- 1Department of Visceral and Digestive Surgery, Fattouma Bourguiba Hospital, University of Monastir, Monastir, Tunisia
- 2Department of Visceral and Digestive Surgery, Institute Mutualist of Montsouris, University of Paris, Paris, France
- 3Department of General Surgery, Great Western Hospitals NHS Foundation Trust, Swindon, United Kingdom
- 4Department of Surgery, Perpignan Hospital Center, Perpignan, France
- 5Department of HPB Surgery and Transplantation, St-Eloi University Hospital, Montpellier, France
- 6Department of Radiology, St-Eloi University Hospital, Montpellier, France
- 7Department of Surgery, Universitäts Medizin Mannheim, Heidelberg University, Mannheim, Germany
Introduction: This systematic review aimed to compare liver venous deprivation (LVD) with portal vein embolization (PVE) in terms of future liver volume, postoperative outcomes, and oncological safety before major hepatectomy.
Methods: We conducted this systematic review and meta-analysis following the PRISMA guidelines 2020 and AMSTAR 2 guidelines. Comparative articles published before November 2022 were retained.
Results: The literature search identified nine eligible comparative studies. They included 557 patients, 207 in the LVD group and 350 in the PVE group. This systematic review and meta-analysis concluded that LVD was associated with higher future liver remnant (FLR) volume after embolization, percentage of FLR hypertrophy, lower failure of resection due to low FLR, faster kinetic growth, higher day 5 prothrombin time, and higher 3 years’ disease-free survival. This study did not find any difference between the LVD and PVE groups in terms of complications related to embolization, FLR percentage of hypertrophy after embolization, failure of resection, 3-month mortality, overall morbidity, major complications, operative time, blood loss, bile leak, ascites, post hepatectomy liver failure, day 5 bilirubin level, hospital stay, and three years’ overall survival.
Conclusion: LVD is as feasible and safe as PVE with encouraging results making some selected patients more suitable for surgery, even with a small FLR.
Systematic review registration: The review protocol was registered in PROSPERO before conducting the study (CRD42021287628).
Introduction
One of the major issues after extended hepatectomy is the insufficient remnant liver volume and liver function. Since its first report in 1986 (1), portal vein embolization (PVE) has been used to enhance future liver remnant volume (FLR) and prevent post-hepatectomy liver failure (PHLF). The principle is to lead to ipsilateral hepatic atrophy and contralateral future liver hypertrophy. Currently, PVE is considered the standard preoperative manipulation to improve the volume of an adequate FLR and reduce the risk of PHLF (2). However, several studies have shown that liver remnant inadequacy also depends on the future liver parenchymal condition (3). PVE allows up to 70–80% of patients to subsequently undergo major hepatectomy (4, 5). However, the major concern regarding PVE is the dropout of up to 36% of patients because of insufficient FLR hypertrophy or tumor progression within 4-to 6-week intervals between PVE and definitive resection (6, 7). In recent years, alternative strategies have been proposed, such as two-stage hepatectomy with portal vein (PV) ligation, additional ligation of the ipsilateral hepatic artery, and associating liver partition and PV ligation for staged hepatectomy (ALPPS) (8). Two-stage hepatectomy with PV ligation induced a similar FLR to PVE, the ipsilateral artery ligation quickly abounded due to a high rate of liver abscess, and the ALPPS was associated with higher postoperative morbidity as well as inferior long-term survival (9). For these reasons, liver venous deprivation (LVD), and a simultaneous embolization of the portal and hepatic ipsilateral veins have been suggested. Different studies comparing LVD and PVE concluded with controversial results. Considering all these findings, we conducted a systematic review and meta-analysis.
This systematic review and meta-analysis aimed to compare LVD and PVE in terms of FLR, postoperative outcomes, and oncological safety.
Methods
We conducted this systematic review and meta-analysis according to the PRISMA 2020 (Preferred Reporting Items for Systematic Review and Meta-analysis) (10) and AMSTAR 2 (assessing the methodological quality of systematic reviews) (11) guidelines. The review protocol was registered in PROSPERO before conducting the study (CRD42021287628).
Electronics searches
The electronic search was conducted on November 30, 2022, with no language restrictions, in the following databases “Cochrane Library,” “PubMed/MEDLINE,” “Excerpta Medica Database,” “Embase,” and “Google Scholar.” Keywords used were: “liver venous deprivation”; “portal vein embolization”; “major hepatectomy”; “future liver remnant”; “liver failure”; “hepatectomy”; “liver failure”; “bilirubin”; “prothrombin”; “surgery”; “outcome,” “morbidity,” and “mortality.” We used the Boolean markers “and” and “or.” The reference lists of the articles obtained were checked for eligible clinical trials.
Study selection: Clinical controlled trials (CCT) comparing LVD with PVE before major liver resection were retained.
Participants/population: Adults operated on for liver disease and candidates for an induction technique of liver regeneration before major hepatectomy.
Intervention group: Single-stage LVD.
Control group: PVE before surgery.
Outcomes: The different outcomes assessed were the remnant future liver volume (complications of embolization, future liver remnant volume and percentage before embolization, future liver remnant volume and percentage after embolization, future liver remnant volume ratio after embolization, future liver remnant hypertrophy, kinetic growth rate, and failure of resection), postoperative outcomes (3-month postoperative mortality, overall morbidity, Clavien-Dindo complications≥ grade III, blood loss, bile leak, ascites, operative time, post hepatectomy liver failure [defined according to ISGLS (International Study Group of Liver Surgery) or “50–50” criteria (12, 13)], day 5 bilirubin level, day 5 prothrombin time, and hospital stay), and oncological outcomes [three years overall survival (OS) and disease-free survival (DFS)].
Assessment of study quality and risk of bias: We used the MINORS (Methodological Index for Non-randomized studies) (14) and the Newcastle-Ottawa Scale (NOS) (15).
Study selection and data extraction: Two authors selected studies and extracted data. Disparities were resolved by the senior author.
Certainly assessment of evidence: We used the GRADE guidelines to rate evidence quality (16). We considered the study limitations in terms of the constancy of effect, imprecision, indirectness, and publication bias. We assessed the certainty of the evidence as high, moderate, low, or very low. If appropriate, we considered the following criteria for upgrading the certainty of evidence: large effect, dose–response gradient, and plausible confounding effect. GRADEpro GDT software was used to prepare the “Summary of findings tables.” We explained the reasons for downgrading or upgrading the included studies using footnotes and comments.
Assessment of heterogeneity: We used the Cochrane Chi2 test (Q-test), Tau2, of true effects (17) and graphical exploration with funnel plots (18).
Evaluation of effect size: We used the Review Manager 5.3.5 statistical package (19). We selected the standardized mean difference (SMD) as an effective measure of continuous data and odds ratios (OR) with 95% confidence intervals (95% CI) for dichotomous data. A random effects model was used. The threshold for significance was set at p < 0.05.
Results
Literature research
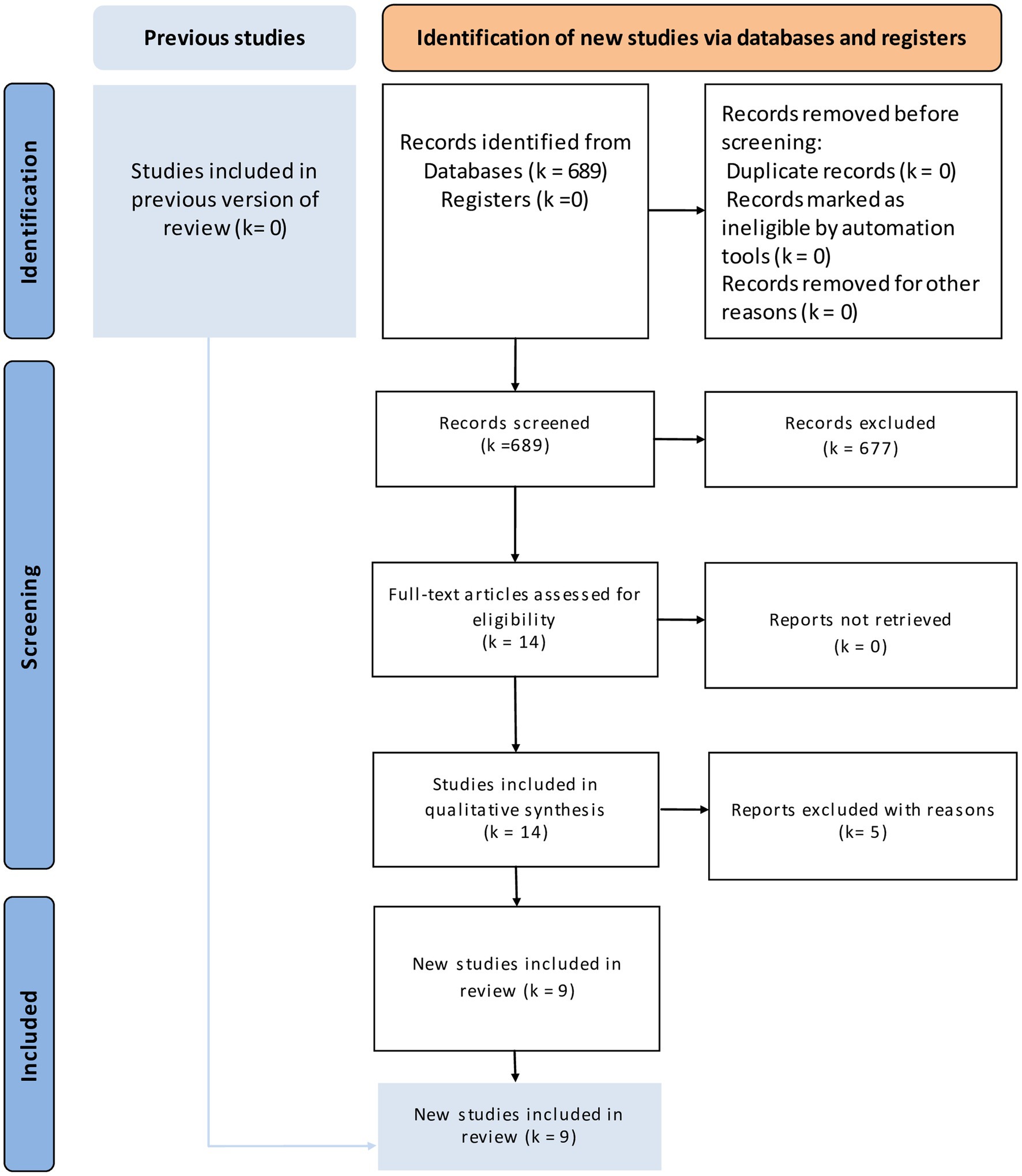
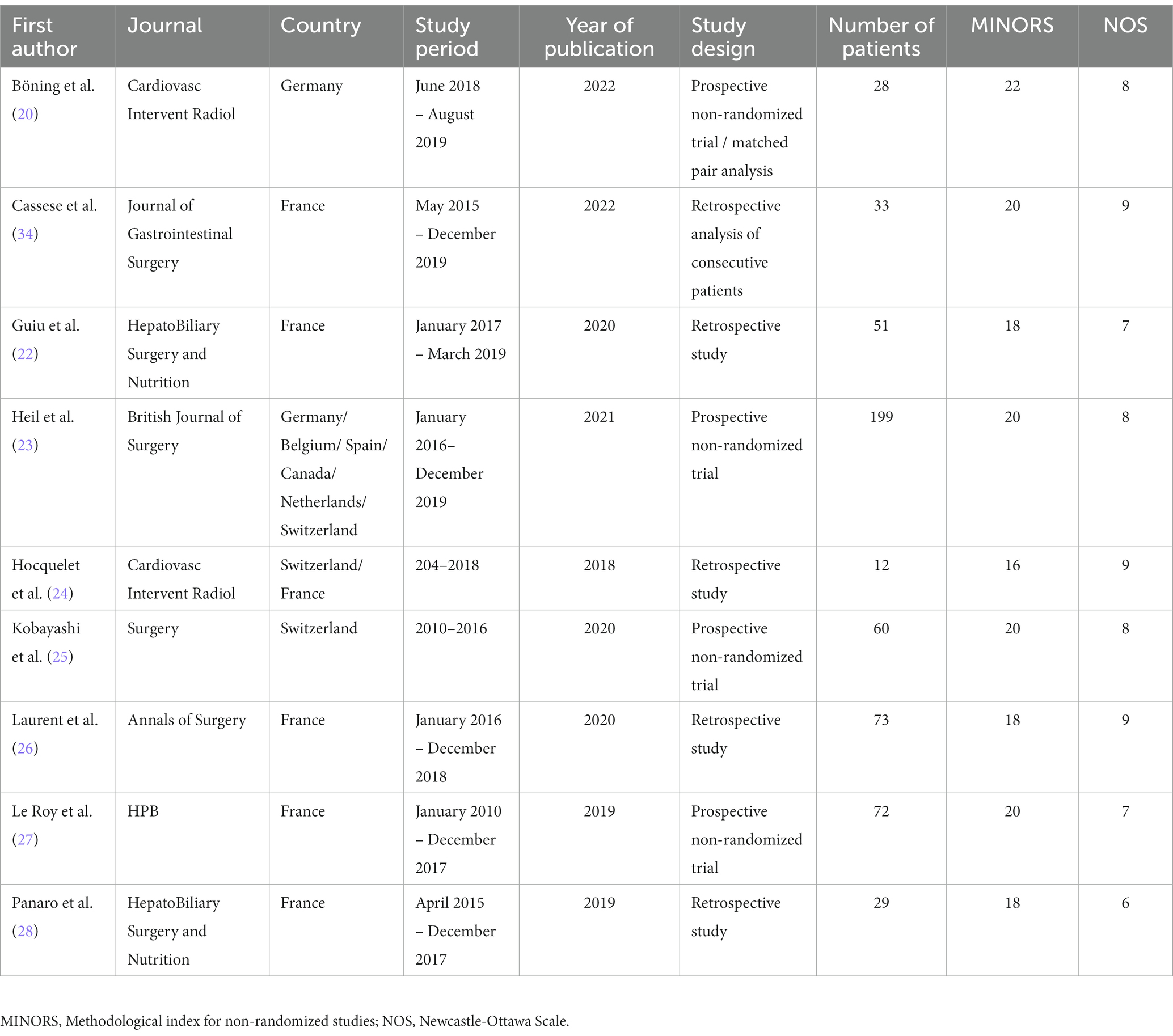
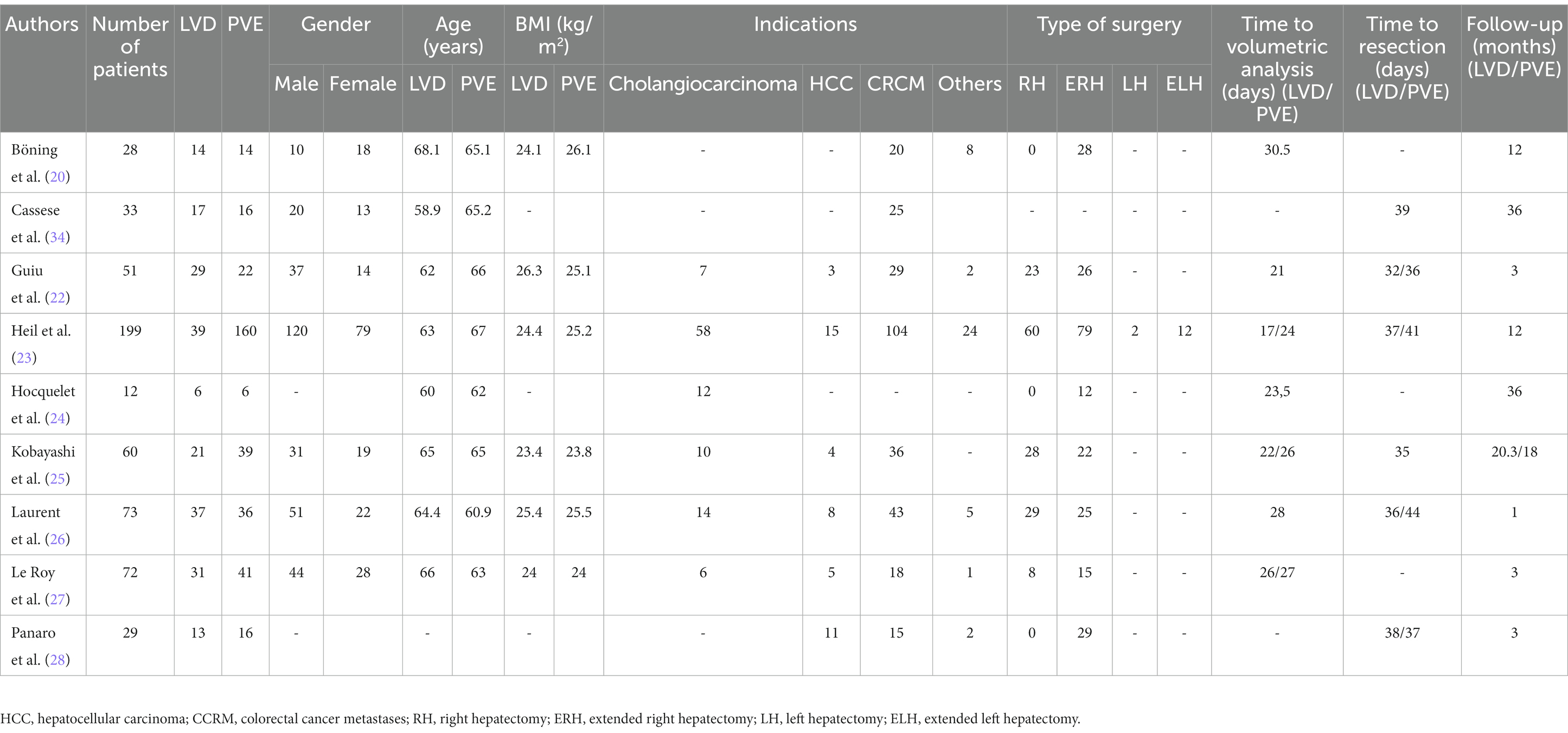
The literature search identified fourteen eligible articles (Figure 1) (20–28). Five articles were excluded for the following reasons: two assessed only liver hypertrophy and pathological changes without post-major hepatectomy outcomes (29, 30), one letter to the editor (31), one non-comparative study (32), and one study protocol (33). Finally only nine studies were included in our study (Table 1). These articles were published between 2018 and 2022. They included 557 patients, 207 in the LVD group and 350 in the PVE group. The demographic data are reported in Table 2. The sex ratio was 1.48. The mean BMI ranged from 23.4 kg/m2 to 26.3 kg/m2 in the LVD group and from 23.8 kg/m2 to 25.5 kg/m2 in the PVE group. Liver metastases of colorectal cancer were the most frequent indication. Extended right hepatectomy was the most frequent procedure. The delay for volumetric analysis ranged from 17 to 30.5 days and for resection ranged from 32 to 44 days and the follow-up ranged from one to 36 months.
Post embolization results
Complications of the embolization
All retained studies reported complication rates after hepatectomy (20–28). This outcome was reported in 16 of 207 patients in the LVD group and 28 of 350 patients in the PVE group. There was no difference between the two groups (OR = 1.44; 95%CI [0.68, 3.03], p = 0.34).
FLR volume before embolization (mL)
Six studies reported the FLR volume before embolization (22–27). This outcome was reported in 163 patients in the LVD group and 264 patients in the PVE group. There was no difference between the two groups (SMD = 0.02; 95%CI [−0.47,0.52], p = 0.92).
FLR percentage before embolization (%)
Six studies reported the FLR percentage before embolization (22–27). This outcome was reported in 149 patients in the LVD group and 279 patients in the PVE group. There was no difference between the two groups (SMD = −0.50; 95%CI [−1.10, 0.09], p = 0.10).
FLR volume after embolization
Five studies reported the FLR volume after embolization (23–27). This outcome was reported in 134 patients in the LVD group and 282 patients in the PVE group. There was a higher FLR volume after embolization in the LVD group (SMD = 0.62; 95%CI [0.06, 1.19], p = 0.03).
FLR percentage after embolization
Five studies reported FLR percentage after embolization (21, 23–26). This outcome was reported in 120 and 257 patients in the LVD and PVE groups, respectively. There was no difference between the two groups (SMD = 0.53; 95% CI [−0.14, 1.21], p = 0.12).
FLR remnant liver hypertrophy (%)
Eight studies reported FLR remnant liver hypertrophy after embolization (20–28). This outcome was reported in 194 patients in the LVD group and 334 patients in the PVE group. The FLR volume after embolization was higher in the LVD group (SMD = 0.80; 95%CI [0.39, 1.21], p = 0.0001).
Kinetic growth
Four studies reported kinetic growth after embolization (21, 23, 25, 27). This outcome was reported in 108 patients in the LVD group and 256 patients in the PVE group. The volume of kinetic growth after embolization was higher in the LVD group (SMD = 2.08; 95%CI [0.61, 3.54], p = 0.005).
Failure of resection
All the retained articles reported the rate of resection failure (20–28). In the LVD group, twenty-five out of 190 patients failed to undergo resection. In the PVE group, eighty-three patients out of the 334 patients failed to undergo resection. There was no difference between the two groups (OR = 0.59; 95%CI [0.33, 1.06], p = 0.08).
Failure of resection due to inadequate FLR
All retained articles reported the rate of resection failure due to inadequate FLR (20–28). Three of 207 patients in the LVD group and 23 of 346 patients in the PVE group. We found a lower rate of resection failure due to low FLR in the LVD group (OR = 0.30; 95%CI [0.09, 0.96], p = 0.04).
Postoperative results
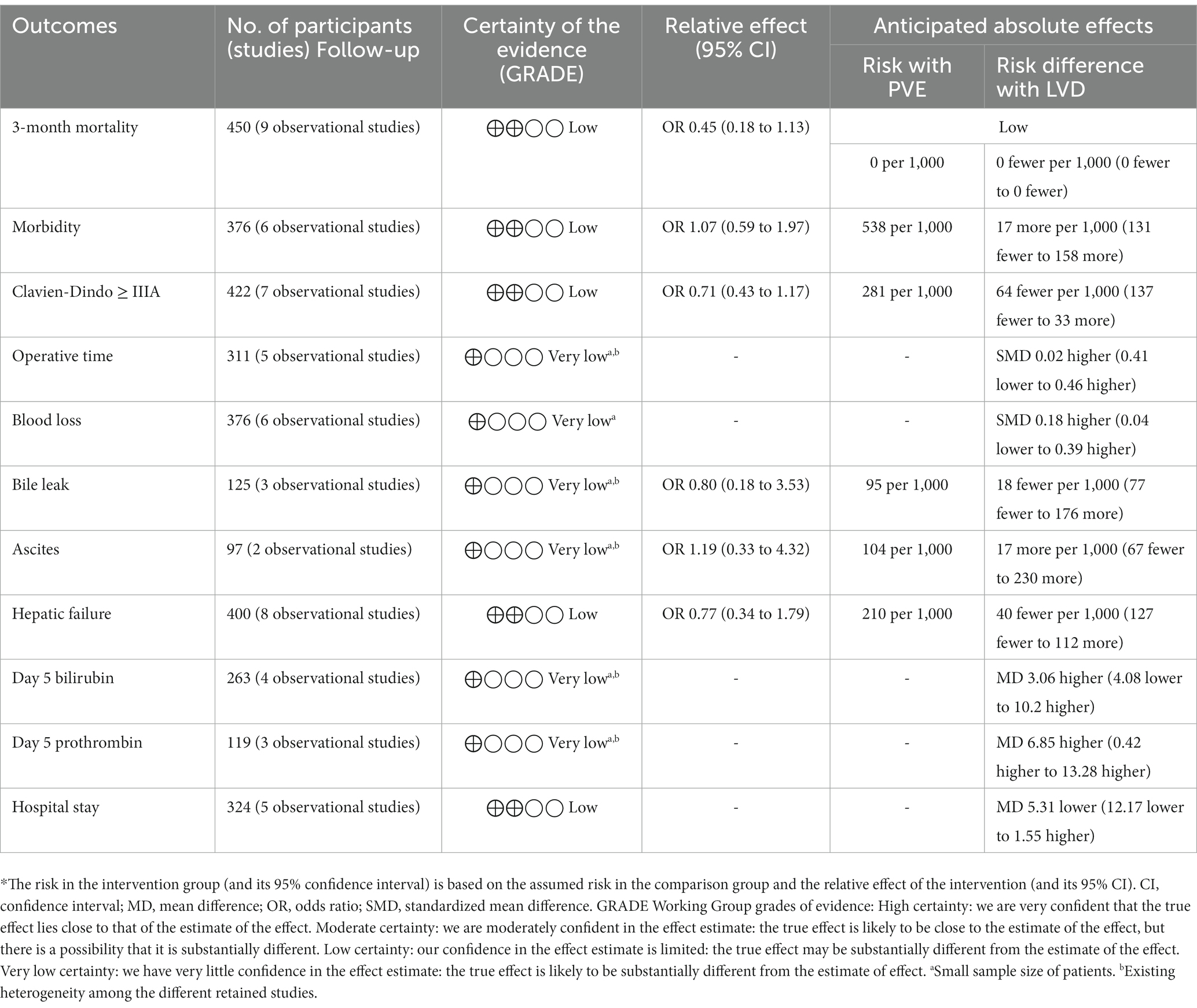
3-month mortality
All retained articles reported the 3-month mortality (20–28). It was found in seven of 182 patients in the LVD group and 29 of 268 patients in the PVE group. There was no difference between the two groups (OR = 0.45; 95%CI [0.18, 1.13], p = 0.09).
Overall morbidity
Six articles reported the overall morbidity (21, 23, 25–28). It was reported in 72 of 142 patients in the LVD group and 126 of 234 patients in the PVE group. There was no difference between the two groups (OR = 1.07; 95%CI [0.59, 1.97], p = 0.82).
Clavien-Dindo ≥ grade III
Seven articles reported postoperative major complications (21–23, 25–28). It was reported in seven of 32 of 169 patients in the LVD group and 71 of 126 patients in the PVE group. There was no difference between the two groups (OR = 0.71; 95%CI [0.43, 1.17], p = 0.18).
Operative time
Five studies reported the operative time (21, 23, 25, 27, 28). This outcome was reported in 110 patients in the LVD group and 201 patients in the PVE group. There was no difference between the two groups (SMD = 0.02; 95%CI [−0.41, 0.46], p = 0.91).
Blood loss
Six studies reported blood loss during hepatectomy (21, 23, 25–28). This outcome was reported in 142 patients in the LVD group and 234 patients in the PVE group. There was no difference between the two groups (SMD = 0.18; 95%CI [−0.04, 0.39], p = 0.11).
Bile leak
Three studies reported the bile leak rates (21, 26, 28). It was reported in five of 62 patients in the LVD group and six in 63 patients in the PVE group. There was no difference between the two groups (OR = 0.80; 95%CI [0.18, 3.53], p = 0.77).
Ascites
Two articles reported on postoperative ascites (21, 26). It was reported in six of 49 patients in the LVD group and five of 48 patients in the PVE group. There was no difference between the two groups (OR = 1.19; 95%CI [0.33, 4.32], p = 0.80).
Post hepatectomy liver failure
The rate of PHLF has been reported in eight studies (20–24, 26–28). Twenty-one of 162 patients in the LVD group presented with PHLF versus 50 of 238 patients in the PVE group. There was no difference between the two groups (OR = 0.77; 95%CI [0.34, 1.79], p = 0.55).
Day 5 bilirubin level
Four studies investigated day 5 bilirubin levels (22–24, 26). They compared 98 and 165 patients in the PVE and HVD groups, respectively. There was no difference between the two groups (SMD = 3.06; 95%CI [−4.08, 10.20], p = 0.40).
Day 5 prothrombin
Three studies reported day 5 prothrombin values (22, 24, 26). They compared 63 and 56 patients in the PVE and LVD groups, respectively. Prothrombin rates were higher in the LVD group (SMD = 6.85; 95%CI [0.42, 13.28], p = 0.04).
Hospital stay
Five studies reported hospital stay (23–27). They compared 116 and 208 patients in the PVE and LVD groups, respectively. There was no difference between the two groups (SMD = −5.31; 95%CI [−12.17, 1.55], p = 0.13).
Oncological outcomes
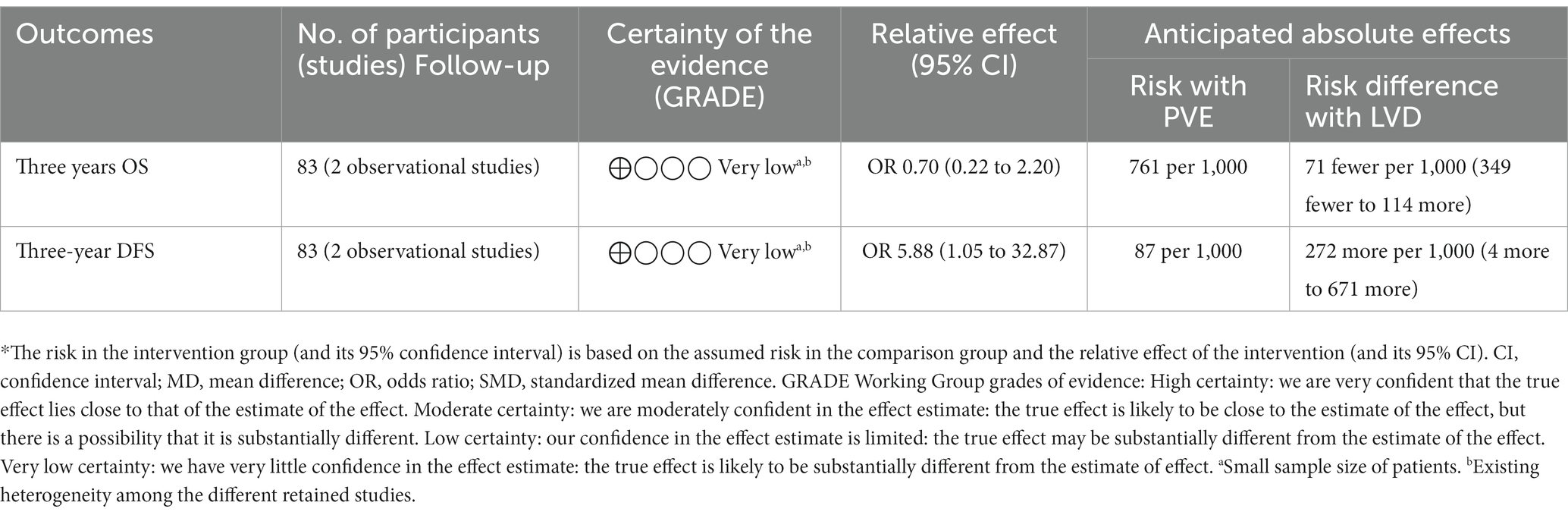
Three years’ OS
Two studies reported the three years’ OS (21, 25). It was reported in 25 of 37 patients in the LVD group and 35 of 46 patients in the PVE group. There was a similar three-year OS (OR = 0.70; 95%CI [0.22, 2.20], p = 0.54).
Three-year DFS
Two studies reported the three-year DFS (21, 25). It was reported in 14 of 37 patients in the LVD group and four of 46 patients in the PVE group. There was a higher three-year DFS in the LVD group (OR = 5.88; 95%CI [1.05, 32.87], p = 0.04).
Quality assessment of the included studies and reporting of the effects of LVD
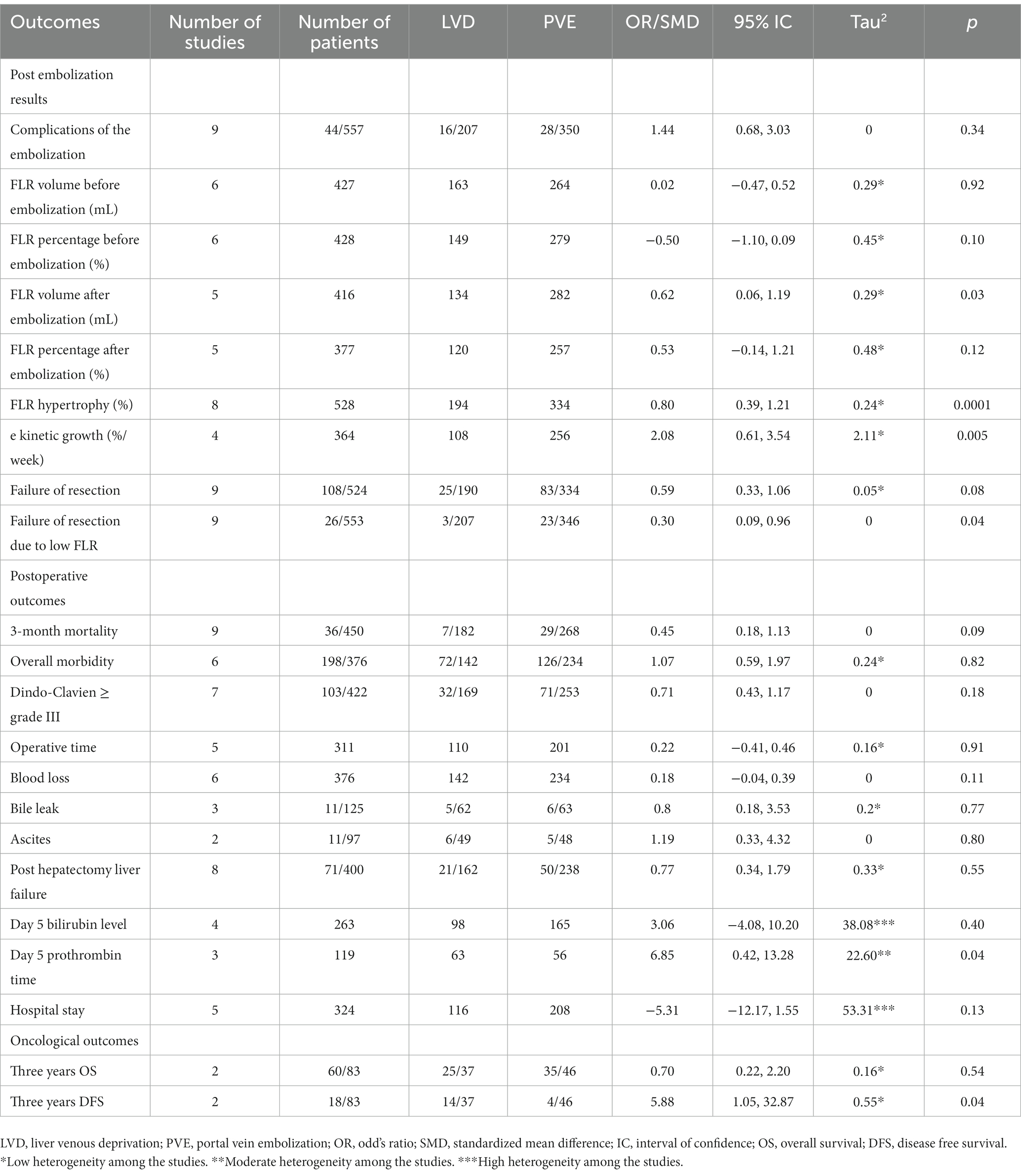
Table 3 summarizes the different findings of the pooled analysis. The MINORS and NOS scores were reported in Table 1. A Summary of the evidence was presented in Tables 4–6. This review showed that when LVD is compared with PVE:
• The post-embolization results (Table 4), it may ensure a higher FLR volume after embolization, a percentage of FLR hypertrophy, lower failure of resection due to low FLR, and faster kinetic growth. We do not know if it leads to additional complications related to the embolization, FLR percentage of hypertrophy after embolization, or failure of resection because the evidence was very uncertain.
• For the postoperative outcomes (Table 5), it may ensure a higher day 5 prothrombin time. We do not know if it leads to additional 3-month mortality, overall morbidity, major complications, operative time, blood loss, bile leak, ascites, post hepatectomy liver failure, day 5 bilirubin level, and hospital stay because the evidence was very uncertain.
• For the oncological outcomes (Table 6), it may ensure a higher 3 years of DFS. We do not know if it leads to higher three-year OS because the evidence was very uncertain.
Discussion
This study concluded that the LVD group was associated with higher FLR volume after embolization, percentage of FLR hypertrophy, lower failure of resection due to low FLR, faster kinetic growth, higher day 5 prothrombin time, and higher 3 years DFS. This study did not find any difference between the LVD and PVE groups in terms of complications related to embolization, FLR percentage of hypertrophy after embolization, failure of resection, 3-month mortality, overall morbidity, major complications, operative time, blood loss, bile leak, ascites, PHLF, day 5 bilirubin level, hospital stay, and three years’ OS.
Mortality and morbidity remain major concerns after an extended hepatectomy. This could be related to technical factors causing bile leakage and abscess, or PHLF causing hypoalbuminemia, cholestasis, and synthetic dysfunction (35). Hwang et al. (36), first, proposed sequential PV and ipsilateral hepatic vein embolization in 42 patients. They found a volume increase of 13.3% after PVE versus 28.9% after sequential PVE followed by hepatic vein embolization. In 2016, Guiu et al. (37) concluded that trans-hepatic LVD is feasible, well tolerated, and provides faster and more important hypertrophy of the FLR. In our study, this safety was confirmed by the similar success and complication rates. Furthermore, most complications after LVD were managed conservatively. Only one of the 16 patients died due to infected tumor necrosis. We concluded that the adjunction of ipsilateral embolization of the liver vein to the PV did not lead to additional mortality or morbidity. Our study demonstrated also that after LVD a small number of patients failed to undergo liver resection. However, when we assessed only resection failure due to an inadequate FLR, we found a lower rate in the LVD group: three out of 25 patients (12%) versus 23 out of 82 patients (28%). These findings were confirmed by reporting a higher FLR hypertrophy percentage and FLR volume after embolization. Even more, heterogeneity among the studies could be explained by including left and right hepatectomies, additional embolization of the segment-4 PV, or middle hepatic vein embolization. In addition, no strict volumetric criteria exist for inclusion like mixed tumor types, and cirrhotic and non-cirrhotic patients. Furthermore, pooled data on multiple factors that could affect FLR growth, such as age, malnutrition, obesity, chronic renal failure, and preoperative chemotherapy were not precise (34). To assess the accuracy of FLR hypertrophy, we have compared FLR volume and percentage data before embolization. Another advantage of LVD was faster kinetic growth (2.08%/week). According to Shindoh et al. (38), the kinetic growth rate is a better predictor of postoperative morbidity and mortality than the standardized future liver remnant and degree of hypertrophy. In a subgroup analysis, a kinetic growth rate < 2%/week was associated with higher major complications, PHLF, and 3-month mortality.
In addition, we found that postoperative outcomes directly depend on the FLR size (39). In the case of a small remnant liver, we have a “small for size” liver syndrome (40), causing an increase in portal pressure and blood flow to the remnant liver and mesentery. Consequently, it causes sinusoidal endothelial and Kupffer cell injury and inflammatory cytokine release. Some authors raised the risk of increased blood loss due to the development of “pseudo-Budd-Chiari syndrome” after LVD (24–26). In our study, we did not find a difference in terms of PHLF and ascites. It seems that the surgical procedure was not more challenging after LVD with similar 30-day mortality, morbidity, major complications, blood loss, and operative time. Furthermore, in studies reporting the Pringle manoeuvre, there was no difference between the LVD and PVE groups. This evidence supported the absence of a “pseudo-Budd-Chari syndrome” after hepatectomy.
Regarding oncological data, our study concluded to similar three-year OS with a higher 3-year DFS. Two other studies, with longer follow-ups, of Azoulay et al. (40) and Elias et al. (41) did not find a difference in 5-year OS. Then, we concluded that LVD ensures at least similar oncological safety.
On the other hand, we should consider a brief comparison between LVD and ALPPS that present an additional option for these area. LVD and ALPPS differ in their approach, speed of liver regeneration, and risk profiles (8). The choice between these techniques depends on various factors including the patient’s condition, extent of liver disease, and the surgeon’s expertise. LVD aims to accelerate liver hypertrophy by blocking the blood supply to the diseased part of the liver, thereby redirecting blood to the healthier part. However, ALPPS seeks to rapidly induce hypertrophy of the future liver remnant by surgically dividing the liver and ligating the blood supply to the diseased portion. Generally, LVD has a slower hypertrophy response compared to ALPPS and ALPPS Known for inducing rapid liver hypertrophy, often within a week or two (9). On the other side, we should consider that LVD potentially reduces the risk of postoperative complications compared to ALPPS, but this can vary based on patient-specific factors and ALPPS is associated with higher morbidity and mortality rates due to the invasiveness of the procedure. For these reasons, LVD is often used in patients where traditional PVE is not effective or in cases with extensive liver disease and ALPPS is typically considered for patients with extensive liver tumors or small future liver remnants where traditional approaches like PVE might not induce sufficient hypertrophy.
Our results should be interpreted within the context of these limitations. Owing to the absence of RCTs, we only included CCTs and our conclusions should be considered with cautions. Comparison of liver volumetry and function should be performed using reproducible and comparable methods, and large amounts of data were required to obtain a definite opinion. One of the missing outcomes to evaluate was the postoperative remnant liver function. Only the comparative study by Guiu et al. (22) assessed FLR function using scintigraphy. In our study, referring to the “50–50” criteria, we have found higher prothrombin time on day 5 post hepatectomy in the LVD group which confirms to some degree these findings of retrospective studies (22, 42). It is difficult to draw strong conclusions regarding the superiority of LVD to PVE because the studies included a small sample size of patients, lacked some outcomes, and presented essentially short-term follow-up. We should wait for more accurate data that will allow us to establish causality from the first French multicenter RCT HYPER-LIV01 trial (NCT03841305) and the Maastricht group DRAGON-2 (NCT05428735) comparing LVD and PVE in colorectal liver metastases. One additional point to consider was that the majority of studies were from Western centers and the translation of results could not be performed only by further studies from Eastern centers.
In conclusion, LVD is as feasible and safe as PVE with encouraging results making some selected patients more suitable for surgery, even with a small FLR. However, this interventional radiological procedure probably helps resolve the volumetric problem, but its effect on tumor progression remains to be better investigated.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
MC: Conceptualization, Data curation, Methodology, Resources, Software, Writing – original draft, Writing – review & editing. AM: Conceptualization, Data curation, Formal analysis, Writing – original draft. AC: Formal analysis, Funding acquisition, Resources, Writing – original draft. MH: Conceptualization, Investigation, Methodology, Resources, Validation, Writing – original draft. AG: Investigation, Resources, Supervision, Validation, Visualization, Writing – original draft. BK: Formal analysis, Funding acquisition, Investigation, Methodology, Resources, Software, Writing – original draft. FP: Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing. BG: Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing. OS: Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing. HO: Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kinoshita, H , Sakai, K , Hirohashi, K , Igawa, S , Yamasaki, O , and Kubo, S . Preoperative portal vein embolization for hepatocellular carcinoma. World J Surg. (1986) 10:803–8. doi: 10.1007/BF01655244
2. Abulkhir, A , Limongelli, P , Healey, AJ , Damrah, O , Tait, P , Jackson, J, et al. Preoperative portal vein embolization for major liver resection: a meta-analysis. Ann Surg Janv. (2008) 247:49–57. doi: 10.1097/SLA.0b013e31815f6e5b
3. Clavien, PA , Petrowsky, H , DeOliveira, ML , and Graf, R . Strategies for safer liver surgery and partial liver transplantation. N Engl J Med. (2007) 356:1545–59. doi: 10.1056/NEJMra065156
4. Alvarez, FA , Castaing, D , Figueroa, R , Allard, MA , Golse, N , Pittau, G, et al. Natural history of portal vein embolization before liver resection: a 23-year analysis of intention-to-treat results. Surgery. (2018) 163:1257–63. doi: 10.1016/j.surg.2017.12.027
5. Golse, N , Nunez, J , Mazzotta, A , Cano, L , Bergeat, D , Sulpice, L, et al. Personalized preoperative nomograms predicting postoperative risks after resection of perihilar cholangiocarcinoma. World J Surg. (2020) 44:3449–60. doi: 10.1007/s00268-020-05618-8
6. Alizai, PH , Haelsig, A , Bruners, P , Ulmer, F , Klink, CD , Dejong, CH, et al. Impact of liver volume and liver function on posthepatectomy liver failure after portal vein embolization–a multivariable cohort analysis. Ann Med Surg. (2018) 25:6–11. doi: 10.1016/j.amsu.2017.12.003
7. Fujii, Y , Shimada, H , Endo, I , Morioka, D , Nagano, Y , Miura, Y, et al. Risk factors of posthepatectomy liver failure after portal vein embolization. J Hepato-Biliary-Pancreat Surg. (2003) 10:226–32. doi: 10.1007/s00534-002-0820-9
8. Chebaro, A , Buc, E , Durin, T , Chiche, L , Brustia, R , Didier, A, et al. Liver venous deprivation or associating liver partition and portal vein ligation for staged hepatectomy?: a retrospective multicentric study. Ann Surg. (2021) 274:874–80. doi: 10.1097/SLA.0000000000005121
9. Gavriilidis, P , Sutcliffe, RP , Roberts, KJ , Pai, M , Spalding, D , Habib, N, et al. No difference in mortality among ALPPS, two-staged hepatectomy, and portal vein embolization/ligation: a systematic review by updated traditional and network meta-analyses. Hepatobiliary Pancreat Dis Int. (2020) 19:411–9. doi: 10.1016/j.hbpd.2020.07.005
10. Page, MJ , McKenzie, JE , Bossuyt, PM , Boutron, I , Hoffmann, TC , Mulrow, CD, et al. Updating guidance for reporting systematic reviews: development of the PRISMA 2020 statement. J Clin Epidemiol. (2021) 134:103–12. doi: 10.1016/j.jclinepi.2021.02.003
11. Shea, BJ , Reeves, BC , Wells, G , Thuku, M , Hamel, C , Moran, J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. (2017):j4008. doi: 10.1136/bmj.j4008
12. Rahbari, NN , Garden, OJ , Padbury, R , Brooke-Smith, M , Crawford, M , Adam, R, et al. Posthepatectomy liver failure: a definition and grading by the international study Group of Liver Surgery (ISGLS). Surgery. (2011) 149:713–24. doi: 10.1016/j.surg.2010.10.001
13. Balzan, S , Belghiti, J , Farges, O , Ogata, S , Sauvanet, A , Delefosse, D, et al. The “50-50 criteria” on postoperative day 5: an accurate predictor of liver failure and death after hepatectomy. Ann Surg. (2005) 242:824–9. doi: 10.1097/01.sla.0000189131.90876.9e
14. Slim, K , Nini, E , Forestier, D , Kwiatkowski, F , Panis, Y , and Chipponi, J . Methodological index for non-randomized studies (MINORS): development and validation of a new instrument. ANZ J Surg. (2003) 73:712–6. doi: 10.1046/j.1445-2197.2003.02748.x
15. Lo, CKL , Mertz, D , and Loeb, M . Newcastle-Ottawa scale: comparing reviewers’ to authors’ assessments. BMC Med Res Methodol. (2014) 14:1–5. doi: 10.1186/1471-2288-14-45
16. Balshem, H , Helfand, M , Schünemann, HJ , Oxman, AD , Kunz, R , Brozek, J, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol avr. (2011) 64:401–6. doi: 10.1016/j.jclinepi.2010.07.015
17. Higgins, JPT , Thompson, SG , Deeks, JJ , and Altman, DG . Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
18. Egger, M , Smith, GD , Schneider, M , and Minder, C . Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
19. Higgins, Julian P. T. , and Green, Sally . (2020). Cochrane handbook for systematic reviews of interventions. Available at: https://handbook-5-1.cochrane.org/
20. Böning, G , Fehrenbach, U , Auer, TA , Neumann, K , Jonczyk, M , Pratschke, J, et al. Liver venous deprivation (LVD) versus portal vein embolization (PVE) alone prior to extended hepatectomy: a matched pair analysis. Cardiovasc Intervent Radiol. (2022) 45:950–7. doi: 10.1007/s00270-022-03107-0
21. Cassese, G , Troisi, RI , Khayat, S , Benoudifa, B , Quenet, F , Guiu, B, et al. Liver venous deprivation versus portal vein embolization before major hepatectomy for colorectal liver metastases: a retrospective comparison of short- and medium-term outcomes. J Gastrointest Surg. (2023) 27:296–305. doi: 10.1007/s11605-022-05551-2
22. Guiu, B , Quenet, F , Panaro, F , Piron, L , Cassinotto, C , Herrerro, A, et al. Liver venous deprivation versus portal vein embolization before major hepatectomy: future liver remnant volumetric and functional changes. Hepatobiliary Surg Nutr. (2020) 9:564–76. doi: 10.21037/hbsn.2020.02.06
23. Heil, J , Korenblik, R , Heid, F , Bechstein, WO , Bemelmans, M , Binkert, C, et al. Preoperative portal vein or portal and hepatic vein embolization: DRAGON collaborative group analysis. Br J Surg. (2021) 108:834–42. doi: 10.1093/bjs/znaa149
24. Hocquelet, A , Sotiriadis, C , Duran, R , Guiu, B , Yamaguchi, T , Halkic, N, et al. Preoperative portal vein embolization alone with biliary drainage compared to a combination of simultaneous portal vein, right hepatic vein embolization and biliary drainage in Klatskin tumor. Cardiovasc Intervent Radiol. (2018) 41:1885–91. doi: 10.1007/s00270-018-2075-0
25. Kobayashi, K , Yamaguchi, T , Denys, A , Perron, L , Halkic, N , Demartines, N, et al. Liver venous deprivation compared to portal vein embolization to induce hypertrophy of the future liver remnant before major hepatectomy: a single center experience. Surgery. (2020) 167:917–23. doi: 10.1016/j.surg.2019.12.006
26. Laurent, C , Fernandez, B , Marichez, A , Adam, JP , Papadopoulos, P , Lapuyade, B, et al. Radiological simultaneous Portohepatic vein embolization (RASPE) before major hepatectomy: a better way to optimize liver hypertrophy compared to portal vein embolization. Ann Surg. (2020) 272:199–205. doi: 10.1097/SLA.0000000000003905
27. Le Roy, B , Gallon, A , Cauchy, F , Pereira, B , Gagnière, J , Lambert, C, et al. Combined biembolization induces higher hypertrophy than portal vein embolization before major liver resection. HPB. (2020) 22:298–305. doi: 10.1016/j.hpb.2019.08.005
28. Panaro, F , Giannone, F , Riviere, B , Sgarbura, O , Cusumano, C , Deshayes, E, et al. Perioperative impact of liver venous deprivation compared with portal venous embolization in patients undergoing right hepatectomy: preliminary results from the pioneer center. HepatoBiliary Surg Nutr. (2019) 8:329–37. doi: 10.21037/hbsn.2019.07.06
29. Schadde, E , Guiu, B , Deal, R , Kalil, J , Arslan, B , Tasse, J, et al. Simultaneous hepatic and portal vein ligation induces rapid liver hypertrophy: a study in pigs. Surgery. (2019) 165:525–33. doi: 10.1016/j.surg.2018.09.001
30. Piron, L , Deshayes, E , Cassinotto, C , Quenet, F , Panaro, F , Hermida, M, et al. Deportalization, venous congestion, venous deprivation: serial measurements of volumes and functions on Morphofunctional 99mTc-Mebrofenin SPECT-CT. Diagnostics. (2021) 11:12. doi: 10.3390/diagnostics11010012
31. Luz, JHM , and Bilhim, T . Portal vein embolization, biembolization, and liver venous deprivation. Radiol Bras. (2021) 54:206–7. doi: 10.1590/0100-3984.2021.0040
32. Bockhorn, M , Benkö, T , Opitz, B , Sheu, SY , Sotiropoulos, GC , Schlaak, JF, et al. Impact of hepatic vein deprivation on liver regeneration and function after major hepatectomy. Langenbeck's Arch Surg. (2007) 393:527–33. doi: 10.1007/s00423-007-0219-9
33. Deshayes, E , Piron, L , Bouvier, A , Lapuyade, B , Lermite, E , Vervueren, L, et al. Study protocol of the HYPER-LIV01 trial: a multicenter phase II, prospective and randomized study comparing simultaneous portal and hepatic vein embolization to portal vein embolization for hypertrophy of the future liver remnant before major hepatectomy for Colo-rectal liver metastases. BMC Cancer. (2020) 20:574. doi: 10.1186/s12885-020-07065-z
34. Cassese, G , Han, HS , Al Farai, A , Guiu, B , Troisi, RI , and Panaro, F . Future remnant liver optimization: preoperative assessment, volume augmentation procedures and management of PVEfailure. Minerva Surg. (2022) 77:368–79. doi: 10.23736/S2724-5691.22.09541-7
35. Baumgartner, R , Gilg, S , Björnsson, B , Hasselgren, K , Ghorbani, P , Sauter, C, et al. Impact of post-hepatectomy liver failure on morbidity and short-and long-term survival after major hepatectomy. BJS Open. (2022) 6:97. doi: 10.1093/bjsopen/zrac097
36. Hwang, S , Lee, SG , Ko, GY , Kim, BS , Sung, KB , Kim, MH, et al. Sequential preoperative ipsilateral hepatic vein embolization after portal vein embolization to induce further liver regeneration in patients with hepatobiliary malignancy. Ann Surg. (2009) 249:608–16. doi: 10.1097/SLA.0b013e31819ecc5c
37. Guiu, B , Chevallier, P , Denys, A , Delhom, E , Pierredon-Foulongne, MA , Rouanet, P, et al. Simultaneous trans-hepatic portal and hepatic vein embolization before major hepatectomy: the liver venous deprivation technique. Eur Radiol. (2016) 26:4259–67. doi: 10.1007/s00330-016-4291-9
38. Shindoh, J , Truty, MJ , Aloia, TA , Curley, SA , Zimmitti, G , Huang, SY, et al. Kinetic growth rate after portal vein embolization predicts posthepatectomy outcomes: toward zero liver-related mortality in patients with colorectal liver metastases and small future liver remnant. J Am Coll Surg. (2013) 216:201–9. doi: 10.1016/j.jamcollsurg.2012.10.018
39. Fernandez, H , Nadalin, S , and Testa, G . Optimizing future remnant liver prior to major hepatectomies: increasing volume while decreasing morbidity and mortality. Hepatobiliary Surg Nutr. (2020) 9:215–8. doi: 10.21037/hbsn.2019.10.24
40. Azoulay, D , Castaing, D , Smail, A , Adam, R , Cailliez, V , Laurent, A, et al. Resection of nonresectable liver metastases from colorectal cancer after percutaneous portal vein embolization. Ann Surg. (2000) 231:480–6. doi: 10.1097/00000658-200004000-00005
41. Elias, D , Ouellet, JF , De Baère, T , Lasser, P , and Roche, A . Preoperative selective portal vein embolization before hepatectomy for liver metastases: long-term results and impact on survival. Surgery. (2002) 131:294–9. doi: 10.1067/msy.2002.120234
Keywords: liver venous deprivation, portal embolization, liver failure, hepatectomy, surgery, remnant liver volume
Citation: Chaouch MA, Mazzotta A, da Costa AC, Hussain MI, Gouader A, Krimi B, Panaro F, Guiu B, Soubrane O and Oweira H (2024) A systematic review and meta-analysis of liver venous deprivation versus portal vein embolization before hepatectomy: future liver volume, postoperative outcomes, and oncological safety. Front. Med. 10:1334661. doi: 10.3389/fmed.2023.1334661
Edited by:
Huan Tong, Sichuan University, ChinaReviewed by:
Mohammadsadegh Nikdad, Universitätsmedizin Greifswald, GermanyTuerhongjiang Tuxun, First Affiliated Hospital of Xinjiang Medical University, China
Copyright © 2024 Chaouch, Mazzotta, da Costa, Hussain, Gouader, Krimi, Panaro, Guiu, Soubrane and Oweira. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohamed Ali Chaouch, ZG9jbWVkYWxpY2hhb3VjaEBnbWFpbC5jb20=
 Mohamed Ali Chaouch
Mohamed Ali Chaouch Alessandro Mazzotta
Alessandro Mazzotta Adriano Carneiro da Costa2
Adriano Carneiro da Costa2 Fabrizio Panaro
Fabrizio Panaro Boris Guiu
Boris Guiu