- 1Department of Epidemiology, School of Public Health, Cheeloo College of Medicine, Shandong University, Jinan, China
- 2Department of Bacteriology and Immunology, Beijing Chest Hospital, Capital Medical University/Beijing Tuberculosis and Thoracic Tumor Research Institute, Beijing, China
Objectives: Tuberculosis (TB) remains a significant concern in terms of public health, necessitating the timely and accurate diagnosis to impede its advancement. The utilization of oral swab analysis (OSA) presents a promising approach for diagnosing pulmonary TB by identifying Mycobacterium tuberculosis (MTB) within oral epithelial cells. Due to disparities in the diagnostic performance of OSA reported in the original studies, we conducted a meticulous meta-analysis to comprehensively assess the diagnostic efficacy of OSA in pulmonary TB.
Methods: We conducted a comprehensive investigation across multiple databases, namely PubMed, Cochrane Library, Embase, Web of Science, ClinicalTrials.gov, Chinese BioMedical Literature Database (CBM), China National Knowledge Infrastructure Database (CNKI), and Wanfang China Science and Technology Journal Database to identify relevant studies. Out search query utilized the following keywords: oral swab, buccal swab, tongue swab, tuberculosis, and TB. Subsequently, we employed STATA 16.0 to compute the combined sensitivity, specificity, positive likelihood ratio, negative likelihood ratio, and diagnostic odds ratio for both the overall and subgroup analyses.
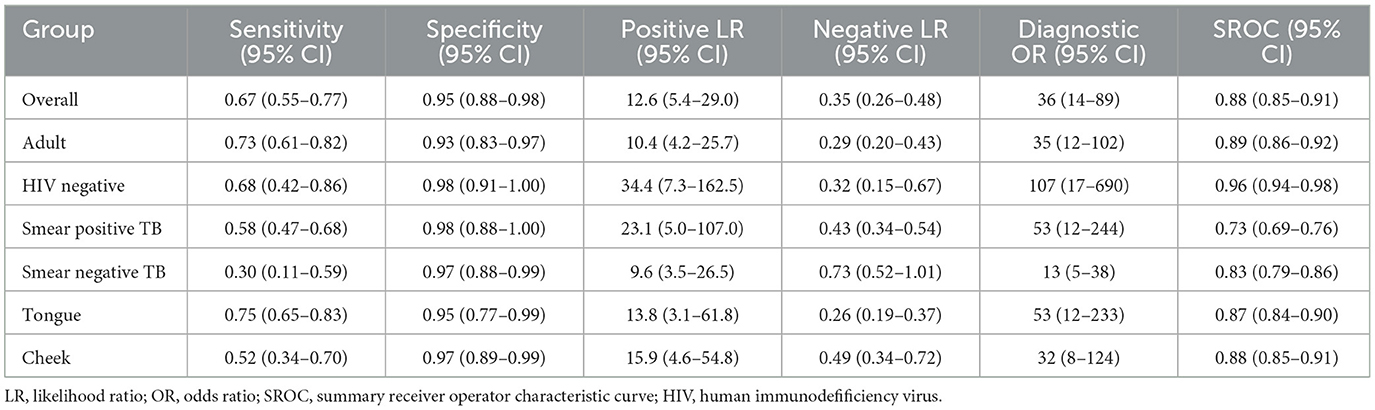
Results: Our findings indicated that OSA has a combined sensitivity of 0.67 and specificity of 0.95 in individuals with pulmonary TB. Subgroup analysis further revealed that among adult individuals with pulmonary TB, the sensitivity and specificity of OSA were 0.73 and 0.93, respectively. In HIV-negative individuals with pulmonary TB, the sensitivity and specificity were 0.68 and 0.98, respectively. The performance of OSA in detecting pulmonary TB correlated with the bacteria load in sputum. Additionally, the sensitivity for diagnosing pulmonary TB using tongue specimens was higher (0.75, 95% CI: 0.65–0.83) compared to cheek specimens (0.52, 95% CI: 0.34–0.70), while both types of specimens demonstrated high specificity.
Conclusions: To conclude, oral swabs serve as a promising alternative for diagnosing pulmonary TB, especially in adult patients. In addition, tongue swabs yield better sensitivity than cheek swabs to identify pulmonary TB patients.
Systematic review registration: identifier: CRD42023421357.
Introduction
Tuberculosis (TB), caused by Mycobacterium tuberculosis (MTB), remains a major significant contributor to mortality resulting from chronic infectious diseases, especially in immunodeficiency patients with HIV (1). The emergence of drug-resistant TB (DR-TB), encompassing extensively drug-resistant TB (XDR-TB) and pre-XDR-TB, has posed a substantial threat to TB control efforts in recent years (2). Timely and early diagnosis, along with prompt treatment, plays a crucial role in preventing the dissemination of drug-resistant TB (3). Currently, the majority of confirmed TB cases rely on the detection of MTB in sputum specimens, such as sputum smear, sputum culture, and MTB molecular in sputum (4–6). However, the reliance on sputum specimens presents limitations, particularly in pediatric patients and those who are unable to produce sputum (7). Additionally, the collection of sputum can cause potentially infectious aerosols that are harmful to healthcare workers and other patients (8). Moreover, the efficacy of TB diagnostic tests is contingent upon the quality of the sputum collected (9). Therefore, there is a pressing need for non-invasive alternatives to sputum-based testing that are easier, safer, and more efficient in diagnosing TB.
Several human samples, including exhaled breath condensate, saliva, urine, blood and stool, have been explored as alternatives to sputum for evaluating the diagnostic performance of TB (10, 11). Unfortunately, these alternative samples have demonstrated lower sensitivity or specificity than sputum. Recently, an oral (buccal) swab has emerged as a more encouraging alternative to sputum specimens for TB diagnosis. Previous studies have shown that oral swab analysis (OSA) can detect MTB DNA present in oral epithelial cells (12–15). OSA involves the utilization of a sterile brush to gently scrape cells from the dorsal surface of the tongue. This procedure is simple, quick, painless, non-invasive and does not generate aerosols, making it suitable for implementation in various healthcare settings, including tertiary hospitals, outpatient clinics, and community settings. Consequently, OSA holds particular value in identifying TB cases within diverse populations.
A series of studies examining the efficacy of OSA for diagnosing pulmonary TB have yielded inconsistent findings. For instance, one study conducted in South Africa evaluated swabs from adult subjects and found that OSA exhibited a sensitivity of 92.8% and specificity of 91.5% when compared to sputum GeneXpert testing (14). Similarly, another study involving 201 South African children with suspected pulmonary TB revealed that OSA demonstrated greater sensitivity than sputum testing in children who were negative for sputum, although its sensitivity was lower in sputum-positive children (16). By contrast, conflicting outcomes were noted in other studies, reporting lower sensitivity for detecting tubercle bacilli using oral swabs (13, 15, 17, 18). The limited sample sizes in previous studies undermine the confidence of these conclusions. Therefore, we conducted a meta-analysis to investigate the diagnostic value of OSA in detecting pulmonary TB while also conducting a comprehensive analysis of its diagnostic accuracy across different populations.
Materials and methods
Protocol registration
This systematic review and meta-analysis were conducted according to the Preferred Reporting Items for Systematic Reviews and meta-Analyses (PRISMA) statement. The protocol has been registered in PROSPERO (Number: CRD42023421357).
Search strategy
We conducted a comprehensive and systematic search for relevant studies in electronic databases, including PubMed, Cochrane Library, Embase, Web of Science, ClinicalTrials.gov, Chinese BioMedical Literature Database (CBM), China National Knowledge Infrastructure Database (CNKI), and Wanfang China Science and Technology Journal Database, based on our review protocol. The most recent searches performed on 12 March 2023 used the following terms: (“oral swab” or “buccal swab” or “tongue swab”) and (“tuberculosis” or “TB”). No geographical or demographic restrictions were imposed during the search process, including the race or age of study participants. We considered studies published in both English and Chinese languages, and only included relevant data for our analysis.
Eligibility criteria
We enrolled records that met the following eligibility criteria, which involved studies designed as diagnostic accuracy studies aiming to assess the diagnostic value of oral swabs for TB in human subjects. We exclusively considered studies that provided sufficient data for computing pooled sensitivity and specificity in human populations, including the count of true positives and negatives, as well as false positives and negatives. Only studies with complete data were included to avoid duplications. We excluded studies that lacked adequate data for calculating effect size or were missing other essential information. Moreover, publications encompassing reviews, case reports, abstracts, guidelines, and recommendations were also excluded, as they did not present primary results. Furthermore, studies lacking research indicators necessary for meta-analysis were also excluded.
Data extraction
The study selection process was conducted independently by two investigators. In case of any discrepancies, a third author reviewed the articles, and a final consensus was reached through discussion. Initially, the investigators screened the literature based on titles and abstracts to exclude articles that did not meet the inclusion criteria. Subsequently, the remaining articles were re-evaluated by reading their full texts. Detailed information and data from the identified studies were extracted by the two investigators using a standardized data extraction form that had been pre-constructed. The following information was extracted for each study: the first author's name, year of publication, place of study, study population, population size, age, and sex distribution of participants, method of diagnosis, TB type, sensitivity, and specificity values with their corresponding 95% confidence intervals (CI), as well as the number of true positives, false positives, false negatives, and true negatives. The investigators extracted data according to various subgroups, including adults and children, TB patients with and without HIV and smear-positive and smear-negative TB. Furthermore, OSA samples collected from the tongue and cheek were evaluated in the diagnosis of TB.
Statistical analysis
The quality of the included studies was evaluated using the widely Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) tool (19). This tool assesses diagnostic accuracy based on four main points: selection of patients, index criteria, reference standard and flow and timing in the preliminary study. The quality rating scale is based on risk bias. Additionally, applicability concerns were taken into account when assessing the first three domains.
For the meta-analysis, STATA software version 16.0 was utilized. Heterogeneity was evaluated using both the chi-square and the I2 statistical tests. A significant level of heterogeneity was considered when P is greater or equal to 0.10, accompanied by an I2 values of 50% or higher. Acceptable inter-study heterogeneity was determined when the I2 value was equal to or < 50%. To mitigate the potential impact of the heterogeneity on the final conclusion, a random effects model was employed combine data from individual studies. This approach allowed for the estimation of sensitivity, specificity, positive/negative likelihood ratio and diagnostic odds ratio. The receiver operator characteristic (ROC) curve was utilized to obtain the area under the curve, providing a measure of the diagnostic accuracy. Publication bias was assessed using Deek's funnel plot methodology.
Results
Study characteristics
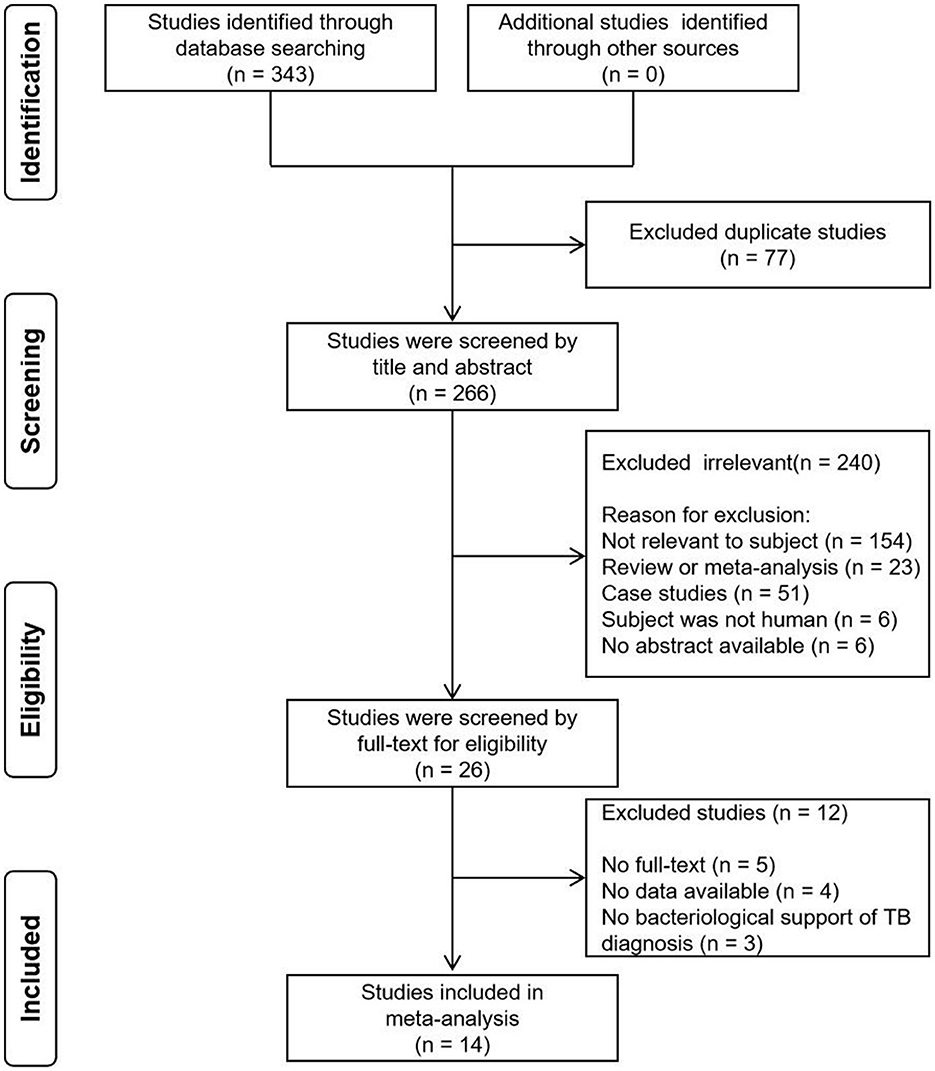
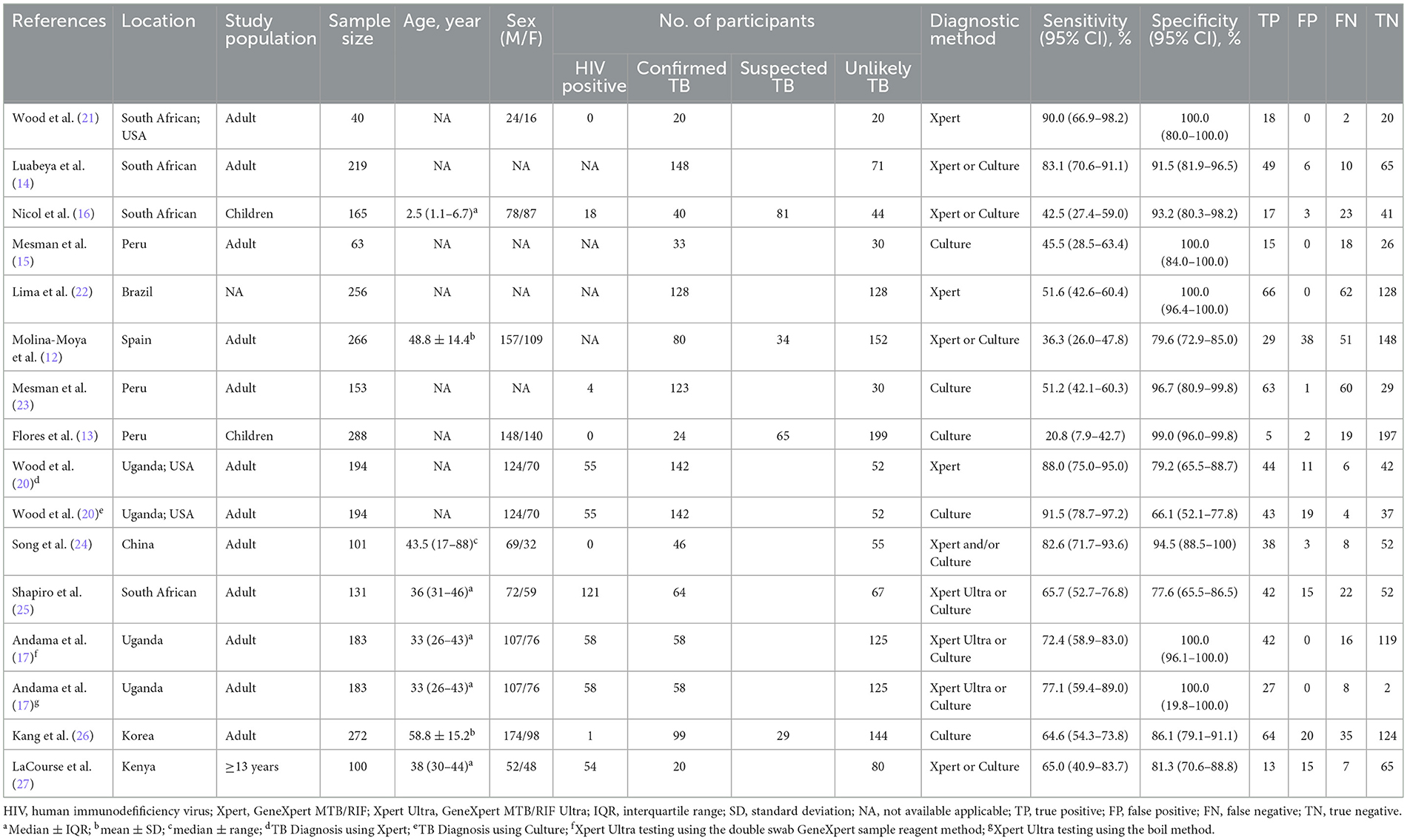
Initially, a total of 343 records were identified, out of which 77 duplicates were removed. Subsequently, a screening process based on titles and abstracts was carried out, resulting in the exclusion of 240 irrelevant records. This narrowed down the selection to 26 articles, which were then assessed based on full-text contents. Among these, 12 articles were excluded due to reasons such as unavailability of full-text, absence of data and lack of bacteriological support of pulmonary TB diagnosis. Eventually, a systematic review was conducted, including 14 studies that fulfilled the inclusion criteria (Figure 1). Notably, two studies were included twice in separate records. In one study, TB was diagnosed using either culture or GeneXpert MTB/RIF as reference standards, resulting in two records being incorporated for this study (20). In the other study, two methods were employed in the OSA experiment. One used the double swab GeneXpert sample reagent method for GeneXpert MTB/RIF Ultra, while the other used the boil method for GeneXpert MTB/RIF Ultra, with two records also being included in this study (17). Among the remaining 16 records, two reported the diagnostic effectiveness of OSA in children with pulmonary TB, twelve explored the diagnostic effectiveness of OSA in adults with pulmonary TB, while the age range of the study population in two records could not be determined. Table 1 provides a concise summary of the key characteristics of the included studies. It is important to note that the diagnosis of pulmonary TB in all the included studies was based on confirmed cases using the reference standard.
Risk of bias within studies
The 14 studies included in this research were assessed for risk of bias by using the QUADAS-2 tool. Among them, ten studies were found to have a low risk of selection bias, while 4 studies had an unclear risk of selection bias. Furthermore, eight studies exhibited a high risk of bias in the index test, as the interpretation of OSA results was influenced by prior knowledge of the reference standard results. In contrast, the reference standard and flow and timing displayed a low risk across all 14 studies. Lastly, two studies had unclear applicability concerns regarding patient selection. A detailed summary of the results can be found in Supplementary Table 1.
Overall diagnostic accuracy of OSA in pulmonary TB
A total of 16 records were examined to assess the diagnostic value of OSA in pulmonary TB. Analysis of the results revealed that despite the heterogeneity resulting from variations in the operational processing of OSA among the studies (see Supplementary Table 3), OSA showed an overall pooled sensitivity of 0.67 (95% CI: 0.55–0.77, I2 = 88.94%) and an overall pooled specificity of 0.95 (95% CI: 0.88–0.96, I2 = 91.09%) in the diagnosis of pulmonary TB (Figure 2). Further subgroup analysis supported the overall findings, indicating a high specificity and diagnostic sensitivity of OSA in TB.
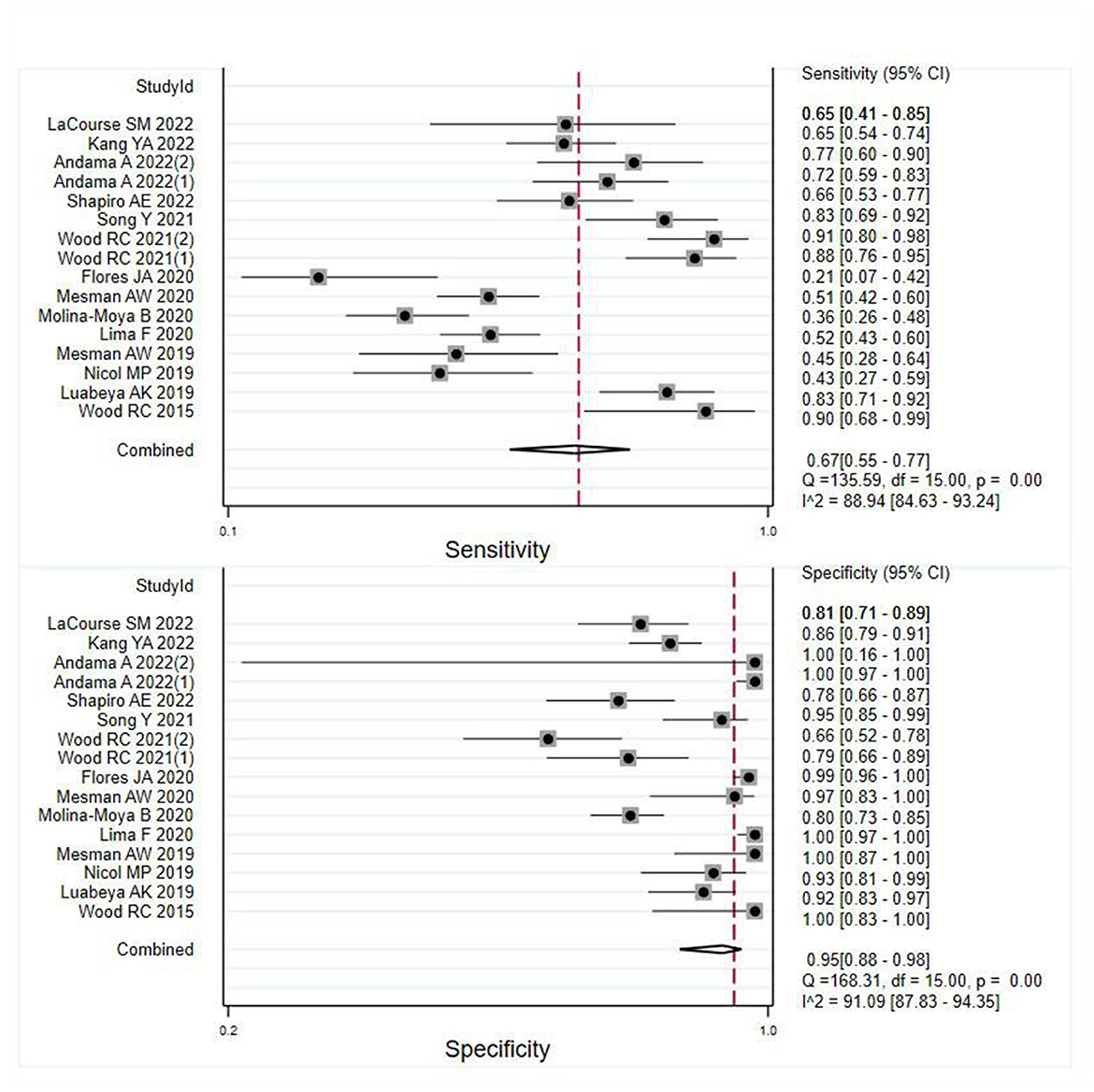
Figure 2. Meta-analysis results of the sensitivity and specificity of OSA in the diagnosis of individuals with pulmonary TB.
Subgroups diagnostic accuracy of OSA in pulmonary TB
The meta-analysis findings demonstrated that OSA exhibits sensitivity of 0.73 (95% CI: 0.61–0.82, I2 = 90.08%) and specificity of 0.93 (95% CI: 0.83–0.97, I2 = 88.07%) for diagnosing adults with pulmonary TB (Figure 3A). In HIV-negative individuals with pulmonary TB, the sensitivity and specificity of OSA were 0.68 (95% CI: 0.42–0.86, I2 = 88.69%) and 0.98 (95% CI: 0.91–1.00, I2 = 90.03%), respectively (Figure 3B). However, due to the limited number of studies available, a meta-analysis could not be conducted to evaluate the effectiveness of OSA in diagnosing children and HIV-positive individuals with pulmonary TB. Therefore, the diagnosis of OSA in these populations remains uncertain. Details are listed in Supplementary Table 2. Furthermore, for individuals with smear-positive pulmonary TB, OSA demonstrated a sensitivity and specificity of 0.58 (95% CI: 0.47–0.68, I2 = 20.99%) and 0.98 (95% CI: 0.88–1.00, I2 = 88.80%), respectively (Figure 3C). For smear-negative individuals with pulmonary TB, OSA exhibited a sensitivity and specificity of 0.30 (95% CI: 0.11–0.59, I2 = 84.78%) and 0.97 (95% CI: 0.88–0.99, I2 = 89.17%), respectively (Figure 3D). Moreover, we investigated the performance of OSA in diagnosing pulmonary TB using tongue and cheek specimens. The sensitivity of tongue and cheek specimens was 0.75 (95% CI: 0.65–0.83, I2 = 85.34%) and 0.52 (95% CI: 0.34–0.70, I2 = 79.28%), while the specificity was 0.95 (95% CI: 0.77–0.99, I2 = 91.81%) and 0.97 (95% CI: 0.89–0.99, I2 = 90.34%), respectively (Figure 4). Supplementary Table 2 displays all subgroup analyses of OSA in diagnosing pulmonary TB. Table 2 provides a summary of the meta-analysis results.
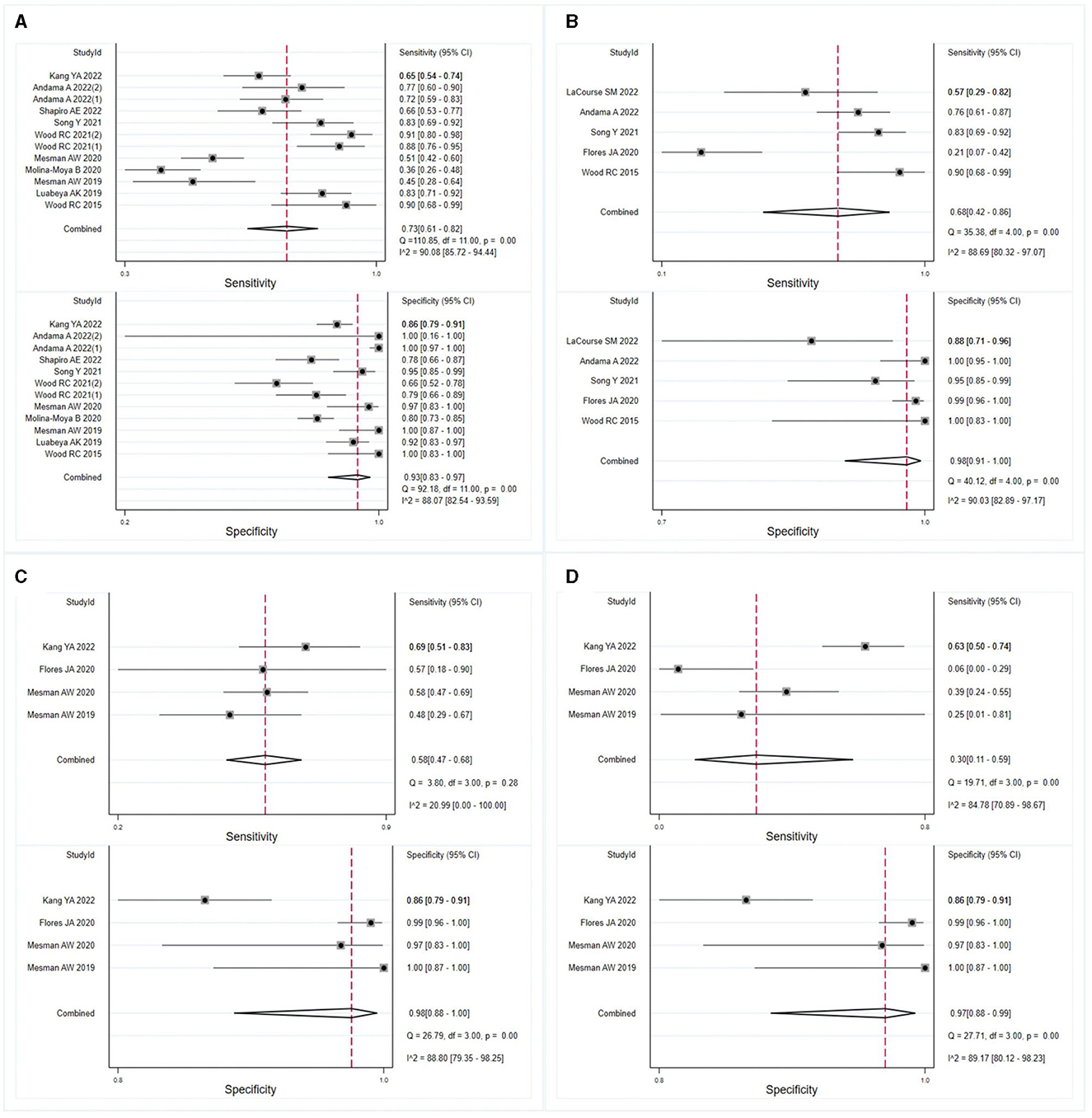
Figure 3. Meta-analysis assessed the sensitivity and specificity of OSA in diagnosing individuals with pulmonary TB. The results were stratified by (A) adult individuals with pulmonary TB, (B) HIV-negative individuals with pulmonary TB, (C) smear-positive individuals with pulmonary TB, and (D) smear-negative individuals with pulmonary TB.

Figure 4. Meta-analysis of the sensitivity and specificity of tongue and cheek specimens of OSA in diagnosing pulmonary TB. (A) Results of meta-analysis on tongue specimens in diagnosing pulmonary TB; (B) results of meta-analysis on cheek specimens in diagnosing pulmonary TB.
Publication bias assessment
To assess publication bias, a funnel plot was employed (Supplementary Figure 1). The funnel plot clearly indicated the absence of significant publication bias in this meta-analysis (P = 0.99).
Discussion
TB continues to pose a significant public health challenge, necessitating early detection and prompt treatment to curb its spread (3, 28). In recent years, researchers have developed oral swabs, as a non-invasive method for detecting MTB DNA, which can be obtained from the tongue, cheek or gums (14). While obtaining samples directly from the lower respiratory tract is of paramount importance and demonstrates superior efficacy in diagnosing TB, oral swabs present a more accessible alternative approach for TB diagnosis (17, 25). They have also been widely utilized in diagnosing TB in animals (29–31). Furthermore, the development of oral swabs aimed to mitigate the health risks associated with collecting sputum, which poses potential hazards for healthcare providers (32). In our meta-analysis, we comprehensively evaluated the diagnostic efficacy of oral swabs for pulmonary TB, providing novel insights into its potential utility. We observed that while the sensitivity of oral swabs for TB diagnosis was moderate, the specificity was notably high, yielding an overall favorable diagnostic effect. These findings suggest that oral swabs may serve as suitable specimens for rule-in testing in the diagnosis of pulmonary TB. Nonetheless, the records included exhibited considerable heterogeneity. This was mainly due to variations in the collection process of oral swabs and the number of participants in the studies. For instance, in a study by Wood et al. (21) found that oral swabs had high sensitivity and specificity for the diagnosis of pulmonary TB. However, the sample size was small, and the set positive threshold was larger than other studies (21). Another study by Wood et al. (20) revealed that increasing the number of participants and lowering the positive threshold of OSA resulted in a reduced specificity and slightly decreased sensitivity for diagnosing pulmonary TB. Nevertheless, the sensitivity still remained high in such instances, with Xpert-positive TB as the reference standard (20). Notably, when using the TB-LAMP method (24) or combining samples from two consecutive days (14) for OSA, both sensitivity and specificity were improved. These results highlight the presence of heterogeneity in the available data, emphasizing the need for further investigation into improving the implementation methodology of OSA and establishing appropriate thresholds to enhance sensitivity and specificity. Consequently, conducting more robust studies in this area becomes imperative.
In this study, we performed a subgroup analysis of various factors related to OSA for diagnosing individuals with pulmonary TB. Our finding revealed a high specificity of OSA for diagnosing pulmonary TB across various subgroups. Specifically, our analysis revealed a higher combined sensitivity of OSA for diagnosing pulmonary TB in adults compared to in children, as observed in studies by Nicol et al. (16) and Flores et al. (13). However, the sensitivity was lower for diagnosing pulmonary TB in HIV-negative individuals compared to HIV-positive individuals, according to LaCourse et al. (27), but higher than in the study by Andama et al. (17). These results suggest that OSA is a reliable method for diagnosing pulmonary TB in adults. However, caution is warranted in the case of HIV-positive individuals with pulmonary TB. Notably, Cox et al. found that the yield of microbiologic confirmation using oral swab specimens in children with pulmonary TB was suboptimal (18). Flores et al. demonstrated that tongue swabs can be employed for diagnosing TB in children who are clinically diagnosed with TB but are unable to produce sputum samples (33). Nevertheless, interpretation of these results requires caution as the number of studies on diagnosing OSA in children with pulmonary TB and HIV-positive individuals with pulmonary TB is limited. Further investigations are necessary to validate these findings. Moreover, in this meta-analysis, we assessed the diagnostic efficacy of OSA in individuals with pulmonary TB who had positive and negative sputum smear test results. Our analysis revealed that OSA exhibited a higher sensitivity in diagnosing pulmonary TB in individuals with positive sputum smear test results compared to those with negative test results. Flores et al. observed a positive correlation between positive sputum smear results and the detection of MTB in oral specimens (13). This suggests that the detection rate of MTB by oral swabs is proportional to the amount of MTB present in sputum. Consequently, oral swabs can serve as a viable substitute for sputum specimens in the diagnosis of TB.
While OSA can be utilized for diagnosing TB, it is important to note that their sensitivity is lower compared to the detection of TB using sputum specimens with the Xpert MTB/RIF and Xpert MTB/RIF Ultra methods, as indicated by previous study (34). However, in children with suspected pulmonary TB, OSA exhibits higher diagnostic sensitivity in comparison to using Xpert MTB/RIF for detecting sputum specimens and is comparable to the sensitivity of Xpert MTB/RIF Ultra for sputum specimen detection (35). Moreover, the sensitivity of OSA in diagnosing HIV-positive individuals with pulmonary TB (27) is similar to that of TB detection using Xpert MTB/RIF and Xpert MTB/RIF Ultra methods for sputum specimens (36). It is crucial to note that these conclusions are based on a limited number of studies examining the use of OSA as a diagnostic tool for pulmonary TB, highlighting the necessity for additional high-quality studies to confirm its utility in the future. To enhance the diagnostic effectiveness of OSA in pulmonary TB, optimization tests can be conducted. Supplementary Table 3 provides a comprehensive overview of the data we used from OSA in this study. Our previous research demonstrated that the TB-LAMP method we utilized to detect MTB in tongue swabs for diagnosing pulmonary TB yielded high sensitivity and specificity (24). Additionally, Andama et al. demonstrated that the sensitivity of Xpert MTB/RIF Ultra method for detecting MTB in tongue swabs was also relatively high, with a specificity of 100% (17). We recommend conducting further studies employing different methods to detect MTB in tongue swabs, thereby improving the sensitivity and specificity of OSA.
Tongue and cheek swabs are oral specimens commonly employed for the diagnosis of TB, although their exact diagnostic value remains uncertain. In this study, we conducted a meta-analysis focusing on tongue and cheek swabs collected via the use of OSA to diagnose pulmonary TB. The results of the meta-analysis revealed that tongue swabs exhibited higher sensitivity than cheek swabs when utilizing OSA for TB diagnosis, while their specificity remained comparable. The summary findings strongly underscore the importance of utilizing tongue swabs for the diagnosis of pulmonary TB, attributing their superior sensitivity to the fact that MTB primarily colonize the epithelial cells on the surface of the tongue. For future developments in the field of OSA-based diagnosis of pulmonary TB, we highly recommend the utilization of tongue swabs. However, it is crucial to note that despite conducting a subgroup analysis, the presence of substantial heterogeneity observed amongst the results emphasizes significant variability across the studies included in the analysis. Hence, there is a pressing need to establish standardized protocols for conducting OSA to ensure consistency and reliability in its diagnostic applications.
A limitation of the current work is the relatively small number of publications available on this particular topic. Despite ongoing and extensive research in the field of OSA for diagnosing TB, only a limited number of articles met our eligibility criteria. Adding complexity to the analysis is the lack of methodological consistency across studies and insufficient reporting of methods and outcome parameters. Additionally, there are several other confounding factors that might have affected the diagnostic effectiveness of OSA in pulmonary TB, including the method of sample collection, swab storage buffer, storage period before testing, DNA extraction method and detection method. Consequently, the heterogeneity among the included studies was significant, thereby impacting the analysis in this study.
In conclusion, our findings suggest that oral swabs serve as a promising alternative for diagnosing pulmonary TB, particularly in adult patients. Notably, tongue swabs exhibit superior sensitivity compared to cheek swabs in detecting individuals with pulmonary TB. However, further rigorous assessment with a larger sample size, focusing on populations such as HIV-positive individuals and children, is warranted to validate the diagnostic performance of OSA. Furthermore, we strongly advocate for future studies to offer comprehensive information regarding collection procedures, enabling the field to better comprehend the necessary trade-offs required for the successful implementation and scalability of OSA in TB diagnosis.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
FZ: Writing – original draft, Data curation, Formal analysis, Investigation, Methodology, Software. YW: Data curation, Formal analysis, Investigation, Validation, Writing – review & editing. XZ: Data curation, Formal analysis, Investigation, Writing – review & editing, Supervision. KL: Formal analysis, Supervision, Writing – review & editing, Methodology. YS: Formal analysis, Methodology, Supervision, Writing – review & editing. WW: Formal analysis, Methodology, Supervision, Writing – review & editing. YL: Formal analysis, Methodology, Supervision, Writing – review & editing. LL: Conceptualization, Funding acquisition, Writing – original draft. YP: Conceptualization, Funding acquisition, Writing – original draft.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Beijing Hospitals Authority Ascent Plan (DFL20191601 & DFL20221401).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2023.1278716/full#supplementary-material
References
1. World Health Organization. Global tuberculosis report 2022. Geneva: World Health Organization (2022). Available online at: https://www.who.int/teams/global-tuberculosis-programme/data (accessed October 27, 2022).
2. Chakaya J, Khan M, Ntoumi F, Aklillu E, Fatima R, Mwaba P, et al. Global Tuberculosis Report 2020 - Reflections on the Global TB burden, treatment and prevention efforts. Int J Infect Dis. (2021) 113:S7–S12. doi: 10.1016/j.ijid.2021.02.107
3. van Cutsem G, Isaakidis P, Farley J, Nardell E, Volchenkov G, Cox H. Infection control for drug-resistant tuberculosis: early diagnosis and treatment is the key. Clin Infect Dis. (2016) 62:S238–S43. doi: 10.1093/cid/ciw012
4. Denkinger CM, Pai M. Point-of-care tuberculosis diagnosis: are we there yet? Lancet Infect Dis. (2012) 12:169–70. doi: 10.1016/S1473-3099(11)70257-2
5. Ryu YJ. Diagnosis of pulmonary tuberculosis: recent advances and diagnostic algorithms. Tuberc Respir Dis. (2015) 78:64–71. doi: 10.4046/trd.2015.78.2.64
6. Acharya B, Acharya A, Gautam S, Ghimire SP, Mishra G, Parajuli N, et al. Advances in diagnosis of Tuberculosis: an update into molecular diagnosis of Mycobacterium tuberculosis. Mol Biol Rep. (2020) 47:4065–75. doi: 10.1007/s11033-020-05413-7
7. Bulterys MA, Wagner B, Redard-Jacot M, Suresh A, Pollock NR, Moreau E, et al. Point-of-care urine LAM tests for tuberculosis diagnosis: a status update. J Clin Med. (2020) 9:111. doi: 10.3390/jcm9010111
8. Klompas M, Baker M, Rhee C. What is an aerosol-generating procedure? JAMA Surg. (2021) 156:113–4. doi: 10.1001/jamasurg.2020.6643
9. Alisjahbana B, van Crevel R, Danusantoso H, Gartinah T, Soemantri ES, Nelwan RHH, et al. Better patient instruction for sputum sampling can improve microscopic tuberculosis diagnosis. Int J Tuberc Lung Dis. (2005) 9:814–7.
10. Shenai S, Amisano D, Ronacher K, Kriel M, Banada PP, Song T, et al. Exploring alternative biomaterials for diagnosis of pulmonary tuberculosis in HIV-negative patients by use of the GeneXpert MTB/RIF assay. J Clin Microbiol. (2013) 51:4161–6. doi: 10.1128/JCM.01743-13
11. Nicol MP, Spiers K, Workman L, Isaacs W, Munro J, Black F, et al. Xpert MTB/RIF testing of stool samples for the diagnosis of pulmonary tuberculosis in children. Clin Infect Dis. (2013) 57:E18–21. doi: 10.1093/cid/cit230
12. Molina-Moya B, Ciobanu N, Hernandez M, Prat-Aymerich C, Crudu V, Adams ER, et al. Molecular detection of Mycobacterium tuberculosis in oral mucosa from patients with presumptive tuberculosis. J Clin Med. (2020) 9:4124. doi: 10.3390/jcm9124124
13. Flores JA, Calderon R, Mesman AW, Soto M, Coit J, Aliaga J, et al. Detection of Mycobacterium tuberculosis DNA in buccal swab samples from children in Lima, Peru. Pediatr Infect Dis J. (2020) 39:E376–E80. doi: 10.1097/INF.0000000000002828
14. Luabeya AK, Wood RC, Shenje J, Filander E, Ontong C, Mabwe S, et al. Noninvasive detection of tuberculosis by oral swab analysis. J Clin Microbiol. (2019) 57:10–1128. doi: 10.1128/JCM.01847-18
15. Mesman AW, Calderon R, Soto M, Coit J, Aliaga J, Mendoza M, et al. Mycobacterium tuberculosis detection from oral swabs with Xpert MTB/RIF ULTRA: a pilot study. BMC Res Notes. (2019) 12:349. doi: 10.1186/s13104-019-4385-y
16. Nicol MP, Wood RC, Workman L, Prins M, Whitman C, Ghebrekristos Y, et al. Microbiological diagnosis of pulmonary tuberculosis in children by oral swab polymerase chain reaction. Sci Rep. (2019) 9:10789. doi: 10.1038/s41598-019-47302-5
17. Andama A, Whitman GR, Crowder R, Reza TF, Jaganath D, Mulondo J, et al. Accuracy of tongue swab testing using Xpert MTB-RIF ultra for tuberculosis diagnosis. J Clin Microbiol. (2022) 60:e0042122. doi: 10.1128/jcm.00421-22
18. Cox H, Workman L, Bateman L, Franckling-Smith Z, Prins M, Luiz J, et al. Oral swab specimens tested with xpert MTB/RIF ultra assay for diagnosis of pulmonary tuberculosis in children: a diagnostic accuracy study. Clin Infect Dis. (2022) 75:2145–52. doi: 10.1093/cid/ciac332
19. Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. (2011) 155:529–36. doi: 10.7326/0003-4819-155-8-201110180-00009
20. Wood RC, Andama A, Hermansky G, Burkot S, Asege L, Job M, et al. Characterization of oral swab samples for diagnosis of pulmonary tuberculosis. PLoS ONE. (2021) 16:e0251422. doi: 10.1371/journal.pone.0251422
21. Wood RC, Luabeya AK, Weigel KM, Wilbur AK, Jones-Engel L, Hatherill M, et al. Detection of Mycobacterium tuberculosis DNA on the oral mucosa of tuberculosis patients. Sci Rep. (2015) 5:8668. doi: 10.1038/srep08668
22. Lima F, Santos AS, Oliveira RD, Silva CCR, Goncalves CCM, Andrews JR, et al. Oral swab testing by Xpert(R) MTB/RIF Ultra for mass tuberculosis screening in prisons. J Clin Tuberc Other Mycobact Dis. (2020) 19:100148. doi: 10.1016/j.jctube.2020.100148
23. Mesman AW, Calderon RI, Pollock NR, Soto M, Mendoza M, Coit J, et al. Molecular detection of Mycobacterium tuberculosis from buccal swabs among adult in Peru. Sci Rep-Uk. (2020) 10:22231. doi: 10.1038/s41598-020-79297-9
24. Song Y, Ma Y, Liu R, Shang Y, Ma L, Huo F, et al. Diagnostic yield of oral swab testing by TB-LAMP for diagnosis of pulmonary tuberculosis. Infect Drug Resist. (2021) 14:89–95. doi: 10.2147/IDR.S284157
25. Shapiro AE, Olson AM, Kidoguchi L, Niu X, Ngcobo Z, Magcaba ZP, et al. Complementary nonsputum diagnostic testing for tuberculosis in people with HIV using oral swab PCR and urine Lipoarabinomannan detection. J Clin Microbiol. (2022) 60:e0043122. doi: 10.1128/jcm.00431-22
26. Kang YA, Koo B, Kim OH, Park JH, Kim HC, Lee HJ, et al. Gene-based diagnosis of tuberculosis from oral swabs with a new generation pathogen enrichment technique. Microbiol Spectr. (2022) 10:e0020722. doi: 10.1128/spectrum.00207-22
27. LaCourse SM, Seko E, Wood R, Bundi W, Ouma GS, Agaya J, et al. Diagnostic performance of oral swabs for non-sputum based TB diagnosis in a TB/HIV endemic setting. PLoS ONE. (2022) 17:e0262123. doi: 10.1371/journal.pone.0262123
28. Jo KW. Preventing the transmission of tuberculosis in health care settings: administrative control. Tuberc Respir Dis. (2017) 80:21–6. doi: 10.4046/trd.2017.80.1.21
29. Wilbur AK, Engel GA, Rompis A, Ig AP, Lee BP, Aggimarangsee N, et al. From the mouths of monkeys: detection of Mycobacterium tuberculosis complex DNA from buccal swabs of synanthropic macaques. Am J Primatol. (2012) 74:676–86. doi: 10.1002/ajp.22022
30. Meesawat S, Warit S, Hamada Y, Malaivijitnond S. Prevalence of Mycobacterium tuberculosis complex among wild rhesus macaques and 2 subspecies of long-tailed macaques, Thailand, 2018-2022. Emerg Infect Dis. (2023) 29:551–60. doi: 10.3201/eid2903.221486
31. Rosenbaum M, Mendoza P, Ghersi BM, Wilbur AK, Perez-Brumer A, Cavero Yong N, et al. Detection of Mycobacterium tuberculosis complex in new world monkeys in Peru. Ecohealth. (2015) 12:288–97. doi: 10.1007/s10393-014-0996-x
32. Valinetz ED, Cangelosi GA. A look inside: oral sampling for detection of non-oral infectious diseases. J Clin Microbiol. (2021) 59:e0236020. doi: 10.1128/JCM.02360-20
33. Ealand C, Peters J, Jacobs O, Sewcharran A, Ghoor A, Golub J, et al. Detection of Mycobacterium tuberculosis complex bacilli and nucleic acids from tongue swabs in young, hospitalized children. Front Cell Infect Microbiol. (2021) 11:696379. doi: 10.3389/fcimb.2021.696379
34. Horne DJ, Kohli M, Zifodya JS, Schiller I, Dendukuri N, Tollefson D, et al. Xpert MTB/RIF and Xpert MTB/RIF Ultra for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev. (2019) 6:CD009593. doi: 10.1002/14651858.CD009593.pub4
35. Pradhan NN, Paradkar MS, Kagal A, Valvi C, Kinikar A, Khwaja S, et al. Performance of Xpert® MTB/RIF and Xpert® Ultra for the diagnosis of tuberculous meningitis in children. Int J Tuberc Lung Dis. (2022) 26:317–25. doi: 10.5588/ijtld.21.0388
Keywords: oral swab analysis, tuberculosis, diagnostic accuracy, systematic review, meta-analysis
Citation: Zhang F, Wang Y, Zhang X, Liu K, Shang Y, Wang W, Liu Y, Li L and Pang Y (2024) Diagnostic accuracy of oral swab for detection of pulmonary tuberculosis: a systematic review and meta-analysis. Front. Med. 10:1278716. doi: 10.3389/fmed.2023.1278716
Received: 23 August 2023; Accepted: 26 December 2023;
Published: 11 March 2024.
Edited by:
Xueqiong Wu, The 8th Medical Center of Chinese PLA General Hospital, ChinaReviewed by:
Quanxin Long, Chongqing Medical University, ChinaJana Amlerova, University Hospital in Pilsen, Czechia
Copyright © 2024 Zhang, Wang, Zhang, Liu, Shang, Wang, Liu, Li and Pang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yu Pang, cGFuZ3l1cG91bmRAMTYzLmNvbQ==; Liang Li, bGlsaWFuZzY5QHRiMTIzLm9yZw==
†These authors have contributed equally to this work and share first authorship
 Fuzhen Zhang1,2†
Fuzhen Zhang1,2† Yilin Wang
Yilin Wang Xuxia Zhang
Xuxia Zhang Liang Li
Liang Li Yu Pang
Yu Pang