- 1First Clinical Medical College, Shandong University of Traditional Chinese Medicine, Jinan, China
- 2Affiliated Hospital of Shandong University of Traditional Chinese Medicine, Jinan, China
Background: In traditional Chinese medicine, Jintiange capsules are frequently used to treat metabolic bone diseases and strengthen bones and tendons. The main component of Jintiange capsules is bionic tiger bone powder. However, the active ingredients and proteins are derived from other animal bones, with chemical profiles similar to that of natural tiger bone. This study aimed to explore the efficacy of Jintiange capsules, a Chinese herbal medicine, in the postoperative treatment of osteoporotic vertebral compression fractures (OVCFs).
Methods: In this systematic review, literature was retrieved using PubMed, the Cochrane Library, the Chinese National Knowledge Infrastructure, the Web of Science, the Wanfang Database, the Chinese Biomedical Literature Database, and the Chinese VIP Database from inception to July 2023. The primary outcome measures were the bone mineral density (BMD) and effective rate. The secondary outcome measures were the visual analog pain score (VAS), Oswestry disability index (ODI), Cobb’s angle, serum osteocalcin, serum alkaline phosphatase, and adverse events. RevMan 5.4 and STATA 17.0 software were used for data analysis.
Results: We enrolled randomized controlled trials (RCTs) focusing on 1,642 patients in the meta-analysis. The meta-analysis illustrated that Jintiange capsules significantly increased the BMD of the lumbar spine (p < 0.00001), femoral neck (p = 0.0005), and whole body (p = 0.01). The subgroup analysis of Jintiange capsules combination therapy showed that the BMD of the lumbar spine and whole body was significantly improved with Jintiange capsules (p < 0.00001). The test for the overall effect showed that Jintiange capsules had a significantly higher effective rate than the control groups (p = 0.003). Additionally, the overall effect test showed that Jintiange capsules decreased the VAS and ODI (p < 0.00001) and Cobb’s angle (p = 0.02), and improved serum OC and ALP (p < 0.00001) compared with the controls. Furthermore, the pooled analysis of adverse reactions showed no serious impacts on the treatment of OVCFs.
Conclusion: Jintiange capsules demonstrate high safety and efficacy in the treatment of OVCFs, including increasing BMD, the lift effect rate, serum OC levels, and pain relief, decreasing the ODI, serum ALP levels, and adverse events, and improving Cobb’s angle. Additional research is required to validate the efficacy of Jintiange capsules for the postoperative treatment of OVCFs.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO.
1 Introduction
Osteoporosis (OP) is a systemic disease characterized by low bone mass, decreased bone strength, increased skeletal fragility, and bone tissue destruction (1). Osteoporosis affects approximately 10 million people aged over 50 years in the United States and 34.65% of those over 50 years of age in China (2). Among older adults, osteoporotic vertebral compression fractures (OVCFs) are one of the most severe manifestations of osteoporosis (3). The clinical symptoms of OVCFs, including severe kyphosis, lower back pain, and frequent relapse, impact the physical and mental health, quality of life, and life expectancy of patients with OVCFs (4). The most common non-surgical treatment of OVCFs is bed rest, analgesics, and estrogen replacement therapy (5). Although beneficial, these have several side effects that prevent pain relief and progression over the long term (6). Although percutaneous vertebroplasty and percutaneous kyphoplasty (PVP/PKP) are frequently used to treat OVCFs, they do not relieve osteoporosis-related pain or prevent adjacent vertebral fractures (7). Therefore, developing alternative medications with fewer adverse effects and novel therapeutic techniques for postoperative OVCF treatment is essential and feasible.
Currently, bisphosphonates, calcium agents, and estrogens are widely used to prevent and treat OVCFs. Although numerous anti-OVCF medications with diverse pharmacological properties are available, the targeted therapeutic effect is not attained in significant numbers of individuals with OVCFs (8). Some studies have shown that bisphosphonates can selectively adhere to and remain within the bone and promote the apoptosis of osteoclasts, but the long-term use of the medication can lead to gastrointestinal side effects and induce bone microdamage accumulation and atypical insufficiency fractures in the skeletal system (9). Calcium has been reported to increase the risk of myocardial infarction in the long term because OVCFs require long-term treatment, in which the harm and benefits of medications are unavoidable and need to be well balanced (10).
Traditional Chinese medicine has long been a treatment for OP, tonifying the kidneys and strengthening the bones in accordance with Chinese medicine treatment principles (11). For the treatment of OP, natural Chinese herbal medicine possesses distinctive advantages that improve bone quality and biomechanical properties and influence the fate of bone cells during bone remodeling (12). Jintiange capsules are a common Chinese medicine, the primary ingredient of which is artificial tiger bone powder (13). Modern pharmacological studies have demonstrated that tiger bone is abundant in collagen, amino acids, and minerals and can play important anti-inflammatory and analgesic roles as well as strengthening tendons and bones (14). Existing evidence shows that natural tiger bone can reduce fracture risk and relieve pain in OVCFs (15). However, the use of natural tiger bone is prohibited because the tiger is a protected animal in China. To meet the medical demand, artificial tiger bone powder has been developed to substitute natural tiger bone, and its structure closely resembles that of natural tiger bone (16, 17). Several clinical studies have indicated that multiple organic constituents of Jintiange capsules, such as amino acids, calcium, magnesium, phosphorus, iron, copper, manganese, and zinc, can improve bone formation, inhibit bone absorption, and accelerate the metabolism to absorb calcium for the postoperative treatment of OVCFs (18, 19). Although Jintiange capsules have been regarded as an effective Chinese patent medicine for patients with OVCFs in China and are recommended in Chinese OP treatment guidelines, there is currently a lack of high-quality evidence regarding the efficacy of Jintiange capsules in the treatment of OVCFs (20). Therefore, it is imperative to conduct this Systematic Review and meta-analysis to evaluate the efficacy of Jintiange capsules in the postoperative management of OVCFs.
2 Materials and methods
This study strictly followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (21). The ethical review was unnecessary because the data from the articles included in this meta-analysis were publicly available. The study was registered on PROSPERO (CRD42023456068).
2.1 Search strategy
We conducted an electronic search of the following seven authoritative literature databases: PubMed, the Cochrane Library, the Chinese National Knowledge Infrastructure (CNKI), the Web of Science, the Wanfang Database, the Chinese Biomedical Literature Database, and the Chinese VIP Database from their inception until July 2023. Furthermore, each database employed a combination of MeSH terms and free words for comprehensive study searches, and the language of the published literature was unrestricted. The relevant keywords for Jintiange were “Jintiange capsule” OR “artificial tiger bone” OR “Jintiange” OR “bionic tiger bone.” We retrieved keywords of OP, including “osteoporosis” OR “postmenopausal osteoporosis” OR “bone mass” OR “bone loss” OR “bone mineral density.” The keywords for OVCFs were “compression fractures” OR “lumbar compression fracture” OR “thoracic vertebral compression fractures” OR “osteoporosis vertebral compression fractures” OR “OVCFs.”
2.2 Eligibility criteria
We only included RCTs that compared Jintiange capsules with conventional western therapies and placebos for the postoperative treatment of OVCFs. The intervention group used Jintiange alone or with other conventional western therapies. The control group received only conventional western therapies or a placebo. Participants were diagnosed with OVCFs regardless of age, gender, dosage, duration, or disease course. Primary outcomes included bone mineral density (BMD) and effective rate; secondary outcomes included visual scale (VAS), the Oswestry Disability Index (ODI), Cobb’s angle, serum osteocalcin (OC), and adverse events. Additionally, all outcome indicators had to specify the results of the pooled data.
2.3 Exclusion criteria
The exclusion criteria included the following: (1) non-randomized controlled trials; (2) participants that were not diagnosed with OVCFs; (3) RCTs with participants who did not receive postoperative treatment; (4) studies that did not use Jintiange capsules or any other Chinese herbals or Traditional Chinese Medicine therapies; and (5) meeting abstracts, reviews, duplicate publications, animal experiments, and insufficient clinical data.
2.4 Data extraction
Two researchers extracted and collated the basic information of the final studies based on inclusion and exclusion criteria. Additionally, a third researcher examined the results independently. For this meta-analysis, author, publication year, study type, sample size, age of participants, operation method, number of study centers, follow-up time, intervening measures, and course of treatment were required as general information about the study. Disagreements between the two researchers about the pooled data were determined and resolved by consulting with a third researcher.
2.5 Quality assessment of the included studies
Two independent authors evaluated the risk of bias for each trial study using the Cochrane systematic reviews. This evaluation tool has seven items: random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and other bias. Each domain can be judged as low bias risk, high bias risk, and unclear bias risk in the literature (22, 23).
2.6 Statistical analysis
All the enrolled studies were conducted on the Review Manager Software (RevMan5.4), and the STATA software (STATA Software Version 17.0) was used to evaluate publication bias and execute sensitivity analysis. The continuous outcome variables were presented as mean difference (MD) with a 95% confidence interval (95% CI), and we measured the same outcomes using standardized mean differences (SMD) of 95% CIs. We extracted the number of positive events based on binary outcome variables and calculated the risk ratio (RR) with 95% confidence intervals for both categories. The heterogeneity of the trial was assessed using I2 statistics, among which p < 0.05 or I2 > 50% was evaluated to have high heterogeneity, and we used a random-effects model. Each article that contributed to heterogeneity was excluded from the sensitivity analysis; otherwise, the fixed-effects model was used when the heterogeneity between the enrolled studies was small (p ≥ 0.05 or I2 ≤ 50%). Furthermore, subgroup analysis was applied to the trial based on Jintiange capsules, separated into Jintiange combined intervention subgroup and Jintiange alone intervention subgroup. A funnel plot was constructed to evaluate potential publication bias, which was analyzed using Egger’s test.
3 Results
3.1 Screening results of the studies
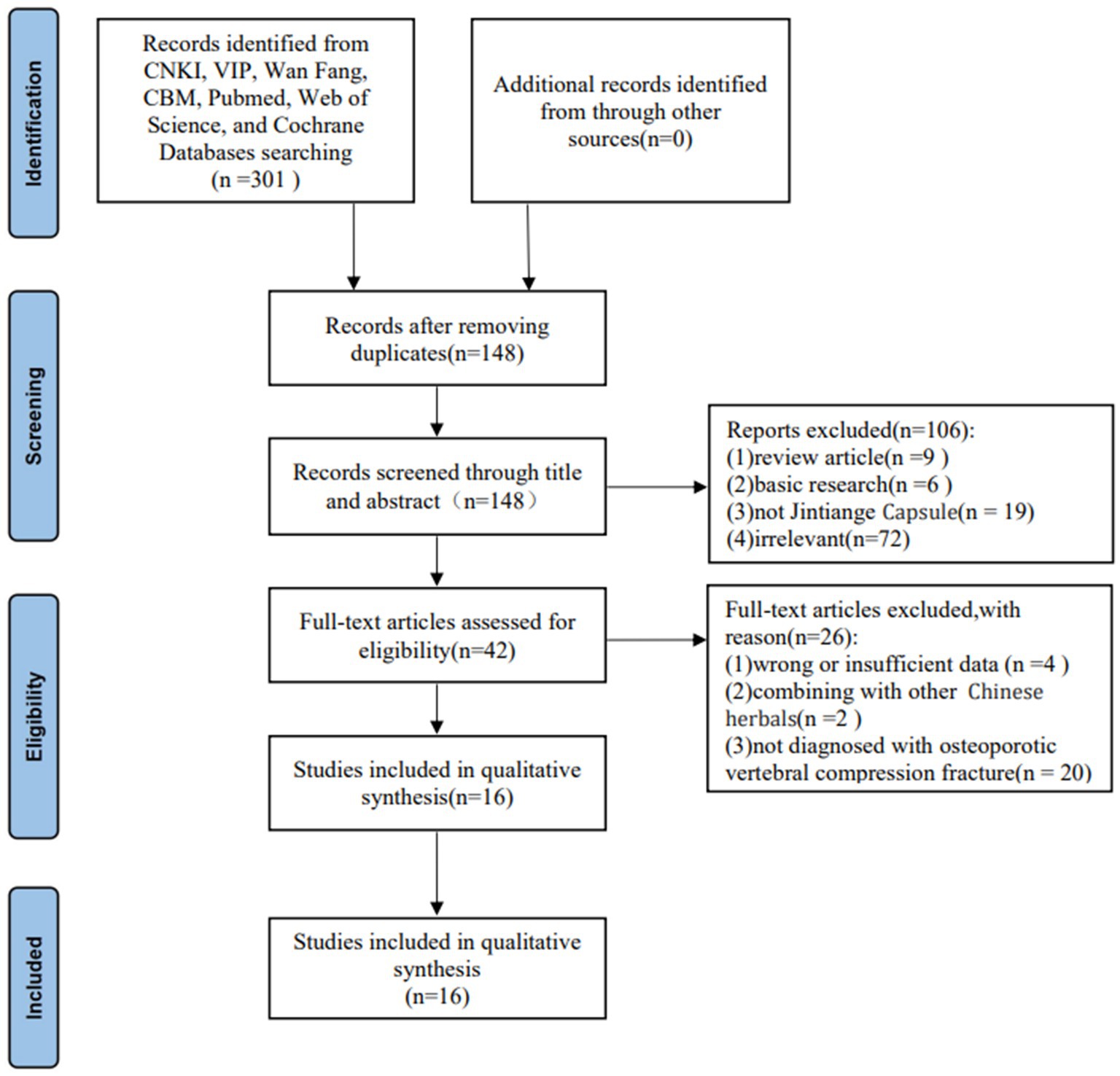
According to the screening strategy, 148 articles were initially identified after removing duplicate literature. Next, we eliminated 102 articles by scanning the titles and abstracts. After applying the inclusion and exclusion criteria, the full text of the 42 studies met the requirement with the Jintiange Capsule, of which 16 studies on the postoperative treatment of patients with OVCFs ultimately met the inclusion criterias of this meta-analysis (18, 24–38). Figure 1 shows the search process and selection details.
3.2 Characteristics of the included studies
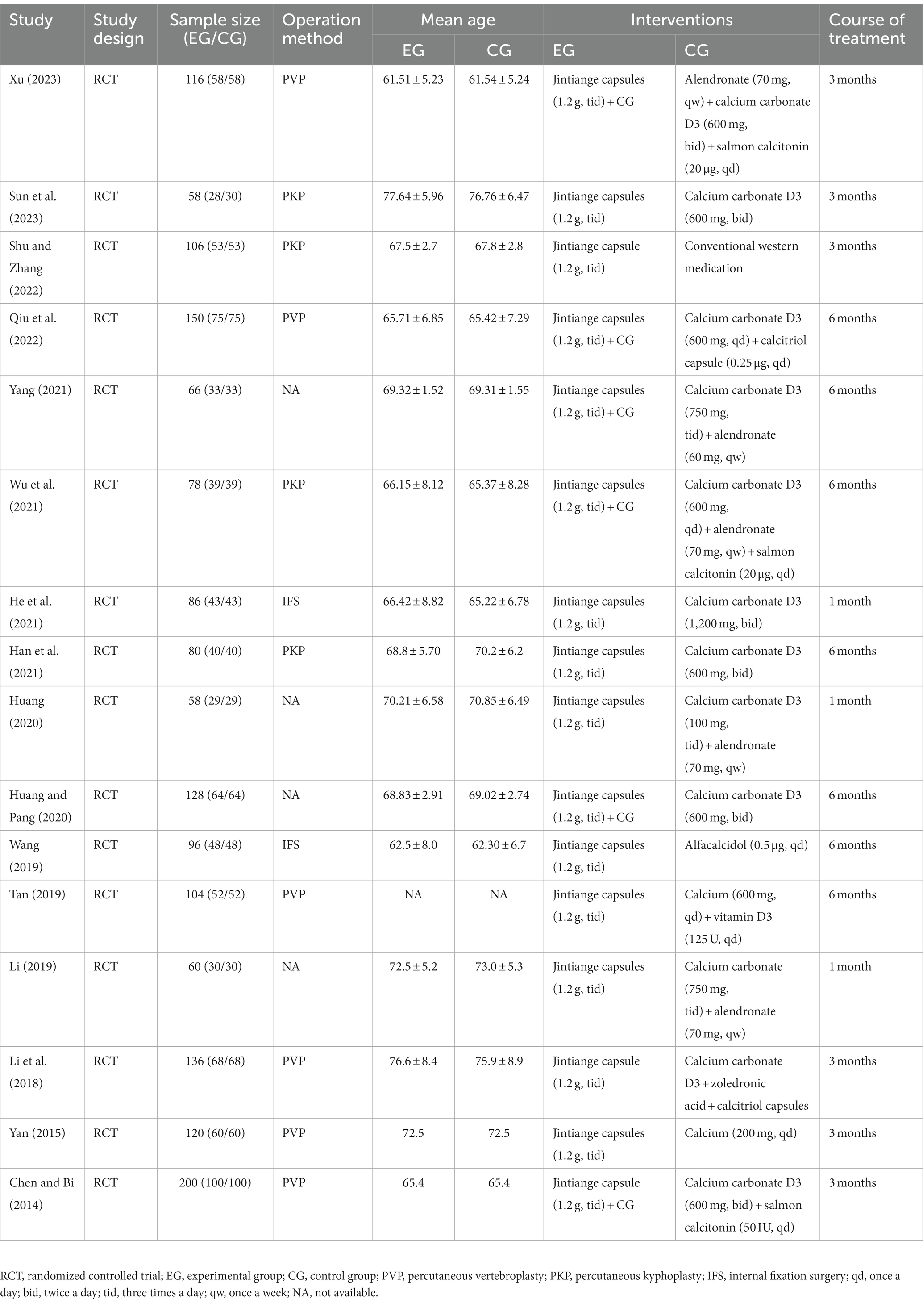
Through searching and screening, this meta-analysis included 16 clinical studies, all of which were RCTs on the postoperative treatment of patients with OVCFs with Jintiange capsules. These enrolled studies were all conducted in China. Overall, 16 RCTs included 1,642 participants, of which 822 were in the intervention group and 820 were in the control group. The year of publication of the included trials ranged from 2014 to 2023. All the reported groups in the studies were matched in terms of age, gender, operation, course of disease, and outcome. The operation methods for OVCFs were PVP, PKP, and DHS. Among all studies of the intervention group, 10 studies involved combined therapies and six were single treatment studies with only Jintiange capsules. The daily dose of Jintiange capsules is 1.2 g. The course of interventions was between 1 month and 12 months. Table 1 shows the specific details and characteristics of all the included studies.
3.3 Literature quality assessment
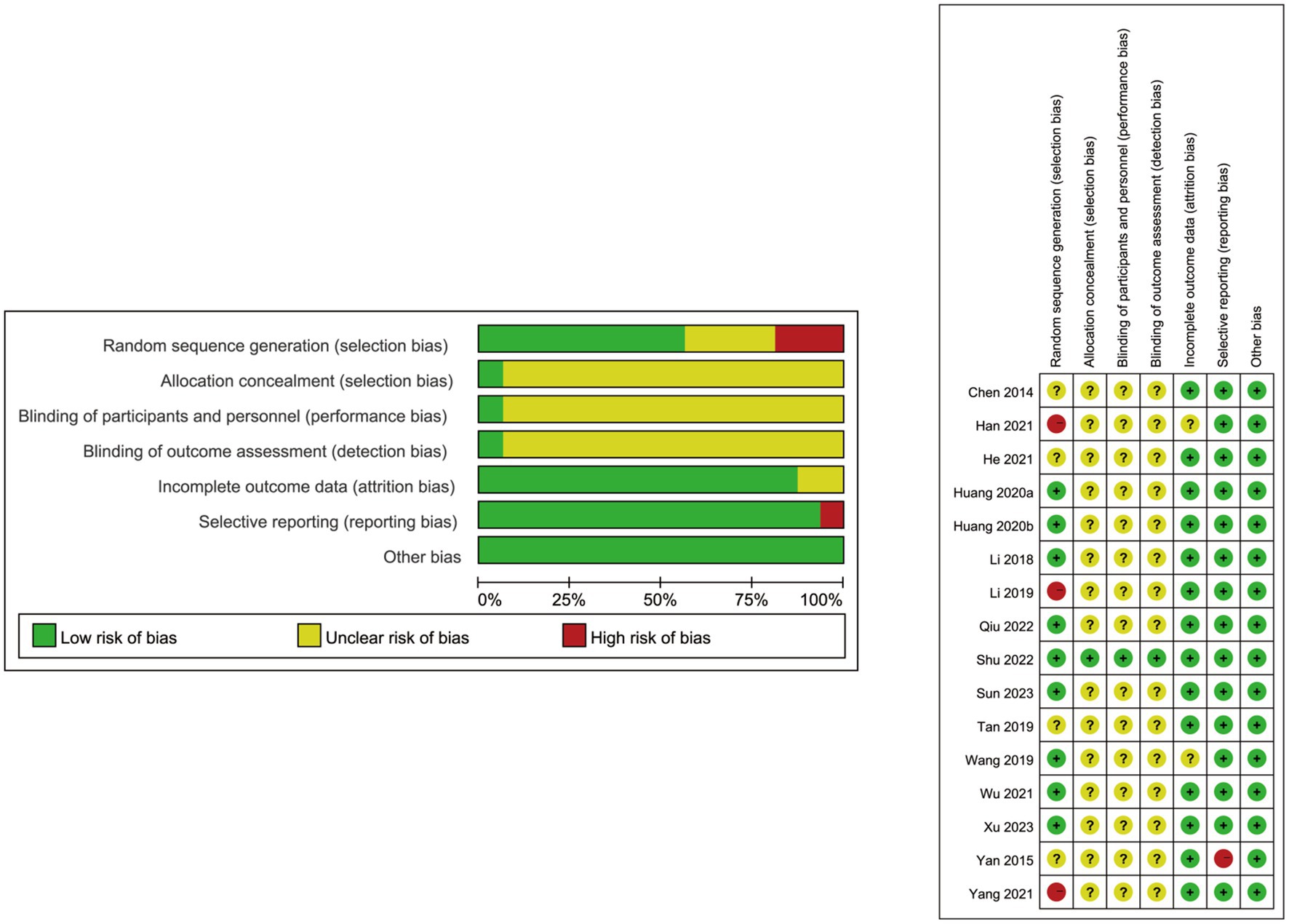
Each RCT employed the Cochrane Collaboration’s tool to evaluate the risk of bias. Most of the 16 articles enrolled in this meta-analysis were randomized controlled trials, among which three studies did not document the random allocation approach (21, 26, 29). Although only one study mentioned the concealment allocation and others did not clearly describe the method (12), it was unlikely to affect the data evaluation. With one of the 16 studies considered to have a low risk of performance bias for the blinding of participants and blinding of outcome assessment (12), the residual studies included remained unclear. Fourteen RCTs did not meet the incomplete outcome data criterion or had no drop-out patient data (12, 18–22, 24, 25, 27–32). One study did not provide a precise explanation of the selective reporting of data (19), which may lead to potential outcomes risk bias. Baseline comparisons were in accordance with the published prespecified analysis plan. No information mentioned that the baseline comparisons were imbalanced, and other biases were not present in the enrolled studies. The details about the risk of bias evaluation for each study are shown in Figure 2.
3.4 Results of the meta-analysis
3.4.1 Primary outcomes
3.4.1.1 BMD of the lumbar spine
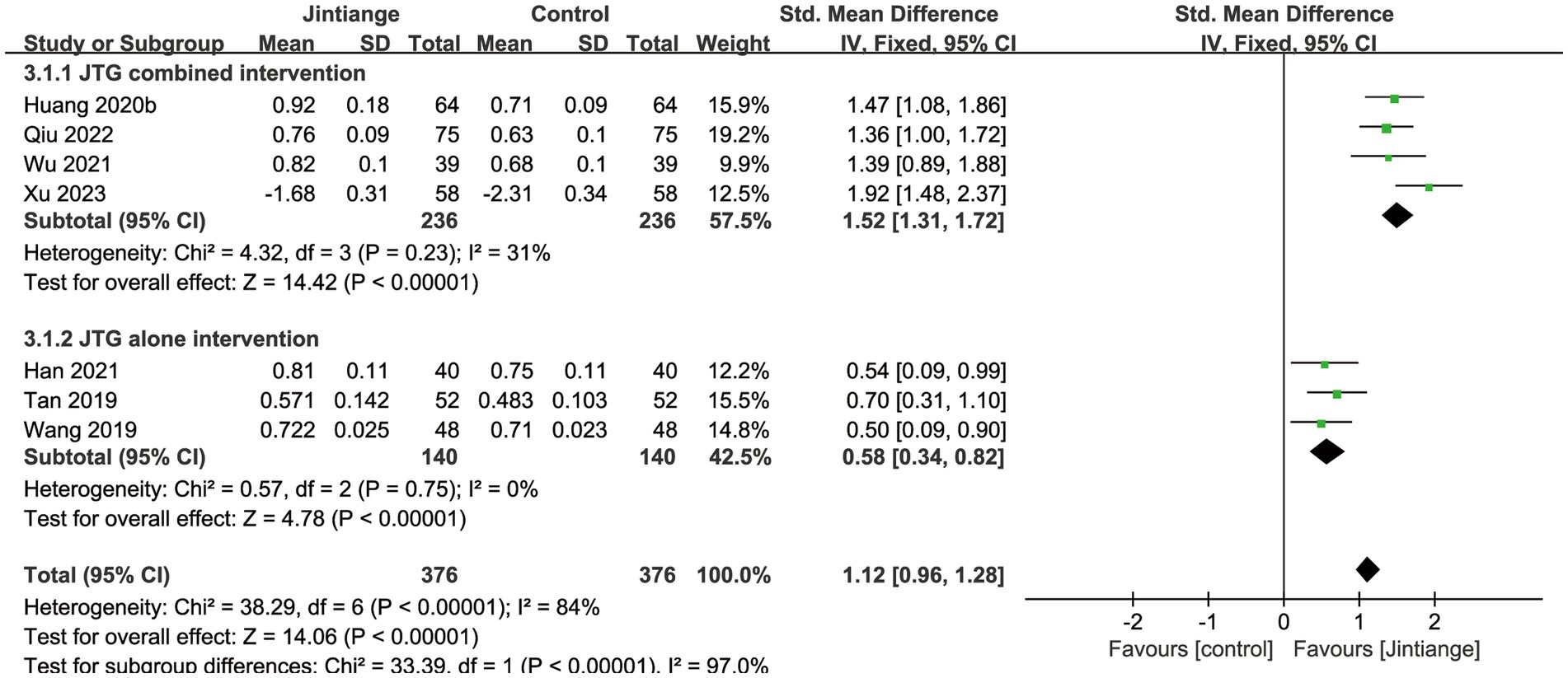
Seven studies (28–30, 32, 34, 36, 38) reported BMD changes at the lumbar spine. These enrolled studies showed that the test for the overall effect of the Jintiange capsules significantly increased the BMD of the lumbar spine compared with the controls (SMD = 1.12, 95% CI: 0.96 to 1.28, p < 0.00001). Subgroup analyses of the Jintiange capsules combined intervention reported that the Z value of the test for overall effect was larger than the Jintiange capsules alone intervention group (Z = 14.42), which further suggests that the Jintiange capsules combined intervention appears to be superior at improving the BMD of the lumbar spine (Figure 3).
3.4.1.2 BMD of the femoral neck
Three studies reported the effects of the Jintiange capsules on the BMD in the femoral neck (24, 34, 36). The pooled data demonstrated a significant difference in lifting BMD at the femoral neck between the Jintiange capsules and control groups (MD = 0.08, 95% CI: 0.04 to 0.13, p = 0.0005) (Figure 4).
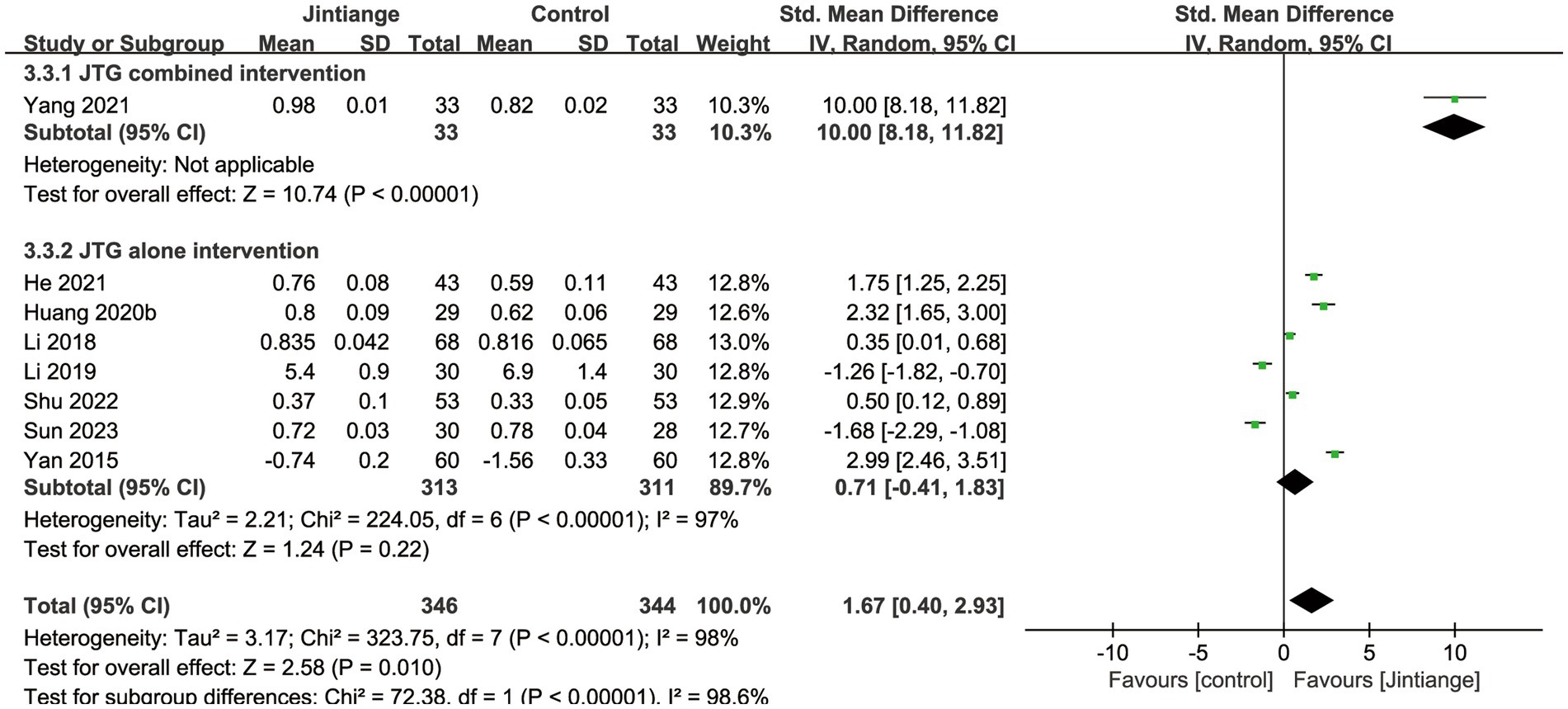
3.4.1.3 BMD of the whole body
Eight studies (18, 25–27, 30, 33, 35, 37) reported percentage changes in whole-body BMD. The available data illustrated that compared with the control groups, the overall effect test of the Jintiange capsules showed a significant difference in the BMD of the whole body (SMD = 1.67, 95% CI: 0.40 to 2.93, p = 0.01). Additionally, the subgroup analysis showed that the Jintiange capsules combined intervention had a superior effect on whole-body BMD relative to the Jintiange capsules alone (SMD =10.0, 95% CI: 8.18 to 11.82, p < 0.00001). However, the pooled results showed no significant difference in whole-body BMD changes between the Jintiange capsules and the control groups (p = 0.22) (Figure 5).
3.4.1.4 Effective rate
Nine studies (18, 24, 25, 27, 31, 33–35, 38) showed percentage changes in the effective rate. The test for the overall effect of these nine studies showed that Jintiange capsules significantly improved the effective rate in contrast with control groups (RR = 1.14, 95% CI: 1.05 to 1.25, p = 0.003). Subgroup analysis of the effective rate showed that the Jintiange capsules alone intervention was also superior to that of the control groups (p = 0.003). However, the pooled results showed that the Jintiange capsules combined intervention did not significantly impact the effective changes between the Jintiange capsules group and the control group (p = 0.12). Therefore, the heterogeneity of the Jintiange capsules combined intervention was eliminated after removing Wu et al. The subgroup analysis demonstrated that the Z value of the Jintiange capsules combined intervention increased (Z = 4.54), the p-value decreased (p < 0.00001), and the 95% confidence interval was narrowed down (1.09 to 1.24). These changes further suggest that the Jintiange capsules combined intervention appears to be better at increasing the effective rate (Figure 6).
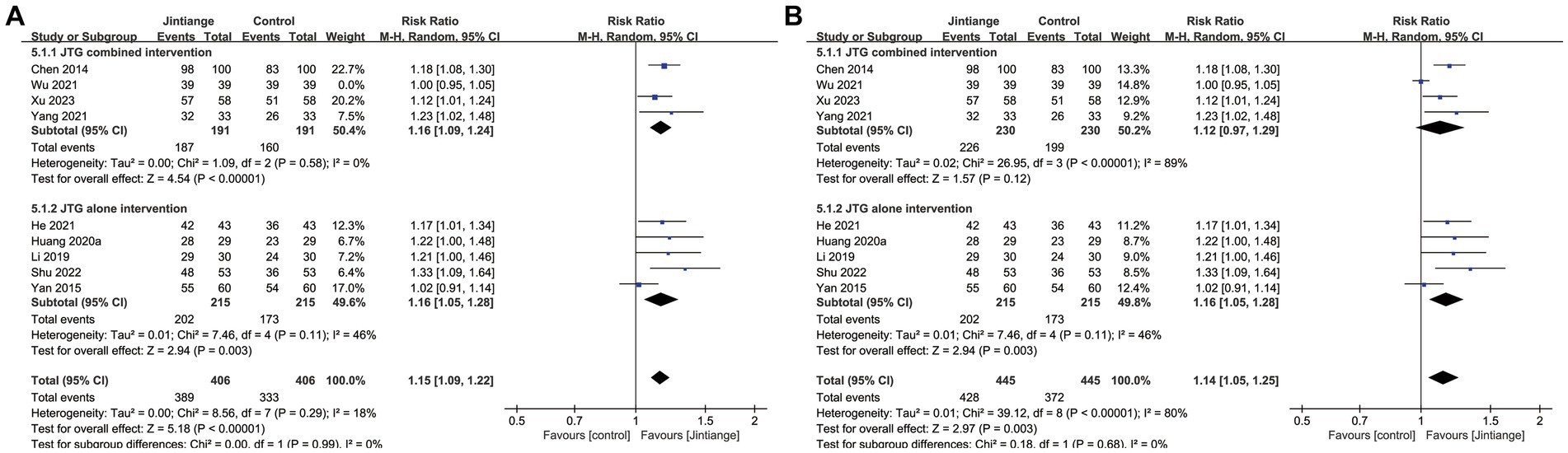
Figure 6. (A) Forest plot of the effective rate. (B) Forest plot of the effective rate after eliminating the study by Wu.
3.4.2 Secondary outcomes
3.4.2.1 VAS
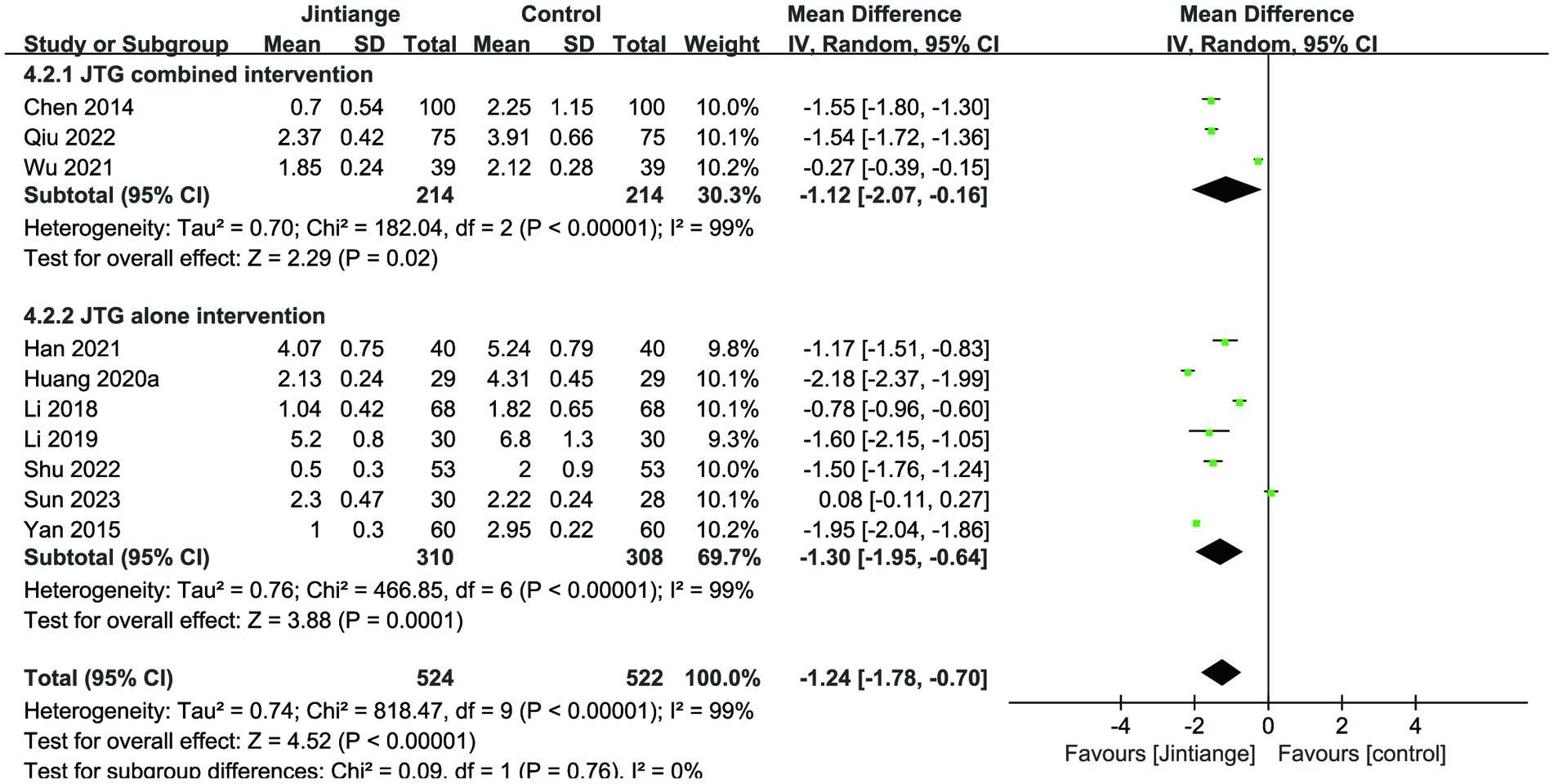
Ten studies showed the assessment of the VAS. Meta-analysis results showed that the overall effect test reported a significant difference between the Jintiange capsules and the control groups (MD = −1.24, 95% CI: −1.78 to −0.70, p < 0.00001) (18, 24–27, 31, 32, 34, 36, 37). Similarly, the subgroup analyses illustrated that both the Jintiange capsules combined and alone interventions remarkably reduced the VAS score relative to controls (p = 0.02, p = 0.0001) (Figure 7).
3.4.2.2 ODI
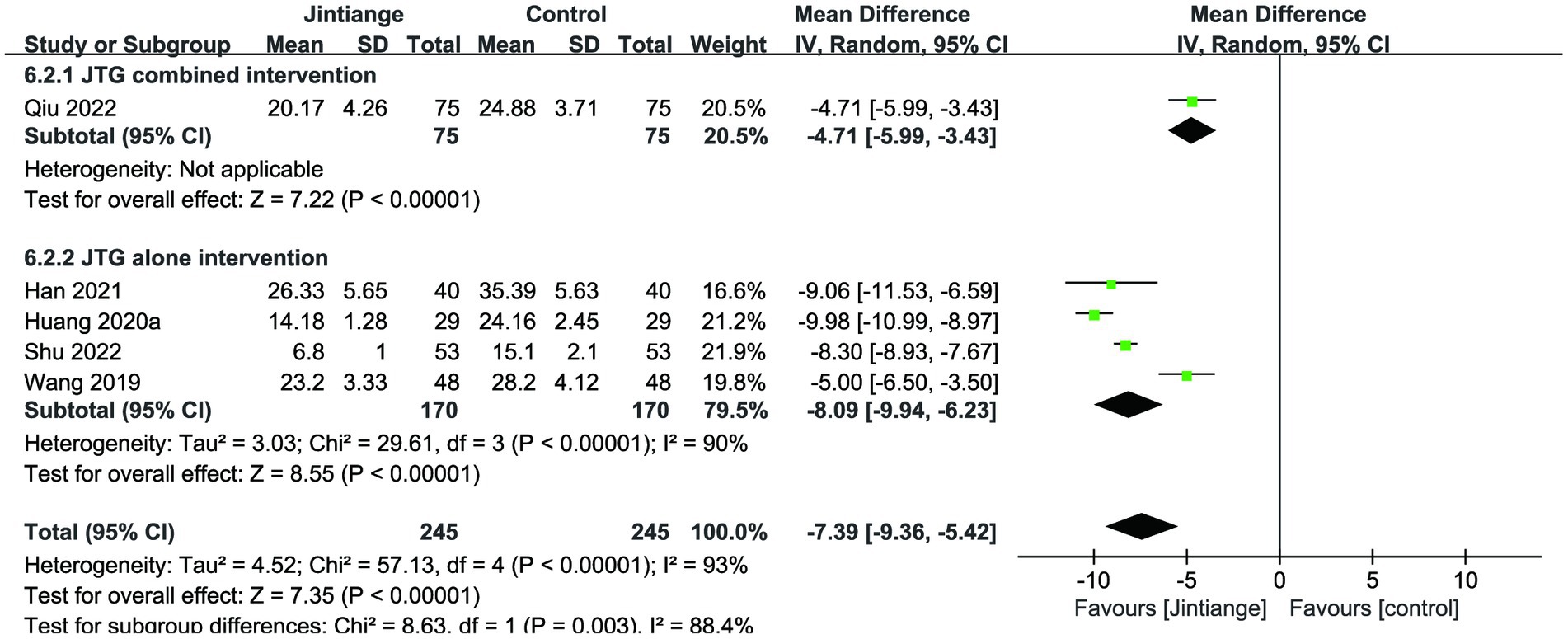
Five studies (18, 29, 31, 32, 36) reported the percentage changes in the ODI. This meta-analysis illustrated that the overall effect test showed a statistically significant difference in the comparison of ODI levels with the control group (MD = −7.39, 95% CI: −9.36 to −5.42, p < 0.00001). The subgroup analysis of the Jintiange capsules combined and alone interventions showed that the Jintiange capsules treatment resulted in a significant decrease in the ODI compared with the control group (p < 0.00001) (Figure 8).
3.4.2.3 Cobb’s angle
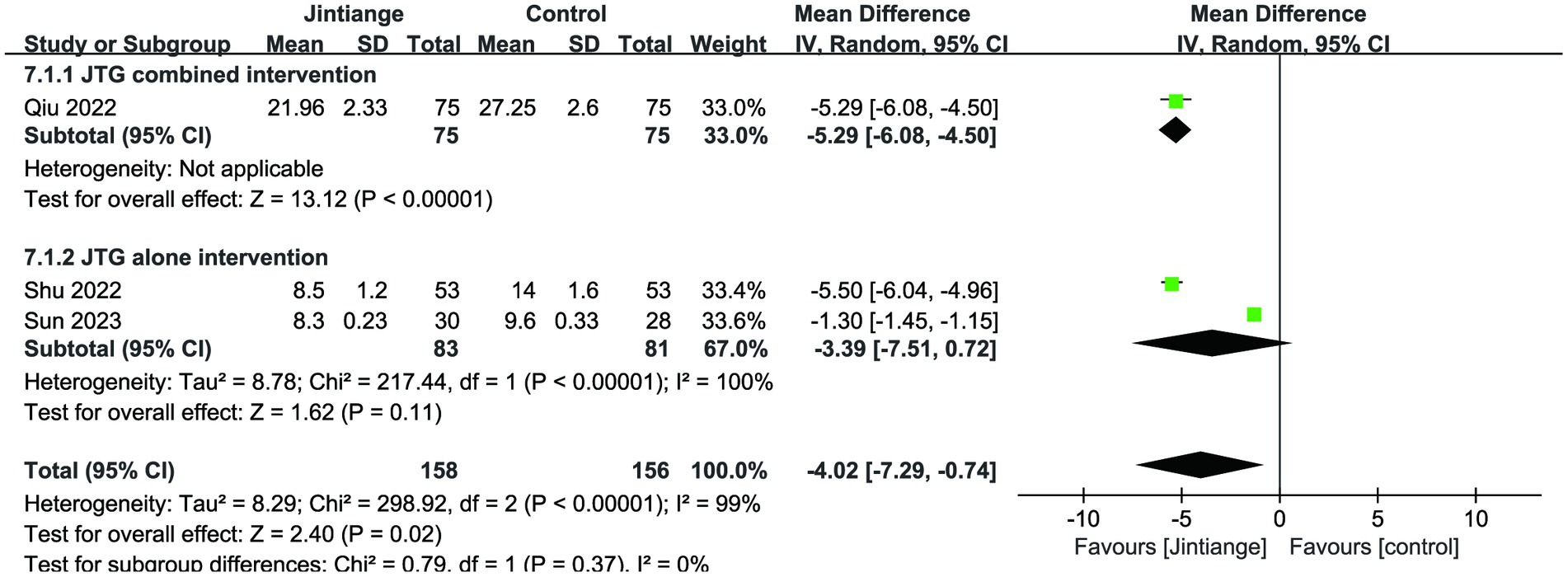
This meta-analysis analyzed three studies related to Cobb’s angle (18, 36, 37). The available data of both overall effect test and the subgroup analysis of the Jintiange capsules combined intervention showed that the Jintiange capsules had a superior effect in improving Cobb’s angle than the control group (p < 0.00001, p = 0.02). However, there was no statistically significant difference between the subgroup of the Jintiange capsules alone and the control condition (p = 0.11) (Figure 9).
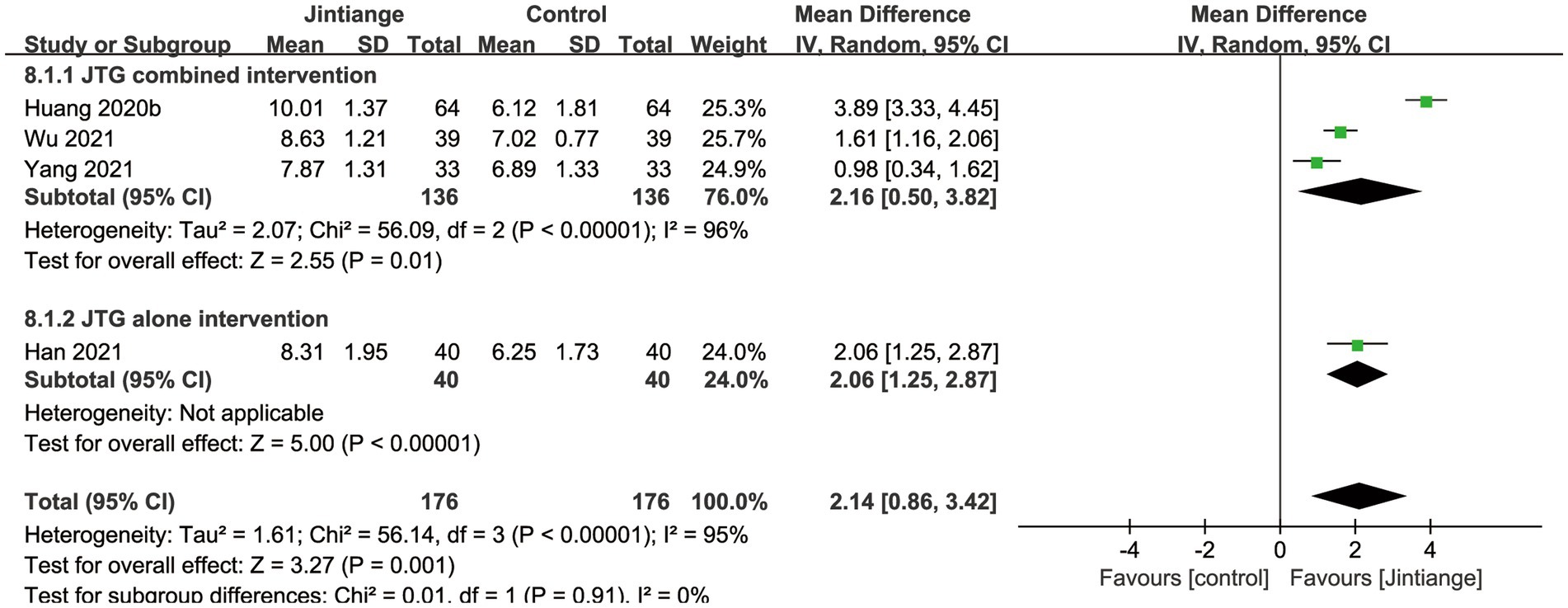
3.4.2.4 Serum OC
Four studies (30, 32, 34, 35) reported the changes in the serum OC. As illustrated in Figure 5, the pooled data depicted that the overall effect test showed a significant difference between the Jintiange capsules and control groups (MD =2.14, 95% CI: 0.86 to 3.42, p = 0.001). The results of the subgroup analysis indicated that the Jintiange capsules combined and alone interventions also significantly increased serum OC in contrast with the control group (p = 0.01, p < 0.00001) (Figure 10).
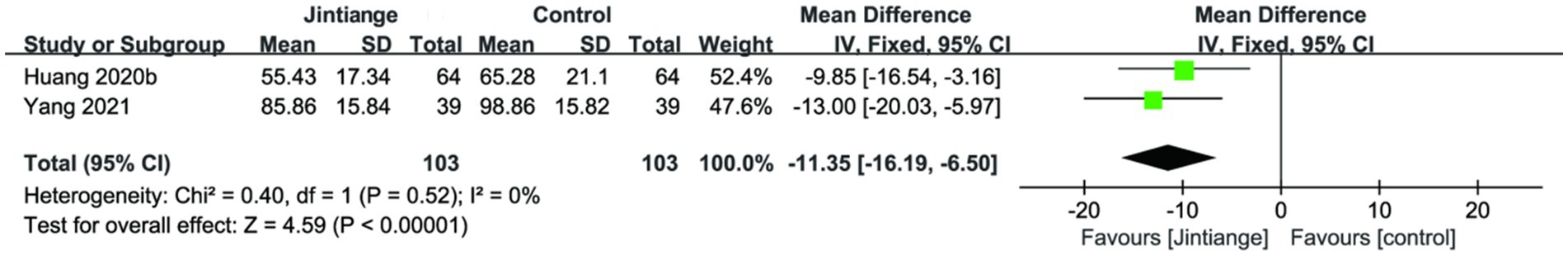
3.4.2.5 Serum ALP
Two studies (30, 35) analyzed the changes in serum ALP. The overall effect test of the pooled data demonstrated that the Jintiange capsules significantly reduced serum ALP in contrast with the control group (SMD = −11.35, 95% CI: −16.19 to −6.50, p < 0.00001). This result further suggested that the Jintiange capsules combined intervention was remarkable in improving the changes in serum ALP (Figure 11).
3.4.3 Adverse events
Six trials reported information on adverse events and 10 did not (21, 24, 25, 27, 29, 32). The incidence of adverse events was 10/238 in the Jintiange capsules group and 45/238 in the control group. The reporting of adverse events included dry mouth, recurrent fractures, constipation, belching, abdominal distension, diarrhea, nausea, and vomiting. Four studies showed that five patients in the Jintiange capsules group and 28 patients from the control group developed mild gastrointestinal symptoms. In the other two studies, 17 subjects developed recurrent fractures in the controls, among which, one study revealed that five subjects developed recurrent fractures in the Jintiange capsules group. These studies evidenced that the frequency of adverse events was mild, and the treatment was less limited than in the control group, with no severe adverse impacts.
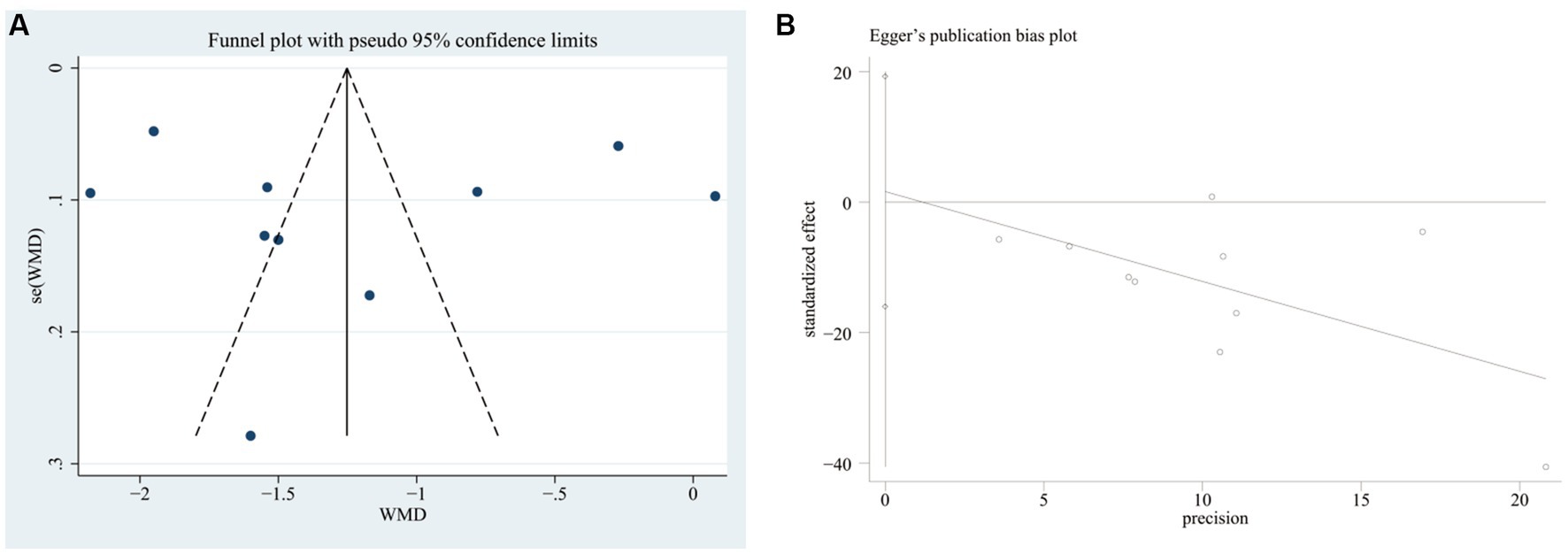
3.5 Publication bias and sensitivity analysis
We plotted the funnel plots along with Egger’s test to evaluate the publication bias of the VAS score in STATA 17 software. The funnel plot showed that these studies were approximately symmetrically distributed on both sides of the regression line. Additionally, the results from Egger’s test showed no significant publication bias for the VAS score (p = 0.838) (Figure 12).
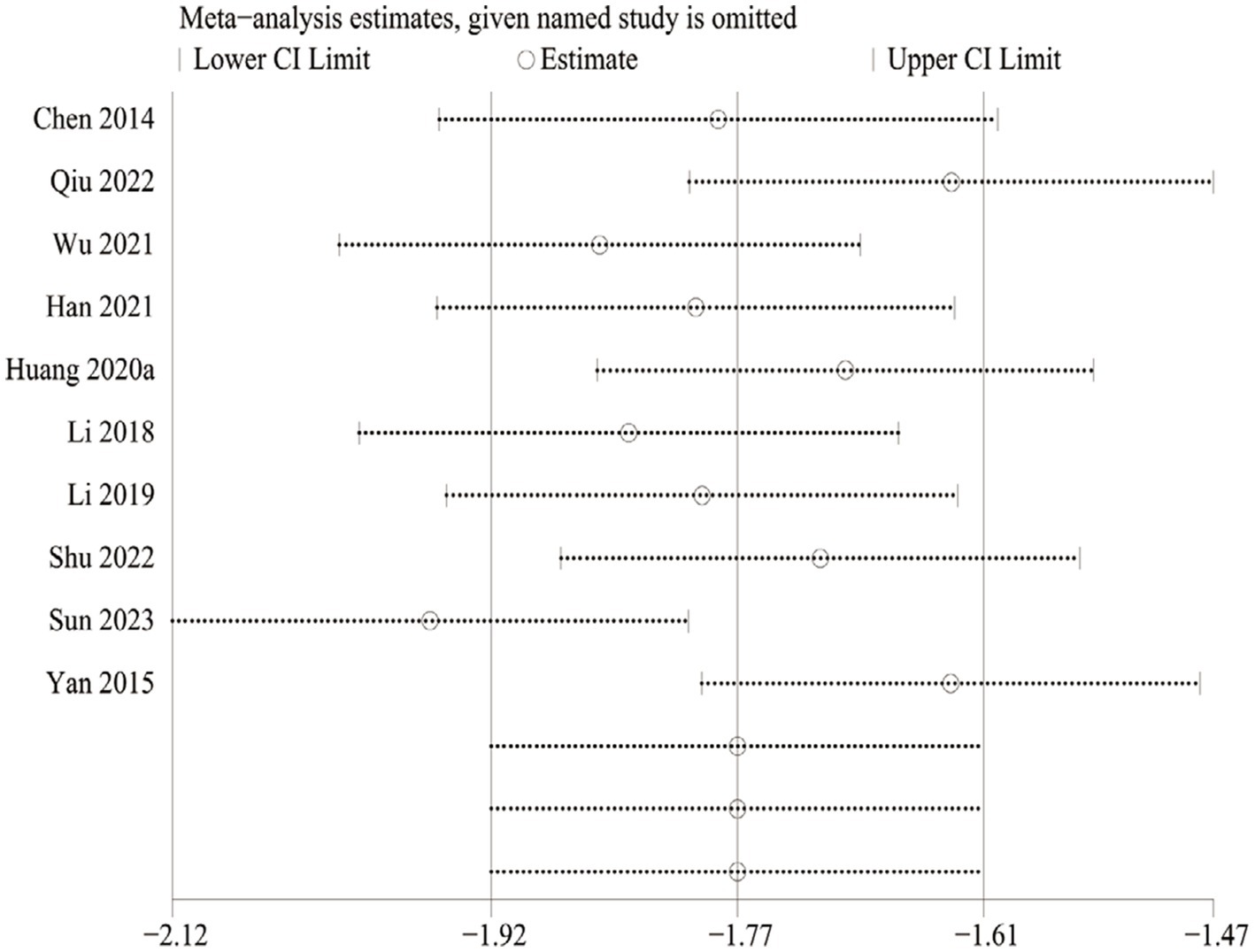
We also performed sensitivity analyses for these 10 studies by excluding each article to validate the stability of this meta-analysis, which revealed that there was no statistical effect on the pooled data when any of the articles were eliminated except Sun 2023. However, this study had to be cautiously interpreted when explaining the prognostic results of the VAS due to Sun 2023 appearing to have a potential impact on the subgroup analysis of the Jintiange capsules alone (Figure 13).
4 Discussion
OP is a metabolic bone disease characterized by bone density and susceptibility to fracture (39). OVCF is a common secondary disease of OP, with an incidence of 0.307% among people over 50 years old, and is closely associated with low-energy trauma and advancing age. OVCFs may cause severe back pain and kyphosis and increase patient mortality (40). Surgery, such as PVP and PKP, are common alternative therapies for patients who fail to restore the height of the vertebral body through conservative treatments. However, there are inevitable adverse events of cement leakage, pulmonary cement embolism, and new vertebral compression fractures caused by PVP or PKP (41, 42). Similarly, current guidelines suggest that calcium and bisphosphonates can be used as routine oral medications for OP, but these medications may cause adverse events with long-term use, such as renal dysfunction and an increased risk of breast cancer (43). Therefore, effective and safe medications for OVCFs are required to improve this situation. The therapeutic effect of Jintiange capsules on OVCFs has received considerable attention, but research on its anti-OVCF mechanism is limited due to the lack of evidence regarding the effectiveness and safety of Jintiange capsule administration.
Our current study demonstrated that Jintiange capsules exert a prominent anti-OVCF effect. The potent anti-OVCF effect of Jintiange capsules can possibly be attributed to its primary ingredient, artificial tiger bone. Tiger bone has been reported to contain abundant calcium, phosphorus, peptides, and proteins (44). Calcium and phosphorus are fundamental bone minerals involved in many biological processes and can promote the development of bone, maintain the stability of the cytoskeleton, and inhibit osteoclast activity (45). The peptides and proteins can degrade into amino acids in vivo, which can potently stimulate increases in intracellular calcium and osteoblast differentiation (46). This meta-analysis indicates that Jintiange capsules maintained and improved the BMD of the lumbar spine, femoral neck, and whole body, and the subgroup analysis of the Jintiange capsules combined intervention showed the same conclusion regarding the lumbar spine and whole-body BMD compared with the control group. After selecting studies with substantial heterogeneity, the overall effect test of subgroup analysis revealed that Jintiange capsules improved the overall effective rate (RR = 1.15, 95% CI: 1.09 to 1.22, p < 0.00001, I2 = 0%). Chronic pain is the major symptom of postoperative treatment for OVCFs (47). In this meta-analysis, the pooled data of the subgroup analysis showed that the Jintiange capsules combined intervention reduced the VAS compared with controls. In addition, Egger’s test revealed no significant publication bias for the VAS. The subgroup analyses of Jintiange capsules also indicated that the Jintiange capsules combined intervention significantly improved ODI and Cobb’s angle compared with the control group. Osteocalcin (OC) is a protein derived from osteoblasts that is vitamin K-dependent and has biological effects on bone metabolism (48). A previous study has shown that Jintiange capsules can prevent bone loss and promote the osteogenic differentiation of BMSCs by regulating the BMP and Wnt-β-catenin pathways in ovariectomized rats, and BMP-2 can increase the expression of osteoblastic markers in cultures of pluripotent cells (49). The result showed that Jintiange capsules significantly raised serum OC levels, and subgroup analyses of the Jintiange capsules alone and combined interventions suggested similar conclusions. Serum ALP is regarded as a diagnostic marker of bone loss in osteoporotic patients and can play an important role in the activation of osteoblasts (50). Empirical evidence in ovariectomized rats has shown that Chinese medicine can significantly increase bone mass and suppress the formation of osteoclasts by inhibiting serum ALP levels (51, 52). The result of this meta-analysis also showed that the Jintiange capsule decreased ALP levels.
Toxicity is the most severe adverse effect of Jintiange capsules. Four studies reported that the adverse effects are relatively mild gastrointestinal symptoms experienced by patients with OVCFs receiving Jintiange capsules, which can be alleviated by stopping treatment. Five participants of the Jintiange capsules group developed recurrent fractures compared with the control group after treatment with Jintiange capsules, which showed a significant reduction in the rate of new vertebral fractures in patients treated with Jintiange capsules. This also showed that Jintiange capsules had a positive effect on reducing severe bone loss in older populations suffering from OVCFs. According to our analysis, the data suggested that Jintiange capsules are safe for the postoperative treatment of OVCFs in the present situation.
Jintiange capsules, the artificial bone tiger powder prepared from several farmed animal skeletons, have similar chemical constituents and pharmacological properties to natural tiger bone, which significantly exhibit anti-inflammatory, analgesic, fracture-healing, and bone metabolism-improving effects that can treat OVCFs (19, 53). An experimental study found that Jintiange capsules can alter the proliferation, differentiation, and mineralization of MC3T3-E1 osteoblasts to increase osteogenesis, which also can inhibit their apoptosis and enhance autophagy by reversing the regulatory effects of the PI3K-AKT and ER stress pathways (54). Another study showed that the main ingredient of tiger bone is calcium phosphate, which can interact with the targets of CALR and CALM1 to promote the synthesis of extracellular phosphate. Therefore, Jintiange capsules can increase bone and cartilage mineralization and regulate multiple signaling pathways (55). Therefore, this meta-analysis showed that Jintiange capsules increased clinical efficacy in improving biochemical markers. Simultaneously, the pooled data showed that Jintiange capsules maintained a relatively high level of serum OC compared with the control treatment, which could be due to the calcium and phosphate in the capsules increasing osteogenesis and the mineralization of osteoblasts, thereby increasing serum OC. Based on the stabilizing regulatory role of Jintiange capsules in bone metabolism, its clinical application deserves further attention in the postoperative treatment of OVCFs.
5 Limitations
The 16 included studies had several limitations that should be considered when interpreting the data. First, the sample sizes of the RCTs were too small, and relevant studies may have been overlooked, although we retrieved seven databases without any restriction on language. Second, there were various methodological quality biases because the report on the assessment methods was uneven for the included studies, such as selection bias, performance bias, and reporting bias. Third, the surgical treatment follow-up period ranged from 1 to 12 months, and thorough groupings with regard to more uniform treatment lengths were not put together. Lastly, there is high heterogeneity of the subgroup analysis in the included articles, indicating that diverse conservative Western medication may be utilized in the Jintiange capsules combined intervention group. Therefore, given the limitations of the designs of the studies, future clinical studies targeting the postoperative treatment of OVCFs with Jintiange capsules should avoid the above situations, as the reliability and evidence-based data on the positive effect of Jintiange capsules on the postoperative treatment of OVCFs remains to be improved.
6 Conclusion
The data from the studies showed that Jintiange capsules are effective in the postoperative treatment of OVCFs when combined with other therapeutic measures or alone. Their mechanism of action can increase BMD, increase lift effect rates, relieve pain, decrease the ODI, improve Cobb’s angle, raise serum OC levels, inhibit serum ALP levels, and lower the incidence of adverse events, making it a potential medication for treating OVCFs with high safety and effectiveness. Given the limitations of this study, the efficacy of Jintiange capsules for the postoperative treatment of OVCFs will need to be validated in future clinical studies.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
YF: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Supervision, Visualization, Writing – original draft, Writing – review & editing. WW: Writing – review & editing, Conceptualization, Funding acquisition, Project administration, Supervision, Visualization. MZ: Writing – review & editing, Formal analysis, Validation. JZ: Software, Validation, Writing – review & editing. MT: Validation, Writing – review & editing, Formal analysis.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Clinical Research Project of the Wu Jieping Medical Foundation (No. 320.6750.18548) and Shandong Geriatric Association 2021 Science and Technology Research Plan Project (No. LKJGG2021Y043).
Acknowledgments
The authors greatly acknowledge the editors and reviewers who participated in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2023.1289818/full#supplementary-material
Abbreviations
OP, Osteoporosis; OVCFs, Osteoporotic vertebral compression fractures; PVP, Percutaneous vertebroplasty; PKP, Percutaneous kyphoplasty; CNKI, China National Knowledge Infrastructure; BMD, Bone mineral density; VAS, Visual analog scale; ODI, Oswestry disability index; OC, Osteocalcin; MD, Mean difference; CI, Confidence interval; RR, Risk ratio
References
1. Jia, Y , Sun, J , Zhao, Y , Tang, K , Zhu, R , Zhao, W, et al. Chinese patent medicine for osteoporosis: a systematic review and meta-analysis. Bioengineered. (2022) 13:5581–97. doi: 10.1080/21655979.2022.2038941
2. Chen, P , Li, Z , and Hu, Y . Prevalence of osteoporosis in China: a meta-analysis and systematic review. BMC Public Health. (2016) 16:1039. doi: 10.1186/s12889-016-3712-7
3. Zhu, J , Yang, S , Cai, K , Wang, S , Qiu, Z , Huang, J, et al. Bioactive poly (methyl methacrylate) bone cement for the treatment of osteoporotic vertebral compression fractures. Theranostics. (2020) 10:6544–60. doi: 10.7150/thno.44428
4. Roux, C , Cortet, B , Bousson, V , and Thomas, T . Vertebroplasty for osteoporotic vertebral fracture. RMD Open. (2021) 7:e001655. doi: 10.1136/rmdopen-2021-001655
5. Li, HM , Zhang, RJ , Gao, H , Jia, CY , Zhang, JX , Dong, FL, et al. New vertebral fractures after osteoporotic vertebral compression fractures between balloon kyphoplasty and nonsurgical treatment PRISMA. Medicine. (2018) 97:e12666. doi: 10.1097/D.0000000000012666
6. Zeng, L , Yang, T , Yang, K , Yu, G , Li, J , Xiang, W, et al. Efficacy and safety of curcumin and Curcuma longa extract in the treatment of arthritis: a systematic review and meta-analysis of randomized controlled trial. Front Immunol. (2022) 13:891822. doi: 10.3389/fimmu.2022.891822
7. Sun, Y , Ma, H , Yang, F , Tang, X , Yi, P , and Tan, M . Clinical efficacy and safety of zoledronic acid combined with PVP/PKP in the treatment of osteoporotic vertebral compression fractures: a systematic review and meta-analysis of randomized controlled trials. Biomed Res Int. (2021) 2021:6650358. doi: 10.1155/2021/6650358
8. Yu, WB , Jiang, XB , Liang, D , Xu, WX , Ye, LQ , and Wang, J . Risk factors and score for recollapse of the augmented vertebrae after percutaneous vertebroplasty in osteoporotic vertebral compression fractures. Osteoporos Int. (2019) 30:423–30. doi: 10.1007/s00198-018-4754-8
9. Barrionuevo, P , Kapoor, E , Asi, N , Alahdab, F , Mohammed, K , Benkhadra, K, et al. Efficacy of pharmacological therapies for the prevention of fractures in postmenopausal women: a network meta-analysis. J Clin Endocrinol Metab. (2019) 104:1623–30. doi: 10.1210/jc.2019-00192
10. Zhao, J , Zeng, L , Wu, M , Huang, H , Liang, G , Yang, W, et al. Efficacy of Chinese patent medicine for primary osteoporosis: a network meta-analysis. Complement Ther Clin Pract. (2021) 44:101419. doi: 10.1016/j.ctcp.2021.101419
11. Wang, S , Yuan, Y , Lin, Q , Zhou, H , Tang, B , Liu, Y, et al. Antiosteoporosis effect of tanshinol in osteoporosis animal models: a systematic review and meta-analysis. Front Pharmacol. (2022) 13:937538. doi: 10.3389/fphar.2022.937538
12. Duan, Y , Su, YT , Ren, J , Zhou, Q , Tang, M , Li, J, et al. Kidney tonifying traditional Chinese medicine: potential implications for the prevention and treatment of osteoporosis. Front Pharmacol. (2023) 13:1063899. doi: 10.3389/fphar.2022.1063899
13. Yan, Z , Li, J , He, X , Wang, Z , Fan, Y , Xiao, J, et al. Jintiange capsule may have a positive effect on pain relief and functional activity in patients with knee osteoarthritis: a meta-analysis of randomized trials. Evid Based Complement Alternat Med. (2021) 2021:7908429–11. doi: 10.1155/2021/7908429
14. Shen, Y , Wang, N , Zhang, Q , Liu, Y , Wu, Q , He, Y, et al. Jin-Tian-Ge ameliorates ovariectomy-induced bone loss in rats and modulates osteoblastogenesis and osteoclastogenesis in vitro. Chin Med. (2022) 17:78. doi: 10.1186/s13020-022-00627-2
15. Gratwicke, B , Mills, J , Dutton, A , Gabriel, G , Long, B , Seidensticker, J, et al. Attitudes toward consumption and conservation of tigers in China. PLoS One. (2008) 3:e2544. doi: 10.1371/journal.pone.0002544
16. Sun, J , Yang, XG , and Hu, YC . Efficacy of Jintiange capsules in the treatment of osteoporosis: a network meta-analysis. Orthop Surg. (2019) 11:176–86. doi: 10.1111/os.12439
17. Ye, X , Wu, XC , He, L , Liu, ZB , Sun, X , Wang, HZ, et al. Research progress on substitutes for endangered animal medicinal materials. Chin J Experiment Formulas. (2022) 28:226–31. doi: 10.13422/j.cnki.syfjx.20220220247
18. Shu, LJ , and Zhang, JY . Effect of artificial Tiger bone powder (Jintiange capsule®) on vertebral height ratio, Cobb’s angle, bone mineral density, and visual analog score. Orthop Surg. (2022) 14:427–34. doi: 10.1111/os.13121
19. Wang, X , Shen, Y , Zhuang, X , Wang, N , Zhang, Q , Zhu, L, et al. Jintiange capsule alleviates rheumatoid arthritis and reverses changes of serum metabolic profile in collagen-induced arthritic rats. J Inflamm Res. (2021) 14:6685–706. doi: 10.2147/JIR.S338107
20. Zhang, BS , and Dong, KF . A meta-analysis of efficacy of two kinds of the Bushen medicine on BMD and VAS in osteoporosis patients. Clin J Chin Med. (2020) 12:139–43.
21. Page, MJ , McKenzie, JE , Bossuyt, PM , Boutron, I , Hoffmann, TC , Mulrow, CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. J Clin Epidemiol. (2021) 134:178–89. doi: 10.1016/j.jclinepi.2021.03.001
22. Hu, J , Xie, S , Li, W , and Zhang, L . Diagnostic and prognostic value of serum S100B in sepsis-associated encephalopathy: a systematic review and meta-analysis. Front Immunol. (2023) 14:1102126. doi: 10.3389/fimmu.2023.1102126
23. Wang, Z , Wang, Y , Wang, C , Li, X , Zhou, Z , Zhang, L, et al. Systematic review and network meta-analysis of acupuncture combined with massage in treating knee osteoarthritis. Biomed Res Int. (2022) 2022:1–21. doi: 10.1155/2022/4048550
24. Chen, YP , and David, B . Clinical analysis of the treatment of osteoporotic vertebral compression fractures with Jintiange capsule combined with mifepristone. Chinese. J Osteoporos. (2014) 20:1326–9. doi: 10.3969/j.issn.1006-7108.2014.11.013
25. Yan, WF . Clinical experience of surgery combined with Jintiange capsule in the treatment of elderly osteoporotic thoracolumbar compression fractures. Henan J Surg. (2015) 21:47–8. doi: 10.16193/j.cnki.hnwk.2015.03.029
26. Li, WQ , Xing, YJ , Yang, ZQ , Dang, PY , Li, YZ , Ma, Q, et al. The application and clinical analysis of Jin Tiange in elderly patients with osteoporotic vertebral fractures after surgery. Mod Biomed Prog. (2018) 18:1116–9. doi: 10.13241/j.cnki.pmb.2018.06.024
27. Li, ZQ . Clinical observation on the treatment of primary osteoporosis with vertebral compression fractures with Jintiange capsules. Guangming Tradit Chin Med. (2019) 34:83–5.
28. Tan, SL , Xu, W , Yang, B , and Sun, J . Analysis of the therapeutic effect of Jintiange capsule on preventing recurrent fractures after PVP surgery for thoracolumbar osteoporotic fractures. J Clin Ration Drug Use. (2019) 12:81–2. doi: 10.15887/j.cnki.13-1389/r.2019.28.046
29. Wang, L . The effect of Jintiange capsule on postoperative internal fixation of osteoporotic thoracolumbar fractures. Shenzhen J Integr Tradit Chin Western Med. (2019) 29:63–4. doi: 10.16458/j.cnki.1007-0893.2019,23.030
30. Huang, HJ , and Pang, ZC . Jintiange capsules combined with calcium carbonate D the effect of 3 chewable tablets (II) on adjacent vertebral re fractures in elderly patients with osteoporotic vertebral compression fractures after surgery. Chin Folk Therapeut. (2020) 28:68–70. doi: 10.19621/j.cnki.11-3555/r.2020.2229
31. Huang, JZ . Clinical observation of the therapeutic effect of Jintiange capsules on primary osteoporotic vertebral compression fractures. Clin Med Eng. (2020) 27:1349–50. doi: 10.3969/j.issn.1674-4659.2020.10.1349
32. Han, CX , Tian, XD , Zhu, GY , Tan, ZT , Ma, S , Du, DF, et al. The effect of Jintiange capsule on bone density, bone metabolism, and quality of life in patients after percutaneous balloon kyphoplasty. Chin J Osteoporosis. (2021) 27:110–3.
33. He, JX , Wu, CH , and Hang, L . The effect of Jintiange capsule on postoperative healing of elderly osteoporotic thoracolumbar compression fractures. New Chin Med. (2021) 53:106–8. doi: 10.13457/j.cnki.jncm.2021.05.027
34. Wu, YY , Liu, GX , and Li, JX . Observation of the therapeutic effect of Jintiange capsule combined with percutaneous kyphoplasty on osteoporotic spinal compression fractures and its impact on bone density. New Chin Med. (2021) 53:93–6. doi: 10.13457/j.cnki.jncm.2021.22.024
35. Yang, ZJ . Clinical efficacy of Jintiange capsules in the treatment of primary osteoporosis with vertebral compression fractures. J Clin Ration Drug Use. (2021) 14:110–2. doi: 10.15887/j.cnki.13-1389/r.2021.13.046
36. Qiu, ZT , Yang, H , Wang, WG , Qin, GQ , and Zhang, BB . Analysis of the application effect of Jintiange capsule combined with calcitriol on patients with osteoporotic vertebral compression fractures. Hebei Med J. (2022) 28:1573–7.
37. Sun, GY , Zheng, H , and Yu, HF . The application effect of Jintiange capsules in postoperative patients with osteoporotic vertebral compression fractures. Chin J Contemp Med. (2023) 30:97–100.
38. Xu, C . Clinical study on percutaneous vertebroplasty assisted by Jintiange capsules for the treatment of thoracolumbar osteoporotic fractures. New Chin Med. (2023) 55:99. doi: 10.13457/j.cnki.jncm.2023.04.021
39. Ma, XL , Xing, D , Ma, JX , Xu, WG , Wang, J , and Chen, Y . Balloon kyphoplasty versus percutaneous vertebroplasty in treating osteoporotic vertebral compression fractures: grading the evidence through a systematic review and meta-analysis. Eur Spine J. (2012) 21:1844–59. doi: 10.1007/s00586-012-2441-6
40. Cheng, Y , Cheng, X , and Wu, H . Risk factors of new vertebral compression fractures after percutaneous vertebroplasty or percutaneous kyphoplasty. Front Endocrinol. (2022) 13:964578. doi: 10.3389/fendo.2022.964578
41. Kamei, S , Noguchi, T , Shida, Y , Okafuji, T , Yokoyama, K , Uchiyama, F, et al. The safety and efficacy of percutaneous vertebroplasty for patients over 90 years old. Jpn J Radiol. (2019) 37:178–85. doi: 10.1007/s11604-018-0797-1
42. Sun, HB , Shan, JL , and Tang, H . Percutaneous vertebral augmentation for osteoporotic vertebral compression fracturess will increase the number of subsequent fractures at adjacent vertebral levels: a systematic review and meta-analysis. Eur Rev Med Pharmacol Sci. (2021) 25:5176–88. doi: 10.26355/eurrev_202108_26531
43. Khan, SN , Craig, L , and Wild, R . Osteoporosis: therapeutic guidelines. Guidelines for practice management of osteoporosis. Clin Obstet Gynecol. (2013) 56:694–702. doi: 10.1097/01.grf.0000437016.19989.61
44. Liu, L , Cheng, L , Long, YY , Zhou, YM , and Yang, DX . Systematic evaluation of the treatment of primary osteoporosis in Jintiange capsules. Chin Tradit Pat Med. (2018) 40:2606–12. doi: 10.3969/j.issn.1001-1528.2018.11.053
45. Calvo, MS , and Uribarri, J . Public health impact of dietary phosphorus excess on bone and cardiovascular health in the general population. Am J Clin Nutr. (2013) 98:6–15. doi: 10.3945/ajcn.112.053934
46. Su, Y , Elshorbagy, A , Turner, C , Refsum, H , Chan, R , and Kwok, T . Circulating amino acids are associated with bone mineral density decline and ten-year major osteoporotic fracture risk in older community-dwelling adults. Bone. (2019) 129:115082. doi: 10.1016/j.bone.2019.115082
47. Tian, J , Xiang, L , Zhou, D , Fan, Q , and Ma, B . The clinical efficacy of vertebroplasty on osteoporotic vertebral compression fractures: a meta-analysis. Int J Surg. (2014) 12:1249–53. doi: 10.1016/j.ijsu.2014.10.027
48. Jagannath, VA , Thaker, V , Chang, AB , and Price, AI . Vitamin K supplementation for cystic fibrosis. Cochrane Database Syst Rev. (2020) 2020:CD008482. doi: 10.1002/14651858.CD008482.pub6
49. Fawzy El-Sayed, KM , Dörfer, C , Ungefroren, H , Kassem, N , Wiltfang, J , and Paris, S . Effect of Emdogain enamel matrix derivative and BMP-2 on the gene expression and mineralized nodule formation of alveolar bone proper-derived stem/progenitor cells. J Craniomaxillofac Surg. (2014) 42:568–76. doi: 10.1016/j.jcms.2013.07.028
50. Kim, MH , Choi, Y , Chung, JY , Kim, EJ , and Yang, WM . Auraptene ameliorates osteoporosis by inhibiting RANKL/NFATc1 pathway-mediated bone resorption based on network pharmacology and experimental evaluation. Bone Joint Res. (2022) 11:304–16. doi: 10.1302/2046-3758.115.BJR-2021-0380.R1
51. Long, ZY , Wu, JM , Xiang, W , Yuan, MX , Wu, YH , Li, J, et al. Research on the mechanism of Liuwei Dihuang decoction for osteoporosis based on systematic biological strategies. Evid Based Complement Alternat Med. (2022) 2022:7017610–22. doi: 10.1155/2022/7017610
52. Cai, P , Lu, Y , Yin, Z , Wang, X , Zhou, X , and Li, Z . Baicalein ameliorates osteoporosis via AKT/FOXO1 signaling. Aging. (2021) 13:17370–9. doi: 10.18632/aging.203227
53. Liang, H , Wang, O , Cheng, Z , Xia, P , Wang, L , Shen, J, et al. Jintiange combined with alfacalcidol improves muscle strength and balance in primary osteoporosis: a randomized, double-blind, double-dummy, positive-controlled, multicenter clinical trial. J Orthop Translat. (2022) 35:53–61. doi: 10.1016/j.jot.2022.05.002
54. Liu, Y , Zhao, L , He, X , Shen, Y , Wang, N , Hu, S, et al. Jintiange proteins promote osteogenesis and inhibit apoptosis of osteoblasts by enhancing autophagy via PI3K/AKT and ER stress pathways. J Ethnopharmacol. (2023) 311:116399. doi: 10.1016/j.jep.2023.116399
Keywords: Chinese herbal medicine, Jintiange capsules, osteoporosis, osteoporotic vertebral compression fractures, systematic review and meta-analysis
Citation: Fu Y, Wang W, Zhao M, Zhao J and Tan M (2023) Efficacy of the Chinese herbal medicine Jintiange capsules in the postoperative treatment of osteoporotic vertebral compression fractures: a systematic review and meta-analysis. Front. Med. 10:1289818. doi: 10.3389/fmed.2023.1289818
Edited by:
Lingfeng Zeng, The Second Affiliated Hospital of Guangzhou University of Chinese Medicine, ChinaReviewed by:
Liu Kang, The Second Affiliated Hospital of Zhejiang University of Traditional Chinese Medicine, ChinaLei Wan, Guangzhou University of Chinese Medicine, China
Maria Cristina Ferrara, University of Milano-Bicocca, Italy
Georgios Mikellides, University of Nicosia, Cyprus
Copyright © 2023 Fu, Wang, Zhao, Zhao and Tan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weiguo Wang, d2pid3dnQGhvdG1haWwuY29t
 Yongsheng Fu
Yongsheng Fu Weiguo Wang
Weiguo Wang Minghua Zhao
Minghua Zhao Jianpeng Zhao1
Jianpeng Zhao1