
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Med., 08 December 2023
Sec. Gastroenterology
Volume 10 - 2023 | https://doi.org/10.3389/fmed.2023.1323113
This article is part of the Research TopicClinical Management of Helicobacter pylori InfectionsView all 7 articles
Background: Helicobacter pylori (H. pylori) infection, a type I carcinogen, affects approximately 50% of the global population, correlating with various gastric pathologies. Notably, diagnostic sensitivities of non-invasive methods, such as the stool antigen test (HpSA), Serology, and Urea Breath Test (UBT), have been suggested to be less effective in older age groups. This study systematically reviews and meta-analyzes the diagnostic accuracy of these tests within the elderly population.
Methods: A comprehensive literature search was performed across multiple databases, including PubMed, Medline, and Web of Science, up to July 2023. Data were pooled and analyzed using random-effects models. Sensitivity, specificity, and Diagnostic Odds Ratios (DOR) were computed for the tests. Heterogeneity and risk of bias were assessed.
Results: Eight studies involving diverse geographic locations and totaling between 46 and 1,441 participants per study were included. The pooled sensitivity and specificity for HpSA were 72.5 and 94.7%, for Serology 83.7 and 73.3%, and for UBT 96.4 and 88.3%, respectively. DOR for UBT, HpSA, and Serology were 94.5, 47.9, and 14.2, respectively. High levels of heterogeneity were observed across the studies.
Conclusion: UBT and HpSA proved effective for diagnosing H. pylori in those over 60, while serology showed lower specificity. Despite methodological variations in available studies, these non-invasive tests offer reliable alternatives, especially for older patients who recently undergone endoscopy or without an indication for it, warranting consideration by healthcare practitioners.
Helicobacter pylori (H. pylori) infection is ubiquitously prevalent in the human population afflicting close to 50% of individuals globally (1). The epidemiological distribution of H. pylori exhibits a marked variability in relation to geographical location, ethnic background, age, and socioeconomic conditions (1). This Gram-negative, type I carcinogen microorganism induces chronic gastric mucosal inflammation, resulting in atrophic and metaplastic alterations. Consequently, H. pylori infection is implicated in a myriad of gastric conditions including but not limited to chronic gastritis, peptic ulcers, and gastric-related malignancies (2, 3).
The pathophysiology of the infection is multifaceted, involving intricate interactions between the virulence factors of the bacterium, host immune responses, and various environmental determinants (3). H. pylori is diagnosed through a variety of both invasive and non-invasive tests. The choice of diagnostic tool depends on individual patient history and local availability (4). Non-invasive tests, such as the urea breath test (UBT), stool antigen test (HpSA), and serological tests, are generally preferred due to their convenience and non-invasivness (4, 5). However, their diagnostic accuracy can vary, particularly in patients over 65 years, and may also be influenced by conditions such as gastric mucosal atrophy or intestinal metaplasia (4, 6).
The elderly demographic (ages above 65), inherently vulnerable to the detrimental effects of H. pylori, encounters a complex clinical scenario (7). The prevalence of H. pylori in this age cohort not only exacerbates the predisposition to atrophic gastritis and intestinal metaplasia but also accelerates the trajectory toward gastric malignancies, thereby necessitating a nuanced, age-specific diagnostic and therapeutic approach (8).
Existing guidelines and expert consensuses conspicuously overlook the elderly, often sidelining the unique challenges and considerations pertinent to managing H. pylori infections within this population (9). The intricacies of eradication therapies, particularly in the context of potential side effects and the necessity for a meticulous risk–benefit analysis, become paramount, especially given the elderly’s often complex clinical and physiological profiles.
Our meta-analysis seeks to elucidate the accuracy of various diagnostic tests for H. pylori within the elderly demographic, aiming to bridge the extant gap in the literature and facilitate the development of robust, individualized management strategies.
This systematic review and meta-analysis study was prospectively registered at PROSPERO (Registration code: CRD42023463706) and was carried out according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (10).
A systematic search was conducted on PubMed, Medline, Web of Science, the Cochrane Library, Embase, Google Scholar, and CINAHL from database inception until July 2023 to look for potentially eligible articles. The search strategy was based on the terms appearing in Appendix 1. All retrieval processes were performed manually and independently by two researchers.
To align with our research objectives, specific eligibility criteria were utilized for study selection. We included observational studies—comprising cohort, case–control, and, where sufficient diagnostic accuracy measures were available, cross-sectional designs—as well as randomized controlled trials. The focus was on studies with a population aged 60 years and above, either by mean age or by exclusive age range of study participants, undergoing evaluation for H. pylori infection. The primary outcome was the diagnostic accuracy of non-invasive tests such as the Urea Breath Test, Stool Antigen Test, and serological tests, compared to the gold standard of invasive endoscopy. Studies were excluded if they fell into categories of case reports, case series, letters, comments, editorials, were not published in English, or involved populations younger than 60 years without specific subgroup analysis. Additionally, studies lacking sufficient data to compute sensitivity and specificity were also excluded to maintain analytical rigor.
A systematic approach was adopted to conduct the study selection process. Two independent reviewers meticulously and manually screened titles and abstracts of retrieved articles against the predefined inclusion criteria, no software was used during the process. Subsequently, full-text articles that showed potential for meeting the eligibility criteria were retrieved for further assessment. During this process, any discrepancies or disagreements between the reviewers were addressed through discussion, and in case of persistent discrepancies, a third reviewer was consulted to ensure objective and unbiased study selection. A standardized data extraction form was employed to facilitate the extraction of relevant data from the studies included. This data extraction form enabled the collection of essential information from each study, ensuring consistency and uniformity in data reporting.
In accordance with rigorous scientific protocols, we employed the Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) tool to evaluate the methodological integrity of the nine studies incorporated into our meta-analysis (11). Two independent reviewers scrutinized each study across four critical domains: patient selection, index test, reference standard, and flow and timing. The assessments were undertaken with an emphasis on both the risk of bias and applicability, with the first three domains being evaluated for the latter as well. Discrepancies between the reviewers were resolved through consensus-driven discussion.
Statistical analyses were conducted using R Studio. A random-effects model was employed to account for significant heterogeneity observed among the included studies. We computed pooled sensitivity, specificity, and Diagnostic Odds Ratios (DOR) for three diagnostic tests: HpSA, Serology, and UBT. Additionally, we performed a Hierarchical Summary Receiver Operating Characteristic (HSROC) model and generated the corresponding curve to assess the overall diagnostic accuracy. Threshold Effect and Spearman’s Correlation Analysis were also conducted to further evaluate the data. All tests of statistical significance were two-sided, adopting an alpha level of 0.05.
A rigorous search strategy was employed across multiple databases to identify studies pertinent to our systematic review and meta-analysis. Initially, the database search yielded 1,642 studies: 811 from PubMed, 365 from Medline, 102 from Web of Science, 36 from Cochrane Library, 62 from Embase, 246 from Google Scholar, and 20 from CINAHL. After eliminating 497 duplicate records, a total of 1,145 studies remained for eligibility assessment. Following an initial screening of titles and abstracts, 673 studies were flagged as potentially eligible and were subjected to full-text review. Upon comprehensive evaluation against our pre-defined eligibility criteria, 8 studies were ultimately included in both the systematic review and meta-analysis. The PRISMA flowchart detailing this process is presented in Figure 1.
This meta-analysis incorporates eight studies (12–19), summarized in Table 1. Published between 1991 and 2020, these studies have a broad geographical coverage, including Turkey, Bulgaria, China, Italy, the UK, and Israel. The total number of participants in the individual studies ranged from as few as 46 to as many as 193. The age of the participants varied widely, with study-specific mean ages ranging from 62.6 years to 80.1 years. Both males and females are represented in these studies, although the gender distribution varies among them.
Various non-invasive diagnostic tests for H. pylori were employed across the studies, including UBT, HpSA test, and serology. All the studies used endoscopy and histology as reference tests for diagnosing H. pylori, except Han et al. (19), which employed [13C] urea breath test as the reference standard. Sensitivity and specificity in the untreated groups varied significantly, with sensitivity ranging from 65.1 to 100%, and specificity ranging from 52 to 98.7%.
The heterogeneity in study design, diagnostic methods, and patient demographics enhances the generalizability and comprehensiveness of our findings. However, it’s worth noting that the follow-up duration was not consistently reported across the studies, with some as short as a three-month period (15).
Our analysis revealed that the included studies generally demonstrated a satisfactory level of methodological quality. Nonetheless, specific domains exhibited varying levels of risk. Two studies posed a high risk of bias in the domain of patient selection due to non-consecutive or non-random enrollment (14, 17). A notable concentration of high-risk bias was identified in the index test domain, specifically attributable to pre-defined threshold levels in four out of the eight studies (12, 13, 17, 19). In contrast, no significant risk of bias was detected in the reference standard domain. However, the domain of flow and timing posed an unclear risk in three studies (13, 15, 17), primarily due to the ambiguous time intervals between the administration of the index test and the reference standard. Regarding applicability, the studies were generally robust, although three presented high applicability concerns in the index test domain (12, 13, 19) (Figures 2, 3).
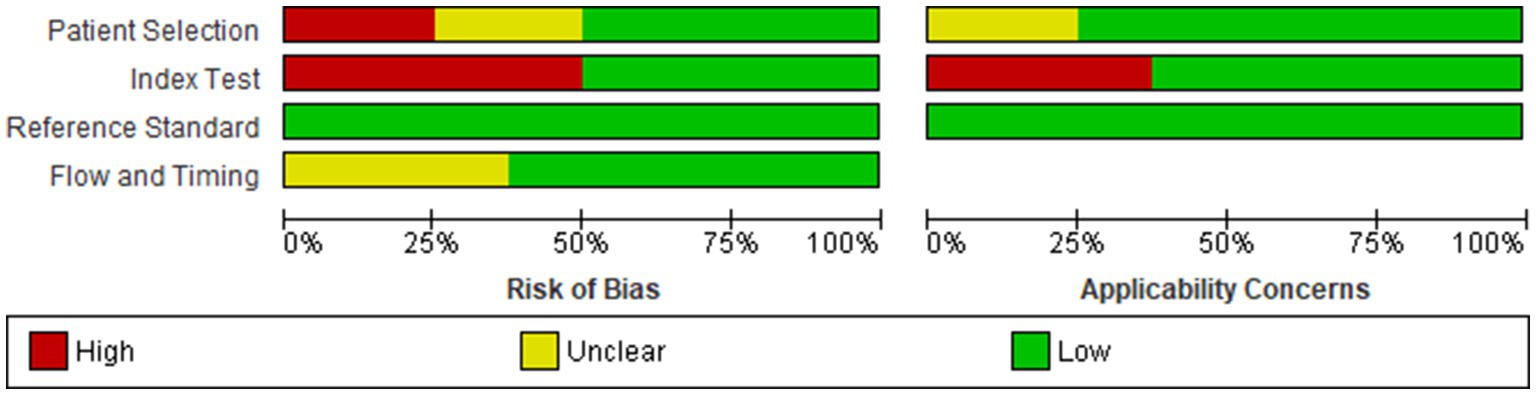
Figure 3. Cumulative assessment of overall risk of bias and applicability concerns across all included studies.
The diagnostic effectiveness of HpSA, Serology, and UBT in detecting H. pylori infection was evaluated through meta-analysis, using the data of the untreated groups. Given the significant heterogeneity among the included studies, random-effects models were employed for the meta-analysis.
The pooled sensitivity for HpSA was 72.5%, with a 95% Confidence Interval (CI) ranging from 65 to 79%. The pooled specificity was 94.7%, with a 95% CI of 80 to 99%. The I^2 statistics for sensitivity and specificity were 91.73 and 99.54%, respectively, indicating a high level of heterogeneity across the studies. This heterogeneity was statistically significant, with a value of p less than 0.0001 for both metrics (Figure 4).
For Serology tests, the pooled sensitivity was 83.7%, with a 95% CI of 73 to 91%. The pooled specificity was 73.3%, with a 95% CI of 37 to 93%. The I^2 statistics for sensitivity and specificity were 96.77 and 99.45%, respectively, which suggests substantial heterogeneity. This heterogeneity was statistically significant, with a value of p less than 0.0001 for both sensitivity and specificity (Figure 5).
The Urea Breath Test (UBT) exhibited a sensitivity of 96.4%, with a 95% CI of 82 to 99%. The specificity was 88.3%, with a 95% CI of 71 to 96%. The I^2 statistics for sensitivity and specificity were 86.14 and 99.15%, respectively, indicating significant heterogeneity. This heterogeneity was confirmed as statistically significant with p-values of 0.0149 for sensitivity and less than 0.0001 for specificity (Figure 6).
We conducted a meta-analysis to estimate the combined Diagnostic Odds Ratios (DOR) for three different tests: HpSA, Serology, and UBT. Diagnostic Odds Ratio (DOR) serves as a single indicator that combines sensitivity and specificity, providing an overall measure of the diagnostic test’s effectiveness. In this study, a higher DOR indicates better discriminative test performance for diagnosing H. pylori infection. The results are presented below.
The meta-analysis yielded a pooled DOR of approximately 47.9 (CI 95%: 14.9–153.2, p < 0.001). The studies included in this analysis exhibited significant heterogeneity, with an I2 value of 99.25%.
The pooled DOR for Serology tests was approximately 14.2 (CI 95%: 1.7–115.4, p = 0.0131). The studies included in this analysis also exhibited significant heterogeneity, with an I2 value of 99.70%.
For the UBT test, the pooled DOR was approximately 94.5 (CI 95%: 22.4–397.5, p < 0.001). The I2 statistic was 99.47%, signifying a high level of heterogeneity among the included studies.
Figure 7 visualizes the DOR’s of the different tests, and Figure 8 represents the findings from the Hierarchical Summary Receiver Operating Characteristic (HSROC) Model Analysis.
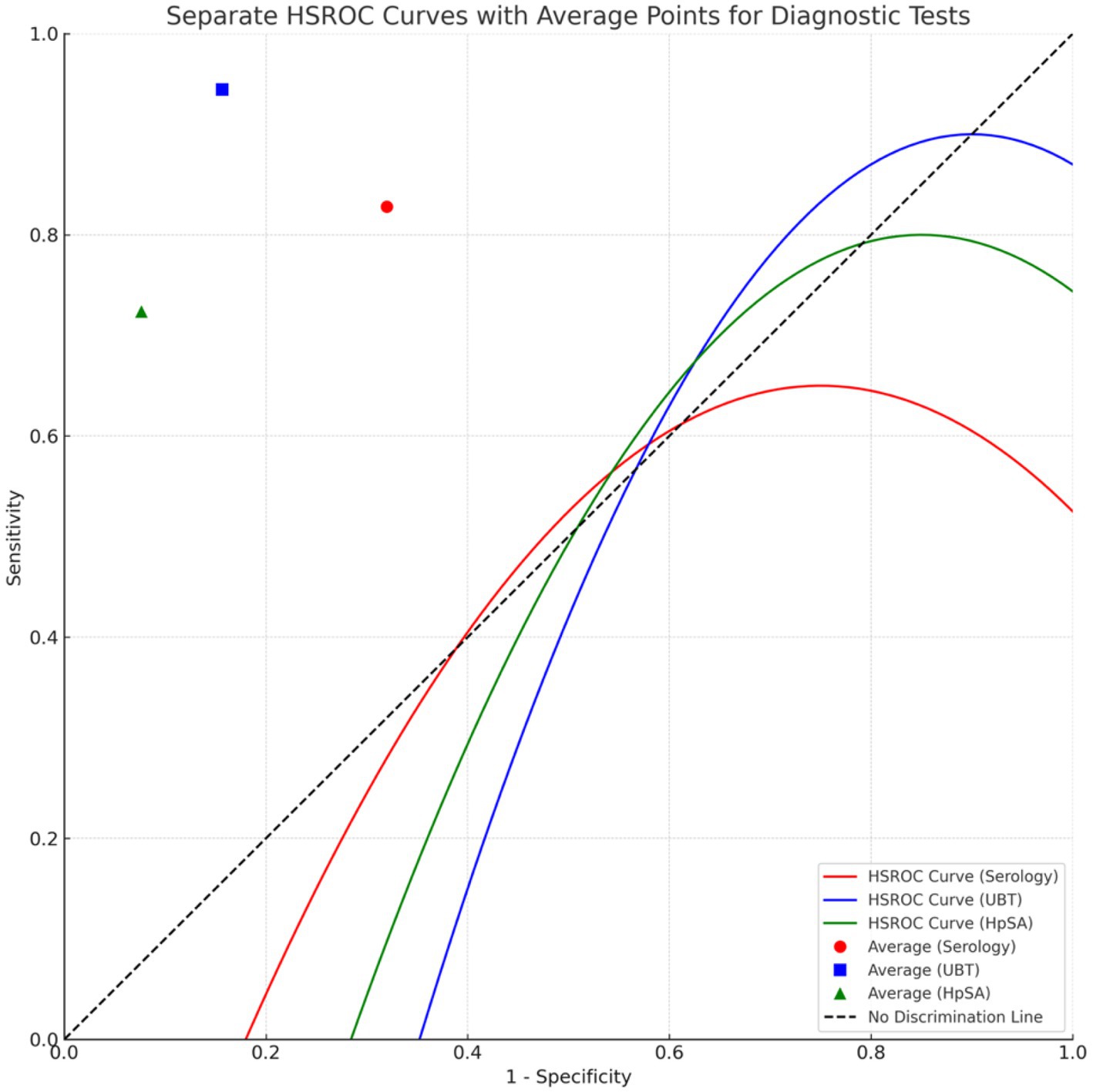
Figure 8. HSROC model illustrating the comparative diagnostic performance of serology, UBT, and HpSA for Helicobacter pylori detection. *Points closer to the top-left corner indicate better test performance.
In the assessment of the threshold effect using Spearman’s Correlation Analysis, the Serology test exhibited a moderate positive correlation between sensitivity and specificity with a rho value of 0.5, though this was not statistically significant (value of p = 1). For the Urea Breath Test (UBT), a rho value of 0.6324555 was observed, indicating a moderate to strong positive correlation between sensitivity and specificity; however, this correlation was not supported by statistical significance (value of p = 0.3675). The Helicobacter pylori Stool Antigen (HpSA) test demonstrated a strong negative correlation between sensitivity and specificity with a rho value of −0.8660254, yet this too lacked statistical significance (value of p = 0.3333).
In the meta-analysis, a Hierarchical Summary Receiver Operating Characteristic (HSROC) model was applied to evaluate the diagnostic accuracy of non-invasive tests for Helicobacter pylori detection in elderly patients. The model was based on 18 data points derived from the included studies. Using the Restricted Maximum Likelihood (REML) estimation method, the model yielded a log odds ratio estimate of 2.3604, with a 95% confidence interval ranging from 1.7272 to 2.9937. This positive value indicates a consistent trend in the sensitivity and specificity results across the studies. The goodness-of-fit statistics were as follows: Deviance = 7704.8069, AIC = 7708.8069, and BIC = 7710.4733. The estimated between-study variance (sigma^2) was 0.8343, suggesting a moderate heterogeneity among the studies. This was further confirmed by the Cochran’s Q-test, which indicated significant variability with a value of p of less than 0.0001. The findings from the Hierarchical Summary Receiver Operating Characteristic (HSROC) Model Analysis are visually represented in Figure 8.
Our meta-analysis assessed the diagnostic accuracy of HpSA, Serology, and Urea Breath Test (UBT) in detecting H. pylori infections in the elderly population. The analysis included four studies for UBT, three for HpSA, and three for Serology. The Urea Breath Test showed the highest pooled sensitivity, while HpSA demonstrated superior specificity. In terms of Diagnostic Odds Ratios (DOR), UBT emerged as the most effective diagnostic tool with a DOR of approximately 94.5. HpSA followed with a DOR of around 47.9, and Serology had a DOR of about 14.2. Interestingly, the correlations observed between sensitivity and specificity across these tests suggest a potential deviation from the typical trade-off seen in diagnostic evaluations. The HSROC model further enriched our understanding, indicating a consistent trend in diagnostic accuracy across studies, as evidenced by the positive log odds ratio. However, the significant heterogeneity observed among the studies underscores the importance of individual study contexts and calls for a nuanced interpretation of results. Collectively, our findings spotlight the accuracy of these non-invasive tests in the elderly demographic but also emphasize the variability across studies, underscoring the need for a judicious approach in clinical applications.
The pooled sensitivity and specificity indicate that UBT appears to be the most reliable for diagnosing H. pylori among the elderly, particularly given its high DOR, which serves as an overall measure of test effectiveness (20). However, the HpSA test also shows promise, especially in terms of specificity. The DOR values signify the clinical utility of each test, with higher values indicating better diagnostic performance (20). Despite these promising results, the high level of heterogeneity across studies (often exceeding 90%) warrants caution in the generalizability of these findings (21). The p-values were consistently less than 0.05 for most metrics, which supports the statistical significance of our results.
Our meta-analysis introduces a nuanced perspective to the burgeoning literature on the diagnostics of H. pylori in elderly patients. The pooled results confirm the high sensitivity and specificity of UBT in general, as our study demonstrated that UBT had a pooled sensitivity of 96.4% and specificity of 88.3%, results which align closely with previously published literature declaring UBT as the gold standard with a diagnostic accuracy of around 96% (22). Furthermore, when contrasting our pooled findings with those observed in the general populace, particularly focusing on sensitivity, with our primary objective is to ascertain the accuracy of non-invasive tests in accurately eliminating the occurrence of false negatives. Our results show that the sensitivity of UBT aligns well with the general population’s, as noted by Leal et al.’s meta-analysis, reporting a sensitivity of 95.9% compared to our 96.4%. However, we did observe a slight decrease in specificity, especially in the elderly, with values at 88.3% versus 95.7% (23). It’s worth noting that the specificity can be influenced by age and other physiological factors, and there is evidence suggesting the method might be less specific among elderly patients (24–26). When looking at the pooled results of the HpSA test, the pooled specificity is comparable to results from existing literature on both the general and pediatric populations (27–30). Specifically, our analysis reveals that, although the pooled sensitivity of 72.5% is significantly lower than the 91% sensitivity reported by Gisbert et al. in a meta-analysis encompassing 89 studies, our pooled specificity is comparably analogous, with a value of 94.7% relative to their 93% (4). The high specificity observed might infer that employing this diagnostic tool in older populations holds potential to curtail over-diagnosis and subsequent over-treatment (31). This could further enable its utilization as a confirmatory assay after other diagnostic methodologies characterized by elevated rates of false positives (31, 32). The reduced sensitivity observed in HpSA test can be attributed to multiple factors such as extended gastrointestinal transit time, which could decelerate the passage of bacteria to the colon, and advanced atrophic gastritis, which could diminish test positivity, a condition prevalently observed in the elderly demographic (8, 33–35). As per serology test, we found a pooled sensitivity of 83.7% and specificity of 73.3%. Our results indicate a higher sensitivity but align with the general trend of lower specificity in the elderly (16, 17). In comparison, according to a study that compared the performance of 29 different serological test by Burucoa et al., in the general population, serological tests have shown sensitivities and specificities ranging from 55.6 to 100% and 59.6 to 97.9%, respectively (36). The results may strengthen the idea that H. pylori antibody tests may yield high percentage of false positive results in the elderly due to the disappearance of H. pylori in advanced gastric mucosal atrophy (37). This implies that in elderly patients with evident signs of H. pylori infection, utilizing alternative diagnostics is prudent, as results advocate for serology primarily to exclude, not confirm, infection (38).
We found that the prevalence of gastric mucosal atrophy, intestinal metaplasia, and other morphological changes in the stomach that increase in older adults, have not impacted the diagnostic accuracy of UBT, when comparing with the general population, as a meta-analysis of UBT diagnostic accuracy shows very similar sensitivity and specificity to our results (6, 8, 35, 39, 40). Our results reveal that such physiological changes may not necessitate the reconsideration of cut-off values in UBT specifically elderly patient, not corroborating previous reports that suggest age-specific adjustments for UBT (24–26, 41, 42), as similar cut-off values were used in the studies assessing UBT diagnostic value (12, 14, 15, 43–45). According to our results, in elderly patients, where there is a high clinical indication of H. pylori infection yet a lack of worrisome symptoms or indication for upper GI endoscopy as stated by guidelines (46), the instigation of a non-invasive diagnostic assessment may be a suitable alternative to immediate gastroscopy (47, 48). This approach might be particularly beneficial for elderly patients who present with new and heightened clinical suspicions of H. pylori infection and have recently undergone endoscopy, especially if they are within the specified intervals between recommended endoscopies (46, 49), which could offer a pragmatic solution for managing suspected cases efficiently, avoiding unnecessary repeat procedures while ensuring accurate diagnosis and timely intervention. Additionally, even in instances where endoscopy is indicated due to concerning features, identifying, and regularly checking for H. pylori via this method can be both time-consuming and expensive (50–52). Thus, employing non-invasive tests can be particularly advantageous in the older population, even when endoscopic examinations are deemed necessary, serving as a cost-effective and efficient alternative for H. pylori regular endoscopic screening and detection. We propose that the UBT be administered concomitantly with HpSA testing to minimize the likelihood of over-diagnosis and to exploit the confirmatory value of the elevated specificity exhibited by the HpSA test in this demographic, especially in older patients that have not been lately treated for H. pylori (53). The amalgamation of multiple diagnostic tests to precisely identify H. pylori infection is a methodology endorsed by Vörhendi et al. within their research conducted on the general population (54).
As our research mainly focuses on individuals aged 60 and above, our findings underscore key age-related physiological changes and their potential impact on test sensitivity and specificity. For instance, the decline in gastric emptying, along with hypoxia and increased levels of reactive oxygen species in aging stomachs, could introduce variations in diagnostic accuracy (40, 55–57). This could explain the significant decline in the sensitivity of HpsA test and the specificity of UBT tests among older adults. The decline in the sensitivity of the serological tests could also be attributed to age-related immunosenescence, affecting immunoglobulin titers, and thereby reducing test accuracy in general (19, 37). The reduction of microbial diversity in the stomach, as people age, may also contribute to variations in test accuracy (58). This may also be attributed to the phenomena known as ‘anorexia of aging’ and post-prandial hypotension, which could potentially exacerbate the condition and impede the accuracy of diagnostic procedures. (39, 59).
One of the major strengths of this meta-analysis is its rigor in methodology, including a comprehensive search strategy and the utilization of dual independent reviewers, thereby minimizing bias, and adhering to PRISMA guidelines (10), with it being the first comprehensive systematic review and meta-analysis in this important topic. However, the significant heterogeneity among studies cannot be overlooked and stands as a substantial limitation (21). The observed extensive heterogeneity could be attributed to numerous elements including the varied prevalence of H. pylori across distinct countries and populations and divergent study designs, encompassing discrepancies in the type or protocol of the reference test; for instance, Han et al. utilized 13C Urea Breath Test (UBT) as a reference (2, 8, 19, 21). Furthermore, the exclusion of non-English studies may have constrained the comprehensiveness of our analysis, particularly given the substantial presence of non-English literature identified during our search (10).
In conclusion, our meta-analysis of 8 studies, which scrutinized three papers on HpSA, three on Serology, and four on UBT, offers a granular and comparative look into the diagnostic accuracy of non-invasive tests for H. pylori in individuals aged 60 and over. Our findings imply that the elevated sensitivity of UBT closely mirrors the values found in the general populace. Moreover, our data might infer that merging UBT’s high sensitivity in older individuals, with the HpsA test’s heightened specificity, can produce precise diagnoses, devoid of the peril of false negatives. This might motivate medical practitioners to employ these non-invasive tests in numerous practical scenarios, to identify and address conditions in the elderly effectively.
Future studies should further validate these findings in the elderly through focused Randomized Controlled Trials (RCTs), particularly evaluating the specificity of HpSA as a potential confirmatory test in this age group. Additionally, the impact of commonly prescribed medications—like Proton Pump Inhibitors (PPIs), Non-Steroidal Anti-Inflammatory Drugs (NSAIDs), and antibiotics—on the outcomes of these diagnostic tests needs exploration, enabling a more refined application of diagnostic methods in older populations.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
MO: Investigation, Writing – original draft. RA-S: Methodology, Validation, Writing – review & editing. RA: Conceptualization, Data curation, Validation, Writing – review & editing. YS: Data curation, Validation, Writing – review & editing. RL: Resources, Visualization, Writing – review & editing. AL: Methodology, Software, Validation, Writing – original draft. KS: Writing – original draft.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Hooi, JKY, Lai, WY, Ng, WK, Suen, MMY, Underwood, FE, Tanyingoh, D, et al. Global prevalence of Helicobacter pylori infection: systematic review and meta-analysis. Gastroenterology. (2017) 153:420–9. doi: 10.1053/j.gastro.2017.04.022
2. Eusebi, LH, Zagari, RM, and Bazzoli, F. Epidemiology of Helicobacter pylori infection. Helicobacter. (2014) 19:1–5. doi: 10.1111/hel.12165
3. Malfertheiner, P, Camargo, MC, El-Omar, E, Liou, JM, Peek, R, Schulz, C, et al. Helicobacter pylori infection. Nat Rev Dis Primers. (2023) 9:19. doi: 10.1038/s41572-023-00431-8
4. Gisbert, JP, and Pajares, JM. Stool antigen test for the diagnosis of Helicobacter pylori infection: a systematic review. Helicobacter. (2004) 9:347–68. doi: 10.1111/j.1083-4389.2004.00235.x
5. Leal, YA, Flores, LL, García-Cortés, LB, Cedillo-Rivera, R, and Torres, J. Antibody-based detection tests for the diagnosis of Helicobacter pylori infection in children: a meta-analysis. PLoS One. (2008) 3:e3751. doi: 10.1371/journal.pone.0003751
6. Huang, Q, Jia, X, Chu, Y, Zhang, X, and Ye, H. Helicobacter pylori infection in geriatric patients: current situation and treatment regimens. Front Med. (2021) 8:713908. doi: 10.3389/fmed.2021.713908
7. Weck, MN, and Brenner, H. Prevalence of chronic atrophic gastritis in different parts of the world. Cancer Epidemiol Biomark Prev. (2006) 15:1083–94. doi: 10.1158/1055-9965.EPI-05-0931
8. Weck, M, Stegmaier, C, Rothenbacher, D, and Brenner, H. Epidemiology of chronic atrophic gastritis: population-based study among 9444 older adults from Germany. Aliment Pharmacol Ther. (2007) 26:879–87. doi: 10.1111/j.1365-2036.2007.03430.x
9. Malfertheiner, P, Megraud, F, Rokkas, T, Gisbert, JP, Liou, JM, Schulz, C, et al. Management of Helicobacter pylori infection: the Maastricht VI/Florence consensus report. Gut. (2022) 71:1724–62. doi: 10.1136/gutjnl-2022-327745
10. Page, MJ, McKenzie, JE, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
11. Whiting, PF. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. (2011) 155:529. doi: 10.7326/0003-4819-155-8-201110180-00009
12. Atli, T, Sahin, S, Arslan, BU, Varli, M, Yalcin, AE, and Aras, S. Comparison of the C14 urea breath test and histopathology in the diagnosis of Helicobacter pylori in the elderly. J Pak Med Assoc. (2012) 62:1061–5.
13. Inelmen, EM, Maccari, T, Enzi, G, Gasparini, G, Fuson, F, Davanzo, B, et al. Helicobacter pylori infection diagnosis in hospitalised elderly patients: the stool antigen test (HpSA) in comparison with other methods. Aging Clin Exp Res. (2004) 16:349–55. doi: 10.1007/BF03324563
14. Newell, DG, Hawtin, PR, Stacey, AR, Mac Dougall, MH, and Ruddle, AC. Estimation of prevalence of Helicobacter pylori infection in an asymptomatic elderly population comparing [14C] urea breath test and serology. J Clin Pathol. (1991) 44:385–7. doi: 10.1136/jcp.44.5.385
15. Pilotto, A, Franceschi, M, Leandro, G, Rassu, M, Zagari, RM, Bozzola, L, et al. Noninvasive diagnosis of Helicobacter pylori infection in older subjects: comparison of the 13C-urea breath test with serology. J Gerontol A Biol Sci Med Sci. (2000) 55:M163–7. doi: 10.1093/gerona/55.3.M163
16. Safe, AF, Warren, B, Corfield, A, McNulty, CA, Watson, B, Mountford, RA, et al. Helicobacter pylori infection in elderly people: correlation between histology and serology. Age Ageing. (1993) 22:215–20. doi: 10.1093/ageing/22.3.215
17. Shirin, H, Bruck, R, Kenet, G, Krepel, Z, Wardi, J, Reif, S, et al. Evaluation of a new immunochromatographic test for Helicobacter pylori IgG antibodies in elderly symptomatic patients. J Gastroenterol. (1999) 34:7–10. doi: 10.1007/s005350050209
18. Inelmen, EM, Gasparini, G, Sergi, G, and Enzi, G. Evaluation of Helicobacter pylori with a stool antigen assay in frail, elderly patients. Scand J Gastroenterol. (2005) 40:794–9. doi: 10.1080/00365520510015638
19. Han, Y, Dai, W, Meng, F, Gan, X, Liu, M, Deng, X, et al. Diagnosis of Helicobacter pylori infection in the elderly using an immunochromatographic assay-based stool antigen test. Microbiology. (2020) 9:e1102. doi: 10.1002/mbo3.1102
20. Glas, AS, Lijmer, JG, Prins, MH, Bonsel, GJ, and Bossuyt, PMM. The diagnostic odds ratio: a single indicator of test performance. J Clin Epidemiol. (2003) 56:1129–35. doi: 10.1016/S0895-4356(03)00177-X
21. Higgins, JPT, and Thompson, SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. doi: 10.1002/sim.1186
22. Goossens, H, Glupczynski, Y, Burette, A, Van den Borre, C, DePrez, C, Bodenmann, J, et al. Evaluation of a commercially available complement fixation test for diagnosis of Helicobacter pylori infection and for follow-up after antimicrobial therapy. J Clin Microbiol. (1992) 30:3230–3. doi: 10.1128/jcm.30.12.3230-3233.1992
23. Leal, YA, Flores, LL, Fuentes-Pananá, EM, Cedillo-Rivera, R, and Torres, J. 13C-urea breath test for the diagnosis of Helicobacter pylori infection in children: a systematic review and Meta-analysis. Helicobacter. (2011) 16:327–37. doi: 10.1111/j.1523-5378.2011.00863.x
24. Zevit, N, Niv, Y, Shirin, H, and Shamir, R. Age and gender differences in urea breath test results. Eur J Clin Investig. (2011) 41:767–72. doi: 10.1111/j.1365-2362.2010.02467.x
25. Chen, X, Haruma, K, Kamada, T, Mihara, M, Komoto, K, Yoshihara, M, et al. Factors that affect results of the 13C urea breath test in Japanese patients. Helicobacter. (2000) 5:98–103. doi: 10.1046/j.1523-5378.2000.00015.x
26. Perets, TT, Gingold-Belfer, R, Leibovitzh, H, Itskoviz, D, Schmilovitz-Weiss, H, Snir, Y, et al. Optimization of 13 C-urea breath test threshold levels for the detection of Helicobacter pylori infection in a national referral laboratory. J Clin Lab Anal. (2019) 33:e22674. doi: 10.1002/jcla.22674
27. Faruqui, AN, Majid, U, Ahmad, L, Khalil, M, and Hassan, MU. Helicobacter pylori stool antigen test (HpSA) for the diagnosis of gastric infection. J Coll Physicians Surg Pak. (2007) 17:316–9.
28. Chang, MC, Wu, MS, Wang, HH, Wang, HP, and Lin, JT. Helicobacter pylori stool antigen (HpSA) test--a simple, accurate and non-invasive test for detection of Helicobacter pylori infection. Hepato-Gastroenterology. (1999) 46:299–302.
29. Yang, HR, and Seo, JK. Helicobacter pylori stool antigen (HpSA) tests in children before and after eradication therapy: comparison of rapid immunochromatographic assay and HpSA ELISA. Dig Dis Sci. (2008) 53:2053–8. doi: 10.1007/s10620-007-0131-8
30. Tanaka, A, and Takahashi, S. Helicobacter pylori stool antigen test. Nihon Rinsho. (2004) 62:464–9.
31. Monaghan, TF, Rahman, SN, Agudelo, CW, Wein, AJ, Lazar, JM, Everaert, K, et al. Foundational statistical principles in medical research: sensitivity, specificity, positive predictive value, and negative predictive value. Medicina. (2021) 57:503. doi: 10.3390/medicina57050503
32. Leeflang, MMG, Rutjes, AWS, Reitsma, JB, Hooft, L, and Bossuyt, PMM. Variation of a test’s sensitivity and specificity with disease prevalence. Can Med Assoc J. (2013) 185:E537–44. doi: 10.1503/cmaj.121286
33. Pilotto, A. Helicobacter pylori infection in older people. World J Gastroenterol. (2014) 20:6364–73. doi: 10.3748/wjg.v20.i21.6364
34. Salles-Montaudon, N, Dertheil, S, Broutet, N, Gras, N, Monteiro, L, De Mascarel, A, et al. Detecting Helicobacter Pylori infection in hospitalized frail older patients: the challenge. J Am Geriatr Soc. (2002) 50:1674–80. doi: 10.1046/j.1532-5415.2002.50459.x
35. Toyokawa, T, Kichiro, S, Miyake, Y, Nakatsu, M, and Ando, M. Eradication of Helicobacter pylori infection improved gastric mucosal atrophy and prevented progression of intestinal metaplasia, especially in the elderly population: a long-term prospective cohort study. J Gastroenterol Hepatol. (2010) 25:544–7. doi: 10.1111/j.1440-1746.2009.05995.x
36. Burucoa, C, Delchier, JC, Courillon-Mallet, A, de Korwin, JD, Mégraud, F, Zerbib, F, et al. Comparative evaluation of 29 commercial Helicobacter pylori serological kits. Helicobacter. (2013) 18:169–79. doi: 10.1111/hel.12030
37. Kato, M, Ota, H, Okuda, M, Kikuchi, S, Satoh, K, Shimoyama, T, et al. Guidelines for the management of Helicobacter pylori infection in Japan: 2016 revised edition. Helicobacter. (2019) 24:e12597. doi: 10.1111/hel.12597
38. Mégraud, F. The most important diagnostic modalities for Helicobacter pylori, now and in the future. Eur J Gastroenterol Hepatol. (2012) 9:S13–5. discussion S15
39. Ferwana, M. Accuracy of urea breath test in Helicobacter pylori infection: meta-analysis. World J Gastroenterol. (2015) 21:1305–14. doi: 10.3748/wjg.v21.i4.1305
40. Tarnawski, AS, and Szabo, I. Apoptosis—programmed cell death and its relevance to gastrointestinal epithelium: survival signal from the matrix. Gastroenterology. (2001) 120:294–8. doi: 10.1053/gast.2001.21402
41. Eisdorfer, I, Shalev, V, Goren, S, Chodick, G, and Muhsen, K. Sex differences in urea breath test results for the diagnosis of Helicobacter pylori infection: a large cross-sectional study. Biol Sex Differ. (2018) 9:1. doi: 10.1186/s13293-017-0161-7
42. Kwon, YH, Kim, N, Yoon, H, Shin, CM, Park, YS, and Lee, DH. Effect of citric acid on accuracy of 13 C-urea breath test after Helicobacter pylori eradication therapy in a region with a high prevalence of atrophic gastritis. Gut Liver. (2019) 13:506–14. doi: 10.5009/gnl18398
43. Atherton, J. Non-endoscopic tests in the diagnosis of Helicobacter pylori infection. Aliment Pharmacol Ther. (1997) 11:11–20. doi: 10.1046/j.1365-2036.11.s1.3.x
44. Mion, F, Rosner, G, Rousseau, M, and Minaire, Y. 13C-urea breath test for Helicobacter pylori: cut-off point determination by cluster analysis. Clin Sci. (1997) 93:3–6. doi: 10.1042/cs0930003
45. Kato, C, Sugiyama, T, Sato, K, Saito, S, Kudara, N, Abiko, Y, et al. Appropriate cut-off value of 13C-urea breath test after eradication of Helicobacter pylori infection in Japan. J Gastroenterol Hepatol. (2003) 18:1379–83. doi: 10.1046/j.1440-1746.2003.03193.x
46. Early, DS, Ben-Menachem, T, Decker, GA, Evans, JA, Fanelli, RD, Fisher, DA, et al. Appropriate use of GI endoscopy. Gastrointest Endosc. (2012) 75:1127–31. doi: 10.1016/j.gie.2012.01.011
47. Mao, LQ, Wang, SS, Zhou, YL, Chen, L, Yu, LM, Li, M, et al. Clinically significant endoscopic findings in patients of dyspepsia with no warning symptoms: a cross-sectional study. World J Clin Cases. (2021) 9:3597–606. doi: 10.12998/wjcc.v9.i15.3597
48. Talley, NJ. Dyspepsia: management guidelines for the millennium. Gut. (2002) 50:iv72–8. doi: 10.1136/gut.50.suppl_4.iv72
49. Kim, JW, Jung, KW, Kwon, JG, Lee, JB, Park, JK, Bang, KB, et al. What is appropriate upper endoscopic interval among dyspeptic patients with previously normal endoscopy? A multicenter study with Bayesian change point analysis. J Neurogastroenterol Motil. (2019) 25:544–50. doi: 10.5056/jnm19063
50. Onders, RP. Detection methods of Helicobacter pylori: accuracy and costs. Am Surg. (1997) 63:665–8.
51. Toulaymat, M, Marconi, S, Garb, J, Otis, C, and Nash, S. Endoscopic biopsy pathology of Helicobacter pylori gastritis. Arch Pathol Lab Med. (1999) 123:778–81. doi: 10.5858/1999-123-0778-EBPOHP
52. Benoit, A, Hoyeau, N, and Fléjou, JF. Diagnostic d’infection à Helicobacter pylori sur biopsies gastriques: coloration standard, coloration spéciale ou immunohistochimie? Ann Pathol. (2018) 38:363–9. doi: 10.1016/j.annpat.2018.03.009
53. Hahn, M, Fennerty, MB, Corless, CL, Magaret, N, Lieberman, DA, and Faigel, DO. Noninvasive tests as a substitute for histology in the diagnosis of Helicobacter pylori infection. Gastrointest Endosc. (2000) 52:20–6. doi: 10.1067/mge.2000.106686
54. Vörhendi, N, Soós, A, Anne Engh, M, Tinusz, B, Szakács, Z, Pécsi, D, et al. Accuracy of the Helicobacter pylori diagnostic tests in patients with peptic ulcer bleeding: a systematic review and network meta-analysis. Ther Adv Gastroenterol. (2020) 13:175628482096532. doi: 10.1177/1756284820965324
55. Soenen, S, Rayner, CK, Horowitz, M, and Jones, KL. Gastric emptying in the elderly. Clin Geriatr Med. (2015) 31:339–53. doi: 10.1016/j.cger.2015.04.003
56. Gomes, AP, Price, NL, Ling, AJY, Moslehi, JJ, Montgomery, MK, Rajman, L, et al. Declining NAD+ induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cells. (2013) 155:1624–38. doi: 10.1016/j.cell.2013.11.037
57. Samoszuk, MK, Walter, J, and Mechetner, E. Improved Immunohistochemical method for detecting hypoxia gradients in mouse tissues and tumors. J Histochem Cytochem. (2004) 52:837–9. doi: 10.1369/jhc.4B6248.2004
58. Shin, CM, Kim, N, Park, JH, and Lee, DH. Changes in gastric corpus microbiota with age and after Helicobacter pylori eradication: a long-term follow-up study. Front Microbiol. (2021) 11:621879. doi: 10.3389/fmicb.2020.621879
59. Pham, H, Phillips, L, Trahair, L, Hatzinikolas, S, Horowitz, M, and Jones, KL. Longitudinal changes in the blood pressure responses to, and gastric emptying of, an oral glucose load in healthy older subjects. J Gerontol A Biol Sci Med Sci. (2019) 75:244–8. doi: 10.1093/gerona/glz014
Keywords: H. pylori, elderly, non-invasive, endoscopy, urea breath test, stool analysis
Citation: Omar M, Abu-Salah R, Agbareia R, Sharif Y, Levin R, Lahat A and Sharif K (2023) A comparative systematic review and meta-analysis on the diagnostic accuracy of non-invasive tests for Helicobacter pylori detection in elderly patients. Front. Med. 10:1323113. doi: 10.3389/fmed.2023.1323113
Received: 17 October 2023; Accepted: 23 November 2023;
Published: 08 December 2023.
Edited by:
Amin Talebi Bezmin Abadi, Tarbiat Modares University, IranReviewed by:
Caroline Tianeze de Castro, Federal University of Bahia, BrazilCopyright © 2023 Omar, Abu-Salah, Agbareia, Sharif, Levin, Lahat and Sharif. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kassem Sharif, a2Fzc2Vtc2hhcmlmQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.