- 1School of Public Health, Ningxia Medical University, Yinchuan, Ningxia, China
- 2Department of Joint Surgery, HongHui Hospital, Xi'an Jiaotong University, Xi'an, Shannxi Province, China
Objective: Osteoarthritis (OA) is associated with cardiovascular disease and represents a persistent economic and physical burden on patients in the United States. This study evaluated the mediating effect of dietary live microbe intake on the association between cardiovascular health [based on Life's Essential 8 (LE8) scores] and osteoarthritis (OA) in adults.
Methods: This cross-sectional study included data from the National Health and Nutrition Examination Survey, 1999–2019 (from patients aged ≥20 years). LE8 scores (0–100) were measured according to the American Heart Association definition and categorized as low (0–49), moderate (50–79), or high (80–100). OA disease status was assessed using self-reported data from patients. The relationships were evaluated using multivariate logistic and restricted cubic spline models. Mediation analysis was used to evaluate the mediating effect of dietary live microbe intake on the association between LE8 and OA risk.
Results: The study included 23,213 participants aged ≥20 years. After adjusting for latent confounders, higher LE8 scores were found to be associated with a lower incidence of OA. The odds ratios (with 95% confidence intervals) for low, moderate, and high OA risk were 0.81 (0.69, 0.96) and 0.55 (0.44, 0.69), respectively; a non-linear dose-response relationship was observed (P-nonlinear = 0.012). Health behavior and health factor scores showed a similar pattern of correlation with OA risk. Low live microbe intake mediated the association between LE8, health behavior, and health factor scores with OA risk and did not appear to reduce OA risk.
Conclusion: Our findings suggest that although higher LE8 scores reduce the risk of developing OA, low live microbe intake may reduce the protective effect of higher scores. It is, therefore, essential to emphasize adherence to a lifestyle that confers high LE8 scores. Individuals should also be advised to reduce the intake of foods with low live microbe content.
1 Introduction
Osteoarthritis (OA) is the most common form of arthritis and is caused by an imbalance between repair and destruction of joint tissue. It entails structural changes in the joints, including cartilage degeneration, synovial inflammation, and inflammation of the capsular ligaments. The United States has the highest age-standardized prevalence rate of OA (1), and the number of affected individuals is expected to increase to 67 million by 2030 (2). Notably, OA is associated with an increased risk of premature death from cardiovascular disease (CVD). Therefore, healthcare professionals need to take particular note of modifiable cardiovascular risk factors (including hypertension, diabetes mellitus, hyperlipidemia, smoking, and physical inactivity) in this population (3). Certain lifestyle-related risk factors have been demonstrated to have a definite biological effect on the development of OA and these include age, gender, smoking, diet, hypertension, sedentary lifestyle, body mass index (BMI), low-density lipoprotein levels, genetics, metformin use, bone mineral density, joint shape abnormalities, joint malalignment, decreased muscle strength/mass, injuries, and joint loading abnormalities (4–6). Although the causes of OA have not been fully elucidated, there is a growing agreement that indispensable environmental factors (health behaviors and diet) are important contributors to this disease (6, 7). In 2010, the American Heart Association recommended Life's Simple 7 as a measure of cardiovascular health (CVH), with the aim of improving the health of the general population (8). Owing to the limitations of the LS7 CVH score, the American Heart Association recently updated the evaluation tool to Life's Essential 8 (LE8) (9). The LE8 scoring system is more sensitive to differences between individuals and emphasizes the role of maintaining or improving CVH. The components of LE8 include diet (updated), physical activity, nicotine exposure (updated), sleep health (new), body mass index, blood lipids (updated), blood glucose (updated), and blood pressure. In this context, epidemiologic studies have shown that conventional risk factors for CVD such as age, hypertension, diabetes mellitus, obesity, and low physical activity are associated with the development and progression of OA (10). However, no studies have evaluated the association between CVH (LE8 scores) and OA risk.
Notably, previous studies have shown the existence of mutual protective factors between OA and CVD (11). In their study, Sanders et al. assessed the number of live microbes consumed in the diet and accordingly categorized foods into low [Lo; < 104 colony forming units (CFU)/g], medium (Med; 104–107 CFU/g), and high (Hi: >107 CFU/g) groups based on the number of live microbes per gram. In this context, certain probiotics (including Bifidobacterium bifidum and Lactobacillus acidophilus) may lower elevated cholesterol levels and aid the prevention and treatment of some CVDs (12, 13). Beneficial symbiotic microbes have also been found to be capable of exerting cholesterol-lowering effects (14). In addition, studies have reported that gut dysbiosis exacerbates OA; this may be explained by disruption of the host–gut microbial balance, which, in turn, triggers the host immune response and activates the “gut–joint axis” (15). Research suggests that LE8 scores and live microbe intake may reduce the risk of OA by reducing oxidative stress, inflammation, and obesity (16). However, the relevance of these factors is limited by the lack of animal and human studies on this association. This cross-sectional study, using data from the National Health and Nutrition Examination Survey (NHANES) 2005–2019 cohort, was therefore performed to evaluate the association between LE8 scores and OA risk. The live microbe content of 9,388 foods listed in the NHANES database was also evaluated; the foods were grouped according to microbial content, and their mediating effects were assessed.
2 Materials and methods
2.1 Database and study subjects
The NHANES utilizes stratified multistage probability sampling methods to select a series of nationally representative samples of non-institutionalized United States adults in 2-year cycles, which started from 1999 to 2000 (http://www.cdc.go/nchs/nhanes.htm). The NHANES program was approved by the Ethics Review Board of the National Center for Health Statistics. All participants provided written informed consent to participate in the survey and agreed to the use of their data in health-related statistical research. The study followed the Strengthening Reporting of Observational Studies in Epidemiology reporting guidelines (17). As shown in Figure 1, data from 23,213 participants (aged 20 years and older with complete data from eight survey cycles spanning from 2005–2006 to 2017–2019) were included in the study.
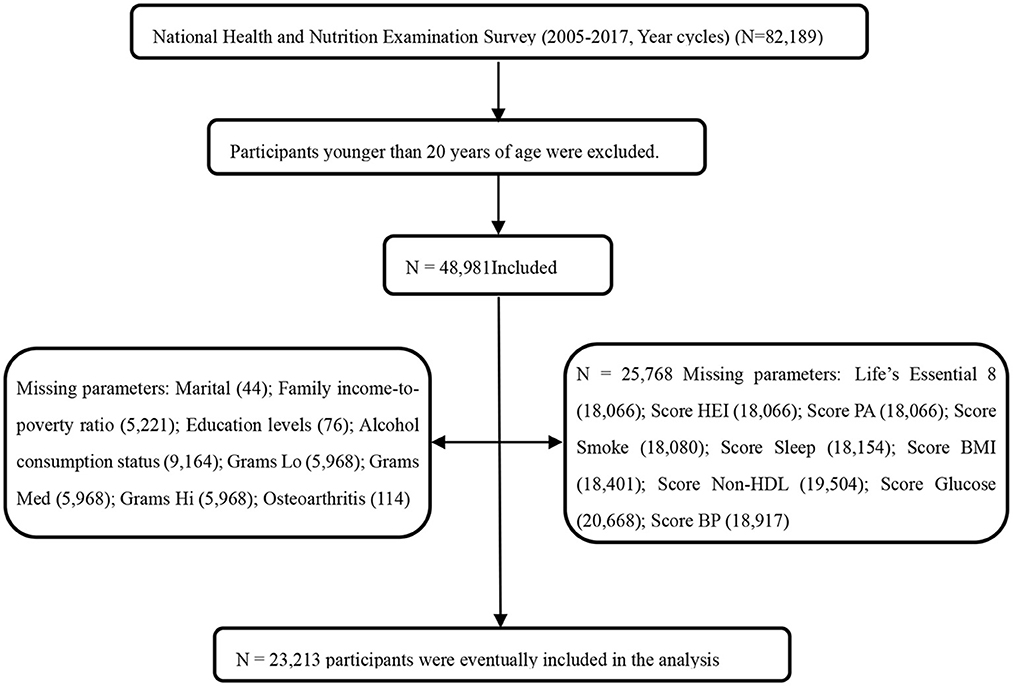
Figure 1. Flowchart of the screening process for the selection of the study population. NHANES, National Health and Nutrition Examination Survey; FBG, fasting blood glucose; HEI score, Healthy Eating Index; Non-HDL, Non-High-Density Lipoprotein Cholesterol; BP, Blood Pressure; Experts assigned foods an estimated level of live microbes per gram [low (Lo), <104 CFU/g; medium (Med), 104–107 CFU/g; or high (Hi), >107 CFU/g]; CVH, cardiovascular health; Life's Essential 8, The components of Life's Essential 8 include diet (updated), physical activity, nicotine exposure (updated), sleep health (new), body mass index, blood lipids (updated), blood glucose (updated), and blood pressure. Each metric has a new scoring algorithm ranging from 0 to 100 points, allowing the generation of a new composite cardiovascular health score (the unweighted average of all components) that also varies from 0 to 100 points. The LE8 scoring algorithm consists of 4 health behaviors (diet, physical activity, nicotine exposure, and sleep duration) and 4 health factors [body mass index (BMI), non-high-density-lipoprotein cholesterol, blood glucose, and blood pressure]. The overall LE8 score, health behavior score, and health factor score were calculated as the unweighted average of the eight metrics. Participants with a LE8 score of 80–100 were considered high CVH; 50–79, moderate CVH; and 0–49 points, low CVH.
2.2 Measurement of LE8 scores
The LE8-scored sub-questionnaire assesses four health behaviors (diet, physical activity, nicotine exposure, and sleep duration) and four health factors (BMI, non-high-density lipoprotein cholesterol, blood glucose, and blood pressure) (9). The scores for the eight CVH metrics range from 0 to 100 (9). The overall LE8 score is calculated as the arithmetic mean of the eight metrics. Participants with LE8 scores of 80–100, 50–79, and 0–49 are considered to have high, moderate, and low CVH, respectively. In this study, dietary indicators were evaluated using the Healthy Eating Index 2015 scores; dietary intakes of participants (obtained from two 24-h dietary recalls) were combined with United States Department of Agriculture Food Pattern Equivalent data to calculate the scores. Self-reported questionnaires were used to obtain information regarding the frequency and duration of vigorous or moderate physical activity over the past 30 days. Smoking habits, sleep duration, history of diabetes, and medication history were also assessed using self-reported questionnaires. Blood pressure, height, and weight were measured during physical examination. The blood pressure was determined by averaging three consecutive measurements, and the BMI was calculated by dividing the weight in kilograms by the square of the height in meters. Blood samples were collected and sent to a central laboratory for analysis of lipid, blood glucose, and glycosylated hemoglobin levels (9).
2.3 OA assessment
A study has shown 81% consistency between self-reported and clinically confirmed diagnoses (18). In the NHANES, OA was diagnosed by a professional, and information was obtained using a questionnaire. All participants (aged ≥20 years) were asked questions related to arthritis. They were asked the following question: “Has a doctor or other health professional ever told you that you have arthritis?” Participants were included in the study if their responses indicated that they were diagnosed with OA (19).
2.4 Definition of live microbes in food
A classification system has been established for defining and estimating the dietary intake of live microbes among United States adults. The NHANES uses food codes for the assigned categories. Among the 9,388 food codes in the NHANES database, 8,954 contain small amounts of live microbes (< 104 CFU/g) (20). Processed foods that usually undergo heat treatment (such as milk; prepared meats including pork, poultry, and seafood dishes; and sauces and gravies) are considered to be very low in microbes and are therefore classified as Lo foods. Raw meats, pork, poultry, and seafood are also classified in the Lo category, based on the presumption that they are cooked prior to consumption (with the exception of a few of these foods that are specified as being eaten raw). Fresh vegetables and fruits are the top two food items allocated to the Med category (accounting for 41 and 39%, respectively). Fresh fruit juices, such as fruit smoothies, are allocated to the Med category; beverages, condiments, and sauces comprise more than 10% of the food allocated to this category. Some fermented foods (e.g., miso and sauerkraut) are also assigned to the Med category. Notably, fermented dairy products comprise the majority of foods assigned to the Hi category. Yogurt and other fermented milks are assigned to this category unless they constitute a component of another food. Codes that contain a significant amount of fermented foods, such as yogurt or sour cream, are assigned to the Hi category. However, foods that contain cheese as a minor component are assigned to the Lo or Med categories, depending on their relative amount in the food (20).
2.5 Defining covariates
Demographic information was obtained using a questionnaire. The attributes included age (20–44, 45–64, and ≥65 years), gender, race (non-Hispanic white, non-Hispanic Black, Hispanic, and others), marital status (married, separated, and never married), and family income to poverty ratio (< 1.30, 1.30– < 3.00, 3.00– < 5.00, and ≥5.00). This ratio represents the proportion of the family income in relation to the federal poverty threshold after adjusting for household size; a higher ratio indicates a higher level of income. Data were also obtained on the level of education [less than high school (grade 11), high school graduate/general education, college, and above college level] and the alcohol consumption status [current heavy drinker (women: ≥three drinks per day, men: ≥four drinks per day, or binge drinking for 5 days or more per month), current moderate drinker (women: ≥two drinks per day, men: ≥three drinks per day, or binge drinking ≥2 days per month), current light/mild alcohol drinkers (not fulfilling the criteria for the previous two categories) (6), former alcohol consumption (previous history of drinking but not a current drinker), and no history of alcohol consumption].
2.6 Statistical analysis
To estimate the statistical data representative of United States adults, oversampling, stratification, and clustering were performed in accordance with the NHANES guidelines; particular emphasis was placed on weight-adjusted statistical tests. Chi-square and t-tests were used to evaluate demographic characteristics, pertaining to OA status. The association between LE8 scores and the risk of OA was evaluated using a multivariate logistic regression model; the results were presented as the odds ratio (OR) with 95% confidence intervals (95% CIs). Stratified analyses were performed by gender, age, and ethnicity to evaluate the association between LE8 scores and OA risk in different groups. The group with low LE8, health behavior, and health factor scores was considered as the reference group. Restricted cubic spline plots were used to evaluate trends among variables that were found to demonstrate the significance of logistic regression. They were also used to determine the presence of any non-linear association between exposure factors and OA risk. The potential mediating role of live microbes (in Lo, Med, and Hi foods) in the association between LE8 scores and OA risk was evaluated using a parallel mediation model. Mediation analysis was performed using the mediation package of R; a quasi-Bayesian Monte Carlo method with 1,000 simulations was used based on the normal approximation. The direct and indirect effects represented the effect of LE8 scores on OA risk without mediators and via the mediator, respectively. The proportion of mediation was calculated as the quotient of the indirect effect divided by the total effect.
All remaining statistical analyses were performed using R software (version 4.2.2, https://cran.r-project.org/bin/windows/base/old/4.2.2/); the following packages were used: nhanesR (version 0.9.2.8), survey, CompareGroups, dplyr, tidyverse, do, MASS, finalfit, Hmisc, lattice, Formula, rms, and foreign. The statistical tests were two-sided, and the results were considered statistically significant when the P-value was < 0.05.
3 Results
3.1 Baseline characteristics
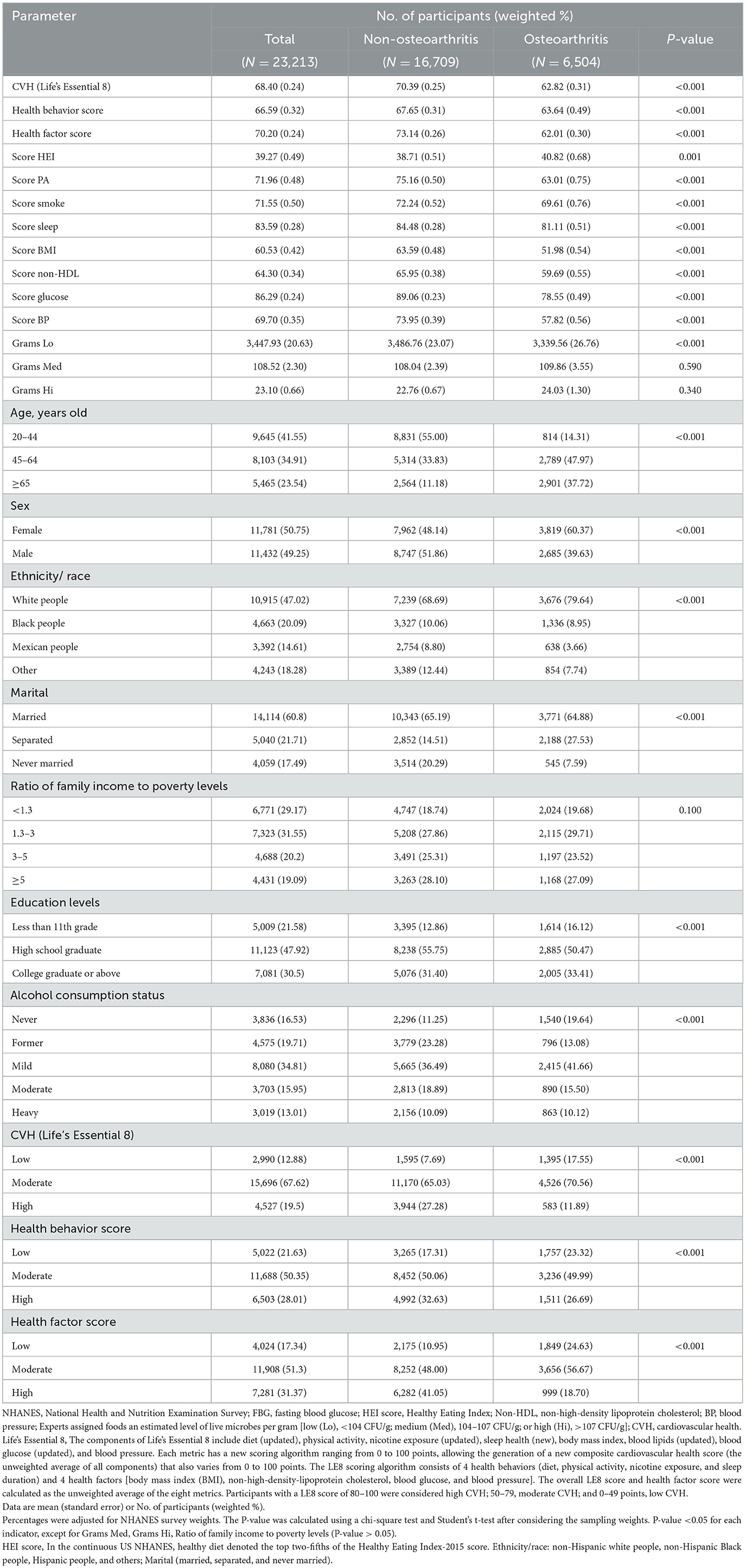
The data from 23,213 participants (aged ≥20 years) were included. The baseline characteristics of the study population (according to OA risk) are presented in Table 1. The weighted percentages of participants aged 20–44, 45–64, and ≥65 years were 41.55%, 47.97%, and 37.72%, respectively. The weighted percentages of the 11,781 female and 11, 432 male individuals were 50.75% and 49.25%, respectively. The mean LE8 score for the total population was 68.40, and the low, moderate, and high scores (weighted %) were 5,022 (21.63), 1,688 (50.35), and 6,503 (28.01), respectively. The scores for those with OA (62.82) was lower than that of participants without OA (70.39). In contrast to those without OA, those with the condition were older, more likely to be women, and more likely to be of non-Hispanic white ethnicity; they also had lower LE8 (and its component metrics) scores, consumed more foods with lower live microbe content, and were mostly married. However, as shown in Table 1, live microbe intake (Med and Hi foods) and the ratio of family income to poverty levels did not differ significantly between the two groups.
3.2 Univariate logistic regression analysis for the association between LE8 scores and OA risk
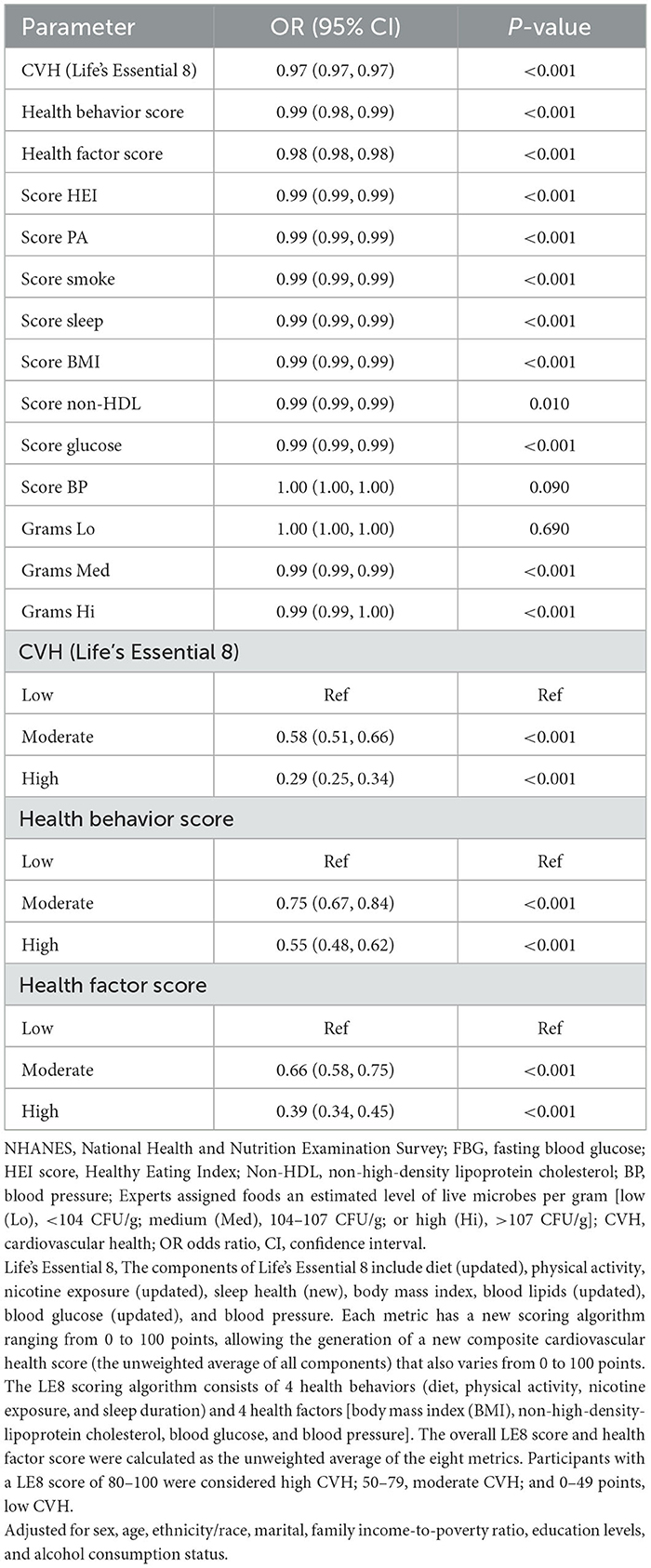
Participants with low CVH (LE8 scores) demonstrated moderate [OR: 0.58; 95% CI: (0.51, 0.66)] and high [OR: 0.29; 95% CI: (0.25,0.34)] propensity for developing OA. Individuals with low health behavior scores also demonstrated moderate [OR: 0.75; 95% CI: (0.67, 0.84)] and high [OR: 0.55; 95% CI: (0.48, 0.62)] OA risk. Additionally, those with low health factor scores showed moderate [OR: 0.66; 95% CI: (0.58, 0.75)] and high [OR: 0.39; 95% CI: (0.34, 0.45)] likelihood of developing OA. The LE8, health behavior, and health factor scores remained significantly associated with OA risk on being used as continuous variables, and this was suggestive of their possible protective role against OA. Live microbe intake was associated with OA risk; in particular, the intake of Med [OR: 0.99; 95% CI: (0.99, 0.99)] and Hi [OR: 0.99; 95% CI: (0.99, 1.00)] category foods appeared to be protective against OA (Table 2).
3.3 Multivariate logistic regression analysis for the association between LE8 scores and OA
After adjusting for multiple latent variables, LE8, health behavior, and health factor scores continued to demonstrate a significant association with OA risk. In model 2, the groups with moderate and high LE8 scores (representing CVH) demonstrated an OR of 0.81 (95% CI: 0.69, 0.96) for developing OA; in comparison, participants with low LE8 scores demonstrated an OR of 0.55 (95% CI: 0.44, 0.69). The groups with moderate and high health behavior scores demonstrated an OR of 0.83 (95% CI: 0.73, 0.95) for OA risk; in the group with low scores, the OR was 0.74 (95% CI: 0.63, 0.87). The groups with moderate and high health factor scores demonstrated an OR of 0.73 (95% CI: 0.64, 0.84), while the group with low scores showed an OR of 0.53 (95% CI: 0.45, 0.61; Table 3). As shown in Figure 2, a non-linear relationship was observed between OA risk and LE8 scores [P-nonlinear < 0.001; minimum threshold value for beneficial association: 66.52 (estimated OR = 1)], health behavior scores [P-nonlinear < 0.001; minimum threshold value for beneficial association: 67.34 (estimated OR = 1)], and health factor scores [P-nonlinear < 0.001; minimum threshold value for beneficial association: 68.36 (estimated OR = 1)].
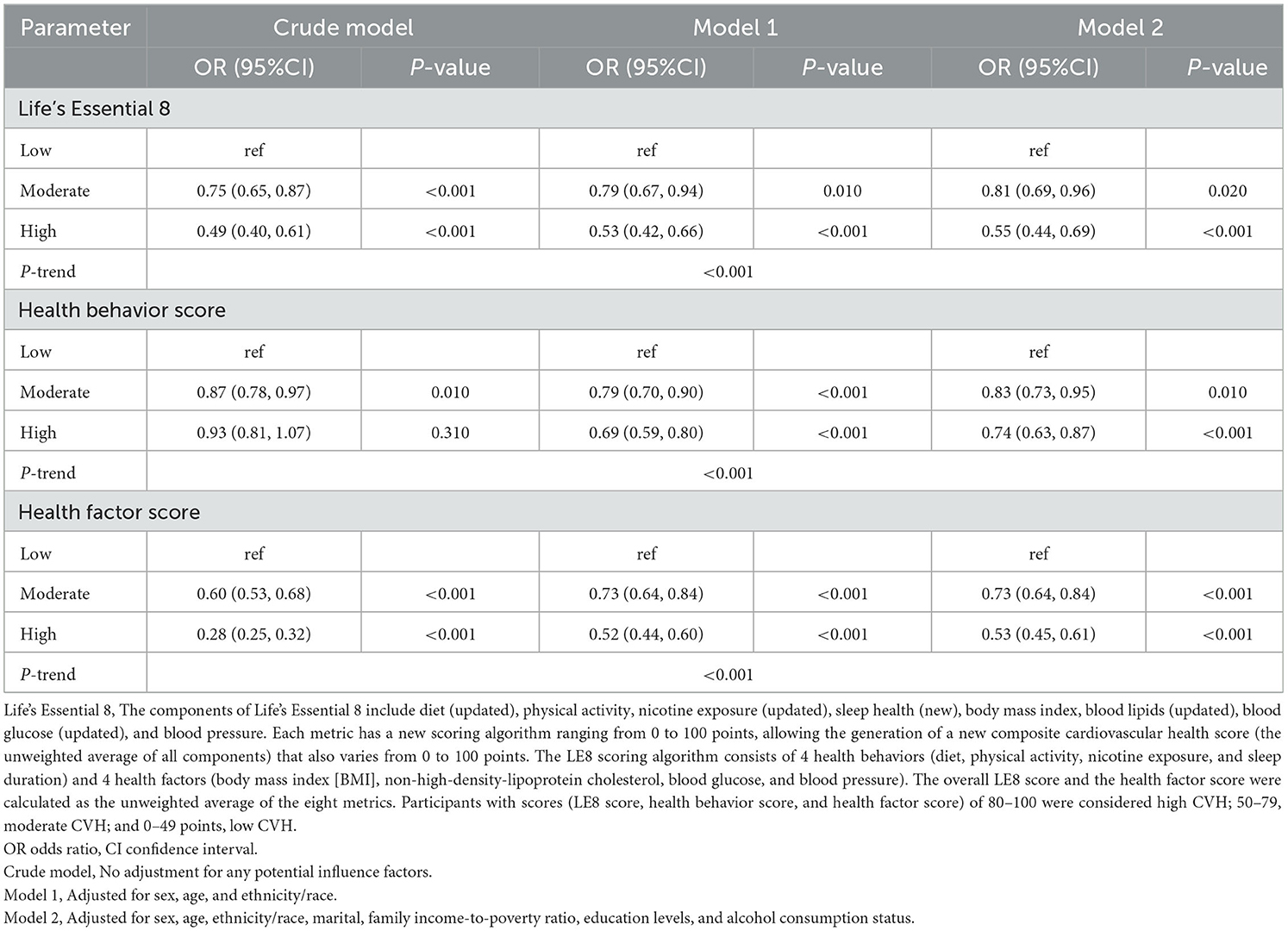
Table 3. Multiple logistic regression models of Life's Essential 8 with osteoarthritis for participants.
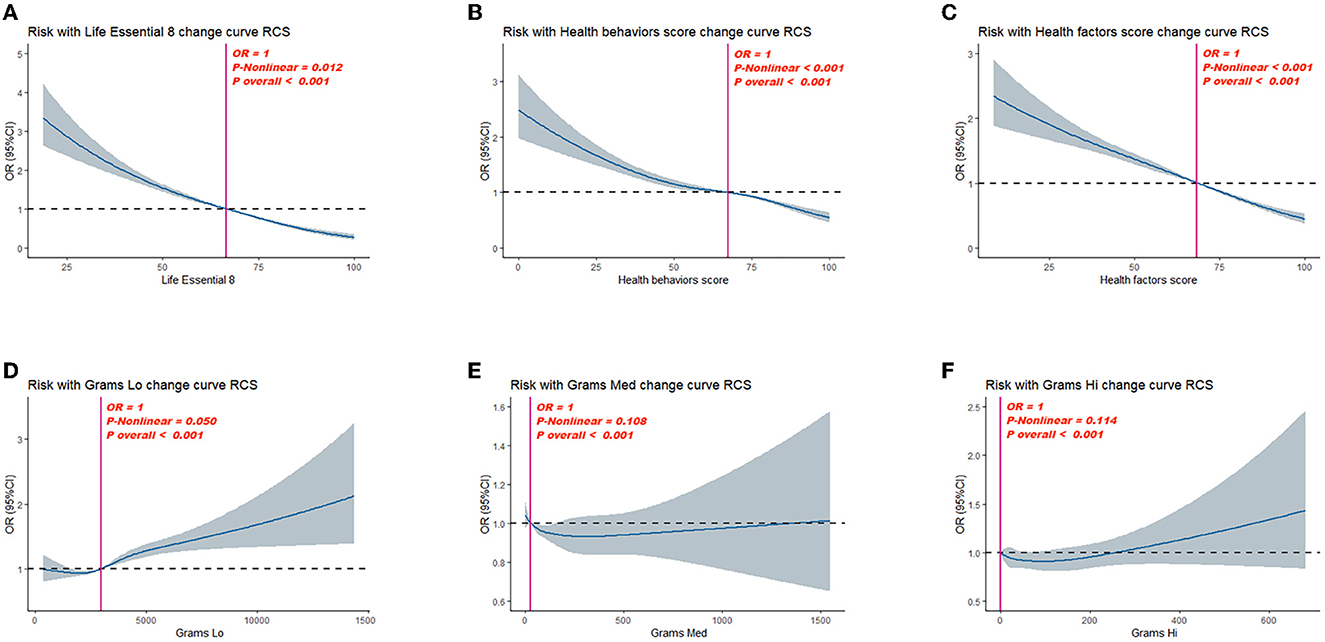
Figure 2. Dose–response relationships between Life's Essential 8 scores (A), health behavior score (B), health factor score (C), Grams Lo (D), Grams Med (E), Grams Hi (F), and Osteoarthritis (OA). OR (95% CI; shaded areas) were adjusted for sex, age, ethnicity/race, marital, family income-to-poverty ratio, education levels, and alcohol consumption status. Vertical red solid lines indicate the minimal threshold for the beneficial association with estimated OR = 1. OR, odds ratio.
3.4 Subgroup analysis for the association between LE8 scores and OA risk
After adjusting for multiple latent variables, the LE8, health behavior, and health factor scores were all found to be significantly correlated with OA risk across the different groups (irrespective of sex, ethnicity/race, and age); therefore, these may serve as protective factors against OA. On comparing the group with low LE8 scores with the group having moderate and high scores, the ORs (with 95% CIs) for each subgroup were as follows: male: 0.55 (0.46, 0.66), 0.30 (0.23,0.38); female: 0.48 (0.40, 0.56), 0.16 (0.14,0.20); white: 0.54 (0.46, 0.64), 0.22 (0.18, 0.27); Black: 0.47 (0.40, 0.55), 0.17 (0.12, 0.24); Mexican: 0.21 (0.14, 0.32), 0.46 (0.34, 0.62); 20–44 years old: 0.37 (0.29, 0.48), 0.20 (0.14, 0.27); 45–64 years old: 0.37 (0.29, 0.48), 0.20 (0.14, 0.27), 0.20 (0.14, 0.27); 45–64 years old: 0.63 (0.53, 0.74), 0.25 (0.20, 0.31); and ≥65 years old: 0.75 (0.60, 0.93), 0.56 (0.41, 0.77). On comparing participants with low health behavior scores with those having moderate and high scores, the ORs (with 95% CIs) for each subgroup were as follows: male: 0.81 (0.69, 0.95), 0.70 (0.57, 0.86); female: 0.73 (0.63, 0.84), 0.55 (0.47, 0.64); white: 0.82 (0.71, 0.94), 0.63 (0.54, 0.74); Black: 0.74 (0.62, 0.90), 0.58 (0.45, 0.74); Mexican: 0.51 (0.40, 0.63), 0.49 (0.37, 0.65); 20–44 years old: 0.56 (0.46, 0.69), 0.41 (0.31, 0.55); 45–64 years old: 0.78 (0.67, 0.90), 0.51 (0.43, 0.61); and ≥65 years old: 1.01 (0.82, 1.23), 0.86 (0.69, 1.08). On comparing participants with low health factor scores with those having moderate and high scores, the ORs (with 95% CIs) for each subgroup were as follows: male: 0.60 (0.50, 0.71), 0.32 (0.26, 0.39); female: 0.52 (0.44, 0.62), 0.19 (0.16, 0.22); white: 0.57 (0.48, 0.68), 0.25 (0.22, 0.30); Black: 0.50 (0.43, 0.58), 0.19 (0.15, 0.24); Mexican: 0.49 (0.38, 0.64), 0.19 (0.14, 0.27); 20–44 years old: 0.46 (0.36, 0.59), 0.24 (0.19, 0.31); 45–64 years old: 0.69 (0.58, 0.81), 0.41 (0.35, 0.49); and ≥65 years old: 0.72 (0.60, 0.88), 0.57 (0.46, 0.72) (Table 4).
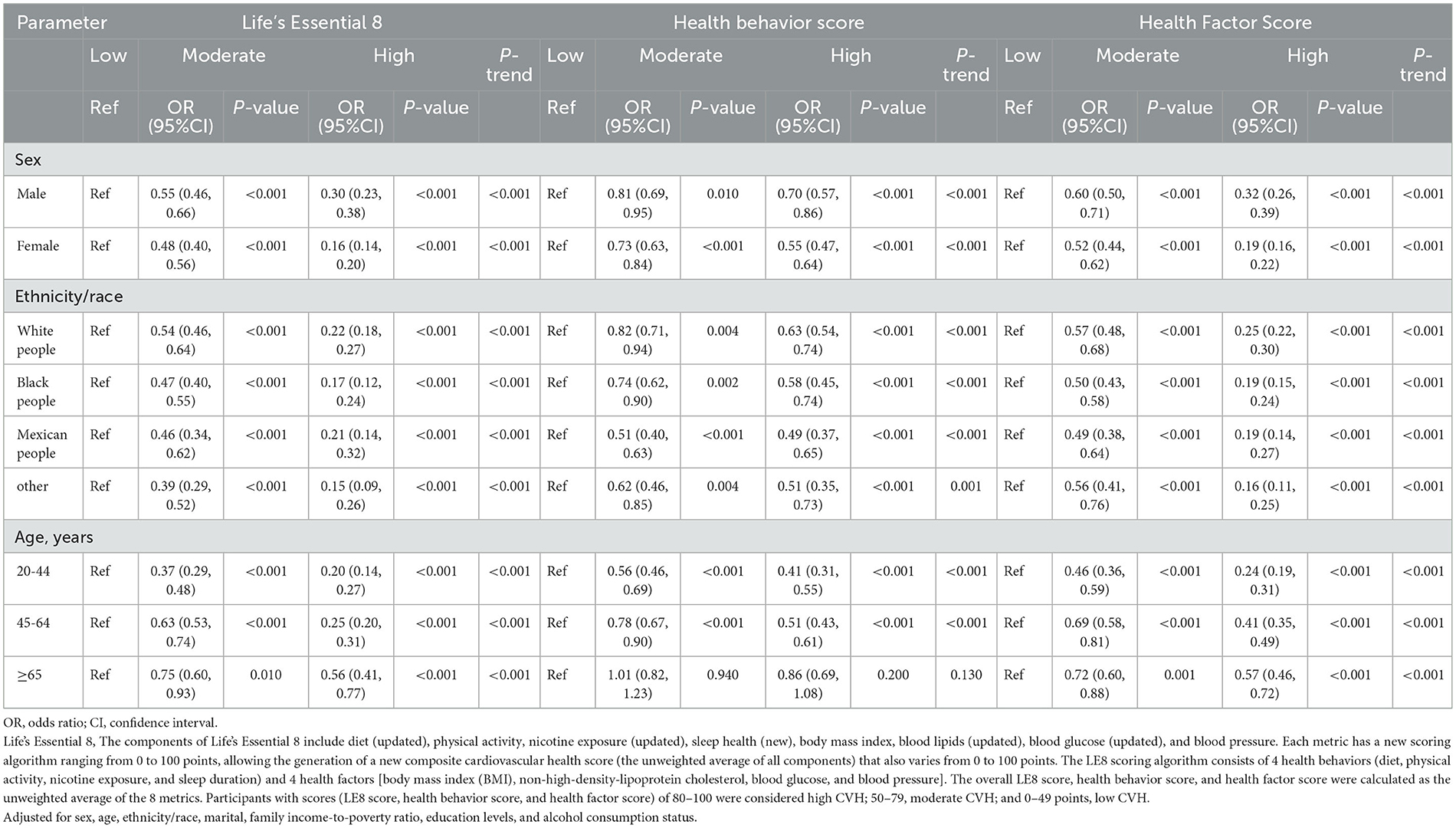
Table 4. Results of multiple logistic regression of participant scores using Life's Essential 8 with Osteoarthritis subgroup analysis.
3.5 Mediation analysis
Parallel mediation analysis was performed to evaluate the potential mediating role of dietary active microorganisms in the association between the LE8, health behavior, and health factor scores and OA risk. Notably, Lo category foods demonstrated a mediating effect on the association between the LE8, health behavior, and health factor scores and OA risk. The mediation proportions were: 0.01%, P ≤ 0.001; 0.01%, P = 0.018; 0.01%; and P ≤ 0.001, respectively. A non-linear relationship was observed between Lo foods and OA risk (P-nonlinear = 0.050; Figure 2). As shown in Figure 3, the maximum threshold for the beneficial association was 2,972.81 CFU/g (estimated OR = 1).
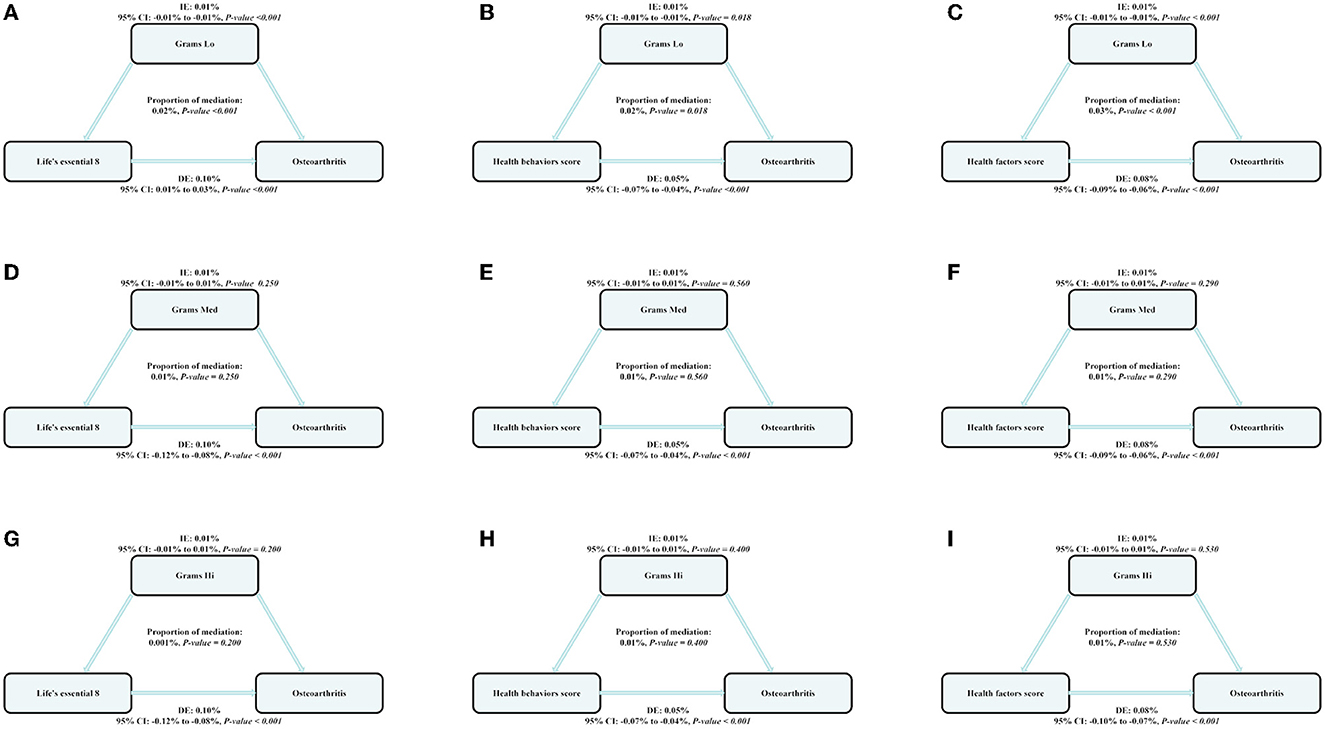
Figure 3. The estimated proportions of the associations between CVH (Life's Essential 8), health behavior score, health factor score, and OA mediated effect by the dose of dietary intake of live microbes. Model adjusted for sex, age, ethnicity/race, marital, family income-to-poverty ratio, education levels, and alcohol consumption status. IE, the estimate of the indirect effect; DE, the estimate of the direct effect; Proportion of mediation = IE/DE + IE, OR, odds ratio. (A, D, G) Shows the relationship between Life's Essential 8 and Osteoarthritis: the mediating effect of dietary live microbe (Grams Lo, Med, Hi) intake. (B, E, H) Shows the relationship between Life's Essential 8 and Osteoarthritis: the mediating effect of dietary live microbe (Grams Lo, Med, Hi) intake. (C, F, I) Shows the relationship between Life's Essential 8 and Osteoarthritis: the mediating effect of dietary live microbe (Grams Lo, Med, Hi) intake.
4 Discussion
The present study provides two new major findings regarding the general population of the United States (based on the NHANES 2005–2019 cohort). First, LE8 scores consistently showed protective effects against OA. Second, Lo foods appeared to increase the risk of developing OA; in particular, they demonstrated a mediating effect in the protection offered by LE8 scores against OA.
Our findings suggest that higher LE8 scores reduce the risk of OA. In addition, the number of healthy LE8 indicators is directly proportional to the reduction in OA risk. In this context, direct research evidence on the association between LE8 scores and the risk of developing OA is currently lacking. Several studies have estimated the association between modifiable lifestyle-related risk factors and the risk of developing OA. Examples of such factors include smoking habits, dietary patterns, hypertension, sedentary lifestyle, BMI, low-density lipoprotein levels, exercise, and sleep (4–6, 21). CVH and OA are the most common causes of joint pain in adults with shared risk factors. As OA and CVD coexist, individuals who are at risk for developing one condition should be advised to undergo testing for the other (22). A study that evaluated four lifestyle factors (blood pressure, cholesterol levels, smoking, and diabetes) associated with CVD-related deaths before the age of 80 years, found that individuals with the best risk factor characteristics demonstrated a significantly lower risk than those with at least two major risk factors (23). Targeting these factors with low-intensity lifestyle interventions may therefore improve joint pain (24). Epidemiological studies have recognized healthy physical activity to be a key factor in the prevention and management of CVD and OA. A study also reported that 20–30 min of exercise performed once a week had a preventive effect on OA, especially in young patients with knee OA (25). Notably, insomnia and shorter sleep duration have an adverse impact on the risk of OA (21). Additionally, smoking, total counts, and triglyceride and low-density lipoprotein levels are risk factors for OA; an elevation in the levels of these laboratory parameters has been found to increase the risk of OA (26, 27). Emerging evidence suggests that hyperglycemia has a detrimental effect on the knee joint (28). The findings from studies have suggested that inflammation (29), lack of exercise (25), and medications (4) may contribute to this interrelationship. In this context, inflammatory mediators (including chemokines and cytokines) (30) play a key role in the pathogenesis of OA. In our study, higher LE8 scores (indicative of better CVH) consistently showed a protective effect against OA. Higher health behavior and factor scores were also found to be protective. This implies that a change in lifestyle habits may effectively reduce the risk of OA, and that CVH (as indicated by LE8 scores) has a non-negligible role in the prevention of OA.
Further mediation analyses were performed based on these findings. Foods in the Lo category demonstrated a significant mediating effect on the association between LE8 scores and OA risk; the mediation ratio was found to be significant at 0.01%. Based on our findings, the intake of Lo category foods with microbe numbers of < 2,972.81 CFU/g is likely to be associated with an increased risk of OA. Previous studies have reported that active probiotics can alleviate the symptoms of OA (31). In this context, interleukin-1β and tumor necrosis factor-α are secreted by synovial fibroblasts and chondrocytes in individuals with OA. These promote the synthesis of protein hydrolases, which degrade the joint extracellular matrix; this, in turn, drives disease progression, worsens disease-related synovial inflammation, and induces cartilage degeneration and the formation of subchondral bone lesions (32–34). Dietary probiotic supplementation promotes balance in the intestinal flora and reduces the inflammatory response, thereby reducing the risk of OA. Probiotics are effective in combating inflammation caused by interleukin-1β and tumor necrosis factor-α. Therefore, these cytokines represent important targets for probiotics (35–37) in the treatment of arthritis. The probiotics inhibit or reduce pro-inflammatory expression, thereby inhibiting joint degeneration (38, 39). In this context, Clostridium butyricum (GKB7 strain) has been found to produce butyrate, which can specifically increase mucin production and prevent microbes and their toxins from entering the circulation, and this reduces systemic inflammation (16). Probiotics may also reduce intestinal damage and inflammation associated with the OA disease process and have been found to reduce pain levels and cartilage destruction in animal models of OA (35, 40, 41). The GKB7 strain of C. butyricum, which is usually found in the environment, has also been found to ameliorate knee OA in rats (16). These findings suggest that dietary active microorganisms play an important role in preventing the development of OA. As foods in the Lo category contain fewer active microorganisms, diets incorporating a high proportion of Lo category foods may increase the risk of OA and weaken the protective effect of higher LE8 scores on OA.
The present study has several strengths. First, it assessed the relationship between LE8 scores and OA risk in a relatively large population. Second, it measured the dietary intake of live microbes and found macroscopic dietary live microbes to have a protective effect against OA and a mediating role in the association between LE8 scores and OA risk. This study also has certain limitations. First, self-reported OA diagnoses may reduce the validity of the results. Second, LE8 scores only represented important factors that influence the development of CVD; potential influences were not considered in this study. Third, estimates of live microbe intake were not measured for every sample, and this may have introduced bias. Fourth, residual and unmeasured confounding and measurement errors may have led to bias in our analysis. Fifth, although we adjusted for the survey period, the time span of our analysis was considerably long, and this may have led to bias. Sixth, we did not consider the influence of genetic factors. In this context, Zhang et al. proposed several central genes as possible biomarkers for OA diagnosis and these include the POSTN, MMP 2, CTSG, ELANE, COL3A1, MPO, COL1A1, and COL1A2 (42) genes. Finally, we performed mediation analyses in a cross-sectional study, and this hindered the inference of causality. Given the limitations of the current study, the results need to be interpreted with caution. Further research is needed to support our findings.
5 Conclusion
In conclusion, LE8 scores play an important role in OA risk reduction. In our study, LE8 scores were found to be associated with live microbe intake, which reduced the risk of developing OA. Adherence to the LE8 recommendations and a reduction in Lo category food intake may be favorable for OA control. Our findings have helped identify protective factors against OA and the potentially detrimental effects of foods with low live microbe content.
Data availability statement
Publicly available datasets were analyzed in this study. All data entered into the analysis were from NHANES, which is publicly accessible to all.
Ethics statement
The surveys were approved by the NCHS Research Ethics Review Board (Protocol #2011-17). All methods were performed in accordance with the relevant guidelines and regulations (Declaration of Helsinki). Informed consent was obtained from all subjects and/or their legal guardian(s) (https://www.cdc.gov/nchs/nhanes/irba98.htm). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
RG: Conceptualization, Data curation, Formal analysis, Methodology, Software, Supervision, Writing—original draft, Writing—review & editing. XC: Supervision, Writing—review & editing. ZL: Supervision, Writing—review & editing. YP: Supervision, Writing—review & editing. GL: Funding acquisition, Supervision, Writing—review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Ningxia Natural Science Foundation Key Project (Grant No. 2022AAC03190) and the Department of Physiology, Basic Medical School (Grant No. 2021AAC02015).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Chen X, Tang H, Lin J, Zeng R. Temporal trends in the disease burden of osteoarthritis from 1990 to 2019, and projections until 2030. PLoS ONE. (2023) 18:e288561. doi: 10.1371/journal.pone.0288561
2. Hootman JM, Helmick CG. Projections of us prevalence of arthritis and associated activity limitations. Arthritis Rheum. (2006) 54:226–9. doi: 10.1002/art.21562
3. Sheng X, Murphy MJ, Macdonald TM, Wei L. Effectiveness of statins on total cholesterol and cardiovascular disease and all-cause mortality in osteoarthritis and rheumatoid arthritis. J Rheumatol. (2012) 39:32–40. doi: 10.3899/jrheum.110318
4. Allen KD, Thoma LM, Golightly YM. Epidemiology of osteoarthritis. Osteoarthritis Cartilage. (2022) 30:184–95. doi: 10.1016/j.joca.2021.04.020
5. Ho J, Mak C, Sharma V, To K, Khan W. Mendelian randomization studies of lifestyle-related risk factors for osteoarthritis: a prisma review and meta-analysis. Int J Mol Sci. (2022) 23:11906. doi: 10.3390/ijms231911906
6. Palazzo C, Nguyen C, Lefevre-Colau MM, Rannou F, Poiraudeau S. Risk factors and burden of osteoarthritis. Ann Phys Rehabil Med. (2016) 59:134–8. doi: 10.1016/j.rehab.2016.01.006
7. Nguyen HD, Oh H, Kim MS. An increased intake of nutrients, fruits, and green vegetables was negatively related to the risk of arthritis and osteoarthritis development in the aging population. Nutr Res. (2022) 99:51–65. doi: 10.1016/j.nutres.2021.11.005
8. Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the american heart association's strategic impact goal through 2020 and beyond. Circulation. (2010) 121:586. doi: 10.1161/CIRCULATIONAHA.109.192703
9. Lloyd-Jones DM, Allen NB, Anderson CAM, Black T, Brewer LC, Foraker RE, et al. (2022). Life's essential 8: updating and enhancing the American Heart Association's construct of cardiovascular health: a presidential advisory from the american heart association. Circulation 146:e18–43. doi: 10.1161/CIR.0000000000001078
10. Erb N, Pace AV, Douglas KM, Banks MJ, Kitas GD. Risk assessment for coronary heart disease in rheumatoid arthritis and osteoarthritis. Scand J Rheumatol. (2004) 33:293–9. doi: 10.1080/03009740410006899
11. Yin M, Xu W, Pang J, Xie S, Xiang M, Shi B, et al. Causal relationship between osteoarthritis with atrial fibrillation and coronary atherosclerosis: a bidirectional Mendelian randomization study of european ancestry. Front Cardiovasc Med. (2023) 10:1213672. doi: 10.3389/fcvm.2023.1213672
12. Gadelha C, Bezerra AN. Effects of probiotics on the lipid profile: systematic review. J Vasc Bras. (2019) 18:e20180124. doi: 10.1590/1677-5449.180124
13. Niamah AK, Sahi AA, Al-Sharifi A. Effect of feeding soy milk fermented by probiotic bacteria on some blood criteria and weight of experimental animals. Probiotics Antimicrob Proteins. (2017) 9:284–91. doi: 10.1007/s12602-017-9265-y
14. Jia B, Zou Y, Han X, Bae JW, Jeon CO. Gut microbiome-mediated mechanisms for reducing cholesterol levels: implications for ameliorating cardiovascular disease. Trends Microbiol. (2023) 31:76–91. doi: 10.1016/j.tim.2022.08.003
15. Sun C, Zhou X, Guo T, Meng J. The immune role of the intestinal microbiome in knee osteoarthritis: a review of the possible mechanisms and therapies. Front Immunol. (2023) 14:1168818. doi: 10.3389/fimmu.2023.1168818
16. Stoeva MK, Garcia-So J, Justice N, Myers J, Tyagi S, Nemchek M, et al. Butyrate-producing human gut symbiont, Clostridium butyricum, and its role in health and disease. Gut Microbes. (2021) 13:1–28. doi: 10.1080/19490976.2021.1907272
17. von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (strobe) statement: guidelines for reporting observational studies. Int J Surg. (2014) 12:1495–9. doi: 10.1016/j.ijsu.2014.07.013
18. March LM, Schwarz JM, Carfrae BH, Bagge E. Clinical validation of self-reported osteoarthritis. Osteoarthritis Cartilage. (1998) 6:87–93. doi: 10.1053/joca.1997.0098
19. Chen L, Zhao Y, Liu F, Chen H, Tan T, Yao P, et al. Biological aging mediates the associations between urinary metals and osteoarthritis among U.S. adults. BMC Med. (2022) 20:207. doi: 10.1186/s12916-022-02403-3
20. Marco ML, Hutkins R, Hill C, Fulgoni VL, Cifelli CJ, Gahche J, et al. A classification system for defining and estimating dietary intake of live microbes in us adults and children. J Nutr. (2022) 152:1729–36. doi: 10.1093/jn/nxac074
21. Ni J, Zhou W, Cen H, Chen G, Huang J, Yin K, et al. Evidence for causal effects of sleep disturbances on risk for osteoarthritis: a univariable and multivariable Mendelian randomization study. Osteoarthritis Cartilage. (2022) 30:443–50. doi: 10.1016/j.joca.2021.11.021
22. Sewell J, Hussain SM, Wang Y, Wluka AE, Lim YZ, Carrington MJ, et al. Association between arthritis and cardiovascular risk factors in community-based adults: an opportunity to target cardiovascular risk. BMC Cardiovasc Disord. (2022) 22:232. doi: 10.1186/s12872-022-02674-x
23. McEvoy JW. Lifetime risks of cardiovascular disease. N Engl J Med 366. (2012) 1642:1642–3. doi: 10.1056/NEJMc1202276
24. Wang Y, Lombard C, Hussain SM, Harrison C, Kozica S, Brady S, et al. Effect of a low-intensity, self-management lifestyle intervention on knee pain in community-based young to middle-aged rural women: a cluster randomised controlled trial. Arthritis Res Ther. (2018) 20:74. doi: 10.1186/s13075-018-1572-5
25. Park D, Park YM, Ko SH, Choi YH, Min DU, Ahn JH, et al. Association between knee osteoarthritis and the risk of cardiovascular disease and the synergistic adverse effects of lack of exercise. Sci Rep. (2023) 13:2777. doi: 10.1038/s41598-023-29581-1
26. Ni J, Wang P, Yin KJ, Huang JX, Tian T, Cen H, et al. Does smoking protect against developing osteoarthritis? Evidence from a genetically informed perspective. Semin Arthritis Rheum. (2022) 55:152013. doi: 10.1016/j.semarthrit.2022.152013
27. Wen MT, Liang XZ, Luo D, Li JC, Yan BZ, Lu BW, et al. Plasma lipids, alcohol intake frequency and risk of osteoarthritis: a Mendelian randomization study. BMC Public Health. (2023) 23:1327. doi: 10.1186/s12889-023-16250-1
28. Zheng J, Huang X, Huang J, Meng B, Li F, Liu H, et al. Association of diabetes mellitus status and hyperglycemia with symptomatic knee osteoarthritis. Arthritis Care Res. (2023) 75:509–18. doi: 10.1002/acr.24872
29. Roman MJ, Devereux RB, Schwartz JE, Lockshin MD, Paget SA, Davis A, et al. Arterial stiffness in chronic inflammatory diseases. Hypertension. (2005) 46:194–9. doi: 10.1161/01.HYP.0000168055.89955.db
30. De Miguel C, Rudemiller NP, Abais JM, Mattson DL. Inflammation and hypertension: new understandings and potential therapeutic targets. Curr Hypertens Rep. (2015) 17:507. doi: 10.1007/s11906-014-0507-z
31. Korotkyi O, Dvorshchenko K, Falalyeyeva T, Sulaieva O, Kobyliak N, Abenavoli L, et al. Combined effects of probiotic and chondroprotector during osteoarthritis in rats. Panminerva Med. (2020) 62:93–101. doi: 10.23736/S0031-0808.20.03841-0
32. Bin ARH, Chew D, Kazezian Z, Bull A. Autologous protein solution: a promising solution for osteoarthritis? Efort Open Rev. (2021) 6:716–26. doi: 10.1302/2058-5241.6.200040
33. Molnar V, Matisic V, Kodvanj I, Bjelica R, Jelec Z, Hudetz D, et al. Cytokines and chemokines involved in osteoarthritis pathogenesis. Int J Mol Sci. (2021) 22:9208. doi: 10.3390/ijms22179208
34. Musumeci G, Aiello FC, Szychlinska MA, Di Rosa M, Castrogiovanni P, Mobasheri A. Osteoarthritis in the xxist century: risk factors and behaviours that influence disease onset and progression. Int J Mol Sci. (2015) 16:6093–112. doi: 10.3390/ijms16036093
35. Jhun J, Cho KH, Lee DH, Kwon JY, Woo JS, Kim J, et al. Oral administration of lactobacillus rhamnosus ameliorates the progression of osteoarthritis by inhibiting joint pain and inflammation. Cells. (2021) 10:1057. doi: 10.21203/rs.3.rs-220741/v1
36. Su CH, Lin CY, Tsai CH, Lee HP, Lo LC, Huang WC, et al. Betulin suppresses TNF-α and il-1β production in osteoarthritis synovial fibroblasts by inhibiting the MEK/ERK/NF-κb pathway. J Funct Foods. (2021) 86:104729. doi: 10.1016/j.jff.2021.104729
37. Warman DJ, Jia H, Kato H. The potential roles of probiotics, resistant starch, and resistant proteins in ameliorating inflammation during aging (inflammaging). Nutrients. (2022) 14:747. doi: 10.3390/nu14040747
38. Jotanovic Z, Mihelic R, Sestan B, Dembic Z. Role of interleukin-1 inhibitors in osteoarthritis: an evidence-based review. Drugs Aging. (2012) 29:343–58. doi: 10.2165/11599350-000000000-00000
39. Li H, Xie S, Qi Y, Li H, Zhang R, Lian Y. TNF-alpha increases the expression of inflammatory factors in synovial fibroblasts by inhibiting the pi3k/akt pathway in a rat model of monosodium iodoacetate-induced osteoarthritis. Exp Ther Med. (2018) 16:4737–44. doi: 10.3892/etm.2018.6770
40. Korotkyi O, Kyriachenko Y, Kobyliak N, Falalyeyeva T, Ostapchenko L. Crosstalk between gut microbiota and osteoarthritis: a critical view. J Funct Foods. (2020) 68:103904. doi: 10.1016/j.jff.2020.103904
41. Lin YY, Chen NF, Yang SN, Jean YH, Kuo HM, Chen PC, et al. Effects of streptococcus thermophilus on anterior cruciate ligament transection-induced early osteoarthritis in rats. Exp Ther Med. (2021) 21:222. doi: 10.3892/etm.2021.9653
Keywords: osteoarthritis, Life's Essential 8, mediation analyses, live microbes, NHANES
Citation: Gou R, Chang X, Li Z, Pan Y and Li G (2023) Association of Life's Essential 8 with osteoarthritis in United States adults: mediating effects of dietary intake of live microbes. Front. Med. 10:1297482. doi: 10.3389/fmed.2023.1297482
Received: 21 September 2023; Accepted: 27 November 2023;
Published: 21 December 2023.
Edited by:
Cong-Qiu Chu, Oregon Health and Science University, United StatesReviewed by:
Ibsen Bellini Coimbra, State University of Campinas, BrazilS. Anand Narayanan, Florida State University, United States
Copyright © 2023 Gou, Chang, Li, Pan and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying Pan, MTg2Mjk0OTQzMjlAMTYzLmNvbQ==; Guanghua Li, Z2hsZWUwNDA0QDE2My5jb20=
 Ruoyu Gou
Ruoyu Gou Xiaoyu Chang1
Xiaoyu Chang1 Guanghua Li
Guanghua Li