- 1Division of Gastroenterology and Hepatology, Department of Medicine, Ramathibodi Hospital, Mahidol University, Bangkok, Thailand
- 2Department of Clinical Epidemiology and Biostatistics, Mahidol University, Bangkok, Thailand
- 3Department of Surgery, Faculty of Medicine Vajira Hospital, Navamindradhiraj University, Bangkok, Thailand
Background: Direct-acting antivirals (DAA) are effective for chronic hepatitis C virus (HCV) treatment. However, their impact on overall survival (OS), hepatocellular carcinoma (HCC) occurrence, HCC-free survival, and liver function in patients with HCV decompensated cirrhosis remains uncertain. This study aimed to evaluate the effects of DAA treatment on this population.
Methods: Studies were identified by searching the MEDLINE, SCOPUS, and CENTRAL databases. OS and HCC-free survival probabilities and time data were extracted from Kaplan-Meier curves. A one-stage meta-analysis using parametric Weibull regression was conducted to estimate the relative treatment effects of DAA vs. no DAA. The primary outcome was the OS rate. The secondary outcomes were HCC-free survival, HCC occurrence rate, and improvement in the Model for End-stage Liver Disease (MELD) score.
Results: Eight cohorts comprising 3,430 participants (2,603 in the DAA group and 1,999 in the no-DAA group) were included. The OS probabilities at 12 and 24 months were 95 and 90% for the DAA group, respectively, compared with 89 and 80% in the no-DAA group, respectively. Hazard ratio (HR) was 0.48 (95% confidence interval (CI): 0.39, 0.60; p < 0.001). The HCC-free survival probabilities at 12 and 24 months were 96 and 90%, respectively, in the former, and 94 and 85%, respectively, in the latter. The HR of HCC occurrence was 0.72 (95% CI: 0.52, 1.00; p = 0.05), which suggests that DAA treatment in decompensated cirrhosis may lead to a 28% lower risk of HCC occurrence. The mean MELD score difference was −7.75 (95% CI: −14.52, −0.98; p = 0.02).
Conclusion: Improvement in OS and MELD score is a long-term benefit of DAA treatment in patients with HCV decompensated cirrhosis, with a marginal effect of the treatment on HCC development.
1. Introduction
Hepatitis C virus (HCV) infection is a common cause of chronic viral hepatitis and end-stage liver disease, worldwide (1). According to the World Health Organization (WHO), approximately 58 million individuals are infected with HCV (2). Chronic HCV infection can progress to decompensated cirrhosis, resulting in significant morbidity and mortality, and the need for liver transplantation (3). Interferon-based therapies are unsuitable for patients with decompensated cirrhosis owing to their severe adverse effects (4). However, with the advent of interferon-free direct-acting antiviral (DAA) agents (5, 6) the therapeutic landscape for HCV infection has dramatically changed. These new agents offer higher sustained virological response (SVR) rates and can effectively eradicate HCV infection (7). Treatment regimens consisting of 12 or 24 weeks of DAAs, with or without ribavirin, have become the cornerstone of chronic HCV treatment (5, 8).
Although the European Association for the Study of the Liver (EASL) guidelines recently recommended the use of DAAs for the treatment of decompensated HCV cirrhosis, the clinical efficacy and safety of these agents in this context have not yet been fully confirmed (9). DAA treatment in decompensated cirrhosis has shown promising results in terms of high SVR rates, although improvements in liver function tests and overall survival (OS) have been limited (10–12). However, a recent study has demonstrated a reduction in both all-cause and liver-related mortality following DAA treatment in patients with decompensated cirrhosis (12, 13). Despite these findings, the long-term benefits of HCV eradication in this patient population warrants further investigation (13).
This systematic review and meta-analysis aimed to assess long-term clinical outcomes such as OS, occurrence of hepatocellular carcinoma (HCC), HCC-free survival, and improvement of liver function, which was defined as a decline in the Model for End-stage Liver Disease (MELD) score in patients with decompensated HCV cirrhosis who received DAA treatment.
2. Materials and methods
The entire process of this systematic review and meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (14). Additionally, the study protocol was registered in PROSPERO for systematic reviews (registration number: CRD42022316276).
2.1. Data sources and searches
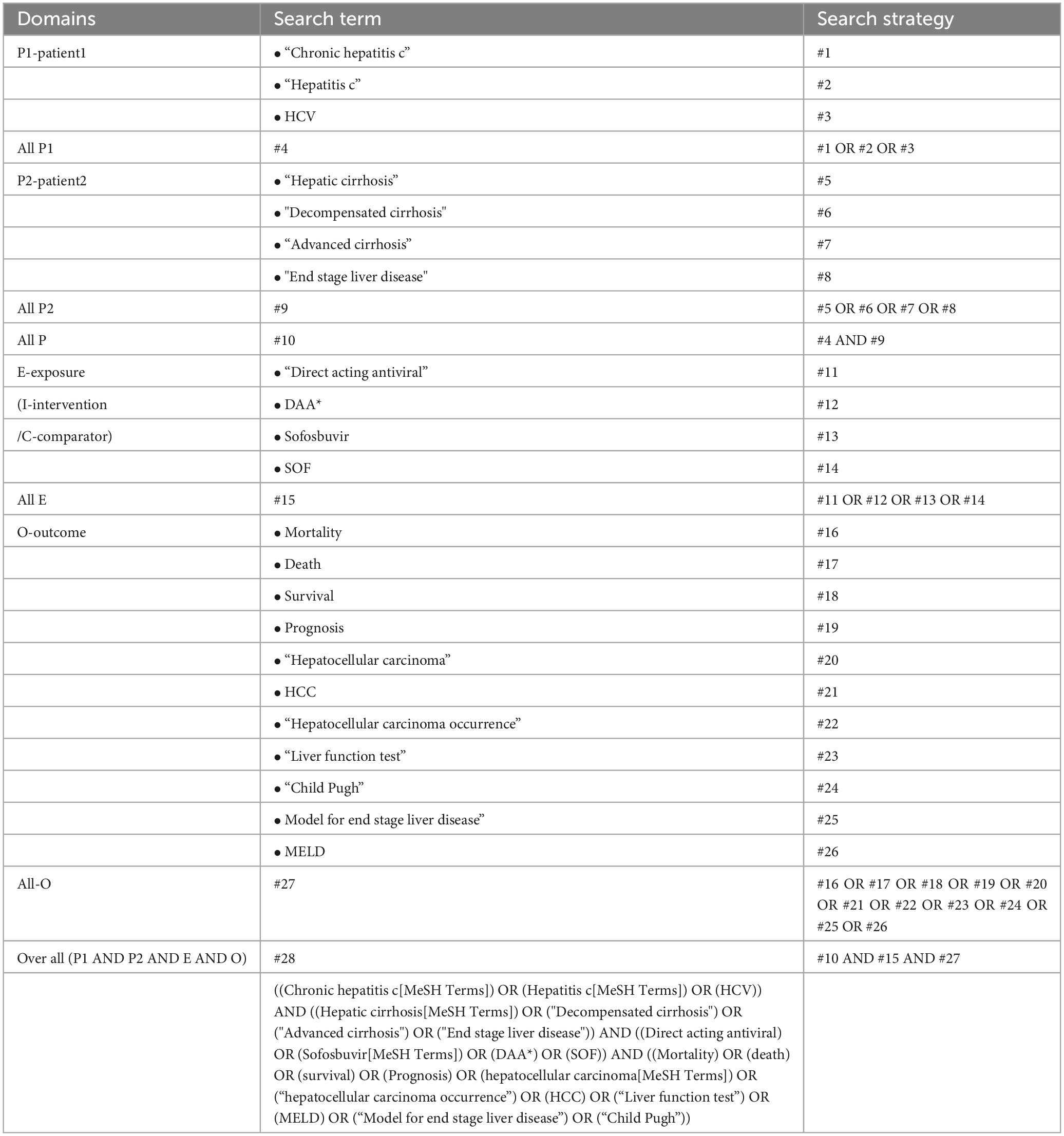
Two investigators (TJ and AS) independently conducted a systematic search for relevant studies in MEDLINE, SCOPUS, and the Cochrane Central Register of Controlled Trials (CENTRAL) from their inception to June 30, 2023. The following search strategy was used: “chronic hepatitis C” OR “hepatitis C” AND “hepatic cirrhosis” OR “decompensated cirrhosis” OR “advanced cirrhosis” OR “end-stage liver disease” AND “direct-acting antiviral” OR “sofosbuvir” OR “DAA” OR “SOF” AND “mortality” OR “death” OR “survival” OR “prognosis” OR “hepatocellular carcinoma” OR “hepatocellular carcinoma occurrence” OR “HCC” OR “liver function test” OR “Model for End-stage Liver Disease” OR “Child-Pugh”(Table 1). Additionally, the reference lists of all the retrieved review articles were examined to identify potentially relevant articles that may have been missed by the search system (15).
2.2. Study selection
Two independent reviewers (TJ and AS) assessed the eligibility of each article by screening titles and abstracts. Full articles were obtained for comprehensive evaluation of cases in which eligibility was unclear. The kappa statistic was used to measure agreement between the two reviewers. In instances of discordant decisions between the two investigators, the articles were subjected to a full-text review. Any discrepancies were resolved through consensus between the two reviewers with the involvement of a third investigator (AT) when necessary.
2.3. Selection criteria
Studies were considered eligible if they fulfilled the following criteria: (1) they had study designs of randomized controlled trials (RCTs), prospective or retrospective cohorts with a sample size greater than 10; (2) they included adult patients aged 18 years and older with decompensated HCV cirrhosis; (3) they included any interferon-free DAA therapy as the treatment group compared with a control group receiving only the standard of care for decompensated cirrhosis; and (4) they reported one or more of the following outcomes: OS, HCC occurrence, liver function tests (LFTs), MELD score, or Child-Pugh score after treatment. The following studies were excluded: (1) duplicated studies; (2) studies involving post-liver transplant patients; (3) studies with insufficient data; and (4) studies in which the authors could not provide the full unpublished article after two separate contact attempts spaced 1 month apart.
2.4. Outcome measures
The primary outcome of this study was OS of patients with decompensated HCV cirrhosis. Secondary outcomes included the occurrence rate of HCC, HCC-free survival, and improvement in liver function, which was defined as a decline in the MELD score. The MELD score was calculated using the following formula: 3.78 × ln[serum bilirubin (mg/dL)] + 11.2 × ln[INR] + 9.57 × ln[serum creatinine (mg/dL)] + 6.43 (16). The MELD scores range from 6 to 40, with higher scores indicating a higher 3-month mortality risk related to liver disease (17).
2.5. Data extraction and quality assessment
Data extraction from the included studies was performed independently by two investigators (TJ and AS). The extracted data included the first author’s name, year of study, country of study population, demographic information, baseline LFT results, and MELD score. Summary statistics, such as hazard ratios (HR) for HCC occurrence and 1-year survival rates were also collected. In cases where these summary statistics were not provided, the GetData Graph Digitizer program version 2.26.0.2017 (18) was used to extract data from Kaplan–Meier curves. Subsequently, Kaplan–Meier data were transformed into individual patient data (IPD) using the “ipdfc” command in STATA 17. Any missing data were sought by contacting the authors of the respective studies via e-mails.
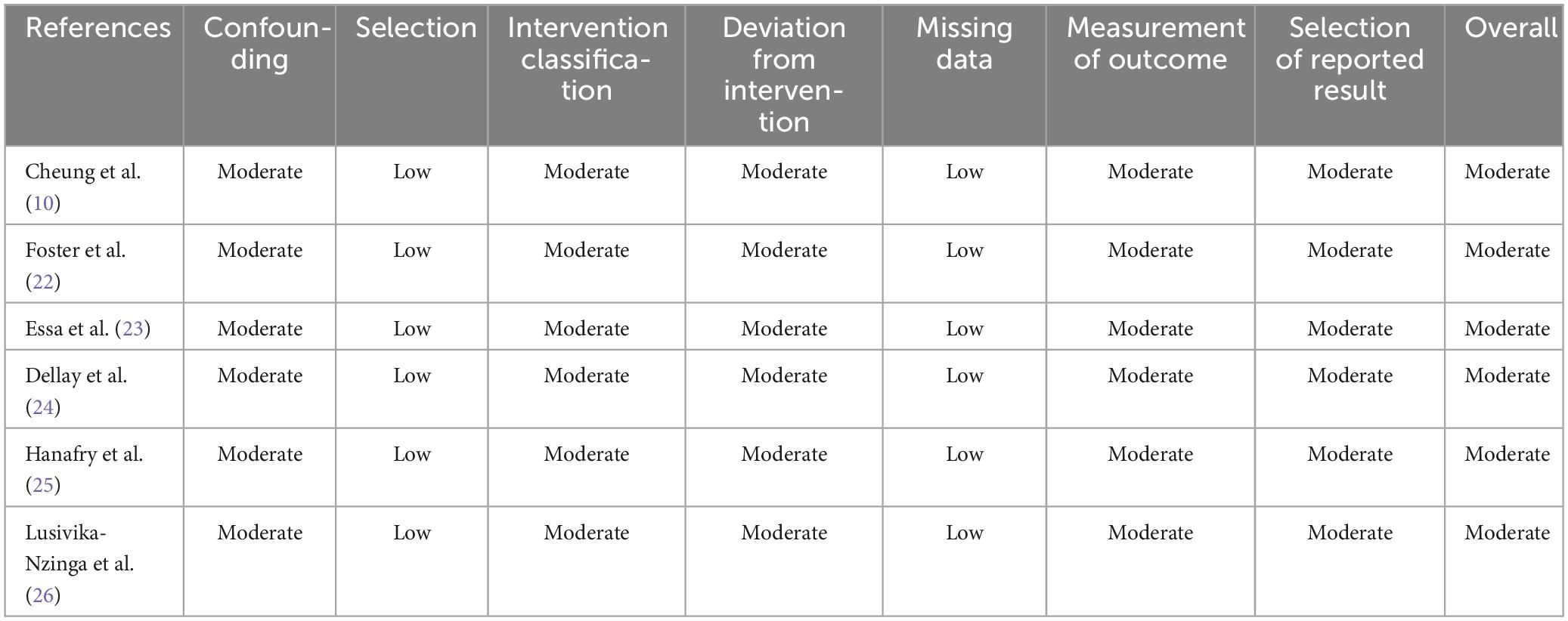
The quality of the included studies was assessed using the Cochrane Risk of Bias in Non-randomized Studies of Interventions (ROBINS-I) tool (19). This tool evaluates seven domains: confounding, study selection, classification of interventions, deviations from intended interventions, missing data, outcome measurements, and selection of reported results. Studies were categorized as having a high risk of bias if at least one domain had a high risk of bias. Conversely, studies were considered to have a low risk of bias if all the domains had a low risk of bias. If some domains had a low risk of bias and others had concerns, the studies were classified as having a moderate risk of bias.
2.6. Statistical analysis
Overall survival and HCC-free survival probabilities and time data were extracted from the Kaplan–Meier curves. A one-stage meta-analysis was conducted using multilevel parametric survival regression with a Weibull distribution (20) to compare the relative treatment effects of DAA compared to the control (no DAA) group for the IPD analysis of overall and HCC-free survival and an improvement in the MELD score. The median survival time for each treatment group and HRs with their corresponding 95% confidence intervals (CIs) (21) were estimated accordingly. For summary statistical data, a meta-analysis was performed using a random-effects model if heterogeneity was present, as indicated by Cochran’s Q-test p-value < 0.1, or an I-square (I2) statistic >25%. A fixed effects model was used in the absence of heterogeneity. Publication bias was assessed using funnel plots and Egger’s test. All statistical analyses were carried out using STATA version 17.0, and a p-value < 0.05 was considered statistically significant.
3. Results
3.1. Search results and study characteristics
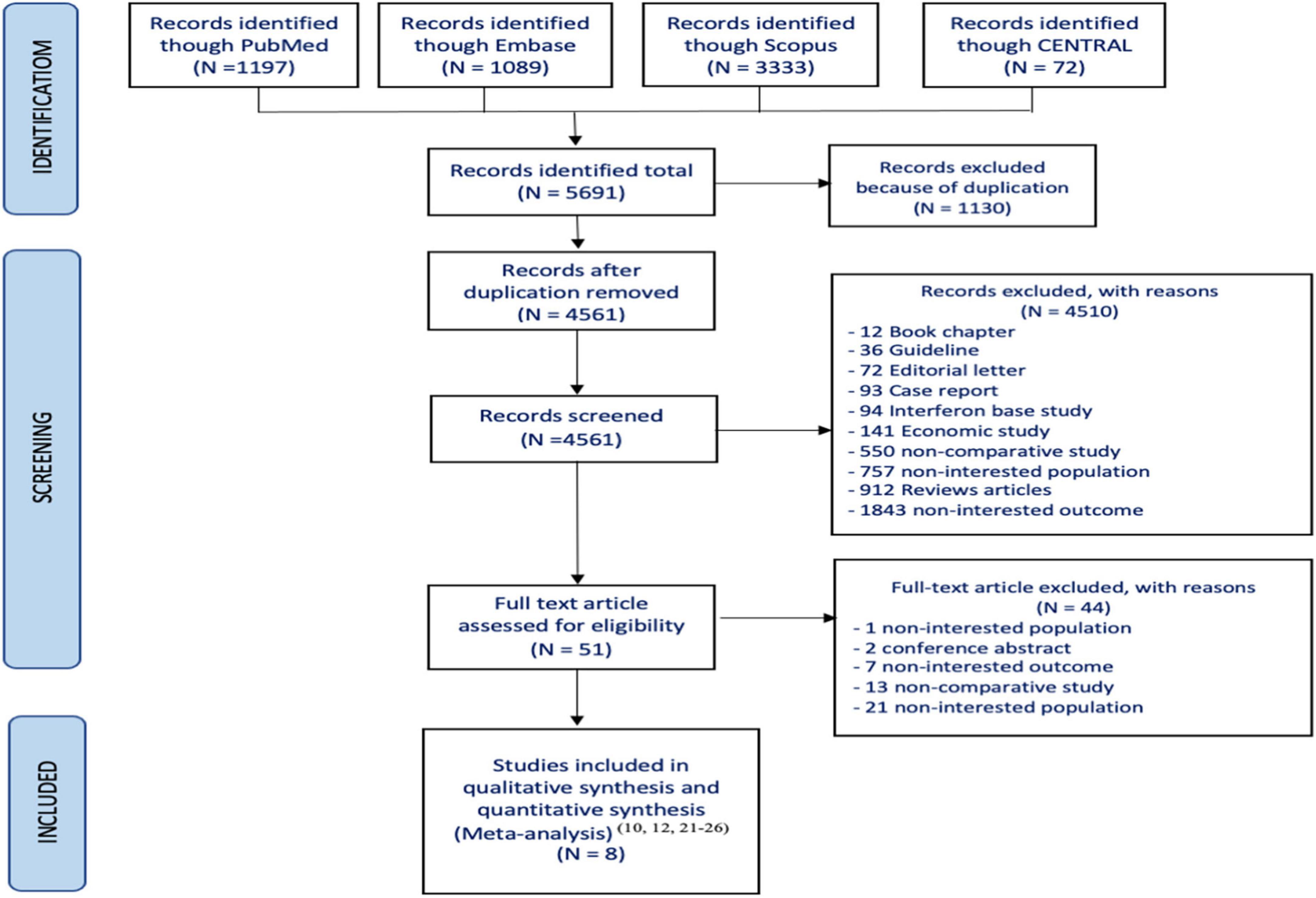
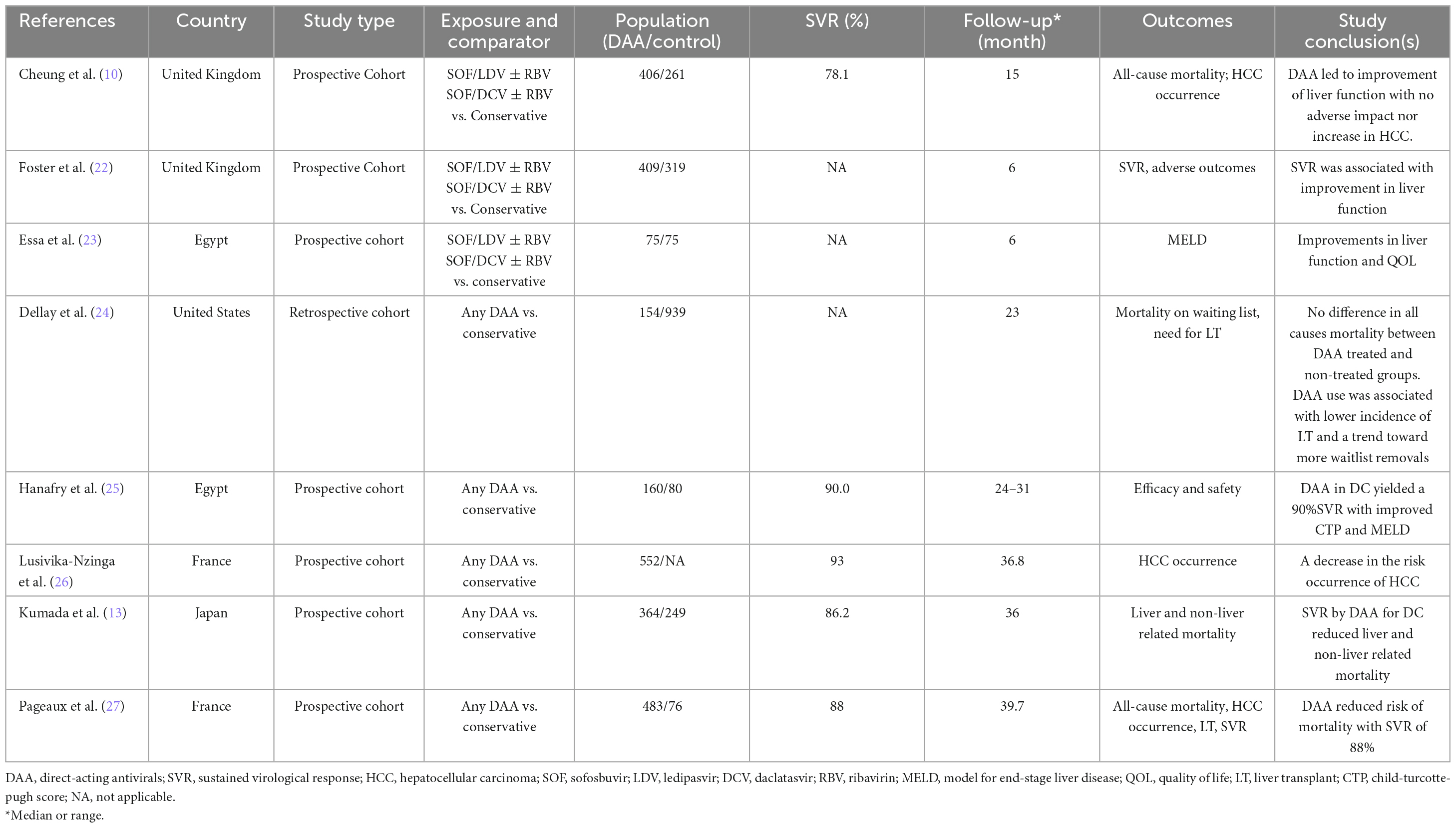
Of the 5,691 articles initially identified, 51 were reviewed for eligibility. However, only 8 studies met the inclusion criteria (10, 13, 22–27) and were included in the systematic review (Figure 1). Among them, five studies reported OS (10, 13, 24, 25, 27) four studies reported HCC occurrence (10, 13, 26, 27) and three reported MELD scores (13, 22, 23). The characteristics of the included studies are summarized in Table 2. All studies had cohort designs, with sample sizes ranging from 150 to 1,093. Eight studies (10, 13, 22–27) which included seven prospective observation cohort studies (10, 13, 22, 23, 25–27) and one retrospective cohort study (24) were enrolled. The mean age of participants ranged from 51 to 59 years, with a mean MELD score of approximately 13. The follow-up time varied from 6 to 40 months, and all studies used interferon-free DAAs regimens with sofosbuvir-based treatments.
The risk of bias assessment is reported in Table 3, which shows that all studies had a moderate risk of bias. All included studies were cohort studies and reported a moderate risk of bias in various domains, including confounding, intervention classification, deviation from intervention, measurement of outcomes, and selection of reported results.
3.2. Overall survival
Five studies (10, 13, 24, 25, 27) reported Kaplan–Meier curves for OS data, allowing the generation of IPD by treatment groups. A one-stage meta-analysis using mixed-effect Weibull regression was employed to analyze the OS in the treatment groups (20). Treatment-specific survival probability curves were constructed (Figure 2). The DAA group exhibited a higher OS than the control group (p < 0.001) (Figure 2A). At 12, 18, and 24 months, the survival rates in the DAA group were 95, 92, and 90%, respectively. In contrast, the corresponding survival probabilities in the control group were 89, 94, and 80%, respectively. The relative treatment effect was estimated with an HR of 0.48 (95% CI: 0.39, 0.60), indicating that patients who received DAAs had a significantly lower risk of death (52% lower) than those who did not. A mixed-effect Weibull regression model was applied, which indicated that the DAA group had a higher probability of HCC-free survival than did the control group. At 12 and 24 months, the HCC-free survival probabilities were 96 and 90% for the DAA group, and 94 and 85% for the control group, respectively (Figure 2B).
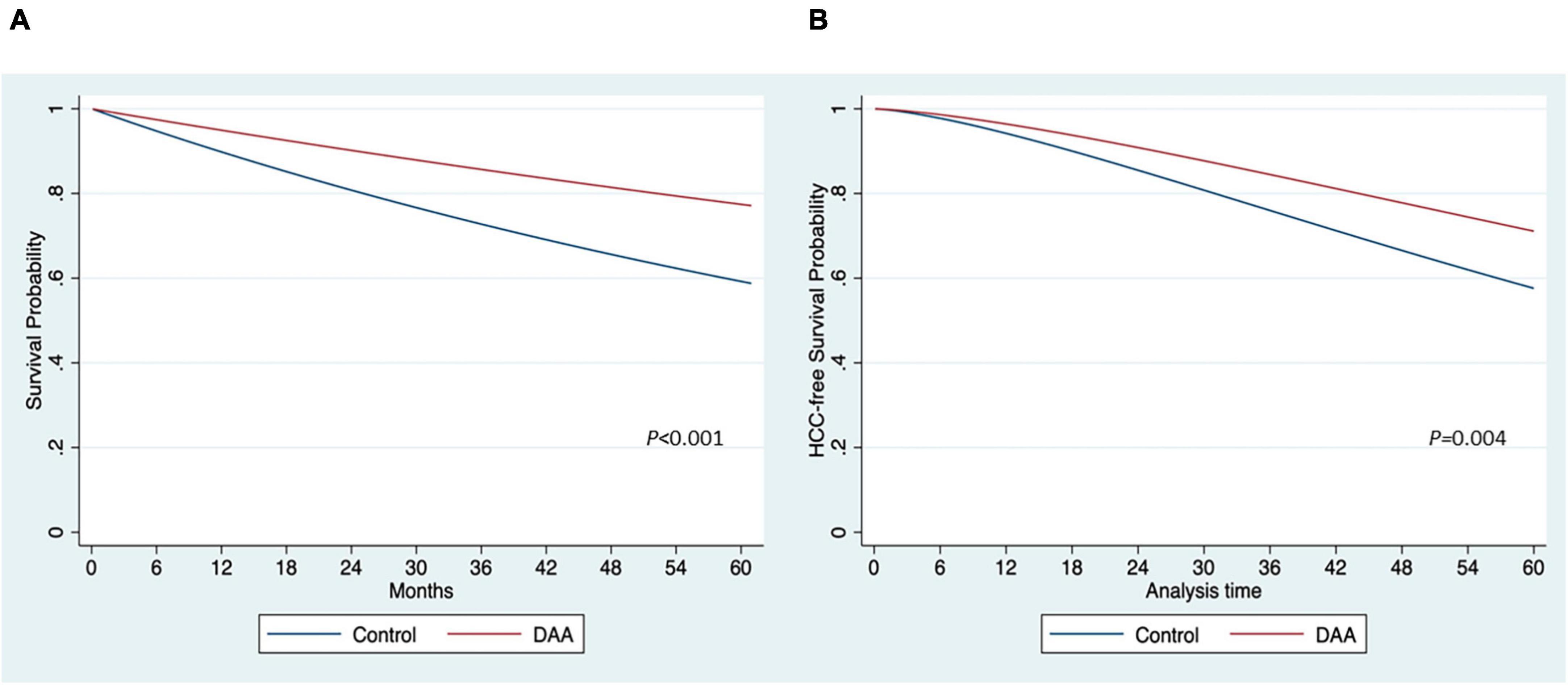
Figure 2. Estimation of survival probability by the treatment of direct-acting antivirals (DAA). (A) Overall survival (B) hepatocellular carcinoma (HCC)-free survival.
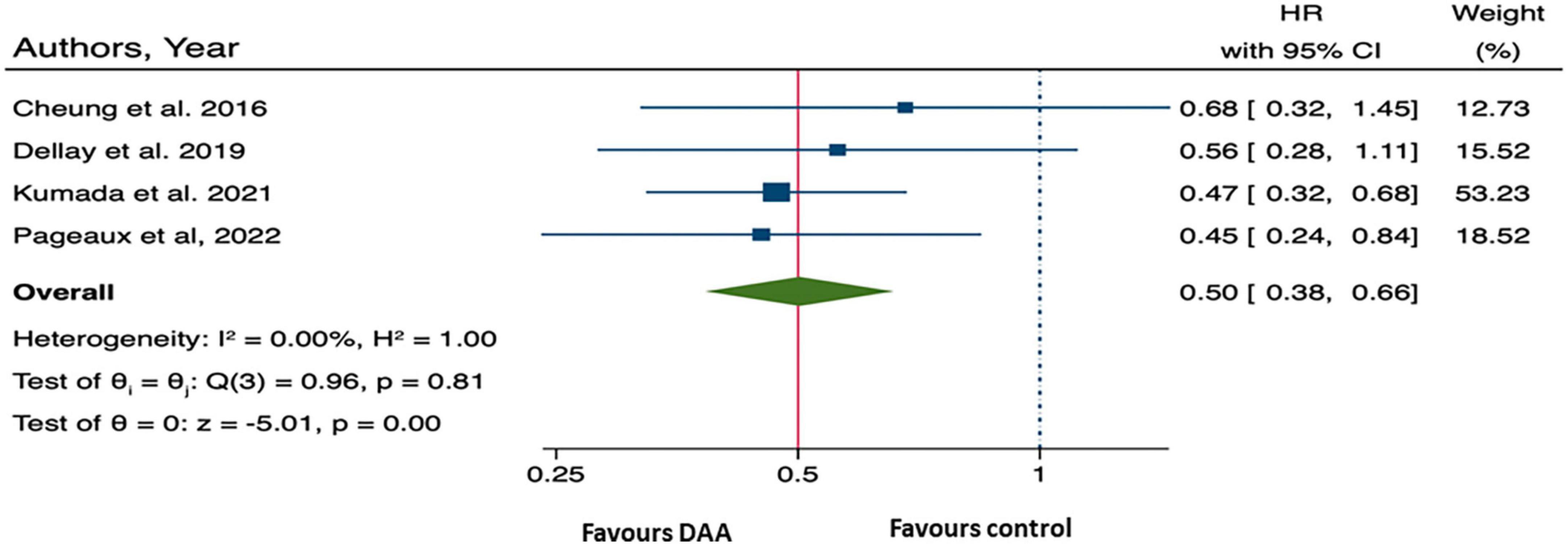
Furthermore, the pooled HR from four studies provided summary statistical data of overall mortality was 0.50 (95% CI: 0.38, 0.66), (10, 13, 25, 27) (Figure 3), suggesting that patients who received DAA treatment had a 50% significantly lower risk of all-cause mortality than those who did not.
3.3. HCC occurrence
Four studies involving 1,832 patients with decompensated HCV cirrhosis were included in the analysis of HCC-free survival following DAA treatment (10, 13, 26, 27). The relative treatment effect or rate of HCC occurrence, as measured by HRs with 95% CI, was 0.72 (0.52, 1.00; p = 0.05) (Figure 4). This suggests that patients with decompensated HCV cirrhosis who received DAA treatment may had a 28% lower risk of HCC than the control patients.
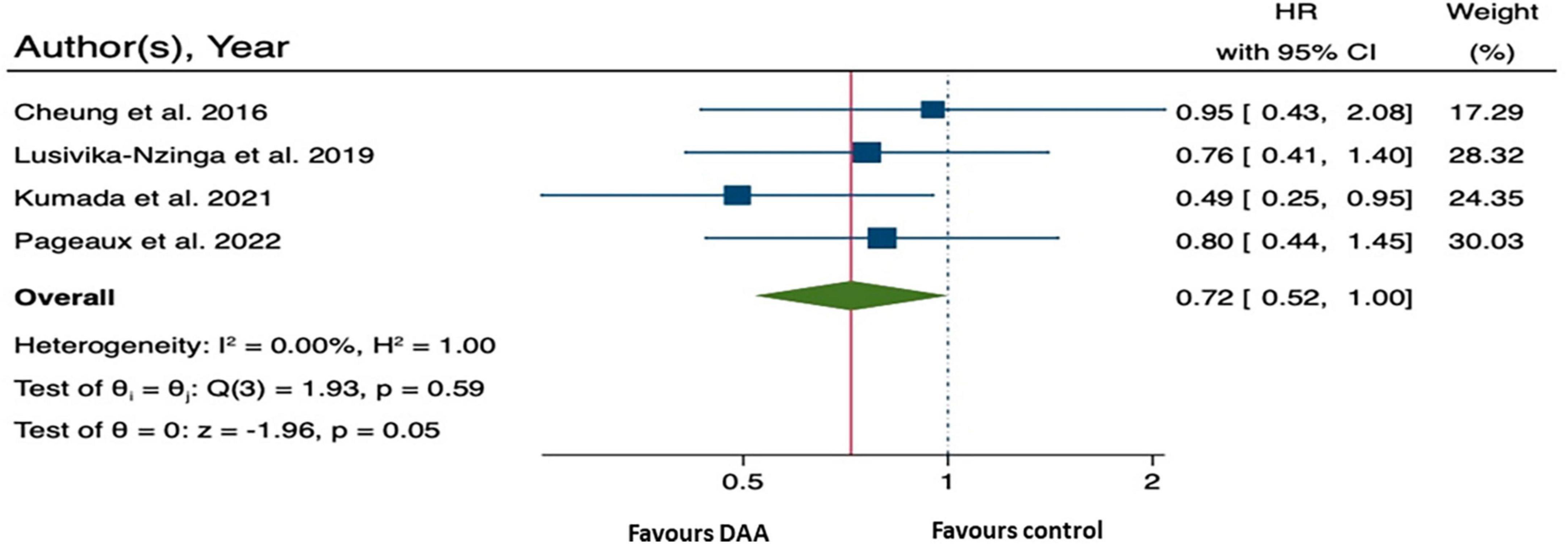
Figure 4. Forest plot of pooling hazard ratios across studies: hepatocellular carcinoma (HCC) occurrence.
3.4. MELD score
Three studies reported the mean MELD scores after treatment at different time points, ranging from 3 to 6 months (13, 22, 23). When pooling the mean differences in MELD scores, it was found that the MELD score was significantly lower in the DAA group than in the control group, with a mean difference of −7.75 (95% CI: −14.52, −0.98; p = 0.020) (Figure 5).
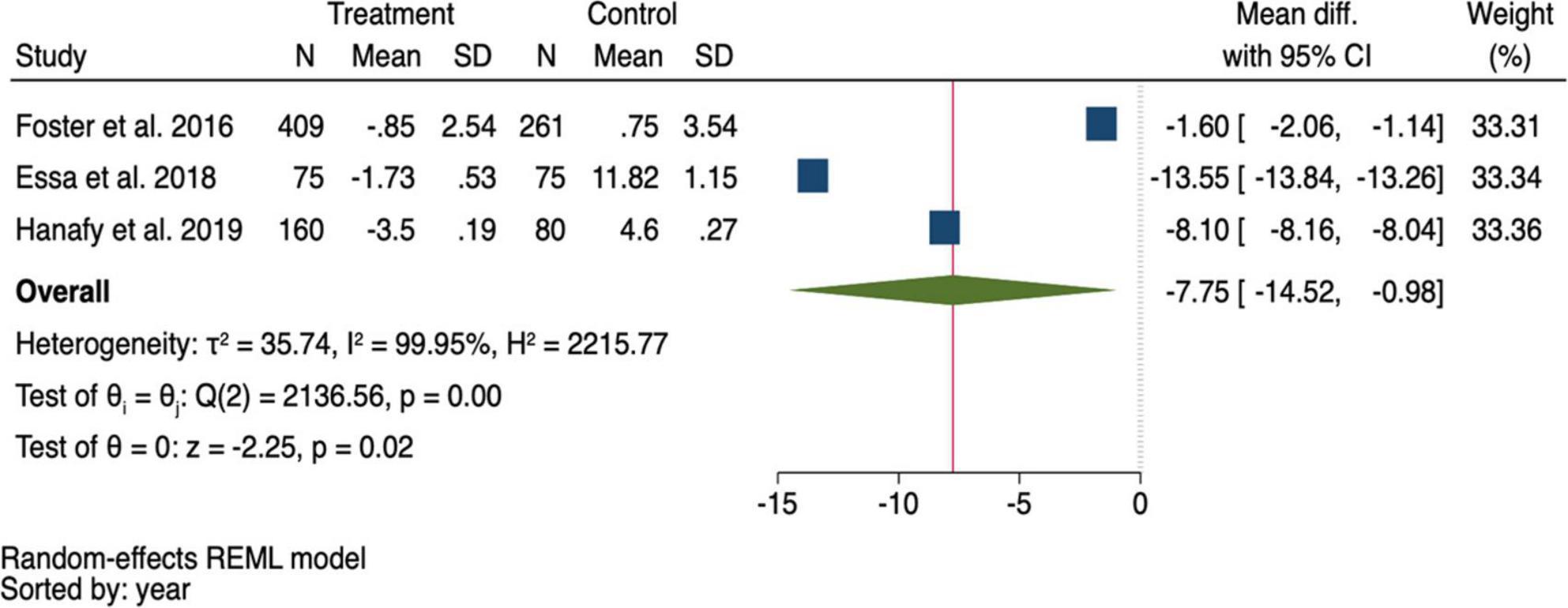
Figure 5. Pooling mean difference of the Model for end-stage liver disease (MELD) score between the direct-acting antivirals (DAA) and control group.
3.5. Heterogeneity and publication bias
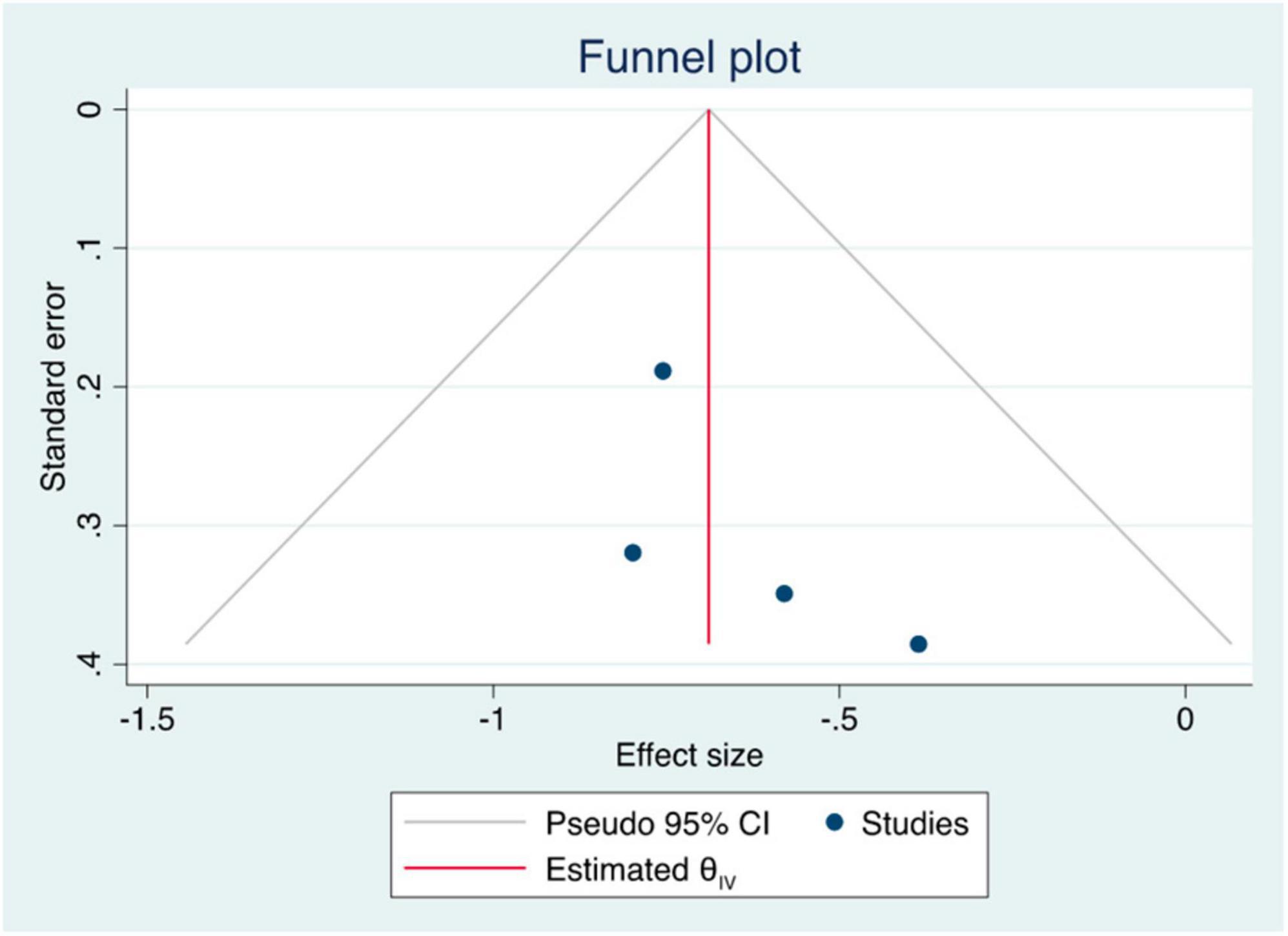
The summary data of the HRs of overall mortality and HCC occurrence revealed no heterogeneity (I2 = 0%) (Figures 3, 4). The mean differences in MELD scores were highly variable across the three studies (13, 22, 23) with an I2 value of 99.95%, indicating substantial heterogeneity (Figure 5). Funnel plots and Egger’s test of the pooled analysis of overall mortality revealed no evidence of publication bias (Egger’s test, p = 0.69) (Figure 6).
4. Discussion
We conducted a systematic review and meta-analysis based on data from eight cohorts (10, 13, 22–27) to evaluate the relative treatment effect of DAA compared with the no-DAA approach. This study was directly compared between patients who received DAA and not received DAA, which meant those who received DAA regardless of SVR achievement were classified into DAA group. Our findings revealed that DAA treatment is associated with improved OS and HCC-free survival. At 24 months, the OS and HCC-free survival probabilities were 90 and 90%, respectively, in the DAA treatment groups and 80 and 85%, respectively, in the standard treatment groups. Patients who received DAAs had a significantly lower risk of death (approximately 50% lower) and lower risk of HCC development (28% lower) than those who did not receive DAAs. Additionally, DAA treatment resulted in a notable improvement in liver function, as demonstrated by a reduction of seven points in the MELD scores. Our study contributes valuable information beyond previous studies (5, 12, 28, 29) that mainly reported SVR rates after DAA treatment. We provided additional clinically relevant insights, including survival rates, HCC-free survival, HCC occurrence rates, and MELD score improvement. The included studies primarily included patients with HCV-related decompensated cirrhosis (10, 12, 21–26).
Our study showed a significant improvement in the MELD score following DAA treatment compared with non-DAA or standard treatment. This finding aligns with that of previous studies (9, 23). However, it is important to note that the pooled post-treatment MELD scores exhibited high heterogeneity due to variations in baseline MELD scores across different studies (13, 22, 23). The results might have shown even greater improvement if additional data on the updated MELD scores were available. Nevertheless, our findings suggest that DAA treatment may contribute to ameliorating disease severity in patients with HCV-related decompensated cirrhosis waiting for liver transplantation. Despite the current guidelines recommending DAA treatment for all patients (9) the outcomes of DAA treatment in decompensated cirrhosis remain unclear, mainly because decompensated cirrhosis is often considered a point of no return (30). However, the findings of this meta-analysis confirmed the benefits of DAA treatment in patients with HCV-related decompensated cirrhosis, highlighting the need for further research that may include a cost-effective analysis of DAAs in this population.
To the best of our knowledge, this study is the first systematic review and meta-analysis to compare the long-term clinical outcomes (OS, HCC-free survival, HCC development, and MELD scores) of DAA treatment with those of no DAA treatment in patients with HCV-related decompensated cirrhosis. However, our study has certain limitations. First, the absence of available RCTs limited our analysis to cohort studies, which inherently carries the risk of confounding and selection bias. This limitation is primarily due to ethical considerations as DAA treatment has become the recommended standard therapy for chronic HCV infections. Second, we could not analyze other outcomes, such as improvement in the Child-Pugh score, fibrosis score, and adverse events, because of the limited number of studies reporting these specific outcomes. To address these limitations, future studies should include large-scale cohort studies that incorporate propensity score analysis. Third, because of the limited number of studies available for each outcome of interest, we could not perform a subgroup analysis based on variables such as the Child-Pugh classification and the benefit of ribavirin in addition to DAAs. Moreover, we could not directly compare the outcomes between DAA-treated patients who achieved SVR and those who did not because the available data were insufficient to explore. However, the impact of DAA-mediated viral elimination on liver-related and non-liver-related mortality in patients with HCV-related decompensated cirrhosis was reported in a propensity score-matching study between the DAA group who achieved SVR and those who did not (13). Further research is needed to investigate the benefits of SVR in patients with HCV-related decompensated cirrhosis treated with DAAs. Lastly, the study by Dellay et al. (24) was conducted in decompensated HCV cirrhosis patients who were listed for liver transplantation while the study was retrospectively compared between patients who received and not received DAA treatment which meant that immortal time bias could occur but we couldn’t exclude this study because population at baseline were matched with our inclusion criteria.
5. Conclusion
Direct-acting antivirals treatment has been demonstrated to be effective in increasing patient survival and HCC-free survival, and potentially reducing the development of HCC in individuals with HCV-related decompensated cirrhosis. Additionally, it was significantly associated with improvements in MELD scores within 6 months of treatment.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
TJ: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Visualization, Writing – original draft, Writing – review and editing. AC: Conceptualization, Formal analysis, Validation, Writing – review and editing. PI: Conceptualization, Validation, Writing – review and editing. ATa: Conceptualization, Data curation, Formal analysis, Validation, Writing – review and editing. AT: Writing – review and editing. AS: Conceptualization, Data curation, Supervision, Validation, Writing – review and editing, Funding acquisition, Visualization.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. AT recieved a grant from the National Reseach Council of Thailand (NRCT) N42A640323. The funder had no role in the design or conduct of the study.
Acknowledgments
We would like to thank The Gastroenterological Association of Thailand.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Alkhatib M, Di Maio V, De Murtas V, Polilli E, Milana M, Teti E, et al. Genetic determinants in a critical domain of NS5A correlate with hepatocellular carcinoma in cirrhotic patients infected with HCV genotype 1b. Viruses. (2021) 13:743. doi: 10.3390/v13050743
2. van Dijk M, Brakenhoff S, Isfordink C, Cheng W, Blokzijl H, Boland G, et al. The Netherlands is on track to meet the World Health Organization hepatitis C elimination targets by 2030. J Clin Med. (2021) 10:4562. doi: 10.3390/jcm10194562
3. Sebastiani G, Gkouvatsos K, Pantopoulos K. Chronic hepatitis C and liver fibrosis. World J Gastroenterol. (2014) 20:11033–53. doi: 10.3748/wjg.v20.i32.11033
4. Xu Y, Qi W, Wang X, Zhao P, Zhang Y, Zhang Q, et al. Pegylated interferon α-2a plus ribavirin for decompensated hepatitis C virus-related cirrhosis: relationship between efficacy and cumulative dose. Liver Int. (2014) 34:1522–31. doi: 10.1111/liv.12417
5. Falade-Nwulia O, Suarez-Cuervo C, Nelson D, Fried M, Segal J, Sulkowski M. Oral direct-acting agent therapy for hepatitis C virus infection: a systematic review. Ann Intern Med. (2017) 166:637–48. doi: 10.7326/M16-2575
6. Abd Alla M, El Awady M, Dawood R, Elhawary M, Al-Azhari S, Galal A. Hepatitis C virus serologic relapse after treatment with direct-acting antivirals is dependent on viral RNA levels in peripheral blood mononuclear cells and the grade of liver cirrhosis. Arch Virol. (2018) 163:2765–74. doi: 10.1007/s00705-018-3922-7
7. European Association for Study of Liver. EASL Clinical Practice Guidelines: management of hepatitis C virus infection. J Hepatol. (2014) 60:392–420. doi: 10.1016/j.jhep.2013.11.003
8. Li D, Chung R. Overview of direct-acting antiviral drugs and drug resistance of hepatitis C Virus. Methods Mol Biol. (2019) 1911:3–32. doi: 10.1007/978-1-4939-8976-8_1
9. European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C: final update of the series ( ). J Hepatol. (2020) 73:1170–218. doi: 10.1016/j.jhep.2020.08.018
). J Hepatol. (2020) 73:1170–218. doi: 10.1016/j.jhep.2020.08.018
10. Cheung M, Walker A, Hudson B, Verma S, McLauchlan J, Mutimer D, et al. Outcomes after successful direct-acting antiviral therapy for patients with chronic hepatitis C and decompensated cirrhosis. J Hepatol. (2016) 65:741–7. doi: 10.1016/j.jhep.2016.06.019
11. Verna E, Morelli G, Terrault N, Lok A, Lim J, Di Bisceglie A, et al. DAA therapy and long-term hepatic function in advanced/decompensated cirrhosis: real-world experience from HCV-TARGET cohort. J Hepatol. (2020) 73:540–8. doi: 10.1016/j.jhep.2020.03.031
12. An J, Park D, Ko M, Ahn S, Yoo J, Jun D, et al. Direct-acting antivirals for HCV treatment in decompensated liver cirrhosis patients: a systematic review and meta-analysis. J Pers Med. (2022) 12:1517. doi: 10.3390/jpm12091517
13. Kumada T, Toyoda H, Yasuda S, Tada T, Tanaka J, Chayama K, et al. Comparison of the prognosis of decompensated cirrhosis in patients with and without eradication of hepatitis C virus. Infect Dis Ther. (2021) 10:1001–13. doi: 10.1007/s40121-021-00441-7
14. Page M, McKenzie J, Bossuyt P, Boutron I, Hoffmann T, Mulrow C, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
15. Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. (2016) 5:210. doi: 10.1186/s13643-016-0384-4
16. Malinchoc M, Kamath P, Gordon F, Peine C, Rank J, ter Borg P. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. (2000) 31:864–71. doi: 10.1053/he.2000.5852
17. Kamath P, Wiesner R, Malinchoc M, Kremers W, Therneau T, Kosberg C, et al. A model to predict survival in patients with end-stage liver disease. Hepatology. (2001) 33:464–70. doi: 10.1053/jhep.2001.22172
18. Rohatgi A. WebPlotDigitizer. (2021). Available online at: https://automeris.io/WebPlotDigitizer [September 16, 2022].
19. Sterne J, Hernán M, Reeves B, Savović J, Berkman N, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. (2016) 355:i4919. doi: 10.1136/bmj.i4919
20. Zhang Z. Parametric regression model for survival data: weibull regression model as an example. Ann Transl Med. (2016) 4:484. doi: 10.21037/atm.2016.08.45
21. Turner P, Ansotegui I, Campbell D, Cardona V, Ebisawa M, El-Gamal Y, et al. COVID-19 vaccine-associated anaphylaxis: a statement of the World Allergy Organization anaphylaxis committee. World Allergy Organ J. (2021) 14:100517. doi: 10.1016/j.waojou.2021.100517
22. Foster G, Irving W, Cheung M, Walker A, Hudson B, Verma S, et al. Impact of direct acting antiviral therapy in patients with chronic hepatitis C and decompensated cirrhosis. J Hepatol. (2016) 64:1224–31. doi: 10.1016/j.jhep.2016.01.029
23. Essa M, Sabry A, Abdelsameea E, Tharwa E, Salama M. Impact of new direct-acting antiviral drugs on hepatitis C virus-related decompensated liver cirrhosis. Eur J Gastroenterol Hepatol. (2019) 31:53–8. doi: 10.1097/MEG.0000000000001250
24. Dellay B, Sexter A, Wang J, Hess G, Ray Kim W, Israni A. Impact of sofosbuvir-based therapy on liver transplant candidates with hepatitis C virus infection. Pharmacotherapy. (2019) 39:424–32. doi: 10.1002/phar.2237
25. Hanafy A, Bassiony M, Basha M. Management of HCV-related decompensated cirrhosis with direct-acting antiviral agents: who should be treated? Hepatol Int. (2019) 13:165–72. doi: 10.1007/s12072-019-09933-8
26. Lusivika-Nzinga C, Fontaine H, Dorival C, Simony M, Pol S, Carrat F, et al. The dynamic effect of direct-acting antiviral treatments on the risk of hepatocellular carcinoma in patients with cirrhosis and chronic hepatitis C. J Viral Hepat. (2019) 26:1489–92. doi: 10.1111/jvh.13186
27. Pageaux G, Nzinga C, Ganne N, Samuel D, Dorival C, Zoulim F, et al. Clinical outcomes after treatment with direct antiviral agents: beyond the virological response in patients with previous HCV-related decompensated cirrhosis. BMC Infect Dis. (2022) 22:94. doi: 10.1186/s12879-022-07076-0
28. Tada T, Kurosaki M, Nakamura S, Hasebe C, Kojima Y, Furuta K, et al. Real-world clinical outcomes of sofosbuvir and velpatasvir treatment in HCV genotype 1- and 2-infected patients with decompensated cirrhosis: a nationwide multicenter study by the Japanese Red Cross Liver Study Group. J Med Virol. (2021) 93:6247–56. doi: 10.1002/jmv.27157
29. Zhang W, Zhang J, Tang S, Liu Y, Du X, Qiu L, et al. Efficacy and safety of sofosbuvir-based regimens in hepatitis C patients with decompensated cirrhosis: a systematic review and meta-analysis. J Clin Transl Hepatol. (2023) 11:144–55. doi: 10.14218/JCTH.2022.00006
Keywords: hepatitis C virus, decompensated cirrhosis, direct-acting antiviral agent, overall survival, hepatocellular carcinoma, MELD score
Citation: Jongraksak T, Chuncharunee A, Intaraprasong P, Tansawet A, Thakkinstian A and Sobhonslidsuk A (2023) Outcomes of direct-acting antivirals in patients with HCV decompensated cirrhosis: a systematic review and meta-analysis. Front. Med. 10:1295857. doi: 10.3389/fmed.2023.1295857
Received: 17 September 2023; Accepted: 30 October 2023;
Published: 29 November 2023.
Edited by:
Naohiko Masaki, Medical Corporation Foundation Kenwakai Yotsugi Clinic, JapanReviewed by:
Masanori Atsukawa, Nippon Medical School, JapanRyosuke Tateishi, The University of Tokyo Hospital, Japan
Copyright © 2023 Jongraksak, Chuncharunee, Intaraprasong, Tansawet, Thakkinstian and Sobhonslidsuk. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abhasnee Sobhonslidsuk, YWJoYXNuZWUuc29iQG1haGlkb2wuYWMudGg=
 Tanawat Jongraksak
Tanawat Jongraksak Alan Chuncharunee
Alan Chuncharunee Pongphob Intaraprasong1
Pongphob Intaraprasong1 Amarit Tansawet
Amarit Tansawet Abhasnee Sobhonslidsuk
Abhasnee Sobhonslidsuk