
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Med. , 22 December 2023
Sec. Intensive Care Medicine and Anesthesiology
Volume 10 - 2023 | https://doi.org/10.3389/fmed.2023.1333209
This article is part of the Research Topic Infections in the Intensive Care Unit, volume II View all 17 articles
Introduction: Restored cardiopulmonary function is efficiently achieved by utilizing extracorporeal membrane oxygenation (ECMO). Nevertheless, the incidence of Clostridioides difficile infection (CDI) associated with ECMO is relatively uncommon.
Case presentation: In this report, we present the case of a 59-year-old male with severe chest pain due to acute myocardial infarction, subsequently necessitating ECMO support. During the first day of hospitalization, pulmonary infections were observed, and piperacillin-tazobactam was prescribed for 7 days at low dosages. However, the patient developed severe diarrhea 4 days later. After ruling out common pathogens, we suspected the occurrence of CDI and performed genetic testing for C. difficile toxin, confirming our diagnosis. The prescription of vancomycin resulted in slight improvement, while fecal microbiota transplantation (FMT) proved to be more effective.
Conclusion: In this case, temporary application of ECMO was applied, and the anti-infective treatment relied on the use of antibiotics at short-term, low-dose, and low CDI risk. Hence, the occurrence of CDI was considered an uncommon event, which may serve as a reference for future cases.
Clostridioides difficile infection (CDI) is a type of bacterial infection that primarily affects intestines. It arises from the disruption of the normal intestinal flora, which facilitates the colonization of C. difficile (1). Although CDI was traditionally considered a hospital-acquired infection, recent studies have indicated a significant proportion of community residents affected by C. difficile (2). Statistics show that approximately half a million individuals in the United States were infected with C. difficile, leading to a substantial annual cost of $4.8–6.3 billion for in-hospital management (3, 4). Several patient-related risk factors contribute to the risk of CDI, with antibiotics exposure being the most significant risk factor. The use of various antibiotics, particularly cephalosporins, clindamycin, fluoroquinolones, and carbapenems, is associated with the development of CDI. The risk of infection also increases with the number and duration of antibiotic applications. Furthermore, CDI is closely associated with advanced age, hospitalization, cancer, chronic kidney diseases, and the usage of immunosuppressants or gastrointestinal interventions like surgeries, nasal feeding, proton pump inhibitor (PPI), histamine-2 receptor antagonist (H2RA) (1, 5). The manifestation of CDI varies widely, ranging from asymptomatic carriage and mild to moderate diarrhea, to severe cases of fulminant colitis that can be fatal (6). Intensive Care Unit (ICU) patients, due to their critical and severe condition, often require concurrent antibiotics, nasogastric tube intubation, and intensive care, placing them at a higher risk of CDI. Therefore, it is crucial to emphasize universal prevention measures and carefully consider CDI as a potential cause when evaluating suspected symptoms.
Extracorporeal membrane oxygenation (ECMO) is a form of extracorporeal life support (ECLS) used for addressing circulatory or respiratory failure in patients. This technique utilizes a mechanical device that provides either short- or long-term extracorporeal life support. The most frequent conditions that warrant ECMO initiation are acute respiratory distress syndrome (ARDS) and cardiogenic shock (7). Despite its frequent utilization in the ICU, ECMO is a highly invasive procedure associated with multiple complications, including large vessel tears, hemorrhage, hemolysis, and infection. It is crucial to acknowledge that patients undergoing ECMO are typically critically ill, malnourished, or immunocompromised, which markedly increases the risk of infection (8). Stefano Biffi et al. (9) reported that lower respiratory tract infection was the most frequent type of infection in adults undergoing ECMO, with bloodstream infections, urinary tract infections, surgical site infections, and intestinal infections being less frequent. Additionally, Wang’s study (10) indicated that gram-negative pathogens, including Acinetobacter baumannii and Klebsiella pneumoniae, were the primary pathogens, while fungal pathogens like Candida albicans and Candida glabrata were also prevalent. In contrast, gram-positive pathogens were rarely observed. Based on clinical experience with ECMO, the incidence of Clostridioides difficile infection following ECMO support is uncommon among ICU patients. It is suspected that surgical interventions and intensive care associated with ECMO may promote the translocation of normal intestinal microbiota and increase the likelihood of C. difficile colonization.
Herein, this study reports on a patient suffered acute myocardial infarction and received ECMO treatment, who subsequently developed C. difficile infection. The clinical data will provide valuable insights for preventing and treating similar cases in the future.
The patient was a 59-year-old male with a history of diabetes and hypertension. On November 9, 2021, at 4 pm, he experienced sudden chest pain and tightness without any apparent triggers, leading to his immediate transfer to the local hospital’s emergency department. An electrocardiogram (ECG) was performed, revealing an acute inferior myocardial infarction and a third-degree atrioventricular block. The patient was then received Alteplase at the standard dose. Shortly after, a follow-up ECG showed slight ST-segment resolution, but the patient had already entered a state of shock. To receive coronary stent implantation, he was immediately transferred to a higher-level hospital. However, during the surgery, the patient’s blood pressure and pulse oxygen saturation remained unstable. Temporary measures were taken, including the administration of norepinephrine (NE) at a rate of 160 ug/min, dopamine (DA) at 10 ug/min, and epinephrine at 20 ug/min. The patient was then admitted to the ICU at Zhongnan Hospital of Wuhan University, where he received tracheal intubations and an intra-aortic balloon pump (IABP). On the November 11 (Day 1), Veno-Arterial ECMO (VA-ECMO) support was provided to avoid deterioration.
The patient’s physical examination revealed body temperature of 36.5°C, heart rate of 72 bpm, SpO2 of 86%, respiration rate of 16 bpm while receiving endotracheal intubation and ventilator support using pressure control mode, with FiO2 set at 100%, frequency (f) set at 16 bmp, positive end-expiratory pressure (PEEP) at 12 cmH2O, and blood pressure reading of 144/99 mmHg (with NE injection at 80 ug/min). The patient’s bilateral pupil diameter was approximately 2.0 mm, with no icteric sclera and retained light reflexes. Cardiac sounds were low and unremarkable, and no other significant physical findings were noted. The laboratory results showed a white blood cell (WBC) count of 11.13 × 109/L, C-reactive protein (CRP) level of 171.2 mg/L, procalcitonin (PCT) level of 46.17 ng/mL, brain natriuretic peptide (BNP) level of 334.8 pg./mL, creatine kinase (CK) level > 4,267 u/L, creatine kinase-MB (CK-MB) level of 823 u/L, myoglobin level > 1200.0 ng/mL, and high-sensitivity troponin I level > 50000.0 pg./mL. The arterial blood gas analysis reflected a pH of 7.21, PaO2 of 57.49 mmHg, PaCO2 of 50.55 mmHg, K+ of 6.04 mmol/L, Na+ of 138.39 mmol/L, HCO3− of 19.8 mmol, and Lac of 4.28 mmol/L. Regarding the patient’s VA-ECMO settings, the speed was set at 7000 rpm/min, blood flow velocity at 3.3 L/min, gas flow velocity at 2 L/min, and fraction of inspired oxygen at 60%.
The patient initially suffered from acute myocardial infarction, despite attempts with medication and emergency surgery, these treatments proved ineffective. Therefore, VA-ECMO was promptly initiated to delay heart failure and provide additional time for cardiac function recovery. Fortunately, after 5 days of ECMO support, the patient’s general condition and heart function returned to normal, as indicated by stable vital signs and cardiac ultrasound (CUS) results. VA-ECMO was discontinued on the sixth day of hospitalization. Table 1 provides a record of the ECMO operating parameters and changes in left ventricular ejection fraction (LVEF). In addition to the primary condition, routine blood tests on day 1 showed abnormal indices: WBC: 11.1 × 109/L, neutrophil percentage (N%): 83.3%, procalcitonin (PCT): 46.2 ng/mL, and interleukin-6 (IL-6): 660 pg./mL, along with a low-grade fever of 37.5°C. These findings suggested the occurrence of an infection, which was confirmed by chest X-ray and computed tomography (CT) showing pulmonary infections. As a result, piperacillin-tazobactam (iv. 4.5 g q8h) was prescribed for temporary control. Although clinical signs of infection were evident, specific bacteria were not detected in the sputum culture, blood culture, or bronchoalveolar lavage fluid (BLF). The infection was gradually improved with the prescribed medication (Figure 1).
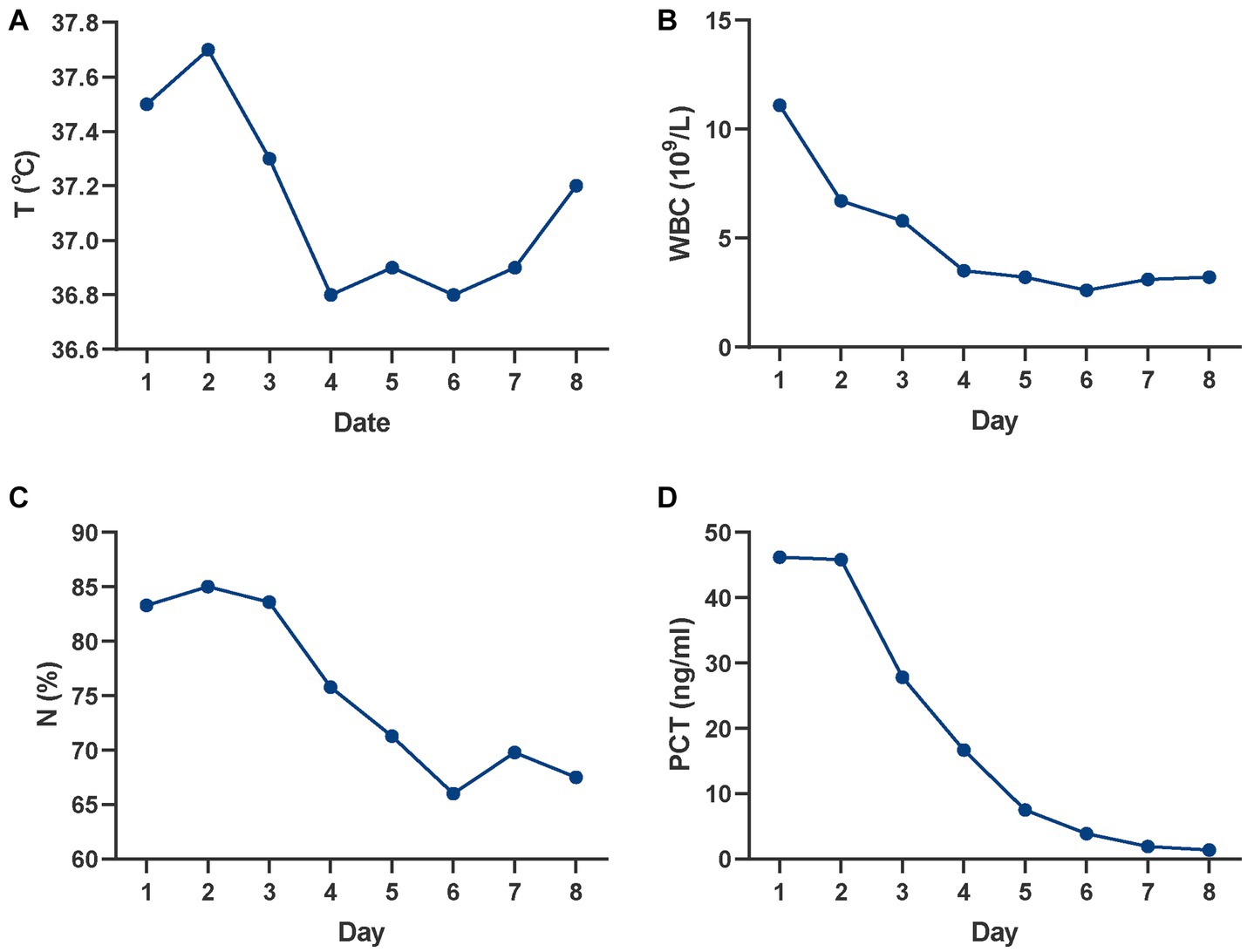
Figure 1. Changes in infectious indicators over time. (A) Changes in T; (B) Changes in WBC; (C) Changes in N%; (D) Changes in PCT. T, temperature; WBC, white blood cell; N%, neutrophil percentage; PCT, procalcitonin.
On Day 12, the patient’s condition deteriorated since he developed diarrhea. The diarrhea was characterized by yellow, water-like stools and the patient defected seven times that day, amounting to approximately 1,100 mL in total. The following day, the patient experienced eight bowel movements, with the volume increasing to 1,320 mL. Despite the prescription of antidiarrheal medications, including berberine and montmorillonite powder, the patient’s condition did not improve. Bacterial culture, biochemical identification, and susceptibility tests were performed on the fecal sample, but they did not yield any useful information. Considering the patient’s medical history of antibiotic administration during the first week of hospitalization, Clostridioides difficile was suspected as the likely cause of the persistent diarrhea. Genetic testing, using a real-time polymerase chain reaction assay for C. difficile toxin, confirmed that the patient tested positive for toxin B. Coupled with a WBC count exceeding 15 × 109/L and creatinine (Cr) above 1.5 mg/dL, the patient was diagnosed with a severe CDI.
According to the ACG Clinical Guidelines (11), the patient was initially treated with vancomycin at a dosage of 125 mg every 6 h via a nasogastric tube. As a result, the patient’s stool frequency decreased to 3 times per day on Day 15, and then stabilized at 5–6 times per day with partially formed stool for the subsequent 4 days. Despite some improvement in diarrhea after 7 days of vancomycin treatment, the patient’s stool consistency did not fully return to a healthy state. Therefore, fecal microbiota transplantation (FMT) was proposed to prevent the progression of intestinal infections. Fecal samples were obtained from the Second Hospital Affiliated with Nanjing Medical University and were stored at a temperature of −20°C after being transported under low temperature conditions. The FMT infusion was administered on Day 20.
Before the operation, the samples were thawed in 37°C water for 1 h, and the patient was assisted to sit upright with intravenous metoclopramide (10 mg). Then, a nasal jejunal feeding tube was pre-placed, and the sample was infused through the tube. The whole procedure was completed within 3–5 min, and normal saline (5 mL) was used to flush the tube. The effect of the FMT was immediate, resulting in a significant reduction in stool frequency (3 times per day with a total volume of 330 mL on Day 21). In the following week, the patient experienced approximately 2–4 bowel movements per day, with a lower volume than before. Furthermore, genetic tests for C. difficile toxin conducted on both Day 24 and Day 28 yielded negative results, indicating a good outcome of FMT therapy for CDI. Detailed data on the development of CDI are presented in Table 2.
After 28 days of ICU treatment, the patient regained consciousness, exhibited a good mood and demonstrated normal cardiac function, and regular bowel movements without any complaints of infections or intestinal discomfort. Comprehensive assessments led to the patient’s transfer from ICU back to the ward, where close monitoring was continued during the early phase of recovery. The whole process is illustrated in Figure 2.
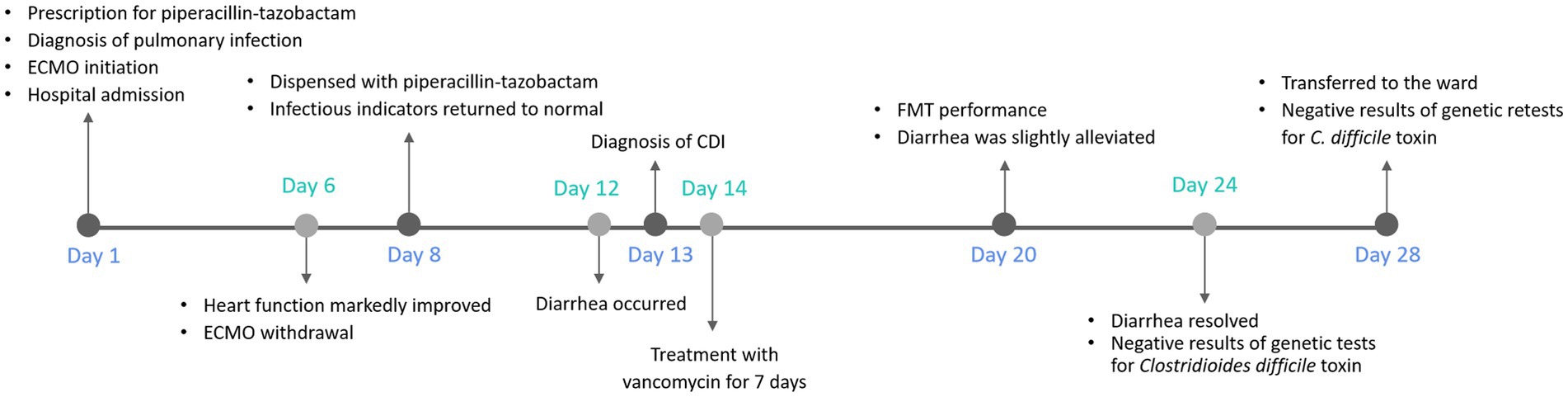
Figure 2. Diagnosis and treatment timeline of the patient. The timeline shows the major clinical events during the patient’s hospitalization. ECMO, extracorporeal membrane oxygenation; CDI, Clostridioides difficile infection; FMT, fecal microbiota transplantation.
ECMO is a crucial intervention for replacing cardiac and pulmonary functions in the short term. VA-ECMO is primarily used to manage heart failure, including cardiogenic shock following myocardial infarction, extracorporeal cardiopulmonary resuscitation (ECPR), and fulminant myocarditis (7). In this case study, the patient was admitted to the hospital due to acute myocardial infarction and initially received empiric treatment with thrombolytic drugs and IABP. However, the effectiveness of the treatment was limited, and the patient’s symptoms worsened. Consequently, ECMO was employed to provide more consummate support. Nonetheless, the duration of ECMO application increased, thereby increasing the likelihood of bacterial infection (12). Thus far, no reports have been published regarding Clostridioides difficile infection in patients undergoing ECMO support.
CDI is a leading cause of infectious diarrhea in medical institutions, with increasing incidence in the ICU (13, 14). This condition occurs from an imbalance in the normal gut flora, primarily due to the abuse of broad-spectrum antibacterial drugs and other contributing factors (15). Disturbances in the gut microbiota may lead to proliferation of C. difficile from both endogenous and exogenous sources. A meta-analysis comparing various antibiotic classes and their association with hospital-acquired CDI found that third-generation cephalosporins had the highest odds, followed by clindamycin, second-generation cephalosporins, and fourth-generation cephalosporins (16). In this report, the patient demonstrated infectious characteristics from the time of admission, including fever, increased leukocyte count, procalcitonin, neutrophils, and a diagnosis of pulmonary inflammation based on imaging. Piperacillin-tazobactam was used, resulting in the normalization of infection indicators, and antibiotics were discontinued after 7 days. Interestingly, numerous studies have shown a higher risk increase for CDI associated with broad-spectrum antimicrobials, such as cefepime and meropenem than with piperacillin-tazobactam (17–19). Kundrapu (20) explains that piperacillin-tazobactam has the potentially to combat the colonization of C. difficile, unlike cefepime and other cephalosporins, which supports a widely accepted hypothesis. Although the patient receiving antibiotics with a lower CDI risk and at a lower dosage for a shorter duration, it did not alter the patient’s CDI status. Several possible reasons were analyzed, including: (1) the spores of C. difficile were widely distributed in hospital settings, (2) the patient’s cardiogenic shock and 5-day ECMO support, rendering him more susceptible to multi-drug-resistant bacteria, like C. difficile, (3) the invasive facilities used in the ICU, such as mechanical ventilation and nasogastric tubes, which were major risk factors for CDI, (4) the disruption of gut flora balance by piperacillin-tazobactam as an antibiotic, leading to the colonization of various opportunistic pathogens, and (5) the patient’s concurrent conditions of hypoxic–ischemic encephalopathy and acute kidney injury during hospitalization, contributing to a decline in overall immune function.
The diagnosis of CDI necessitates a thorough evaluation of clinical symptoms and confirmatory tests. First, CDI should be suspected when patients present with acute diarrhea, defined as loose stools occurring three or more times in a 24-h period. The severity of symptoms can vary, with mild cases characterized by watery diarrhea and mild abdominal cramping or tenderness. Severe cases can present with extremely frequent voiding (10–15 times/day), intense abdominal pain, nausea, fever, and potentially life-threatening complications such as toxic megacolon, sepsis, and multiorgan failure. Second, the diagnosis of CDI requires positive genetic evidence or colonoscopic confirmation of pseudomembranous colitis (6, 21–23). According to the ACG clinical guidelines (11), a WBC count greater than 15 × 109/L and a serum Cr level greater than 1.5 mg/dL classify the patient as having severe CDI.
Regarding the treatment of CDI, it is crucial to evaluate the discontinuation of antibiotic therapy for all patients diagnosed with CDI, unless it hampers the recovery of other conditions. Vancomycin is the preferred treatment for mild to severe CDI, with evidence supporting the use of fidaxomicin as an alternative. Metronidazole is suggested solely for the first episode of mild or moderate CDI if vancomycin or fidaxomicin are not available (24, 25). In this report, the patient was diagnosed with severe CDI and received vancomycin, which proved ineffective. The lack of effectiveness against the infection may be attributed to common antibiotic resistance.
CDI is rooted in gut-induced dysbiosis, leading to extensive research on microbial therapies. FMT is an advanced approach that restores the complete spectrum of microorganisms constituting normal colonic flora by transferring the colonic microbiome from a healthy individual. When standard medical therapy for severe CDI is ineffective, FMT should be considered a treatment option (26, 27). Serious adverse events associated with FMT are rare, and gastrointestinal symptoms such as nausea, belching, or bloating are the most commonly reported issues, particularly when FMT is administered through the upper gastrointestinal route (28, 29). The risk of infection transmission, such as enteropathogenic Escherichia coli (EPEC) or Shiga toxin-producing Escherichia coli (STEC), can be mitigated through careful donor selection and screening. Nevertheless, the acceptability of this application method to patients and the safety of transportation during the coronavirus disease 2019 (COVID-19) pandemic should be taken into account (30–32). In this report, FMT was used to treat CDI after unsatisfactory outcomes with vancomycin, resulting in significant declines in stool frequency and volume, as well as the absence of the toxin B gene detected in two subsequent tests conducted a few days later.
The prevention strategies for CDI hold paramount importance, with the primary approach being the restriction of antibiotic use, particularly high-risk antibiotics. In case where elderly individuals (aged 65 years or older), patients with a history of healthcare exposure or other inevitable risk factors for CDI, it is crucial to minimize the duration of antibiotic administrations as much as possible (33). Second, decontamination of hospital environments is imperative to prevent the transmission of CDI, as the spores of C. difficile are largely resistant and actively released from infected patients (34). Alongside ensuring high-quality disinfection and sterilization in hospitals, particular attention should be given to hand hygiene. Healthcare personnel in close proximity to patients must adhere to recommended procedures for thorough hand disinfection, using running water (35). It is worth noting that, at present, there is insufficient evidence to recommend any probiotics for the primary or secondary prevention of CDI in most patients (11).
In the ICU, ECMO stands as an easily accessible medical advancement. However, it is important to acknowledge that patients requiring ECMO support often suffer from critical and emergent illnesses, which increase their likelihood of antibiotic use, complications, and immune system impairment. Under these conditions, the intestinal flora becomes particularly vulnerable to dysregulation, potentially leading to the development of CDI. Therefore, clinicians should prioritize the preservation and enhancement of gastrointestinal function, recognizing the paramount significance of implementing all reasonable precautions and treatments to positively influence therapy, prognosis, and healthcare costs. Both healthcare professionals and patients share a common goal of efficiently combatting the risk of CDI.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
YH: Conceptualization, Investigation, Writing – original draft. CH: Methodology, Supervision, Writing – original draft. JJ: Project administration, Writing – original draft. JZ: Resources. YL: Formal analysis, Supervision, Writing – review & editing. ZP: Funding acquisition, Resources, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (ZP, No. 81971816 and 82272208) and the Subject Cultivation Project of Zhongnan Hospital of Wuhan University (ZP, No. ZNXKPY2021001).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Leffler, DA, and Lamont, JT. Clostridium difficile infection. N Engl J Med. (2015) 372:1539–48. doi: 10.1056/NEJMra1403772
2. Lessa, FC. Community-associated Clostridium difficile infection: how real is it? Anaerobe. (2013) 24:121–3. doi: 10.1016/j.anaerobe.2013.01.006
3. Spigaglia, P. Recent advances in the understanding of antibiotic resistance in Clostridium difficile infection. Therapeutic Advan Infect Dis. (2016) 3:23–42. doi: 10.1177/2049936115622891
4. Zhang, S, Palazuelos-Munoz, S, Balsells, EM, Nair, H, Chit, A, and Kyaw, MH. Cost of hospital management of Clostridium difficile infection in United States-a meta-analysis and modelling study. BMC Infect Dis. (2016) 16:447. doi: 10.1186/s12879-016-1786-6
5. Chitnis, AS, Holzbauer, SM, Belflower, RM, Winston, LG, Bamberg, WM, Lyons, C, et al. Epidemiology of community-associated Clostridium difficile infection, 2009 through 2011. JAMA Intern Med. (2013) 173:1359–67. doi: 10.1001/jamainternmed.2013.7056
6. McDonald, LC, Gerding, DN, Johnson, S, Bakken, JS, Carroll, KC, Coffin, SE, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. (2018) 66:e1–e48. doi: 10.1093/cid/cix1085
7. Mosier, JM, Kelsey, M, Raz, Y, Gunnerson, KJ, Meyer, R, Hypes, CD, et al. Extracorporeal membrane oxygenation (ECMO) for critically ill adults in the emergency department: history, current applications, and future directions. Crit Care. (2015) 19:431. doi: 10.1186/s13054-015-1155-7
8. Burket, JS, Bartlett, RH, Hyde, KV, and Chenoweth, CE. Nosocomial infections in adult patients undergoing extracorporeal membrane oxygenation. Clin Infect Dis. (1999) 28:828–33. doi: 10.1086/515200
9. Biffi, S, Di Bella, S, Scaravilli, V, Peri, AM, Grasselli, G, Alagna, L, et al. Infections during extracorporeal membrane oxygenation: epidemiology, risk factors, pathogenesis and prevention. Int J Antimicrob Agents. (2017) 50:9–16. doi: 10.1016/j.ijantimicag.2017.02.025
10. Wang, C, Li, S, Wang, F, Yang, J, Yan, W, Gao, X, et al. Nosocomial infections during extracorporeal membrane oxygenation in pediatric patients: a multicenter retrospective study. Front Pediatr. (2022) 10:873577. doi: 10.3389/fped.2022.873577
11. Kelly, CR, Fischer, M, Allegretti, JR, LaPlante, K, Stewart, DB, Limketkai, BN, et al. ACG clinical guidelines: prevention, diagnosis, and treatment of Clostridioides difficile infections. Am J Gastroenterol. (2021) 116:1124–47. doi: 10.14309/ajg.0000000000001278
12. Millar, JE, Fanning, JP, McDonald, CI, McAuley, DF, and Fraser, JF. The inflammatory response to extracorporeal membrane oxygenation (ECMO): a review of the pathophysiology. Crit Care. (2016) 20:387. doi: 10.1186/s13054-016-1570-4
13. Balsells, E, Shi, T, Leese, C, Lyell, I, Burrows, J, Wiuff, C, et al. Global burden of Clostridium difficile infections: a systematic review and meta-analysis. J Glob Health. (2019) 9:010407. doi: 10.7189/jogh.09.010407
14. Kwon, JH, Olsen, MA, and Dubberke, ER. The morbidity, mortality, and costs associated with Clostridium difficile infection. Infect Dis Clin North Am. (2015) 29:123–34. doi: 10.1016/j.idc.2014.11.003
15. Jenior, ML, Leslie, JL, Young, VB, and Schloss, PD. Clostridium difficile alters the structure and metabolism of distinct Cecal microbiomes during initial infection to promote sustained colonization. mSphere. (2018) 3:e00261–18. doi: 10.1128/mSphere.00261-18
16. Slimings, C, and Riley, TV. Antibiotics and hospital-acquired Clostridium difficile infection: update of systematic review and meta-analysis. J Antimicrob Chemother. (2014) 69:881–91. doi: 10.1093/jac/dkt477
17. Muldoon, EG, Epstein, L, Logvinenko, T, Murray, S, Doron, SI, and Snydman, DR. The impact of cefepime as first line therapy for neutropenic fever on Clostridium difficile rates among hematology and oncology patients. Anaerobe. (2013) 24:79–81. doi: 10.1016/j.anaerobe.2013.10.001
18. Brown, KA, Khanafer, N, Daneman, N, and Fisman, DN. Meta-analysis of antibiotics and the risk of community-associated Clostridium difficile infection. Antimicrob Agents Chemother. (2013) 57:2326–32. doi: 10.1128/AAC.02176-12
19. Hung, YP, Lin, HJ, Wu, TC, Liu, HC, Lee, JC, Lee, CI, et al. Risk factors of fecal toxigenic or non-toxigenic Clostridium difficile colonization: impact of toll-like receptor polymorphisms and prior antibiotic exposure. PloS One. (2013) 8:e69577. doi: 10.1371/journal.pone.0069577
20. Kundrapu, S, Sunkesula, VC, Jury, LA, Cadnum, JL, Nerandzic, MM, Musuuza, JS, et al. Do piperacillin/tazobactam and other antibiotics with inhibitory activity against Clostridium difficile reduce the risk for acquisition of C. difficile colonization? BMC Infect Dis. (2016) 16:159. doi: 10.1186/s12879-016-1514-2
21. Bauer, MP, Notermans, DW, van Benthem, BHB, Brazier, JS, Wilcox, MH, Rupnik, M, et al. Clostridium difficile infection in Europe: a hospital-based survey. Lancet. (2011) 377:63–73. doi: 10.1016/S0140-6736(10)61266-4
22. Bauer, MPKE, and van Dissel, JT. European Society of Clinical Microbiology and Infectious Diseases (ESCMID): treatment guidance document for Clostridium difficile infection (CDI). Clin Microbiol Infect. (2009) 15:1067–79. doi: 10.1111/j.1469-0691.2009.03099.x
23. Bartlett, JG, and Gerding, DN. Clinical recognition and diagnosis of Clostridium difficile infection. Clin Infect Dis. (2008) 46:S12–8. doi: 10.1086/521863
24. Surawicz, CM, Brandt, LJ, Binion, DG, Ananthakrishnan, AN, Curry, SR, Gilligan, PH, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol. (2013) 108:478–98. doi: 10.1038/ajg.2013.4
25. van Prehn, J, Reigadas, E, Vogelzang, EH, Bouza, E, Hristea, A, Guery, B, et al. European Society of Clinical Microbiology and Infectious Diseases: 2021 update on the treatment guidance document for Clostridioides difficile infection in adults. Clin Microbiol Infect. (2021) 27:S1–S21. doi: 10.1016/j.cmi.2021.09.038
26. Zainah, H, Hassan, M, Shiekh-Sroujieh, L, Hassan, S, Alangaden, G, and Ramesh, M. Intestinal microbiota transplantation, a simple and effective treatment for severe and refractory Clostridium difficile infection. Dig Dis Sci. (2015) 60:181–5. doi: 10.1007/s10620-014-3296-y
27. Agrawal, M, Aroniadis, OC, Brandt, LJ, Kelly, C, Freeman, S, Surawicz, C, et al. The long-term efficacy and safety of fecal microbiota transplant for recurrent, severe, and complicated Clostridium difficile infection in 146 elderly individuals. J Clin Gastroenterol. (2016) 50:403–7. doi: 10.1097/MCG.0000000000000410
28. Khanna, S, Pardi, DS, Kelly, CR, Kraft, CS, Dhere, T, Henn, MR, et al. A novel microbiome therapeutic increases gut microbial diversity and prevents recurrent Clostridium difficile infection. J Infect Dis. (2016) 214:173–81. doi: 10.1093/infdis/jiv766
29. Youngster, I, Russell, GH, Pindar, C, Ziv-Baran, T, Sauk, J, and Hohmann, EL. Oral, capsulized, frozen fecal microbiota transplantation for relapsing Clostridium difficile infection. JAMA. (2014) 312:1772–8. doi: 10.1001/jama.2014.13875
30. Kassam, Z, Dubois, N, Ramakrishna, B, Ling, K, Qazi, T, Smith, M, et al. Donor screening for fecal microbiota transplantation. N Engl J Med. (2019) 381:2070–2. doi: 10.1056/NEJMc1913670
31. Cammarota, G, Ianiro, G, Kelly, CR, Mullish, BH, Allegretti, JR, Kassam, Z, et al. International consensus conference on stool banking for faecal microbiota transplantation in clinical practice. Gut. (2019) 68:2111–21. doi: 10.1136/gutjnl-2019-319548
32. Khanna, S, Tande, A, Rubin, DT, Khoruts, A, Kahn, SA, and Pardi, DS. Fecal microbiota transplantation for recurrent C difficile infection during the COVID-19 pandemic: experience and recommendations. Mayo Clin Proc. (2021) 96:1418–25. doi: 10.1016/j.mayocp.2021.04.005
33. Ziakas, PD, Zacharioudakis, IM, Zervou, FN, Grigoras, C, Pliakos, EE, and Mylonakis, E. Asymptomatic carriers of toxigenic C. difficile in long-term care facilities: a meta-analysis of prevalence and risk factors. PloS One. (2015) 10:e0117195. doi: 10.1371/journal.pone.0117195
34. Lawley, TD, Clare, S, Deakin, LJ, Goulding, D, Yen, JL, Raisen, C, et al. Use of purified Clostridium difficile spores to facilitate evaluation of health care disinfection regimens. Appl Environ Microbiol. (2010) 76:6895–900. doi: 10.1128/AEM.00718-10
35. Prasad, P, Brown, L, Ma, S, McDavid, A, Rudmann, A, Lent, D, et al. “if the glove fits”: hospital-wide universal gloving is associated with improved hand hygiene and may reduce Clostridioides difficile infection. Infect Control Hosp Epidemiol. (2021) 42:1351–5. doi: 10.1017/ice.2020.1422
Keywords: Clostridioides difficile, Clostridioides difficile infection, fecal microbiota transplantation, extracorporeal membrane oxygenation, case report
Citation: Hu Y, Hu C, Jiang J, Zhang J, Li Y and Peng Z (2023) Clostridioides difficile infection after extracorporeal membrane oxygenation support for acute myocardial infarction: a case report. Front. Med. 10:1333209. doi: 10.3389/fmed.2023.1333209
Received: 04 November 2023; Accepted: 13 December 2023;
Published: 22 December 2023.
Edited by:
Yuetian Yu, Shanghai Jiao Tong University, ChinaReviewed by:
M. Jahangir Alam, University of Houston, United StatesCopyright © 2023 Hu, Hu, Jiang, Zhang, Li and Peng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yiming Li, bHltLWZseUB3aHUuZWR1LmNu; Zhiyong Peng, em4wMDE1OTBAd2h1LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.