- Department of Gastroenterology, Shengjing Hospital of China Medical University, Shenyang, Liaoning, China
Endoscopic ultrasound (EUS) is an integrated diagnostic technique merging endoscope and ultrasound to examine the digestive system. EUS has emerged as a primary diagnostic method for pancreatic diseases due to its distinctive benefits. Over the past four decades, EUS has undergone a transformation, shifting its role from primarily diagnostic to increasingly therapeutic. Additionally, in recent years, EUS has emerged as an increasingly prominent adjunctive or alternative approach to conventional surgical interventions. This review provides a comprehensive analysis of current technological approaches in the treatment of pancreatic diseases. The dynamic interplay with diverse therapeutic approaches has reinvigorated EUS and shaped its trajectory in the management of pancreatic diseases.
1 Introduction
Endoscopic ultrasound (EUS) (1) is an integrated diagnostic technique merging endoscope and ultrasound to examine the digestive system. It involves a miniature high-frequency ultrasound probe at the distal end of the endoscope. The endoscope enables direct visualization of mucosal lesions in the digestive system. Moreover, the ultrasound facilitates real-time scanning to generate images of the gastrointestinal structures and surrounding extramural structures, thereby augmenting the diagnostic capabilities. EUS presents distinctive advantages when compared to other techniques: it provides close proximity to the tissue, reducing interference from subcutaneous tissue, bones and gas. The utilization of high-resolution, real-time imaging facilitates the identification of minute lesions (2). As a consequence of these unparalleled advantages, EUS has emerged as a primary diagnostic approach for pancreatic lesions. Over the past four decades, EUS has undergone a transformation, shifting its role from primarily diagnostic to increasingly therapeutic. Additionally, in recent years, EUS has emerged as an increasingly prominent adjunctive or alternative approach to conventional surgical interventions. Currently, the therapy for pancreatic diseases comprises: Endoscopic ultrasound-guided drainage of pancreatic fluid collections, Endoscopic ultrasound-guided fine-needle injection (EUS-FNI), Endoscopic ultrasound-guided ablation, Endoscopic ultrasound-guided fiducial implantation and Endoscopic ultrasound-guided brachytherapy, Endoscopic ultrasound-guided celiac plexus block and neurolysis (EUS-CPB/CPN). This review provides a comprehensive interpretation of the current technological approaches, status, and developments in the field.
2 Endoscopic ultrasound-guided drainage of pancreatic fluid collections
Acute or chronic pancreatitis may give rise to the development of fluid collections within the pancreas and/or the peripancreatic area. Pancreatic fluid collections (PFCs) that persist for >4 weeks encompass pseudocysts, characterized by fluid accumulation, and walled-off necrosis (WON), defined by the presence of solid necrotic debris (3). When symptoms such as abdominal pain, nausea, vomiting, and infection occur, intervention is essential. Management modalities comprise EUS-guided drainage, esophagogastroduodenoscopy (EGD) -guided drainage, percutaneous drainage, and surgical intervention (4). EUS-guided drainage has been found to demonstrate comparable or potentially lower mortality rates and adverse events (AEs) compared to alternative approaches, making it the front-line treatment for pseudocysts and WON (5–11).
The stents used for drainage comprise double pigtail plastic stents (DPPS), self-expanding metal stent (SEMS) and lumen-apposing metal stent (LAMS). LAMS, featuring a dumbbell-shaped structure, offers ease of use compared to SEMS, leading to decreased procedure time and lower stent dislodgment (12). Moreover, LAMS provides a larger diameter that facilitates direct endoscopic necrosectomy (13). Considering the aforementioned advantages, LAMS has emerged as the preferred stent choice for endoscopic drainage among a significant number of gastroenterologists (14–16). Although possessing notable advantages, LAMS exhibits certain limitations. In comparison to DPPS, LAMS is linked to an increased susceptibility to bleeding, especially in the form of delayed bleeding manifesting 3–5 weeks post-intervention (17–20). Incorporating color Doppler ultrasound guidance during procedures has the potential to reduce the risk of intraoperative bleeding, however, it does not have any impact on the occurrence of delayed bleeding (21). Two potential explanations exist for the elevated bleeding rate observed in association with LAMS. One explanation postulates that while the lesion diminishes in size during drainage, the LAMS remains in place, causing friction between the stent and adjacent blood vessels, leading to the development of pseudoaneurysms and subsequent hemorrhage (22). Another explanation posits that the broader lumen diameter of LAMS facilitates the entry of low pH gastric acid into the cystic cavity, consequently stimulating the exposed intraluminal blood vessels and heightening the susceptibility to bleeding (23). Consequently, the European Society of Gastrointestinal Endoscopy (ESGE) guideline advocate removing stent within 4 weeks to reduce the risk of complications (24). Nonetheless, several studies have demonstrated that the incidence of AEs associated with LAMS is either unchanged or potentially reduced (25–27). Additionally, Amato et al. (28) demonstrated that a longer removal time did not increase the risk of AEs. Recent randomized controlled trials (RCTs) have not shown the superiority of LAMS over DPPS or an increased bleeding risk for WON (29–31), nor have they demonstrated superiority of LAMS over SEMS for PFCs (32).
The current literature provides evidence that the placement of LAMS with coaxial DPPS for the drainage of pseudocysts results in a noticeably elevated clinical success rate (33), reduced bleeding (34), infection rate (0% vs. 17%) (35), stent occlusion compared to LAMS without DPPS (36). Nevertheless, Shamah et al. (37) exhibit divergent perspectives on the results. They consider that LAMS with or without DPPS does not impact the clinical outcomes, AEs, and reintervention rates associated with PFCs drainage. With respect to the time of drainage, American Society for Gastrointestinal Endoscopy (ASGE) recommends avoiding drainage during the acute phase (< 4 weeks) and advocating for the procedure after the maturation of the cyst wall (≥ 4 weeks) to enhance safety (38). However, a recent meta-analysis revealed that there is no statistical difference in technical and clinical success rates between early drainage (< 4 weeks) and late drainage (39). More RCTs are needed to further compare the safety and efficacy of different stents as well as the time of drainage.
3 Endoscopic ultrasound-guided fine-needle injection (EUS-FNI)
EUS-FNI is a critical component of targeted tumor therapy, providing several benefits including the reduction of systemic AEs and enhancement of intratumoral drug concentrations. It can be employed for the treatment of pancreatic solid tumors and cystic lesions, encompassing specific applications such as chemotherapy, immunotherapy, and gene therapy (40) (Table 1).
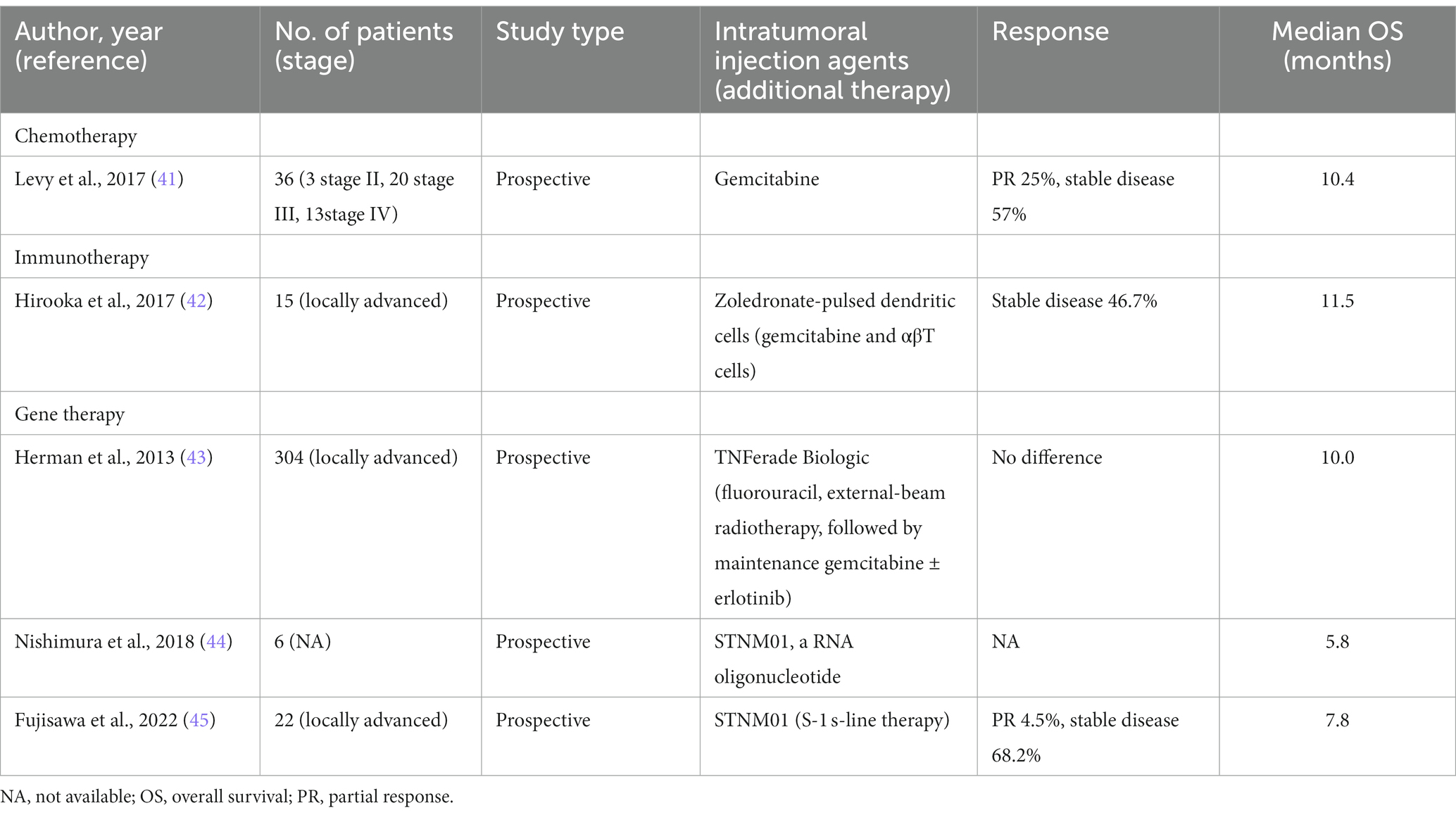
Table 1. Characteristics of recently available studies evaluating EUS-FNI in patients with pancreatic solid tumors.
Chemotherapy involves the application of chemotherapeutic agents such as gemcitabine as well as oncolytic viruses (46). Gemcitabine has been utilized in the treatment of pancreatic cancer for more than two decades. EUS-FNI of gemcitabine has demonstrated favorable feasibility and safety in pancreatic cancer (41). However, its effectiveness in pancreatic cancer is constrained by its rapid clearance. To surmount this impediment, drug nanocarriers have been devised, incorporating active targeting to recognize tumor cells and passive targeting to improve drug penetration and retention at the tumor. This synergistic approach yields heightened anticancer effects and mitigates the emergence of drug resistance resulting from repeated administration (47). Various drug delivery systems, such as hydrogels, microspheres, nanoparticles, micelles, and drug eluting devices, have received considerable attention (48–52). Recently, a nanomedicine delivery system comprising gemcitabine and thermosensitive hydrogels has been developed. The therapeutic efficacy of this system was assessed in a murine model of pancreatic cancer through EUS-FNI. The results indicate that the system enables sustained drug release for 7 days, leading to a significant reduction in pancreatic cancer cell proliferation, invasion, and migration. It promotes cell apoptosis, resulting in a remarkable tumor weight reduction of up to 75.96%. Furthermore, it extends the overall survival (OS)of mice by 14.4 days, with minor AEs and no systemic toxicity based on pathology (53).
Immunotherapy is a therapeutic approach aimed at stimulating immune cells to induce tumor-specific immune responses, thereby indirectly inhibiting and eliminating tumor cells. Immune stimulation of lymphocytes and dendritic cells can be accomplished through EUS-FNI (54, 55). The results of a study assessing the therapeutic efficacy of comprehensive immunotherapy combined with intratumoral injection of zoledronate-pulsed dendritic cells, intravenous adoptive activated T lymphocyte and gemcitabine in unresectable locally advanced pancreatic carcinoma. Fifteen patients are involved, 7 experienced disease stabilization, with a majority demonstrating sustained clinical responses. Notably, patients with disease stability exhibited a significant increase in the CD8+/Treg ratio. The median survival time (MST) and progression-free survival (PFS) for the 15 patients were 12.0 and 5.5 months, respectively. Moreover, this study not only affirms the safety and feasibility of EUS-FNI but also elucidates its potential in inducing immune responses against pancreatic cancer (42).
EUS-FNI gene therapy includes both DNA and RNA (46). STNM01, an artificially synthesized double-stranded RNA oligonucleotide, exhibits selective inhibition of carbohydrate sulfotransferase-15 (CHST-15) expression. Previous studies have implicated an association between CHST-15 and unfavorable prognosis in pancreatic cancer (56). Nishimura et al. (44) conducted a study where they administered 250 nM of STNM01 to a cohort of 6 patients diagnosed with pancreatic cancer. The study observed reductions in tumor size and demonstrated no AEs, leading to an extension in OS. Nonetheless, the MST was observed to be a mere 5.8 months, and a decline in CHST-15 levels was observed in 50% of the patients. In a recent phase I/II clinical trial, a cohort comprising 22 patients diagnosed with pancreatic cancer was prospectively enrolled. Following a cyclical pattern with a biweekly dosing interval, the patients received STNM01 at varying concentrations of 250, 1,000, 2,500, or 10,000 nM. Encouragingly, no AEs were reported, and the study demonstrated a MST of 7.8 months. These significant findings substantiate the safety and feasibility of administration of STNM01 through EUS-FNI as an effective therapeutic modality for pancreatic cancer (45). All approaches provided evidence supporting the safety and efficacy of EUS-FNI as a treatment for pancreatic cancer. Significantly, there have been no RCTs that have shown any advantages of EUS-FNI compared to conventional therapy in the treatment of pancreatic cancer and the final results of a single large-scale RCT comparing the standard of care plus TNFerade Biologic versus standard of care alone, found that no difference between two groups (43). Future prospective research is needed to further compare the differences between various methods.
4 Endoscopic ultrasound-guided ablation
The field of EUS has made significant progress in the direction of intraluminal invasive therapeutic procedures. Notably, EUS-guided ablation has become an essential component in its developmental trajectory. EUS-guided ablation encompasses a wide array of procedures, such as EUS-guided ethanol ablation (EUS-EA), EUS-guided radiofrequency ablation (EUS-RFA), EUS-guided cryothermal ablation (EUS-CTA), EUS-guided photodynamic therapy (EUS-PDT), and EUS-guided laser ablation (EUS-LA). Collectively, these procedures offer a diverse range of therapeutic modalities guided by EUS (57) (Table 2).
The early use of ethanol ablation by GAN et al. (58) and Jürgensen et al. (70) demonstrated the safety and feasibility of treating pancreatic cystic lesions (PCLs) and neuroendocrine tumors (NETs). A propensity score-matching (PSM) study was conducted to investigate the impact of EUS-EA on PCLs. The study included patients with enlarging suspected branch-duct intraductal papillary mucinous neoplasms (BD-IPMNs) or PCLs >3 cm. The findings revealed that EUS-EA was associated with decreased 10-year cumulative incidence rates of BD-IPMNs and a declining trend in surgical resection (SR), whereas its 10-year OS and disease-specific survival (DSS) were comparable to controls (69). In addition, paclitaxel, lipiodol, lauromacrogol, and gemcitabine, either alone or in combination, have been employed for the ablation of PCLs (61, 62, 67, 71). A meta-analysis included 15 studies comprising 524 patients and aimed to evaluate the efficacy of ethanol as a standalone agent compared to its combination with paclitaxel in ablating PCLs. The results demonstrated similar efficiency between the two approaches, yet the latter approach was associated with a higher occurrence of AEs (72). But a recent meta-analysis demonstrated opposite result (73). Due to the unpredictable malignant potential, the management of non-functional NETs measuring <2 cm has stirred controversy. So et al. (68) conducted a PSM study to evaluate the safety and efficacy of EUS-EA and surgery for non-functional NETs measuring <2 cm. The study findings revealed that EUS-EA exhibited a reduced incidence of complications, a shorter duration of hospitalization, and comparable OS and DSS. The results suggest that EUS-EA may serve as a preferred alternative for patients with non-functional small pancreatic NETs (66). It can also be used for pancreatic cancer ablation (74), but it is limited in acquiring pathology for further elucidation of the disease type.
EUS-RFA is a thermal therapy that employs a high-frequency electrical probe, with the purpose of generating and delivering heat to induce coagulative necrosis within the targeted tissue, ultimately resulting in the ablation of the targeted lesion (65). Existing literature suggests that RFA has the potential to generate cellular fragments, facilitate antigen presentation by lymphocytes, stimulate tumor-specific T cells, and activate systemic immunity, thereby enhancing the anti-tumor effect (75). It can be employed for the treatment of pancreatic cancers, PCLs, and NETs. In a meta-analysis, EUS-RFA exhibited technical and clinical success rates of 100 and 91.5%, respectively, for malignant pancreatic tumors and NETs, alongside an AE rate of 15% (76). In a study conducted by Jiang et al. (77) patients with advanced pancreatic cancer were treated by EUS-RFA. One month after the procedure, tumor size reduced and no significant early AEs. However, this study included a limited number of patients. A recent article focused on analyzing the safety of EUS-RFA, revealing an AE rate of 8%, with 1% identified as severe AEs. There was no reported mortality (78). It has been demonstrated that RFA is safe and feasible. A study compared the use of EUS-RFA in combination with systemic chemotherapy versus chemotherapy alone for the treatment of unresectable pancreatic cancer. The findings indicated that the combination therapy resulted in a higher tumor necrosis rate and reduced the need for pain relief. Although there was no statistically significant difference in the average tumor diameter between the two groups before and after treatment, the average tumor diameter increased after treatment in the chemotherapy alone group, suggesting that the combination therapy has an inhibitory effect on tumor growth (79). In terms of functional pancreatic NETs, a PSM study comparing EUS-RFA to surgery revealed a significantly lower rate of AEs in the EUS-RFA group compared to the surgical group (18.0% vs. 61.8%, p < 0.0001), while maintaining a similarly high clinical efficacy (95.5% vs. 100%) (80). Both EUS-EA and RFA can be utilized for ablating NETs and cystic lesions within the pancreas. Garg et al. (81) conducted a comparative analysis of the clinical and technical success rates, as well as AEs, associated with the two techniques for pancreatic NETs, revealing no substantial differences. However, EUS-RFA demonstrates a higher complete response (CR) rate for tumors (82). Conversely, EUS-EA exhibits a higher CR rate but has a greater frequency of AEs in cases of cystic lesions (73). Currently, there is limited evidence to determine which is better, and more research is needed to prove this point. Both methods are progressively emerging as complementary treatments for the multidisciplinary management of pancreatic tumors (83). The widespread application of EUS-RFA in the treatment of pancreatic tumors has been hindered by the occurrence of AEs, including acute pancreatitis, thermal injury to organs, as well as technical limitations.
In contrast to the thermal ablation of EUS-RFA, the utilization of hybrid bipolar cryoprobes has revealed the potential for combining RFA with low-temperature induction, resulting in anti-tumor effects. Petrone et al. (84) were the first to demonstrate the application of this technique, known as EUS-CTA, in a vitro study. Subsequent study has confirmed the safety and efficacy of it in patients with advanced pancreatic cancer (59).
EUS-PDT operates on the principle of employing EUS to precisely deliver photosensitizing drugs, which induces the generation of reactive oxygen species (ROS), consequently promoting cellular apoptosis and augmenting the permeability of tumor tissue (85). Choi et al. (60) in 2015, used EUS-PDT in patients with advanced pancreaticobiliary malignancy, whereby they achieved a median necrotic volume of 4 cm3. DeWitt et al. (64) conducted a prospective study involving 12 patients with advanced pancreatic cancer who received combined treatment of EUS-PDT with paclitaxel or gemcitabine. Out of the cohort, 6 patients had focal tumor necrosis, of whom one patient achieved CR as confirmed by postoperative pathology, no complications were reported. Although limited clinical data support its role as a palliative treatment method for advanced pancreatic cancer, current research indicates that EUS-PDT is a safe and feasible intervention for pancreatic cancer.
Di Matteo et al. (63) pioneered the use of EUS-LA to treat patients with unresectable pancreatic cancer. In their study, they performed EUS-LA on a cohort of 9 patients, utilizing a power range of 2–4 watts and delivering energy in the range of 800–1,200 Joules. The largest observed ablation area, measuring 6.4 cm3, was achieved at the power setting of 4 watts/1000 Joules, among the patients who underwent the procedure. Notably, no AEs occurred during the treatment, affirming the feasibility of this approach. Following this, Lim et al. (86) conducted a study using a 1,064 nm laser system that emitted a laser output of 5 watts in a porcine model of pancreatic cancer. They found that EUS-LA induced acute coagulation necrosis in the targeted pancreatic tissue. The size of the ablation zone showed a linear correlation with the total energy delivered. Initially, the effective ablation area demonstrated an increasing trend with the rise in total energy, eventually reaching saturation at 600 Joules. Nevertheless, additional experimental evidence is necessary to assess the presence of inflammatory reactions in the surrounding normal pancreatic tissue, as this finding would be pivotal in establishing the clinical safety and efficacy of EUS-LA.
5 Endoscopic ultrasound-guided fiducial implantation and endoscopic ultrasound-guided brachytherapy
Radiotherapy is a crucial therapeutic modality for pancreatic cancer, but its high-dose radiation may result in damage to nearby organs. To mitigate this potential harm, markers can be used for localization in pancreatic cancer radiotherapy. These markers, comprised of gold, platinum, or carbon, can be surgically or percutaneously inserted into the target lesion with the assistance of ultrasound, CT, or EUS. Subsequently, these markers can undergo low-dose gamma radiation, X-ray, or beta particle irradiation, resulting in localized tissue injury and tumor ablation (87). EUS is widely regarded as the preferred technique for marker implantation in pancreatic lesions due to its minimal invasiveness and ability to achieve the closest proximity to the pancreas (88–90). A meta-analysis demonstrated an overall success rate of 96.27%, with a particle migration rate of 4.33% and an AE rate of 4.85% for marker implantation guided by EUS (91). The conventional approach to marker implantation entails an initial lesion puncture using a biopsy needle, followed by subsequent marker implantation through the same needle. A study was conducted to compare the utilization of a preloaded 22-gauge (22G) needle with two markers to the conventional approach of placing a single marker using a 19G needle, assessing the technical success rate, clinical success rate, average procedure time, and occurrence of AEs. Similar success rates and AEs rates were observed between the two approaches, but the 22G needle enabled more markers, with 78% of patients receiving four markers, as opposed to only 23% with the 19G needle. These findings provide evidence of the feasibility and safety associated with the utilization of the preloaded 22G needle (92). However, further research is needed to establish the benefits of mortality reduction between two methods.
EUS-guided brachytherapy is the intratumoral administration of radioactive particles. Previously, the radioactive particle used was 125I, but later on, 32P started to be applied in advanced pancreatic cancer (93, 94). In a study involving 23 patients with advanced pancreatic cancer, 32P with gemcitabine exhibited favorable tolerability and feasibility (95). An ongoing study exploring the efficacy of combining 32P, gemcitabine, with or without paclitaxel in advanced pancreatic cancer has demonstrated promising therapeutic outcomes, involving a total of 9 patients, all of whom underwent successful implantation of radioactive particles without any severe AEs (96). Further data is needed, but the study has successfully demonstrated the feasibility of EUS-guided fiducial implantation and its potential for combination with other treatment modalities. Recently, a multicenter prospective study assessed the efficacy of combining 32P with 5-fluorouracil, leucovorin, irinotecan and oxaliplatin (FOLFIRINOX) or gemcitabine/nab-paclitaxel chemotherapy in patients with advanced unresectable pancreatic cancer. The study showed no significant AEs associated with 32P. Importantly, the tumor size, metabolic response, and CA19-9 levels significantly decreased, resulting in a local disease control rate of 90.5% after 16 weeks. Encouragingly, 23.8% of patients achieved a transition from unresectable to potentially operable after 32P treatment, with a MST of 15.5 months, surpassing that of other studies employing percutaneous implantation of 32P (94). However, the absence of a control group in this study necessitates further research to evaluate OS.
6 Endoscopic ultrasound-guided celiac plexus block and neurolysis (EUS-CPB/CPN)
The selection of analgesic therapy for chronic pancreatitis and pancreatic malignancy is a frequent dilemma in clinical practice. Despite the frequently inadequate results of drugs, EUS-CPB and CPN are effective therapeutic options for pain management in patients suffering from chronic pain. The transmission of pancreatic pain occurs through the celiac plexus, which conveys pain signals from the periphery to the central nervous system, leading to pain perception. CPB and CPN are performed using a linear echoendoscope through which a 19 or 22 G needle is passed and used for drug delivery. CPB administrates corticosteroids and long-acting local anesthetics via injection into the celiac plexus. Conversely, CPN typically involves the injection of substances, such as alcohol or phenol, that exert a more prolonged effect (97). For EUS-CPB in chronic pancreatitis, the American Gastroenterological Association (AGA) guideline does not recommend routine implementation. However, for patients with debilitating pain that remains unresponsive to other therapy, individual considerations may be warranted. Nevertheless, CPB should only be made after a thorough discussion of the uncertain outcomes and procedural risks associated with this intervention (98). Currently, CPN primarily includes bilateral approach, central approach, direct ganglion injection, and broad approach (99–101).
The guideline recommends the bilateral approach as the preferred method for CPN, but technical feasibility and operator comfort justify central injection as an acceptable option (strong recommendation, moderate-quality evidence) (102). Comparative studies have shown that both the bilateral and central approaches yield similar outcomes in terms of pain control. However, most research indicates a substantial reduction in opioid dosage with the bilateral approach (103–105). Some studies with conflicting opinions, indicating that the bilateral approach does not reduce the opioid dosage and the central approach exhibits a higher rate of pain relief compared to the bilateral approach (66% vs. 57%) (106, 107). In a prospective study comparing direct ganglion injection with the central approach, it was found that patients receiving direct ganglion injection had a shorter MST of 5.59 months compared to 10.46 months in the central approach, but no significant improvements were observed in pain relief rates, quality of life, or AEs (108). According to a meta-analysis, ganglion injection demonstrates superior pain control in the short term when compared to alternative methods in EUS-CP. However, it may lead to decrease in OS, and the long-term efficacy of pain control remains inconclusive (109). Combining ganglion injection with the central approach demonstrates superior rates of pain relief compared to the central approach alone (101). Furthermore, the broad approach exhibits great pain control compared to the bilateral approach (110), and when combined with ganglion injection, it demonstrates even better pain control (111). Koulouris et al. (100) conducted a systematic review and meta-analysis assessing the efficacy of the first three EUS-CPN techniques in pain control. At the 2-week follow-up, 68% of patients achieved pain control, while at the 4-week follow-up, this percentage decreased to 53%. Although there were no significant differences in success rates among the three methods, the central approach stood out as the only one without severe complications. It may indicate that central approach is the simplest way to operate. However, this review may have significant bias due to the inclusion of low-quality non-randomized studies and the absence of assessment regarding other treatments effects (e.g., opioid medications or chemotherapy).
The optimal timing for EUS-CPN remains unclear. If patients experience effective pain relief with opioid medications, CPN may not be necessary. On the contrary, early combination with CPN may be considered (112). The optimal timing should be based on a comprehensive assessment of the patient’s risks and benefits, expectations for pain control, stages of the disease, and individual clinical circumstances. The effectiveness of EUS-CPN in pain control varies from 50 to 94% in different studies, with a duration of pain relief ranging from 4 to 8 weeks (99). Recently, a tertiary hospital in India demonstrated EUS-CPN with a 100% technical success rate, suggesting that this procedure is easy and effective, rendering it suitable for broad implementation (113).
7 Discussion
EUS provides close proximity to the tissue, reducing interference from subcutaneous tissue, bones and gas. The utilization of high-resolution, real-time imaging facilitates the identification of minute lesions. As a consequence of these unparalleled advantages, EUS has emerged as a primary diagnostic approach for pancreatic lesions (2).
With the continuous development of endoscopic technology and equipment, EUS has transitioned from a diagnostic method to a minimally invasive intervention. In the management of PFCs drainage, plastic stents are progressively being substituted by barbell-shaped metal stents. Presently, a electrocautery-enhanced LAMS (Hot-Axios) has been employed in clinical practice (114). This stent allows stent deployment in a 1-step procedure guided by EUS. This system avoids utilization of additional accessories (such as needles, guidewires, or dilator devices), thereby reducing the overall procedural complexity and minimizing the risk of AEs (115, 116). The effectiveness and safety of it have been demonstrated in PFCs drainage, with technical and clinical success rates both reaching 96% (117). Recently introduced in China, this stent has been employed by Chinese researchers in 15 patients with pseudocysts and 15 patients with walled-off necrosis, resulting in a technical success rate of 100% and a clinical success rate of 93.3%. After one month, the stent was successfully removed in 73.3% of patients. Patients with a history of pancreatitis lasting more than 6 months are more likely to achieve complete resolution of the lesion within one month of undergoing AXIOS treatment (118). Another electrocautery-enhanced LAMS (Hot-Spaxus), has been utilized for draining PFCs. It demonstrates similar clinical and technical success rates as Hot-Axios, but its advantages stem from its prolonged length and reduced risk of bleeding (119). Larghi et al. (120) deployed Hot-Spaxus for PFCs drainage, achieving technical success in all cases without any AEs. However, there have been few comparative studies on this topic, and there is a lack of clear evidence associating other types of LAMS with an increased risk of bleeding. With the advent of new stents, drainage is no longer limited to lesions within 10 mm of the gastric or duodenal wall. LAMS can effectively drain even lesions measuring 10-14 mm, providing a clinical and technical success rate of 97% (121). The debate over the potential risk reduction of AEs, such as bleeding, with the coaxial placement of metal and plastic stents remains unsettled, warranting further prospective studies to provide substantiating evidence (34, 37).
EUS-guided ablation techniques have primarily been utilized in the management of PCLs and neuroendocrine tumors, employing a diverse range of procedural approaches, amidst the progress of precision medicine and translational research. In specific cases of non-functional small pancreatic neuroendocrine tumors, EUS-EA may be preferred over SR (66). Nevertheless, EUS-EA cannot obtain histopathological confirmation of the disease type. Continued advancements are required to optimize needle size, ethanol injection concentration, ablation techniques, and treatment effect, aiming to enhance efficiency while minimizing AEs. Moreover, its combination with chemotherapeutic agents in conjunction with laser ablation or photodynamic therapy has been explored for the management of advanced pancreatic malignancy, despite limited evidence supporting its feasibility (63, 64). The differences in treatment CR and AEs between EUS-EA and EUS-RFA for solid pancreatic tumors and cystic lesions may be associated with the limitedly existing research on cystic lesions, thus necessitating additional literature to provide further substantiation. Lim et al. (86) recently reported that EUS-LA induces acute coagulative necrosis in the targeted tissues of porcine pancreatic cancer. More study is required to verify if the normal pancreatic tissue exhibits inflammatory reactions, crucial in determining the clinical safety of the procedure. Nevertheless, the efficacy of this procedure for pancreatic lesions has already been established. In the management of advanced pancreatic tumors, precise therapies such as EUS-FNI, EUS- guided fiducial implantation, and EUS-guided brachytherapy are progressively being integrated into clinical practice, aiming to reduce radiation and enhance treatment precision. Recent studies have proved the effectiveness of EUS-FNI in alleviating adverse events and exhibiting promising therapeutic outcomes for pancreatic cancer treatment (46). In addition, EUS-guided brachytherapy has demonstrated a disease control rate of over 90% and provides a surgical option for unresectable pancreatic cancer (94). Recent studies have shown the efficacy of a combination therapy in a mouse model of pancreatic cancer, where thermally responsive elastin-like polypeptide (ELP) conjugated with iodine-131 radionuclides (131I-ELP) were delivered through nanoparticle albumin-bound paclitaxel. This intervention induces alterations in the expression of intercellular collagen and adhesion proteins within the tumor microenvironment, leading to complete regression of pancreatic cancer (122). However, pancreatic cancer is a systemic disease, and it is highly improbable that the aforementioned treatments will completely replace current therapies for pancreatic cancer. Nevertheless, considering the safety and feasibility of the mentioned methods, as well as the expansion of translational medicine, they might become viable adjunctive approaches in the future. Further development of these approaches could involve the injection of alternative drugs, combination of brachytherapy and chemotherapy, among other possibilities. They are hopeful for precision treatment of pancreatic cancer patients. It is hoped that future clinical research can increase the proportion of pancreatic cancer patients who can undergo SR or achieve CR.
Effective pain management is crucial for patients with advanced pancreatic tumors and chronic pancreatitis, as it can improve the quality of life for those with cancer and alleviate suffering in individuals with chronic diseases. An ongoing debate persists regarding the optimal approach to achieve pain relief in EUS-CPB/CPN. Current studies suggest that central with ganglion injection could potentially be the most appropriate method (101). According to the AGA guideline, EUS-CPB and CPN are not routinely recommended for pain management in patients with chronic pancreatitis and advanced pancreatic cancer. However, they can serve as adjunctive methods for patients who prefer not to undergo surgery or wish to reduce their use of opioid medications (98). Some researches indicate that the utilization of opioid medications can prolong the MST in pancreatic cancer patients from 4 months to 6 months (123). Conversely, patients who receive CPN exhibit a shorter survival time (4 months) in contrast to those who use opioid medications (7 months) (124). Consequently, the EUS-CPN remains a topic of debate.
In general, the development of EUS has made remarkable strides in the diagnosis and treatment of pancreatic diseases. In recent decades, a variety of EUS-related techniques have undergone continuous updates and evolution, with the incorporation of multiple therapies propelling technological advancements. This dynamic interplay with diverse therapeutic approaches has reinvigorated EUS and shaped its trajectory in the management of pancreatic diseases. The aforementioned treatment approaches have been advancing toward simplicity and minimally invasive procedures, aiming to enhance patients’ quality of life and survival rate, thereby yielding substantial accomplishments. Moving forward, EUS will require endoscopists to attain higher levels of proficiency. Despite the current limited long-term evidence or prognostic data on certain EUS-related techniques, their application in pancreatic diseases is anticipated to gain broader acceptance as they evolve, fostering deeper integration and dissemination of various adjunctive technologies, ultimately resulting in increased patient benefits.
Author contributions
RX: Writing – original draft. KZ: Writing – review & editing. NG: Writing – review & editing. SS: Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The author would like to express her sincere gratitude to the following experts for their invaluable assistance: SS for providing topic guidance and feedback, and NG and KZ for providing valuable suggestions on revisions. Their contributions are greatly appreciated.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Teshima, CW , and Poley, JW . Endoscopic ultrasonography. Endoscopy. (2010) 42:944–9. doi: 10.1055/s-0030-1255795
2. Simons-Linares, CR , Wander, P , Vargo, J , and Chahal, P . Endoscopic ultrasonography: an inside view. Cleve Clin J Med. (2020) 87:175–83. doi: 10.3949/ccjm.87a.19003
3. Banks, PA , Bollen, TL , Dervenis, C , Gooszen, HG , Johnson, CD , Sarr, MG, et al. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. (2013) 62:102–11. doi: 10.1136/gutjnl-2012-302779
4. Thoeni, RF . The revised Atlanta classification of acute pancreatitis: its importance for the radiologist and its effect on treatment. Radiology. (2012) 262:751–64. doi: 10.1148/radiol.11110947
5. van Brunschot, S , Hollemans, RA , Bakker, OJ , Besselink, MG , Baron, TH , Beger, HG, et al. Minimally invasive and endoscopic versus open necrosectomy for necrotising pancreatitis: a pooled analysis of individual data for 1980 patients. Gut. (2018) 67:697–706. doi: 10.1136/gutjnl-2016-313341
6. van Brunschot, S , van Grinsven, J , van Santvoort, HC , Bakker, OJ , Besselink, MG , Boermeester, MA, et al. Endoscopic or surgical step-up approach for infected necrotising pancreatitis: a multicentre randomised trial. Lancet. (2018) 391:51–8. doi: 10.1016/S0140-6736(17)32404-2
7. Rana, SS . An overview of walled-off pancreatic necrosis for clinicians. Expert Rev Gastroenterol Hepatol. (2019) 13:331–43. doi: 10.1080/17474124.2019.1574568
8. Zhao, X , Feng, T , and Ji, W . Endoscopic versus surgical treatment for pancreatic pseudocyst. Dig Endosc. (2016) 28:83–91. doi: 10.1111/den.12542
9. Theerasuwipakorn, N , Tasneem, AA , Kongkam, P , Angsuwatcharakon, P , Ridtitid, W , Navicharern, P, et al. Walled-off Peripancreatic fluid collections in Asian population: paradigm shift from surgical and percutaneous to endoscopic drainage. J Transl Internal Med. (2019) 7:170–7. doi: 10.2478/jtim-2019-0032
10. Varadarajulu, S , Christein, JD , Tamhane, A , Drelichman, ER , and Wilcox, CM . Prospective randomized trial comparing EUS and EGD for transmural drainage of pancreatic pseudocysts (with videos). Gastrointest Endosc. (2008) 68:1102–11. doi: 10.1016/j.gie.2008.04.028
11. Sauer, B , and Kahaleh, M . Prospective randomized trial comparing EUS and EGD for transmural drainage of pancreatic pseudocysts: a need for a large randomized study. Gastrointest Endosc. (2010) 71:432. doi: 10.1016/j.gie.2009.05.026
12. de Paredes, AGG , Martin, JAG , Olcina, JRF , Lucas, DJ , Panizo, FG , Duran, SL, et al. Lumen-apposing metal stents versus biliary fully-covered metal stents for EUS-guided drainage of pancreatic fluid collections: a case control study: meeting presentations: European Society of Gastrointestinal Endoscopy ESGE days 2018. Endosc Int Open. (2020) 8:E6–E12. doi: 10.1055/a-1031-9295
13. Hammad, T , Khan, MA , Alastal, Y , Lee, W , Nawras, A , Ismail, MK, et al. Efficacy and safety of lumen-apposing metal stents in Management of Pancreatic Fluid Collections: are they better than plastic stents? A systematic review and Meta-analysis. Dig Dis Sci. (2018) 63:289–301. doi: 10.1007/s10620-017-4851-0
14. Ali, SE , Benrajab, K , Mardini, H , Su, L , Gabr, M , and Frandah, WM . Anchoring lumen-apposing metal stent with coaxial plastic stent for endoscopic ultrasound-guided drainage of pancreatic fluid collections: any benefit? Ann Gastroenterol. (2019) 32:620–5. doi: 10.20524/aog.2019.0414
15. Saunders, R , Ramesh, J , Cicconi, S , Evans, J , Yip, VS , Raraty, M, et al. A systematic review and meta-analysis of metal versus plastic stents for drainage of pancreatic fluid collections: metal stents are advantageous. Surg Endosc. (2019) 33:1412–25. doi: 10.1007/s00464-018-6416-5
16. Al Lehibi, A , Al Jabri, A , Abbarh, S , Al Balkhi, A , Al Otaibi, N , Almasoudi, T, et al. Peripancreatic fluid collections, plastic stents, and different sub-types of metal stents: where does the evidence land? Saudi J Gastroenterol. (2021) 27:85–90. doi: 10.4103/sjg.SJG_244_20
17. Lang, GD , Fritz, C , Bhat, T , Das, KK , Murad, FM , Early, DS, et al. EUS-guided drainage of peripancreatic fluid collections with lumen-apposing metal stents and plastic double-pigtail stents: comparison of efficacy and adverse event rates. Gastrointest Endosc. (2018) 87:150–7. doi: 10.1016/j.gie.2017.06.029
18. Park, CH , Park, SW , Nam, E , Jung, JH , and Jo, JH . Comparative efficacy of stents in endoscopic ultrasonography-guided peripancreatic fluid collection drainage: a systematic review and network meta-analysis. J Gastroenterol Hepatol. (2020) 35:941–52. doi: 10.1111/jgh.14960
19. Brimhall, B , Han, S , Tatman, PD , Clark, TJ , Wani, S , Brauer, B, et al. Increased incidence of Pseudoaneurysm bleeding with lumen-apposing metal stents compared to double-pigtail plastic stents in patients with Peripancreatic fluid collections. Clin Gastroenterol Hepatol. (2018) 16:1521–8. doi: 10.1016/j.cgh.2018.02.021
20. Ramai, D , Facciorusso, A , DeLuca, M , Barakat, M , and Adler, DG . Adverse events associated with AXIOS stents: insights from the manufacturer and user facility device experience database. Endosc Ultrasound. (2022) 11:231–6. doi: 10.4103/EUS-D-21-00096
21. Donatelli, G , Dumont, JL , Cereatti, F , Randone, B , Meduri, B , Lainas, P, et al. EUS-guided Transrectal evacuation of organized pelvic collection following roux-en-Y gastric bypass after failure of radiological and surgical approach. Obes Surg. (2018) 28:595–6. doi: 10.1007/s11695-017-3031-9
22. Bang, JY , Hasan, M , Navaneethan, U , Hawes, R , and Varadarajulu, S . Lumen-apposing metal stents (LAMS) for pancreatic fluid collection (PFC) drainage: may not be business as usual. Gut. (2017) 66:2054–6. doi: 10.1136/gutjnl-2016-312812
23. Seewald, S , Ang, TL , Richter, H , Teng, KY , Zhong, Y , Groth, S, et al. Long-term results after endoscopic drainage and necrosectomy of symptomatic pancreatic fluid collections. Dig Endosc. (2012) 24:36–41. doi: 10.1111/j.1443-1661.2011.01162.x
24. Arvanitakis, M , Dumonceau, JM , Albert, J , Badaoui, A , Bali, MA , Barthet, M, et al. Endoscopic management of acute necrotizing pancreatitis: European Society of Gastrointestinal Endoscopy (ESGE) evidence-based multidisciplinary guidelines. Endoscopy. (2018) 50:524–46. doi: 10.1055/a-0588-5365
25. Guzmán-Calderón, E , Chacaltana, A , Díaz, R , Li, B , Martinez-Moreno, B , and Aparicio, JR . Head-to-head comparison between endoscopic ultrasound guided lumen apposing metal stent and plastic stents for the treatment of pancreatic fluid collections: a systematic review and meta-analysis. J Hepatobiliary Pancreat Sci. (2022) 29:198–211. doi: 10.1002/jhbp.1008
26. Calo, NC , Bishay, K , Yaghoobi, M , Yuan, Y , Mosko, J , May, G, et al. Comparative effectiveness of lumen-apposing metal stents and plastic stents for the treatment of pancreatic walled-off necrosis: a meta-analysis. J Canadian Assoc Gastroenterol. (2022) 5:68–78. doi: 10.1093/jcag/gwab024
27. Zhou, X , Lin, H , Su, X , Zhang, P , Fu, C , Kong, X, et al. Metal versus plastic stents for pancreatic fluid collection drainage: a systematic review and Meta-analysis. J Clin Gastroenterol. (2021) 55:652–60. doi: 10.1097/MCG.0000000000001539
28. Amato, A , Tarantino, I , Facciorusso, A , Binda, C , Crinò, SF , Fugazza, A, et al. Real-life multicentre study of lumen-apposing metal stent for EUS-guided drainage of pancreatic fluid collections. Gut. (2022) 71:1050–2. doi: 10.1136/gutjnl-2022-326880
29. Valente, R , Zarantonello, L , Del Chiaro, M , Vujasinovic, M , Baldaque Silva, F , Scandavini, CM, et al. Lumen apposing metal stents vs double pigtail plastic stents for the drainage of pancreatic walled-off necrosis. Minerva Gastroenterol. (2022). doi: 10.23736/S2724-5985.22.03055-8
30. Karstensen, JG , Novovic, S , Hansen, EF , Jensen, AB , Jorgensen, HL , Lauritsen, ML, et al. EUS-guided drainage of large walled-off pancreatic necroses using plastic versus lumen-apposing metal stents: a single-Centre randomised controlled trial. Gut. (2022) 72:1167–73. doi: 10.1136/gutjnl-2022-328225
31. Boxhoorn, L , Verdonk, RC , Besselink, MG , Boermeester, M , Bollen, TL , Bouwense, SA, et al. Comparison of lumen-apposing metal stents versus double-pigtail plastic stents for infected necrotising pancreatitis. Gut. (2023) 72:66–72. doi: 10.1136/gutjnl-2021-325632
32. Lera Dos Santos, ME , Proença, IM , de Moura, DTH , Ribeiro, IB , Matuguma, SE , Cheng, S, et al. Self-expandable metal stent (SEMS) versus lumen-apposing metal stent (LAMS) for drainage of pancreatic fluid collections: a randomized clinical trial. Cureus. (2023) 15:e37731. doi: 10.7759/cureus.37731
33. Bang, JY , Wilcox, CM , Arnoletti, JP , Peter, S , Christein, J , Navaneethan, U, et al. Validation of the Orlando protocol for endoscopic management of pancreatic fluid collections in the era of lumen-apposing metal stents. Dig Endosc. (2022) 34:612–21. doi: 10.1111/den.14099
34. Garg, R , Chaar, A , Szpunar, S , Mohan, BP , and Barawi, M . Efficacy and safety of lumen-apposing stents for Management of Pancreatic Fluid Collections in a community hospital setting. Clinical Endoscopy. (2020) 53:480–6. doi: 10.5946/ce.2019.116
35. Aburajab, M , Smith, Z , Khan, A , and Dua, K . Safety and efficacy of lumen-apposing metal stents with and without simultaneous double-pigtail plastic stents for draining pancreatic pseudocyst. Gastrointest Endosc. (2018) 87:1248–55. doi: 10.1016/j.gie.2017.11.033
36. Vanek, P , Falt, P , Vitek, P , Zoundjiekpon, V , Horinkova, M , Zapletalova, J, et al. Endoscopic ultrasound-guided transluminal drainage using lumen-apposing metal stent with or without coaxial plastic stent for treatment of walled-off necrotizing pancreatitis: a prospective bicentric randomized controlled trial. Gastrointest Endosc. (2023) 97:1070–80. doi: 10.1016/j.gie.2022.12.026
37. Shamah, SP , Sahakian, AB , Chapman, CG , Buxbaum, JL , Muniraj, T , Aslanian, HA, et al. Double pigtail stent placement as an adjunct to lumen-apposing metal stentsfor drainage of pancreatic fluid collections may not affect outcomes: a multicenter experience. Endosc Ultrasound. (2022) 11:53–8. doi: 10.4103/EUS-D-21-00030
38. Baron, TH , DiMaio, CJ , Wang, AY , and Morgan, KA . American Gastroenterological Association clinical practice update: management of pancreatic necrosis. Gastroenterology. (2020) 158:67–75.e1. doi: 10.1053/j.gastro.2019.07.064
39. Ramai, D , Enofe, I , Deliwala, SS , Mozell, D , Facciorusso, A , Gkolfakis, P, et al. Early (<4 weeks) versus standard (≥4 weeks) endoscopic drainage of pancreatic walled-off fluid collections: a systematic review and meta-analysis. Gastrointest Endosc. (2023) 97:415–421.e5. doi: 10.1016/j.gie.2022.11.003
40. Larghi, A , Rimbaș, M , Rizzatti, G , Carbone, C , Gasbarrini, A , Costamagna, G, et al. Endoscopic ultrasound-guided therapies for pancreatic solid tumors: an overview. Semin Oncol. (2021) 48:95–105. doi: 10.1053/j.seminoncol.2021.01.004
41. Levy, MJ , Alberts, SR , Bamlet, WR , Burch, PA , Farnell, MB , Gleeson, FC, et al. EUS-guided fine-needle injection of gemcitabine for locally advanced and metastatic pancreatic cancer. Gastrointest Endosc. (2017) 86:161–9. doi: 10.1016/j.gie.2016.11.014
42. Hirooka, Y , Kawashima, H , Ohno, E , Ishikawa, T , Kamigaki, T , Goto, S, et al. Comprehensive immunotherapy combined with intratumoral injection of zoledronate-pulsed dendritic cells, intravenous adoptive activated T lymphocyte and gemcitabine in unresectable locally advanced pancreatic carcinoma: a phase I/II trial. Oncotarget. (2018) 9:2838–47. doi: 10.18632/oncotarget.22974
43. Herman, JM , Wild, AT , Wang, H , Tran, PT , Chang, KJ , Taylor, GE, et al. Randomized phase III multi-institutional study of TNFerade biologic with fluorouracil and radiotherapy for locally advanced pancreatic cancer: final results. J Clin Oncol. (2013) 31:886–94. doi: 10.1200/JCO.2012.44.7516
44. Nishimura, M , Matsukawa, M , Fujii, Y , Matsuda, Y , Arai, T , Ochiai, Y, et al. Effects of EUS-guided intratumoral injection of oligonucleotide STNM01 on tumor growth, histology, and overall survival in patients with unresectable pancreatic cancer. Gastrointest Endosc. (2018) 87:1126–31. doi: 10.1016/j.gie.2017.10.030
45. Fujisawa, T , Tsuchiya, T , Kato, M , Mizuide, M , Takakura, K , Nishimura, M, et al. STNM01, the RNA oligonucleotide targeting carbohydrate sulfotransferase 15, as second-line therapy for chemotherapy-refractory patients with unresectable pancreatic cancer: an open label, phase I/IIa trial. EClinicalMedicine. (2023) 55:101731. doi: 10.1016/j.eclinm.2022.101731
46. Kaur, J , Jaruvongvanich, V , and Chandrasekhara, V . Endoscopic ultrasound-guided injectable therapy for pancreatic cancer: a systematic review. World J Gastroenterol. (2022) 28:2383–95. doi: 10.3748/wjg.v28.i21.2383
47. Akhter, MH , Rizwanullah, M , Ahmad, J , Ahsan, MJ , Mujtaba, MA , and Amin, S . Nanocarriers in advanced drug targeting: setting novel paradigm in cancer therapeutics. Artificial Cells Nanomed Biotechnol. (2018) 46:873–84. doi: 10.1080/21691401.2017.1366333
48. Wei, W , Tang, J , Li, H , Huang, Y , Yin, C , Li, D, et al. Antitumor effects of self-assembling peptide-Emodin in situ hydrogels in vitro and in vivo. Int J Nanomedicine. (2021) 16:47–60. doi: 10.2147/IJN.S282154
49. Jo, YK , and Lee, D . Biopolymer microparticles prepared by microfluidics for biomedical applications. Small. (2020) 16:e1903736. doi: 10.1002/smll.201903736
50. Mitchell, MJ , Billingsley, MM , Haley, RM , Wechsler, ME , Peppas, NA , and Langer, R . Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discov. (2021) 20:101–24. doi: 10.1038/s41573-020-0090-8
51. Chen, Q , Wang, Q , Wang, Y , Chu, Y , Luo, Y , You, H, et al. Penetrating micelle for reversing immunosuppression and drug resistance in pancreatic Cancer treatment. Small. (2022) 18:e2107712. doi: 10.1002/smll.202107712
52. Qian, Y , Liu, Q , Li, P , Han, Y , Zhang, J , Xu, J, et al. Highly tumor-specific and long-acting Iodine-131 microbeads for enhanced treatment of hepatocellular carcinoma with low-dose radio-chemoembolization. ACS Nano. (2021) 15:2933–46. doi: 10.1021/acsnano.0c09122
53. Yang, D , Ning, J , Liao, X , Jiang, H , and Qin, S . Local sustained chemotherapy of pancreatic Cancer using endoscopic ultrasound-guided injection of biodegradable Thermo-sensitive hydrogel. Int J Nanomed. (2023) 18:3989–4005. doi: 10.2147/IJN.S417445
54. Chang, KJ , Nguyen, PT , Thompson, JA , Kurosaki, TT , Casey, LR , Leung, EC, et al. Phase I clinical trial of allogeneic mixed lymphocyte culture (cytoimplant) delivered by endoscopic ultrasound-guided fine-needle injection in patients with advanced pancreatic carcinoma. Cancer. (2000) 88:1325–35. doi: 10.1002/(SICI)1097-0142(20000315)88:6<1325::AID-CNCR8>3.0.CO;2-T
55. Laureano, RS , Sprooten, J , Vanmeerbeerk, I , Borras, DM , Govaerts, J , Naulaerts, S, et al. Trial watch: dendritic cell (DC)-based immunotherapy for cancer. Onco Targets Ther. (2022) 11:2096363. doi: 10.1080/2162402X.2022.2096363
56. Ito, Z , Takakura, K , Suka, M , Kanai, T , Saito, R , Fujioka, S, et al. Prognostic impact of carbohydrate sulfotransferase 15 in patients with pancreatic ductal adenocarcinoma. Oncol Lett. (2017) 13:4799–805. doi: 10.3892/ol.2017.6071
57. Ardeshna, DR , Woods, E , Tsung, A , and Krishna, SG . An update on EUS-guided ablative techniques for pancreatic cystic lesions. Endosc Ultrasound. (2022) 11:432–41. doi: 10.4103/EUS-D-21-00178
58. Gan, SI , Thompson, CC , Lauwers, GY , Bounds, BC , and Brugge, WR . Ethanol lavage of pancreatic cystic lesions: initial pilot study. Gastrointest Endosc. (2005) 61:746–52. doi: 10.1016/S0016-5107(05)00320-2
59. Arcidiacono, PG , Carrara, S , Reni, M , Petrone, MC , Cappio, S , Balzano, G, et al. Feasibility and safety of EUS-guided cryothermal ablation in patients with locally advanced pancreatic cancer. Gastrointest Endosc. (2012) 76:1142–51. doi: 10.1016/j.gie.2012.08.006
60. Choi, JH , Oh, D , Lee, JH , Park, JH , Kim, KP , Lee, SS, et al. Initial human experience of endoscopic ultrasound-guided photodynamic therapy with a novel photosensitizer and a flexible laser-light catheter. Endoscopy. (2015) 47:1035–8. doi: 10.1055/s-0034-1392150
61. Linghu, E , Du, C , Chai, N , Li, H , Wang, Z , Sun, Y, et al. A prospective study on the safety and effectiveness of using lauromacrogol for ablation of pancreatic cystic neoplasms with the aid of EUS. Gastrointest Endosc. (2017) 86:872–80. doi: 10.1016/j.gie.2017.03.1525
62. Choi, JH , Park, DH , Kim, MH , Hwang, HS , Hong, SM , Song, TJ, et al. Outcomes after endoscopic ultrasound-guided ethanol-lipiodol ablation of small pancreatic neuroendocrine tumors. Dig Endosc. (2018) 30:652–8. doi: 10.1111/den.13058
63. Di Matteo, FM , Saccomandi, P , Martino, M , Pandolfi, M , Pizzicannella, M , Balassone, V, et al. Feasibility of EUS-guided Nd:YAG laser ablation of unresectable pancreatic adenocarcinoma. Gastrointest Endosc. (2018) 88:168–174.e1. doi: 10.1016/j.gie.2018.02.007
64. DeWitt, JM , Sandrasegaran, K , O'Neil, B , House, MG , Zyromski, NJ , Sehdev, A, et al. Phase 1 study of EUS-guided photodynamic therapy for locally advanced pancreatic cancer. Gastrointest Endosc. (2019) 89:390–8. doi: 10.1016/j.gie.2018.09.007
65. Bang, JY , Sutton, B , Hawes, RH , and Varadarajulu, S . EUS-guided celiac ganglion radiofrequency ablation versus celiac plexus neurolysis for palliation of pain in pancreatic cancer: a randomized controlled trial (with videos). Gastrointest Endosc. (2019) 89:58–66.e3. doi: 10.1016/j.gie.2018.08.005
66. Matsumoto, K , Kato, H , Kawano, S , Fujiwara, H , Nishida, K , Harada, R, et al. Efficacy and safety of scheduled early endoscopic ultrasonography-guided ethanol reinjection for patients with pancreatic neuroendocrine tumors: prospective pilot study. Dig Endosc. (2020) 32:425–30. doi: 10.1111/den.13552
67. Du, C , Chai, N , Linghu, E , Li, H , Feng, X , Ning, B, et al. Long-term outcomes of EUS-guided lauromacrogol ablation for the treatment of pancreatic cystic neoplasms: 5 years of experience. Endosc Ultrasound. (2022) 11:44–52. doi: 10.4103/EUS-D-20-00231
68. So, H , Ko, SW , Shin, SH , Kim, EH , Son, J , Ha, S, et al. Comparison of EUS-guided ablation and surgical resection for nonfunctioning small pancreatic neuroendocrine tumors: a propensity score-matching study. Gastrointest Endosc. (2023) 97:741–751.e1. doi: 10.1016/j.gie.2022.11.004
69. Song, YJ , Huh, G , Kim, EH , Lee, JB , and Park, DH . Comparison of outcomes of EUS-guided ablation and surveillance only for pancreatic cystic lesions: a propensity score-matching study (with videos). Gastrointest Endosc. (2023) 98:585–596.e3. doi: 10.1016/j.gie.2023.05.049
70. Jürgensen, C , Schuppan, D , Neser, F , Ernstberger, J , Junghans, U , and Stölzel, U . EUS-guided alcohol ablation of an insulinoma. Gastrointest Endosc. (2006) 63:1059–62. doi: 10.1016/j.gie.2005.10.034
71. Du, C , Chai, NL , Linghu, EQ , Li, HK , and Feng, XX . Endoscopic ultrasound-guided injective ablative treatment of pancreatic cystic neoplasms. World J Gastroenterol. (2020) 26:3213–24. doi: 10.3748/wjg.v26.i23.3213
72. Saghir, SM , Dhindsa, BS , Daid, SGS , Naga, Y , Dhaliwal, A , Mashiana, HS, et al. Safety and efficacy of EUS-guided ablation of pancreatic lesions with ethanol versus ethanol with paclitaxel: a systematic review and meta-analysis. Endosc Ultrasound. (2022) 11:371–6. doi: 10.4103/EUS-D-20-00185
73. Papaefthymiou, A , Johnson, GJ , Maida, M , Gkolfakis, P , Ramai, D , Facciorusso, A, et al. Performance and safety of EUS ablation techniques for pancreatic cystic lesions: a systematic review and meta-analysis. Cancers. (2023) 15:2627. doi: 10.3390/cancers15092627
74. Zhang, L , Tan, S , Huang, S , Zhong, C , Lü, M , Peng, Y, et al. The safety and efficacy of endoscopic ultrasound-guided ablation therapy for solid pancreatic tumors: a systematic review. Scand J Gastroenterol. (2020) 55:1121–31. doi: 10.1080/00365521.2020.1797870
75. Faraoni, EY , O'Brien, BJ , Strickland, LN , Osborn, BK , Mota, V , Chaney, J, et al. Radiofrequency ablation remodels the tumor microenvironment and promotes neutrophil-mediated Abscopal immunomodulation in pancreatic Cancer. Cancer Immunol Res. (2023) 11:4–12. doi: 10.1158/2326-6066.CIR-22-0379
76. Dhaliwal, A , Kolli, S , Dhindsa, BS , Choa, J , Mashiana, HS , Ramai, D, et al. Efficacy of EUS-RFA in pancreatic tumors: is it ready for prime time? A systematic review and meta-analysis. Endoscopy Int Open. (2020) 8:E1243–e1251. doi: 10.1055/a-1221-5012
77. Jiang, J , Lou, Q , Yang, J , and Zhang, X . Feasibility and safety of EUS-guided radiofrequency ablation in treatment of locally advanced, unresectable pancreatic cancer. Endosc Ultrasound. (2021) 10:398–9. doi: 10.4103/EUS-D-21-00013
78. Spadaccini, M , Di Leo, M , Iannone, A , von den Hoff, D , Fugazza, A , Galtieri, PA, et al. Endoscopic ultrasound-guided ablation of solid pancreatic lesions: a systematic review of early outcomes with pooled analysis. World J Gastrointest Oncol. (2022) 14:533–42. doi: 10.4251/wjgo.v14.i2.533
79. Kongkam, P , Tiankanon, K , Seo, DW , Luangsukrerk, T , Sriuranpong, V , Nantavithya, C, et al. EUS-guided radiofrequency ablation plus chemotherapy versus chemotherapy alone for pancreatic cancer (ERAP): an observational open-label pilot study. Endosc Ultrasound. (2023) 12:402–8. doi: 10.1097/eus.0000000000000003
80. Crinò, SF , Napoleon, B , Facciorusso, A , Lakhtakia, S , Borbath, I , Caillol, F, et al. Endoscopic ultrasound-guided radiofrequency ablation versus surgical resection for treatment of pancreatic Insulinoma. Clin Gastroenterol Hepatol. (2023) 21:2834–2843.e2. doi: 10.1016/j.cgh.2023.02.022
81. Garg, R , Mohammed, A , Singh, A , Harnegie, MP , Rustagi, T , Stevens, T, et al. EUS-guided radiofrequency and ethanol ablation for pancreatic neuroendocrine tumors: a systematic review and meta-analysis. Endosc Ultrasound. (2022) 11:170–85. doi: 10.4103/EUS-D-21-00044
82. Matsumoto, K , and Kato, H . Endoscopic ablation therapy for the pancreatic neoplasms. Dig Endosc. (2022) 35:430–42. doi: 10.1111/den.14468
83. Lakhtakia, S , and Seo, DW . Endoscopic ultrasonography-guided tumor ablation. Dig Endosc. (2017) 29:486–94. doi: 10.1111/den.12833
84. Petrone, MC , Arcidiacono, PG , Carrara, S , Albarello, L , Enderle, MD , Neugebauer, A, et al. US-guided application of a new hybrid probe in human pancreatic adenocarcinoma: an ex vivo study. Gastrointest Endosc. (2010) 71:1294–7. doi: 10.1016/j.gie.2010.02.014
85. Carigga Gutierrez, NM , Pujol-Solé, N , Arifi, Q , Coll, JL , le Clainche, T , and Broekgaarden, M . Increasing cancer permeability by photodynamic priming: from microenvironment to mechanotransduction signaling. Cancer Metastasis Rev. (2022) 41:899–934. doi: 10.1007/s10555-022-10064-0
86. Lim, S , Truong, VG , Choi, J , Jeong, HJ , Oh, SJ , Park, JS, et al. Endoscopic ultrasound-guided laser ablation using a diffusing applicator for locally advanced pancreatic cancer treatment. Cancers. (2022) 14:2274. doi: 10.3390/cancers14092274
87. Kim, SH , and Shin, EJ . Endoscopic ultrasound-guided fiducial placement for stereotactic body radiation therapy in pancreatic malignancy. Clin Endoscopy. (2021) 54:314–23. doi: 10.5946/ce.2021.102
88. Coronel, E , Singh, BS , Cazacu, IM , Moningi, S , Romero, L , Taniguchi, C, et al. EUS-guided placement of fiducial markers for the treatment of pancreatic cancer. VideoGIE. (2019) 4:403–6. doi: 10.1016/j.vgie.2019.05.007
89. Ashida, R , Fukutake, N , Takada, R , Ioka, T , Ohkawa, K , Katayama, K, et al. Endoscopic ultrasound-guided fiducial marker placement for neoadjuvant chemoradiation therapy for resectable pancreatic cancer. World J Gastrointest Oncol. (2020) 12:768–81. doi: 10.4251/wjgo.v12.i7.768
90. Staudenmann, D , Mudaliar, S , Kaffes, AJ , and Saxena, P . Lumen-apposing metal stents salvage that accidentally dislodged during a necrosectomy of a WON (with video). Endosc Ultrasound. (2022) 11:147–8. doi: 10.4103/EUS-D-21-00054
91. Patel, JB , Revanur, V , Forcione, DG , Bechtold, ML , and Puli, SR . Endoscopic ultrasound-guided fiducial marker placement in pancreatic cancer: a systematic review and meta-analysis. World J Gastrointest Endoscopy. (2020) 12:231–40. doi: 10.4253/wjge.v12.i8.231
92. Glissen Brown, JR , Perumpail, RB , Duran, JF , Bharadwaj, S , Baran, B , Becq, A, et al. Preloaded 22-gauge fine-needle system facilitates placement of a higher number of fiducials for image-guided radiation therapy compared with traditional backloaded 19-gauge approach. Gastrointest Endosc. (2021) 94:953–8. doi: 10.1016/j.gie.2021.05.035
93. Sun, X , Lu, Z , Wu, Y , Min, M , Bi, Y , Shen, W, et al. An endoscopic ultrasonography-guided interstitial brachytherapy based special treatment-planning system for unresectable pancreatic cancer. Oncotarget. (2017) 8:79099–110. doi: 10.18632/oncotarget.15763
94. Ross, PJ , Wasan, HS , Croagh, D , Nikfarjam, M , Nguyen, N , Aghmesheh, M, et al. Results of a single-arm pilot study of (32)P microparticles in unresectable locally advanced pancreatic adenocarcinoma with gemcitabine/nab-paclitaxel or FOLFIRINOX chemotherapy. ESMO Open. (2022) 7:100356. doi: 10.1016/j.esmoop.2021.100356
95. Bhutani, MS , Cazacu, IM , Luzuriaga Chavez, AA , Singh, BS , Wong, FCL , Erwin, WD, et al. Novel EUS-guided brachytherapy treatment of pancreatic cancer with phosphorus-32 microparticles: first United States experience. VideoGIE. (2019) 4:223–5. doi: 10.1016/j.vgie.2019.02.009
96. Bhutani, MS , Klapman, JB , Tuli, R , El-Haddad, G , Hoffe, S , Wong, FCL, et al. An open-label, single-arm pilot study of EUS-guided brachytherapy with phosphorus-32 microparticles in combination with gemcitabine +/− nab-paclitaxel in unresectable locally advanced pancreatic cancer (OncoPaC-1): technical details and study protocol. Endosc Ultrasound. (2020) 9:24–30. doi: 10.4103/eus.eus_44_19
97. Sachdev, AH , and Gress, FG . Celiac plexus block and Neurolysis: a review. Gastrointest Endosc Clin N Am. (2018) 28:579–86. doi: 10.1016/j.giec.2018.06.004
98. Strand, DS , Law, RJ , Yang, D , and Elmunzer, BJ . AGA clinical practice update on the endoscopic approach to recurrent acute and chronic pancreatitis: expert review. Gastroenterology. (2022) 163:1107–14. doi: 10.1053/j.gastro.2022.07.079
99. Pérez-Aguado, G , de la Mata, DM , Valenciano, CM , and Sainz, IF . Endoscopic ultrasonography-guided celiac plexus neurolysis in patients with unresectable pancreatic cancer: an update. World J Gastrointest Endosc. (2021) 13:460–72. doi: 10.4253/wjge.v13.i10.460
100. Koulouris, AI , Alexandre, L , Hart, AR , and Clark, A . Endoscopic ultrasound-guided celiac plexus neurolysis (EUS-CPN) technique and analgesic efficacy in patients with pancreatic cancer: a systematic review and meta-analysis. Pancreatology. (2021) 21:434–42. doi: 10.1016/j.pan.2020.12.016
101. Kamata, K , Kinoshita, M , Kinoshita, I , Imai, H , Ogura, T , Matsumoto, H, et al. Efficacy of EUS-guided celiac plexus neurolysis in combination with EUS-guided celiac ganglia neurolysis for pancreatic cancer-associated pain: a multicenter prospective trial. Int J Clin Oncol. (2022) 27:1196–201. doi: 10.1007/s10147-022-02160-6
102. Wyse, JM , Battat, R , Sun, S , Saftoiu, A , Siddiqui, AA , Leong, AT, et al. Practice guidelines for endoscopic ultrasound-guided celiac plexus neurolysis. Endosc Ultrasound. (2017) 6:369–75. doi: 10.4103/eus.eus_97_17
103. Lu, F , Dong, J , Tang, Y , Huang, H , Liu, H , Song, L, et al. Bilateral vs. unilateral endoscopic ultrasound-guided celiac plexus neurolysis for abdominal pain management in patients with pancreatic malignancy: a systematic review and meta-analysis. Supportive Care Cancer. (2018) 26:353–9. doi: 10.1007/s00520-017-3888-0
104. LeBlanc, JK , Al-Haddad, M , McHenry, L , Sherman, S , Juan, M , McGreevy, K, et al. A prospective, randomized study of EUS-guided celiac plexus neurolysis for pancreatic cancer: one injection or two? Gastrointest Endosc. (2011) 74:1300–7. doi: 10.1016/j.gie.2011.07.073
105. Arcidiacono, PG , Calori, G , Carrara, S , McNicol, ED , and Testoni, PA . Celiac plexus block for pancreatic cancer pain in adults. Cochrane Database Syst Rev. (2011) 2019:Cd007519. doi: 10.1002/14651858.CD007519.pub2
106. Kanno, Y , Koshita, S , Masu, K , Ogawa, T , Kusunose, H , Murabayashi, T, et al. Efficacy of EUS-guided celiac plexus neurolysis compared with medication alone for unresectable pancreatic cancer in the oxycodone/fentanyl era: a prospective randomized control study. Gastrointest Endosc. (2020) 92:120–30. doi: 10.1016/j.gie.2020.01.011
107. Asif, AA , Walayat, SK , Bechtold, ML , Revanur, V , and Puli, SR . EUS-guided celiac plexus neurolysis for pain in pancreatic cancer patients – a meta-analysis and systematic review. J Commun Hospital Internal Med Persp. (2021) 11:536–42. doi: 10.1080/20009666.2021.1929049
108. Levy, MJ , Gleeson, FC , Topazian, MD , Fujii-Lau, LL , Enders, FT , Larson, JJ, et al. Combined celiac ganglia and plexus Neurolysis shortens survival, without benefit, vs plexus Neurolysis alone. Clin Gastroenterol Hepatol. (2019) 17:728–738.e9. doi: 10.1016/j.cgh.2018.08.040
109. Li, M , Wang, Z , Chen, Y , Wu, Z , Huang, X , Wu, C, et al. EUS-CGN versus EUS-CPN in pancreatic cancer: a qualitative systematic review. Medicine. (2021) 100:e27103. doi: 10.1097/MD.0000000000027103
110. Sakamoto, H , Kitano, M , Kamata, K , Komaki, T , Imai, H , Chikugo, T, et al. EUS-guided broad plexus neurolysis over the superior mesenteric artery using a 25-gauge needle. Am J Gastroenterol. (2010) 105:2599–606. doi: 10.1038/ajg.2010.339
111. Minaga, K , Kitano, M , Sakamoto, H , Miyata, T , Imai, H , Yamao, K, et al. Predictors of pain response in patients undergoing endoscopic ultrasound-guided neurolysis for abdominal pain caused by pancreatic cancer. Ther Adv Gastroenterol. (2016) 9:483–94. doi: 10.1177/1756283X16644248
112. Mercadante, S . Commentary: interpreting data of celiac plexus block in patients with pancreatic pain: timing, patients. Survival Pain Ther. (2022) 11:747–51. doi: 10.1007/s40122-022-00396-8
113. Maulahela, H , Annisa, NG , Fauzi, A , Renaldi, K , Abdullah, M , Simadibrata, M, et al. Role of interventional endoscopic ultrasound in a developing country. Clin Endosc. (2023) 56:100–6. doi: 10.5946/ce.2022.058
114. Rana, SS , Bush, N , and Gupta, R . Cystotome or nonelectrocautery dilating catheters for fistula tract dilatation during endoscopic transmural drainage of pancreatic fluid collections. Endosc Ultrasound. (2021) 10:483–4. doi: 10.4103/EUS-D-21-00097
115. Rinninella, E , Kunda, R , Dollhopf, M , Sanchez-Yague, A , Will, U , Tarantino, I, et al. EUS-guided drainage of pancreatic fluid collections using a novel lumen-apposing metal stent on an electrocautery-enhanced delivery system: a large retrospective study (with video). Gastrointest Endosc. (2015) 82:1039–46. doi: 10.1016/j.gie.2015.04.006
116. Siddiqui, AA , Adler, DG , Nieto, J , Shah, JN , Binmoeller, KF , Kane, S, et al. EUS-guided drainage of peripancreatic fluid collections and necrosis by using a novel lumen-apposing stent: a large retrospective, multicenter U.S. experience (with videos). Gastrointest Endosc. (2016) 83:699–707. doi: 10.1016/j.gie.2015.10.020
117. Schawkat, K , Luo, M , Lee, K , Beker, K , Meir, M , Berzin, TM, et al. Lumen-apposing covered self-expanding metallic stent for symptomatic pancreatic fluid collections: assessment of outcomes and complications with CT and MRI. Abdominal Radiol. (2021) 46:757–67. doi: 10.1007/s00261-020-02638-9
118. Li, P , Zhang, Z , Wang, S , Jin, Z , Du, Y , Yang, A, et al. A Chinese prospective multicenter cohort study evaluating EUS-guided drainage of pancreatic fluid collections using the hot AXIOS system. Endosc Ultrasound. (2023) 12:259–65. doi: 10.4103/EUS-D-22-00058
119. Mangiavillano, B , Moon, JH , Crinò, SF , Larghi, A , Pham, KD , Teoh, AYB, et al. Safety and efficacy of a novel electrocautery-enhanced lumen-apposing metal stent in interventional EUS procedures (with video). Gastrointest Endosc. (2022) 95:115–22. doi: 10.1016/j.gie.2021.07.021
120. Larghi, A , Crinò, SF , Vanella, G , Rizzatti, G , Bernardoni, L , and Arcidiacono, PG . Preliminary experience of EUS-guided pancreatic fluid collections drainage using a new lumen-apposing metal stent mounted on a cautery device. Endosc Ultrasound. (2022) 11:84–5. doi: 10.4103/EUS-D-21-00033
121. Zhang, LY , Kunda, R , Aerts, M , Messaoudi, N , Pawa, R , Pawa, S, et al. Novel 15-mm-long lumen-apposing metal stent for endoscopic ultrasound-guided drainage of pancreatic fluid collections located ≥10 mm from the luminal wall. Endoscopy. (2022) 54:706–11. doi: 10.1055/a-1682-7095
122. Schaal, JL , Bhattacharyya, J , Brownstein, J , Strickland, KC , Kelly, G , Saha, S, et al. Brachytherapy via a depot of biopolymer-bound (131)I synergizes with nanoparticle paclitaxel in therapy-resistant pancreatic tumours. Nature Biomed Eng. (2022) 6:1148–66. doi: 10.1038/s41551-022-00949-4
123. Zylberberg, HM , Woodrell, C , Rustgi, SD , Aronson, A , Kessel, E , Amin, S, et al. Opioid prescription is associated with increased survival in older adult patients with pancreatic cancer in the United States: a propensity score analysis. JCO Oncol Practice. (2022) 18:e659–68. doi: 10.1200/OP.21.00488
124. Zylberberg, HM , Nagula, S , Rustgi, SD , Aronson, A , Kessel, E , Kumta, NA, et al. Celiac plexus neurolysis is associated with decreased survival in patients with pancreatic cancer: a propensity score analysis. Pancreas. (2022) 51:153–8. doi: 10.1097/MPA.0000000000001992
Glossary
Keywords: EUS, pancreatic diseases, treatment, drainage, ablation, celiac plexus neurolysis
Citation: Xu R, Zhang K, Ge N and Sun S (2024) EUS-guided interventional therapies for pancreatic diseases. Front. Med. 10:1329676. doi: 10.3389/fmed.2023.1329676
Edited by:
Stefano Francesco Crinò, University of Verona, ItalyReviewed by:
Andrea Anderloni, San Matteo Hospital Foundation (IRCCS), ItalyYousuke Nakai, The University of Tokyo, Japan
Sabrina Gloria Giulia Testoni, San Raffaele Hospital (IRCCS), Italy
Copyright © 2024 Xu, Zhang, Ge and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Siyu Sun, c3VuLXNpeXVAMTYzLmNvbQ==
 Rongmin Xu
Rongmin Xu Kai Zhang
Kai Zhang Siyu Sun
Siyu Sun