
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 24 January 2024
Sec. Regulatory Science
Volume 10 - 2023 | https://doi.org/10.3389/fmed.2023.1294696
 Takashi Waki1
Takashi Waki1 Yusuke Okada1
Yusuke Okada1 Yuki Kinoshita1
Yuki Kinoshita1 Kazuhiro Kajiyama1
Kazuhiro Kajiyama1 Chieko Ishiguro1†
Chieko Ishiguro1† Yuki Nakazato2
Yuki Nakazato2 Ryota Kimura2†
Ryota Kimura2† Harumi Maniwa2
Harumi Maniwa2 Naoya Horiuchi2
Naoya Horiuchi2 Toyotaka Iguchi3
Toyotaka Iguchi3 Yoshiaki Uyama1*
Yoshiaki Uyama1*Introduction: This study was conducted to understand the impact of package insert (PI) revision in Japan on 18 June 2019 to allow metformin use for patients with moderately decreased kidney function (30 ≤ estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2).
Methods: A new user cohort design was employed to examine the prescription trend and the occurrence of lactic acidosis in patients prescribed metformin before and after PI revision using the Medical Information Database Network (MID-NET®).
Results: From 12 May 2016 to 31 March 2020, 5,874 patients (before, n = 4,702; after, n = 1,172) were identified as new metformin users, including 1,145 patients (before, n = 914; after, n = 231) with moderately decreased kidney function. Although no marked changes in metformin prescription were observed before and after PI revision, the daily metformin dose at the first prescription decreased after PI revision. For both before and after PI revision, less than 10 cases of lactic acidosis occurred in all patients prescribed metformin, and no lactic acidosis was observed in patients with moderately decreased kidney function.
Conclusion: The results of this study are useful for understanding the safety of metformin use in patients with decreased kidney function and suggest no worse impacts of PI revision in Japan, indicating no further safety concerns on metformin use in patients with moderately decreased kidney function under the situation with careful use and safety monitoring of metformin.
Lactic acidosis is well known as one of the adverse reactions during the administration of metformin (1, 2). Previously, metformin was contraindicated in the USA, EU, and Japan for patients with moderately or severely decreased kidney function (3, 4), owing to the augmented risk of lactic acidosis depending on kidney function (5) and increased blood concentration of metformin caused by delayed excretion (6).
In 2016, based on accumulated scientific evidence, the package insert (PI) of metformin was revised to limit the contraindication to patients with severely decreased kidney function whose estimated glomerular filtration rate (eGFR) was <30 mL/min/1.73 m2 in the USA and EU (4, 7). A similar revision in the PI of metformin was implemented on 18 June 2019 in Japan (3). The revised PI in Japan also states that the safety of metformin should be carefully monitored by initiating at a low dose and by frequent monitoring of eGFR, based on a possibility such as higher blood concentration of metformin in the Japanese population than in the non-Japanese population (8). In these conditions, metformin use is allowed in patients with moderately decreased kidney function (30 ≤ eGFR <60 mL/min/1.73 m2).
After PI revision in Japan, metformin is expected to be used in patients with moderately decreased kidney function (30 ≤ eGFR <60 mL/min/1.73 m2), but the actual impacts of PI revision have not been evaluated. Therefore, the PMDA decided to conduct a pharmacoepidemiological study to understand the impacts of PI revision on the prescription trend and the occurrence of lactic acidosis in patients prescribed metformin before and after PI revision in Japan.
In this study, real-world data (RWD) from MID-NET®, a reliable and valuable database in Japan (9, 10), were used for the analysis because MID-NET® stores electronic medical records, administrative claim data, and diagnosis procedure combination (DPC) data of more than 6.05 million patients (as of December 2022) in cooperation with 10 healthcare organizations, including 23 university hospitals and regional core hospitals. In this database, data on the eGFR, blood lactic acid concentration, and blood pH, which are useful for detecting lactic acidosis and kidney function, were available for analysis. The study period spanned from 1 January 2009 to 31 March 2020.
The utilization of MID-NET® for this study was approved on 19 February 2020 through a discussion with the expert committee of MID-NET® (11), and the actual data extraction from MID-NET® for the analysis was performed on the week of 26 April 2021.
A new user cohort design was employed to evaluate the number of patients prescribed metformin and the occurrence of lactic acidosis (see Supplementary Figure S1 for details of the study design). The source cohort comprised patients who were newly prescribed metformin [i.e., A10BA (metformin) or A10BD (combination of metformin and pioglitazone) of the anatomical therapeutic chemical (ATC) classification (World Health Organization)] or dipeptidyl peptidase IV (DPP-4) inhibitors [A10BD (combinations of alogliptin and pioglitazone, linagliptin and empagliflozin, sitagliptin and ipragliflozin, and teneligliptin and canagliflozin) or A10BH (alogliptin, anagliptin, linagliptin, omarigliptin, saxagliptin, sitagliptin, teneligliptin, trelagliptin, and vildagliptin) of the ATC code (World Health Organization)] (12) from 12 May 2016 to 31 March 2020.
Patients prescribed those drugs on and after 12 May 2016 were only selected to eliminate the effects of the recommendation by the Japan Diabetes Society on 12 May 2016, which described the contraindicated use of metformin in patients with severely decreased kidney function (eGFR <30 mL/min/1.73 m2) and the need for careful administration of metformin to patients with decreased kidney function in the eGFR range of 30–60 mL/min/1.73 m2 (13). During the identification of patients in the source cohort, the following patients were excluded: (1) patients prescribed metformin or DPP-4 inhibitors before 12 May 2016; (2) patients concomitantly prescribed both metformin and DPP-4 inhibitors at t0 (the first prescription date of metformin or DPP-4 inhibitors from 12 May 2016 to 31 March 2020), or patients with the first medical records (electronic medical records, administrative claim data, or DPC data) within 180 days before t0; and (3) patients prescribed metformin or DPP-4 inhibitors within 180 days before t0.
To create the primary cohort for analysis, the following patients were excluded from the source cohort: (a) patients with records of a lactic acid concentration of ≥4 mmol/L (36 mg/dL) within 180 days before t0 and (b) patients without any medical record after t0. In the primary cohort, the patients prescribed metformin were divided into two groups based on t0 before and after PI revision on 18 June 2019. To select patients with moderately decreased kidney function at baseline, eGFR calculated on the day closest to t0 within 180 days before and at t0 were used. The eGFR was calculated based on the following formula: 194 × serum creatinine−1.094 × age−0.287 (×0.739 for women) (mL/min/1.73 m2). For analysis, kidney function was categorized as normal or mild for eGFR ≥60 mL/min/1.73 m2, moderate for 30 ≤ eGFR <60 mL/min/1.73 m2, and severe for eGFR <30 mL/min/1.73 m2. If the eGFR was unavailable within 180 days before or at t0, the kidney function of such patients was categorized as “unknown.”
Similarly, patients with moderately decreased kidney function who were newly prescribed DPP-4 inhibitors for the treatment of type 2 diabetes after 18 June 2019 [the date of the PI revision (3)] were enrolled to create the reference cohort because DPP-4 inhibitors were reported as the most prescribed medication for type 2 diabetes in Japan (14). This reference cohort was used as a reference in the analysis for the appropriate interpretation of the results in patients prescribed metformin (see Supplementary Figure S2).
The follow-up period of the cohort started 1 day after t0 and ended at an earlier date according to the following: (1) end date of the treatment period, (2) date of another drug prescription (metformin or DPP-4 inhibitors), (3) date of the last medical record entry within the study period, (4) day before the date of the PI revision (18 June 2019), which was only applied for patients whose t0 was before 18 June 2019, or (5) date of outcome occurrence. The treatment period comprised the start date and duration of the prescription with a 30-day gap and a 30-day grace period. Thus, two prescriptions for the same drug were considered continuous if the later prescription date was within 30 days of the former prescription date.
In this study, the definition of lactic acidosis was based on the clinical evaluation guideline in Japan (15). It was defined as a case in which the following criteria were met on the same day during the follow-up period: (1) lactic acid concentration ≥ 5 mmol/L (45 mg/dL) and (2) blood pH < 7.35. Hyperlactacidemia, which was used in the sensitivity analysis, was defined as ≥4 mmol/L (36 mg/dL) of lactic acid concentration.
The prescription trend was evaluated by counting the number of patients prescribed metformin per month before and after PI revision and calculating the percentage of patients prescribed metformin with moderately decreased kidney function among all patients in each month. Furthermore, the number and percentage of patients who had a daily metformin dose of ≤500 mg at the first prescription were calculated before and after PI revision.
In the primary cohort, the number of patients who were newly prescribed metformin per month was counted. The percentage of patients with moderately decreased kidney function (30 ≤ eGFR <60 mL/min/1.73 m2) was also calculated every month.
Patient background such as eGFR (within 180 days before or at t0), age and sex (at t0), concomitant medication (within 180 days before t0; diuretics and antidiabetic drugs other than metformin and DPP-4 inhibitors), and comorbidities (within 180 days before t0) such as heart diseases (e.g., heart failure, angina pectoris, arrhythmia, and cardiac infarction), diabetic complications (e.g., diabetic neuropathy, diabetic kidney disease, and diabetic eye disease), and pulmonary diseases (e.g., chronic obstructive pulmonary disease and bronchitis) were tabulated for all patients, regardless of their kidney function, and for patients with moderately decreased kidney function prescribed metformin or DPP-4 inhibitors, separately. When a patient had two or more concomitant medications or comorbidities, the number of patients was separately counted for each item, resulting in a duplicate count of a patient.
Among metformin-prescribed patients with moderately decreased kidney function, the prescribed daily dose of metformin at the first prescription before and after PI revision was evaluated in patients with eGFR of 45 ≤ eGFR <60 mL/min/1.73 m2 and patients with eGFR of 30 ≤ eGFR <45 mL/min/1.73 m2, separately.
In the primary cohort, the occurrence of lactic acidosis was counted before and after PI revision in all patients prescribed metformin, regardless of their kidney function, and patients prescribed metformin with moderately decreased kidney function (30 ≤ eGFR <60 mL/min/1.73 m2). Then, the adjusted incidence rate ratio (aIRR) in these patients was calculated after adjusting for sex and age using Poisson regression to compare the occurrence of lactic acidosis before and after PI revision. A similar sensitivity analysis was conducted for hyperlactacidemia.
SAS version 9.4 (SAS Institute, Cary, NC, USA) was used for all analyses. In presenting data with <10 patients, such data were shown as an aggregated value considering privacy based on the MID-NET® publication rule (Section 13–3) (16).
In total, 29,244 patients were identified as new users of metformin or DPP-4 inhibitors in the source cohort. Of these patients, 5,874 patients prescribed metformin, including 4,702 patients before and 1,172 patients after PI revision, were included in the primary cohort. Among metformin-prescribed patients with moderately decreased kidney function, 914 and 231 patients (1,145 patients in total) were identified before and after PI revision, respectively. In the reference cohort, 1,268 patients were identified as the new users of DPP-4 inhibitors with moderately decreased kidney function after PI revision (see Supplementary Figure S2 for more details).
Table 1 shows the background of patients prescribed metformin or DPP-4 inhibitors. The population with metformin prescription in the primary cohort mainly comprised patients with normal or mildly decreased kidney function (eGFR ≥60 mL/min/1.73 m2) and those aged ≤65 years. Among these patients, there were slightly more number of male than female ones. These patients were frequently coprescribed with insulin or diuretics and usually had comorbidities such as heart diseases or diabetic complications. No differences were observed between patients prescribed metformin before and after PI revision. More patients prescribed metformin with moderately decreased kidney function were more than 65 years old or had comorbidities of heart disease relative to all patients. However, no major differences were found among patients prescribed metformin before and after PI revision.
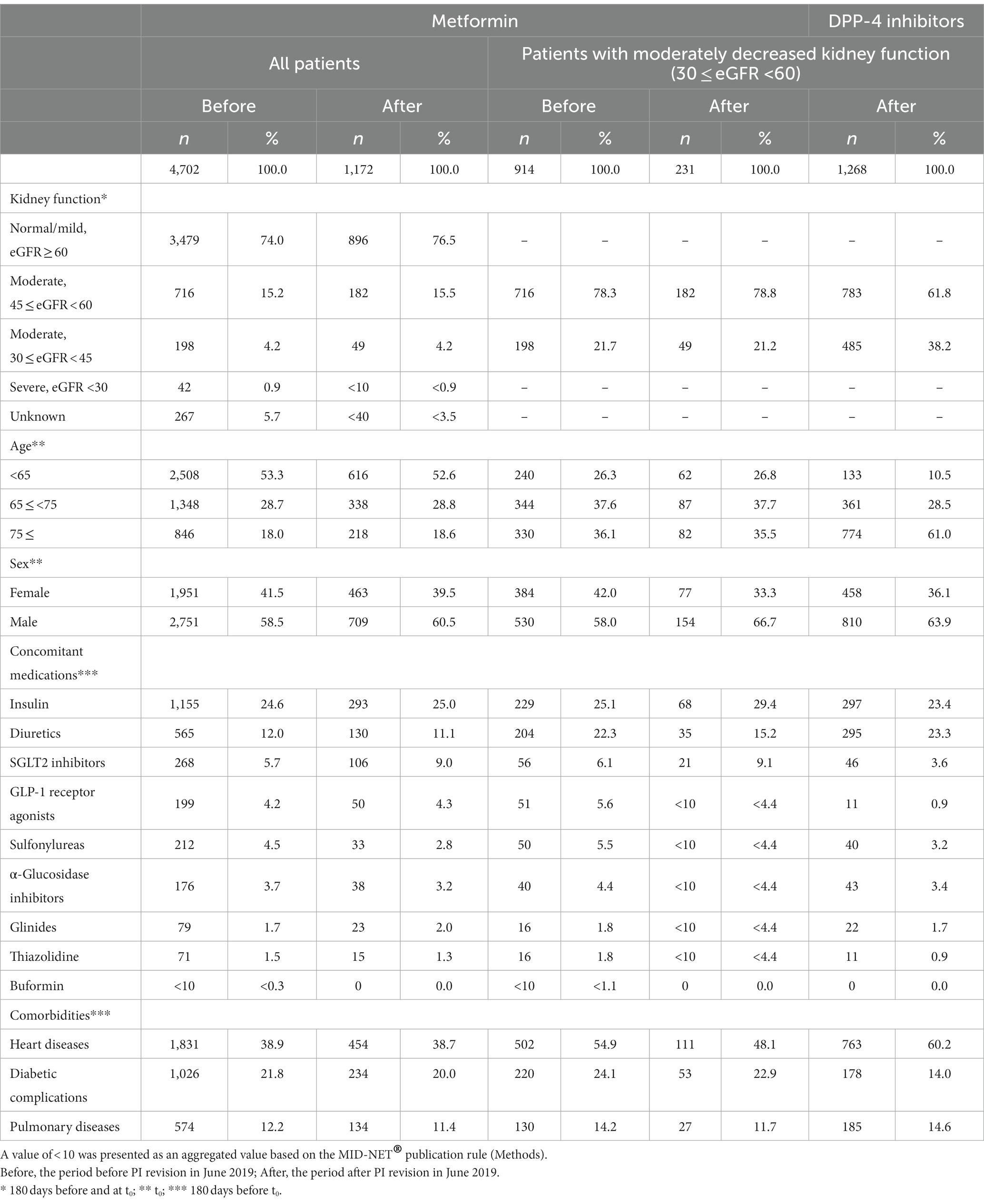
Table 1. Background of patients prescribed metformin (primary cohort) or DPP-4 inhibitors (reference cohort).
Figure 1 shows the number of patients newly prescribed metformin before and after PI revision in June 2019. Metformin was prescribed to approximately 100–150 patients per month, and 10–30% of these patients had moderately decreased kidney function. No marked changes in the number of patients or the percentages were found before and after PI revision.
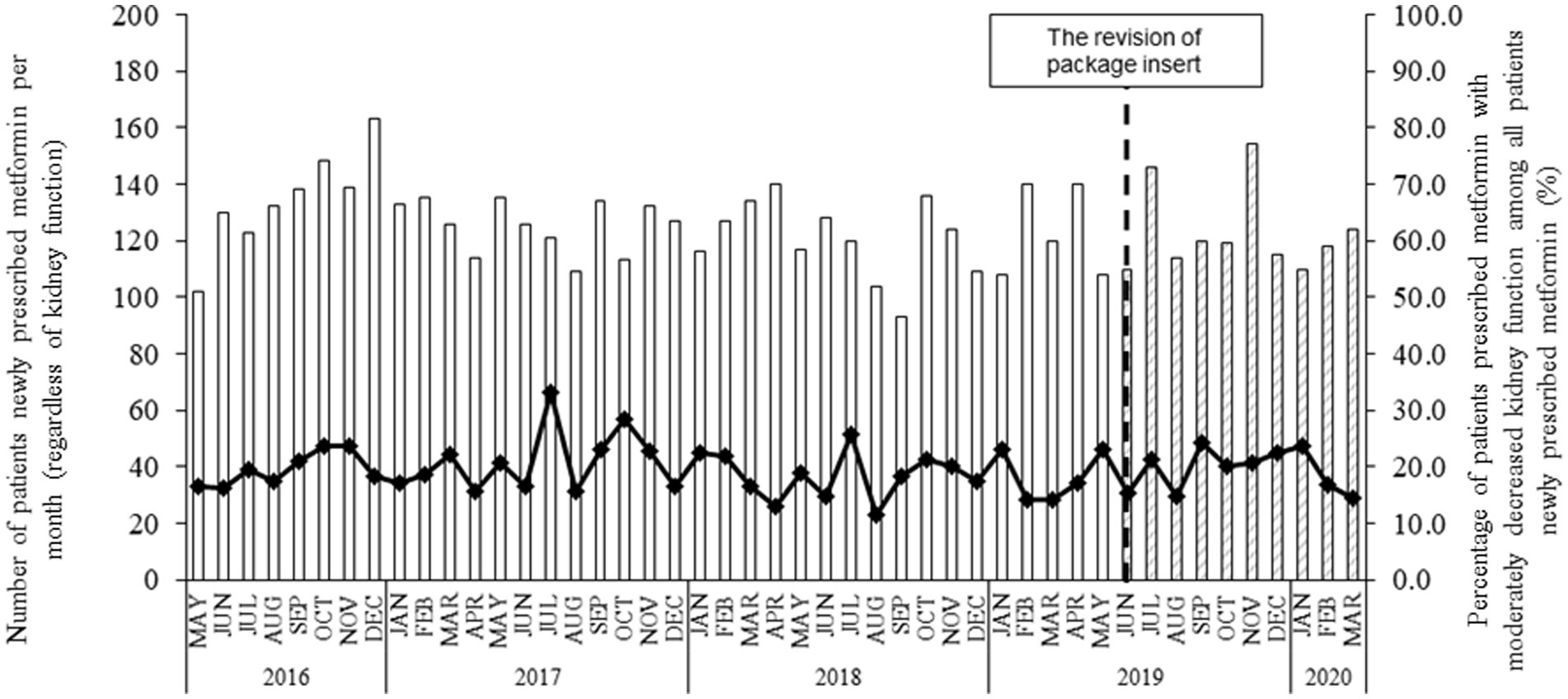
Figure 1. The number of patients newly prescribed metformin and the percentage of patients with moderately decreased kidney function before and after the PI revision in June 2019. Each bar indicates the number of patients prescribed metformin per month, regardless of their kidney function since 12 May 2016. “Blank” and “shaded” bars present the patient number before and after the PI revision in June 2019, respectively. The symbol with the line indicates the percentage of patients with moderately decreased kidney function among all patients prescribed metformin.
Interestingly, at the first prescription, the percentage of daily metformin dose ≤500 mg increased from 65.2% before PI revision to 75.5% after PI revision in patients with eGFR of 30 ≤ eGFR <45 mL/min/1.73m2 (Table 2A) and from 63.8% before PI revision to 68.7% after PI revision in patients with eGFR of 45 ≤ eGFR <60 mL/min/1.73 m2 (Table 2B).
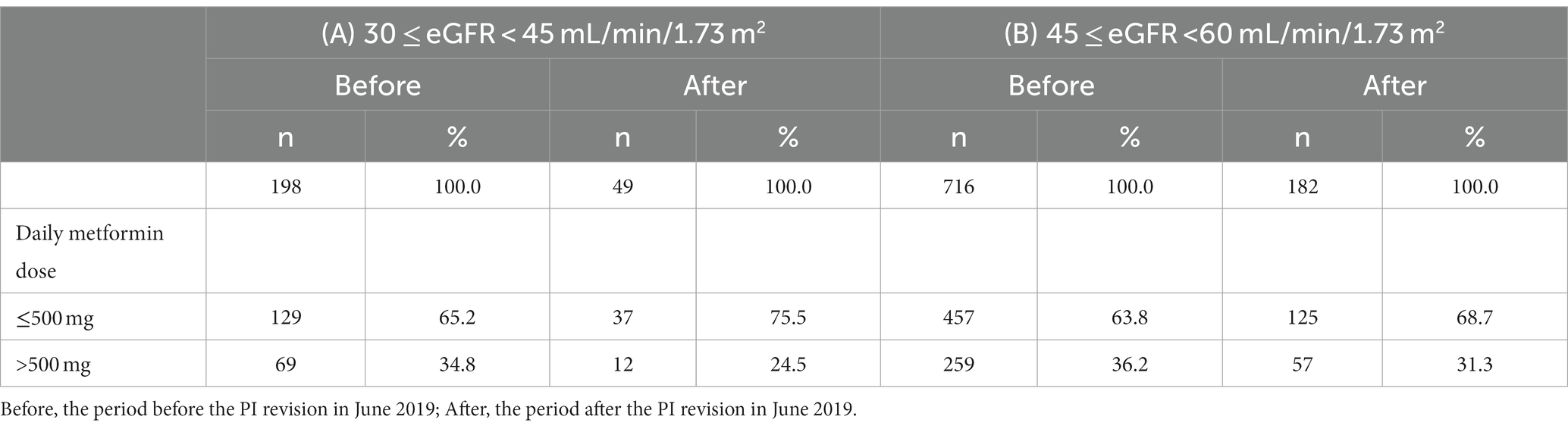
Table 2. Daily metformin dose at the first prescription in patients with moderately decreased kidney function.
In all patients prescribed metformin, regardless of their kidney function in the primary cohort, fewer than 10 cases (as an aggregated value, see the “Methods” section) of lactic acidosis occurred both before and after PI revision, and the aIRR was 4.85 with a markedly large confidence interval (CI) because of the limited occurrence of lactic acidosis (95% CI, 0.44–53.63) (Table 3A). In the patients prescribed metformin with moderately decreased kidney function (30 ≤ eGFR <60 mL/min/1.73 m2) in the primary cohort, no cases of lactic acidosis were observed both before and after PI revision (Table 3B). In addition, fewer than 10 cases were identified in patients prescribed DPP-4 inhibitors with moderately decreased kidney function (30 ≤ eGFR <60 mL/min/1.73 m2) after PI revision in the reference cohort (Table 3C). In the sensitivity analysis of hyperlactacidemia, the aIRR was 2.38 with a large CI (95% CI, 0.27–21.29) compared with those before and after PI revision in all patients prescribed metformin, regardless of their kidney function in the primary cohort. No occurrence of hyperlactacidemia was observed after PI revision in patients prescribed metformin with moderately decreased kidney function (30 ≤ eGFR <60 mL/min/1.73 m2) in the primary cohort (data not shown).
In this study, the impacts of PI revision on prescription trends and the occurrence of lactic acidosis in patients prescribed metformin before and after PI revision were examined using RWD from MID-NET®. No changes in the number of patients prescribed metformin and the percentage of patients with moderately decreased kidney function in the primary cohort were observed before and after PI revision, indicating that PI revision has no impacts on prescription trends in patients with metformin in Japan. In fact, in the period before PI revision in Japan, a certain number of metformin prescriptions in patients with moderately decreased kidney function were identified in this study, which may be due to the potential effect of the 2016 PI revision in the USA and/or EU (4, 7), as well as the 2016 recommendation by the Japan Diabetes Society, which was basically based on the 2016 PI revision in the USA (13) because the 2016 PI revision in the USA and/or EU increased the number of metformin prescriptions to patients, which could bring to a similar situation in Japan (17, 18).
However, the increased percentage of patients who were prescribed a daily metformin dose of ≤500 mg as the first prescription after PI revision in the primary cohort suggests an effect of PI revision for appropriate selection of patients for the treatment with metformin and a more careful use of metformin by initiating at lower dose.
Regarding lactic acidosis in the primary cohort, compared with before PI revision, a higher point estimate was observed after PI revision in all patients prescribed metformin, regardless of their kidney function. However, this higher value may not necessarily indicate the increased occurrence after PI revision as the CI was markedly large owing to the limited number of occurrences of lactic acidosis (95% CI, 0.44–53.63). In fact, lactic acidosis was not observed in patients with moderately decreased kidney function (30 ≤ eGFR <60 mL/min/1.73 m2), despite the expected higher occurrence in this group (5). In addition, lactic acidosis occurred in the patients prescribed DPP-4 inhibitors after PI revision but not in patients prescribed metformin with moderately decreased kidney function (30 ≤ eGFR <60 mL/min/1.73m2) before and after PI revision. Previous studies have also reported that metformin use with dose adjustment based on renal function was safe without hyperlactatemia in patients with moderately or severely decreased kidney function (1), the occurrence of lactic acidosis between patients with type 2 diabetes using metformin and other antidiabetic drugs in Japan was not significantly different (19), and the occurrence of lactic acidosis in patients with an eGFR of 30–60 mL/min/1.73 m2 was not associated with metformin (20). Accordingly, the observed lactic acidosis in patients prescribed metformin in this study would not cause significant safety concerns when continuing the current safety measures such as careful use and safety monitoring of metformin.
A strength of this study was the utilization of the laboratory test results of lactic acid concentration and blood pH as the outcome of lactic acidosis from MID-NET® (9, 10). However, the following limitations should be considered when evaluating the study results. First, the target population in this study may have been smaller than that in clinical practice because data from clinics were not included in MID-NET® (10). Second, the results may be affected by other potential confounders such as dehydration, sick day, and lifestyle factors (e.g., alcohol consumption), other comorbidities (e.g., myocardial infarction, sepsis, and excessive bleeding), and other concomitant medications that were not included in this study. Third, the study period after PI revision (approximately 6 months) may not be sufficient relative to the period before PI revision (approximately 3 years), which would lead to the absence or limited occurrence of lactic acidosis in this study, although the study objective was to examine changes in the period early after PI revision. Further studies may be necessary to evaluate the occurrence of lactic acidosis caused by metformin over a longer period.
The PMDA conducted a safety assessment on metformin-related lactic acidosis based on the results of this study and other information, including case reports and related literature, and concluded that PI revision had no worse impacts on the occurrence of lactic acidosis in patients prescribed metformin with moderately decreased kidney function. Careful use and safety monitoring of metformin should be continued in clinical practice to manage the risk.
In conclusion, the results of this study suggest no further safety concerns on metformin use in patients with moderately decreased kidney function (30 ≤ eGFR <60 mL/min/1.73 m2) under the situation with careful use and safety monitoring of metformin after PI revision in Japan.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethical approval was not required for the studies involving humans because, since this study was conducted as an official activity of the PMDA under the Pharmaceuticals and Medical Devices Agency Law (Article 15–5–(c) and (f)) (Pharmaceuticals and Medical Devices Agency), it was not subject to review by institutional review boards (Pharmaceuticals and Medical Devices Agency) (21, 22). The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from a by-product of routine care or industry. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
TW: Conceptualization, Methodology, Writing – original draft, Writing – review & editing, Formal analysis, Investigation. YO: Conceptualization, Investigation, Methodology, Writing – original draft, Writing – review & editing. YK: Conceptualization, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. KK: Conceptualization, Methodology, Project administration, Writing – original draft, Writing – review & editing. CI: Conceptualization, Methodology, Writing – review & editing. YN: Writing – original draft, Writing – review & editing. RK: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. HM: Writing – original draft, Writing – review & editing. NH: Methodology, Writing – original draft, Writing – review & editing. TI: Methodology, Writing – original draft, Writing – review & editing. YU: Conceptualization, Methodology, Supervision, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
We thank all project members of MID-NETⓇ for their continuous efforts to maintain the reliability of MID-NETⓇ. A portion of this article was included in the official Pharmaceuticals and Medical Devices Agency (PMDA) report which is available at the PMDA website.1
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2023.1294696/full#supplementary-material
1. Lalau, J-D, Kajbaf, F, Bennis, Y, Hurtel-Lemaire, A-S, Belpaire, F, and De Broe, ME. Metformin treatment in patients with type 2 diabetes and chronic kidney disease stages 3A, 3B, or 4. Diabetes Care. (2018) 41:547–53. doi: 10.2337/dc17-2231
2. Powers, A, Fowler, M, and Rickels, M. 404 diabetes mellitus: managment and therapies. 21st ed. Mc Graw Hill: Harrison's Principles of Internal Medicine (2022).
3. PMDA. Revisions of precautions in Fy2019. (2019). Available at: https://www.pmda.go.jp/english/safety/info-services/drugs/revision-of-precautions/0007.html (Accessed May 15 2023).
4. US-FDA. Drug safety communications. (2016). Available at: https://www.fda.gov/media/96771/download (Accessed May 15 2023).
5. Eppenga, WL, Lalmohamed, A, Geerts, AF, Derijks, HJ, Wensing, M, Egberts, A, et al. Risk of lactic acidosis or elevated lactate concentrations in metformin users with renal impairment: a population-based cohort study. Diabetes Care. (2014) 37:2218–24. doi: 10.2337/dc13-3023
6. Defronzo, R, Fleming, GA, Chen, K, and Bicsak, TA. Metformin-associated lactic acidosis: current perspectives on causes and risk. Metabolism. (2016) 65:20–9. doi: 10.1016/j.metabol.2015.10.014
7. EMA. Use of metformin to treat diabetes now expanded to patients with moderately reduced kidney function. (2016). Available at: https://www.ema.europa.eu/en/documents/referral/metformin-article-31-referral-use-metformin-treat-diabetes-now-expanded-patients-moderately-reduced_en.pdf (Accessed May 15 2023).
8. PMDA. 2019 report on investigation results. (2019). Available at: https://www.pmda.go.jp/files/000230001.pdf (Accessed).
9. Yamada, K, Itoh, M, Fujimura, Y, Kimura, M, Murata, K, Nakashima, N, et al. The utilization and challenges of Japan’s mid-net® medical information database network in post-marketing drug safety assessments: a summary of pilot pharmacoepidemiological studies. Pharmacoepidemiol Drug Saf. (2019) 28:601–8. doi: 10.1002/pds.4777
10. Yamaguchi, M, Inomata, S, Harada, S, Matsuzaki, Y, Kawaguchi, M, Ujibe, M, et al. Establishment of the mid-net® medical information database network as a reliable and valuable database for drug safety assessments in Japan. Pharmacoepidemiol Drug Saf. (2019) 28:1395–404. doi: 10.1002/pds.4879
11. PMDA. Information for studies approved by the expert committee of mid-net® [in Japanese]. Pharmaceuticals and Medical Devices Agency. (2020). Available at: https://www.pmda.go.jp/safety/mid-net/0010.html (Accessed May 15, 2023).
12. World Health Organization. Anatomical therapeutic chemical (Atc) classification. Available at: https://www.whocc.no/atc_ddd_index/ (Accessed May 15, 2023).
13. The Japan Diabetes Society. Recommendation for proper use of metformin. (2016). Available at: https://www.nittokyo.or.jp/uploads/files/recommendation_metformin.pdf (Accessed May 15, 2023).
14. Bouchi, R, Sugiyama, T, Goto, A, Imai, K, Ihana-Sugiyama, N, Ohsugi, M, et al. Retrospective nationwide study on the trends in first-line antidiabetic medication for patients with type 2 diabetes in Japan. J Diabetes Investig. (2022) 13:280–91. doi: 10.1111/jdi.13636
15. Araki, E, Goto, A, Kondo, T, Noda, M, Noto, H, Origasa, H, et al. Japanese clinical practice guideline for diabetes 2019. Diabetol Int. (2020) 11:165–223. doi: 10.1007/s13340-020-00439-5
16. PMDA. Mid-Net utilization rule. (2023). Available at: https://www.pmda.go.jp/files/000264724.pdf (Accessed Novermber 6, 2023).
17. Engler, C, Leo, M, Pfeifer, B, Juchum, M, Chen-Koenig, D, Poelzl, K, et al. Long-term trends in the prescription of antidiabetic drugs: real-world evidence from the diabetes registry Tyrol 2012-2018. BMJ Open Diabetes Res Care. (2020) 8:e001279. doi: 10.1136/bmjdrc-2020-001279
18. Huang, A, Wu, X, Orloff, J, Min, JY, and Flory, J. Rates of metformin use in stage 3b chronic kidney disease rose after FDA label change. J Gen Intern Med. (2021) 36:3261–3. doi: 10.1007/s11606-020-06380-2
19. Chang, CH, Sakaguchi, M, and Dolin, P. Epidemiology of lactic acidosis in type 2 diabetes patients with metformin in Japan. Pharmacoepidemiol Drug Saf. (2016) 25:1196–203. doi: 10.1002/pds.4030
20. Lazarus, B, Wu, A, Shin, J-I, Sang, Y, Alexander, GC, Secora, A, et al. Association of Metformin use with risk of lactic acidosis across the range of kidney function: a community-based cohort study. JAMA Intern Med. (2018) 178:903–10. doi: 10.1001/jamainternmed.2018.0292
21. PMDA. Act on the pharmaceuticals and medical devices agency (act no. 192 of 2002). [in Japanese]. (2002). Available at: https://elaws.e-gov.go.jp/document?lawid=414ac0000000192 (Accessed May 15, 2023).
22. PMDA. Pharmacoepidemiological studies for drug safety assessment under Mihari framework (Japanese only). (2023). Available at: https://www.pmda.go.jp/safety/surveillance-analysis/0045.html (Accessed May 15, 2023).
Keywords: metformin, lactic acidosis, drug prescription, safety action, pharmacoepidemiology
Citation: Waki T, Okada Y, Kinoshita Y, Kajiyama K, Ishiguro C, Nakazato Y, Kimura R, Maniwa H, Horiuchi N, Iguchi T and Uyama Y (2024) Prescription trend and lactic acidosis in patients prescribed metformin before and after the revision of package insert for allowing metformin administration to patients with moderately decreased kidney function based on real-world data from MID-NET® in Japan. Front. Med. 10:1294696. doi: 10.3389/fmed.2023.1294696
Received: 15 September 2023; Accepted: 31 December 2023;
Published: 24 January 2024.
Edited by:
Yurong Lai, Gilead, United StatesReviewed by:
Katsura Tsukamoto, Gifu Pharmaceutical University, JapanCopyright © 2024 Waki, Okada, Kinoshita, Kajiyama, Ishiguro, Nakazato, Kimura, Maniwa, Horiuchi, Iguchi and Uyama. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yoshiaki Uyama, dXlhbWEteW9zaGlha2lAcG1kYS5nby5qcA==
†Present ADDRESSES
Chieko Ishiguro, Section of Clinical Epidemiology, Department of Data Science, Center for Clinical Sciences, National Center for Global Health and Medicine, Tokyo, Japan
Ryota Kimura, Office of New Drug I, Pharmaceuticals and Medical Devices Agency, Tokyo, Japan
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.