treatment method because of its highly scientific nature and safety. Chinese orthopedic rehabilitation experts also believe that Tuina is significantly better than conventional treatment in alleviating the pain of lumbar disc herniation (7).
The guidelines based on the evidence of SR are recommendations that could provide patients with reliable healthcare services after balancing the pros and cons of various interventions and help clinicians make medical decisions. Only high-quality guidelines can effectively fulfill their role in clinical guidance; however, the uneven quality of the current clinical practice guidelines (CPGs) makes it necessary to conduct a quality evaluation of the guidelines in a timely manner (8). As the world's attention to Tuina is increasing, more and more clinical guidelines become available. However, these guidelines have not yet been standardized, and the definition and use of Tuina vary in China and in other countries. In addition, the quality of clinical guidelines in Tuina is uncertain, and the tools currently in use cannot accurately address quality assessment and reporting issues in a single statement.
Appraisal of Guidelines for Research and Evaluation II (AGREE II), which is developed by a group of experts, is used for quality assessment and reporting (9, 10). AGREE II has become an international standard for evaluating the methodological quality and transparency of CPGs, and several organizations have incorporated AGREE as part of their CPG programs (11). Therefore, the quality of guidelines determines whether they are suitable for clinical application and promotion. This study aims to conduct methodological and report quality evaluation by the international guidelines for quality evaluation tool AGREE II to evaluate the published clinical practice guidelines for Tuina. It provides a reference for the formulation and update of future guidelines, to enhance the quality of domestic and international guidelines so as to better play its guiding role.
2. Methods
2.1. Literature search
Guidelines meeting the eligibility criteria were searched in English and Chinese using a computer program to avoid subjective interpretation. A total of 11 databases, including the National Institute for Health and Care Excellence (NICE), Agency for Healthcare Research and Quality (AHRQ), PubMed, Embase, AMED (Allied and Alternative Medicine), Cumulative Index to Nursing and Allied Health Literature (CINAHL), WanFang Data, China National Knowledge Infrastructure (CNKI), Chinese Technical Periodicals (VIP), and Chinese Biomedical Literature Database (CBM), collection of clinical practice guidelines for Tuina at CHINA and abroad, were searched for articles from inception until March 2021. At the same time, the Google Academic and the Yimaitong databases were searched to supplement the acquisition of relevant guidelines. The search adopts a combination of subject terms and free words. The search terms include “massage” or “Tuina” or “Chinese manual therapy” AND “guideline” or “guidance” or “recommendation” or “consensus” or “policy”.
2.2. Selection criteria
We included clinical practice guidelines published by international, national, or regional groups for the application of Tuina. The guidelines were included if they met the following criteria: (1) published in English or Chinese language, (2) peer-review publications, and (3) published between 2000 and 2021, and we also excluded patient-used guidelines, guideline commentaries, guideline interpretations, and translated versions of original guidelines.
2.3. Data extraction
Two researchers (Mingwang Qiu and Yue Zhang) used the document management software EndNote X9 to screen the documents and extract data independently. Disagreement between the two parties shall be resolved through a discussion by a third party (Fan Huang).
2.4. Quality evaluation
In total, four researchers (Mingwang Qiu, Yue Zhang, Fan Huang, and Siyi Zhao) used AGREE II to evaluate the quality of the inclusion guidelines, including 23 items in 6 areas and 2 overall assessment items. The minimum score for each item was 1 point, and the maximum score was 7 points (9). We have calculated the final score of each field according to the formula as follows: each field score = (actual score-minimum possible score)/(maximum possible score-minimum possible score) × 100% (9).
The AGREE II instrument does not offer guidance on the cutoff scores to determine the quality of each domain; to determine the overall quality and recommendation level, we based our approach on the methods of previous research, guidelines were classified as strongly recommended when the standardized percentages of all six domains were above 60% and recommended when the standardized percentages ranged from 30% to 60% in more than three domains. However, guidelines are not recommended when the standardized percentages were < 30% in more than three domains.
Before the formal evaluation, all investigators were trained, and one guide was independently pre-scored. Then the group discussed and negotiated to ensure that the four evaluators had basically the same understanding of each item and had the same evaluation criteria.
2.5. Statistical analysis
Statistical analysis used IBM SPSS Statistics 25 software to calculate the ICC value to verify the consistency of the evaluators when using the evaluation tool. Using Excel 2016 for calculating the scores of the AGREE II tool, the mean and standard deviation of the scores in each field and the proportion of each part were calculated. The agreement between the four reviewers was measured by the intra-group correlation coefficient (ICC) and 95% confidence interval (CI). The degree of agreement between 0.01 and 0.20 was considered minor, the degree of proportionality between 0.21 and 0.40 was considered moderate, the degree of proportionality between 0.41 and 0.60, the substantive degree between 0.61 and 0.80, and the agreement between 0.81 and 1.00 were considered very good. P < 0.05 indicates statistical significance. All tests were double-sided. We used SPSS version 25.0 for statistical analysis. The ICC of the scores given by the four evaluators would be analyze.
2.6. Patient and public involvement
No patient was involved.
3. Results
3.1. Selection of studies
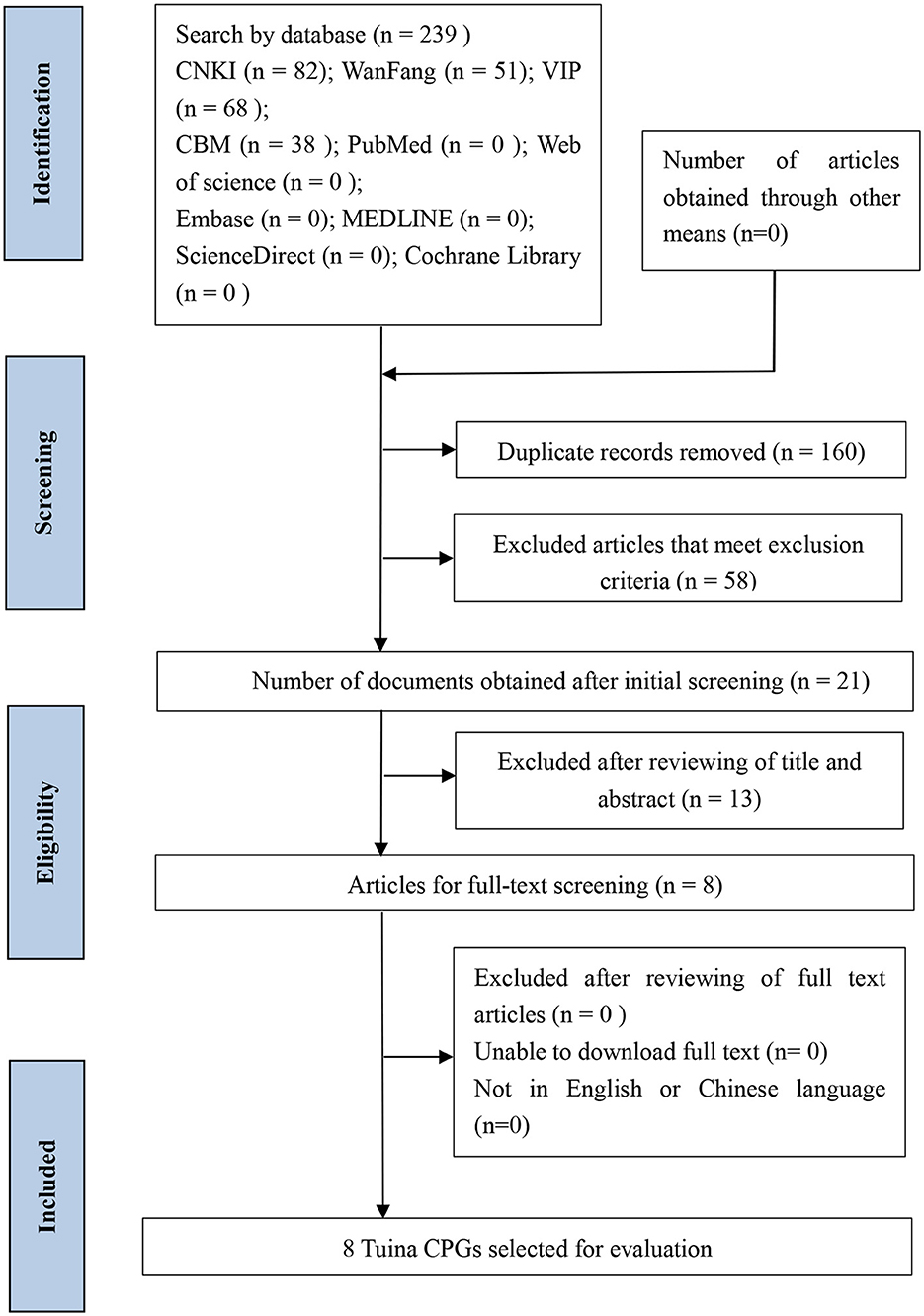
The initial search detected 239 related publications, and EndNote X9 excluded 160 duplicate records. After reading the title and abstract, 13 records were excluded from the preliminary screening. After the full-text screening, a total of eight Tuina guidelines were included (12–19) for SR through AGREE II. The literature search and screening process are shown in Figure 1.
3.2. General characteristics
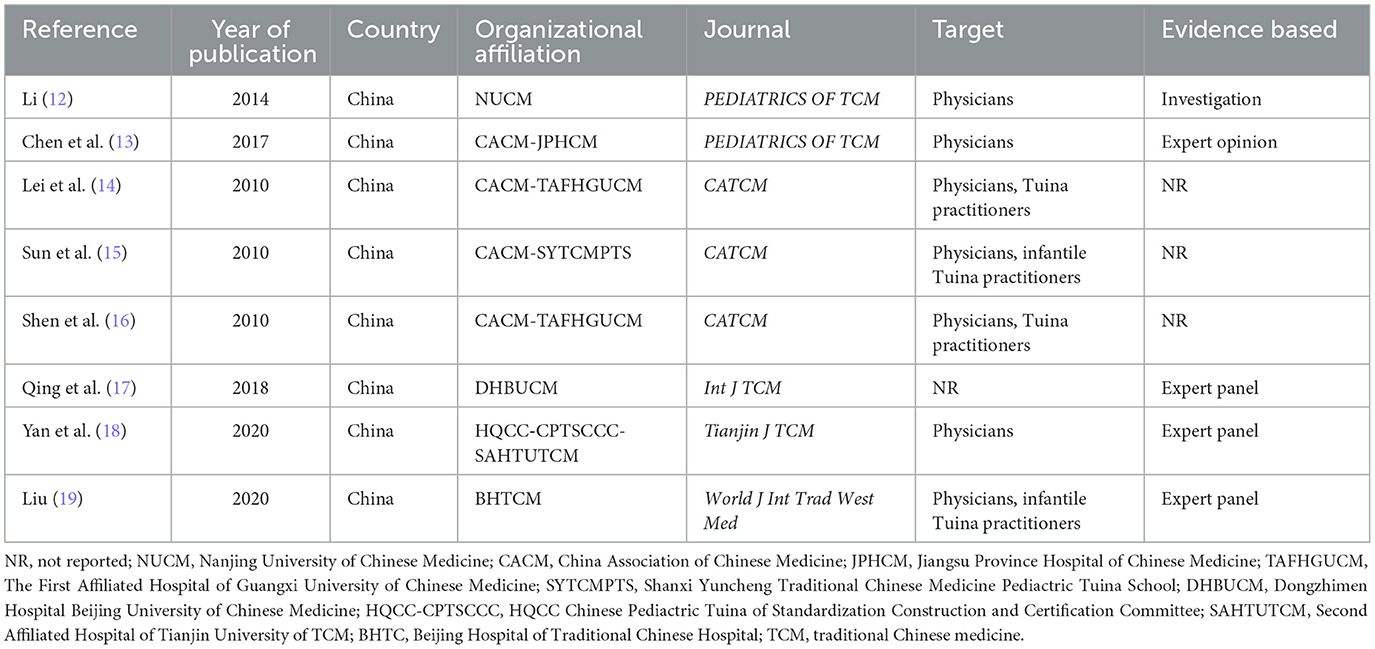
As shown in Table 1, all the guidelines evaluated in this study originated from China and were published from 2010 to 2020. Three of the guidelines (14–16) were published in the same journal in 2010. However, a major shortcoming of the former three articles was that there is no evidence of the source of the guideline writing. In total, seven of the eight guidelines (9, 11–16, 18, 19) were aimed at clinicians or Tuina practitioners specifically. Seven (12–17, 19) guidelines were from different Chinese medicine universities or affiliated hospitals, and the remaining one (18) was from the China HQCC.
3.3. Quality assessment of guidelines and strength of recommendation
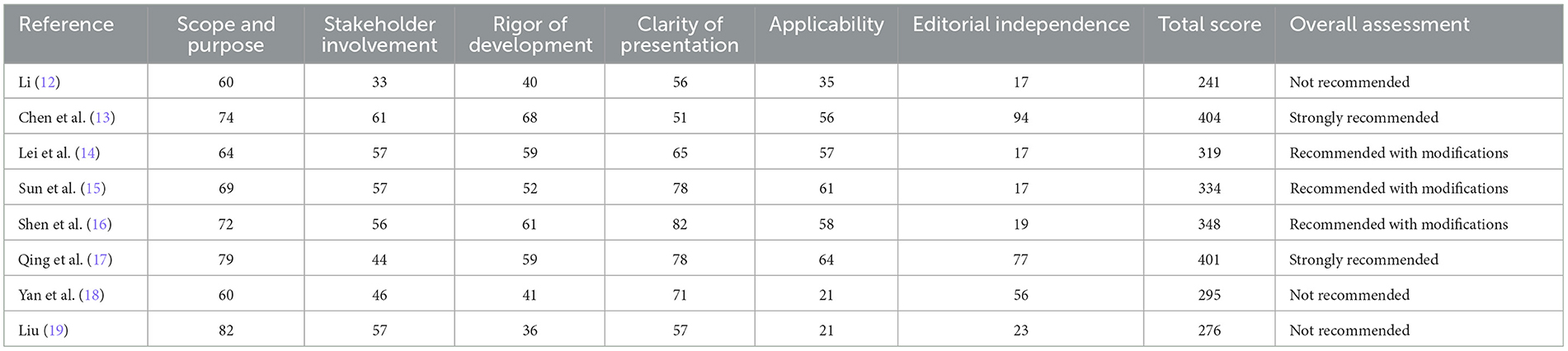
Table 2 shows the AGREE II standardization field score for each Tuina CPG and its overall recommendations. The scope and purpose of the field and the clarity and presentation achieved the highest average scores of 70% and 67% (ranged 60–82% and 51–82%, respectively). The average score for stakeholder participation in the domain was 51% (ranged 33–61%), and only one guide scored more than 60%. The largest score range was editorial independence (17–94%). A total of three guides (37.5%) scored 17, and five guides (62.5%) scored <50%. Editorial independence and applicability produced the lowest average scores of 40% and 47% (ranged 17–94% and 21–64%, respectively). Unexpectedly, the four guidelines (26.7%) had the lowest score due to the failure to describe the criteria for selecting evidence and making recommendations clearly.
In general, the Practical Guidelines for Treating Prophylactic Diseases of Tuina Intervention of Spleen Deficiency (13) in Children's Guide had high scores in all areas and is listed as “strongly recommended” in clinical practice, “recommendation of 3 types of Tuina CPG (37.5%),” and three types (37.5%) “not recommended.” The agreement of the overall reviewer was very good (ICC:0.901, 95% CI).
After four reviewers' SR, we obtained the AGREE II scores and total scores in each field of the eight guides, as shown in Table 3. Chen et al. (13) and Qin et al. (17) served as the first author's guide for a total score of more than 400; therefore, we can strongly recommend using these two Tuina guidelines. The guidelines with Li (12), Yan et al. (18), and Liu (19) as the first authors had a total score of < 300, so we do not recommend using these three guidelines. The guide, led by Lei et al. (14), Sun et al. (15), and Shen et al. (16) as the first author, scored between 300 and 400. These three guides can be recommended after upgrading and improving modifications.
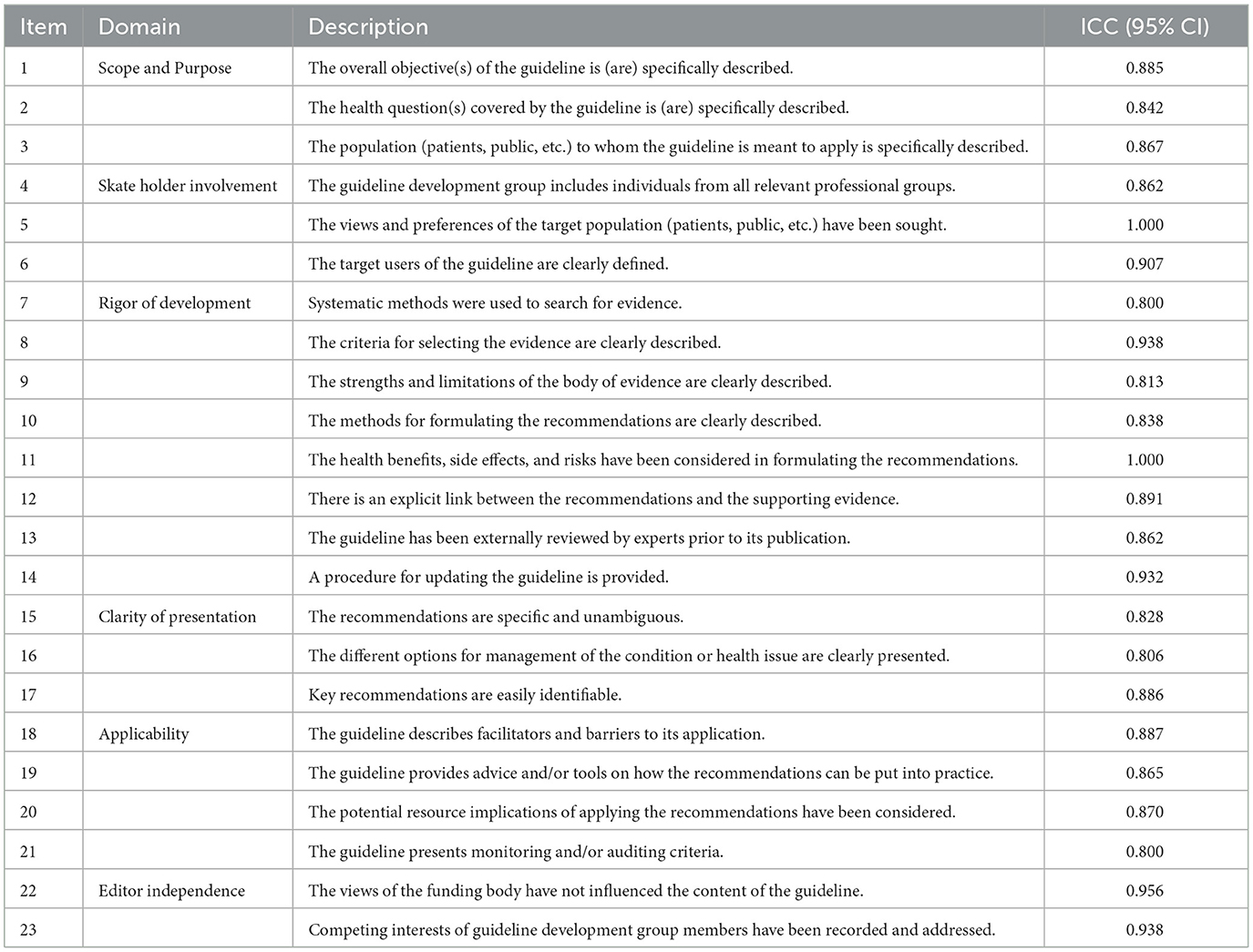
The agreement between the four reviewers was measured by the intra-group correlation coefficient (ICC) and 95% confidence interval (CI). The degree of agreement between 0.01 and 0.20 was considered minor; the degree of proportionality between 0.21 and 0.40 was moderate; and the degree of proportionality between 0.41 and 0.60, the substantive degree between 0.61 and 0.80, and the agreement between 0.81 and 1.00 were very good. P < 0.05 indicates statistical significance. All tests were double-sided. We used SPSS version 25.0 for statistical analysis. By analyzing the intra-group correlation coefficients of the scores given by the four evaluators, we can see that the ICC value of each field was >0.81, as shown in Table 3. It can be considered that the scores given by the evaluators within the group were highly consistent.
3.4. Frequency statistics of disease
As shown in Figure 2, the abscissa axis is the names of the diseases included in the literature, and the ordinate axis is the frequency with which they appear in the literature. A total of 35 diseases were included; of which, 13 were specific to children.

Figure 2. Names of diseases included in the literature (abscissa axis) and their frequency (ordinate axis).
4. Discussion
This study systematically compared the strengths and weaknesses of eight existing guidelines with AGREE II reporting checklist to provide a reference for the development of Tuina CPGs.
The results of this study highlighted the poor applicability of current Tuina CPGs. Meanwhile, unclear articulation of the strengths and weaknesses of the recommendations, the lack of supporting tools, potential resources, and monitoring and auditing criteria may be direct causes of the low scores in this area.
Clinical practice guidelines are defined as systematically developed statements to assist practitioner and patient decisions about appropriate healthcare for specific clinical circumstances (20). As society gradually recognized the therapeutic effect of Tuina, CPGs will be in increasing demand globally. In the present study, systematic research on CPGs for Tuina was performed, and key messages were summarized. We found it difficult to evaluate the individual and community as the available CPGs for Tuina lack reports on obtaining evidence and reaching recommendations.
The implication of this study is that, as a result of our findings, a careful reassessment of the quality standards of existing Tuina guidelines can be called for, and quality improvements can be made in practice to facilitate the development and reporting of more Tuina guidelines in the future. In this study, the quality of massage guidelines was highly heterogeneous across domains. The level of evidence and strength of recommendations also varied widely across the categories of guidelines. With regard to the evidence base for the inclusion of massage guidelines in this study, our findings suggest that many guidelines are based primarily on a low level of evidence or expert opinion, offering a lack of quality evidence and guidelines that do not incorporate evidence.
At present, the quality of Chinese domestic guidelines is generally low, and low-quality guidelines not only fail to guide clinical practice but also may even hinder it. In this study, we chose to use the international guideline quality evaluation tool AGREE II to evaluate the quality of Chinese domestic journals publishing clinical practice guidelines for Tuina in recent years, to monitor the changes in the quality of Tuina clinical guidelines, to provide a reference for the development and updating of guidelines in the future, and to improve the quality of domestic guidelines so that they can better play their guiding role. Compared with the Reporting Items for Practice Guidelines in healthcare (RIGHT) statement completed by the Chinese lead, we use AGREE II to evaluate the guidelines because the international common AGREE II to assess the quality of guidelines, AGREE II has been supported and recognized by many healthcare organizations, which can help Tuina better align with international standards (21). By comparing the original AGREE with the AGREE II program, we chose the latest AGREE II tool for guideline evaluation because the AGREE II program changes the original 12 entries from AGREE to assess guideline quality better as well as provide a methodological strategy for guideline development, informing guideline developers on what to report in the guideline, what information to report, and how to report it (9).
The following part leads to the limitations of our study. First, due to language limitations, this study only collects guidelines written in Chinese and English, which could not include documents from a few countries and regions. Second, the scores of AGREE II are not weighted, so the guideline' recommendation level is only based on the number of fields that meet the standard. There may be cases where the recommended results are not in conformity with the quality of the guideline. Third, the AGREE II score was based on the reports of CPG developers, and low domain scores might be due to poor methodology in the development process of CPGs. Fourth, some guidelines may be in development and thus were not accessible to our research team.
The total quality of CPGs is not high in our country (22), and therefore, how to develop a high-required guideline is an issue that guideline makers need to consider. It is expected that Tuina practitioners can institute high-level CPGs that are suitable for China based on the methodology and normative reports formulated by the guidelines.
5. Conclusion
The number of CPGs for Tuina has proliferated in recent years, but their average quality is unsatisfactory, especially in the domain of stakeholder participation, editorial independence, and the description of the criteria for selecting evidence and making recommendations. In the subsequent development of CPGs in Tuina, guideline developers need to further improve guideline methodology and reporting specifications of the domain mentioned earlier. Evaluation tools for guidelines in Tuina and CPGs for Tuina should be developed according to standardized guideline development methods that are consistent with Chinese national conditions. Emphasis should be placed on strengthening the promotion and application of Tuina guidelines.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This work was supported by the Lin's Bone-setting Massage School Inheritance Studio (E43611) and the Guangdong Provincial Department of Finance Project [Grant (2016) No. 387].
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2023.961886/full#supplementary-material
References
1. Lee NW, Kim GH, Heo I, Kim KW, Ha IH, Lee JH, et al. Chuna (or Tuina) manual therapy for musculoskeletal disorders: a systematic review and meta-analysis of randomized controlled trials. Evid Based Compl Alternat Med. (2017) 2017:8218139. doi: 10.1155/2017/8218139
2. Cheng YH, Huang GC. Efficacy of massage therapy on pain and dysfunction in patients with neck pain: a systematic review and meta-analysis. Evid Based Compl Alternat Med. (2014) 2014:204360. doi: 10.1155/2014/204360
3. Tian Y, Tang D, Tian C, Zhang C, Zhang Y. Clinical observation of ankylosing spondylitis treated with moxibustion along the governor vessel and the conception vessel and salazosulfapyridine. Zhongguo Zhen Jiu. (2016) 36:1037–40. doi: 10.13703/j.0255-2930.2016.10.009
4. Huang F, Zhao S, Dai L, Feng Z, Wu Z, Chen J, et al. Tuina for cervical vertigo: a systematic review and meta-analysis of randomized controlled trials. Compl Ther Clin Pract. (2020) 39:101115. doi: 10.1016/j.ctcp.2020.101115
5. Kong LJ, Fang M, Zhan HS, Yuan WA, Pu JH, Cheng YW, et al. Tuina-focused integrative Chinese medical therapies for inpatients with low back pain: a systematic review and meta-analysis. Evid Based Compl Alternat Med. (2012) 2012:578305. doi: 10.1155/2012/578305
6. Task Force on the Low Back Pain Clinical Practice Guidelines. American osteopathic association guidelines FOVR osteopathic manipulative treatment (OMT) for patients with low back pain. J Am Osteopath Assoc. (2016) 116:536–49. doi: 10.7556/jaoa.2016.107
7. Zhou MW, Yue SW, He CQ. “Rehabilitation treatment of lumbar disc herniation” Chinese expert consensus. Chin J Rehabil Med. (2017) 32:129–35.
8. Murad MH. Clinical practice guidelines: a primer on development and dissemination. Mayo Clin Proc. (2017) 92:423–33. doi: 10.1016/j.mayocp.2017.01.001
9. Brouwers MC, Kerkvliet K, Spithoff K, AGREE Next Steps Consortium. The AGREE reporting checklist: a tool to improve reporting of clinical practice guidelines. BMJ. (2016) 352:i1152. doi: 10.1136/bmj.i1152
10. AGREE Collaboration. Development and validation of an international appraisal instrument for assessing the quality of clinical practice guidelines: the AGREE project. Qual Saf Health Care. (2003) 12:18–23. doi: 10.1136/qhc.12.1.18
11. Makarski J, Brouwers MC, AGREE Enterprise. The AGREE enterprise: a decade of advancing clinical practice guidelines. Implement Sci. (2014) 9:103. doi: 10.1186/s13012-014-0103-2
12. Li JQ. Standardized manipulations of the technique in pediatrics of Chinese medicine: infantile Tuina (exposure draft). J Pediat TCM. (2014) 10:1–5.
13. Chen XZ, Wu YC, Wang JJ, Qiu M, Zhao S, Liang J, et al. Practical guidelines for treating prophylactic diseases of traditional chinese medicine·Tuina intervention of spleen deficiency in children. J Pediat TCM. (2017) 13:5–8. doi: 10.16840/j.issn1673-4297.2017.02.02
14. Lei LM, Pang J, Lun SF, Qiu M, Zhao S, Liang J, et al. Technical Specifications for Health Care and Health Care of Traditional Chinese Medicine-Whole Body Tuina. Beijing: China Association of Chinese Medicine (2010).
15. Sun DR, Wang JH, He L, Lei S, Sun J, Zhou R, et al. Technical Specification of Health Preservation and Prevention of Traditional Chinese Medicine: Tuina for Children. Beijing: China Association of Chinese Medicine (2010).
16. Shen T, Xu ZR, Sang BS. Technical Specification of Health Preservation and Prevention of Traditional Chinese Medicine—Chiropractic. Beijing: China Association of Chinese Medicine (2010).
17. Qing LX, Wang B, Liu JQ Li D, Jiang H, Yang X, et al. Expert consensus on the comprehensive individualized protocol of Tuina therapy for knee osteoarthritis. Int J TCM. (2018) 40:385–9.
18. Yan GW, Sun WQ, Qiang Z, Xiong Y, Li L, Ma Y, et al. Children's new coronavirus pneumonia pediatric Tuina intervention expert consensus (first edition). Tianjin J TCM. (2020) 37:1114–8.
19. Liu F. Expert consensus on intervention in recurrent respiratory tract infection in children with Tuina guided by “treatment before its onset” in TCM. World J Int Trad West Med. (2020) 15:767–84. doi: 10.13935/j.cnki.sjzx.200446
20. Dagens A, Sigfrid L, Cai E, Lipworth S, Cheng V, Harris E, et al. Scope, quality, and inclusivity of clinical guidelines produced early in the covid-19 pandemic: rapid review. BMJ. (2020) 369:m1936. doi: 10.1136/bmj.m1936
21. Chen Y, Yang K, Marušić A, Qaseem A, Meerpohl JJ, Flottorp S, et al. Ein Instrument zur Erstellung von Leitlinienberichten: das RIGHT-Statement [A reporting tool for practice guidelines in health care: the RIGHT statement]. Z Evid Fortbild Qual Gesundhwes. (2017) 127–128:3–10. doi: 10.1016/j.zefq.2017.10.008
Keywords: traditional Chinese medicine, Tuina, evaluation II instrument, guidelines, AGREE II instrument
Citation: Huang F, Zhang Y, Huang C, Qiu M, Zhao S, Liang J, Fan Z and Wu S (2023) Using AGREE II reporting checklist to evaluate the quality of Tuina clinical practice guidelines. Front. Med. 10:961886. doi: 10.3389/fmed.2023.961886
Received: 30 September 2022; Accepted: 10 March 2023;
Published: 18 April 2023.
Edited by:
Jorge Pereira Machado, University of Porto, PortugalReviewed by:
Henry Johannes Greten, TCM Research Centre, Piaget Institute, PortugalDiogo Amorim, Dr. Diogo Amorim Integrative Medicine Institute, Portugal
Maria Begoña Criado, Cooperativa de Ensino Superior Politécnico e Universitário, Portugal
Copyright © 2023 Huang, Zhang, Huang, Qiu, Zhao, Liang, Fan and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhiyong Fan, Znp5c3Ryb25nQDE2My5jb20=; Shan Wu, d3VzaGFuNjg2NkBzaW5hLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Fan Huang
Fan Huang Yue Zhang2†
Yue Zhang2† Siyi Zhao
Siyi Zhao Junquan Liang
Junquan Liang