- 1Department of Gynecology, Xishan People's Hospital of Wuxi City, Wuxi, China
- 2Department of Gynecological Oncology, Wuxi Maternal and Child Health Hospital, Wuxi, China
Background and aims: The residual lesions after Loop Electrosurgical Excision Procedure (LEEP) contributes to poor prognosis in patients with Cervical Intraepithelial Neoplasia Grade 3 (CIN3). The aim of this study is to establish an effective clinical predictive model for residual lesions in CIN3 patients after LEEP.
Methods: A retrospective analysis was performed on 436 CIN3 patients who underwent total hysterectomy within 3 months after LEEP. Based on the post-hysterectomy pathologic, the patients were divided into the no residual group and residual group. Clinical parameters were compared between the two groups, and univariate and multivariate logistic regression analyses were conducted to identify independent risk factors for residual lesions in CIN3 patients after LEEP. Using R software, a nomogram model was established and its effectiveness was evaluated using calibration plots.
Results: There were 178 cases in the residual group and 258 cases in the no residual group. The two groups had no significant difference in general characteristics (p > 0.05). It was found that Post-LEEP follow-up HPV, Post-LEEP follow-up TCT, and the Gland involvement were independent risk factors for residual lesions in CIN3 patients after LEEP (all p < 0.05). The consistency index (C-index) of the nomogram model for predicting residual lesions was 0.975 (0.962–0.988).
Conclusion: The Post-LEEP follow-up HPV, Post-LEEP follow-up TCT, and Gland involvement are independent risk factors related to residual tissue after LEEP surgery in CIN3 patients. The constructed nomogram can effectively predict the presence of residual tissue after LEEP surgery in CIN3 patients and has good practical value.
1 Introduction
Cervical cancer has now become one of the most common malignant tumors among women worldwide. Its incidence and mortality rate rank fourth, accounting for 6.5 and 7.7%, respectively (1). Cervical intraepithelial neoplasia (CIN) is a precursor lesion of cervical cancer. Interventions and treatments at this stage can effectively reduce the incidence of cervical cancer (2). Currently, the preferred treatment option for CIN is LEEP which offers advantages such as minimal invasiveness, simplicity in operation, and the ability to preserve fertility (3). However, residual lesions after LEEP surgery pose a challenging clinical problem. Recent studies have shown that the residual rate of CIN after LEEP surgery remains as high as 18.2–31.1% (4, 5). Currently, there are no effective indicators available for predicting residual lesions after LEEP surgery (6). Building an effective predictive evaluation model can offer valuable guidance for the treatment of patients with CIN after LEEP surgery (7). Therefore, it would be beneficial for the clinical treatment of CIN and improve patients’ prognosis to investigate the independent risk factors for residual CIN after LEEP surgery and establish an effective clinical predictive model providing more intuitive and personalized prediction results. For this purpose, this study utilized total hysterectomy specimens to analyze the independent risk factors for residual lesions after LEEP surgery in CIN3 patients and established an effective nomogram model. The results are reported as follows.
2 Materials and methods
2.1 Study design
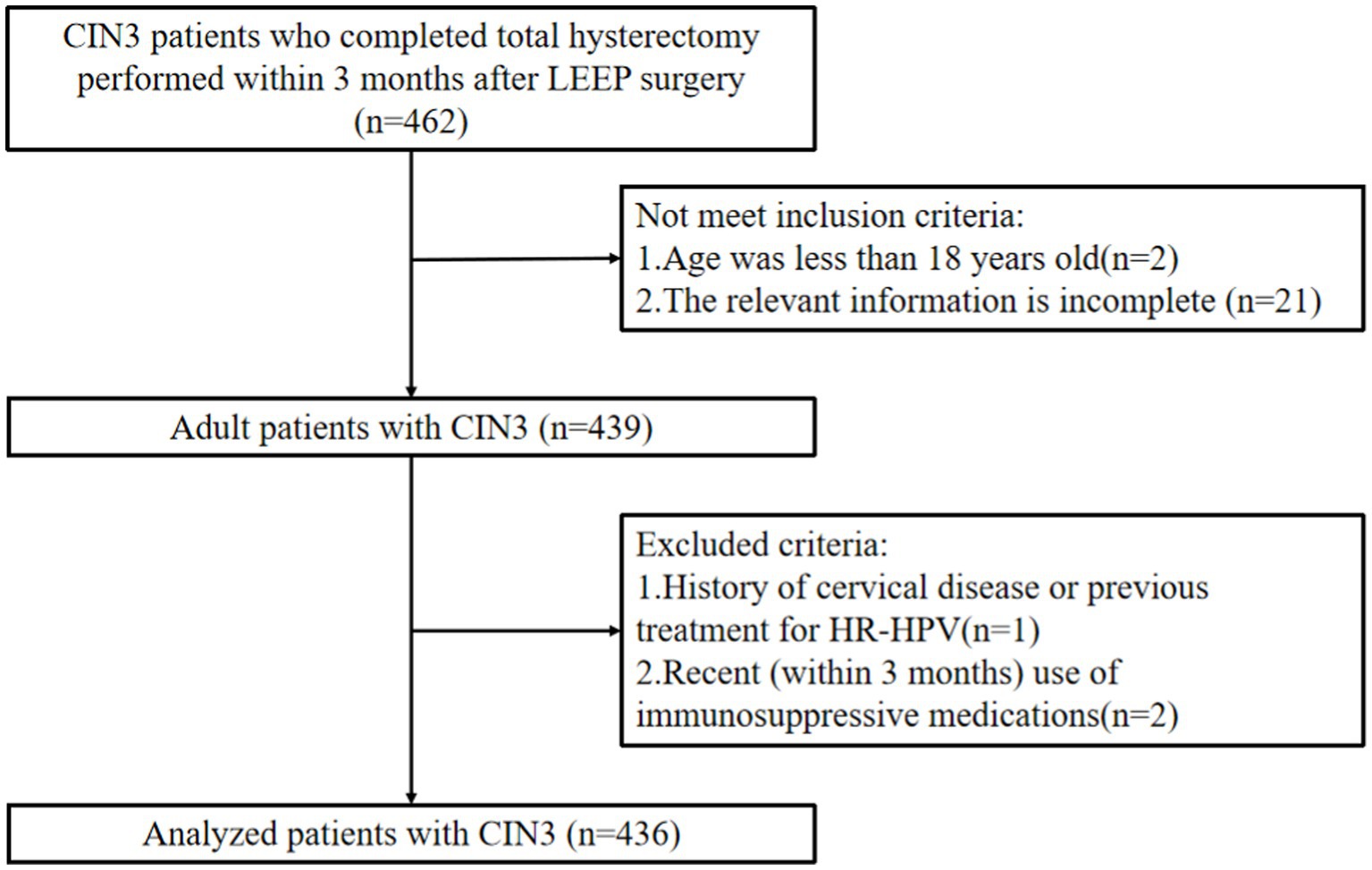
The retrospective analysis was performed on 436 CIN3 patients who underwent total hysterectomy within 3 months after LEEP at Wuxi Xishan People’s Hospital and Wuxi Maternal and Child Health Hospital from January 2009 to December 2021 (see Figure 1). Inclusion criteria were as follows: (1) Patients with CIN3 who underwent LEEP surgery followed by total hysterectomy within 3 months. (2) Smooth surgical process without significant intraoperative or postoperative complications. (3) Complete relevant data available. (4) Age > 18 years. (5) Willing to participate in long-term follow-up. Exclusion criteria were as follows: (1) Pregnant or breastfeeding women. (2) History of cervical diseases or previous treatment for high-risk HPV. (3) Recent use of immunosuppressive drugs within the past 3 months. This study has obtained approval from the Independent Ethics Committee for Clinical Research of Xishan People’s Hospital (Ethics Approval Number: xs2021ky005), and informed consent has been obtained from all patients.
2.2 Data collection
After the patient is admitted, relevant examinations should be completed and the patient’s pre-and post-LEEP treatment hospitalization information, as well as general information, should be collected. This includes testing for HR-HPV and ThinPrep Cytology Test (TCT). TCT is divided into the following categories: A typical squamous cells of undetermined significance (ASC-US), Atypical squamous cells, cannot exclude high-grade squamous intraepithelial lesion (ASC-H), Low-grade squamous intraepithelial lesion (LSIL), High-grade squamous intraepithelial lesion (HSIL), No evidence of lesion or malignancy (NILM). For all patients who undergo hysterectomy, a pathological examination of the uterus should be performed and sent for assessment. The diagnosis of residual CIN is confirmed based on the pathological histology after hysterectomy.
2.3 Nomogram for individualized prediction
We used the bidirectional stepwise selection method based on Akaike’s information criteria to perform multivariable logistic regression analysis on the included CIN3 patients to identify independent risk factors for residual lesions after LEEP procedure. Variables with a p-value < 0.05 were defined as independent predictive factors. A random forest plot was created to display the accuracy and importance of the predictive variables, and a nomogram was constructed for individualized prediction of residual lesions after LEEP procedure. To validate the nomogram, the area under the curve (AUC) was calculated (with 1,000 bootstrap resamples) to evaluate discrimination, and calibration curve analysis (with 1,000 bootstrap resamples) was conducted to assess calibration. The calibration was statistically evaluated using the Hosmer-Lemeshow test. To assess the clinical utility of the line chart, decision curve analysis was performed to calculate the standardized net benefit at different threshold probabilities, using post-hysterectomy pathological results as the comparator for the line chart model.
2.4 Statistical methods
The data processing will be carried out using SPSS 20.0 statistical software. For continuous variables, they will be represented by mean ± standard deviation or median (interquartile range, IQR), depending on whether the data follows a normal distribution. To compare the means or medians between different groups, t-tests or Mann–Whitney U tests will be used. For categorical variables, frequencies and percentages will be provided, and Pearson’s chi-square test will be used for group comparisons. The results will be presented as odds ratios (ORs) with 95% confidence intervals (CIs). Logistic regression analysis will be used to analyze independent risk factors for Postoperative residual lesion after LEEP, considering differences with a statistical significance of p < 0.05. R (x64 for Windows, version 3.6.1) will be used to establish a nomogram model.
3 Results
3.1 Baseline data characteristics of the patient
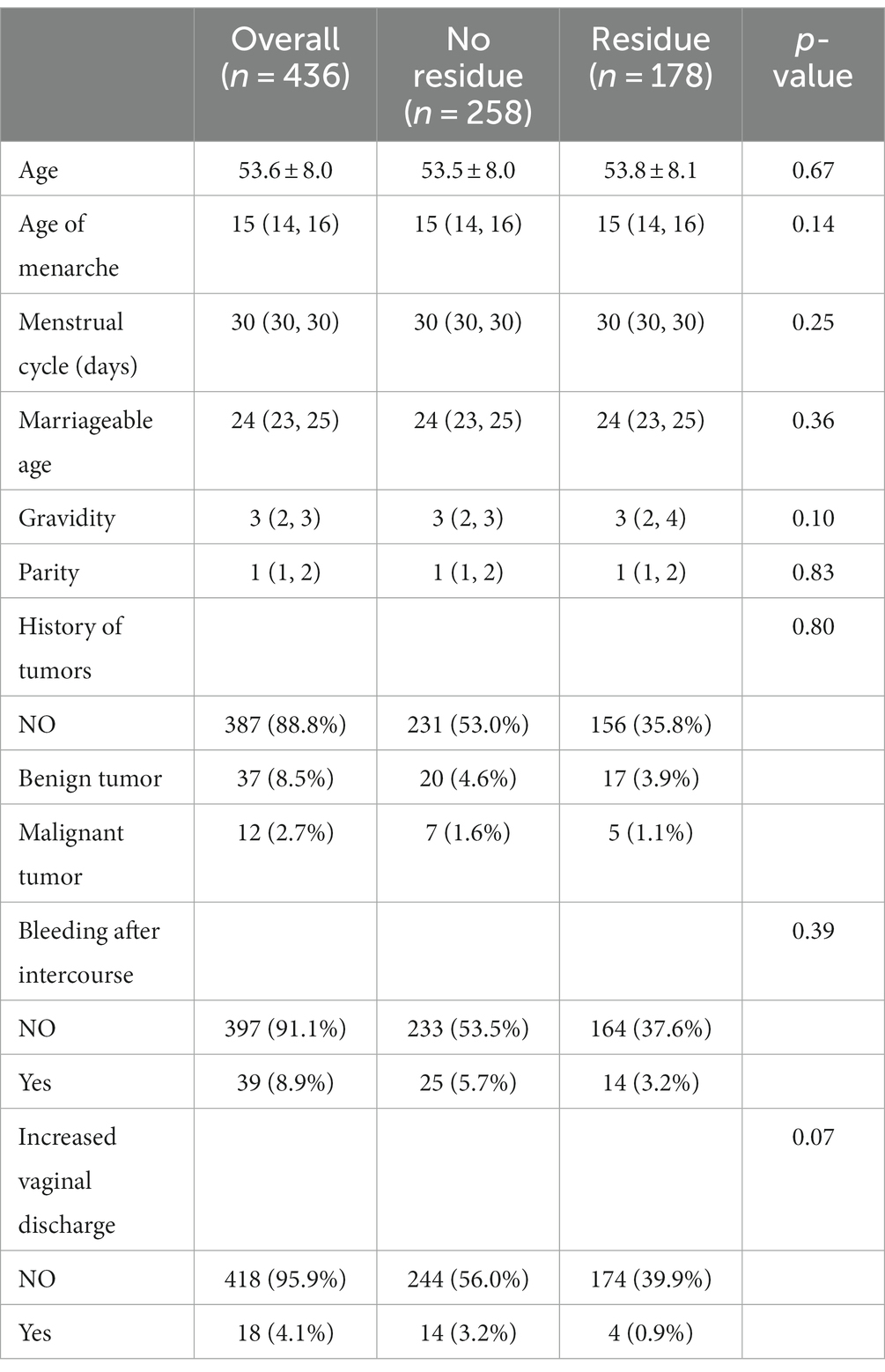
This study included a total of 436 patients with CIN3 who underwent hysterectomy after LEEP. The average age of the patients was 53.6 ± 8.0 years. Among them, there were 178 cases in the group with confirmed residual lesions, with an average age of 53.8 ± 8.1 years. In the group without residual lesions after LEEP, there were 258 cases, with an average age of 53.5 ± 8.0 years. There were no statistically significant differences between the two groups in terms of age at menarche, menstrual cycle, age at marriage, parity, or pregnancy history. There were also no statistically significant differences between the two groups in terms of the presence of previous tumor history (Table 1).
3.2 Postoperative condition after LEEP
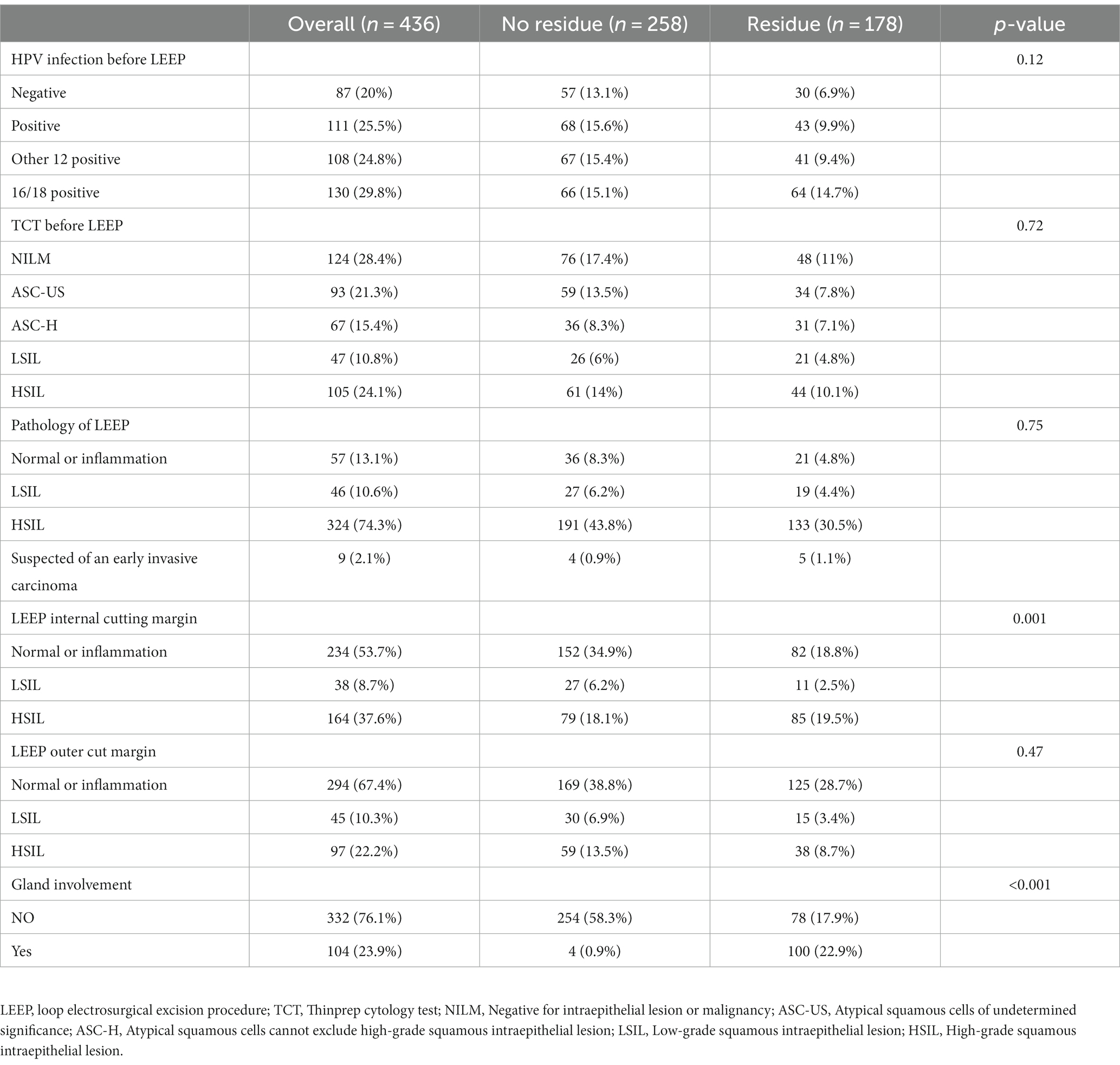
There were no statistically significant differences (p > 0.05) between the residual group and the non-residual group in terms of HPV status, TCT examination, LEEP pathology, and margin status prior to LEEP treatment. However, the number of cases with glandular involvement in the residual group was significantly higher compared to the non-residual group (n = 100 vs. 4), with a statistically significant difference (p < 0.001). The margin status within the LEEP specimen in the residual group also showed a statistically significant difference compared to the non-residual group (p = 0.001) (refer to Table 2 for more details).
3.3 Independent factors associated with residual lesions after LEEP
Univariable and multivariable logistic regression analysis were performed on the two groups of patients (Table 3). According to the Univariable logistic regression analysis, factors such as preoperative ECC pathology, LEEP margin status, Post-LEEP follow-up HPV, Post-LEEP follow-up TCT, and the Gland involvement are potential independent risk factors for residual lesions in patients with CIN3 (all with p < 0.05). These variables were included in the multivariate logistic regression analysis to identify the independent factors associated with residual lesions after LEEP in CIN3 patients, including Post-LEEP follow-up HPV, Post-LEEP follow-up TCT, and the involvement of glandular lesions. To visualize the accuracy and importance of these predicting factors, a random forest plot was generated (Figure 2).
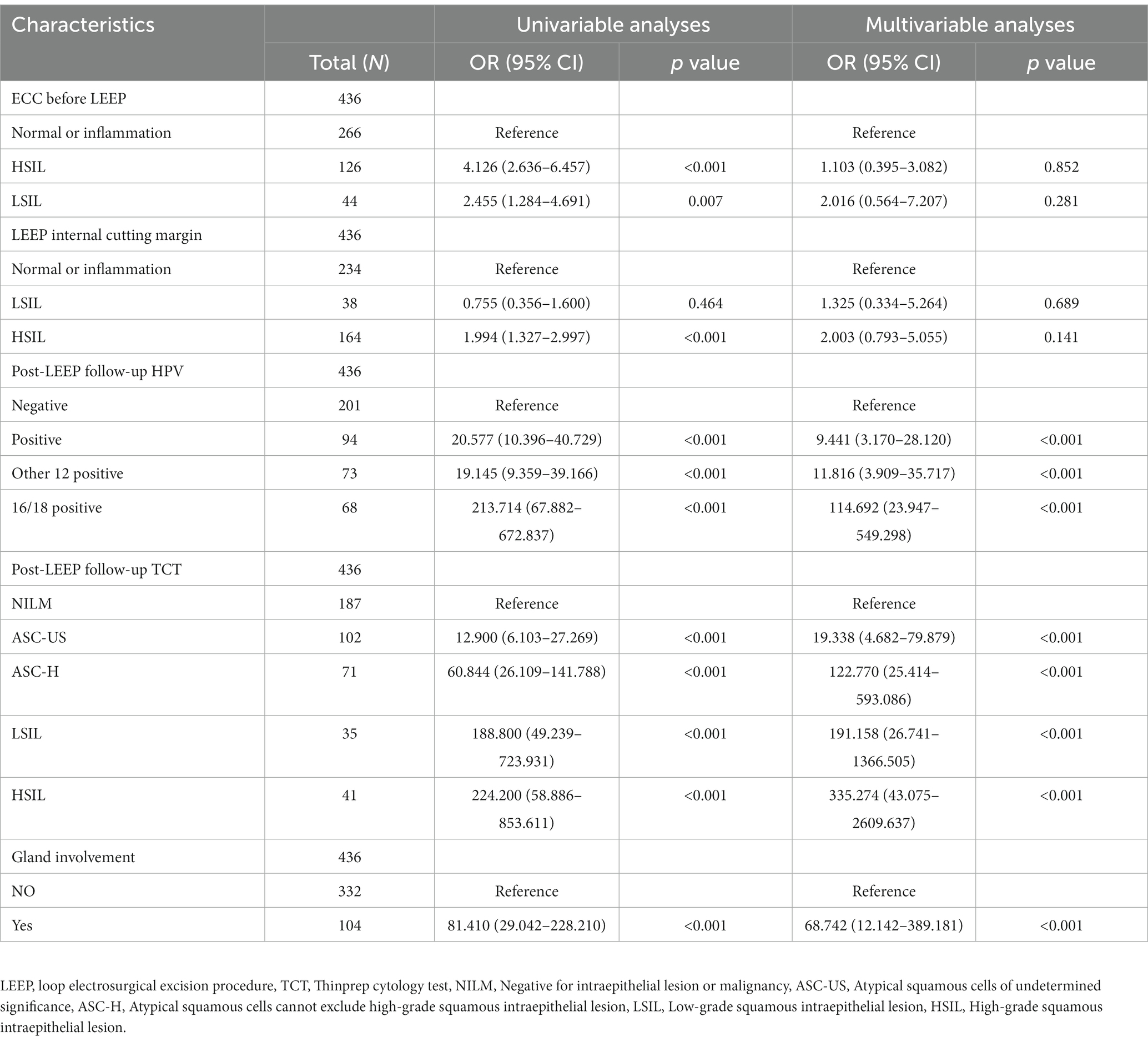
Table 3. Univariable and multivariable logistic regression analysis of residual factors following LEEP procedure in CIN3 patients.
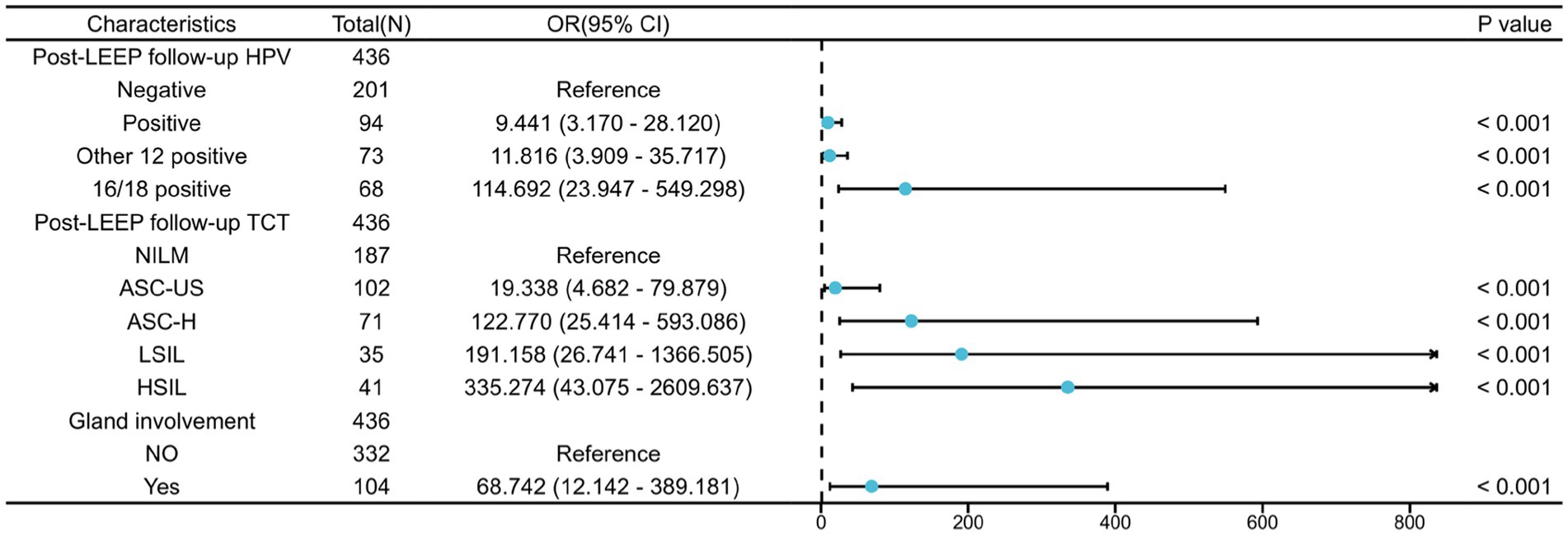
Figure 2. Multifactorial logistic regression analysis of residual lesions after LEEP in CIN3 patients.
3.4 Establishment and evaluation of the nomogram model
Based on the results of the multivariable logistic regression analysis, a nomogram model for predicting residual lesions after LEEP in CIN3 patients was established using the R software (Figure 3A). The model’s discrimination was evaluated by plotting the receiver operating characteristic curve (ROC) (Figure 3B) and calculating the area under the ROC curve (AUC) and C-index. The calibration curve was also plotted to assess the consistency of the nomogram model in predicting the probability of residual lesions after LEEP. The study samples were used as the training set, and internal validation was performed using 1,000 bootstrap samples. The decision curve analysis (DCA) was conducted to evaluate the clinical benefits of the model. The results showed that the AUC values for predicting residual lesions in CIN3 patients after LEEP were 0.820 for Post-LEEP follow-up HPV, 0.894 for Post-LEEP follow-up TCT, and 0.773 for the involvement of glandular lesions. However, the nomogram model had a C-index and AUC of 0.975, indicating better predictive performance compared to individual risk factors. The calibration curve demonstrated good consistency of the model (Figure 3C). The decision curve for the training set (Figure 3D) showed that when the high-risk threshold was set to >0.18, using the nomogram model provided more benefits compared to no treatment or treating all patients. Therefore, the nomogram model has clinical value in predicting residual lesions after LEEP.
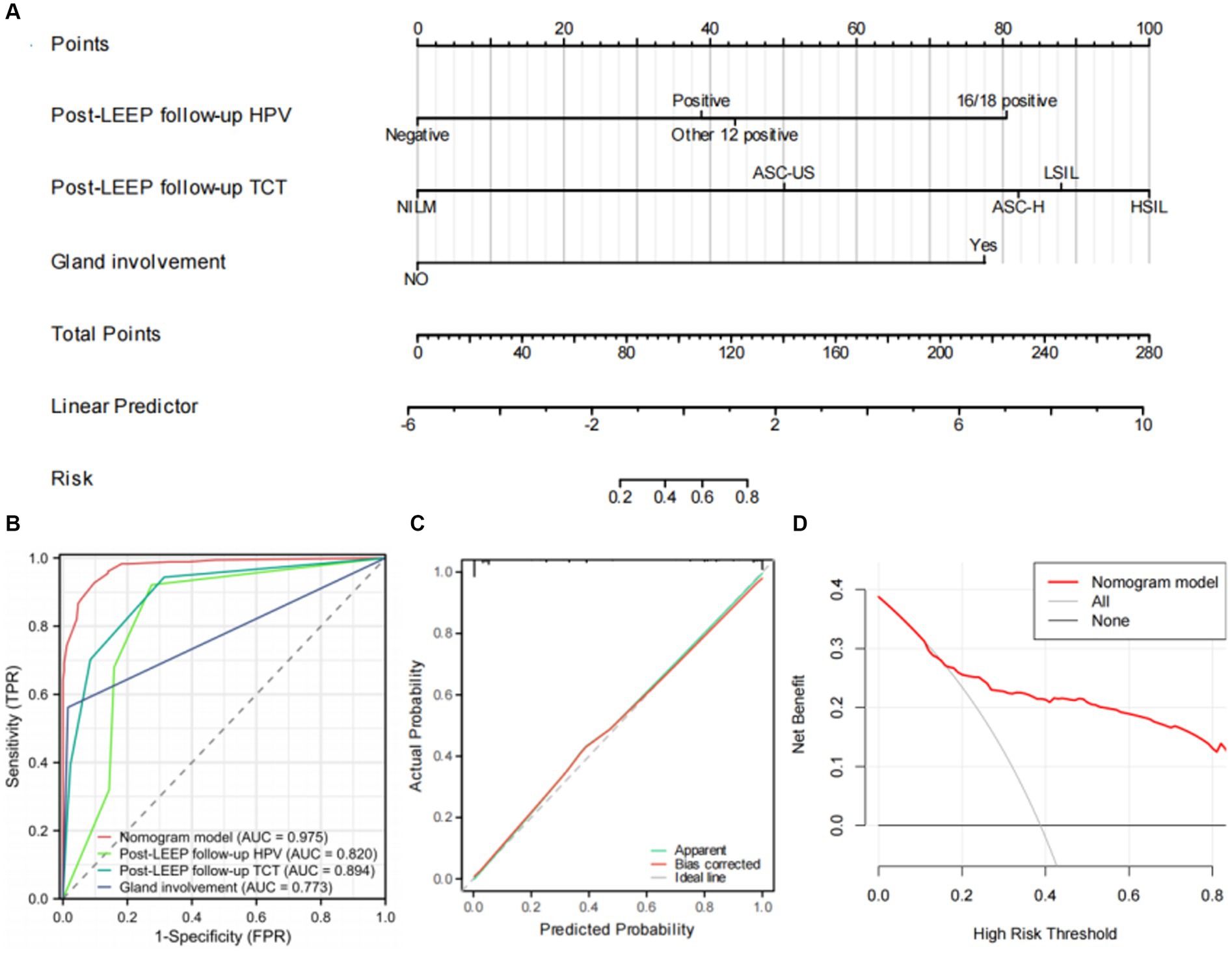
Figure 3. Establishment and evaluation of the nomogram. (A) The nomogram for predicting residual lesions after LEEP in CIN3 patients. (B) Area under the ROC curves (AUC) for the diagnosis of the residual lesions using the nomogram in CIN3 patients. (C) The calibration curve for the risk of residual lesions after LEEP surgery in CIN3 patients. The nomogram-predicted probability of residual lesions is plotted on the x-axis; the actual risk of residual lesions is plotted on the y-axis. (D) Decision curve analysis of the nomogram (red line).
4 Discussion
CIN is a precancerous condition of the cervix and is one of the most common gynecological diseases among women of childbearing age. The natural course of CIN is unpredictable, and if left untreated, it can progress to invasive cervical cancer (ICC) (8). Conization of uterine cervix is a key method for diagnosing and treating CIN. The two most common techniques for conization of uterine cervix are LEEP and cold knife conization (CKC) (9). The principle of LEEP involves the use of targeted radiofrequency waves to break molecular bonds and generate heat within the tissue. This results in cutting and cauterization effects, making LEEP a minimally invasive procedure with faster recovery compared to other treatment methods (10). LEEP is highly effective for treating CIN. However, for high-grade CIN, due to its multifocal nature, complete excision through local LEEP can be challenging. This can lead to further disease progression (11). There is evidence suggesting that women who have residual lesions after treatment have a approximately 5-fold higher risk of developing invasive cervical cancer (ICC) compared to the general population (12). However, excessive excision can lead to negative consequences such as increased risk of premature births in young women, adverse outcomes for newborns, and impacts on sexual health (13, 14). Therefore, it is of great significance to establish an effective predictive model for residual lesions after LEEP to guide the treatment and prognosis of CIN patients. Currently, there is no effective evaluation system for predicting CIN residuals. The nomogram model can present the relevant factors in regression analysis in a graphical form, providing approximate probability values while integrating multiple related factors. It has good visual and operational characteristics (15).
Currently, in most studies, “positive surgical margins” and “persistent HPV infection” after LEEP are identified as risk factors for residual lesions (16, 17). Although positive surgical margins may reflect the characteristics of the disease, multiple studies have also confirmed its correlation with CIN recurrence (18, 19). However, nearly half of the cases with positive surgical margins do not experience recurrence or residual disease, and even patients with negative margins may still have residual disease (20, 21). Furthermore, in the results of this study, there was no correlation between the status of the inner and outer surgical margins after LEEP and the presence of residual lesions. Although there was a statistical difference in the status of the inner surgical margins after LEEP between the residual group and the non-residual group (see Table 2), multivariable logistic regression analysis showed that the status of the inner surgical margins after LEEP was not an independent risk factor for residual lesions (see Table 3). This study speculates whether the absence of residual lesions after hysterectomy following LEEP with positive surgical margins is related to the use of electrosurgical coagulation and hemostasis during LEEP. Other way, we do not rule out the possibility that this could be caused by collinearity between the variables, as well as potential selection bias and a relatively short follow-up period (18).
HPV infection has been confirmed by multiple studies as an important predictive indicator for residual CIN lesions (5, 22, 23). In this study, the post-LEEP follow-up HPV infection status was closely related to the presence of residual CIN3 lesions after LEEP, and it can serve as an independent predictive indicator for residual lesions in CIN3 patients undergoing LEEP. These findings are consistent with previous research. Among the 150+ known HPV subtypes, more than 40 subtypes are known to be associated with genital infections. HPV infection, especially high-risk (HR) HPV infection, is a risk factor for various gynecological diseases (24). The results of the multifactor logistic regression analysis showed that post-LEEP follow-up HPV infection, follow-up TCT results, and involvement of glandular lesions were independent risk factors for residual lesions after LEEP (p < 0.05, Table 3). TCT has now replaced conventional cytology as an important method for cervical cancer screening. It has high sensitivity in the diagnosis of CIN lesions (25). Based on the above results, we have established a waterfall plot model (Figure 3A) to predict the presence of residual lesions after LEEP in CIN3 patients. The nomogram model shows that using post-LEEP follow-up HPV infection, follow-up TCT results, and involvement of glandular lesions as predictive indicators has a good C-index level (0.975, 95% CI = 0.962–0.988). The calibration curve further confirmed the good correlation between the model and the actual occurrence of residual lesions in CIN3 patients post-LEEP. This indicates that the model can effectively predict the presence of residual lesions after LEEP in CIN3 patients. Additionally, clinical decision analysis shows that this model provides more benefits than having no patient treatment plan or treating all patients. It can provide valuable guidance for clinicians in preventing residual lesions after LEEP in CIN3 patients, demonstrating good clinical utility.
This study has the following limitations: the model lacks external data for evaluation and validation, which reduces the reliability and limits the generalizability of the nomogram model. Additionally, this study is a single-center retrospective study, which may result in lower generalizability of the findings. Therefore, further clinical research is needed to validate the nomogram model using multicenter, prospective studies with larger sample sizes and external data. Furthermore, the assessment of HPV-mRNA appears to serve as a prognostic biomarker for monitoring residual disease progression in women undergoing LEEP for CIN3 (26). This biomarker will be further examined and analyzed in our upcoming research endeavors.
In conclusion, the nomogram model constructed in this study based on post-LEEP follow-up HPV infection, follow-up TCT results, and Gland involvement has demonstrated some predictive performance for residual CIN3 lesions after LEEP. After further validation, it may have good clinical application prospects. Additionally, the results of this study also highlight the importance of the postoperative follow-up examination after LEEP.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by IEC for Clinical Research of Xishan People’s Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
LD: Data curation, Formal Analysis, Resources, Writing – original draft. TW: Data curation, Resources, Writing – original draft. YC: Data curation, Resources, Writing – original draft. XT: Conceptualization, Investigation, Methodology, Project administration, Resources, Software, Validation, Visualization, Writing – review & editing. DX: Conceptualization, Funding acquisition, Investigation, Project administration, Resources, Software, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was funded by the Wuxi Municipal Health Commission Maternal and child health scientific research project (FYKY202009).
Acknowledgments
The authors express their gratitude to the clinical staff at the participating hospitals for their valuable contributions and dedication to patient care. The authors would also like to extend their sincere appreciation to the patients who participated in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung, H, Ferlay, J, Siegel, RL, Laversanne, M, Soerjomataram, I, Jemal, A, et al. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Kyrgiou, M, Athanasiou, A, Arbyn, M, Lax, SF, Raspollini, MR, Nieminen, P, et al. Terminology for cone dimensions after local conservative treatment for cervical intraepithelial neoplasia and early invasive cervical cancer: 2022 consensus recommendations from ESGO, EFC, IFCPC, and ESP. Lancet Oncol. (2022) 23:e385–92. doi: 10.1016/S1470-2045(22)00191-7
3. Terzic, M, Makhadiyeva, D, Bila, J, Andjic, M, Dotlic, J, Aimagambetova, G, et al. Reproductive and obstetric outcomes after fertility-sparing treatments for cervical Cancer: current approach and future directions. J Clin Med. (2023) 12:2614. doi: 10.3390/jcm12072614
4. Bruno, MT, Bonanno, G, Sgalambro, F, Cavallaro, A, and Boemi, S. Overexpression of E6/E7 mRNA HPV is a prognostic biomarker for residual disease progression in women undergoing LEEP for cervical intraepithelial neoplasia 3. Cancers (Basel). (2023) 15:4203. doi: 10.3390/cancers15174203
5. Jing, L, Dan, W, Zhunan, L, Ying, X, and Yi, C. Residual lesions in uterine specimens after loop electrosurgical excision procedure in patients with CIN. Arch Gynecol Obstet. (2018) 298:805–12. doi: 10.1007/s00404-018-4881-7
6. Huang, G, Gao, H, Chen, Y, Lin, W, Shen, J, Xu, S, et al. Platelet-to-lymphocyte ratio (PLR) as the prognostic factor for recurrence/residual disease in HSIL patients after LEEP. J Inflamm Res. (2023) 16:1923–36. doi: 10.2147/JIR.S406082
7. Bogani, G, Donato, DI, Sopracordevole, F, Ciavattini, A, Ghelardi, A, Lopez, S, et al. Recurrence rate after loop electrosurgical excision procedure (LEEP) and laser Conization: a 5-year follow-up study. Gynecol Oncol. (2020) 159:636–41. doi: 10.1016/j.ygyno.2020.08.025
8. Mitra, A, Tzafetas, M, Lyons, D, Fotopoulou, C, Paraskevaidis, E, and Kyrgiou, M. Cervical intraepithelial neoplasia: screening and management. Br J Hosp Med (Lond). (2016) 77:C118–23. doi: 10.12968/hmed.2016.77.8.C118
9. Zhang, L, Sauvaget, C, Mosquera, I, and Basu, P. Efficacy, acceptability and safety of ablative versus excisional procedure in the treatment of histologically confirmed CIN2/3: a systematic review. BJOG. (2023) 130:153–61. doi: 10.1111/1471-0528.17251
10. Sikorska, M, Pawlowska, A, Antosik-Wojcinska, A, Zyguła, A, Suchońska, B, Dominiak, M, et al. The impact of HPV diagnosis and the electrosurgical excision procedure (LEEP) on mental health and sexual functioning: a systematic review. Cancers (Basel). (2023) 15:2226. doi: 10.3390/cancers15082226
11. Bruno, MT, Cassaro, N, Garofalo, S, and Boemi, S. HPV16 persistent infection and recurrent disease after LEEP. Virol J. (2019) 16:148. doi: 10.1186/s12985-019-1252-3
12. Melnikow, J, McGahan, C, Sawaya, GF, Ehlen, T, and Coldman, A. Cervical intraepithelial neoplasia outcomes after treatment: long-term follow-up from the British Columbia cohort study. J Natl Cancer Inst. (2009) 101:721–8. doi: 10.1093/jnci/djp089
13. Bevis, KS, and Biggio, JR. Cervical conization and the risk of preterm delivery. Am J Obstet Gynecol. (2011) 205:19–27. doi: 10.1016/j.ajog.2011.01.003
14. Caruso, S, Bruno, MT, Cianci, S, di Pasqua, S, Minona, P, and Cianci, A. Sexual behavior of women with diagnosed HPV. J Sex Marital Ther. (2019) 45:569–73. doi: 10.1080/0092623X.2019.1586019
15. Balachandran, VP, Gonen, M, Smith, JJ, and DeMatteo, RP. Nomograms in oncology: more than meets the eye. Lancet Oncol. (2015) 16:e173–80. doi: 10.1016/S1470-2045(14)71116-7
16. Ayhan, A, Tuncer, HA, Reyhan, NH, Kuscu, E, and Dursun, P. Risk factors for residual disease after cervical conization in patients with cervical intraepithelial neoplasia grades 2 and 3 and positive surgical margins. Eur J Obstet Gynecol Reprod Biol. (2016) 201:1–6. doi: 10.1016/j.ejogrb.2016.03.021
17. Ouh, YT, Cho, HW, Kim, SM, Min, KJ, Lee, SH, Song, JY, et al. Risk factors for type-specific persistence of high-risk human papillomavirus and residual/recurrent cervical intraepithelial neoplasia after surgical treatment. Obstet Gynecol Sci. (2020) 63:631–42. doi: 10.5468/ogs.20049
18. Papakonstantinou, K, Kyrgiou, M, Lyons, D, Soutter, WP, and Ghaem-Maghami, S. Management of stage Ia1 squamous cervical cancer and the importance of excision margins: a retrospective study of long-term outcome after 25 years of follow-up. Am J Obstet Gynecol. (2014) 211:625.e1–6. doi: 10.1016/j.ajog.2014.06.032
19. Alder, S, Megyessi, D, Sundstrom, K, Östensson, E, Mints, M, Belkić, K, et al. Incomplete excision of cervical intraepithelial neoplasia as a predictor of the risk of recurrent disease-a 16-year follow-up study. Am J Obstet Gynecol. (2020) 222:172.e1–172.e12. doi: 10.1016/j.ajog.2019.08.042
20. Dou, Y, Zhang, X, Li, Y, Wang, F, Xie, X, and Wang, X. Triage for management of cervical high-grade squamous intraepithelial lesion patients with positive margin by conization: a retrospective analysis. Front Med. (2017) 11:223–8. doi: 10.1007/s11684-017-0517-8
21. Kwok, ST, Chan, K, Tse, KY, Chu, MMY, Lau, LSK, Ngan, HYS, et al. Outcome after loop electrosurgical excision procedure for cervical high-grade squamous intraepithelial lesion. Taiwan J Obstet Gynecol. (2023) 62:45–9. doi: 10.1016/j.tjog.2022.10.004
22. Arbyn, M, Redman, C, Verdoodt, F, Kyrgiou, M, Tzafetas, M, Ghaem-Maghami, S, et al. Incomplete excision of cervical precancer as a predictor of treatment failure: a systematic review and meta-analysis. Lancet Oncol. (2017) 18:1665–79. doi: 10.1016/S1470-2045(17)30700-3
23. Feng, C, Gu, L, Wei, Y, Niu, J, Yang, H, Hong, Z, et al. Analysis of outcomes following loop electrosurgical excision and clinical features of patients with cervical high-grade squamous intraepithelial lesions with abnormal preoperative endocervical curettage. World J Surg Oncol. (2023) 21:237. doi: 10.1186/s12957-023-03088-5
24. Loopik, DL, Ebisch, RM, Int Hout, J, Melchers, WJ, Massuger, LF, Bekkers, RL, et al. The relative risk of noncervical high-risk human papillomavirus-related (pre) malignancies after recurrent cervical intraepithelial neoplasia grade 3: a population-based study. Int J Cancer. (2020) 147:897–900. doi: 10.1002/ijc.32834
25. Rozemeijer, K, Naber, SK, Penning, C, Overbeek, LIH, Looman, CWN, de Kok, IMCM, et al. Cervical cancer incidence after normal cytological sample in routine screening using sure path, ThinPrep, and conventional cytology: population based study. BMJ. (2017) 356:j504. doi: 10.1136/bmj.j504
Keywords: LEEP, post-total hysterectomy, cervical intraepithelial neoplasia grade III, predictive model, nomogram LEEP
Citation: Deng L, Wang T, Chen Y, Tang X and Xiang D (2023) A predictive model for residual lesions after LEEP surgery in CIN III patients. Front. Med. 10:1326833. doi: 10.3389/fmed.2023.1326833
Edited by:
Long Sui, Fudan University, ChinaReviewed by:
Manuel Maria Ianieri, Agostino Gemelli University Polyclinic (IRCCS), ItalyShen Yang, Southeast University, China
Copyright © 2023 Deng, Wang, Chen, Tang and Xiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xueli Tang, d3VkaXRhbmdqaWFAMTYzLmNvbQ==; Dajun Xiang, eGlhbmdkanhzaG9zcGl0YWxAeWVhaC5uZXQ=
 Lihui Deng1
Lihui Deng1 Xueli Tang
Xueli Tang