- Intensive Care Unit, Dongyang Hospital of Wenzhou Medical University, Jinhua, Zhejiang,China
Asthma, a chronic respiratory ailment, affects millions worldwide. Extracorporeal membrane oxygenation (ECMO) has gained traction as a life-saving intervention for patients with severe asthma who are unresponsive to conventional treatments. However, complications associated with ECMO, including electrolyte imbalances and hemorrhage, can have significant clinical implications. This case report highlights a 49 years-old male patient with severe asthma who developed pronounced hypokalemia and hemorrhage following venovenous ECMO (VVECMO) therapy. Despite potassium supplementation, serum potassium levels continued declining before normalizing after 24 h. The patient subsequently experienced gastrointestinal bleeding, cerebral hemorrhage, and extensive cerebral infarction, ultimately resulting in a deep coma. Hypokalemia during ECMO therapy can result from a rapid reduction of carbon dioxide, β-receptor agonist use, corticosteroid use, and diuretic administration. Hemorrhage is another common ECMO complication, often linked to heparin anticoagulation therapy. Clinicians should be aware of potential complications and adopt appropriate prevention and management strategies when using ECMO in patients with severe asthma.
1 Introduction
Asthma, a widespread chronic respiratory disease, affects hundreds of millions worldwide (1, 2). It is characterized by airway inflammation, bronchoconstriction, and increased mucus secretion, resulting in episodic symptoms such as wheezing, coughing, chest tightness, and dyspnea. Extracorporeal membrane oxygenation (ECMO) is a life-saving intervention for patients with severe asthma who are unresponsive to conventional treatments (3). This extracorporeal life support temporarily sustains cardiopulmonary function by externally removing carbon dioxide and oxygenating blood (4). In contemporary times, ECMO has gained significant attention as a viable therapeutic alternative for severe respiratory failure arising from diverse etiologies, including acute respiratory distress syndrome, pneumonia, and exacerbated asthma (3, 5, 6). With the widespread adoption of ECMO technology in intensive care units, its application in managing patients with severe asthma has also increased. Several cases have been reported where ECMO has been successfully used to manage severe asthma exacerbations that were refractory to traditional treatments. These cases highlight the potential of ECMO as a therapeutic option in critical asthma management, especially when other interventions have failed (7–9). Despite these potential advantages, ECMO is associated with several complications, including hemorrhage, infection, thrombosis, and electrolyte imbalance (10). Electrolyte imbalance is common in ECMO and is often treated with continuous renal replacement therapy. However, during the literature search, we did not find any report on ECMO-related severe hypokalemia in patients with asthma, which is infrequently documented but can have significant clinical implications.
This case report presents a patient with severe asthma who, following venovenous extracorporeal membrane oxygenation (VVECMO) therapy, manifested a rare and grave complication: pronounced hypokalemia and hemorrhage. This occurrence emphasizes the potential risks of ECMO treatment for patients with severe asthma. It underscores the criticality of closely monitoring and managing electrolyte imbalances and coagulation function in critically ill patients.
2 Case description
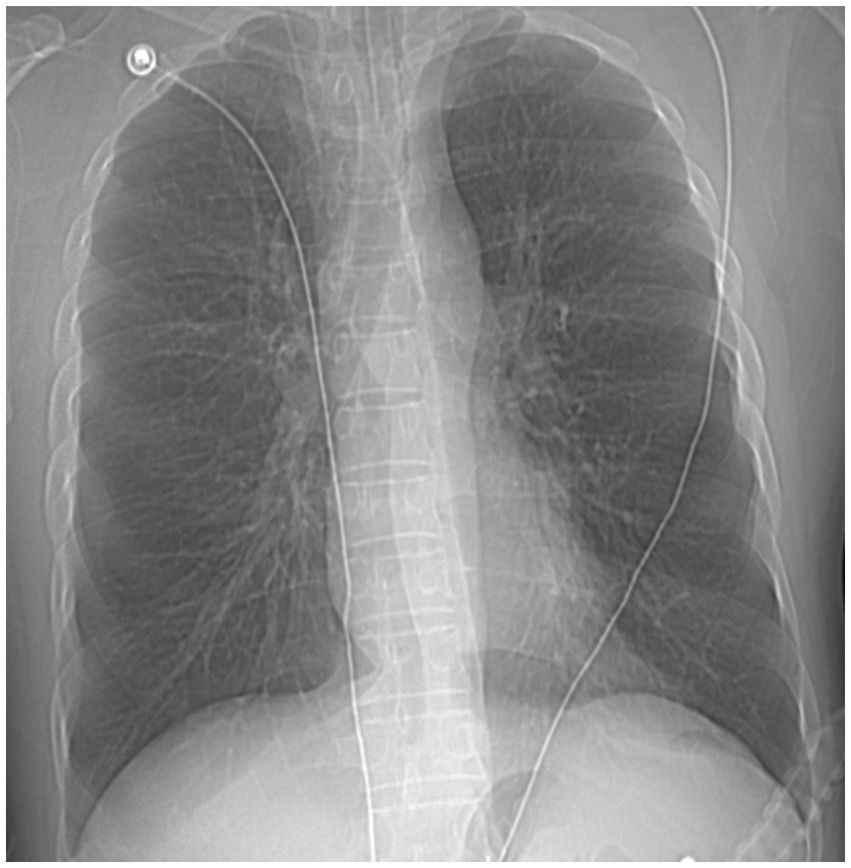
A 49 years-old man was admitted to our hospital with recurrent chest tightness and dyspnea for over 20 years, exacerbated for 16 h. The patient had a history of asthma for >20 years, intermittent use of traditional Chinese medicine to alleviate the disease (details unknown), which had not been adequately treated, and a history of smoking and drinking for over two decades. Upon emergency admission, the patient presented with a body temperature of 37.5°C, heart rate of 156 beats per minute, respiratory rate of 30 breaths per minute, blood pressure of 185/100 mmHg, and peripheral oxygen saturation of 56%. Other observations included scattered wheezing sounds in both lungs, a regular rhythm, no pathological murmurs detected upon auscultation of each valve, no abdominal tenderness, and no limb edema. Arterial blood gas analysis revealed respiratory acidosis [pH, 7.20; partial pressure of carbon dioxide (pCO2), 74 mmHg; partial pressure of oxygen (pO2), 54.6 mmHg; bicarbonate (HCO3) level, 29.3 mmol/L; and lactate level, 3.8 mmol/L]. The nucleic acid test for severe acute respiratory syndrome coronavirus yielded negative results. Chest radiography revealed no abnormalities in either lung (Figure 1).
The patient was diagnosed with asthma and subsequently treated with nebulized budesonide 1 mg, isopropyl bromide 0.5 mg twice daily, ceftriaxone sodium 2 g intravenous drip once daily for anti-infective therapy, methacrylate sodium succinate 40 mg every 6 h, epinephrine 0.2 mg/h via a micropump, and atracurium 20 mg/h via a micropump injection in conjunction, all of which were ineffective. The patient required immediate intubation and mechanical ventilation and was subsequently transferred to the intensive care unit for further monitoring and treatment. Following mechanical ventilation using the pressure control ventilation mode (frequency: 14 breaths/min, control pressure: 28 cm H2O, inspiratory time: 1 s, positive end-expiratory pressure: 10 cm H2O, and fraction of inspired oxygen: 100%), and in combination with a comprehensive 3 days regimen of sedation/analgesia, the patient exhibited a progressive decrease in tidal volume to approximately 200 mL, with peak airway pressure reaching 38 mmHg and persistent acidosis. Subsequent blood gas analysis revealed a pH of 6.86, a pCO2 of 218 mmHg, a pO2 of 194 mmHg, and an HCO3 level of 6.9 mmol/L.
After extensive consultations with family members, the patient underwent VVECMO. Additionally, intravenous heparin was administered at 250 U/h for anticoagulation, and the activated partial thromboplastin time was monitored every 4 h to maintain a range of 60–80 s. At the 1 h mark following ECMO, a significant improvement in acidosis was observed, as evidenced by a blood gas pH of 7.36 and PaCO2 of 54.8 mmHg. However, hypokalemia was detected at 2.6 mmol/L, prompting the administration of large amounts of rapid intravenous potassium supplementation. Despite this intervention, the serum potassium levels continued to decline, reaching a nadir of 1.7 mmol/L within 7 h before ultimately normalizing after 24 h.
On hospitalization day 4, the patient experienced gastrointestinal bleeding, followed by cerebral hemorrhage and extensive cerebral infarction. After consultation, the family opted for conservative treatment. However, 9 days after admission, the patient’s consciousness remained unimproved, and he was in a deep coma. The family consequently decided to terminate further treatment, and the patient succumbed shortly following his release from medical care.
3 Discussion
This case report highlights the potential complications that patients with severe asthma may experience during ECMO therapy, particularly the pronounced hypokalemia and hemorrhage that occurred after VVECMO treatment. Hypokalemia can result in severe consequences, such as life-threatening arrhythmias, muscle weakness, and paralysis, emphasizing the importance of meticulously monitoring and managing electrolyte imbalances in critically ill patients (11).
The principal criterion for implementing ECMO in individuals with asthma is refractory hypercapnic respiratory failure, distinguished by persistently elevated carbon dioxide levels in the bloodstream despite intensive mechanical ventilation and other ancillary interventions (12). Hypokalemia is associated with a rapid reduction in carbon dioxide levels, leading to an expedited exchange of potassium ions between the intracellular and extracellular environments. In particular, the augmented rate of potassium and hydrogen ion exchange causes the translocation of potassium ions into cells as hydrogen ions migrate outward, thereby leading to secondary hypokalemia (13). Hence, it is recommended that the ECMO gas flow be slowly adjusted in patients with persistent hypercapnia if the condition permits, facilitating a gradual reduction in carbon dioxide levels to facilitate the body’s adaptation to alterations in acidity, while closely monitoring electrolyte levels and clinical status (14).
Furthermore, hypokalemia in patients with asthma may be attributed to various factors (Table 1) (15). In particular, patients with asthma frequently use β-receptor agonists such as albuterol that can trigger intracellular potassium ion shifts and consequent hypokalemia (16). The use of corticosteroids, crucial medications in asthma management, may cause hypokalemia through augmented urinary potassium excretion (17). Additionally, diuretic administration during ECMO may contribute to the development of hypokalemia (18). Hemorrhage is a prevalent complication of ECMO; 40% of patients on VVECMO had one or more bleeding events. Hemorrhage is frequently associated with heparin anticoagulation therapy (19, 20). In patients with severe asthma undergoing ECMO therapy, coagulation management should be an essential aspect of the comprehensive treatment plan (21, 22). This case highlights the importance of judicious patient selection, vigilant monitoring, and effective management of complications during ECMO therapy.
In conclusion, ECMO therapy represents a critical intervention for patients experiencing severe asthma and refractory respiratory failure. However, the rapid reduction of carbon dioxide levels after ECMO treatment may lead to severe hypokalemia. In such cases, if the patient’s condition allows, it is advisable to regulate the carbon dioxide reduction rate. As the use of ECMO in patients with asthma becomes increasingly prevalent, clinicians should be cognizant of potential complications, including hypokalemia and hemorrhage, and adopt appropriate measures for prevention and management.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Dongyang People’s Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YW: Writing – original draft. WZ: Conceptualization, Writing – review & editing. XiC: Data curation, Writing – review & editing. XuC: Conceptualization, Writing – review & editing. XJ: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors would like to thank Editage (www.editage.cn) for English language editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Porsbjerg, C, Melén, E, Lehtimäki, L, and Shaw, D. Asthma. Lancet. (2023) 401:858–73. doi: 10.1016/S0140-6736(22)02125-0
2. Asher, MI, García-Marcos, L, Pearce, NE, and Strachan, DP. Trends in worldwide asthma prevalence. Eur Respir J. (2020) 56:2002094. doi: 10.1183/13993003.02094-2020
3. Lozano-Espinosa, M, Antolín-Amérigo, D, Riera Del Brío, J, Gordo Vidal, F, Quirce, S, and Álvarez, RJ. Extracorporeal membrane oxygenation (ECMO) and beyond in near fatal asthma: a comprehensive review. Respir Med. (2023) 215:107246. doi: 10.1016/j.rmed.2023.107246
4. Guglin, M, Zucker, MJ, Bazan, VM, Bozkurt, B, El Banayosy, A, Estep, JD, et al. Venoarterial ECMO for adults: JACC Scientific Expert Panel. J Am Coll Cardiol. (2019) 73:698–716. doi: 10.1016/j.jacc.2018.11.038
5. Combes, A, Peek, GJ, Hajage, D, Hardy, P, Abrams, D, Schmidt, M, et al. ECMO for severe ARDS: systematic review and individual patient data meta-analysis. Intensive Care Med. (2020) 46:2048–57. doi: 10.1007/s00134-020-06248-3
6. Urner, M, Barnett, AG, Bassi, GL, Brodie, D, Dalton, HJ, Ferguson, ND, et al. Venovenous extracorporeal membrane oxygenation in patients with acute COVID-19 associated respiratory failure: comparative effectiveness study. BMJ. (2022) 377:e068723. doi: 10.1136/bmj-2021-068723
7. Manasrah, N, Abdelazeem, B, Al Qasem, S, Kandah, E, and Chaudhary, AJ. Extracorporeal membrane oxygenation (ECMO): a life saver in near-fatal asthma. Cureus. (2021) 13:e20117. doi: 10.7759/cureus.20117
8. Yeo, HJ, Kim, D, Jeon, D, Kim, YS, Rycus, P, and Cho, WH. Extracorporeal membrane oxygenation for life-threatening asthma refractory to mechanical ventilation: analysis of the extracorporeal life support organization registry. Crit Care. (2017) 21:297. doi: 10.1186/s13054-017-1886-8
9. McLellan, T, Patvardhan, C, Rassl, D, Jenkins, HS, Knolle, M, and Thillai, M. Asthma, ECMO and eosinophils. Thorax. (2021) 76:737–9. doi: 10.1136/thoraxjnl-2020-216052
10. Teijeiro-Paradis, R, Gannon, WD, and Fan, E. Complications associated with venovenous extracorporeal membrane oxygenation-what can go wrong? Crit Care Med. (2022) 50:1809–18. doi: 10.1097/CCM.0000000000005673
11. Skogestad, J, and Aronsen, JM. Hypokalemia-induced arrhythmias and heart failure: new insights and implications for therapy. Front Physiol. (2018) 9:1500. doi: 10.3389/fphys.2018.01500
12. Braune, S, Sieweke, A, Brettner, F, Staudinger, T, Joannidis, M, Verbrugge, S, et al. The feasibility and safety of extracorporeal carbon dioxide removal to avoid intubation in patients with COPD unresponsive to noninvasive ventilation for acute hypercapnic respiratory failure (ECLAIR study): multicentre case-control study. Intensive Care Med. (2016) 42:1437–44. doi: 10.1007/s00134-016-4452-y
13. Do, C, Vasquez, PC, and Soleimani, M. Metabolic alkalosis pathogenesis, diagnosis, and treatment: core curriculum 2022. Am J Kidney Dis. (2022) 80:536–51. doi: 10.1053/j.ajkd.2021.12.016
14. Brodie, D, and Bacchetta, M. Extracorporeal membrane oxygenation for ARDS in adults. N Engl J Med. (2011) 365:1905–14. doi: 10.1056/NEJMct1103720
15. Kardalas, E, Paschou, SA, Anagnostis, P, Muscogiuri, G, Siasos, G, and Vryonidou, A. Hypokalemia: a clinical update. Endocr Connect. (2018) 7:R135–46. doi: 10.1530/EC-18-0109
16. Cox, C, Patel, K, Cantu, R, Akmyradov, C, and Irby, K. Hypokalemia measurement and management in patients with status asthmaticus on continuous albuterol. Hosp Pediatr. (2022) 12:198–204. doi: 10.1542/hpeds.2021-006265
17. Ryu, S, Yu, TY, Kim, HY, and Cho, CG. Low-dose glucocorticoid can lead to hypokalemic paralysis. Endocrine. (2020) 67:494–5. doi: 10.1007/s12020-019-02133-2
18. Lin, Z, Wong, LYF, and Cheung, BMY. Diuretic-induced hypokalaemia: an updated review. Postgrad Med J. (2022) 98:477–82. doi: 10.1136/postgradmedj-2020-139701
19. Chlebowski, MM, Baltagi, S, Carlson, M, Levy, JH, and Spinella, PC. Clinical controversies in anticoagulation monitoring and antithrombin supplementation for ECMO. Crit Care. (2020) 24:19. doi: 10.1186/s13054-020-2726-9
20. Nunez, JI, Gosling, AF, O’Gara, B, Kennedy, KF, Rycus, P, Abrams, D, et al. Bleeding and thrombotic events in adults supported with venovenous extracorporeal membrane oxygenation: an ELSO registry analysis. Intensive Care Med. (2022) 48:213–24. doi: 10.1007/s00134-021-06593-x
21. Levy, JH, Staudinger, T, and Steiner, ME. How to manage anticoagulation during extracorporeal membrane oxygenation. Intensive Care Med. (2022) 48:1076–9. doi: 10.1007/s00134-022-06723-z
Keywords: asthma, extracorporeal membrane oxygenation, hemorrhage, hypokalemia, respiratory acidosis
Citation: Wang Y, Zhang W, Chen X, Cheng X and Jiang X (2023) Extracorporeal membrane oxygenation treatment for severe asthma had unexpected adverse effects: a case report. Front. Med. 10:1294421. doi: 10.3389/fmed.2023.1294421
Edited by:
Guo-wei Tu, Fudan University, ChinaReviewed by:
Andrea Glotta, Ospedale Regionale di Lugano, SwitzerlandSerghei Covantsev, S.P. Botkin Clinical Hospital, Russia
Copyright © 2023 Wang, Zhang, Chen, Cheng and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xuandong Jiang, bHhxamlhbmdAaG90bWFpbC5jb20=
 Yun Wang
Yun Wang Weimin Zhang
Weimin Zhang Xuandong Jiang
Xuandong Jiang