- Department of Translational Biomedicine and Neuroscience, University of Bari Medical School, Bari, Italy
Mast cells localized at the level of the mucosal barrier in the skin, lung, and gastrointestinal tract, intervene in the modulation of the function of the epithelial cells and are involved in innate and adaptive defensive responses. In this context, mast cells intervene in the recognition and clearance of microbial pathogens. This mini-review article discusses the role of mast cells in these barrier systems.
Introduction
In rodents, mast cells are classified into mucosal mast cells (MMCs) or connective tissue mast cells (CTMCs) (1). MMCs are localized between epithelial cells in the mucosa of the lung and gastrointestinal tract. In their cytoplasmatic granules, chondroitin sulfate and a few amounts of histamine and carboxypeptidase, are present (2), whereas CTMCs are localized in the intestinal submucosa, skin, peritoneal cavity, and generally occupy a perivascular position surrounding blood vessels. In their granules heparin and large amounts of histamine and carboxypeptidase are present (3).
Another possibility to classify mast cells in humans is founded on the presence in their granules of high levels of tryptase but little or no chymase (MCT) in intestinal and pulmonary mucosa, predominantly found at mucosal sites, resembling rodent MMCs, or mast cells containing chymase, and little or no tryptase (MCC), and finally, mast cells containing tryptase, chymase, and carboxypeptidase (MCTC), resembling rodent CTMCs, predominantly found in the skin, lymph nodes, and lung and gut submucosa (4, 5). In the human small intestine, MCT constitutes ∼98% of all mast cells in the mucosa, while ∼13% of MCs in the submucosa are MCT (6). The protease content of mast cells is strictly correlated with heterogeneous cytokines and receptor expression.
More recently, Tauber et al. (7) using in silico investigation, have analyzed single-cell profiles of mast cells from different organs in mice and humans. In these latter, they identified seven mast cell subsets distributed in 12 organs with different transcriptomic core signatures. MC1 is preferentially localized in the bladder, MC2 in the lung, and MC4, MC6, and MC7 in the skin. MC3 and MC5 are localized in different organs, but not in the skin.
The contribution of mast cells to mucosal barrier control is mediated through the modulation of epithelial function and innate and adaptive immunological responses. The skin, lung, and gastrointestinal tract are potential portals of entry for foreign agents and are involved in the recognition and clearance of microbial pathogens (8), through the modulation of defense functions in these strategically important sites (9, 10). Activation of mast cells in the skin, gastrointestinal tract, and airways mediates the release of several mediators, responsible for different symptoms (Table 1).
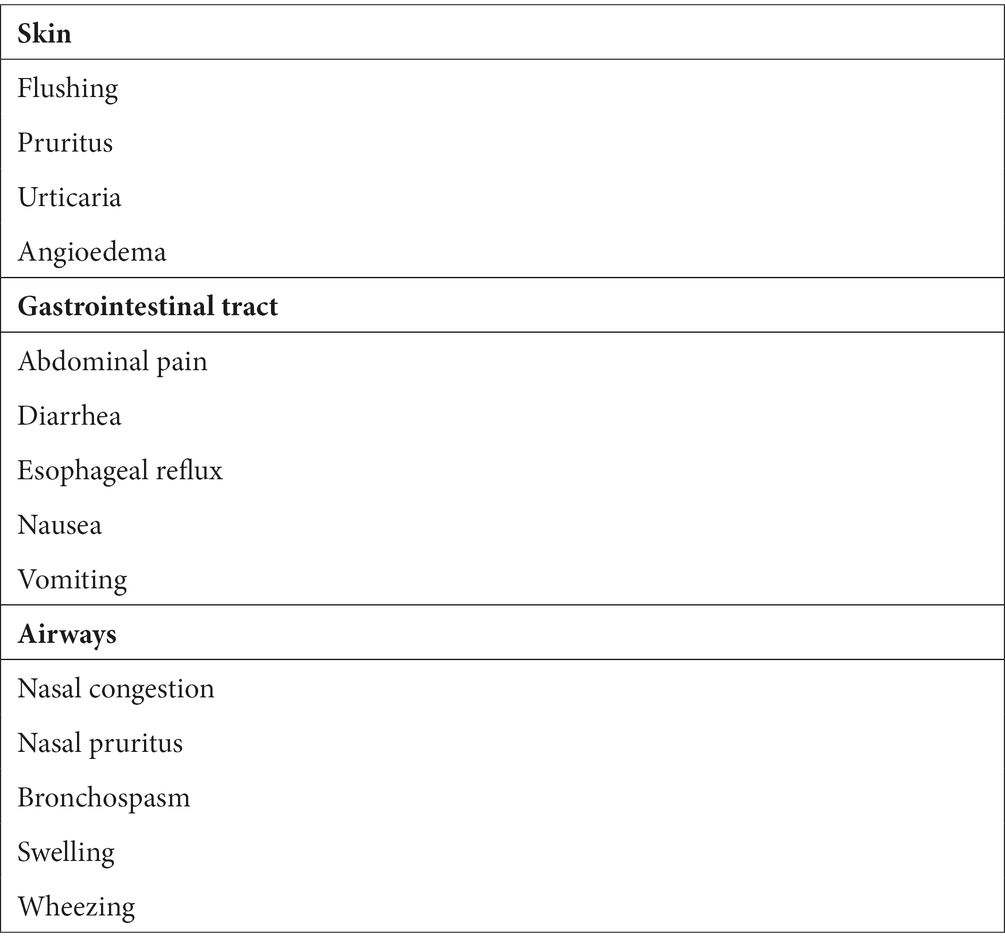
Table 1. Different symptoms induced by the release of mast cell mediators in the skin, gastrointestinal tract, and airways.
Mast cells in the skin
The most important skin function is to protect the host from invasion, using physical barriers and a complex network of resident immune cells, including macrophages, T and B lymphocytes, mast cells, neutrophils, eosinophils, and Langerhans cells, and non-immune cells. Skin mast cells are regulated by the skin microbiota extending from the surface to the dermis and dermal adipose tissue. In germ-free mice, few mast cells are recognizable in the dermis, and the intradermal injection in these mice of staphylococcal-derived lipoteichoic acid is responsible for the induction of the expression of stem cell factor (SCF) in keratinocytes, which induces the rescue of the dermal mast cells (11).
Skin mast cells are formed by three distinct cell populations, derived from the yolk sac and the aorta–gonad–mesonephros–derived hematopoietic stem cells in pre-natal life, and bone marrow in post-natal life (12, 13). SCF is secreted by different cells in the skin, and promotes skin mast cell differentiation (11, 14–16).
The number of skin mast cells involved in immune response and host defense (17) varies between 5,120 and 9,472 per mm3 in physiological conditions, to 260,000/380,000 per mm3 in pathological conditions, such as mastocytosis (18). Moreover, their number is greatest in the skin’s superficial layers as compared with the deep layers (19), and are localized around hair follicles (20), sebaceous and sweat glands, near small blood vessels, and near nerve fibers positive to vasoactive intestinal peptide (VIP), which suppresses mast cell degranulation (21). Small mast cells are found in the subepithelial layer of the skin, and their size and granule content gradually increase in the deep layers.
Skin mast cells are a source of cytokines, chemokines, and growth factors (22, 23). Mast cells induce skin fibroblast proliferation via interleukin (IL)-4 (24, 25), IL-13 (26), vascular endothelial growth factor (VEGF), and basic fibroblast growth factor (bFGF) (27–29). Moreover, mast cells contribute and interact with keratinocytes (30, 31), which, in turn, produce SCF (11, 32). Mast cells synthesize and release keratinocyte growth factor (KGF) (33) and platelet-activating factor (PAF) (31, 34) that activate keratinocytes, and in the meantime, they release histamine, heparin, which inhibit keratinocyte proliferation, controlling epidermal regeneration (35, 36). Finally, tryptase and chymase stimulate fibroblast, inhibit keratinocyte proliferation (37), and regulate epidermal differentiation complex genes, modulating epidermal barrier integrity (38).
Mast cells are involved in many different pathological conditions, including acute and chronic contact hypersensitivity, psoriasis, wound healing, and fibrosis. In acute contact hypersensitivity, mast cell-derived tumor necrosis factor-alpha (TNFα) enhances skin dendritic cell migration to draining lymph nodes, inducing Th-cell expansion in the sensitization phase of contact hypersensitivity (39). In chronic contact hypersensitivity, crosslinked hapten/hapten-specific IgG complexes stimulate mast cells to synthesize IL-10, which, in turn, suppresses the inflammatory reaction (40). In psoriasis, mast cells synthesize TNFα, which promotes dendritic cell migration from the skin to draining lymph nodes (41). In wound healing and fibrosis, mast cells degranulate and release mediators enhances vascular permeability, and recruit inflammatory cells in injured sites. Moreover, mast cell mediators activate fibroblasts to release collagen-promoting fibrosis (42, 43).
Mast cells in the gastrointestinal tract
The intestinal mucosa is lined with enterocytes, separating the lumen from the internal milieu, avoiding the passage of harmful substances, and allowing nutrient and electrolyte absorption. The gut may be considered as a barrier with a defensive function exerted through immune responses, and the acquisition of tolerance against food antigens and the microbiota. The intestinal leakage allows the bacteria to enter the bloodstream, activate pattern recognition receptors, and trigger an immune response (44).
Mast cells are present in all layers of the gastrointestinal tract; their number is higher in the mucosa’s lamina propria of the duodenum and colon, as compared to the submucosa (5, 45). Mast cells are preferentially localized next to nerve terminals of the lamina propria, and are activated by substance P, which stimulates mast cells to release inflammatory mediators, including serotonin and proteases, and pro-inflammatory cytokines.
Intestinal mast cells perform intervene in the regulation of ion and water secretion and permeability, blood flow, coagulation, vascular permeability, wound healing, fibrosis, neuro-immune interactions, peristalsis, pain perception, bacterial, viral, and parasitic infections, and innate and adaptive immunity reactions (4, 46–48). All these activities are exerted by intestinal mast cells in physiological conditions and explain their involvement in many pathological conditions including allergic disorders, gastrointestinal infections, chronic inflammatory diseases, and colon cancer (49).
An increased number of mast cells in inflamed segments of the bowel compared with the non-inflamed segments has been demonstrated in patients with chronic inflammatory bowel disease, including ulcerative colitis and Crohn’s disease. An impaired balance between pro- and anti-inflammatory mediators released by mast cells causes excessive inflammatory reactions and subsequently symptoms such as abdominal cramps, diarrhea, and rectal bleeding (50). Mast cells are involved in the inflammatory process in celiac disease. Intestinal bioptic specimens of patients with celiac disease show a correlation between mast cell density and disease severity (51). Gastrointestinal mastocytosis is characterized by an accumulation of mast cells in the gastrointestinal tissue, which may occur during systemic mastocytosis involving other organs such as bone marrow, liver, and skin. Mast-cell infiltration can result in organ dysfunction in aggressive systemic mastocytosis, and gastrointestinal symptoms, including abdominal pain and diarrhea, are present in 60–80% of cases (52). The term mastocytic enterocolitis was coined to describe an increase in mucosal mast cells in patients with chronic diarrhea (53).
Mast cells in the airways
Each of the MCT and MCTC populations can be further differentiated based on their size, shape, and molecular expression profiles in different lung compartments (54, 55). More than 90% of the mast cells located in bronchi, bronchioles, and alveolar walls are MCT, whereas MCTC is localized in the subepithelial regions and the connective tissue (56). The higher number of mast cells are localized in the alveoli, where they are involved in the homeostatic regulation of blood flow and act as immune modulators and pro-fibrotic cells.
Bronchial MCT expresses more histidine decarboxylase than alveolar MCT. In contrast, in both MCTC and MCT, the high-affinity receptor for IgE (FcεRI) is expressed in conducting airways but absent in alveolar parenchyma (54). IgE-mediated mast cell degranulation is an established hallmark of allergic respiratory conditions, and none of the mast cell effector functions executed in allergy and asthma take place in the alveolar parenchyma.
In addition to the evidence presented for the role of mast cells in the pathophysiology of asthma (57), there is growing evidence that mast cells may also play roles in other diseases of the airways, such as chronic obstructive pulmonary disease (COPD) (58). Mast cell numbers in the lungs of patients with fibrotic disease are increased compared to control subjects and correlate with the severity of fibrosis (59). Patients with idiopathic pulmonary arterial hypertension have marked infiltration of Kit+ mast cells near remodeled pulmonary arteries with a predominant perivascular distribution (60). Initial stages of viral rhinitis are characterized by the high presence of mast cells which, together with other immune cells such as T and B lymphocytes, start the inflammatory cascade in the upper airways (61). Histological examination of the lungs of patients who died from SARS-CoV-2 infection revealed extensive infiltration of mast cells in the interstitium and alveoli (62).
Concluding remarks
Mast cells are involved in the first line of defense against foreign pathogens, producing antimicrobial peptides and releasing different pre-formed and newly synthesized mediators. Mast cells exert protection against systemic or localized bacterial infections in the skin, gastrointestinal tract, and airways by facilitating the clearance of enterobacteria from these tissues.
Mast cells exert this action by engulfing bacteria and secrete inflammatory mediators that, in turn, recruit and activate phagocytes, such as neutrophils through the secretion of TNFα, leukotrienes B4 and C4, and are implicated in the protective response (63). The local increase in mast cell number at the site of infection mediated by the c-kit ligand SCF amplifies mast cell-mediated immune response (64). Mast cells themselves are involved in phagocytosis and the production of nitric oxide, superoxide radicals, and antimicrobial peptides. As concerns viral infection, mast cell number increases during pulmonary viral infections in humans (65), and toll-like receptor 3 expression by human mast cells is implicated in the production of interferon-alpha in response to viral exposure (66).
Author contributions
DR: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by Associazione Italiana contro le Leucemie, Linfomi, e Mieloma (AIL), Bari, Italy.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Irani, AM, and Schwartz, LB. Mast cell heterogeneity. Clin Exp Allergy. (1989) 19:143–55. doi: 10.1111/j.1365-2222.1989.tb02357.x
2. Katz, HR, Stevens, RL, and Austen, KF. Heterogeneity of mammalian mast cells differentiated in vivo and in vitro. J Allergy Clin Immunol. (1985) 76:250–9. doi: 10.1016/0091-6749(85)90638-4
3. Lowman, MA, Rees, PH, Benyon, RC, and Church, MK. Human mast cell heterogeneity: histamine release from mast cells dispersed from skin, lung adenoids, tonsils, and colon in response to IgE-dependent and nonimmunologic stimuli. J Allergy Clin Immunol. (1988) 81:590–7.
4. Bischoff, SC. Role of mast cells in allergic and non-allergic immune responses: comparison of human and murine data. Nat Rev Immunol. (2007) 7:93–104. doi: 10.1038/nri2018
5. Irani, A, Schechter, N, Craig, S, DeBlois, G, and Schwartz, L. Two types of human mast cells that have distinct neutral protease compositions. Proc Natl Acad Sci U S A. (1986) 83:4464–8. doi: 10.1073/pnas.83.12.4464
6. Reber, LL, Sibilano, R, Mukai, K, and Galli, SJ. Potential effector and immunoregulatory functions of mast cells in mucosal immunity. Mucosal Immunol. (2015) 8:444–63. doi: 10.1038/mi.2014.131
7. Tauber, M, Basso, L, Martin, J, Bostan, L, Magalhaes Pinto, M, Thierry, GR, et al. Landscape of mast cell populations across organs in mice and humans. J Exp Med. (2023) 220:e20230570. doi: 10.1084/jem.20230570
8. Jimenez, M, Cervantes-García, D, Córdova-Dávalos, LE, Pérez-Rodríguez, MJ, Gonzalez-Espinosa, C, and Salinas, E. Responses of mast cells to pathogens: beneficial and detrimental roles. Front Immunol. (2021) 12:685865. doi: 10.3389/fimmu.2021.685865
9. Ferguson, A, Cummins, AG, Munro, GH, Gibson, S, and Miller, HR. Role of mucosal mast cells in intestinal cell-mediated immunity. Ann Allergy. (1987) 59:40–3.
10. Fonseca, E, and Solis, J. Mast cells in the skin: progressive systemic sclerosis and the toxic oil syndrome. Ann Int Med. (1985) 102:864–5. doi: 10.7326/0003-4819-102-6-864_2
11. Wang, Z, Mascarenhas, N, Eckmann, L, Miyamoto, Y, Sun, X, Kawakami, T, et al. Skin microbiome promotes mast cell maturation by triggering stem cell factor production in keratinocytes. J Allergy Clin Immunol. (2017) 139:1205–1216.e6. doi: 10.1016/j.jaci.2016.09.019
12. Gentek, R, Ghigo, C, Hoeffel, G, Bulle, MJ, Msallam, R, Gautier, G, et al. Hemogenic endothelial fate mapping reveals dual developmental origin of mast cells. Immunity. (2018) 48:1160–1171.e5. doi: 10.1016/j.immuni.2018.04.025
13. Li, Z, Liu, S, Xu, J, Zhang, X, Han, D, Liu, J, et al. Adult connective tissue-resident mast cells originate from late erythro-myeloid progenitors. Immunity. (2018) 49:640–653.e5. doi: 10.1016/j.immuni.2018.09.023
14. Cicek, F, Cekic, S, and Kilic, SS. Infliximab therapy in an infant with Netherton syndrome. Pediatr Dermatol. (2021) 38:714–6. doi: 10.1111/pde.14590
15. Kambe, N, Kambe, M, Kochan, JP, and Schwartz, LB. Human skin derived mast cells can proliferate while retaining their characteristic functional and protease phenotypes. Blood. (2001) 97:2045–52. doi: 10.1182/blood.V97.7.2045
16. Salducci, M, André, N, Guéré, C, Martin, M, Fitoussi, R, Vié, KM, et al. Factors secreted by irradiated aged fibroblasts induce solar lentigo in pigmented reconstructed epidermis. Pigment Cell Melanoma Res. (2014) 2014:502–4. doi: 10.1111/pcmr.12234
17. Henz, BM, Mauer, M, Lippert, U, Worm, M, and Babina, M. Mast cells as initiators of immunity and host defense. Exp Dermatol. (2001) 10:1–10. doi: 10.1034/j.1600-0625.2001.100101.x
18. Mikhail, GR, and Milinska, A. Mast cell population in human skin. J Invest Dermatol. (1964) 43:249–54. doi: 10.1038/jid.1964.152
19. Weber, A, Knop, J, and Maurer, M. Pattern analysis of human cutaneous mast cell populations by total body surface mapping. Br J Dermatol. (2003) 148:224–8. doi: 10.1046/j.1365-2133.2003.05090.x
20. Ito, N, Sugawara, K, Bodó, E, Takigawa, M, van Beek, N, Ito, T, et al. Corticotropin-releasing hormone stimulates the in situ generation of mast cells from precursors in the human hair follicle mesenchyme. J Invest Dermatol. (2010) 130:995–1004. doi: 10.1038/jid.2009.387
21. Pilkington, SM, Barron, MJ, Watson, REB, Grifths, CEM, and Bulfone-Paus, S. Aged human skin accumulates mast cells with altered functionality that localize to macrophages and vasoactive intestinal peptide-positive nerve fibers. Br J Dermatol. (2019) 180:849–58. doi: 10.1111/bjd.17268
22. Mukai, K, Tsai, M, Saito, H, and Galli, SJ. Mast cells as sources of cytokines, chemokines, and growth factors. Immunol Rev. (2018) 282:121–50. doi: 10.1111/imr.12634
23. Tan, SY, Roediger, B, and Weninger, W. The role of chemokines in cutaneous immunosurveillance. Immunol Cell Biol. (2015) 93:337–46. doi: 10.1038/icb.2015.16
24. Richter, A, Puddicombe, SM, Lordan, JL, Bucchieri, F, Wilson, SJ, Djukanovic, R, et al. The contribution of interleukin (IL)-4 and IL-13 to the epithelial-mesenchymal trophic unit in asthma. Am J Respir Cell Mol Biol. (2001) 25:385–91. doi: 10.1165/ajrcmb.25.3.4437
25. Trautmann, A, Toksoy, A, Engelhardt, E, Bröcker, EB, and Gillitzer, R. Mast cell involvement in normal human skin wound healing: expression of monocyte chemoattractant protein-1 is correlated with recruitment of mast cells which synthesize interleukin-4 in vivo. J Pathol. (2000) 190:100–6. doi: 10.1002/(SICI)1096-9896(200001)190:1<100::AID-PATH496>3.0.CO;2-Q
26. Brown Lobbins, ML, Shivakumar, BR, Postlethwaite, AE, and Hasty, KA. Chronic exposure of interleukin-13 suppress the induction of matrix metalloproteinase-1 by tumour necrosis factor α in normal and scleroderma dermal fibroblasts through protein kinase B/Akt. Clin Exp Immunol. (2018) 191:84–95. doi: 10.1111/cei.13045
27. Komi, DEA, Khomtchouk, K, and Santa Maria, PL. A review of the contribution of mast cells in wound healing: involved molecular and cellular mechanisms. Clin Rev Allergy Immunol. (2020) 58:298–312. doi: 10.1007/s12016-019-08729-w
28. Qu, Z, Huang, X, Ahmadi, P, Stenberg, P, Liebler, JM, Le, AC, et al. Synthesis of basic fibroblast growth factor by murine mast cells. Regulation by transforming growth factor beta, tumor necrosis factor alpha, and stem cell factor. Int Arch Allergy Immunol. (1998) 115:47–54. doi: 10.1159/000023829
29. Tellechea, A, Leal, EC, Kafanas, A, Auster, ME, Kuchibhotla, S, Ostrovsky, Y, et al. Mast cells regulate wound healing in diabetes. Diabetes. (2016) 65:2006–19. doi: 10.2337/db15-0340
30. Nguyen, AV, and Soulika, AM. The dynamics of the skin’s immune system. Int J Mol Sci. (2019) 20:1811. doi: 10.3390/ijms20081811
31. Varricchi, G, Rossi, FW, Galdiero, MR, Granata, F, Criscuolo, G, Spadaro, G, et al. Physiological roles of mast cells: collegium Internationale Allergologicum update 2019. Int Arch Allergy Immunol. (2019) 179:247–61. doi: 10.1159/000500088
32. Cho, KA, Park, M, Kim, YH, and Woo, SY. Th17 cell-mediated immune responses promote mast cell proliferation by triggering stem cell factor in keratinocytes. Biochem Biophys Res Commun. (2017) 487:856–61. doi: 10.1016/j.bbrc.2017.04.141
33. Cho, KA, Kim, HJ, Kim, YH, Park, M, and Woo, SY. Dexamethasone promotes keratinocyte proliferation by triggering keratinocyte growth factor in mast cells. Int Arch Allergy Immunol. (2019) 179:53–61. doi: 10.1159/000494624
34. Feuerherm, AJ, Jørgensen, KM, Sommerfelt, RM, Eidem, LE, Lægreid, A, and Johansen, B. Platelet-activating factor induces proliferation in differentiated keratinocytes. Mol Cell Biochem. (2013) 384:83–94. doi: 10.1007/s11010-013-1784-6
35. Gschwandtner, M, Mildner, M, Mlitz, V, Gruber, F, Eckhart, L, Werfel, T, et al. Histamine suppresses epidermal keratinocyte differentiation and impairs skin barrier function in a human skin model. Allergy. (2013) 68:37–47. doi: 10.1111/all.12051
36. Huttunen, M, Hyttinen, M, Nilsson, G, Butterfield, JH, Horsmanheimo, M, and Harvima, IT. Inhibition of keratinocyte growth in cell culture and whole skin culture by mast cell mediators. Exp Dermatol. (2001) 10:184–92. doi: 10.1034/j.1600-0625.2001.010003184.x
37. Artuc, M, Steckelings, UM, Grützkau, A, Smorodchenko, A, and Henz, BM. A long-term coculture model for the study of mast cell-keratinocyte interactions. J Investig Dermatol. (2002) 119:411–5. doi: 10.1046/j.1523-1747.2002.01838.x
38. Sehra, S, Serezani, APM, Ocaña, JA, Travers, JB, and Kaplan, MH. Mast cells regulate epidermal barrier function and the development of allergic skin inflammation. J Investig Dermatol. (2016) 136:1429–37. doi: 10.1016/j.jid.2016.03.019
39. Gaudenzio, N, Marichal, T, Galli, SJ, and Reber, LL. Genetic and imaging approaches reveal pro-inflammatory and immunoregulatory roles of mast cells in contact hypersensitivity. Front Immunol. (2018) 9:1275. doi: 10.3389/fimmu.2018.01275
40. Grimbaldeston, MA, Nakae, S, Kalesnikoff, J, Tsai, M, and Galli, SJ. Mast cell-derived interleukin 10 limits skin pathology in contact dermatitis and chronic irradiation with ultraviolet B. Nat Immunol. (2007) 8:1095–104. doi: 10.1038/ni1503
41. Heib, V, Becker, M, Warger, T, Rechtsteiner, G, Tertilt, C, Klein, M, et al. Mast cells are crucial for early inflammation, migration of Langerhans cells, and Ctl responses following topical application of Tlr7 ligand in mice. Blood. (2007) 110:946–53. doi: 10.1182/blood-2006-07-036889
42. Levi-Schaffer, F, and Kupietzky, A. Mast cells enhance migration and proliferation of fibroblasts into an in vitro wound. Exp Cell Res. (1990) 188:42–9. doi: 10.1016/0014-4827(90)90275-F
43. Wulff, BC, and Wilgus, TA. Mast cell activity in the healing wound: more than meets the eye? Exp Dermatol. (2013) 22:507–10. doi: 10.1111/exd.12169
44. Levy, M, Kolodziejczyk, AA, Thaiss, CA, and Elinav, E. Dysbiosis and the immune system. Nat Rev Immunol. (2017) 17:219–32. doi: 10.1038/nri.2017.7
45. Bischoff, SC, Wedemeyer, J, Herrmann, A, Meier, PN, Trautwein, C, Cetin, Y, et al. Quantitative assessment of intestinal eosinophils and mast cells in inflammatory bowel disease. Histopathology. (1996) 28:1–13. doi: 10.1046/j.1365-2559.1996.262309.x
46. Alpers, DH, Andrews, PL, and Wood, JD. Fundamental of gastroenterology. Gut. (1999) 45:6–7. doi: 10.1136/gut.45.1.6
47. Bischoff, SC. Physiological and pathophysiological functions of intestinal mast cells. Semin Immunopathol. (2009) 31:185–205. doi: 10.1007/s00281-009-0165-4
48. Crowe, SE, Luthra, G, and Perdue, M. Mast cell mediated ion transport in intestine from patients with and without inflammatory bowel disease. Gut. (1997) 41:785–92. doi: 10.1136/gut.41.6.785
49. Bischoff, SC. Mast cells in gastrointestinal disorders. Eur J Pharmacol. (2016) 778:139–45. doi: 10.1016/j.ejphar.2016.02.018
50. Nishida, Y, Murase, K, Isomoto, H, Furusu, H, Mizuta, Y, Riddell, RH, et al. Different distribution of mast cells and macrophages in colonic mucosa of patients with collagenous colitis and inflammatory bowel disease. Hepatogastroenterology. (2002) 49:678–82.
51. Frossi, B, Tripodo, C, Guarnotta, C, Carroccio, A, De Carli, M, De Carli, S, et al. Mast cells are associated with the onset and progression of celiac disease. J Allergy Clin Immunol. (2017) 139:1266–1274.e1. doi: 10.1016/j.jaci.2016.08.011
52. Sokol, H, Georgin-Lavialle, S, Grandpeix-Guyodo, C, Canioni, D, Barete, S, Dubreuil, P, et al. Gastrointestinal involvement and manifestations in systemic mastocytosis. Inflamm Bowel Dis. (2010) 16:1247–53. doi: 10.1002/ibd.21218
53. Jakate, S, Demeo, M, John, R, Tobin, M, and Keshavarzian, A. Mastocytic enterocolitis: increased mucosal mast cells in chronic intractable diarrhea. Arch Pathol Lab Med. (2006) 130:362–7. doi: 10.5858/2006-130-362-MEIMMC
54. Andersson, CK, Mori, M, Bjermer, L, Löfdahl, CG, and Erjefält, JS. Novel site-specific mast cell subpopulations in the human lung. Thorax. (2009) 64:297–305. doi: 10.1136/thx.2008.101683
55. Bradding, P. Human lung mast cell heterogeneity. Thorax. (2009) 64:278–80. doi: 10.1136/thx.2008.106427
56. Schwartz, LB, Bradford, TR, Irani, AM, DeBlois, G, and Craig, SS. The major enzymes of human mast cell secretory granules. Am Rev Respir Dis. (1987) 135:1186–9.
57. Bradding, P, Walls, AF, and Holgate, ST. The role of the mast cell in the pathophysiology of asthma. J Allergy Clin Immunol. (2006) 117:1277–84. doi: 10.1016/j.jaci.2006.02.039
58. Andersson, CK, Mori, M, Bjermer, L, Lofdahl, CG, and Erjefalt, JS. Alterations in lung mast cell populations in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. (2010) 181:206–17. doi: 10.1164/rccm.200906-0932OC
59. Pesci, A, Bertorelli, G, Gabrielli, M, and Olivieri, D. Mast cells in fibrotic lung disorders. Chest. (1993) 103:989–96. doi: 10.1378/chest.103.4.989
60. Montani, D, Perros, F, Gambaryan, N, Girerd, B, Dorfmuller, P, Price, LC, et al. C-kit-positive cells accumulate in remodeled vessels of idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med. (2011) 184:116–23. doi: 10.1164/rccm.201006-0905OC
61. Piliponsky, AM, Acharya, M, and Shubin, NJ. Mast cells in viral, bacterial, and fungal infection immunity. Int J Mol Sci. (2019) 20:2851. doi: 10.3390/ijms20122851
62. Conti, P, Caraffa, A, Tete, G, Gallenga, CE, Ross, R, Kritas, SK, et al. Mast cells activated by SARS-CoV-2 release histamine which increases IL-1 levels causing cytokine storm and inflammatory reaction in COVID-19. J Biol Regul Homeost Agents. (2020) 34:1629–32. doi: 10.23812/20-2EDIT
63. Marshall, JS. Mast cell responses to pathogens. Nat Rev Immunol. (2004) 4:787–99. doi: 10.1038/nri1460
64. Maurer, M, Echtenacher, B, Hültner, L, Kollias, G, Männel, DN, Langley, KE, et al. The c-kit ligand, stem cell factor, can enhance innate immunity through effects on mast cells. J Exp Med. (1998) 188:2343–8. doi: 10.1084/jem.188.12.2343
65. Casleman, WL, Sorkness, RL, Lemanske, RF Jr, and McAllister, PK. Viral bronchiolitis during early life induces increased numbers of bronchiolar mast cells and airway hyperresponsiveness. Am J Pathol. (1990) 137:821–31.
Keywords: mast cells, gastrointestinal tract, lung, skin, barrier system
Citation: Ribatti D (2024) Mast cells are at the interface between the external environment and the inner organism. Front. Med. 10:1332047. doi: 10.3389/fmed.2023.1332047
Edited by:
Monica Catarina Botelho, Universidade do Porto, PortugalReviewed by:
Satoshi Tanaka, Kyoto Pharmaceutical University, JapanCopyright © 2024 Ribatti. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Domenico Ribatti, ZG9tZW5pY28ucmliYXR0aUB1bmliYS5pdA==
 Domenico Ribatti
Domenico Ribatti