- 1WHO Collaborating Centre for Tuberculosis and the Sydney Infectious Diseases Institute (Sydney ID), The University of Sydney, Sydney, NSW, Australia
- 2Centenary Institute, The University of Sydney, Sydney, NSW, Australia
- 3Woolcock Institute of Medical Research, Hanoi, Vietnam
- 4Faculty of Medicine and Health, The University of Sydney, Sydney, NSW, Australia
- 5School of Public Health and Preventive Medicine, Monash University, Melbourne, VIC, Australia
- 6Woolcock Institute of Medical Research, Sydney, NSW, Australia
- 7Department of Medicine and Health, University of New South Wales, Sydney, NSW, Australia
In recognition of the high rates of undetected tuberculosis in the community, the World Health Organization (WHO) encourages targeted active case finding (ACF) among “high-risk” populations. While this strategy has led to increased case detection in these populations, the epidemic impact of these interventions has not been demonstrated. Historical data suggest that population-wide (untargeted) ACF can interrupt transmission in high-incidence settings, but implementation remains lacking, despite recent advances in screening tools. The reservoir of latent infection—affecting up to a quarter of the global population –complicates elimination efforts by acting as a pool from which future tuberculosis cases may emerge, even after all active cases have been treated. A holistic case finding strategy that addresses both active disease and latent infection is likely to be the optimal approach for rapidly achieving sustainable progress toward TB elimination in a durable way, but safety and cost effectiveness have not been demonstrated. Sensitive, symptom-agnostic community screening, combined with effective tuberculosis treatment and prevention, should eliminate all infectious cases in the community, whilst identifying and treating people with latent infection will also eliminate tomorrow’s tuberculosis cases. If real strides toward global tuberculosis elimination are to be made, bold strategies are required using the best available tools and a long horizon for cost-benefit assessment.
Introduction
After the Second World War the tuberculosis control agenda in most high-income countries was strongly focused on active case finding (ACF). This focus led to a twenty-fold decline in tuberculosis incidence and near elimination in many areas that formerly had a high tuberculosis incidence (1–5). Once tuberculosis was considered “conquered”—with low disease rates in high-income countries and effective treatment widely available—governments shifted their investment and the health policy focus to other priorities. However, tuberculosis has continued to plague low-income countries where it has never been “conquered” and where community transmission often remains uncontrolled. The DOTS (directly observed therapy short course) strategy of rolling out passive case finding linked to effective treatment of sputum smear-positive tuberculosis has contributed to substantial reductions in tuberculosis-related mortality over the past 20 years. However, this strategy had limited epidemic impact (6). Nevertheless, the implementation of better tuberculosis data collection and reporting systems is an important legacy of the DOTS strategy. Today, progress envisioned by the 2014 World Health Organization (WHO) End TB strategy has been hampered by major setbacks in global tuberculosis control resulting from the coronavirus 2019 (COVID-19) pandemic. Therefore, it is now imperative to reevaluate what would be required to truly “move the dial” toward reducing tuberculosis transmission in high incidence settings and achieving global tuberculosis elimination.
It is increasingly appreciated that asymptomatic or “subclinical” tuberculosis (where people are infectious but do not feel ill enough to present to the healthcare system) contributes to a large proportion of community transmission (7). This implies that traditional passive case finding, where programs wait on a symptomatic patient to present to the healthcare system for diagnosis and treatment, is simply insufficient to have a community-wide transmission impact (8). Furthermore, it is estimated that more than a third of active tuberculosis cases every year remain undetected and so are absent from surveillance data (9). In addition, latent tuberculosis infection is now considered a dynamic state, at least in some people, with waxing and waning bacilli numbers and highly variable risk of reactivation (10–13). Under an elimination paradigm, it is prudent to regard latent tuberculosis infection as a “risk state” that carries the potential both to lead to individual harm and to perpetuate community transmission (14, 15). This multifaceted challenge of asymptomatic tuberculosis transmission combined with persistent low rates of disease detection and the risk of reactivation from a latent reservoir constitutes a complex epidemiological reality. This complexity may explain why simple strategies for global tuberculosis elimination remain elusive. Holistic, combined approaches targeting both active and latent disease in a symptom-agnostic way may provide an opportunity to recapture the successes of the past in the modern era.
Population-wide active case finding
Interventions that increase timely access to healthcare for tuberculosis patients, together with good oversight and quality control systems, have typically been emphasized to ensure that people with tuberculosis are diagnosed early and treated appropriately. However, the 2022 WHO Global Report indicates that—despite this long-held emphasis—the decline in tuberculosis incidence is far below End TB targets for most high burden countries (9). In these settings, people with “classic” tuberculosis symptoms do not always seek healthcare and even if they do so, poor access to healthcare, insensitive tuberculosis testing, social stigma and financial insecurities often impede timely and effective treatment (16). The WHO has estimated that in 2021, only 60% of tuberculosis patients globally were identified and notified to the healthcare system (9). Hence, active case finding has been strongly recommended to find and treat unreached tuberculosis patients (17). The primary focus has been on specific high-risk populations, including household contacts of index patients, people living with HIV, workers exposed to silica and incarcerated people (18–24). Although active case finding (ACF) among these “risk groups” is effective in finding new cases, such activities are insufficient to effectively interrupt tuberculosis transmission or reduce disease prevalence on a population scale (25). This is because a significant proportion of infectious tuberculosis cases, particularly in high incidence settings, occur in patients with none of these identifiable risk factors (7, 26, 27). In addition, such targeted case finding fails to address the large pool of people with latent tuberculosis infection, representing a reservoir which can continue to seed new active tuberculosis cases and perpetuate transmission for decades to come (28, 29).
Settings in which transmission had historically been high and where mass radiography screening was subsequently conducted (such as North America, Australia, and the United Kingdom) observed dramatic reductions in tuberculosis incidence and mortality following the introduction of these programs (1–5). Recently, a community-based cluster randomized controlled trial of active case finding for tuberculosis (the ACT3 study) was conducted in Ca Mau, a rural province in the Mekong Delta region of Vietnam (30). Starting in 2014, ACT3 aimed to evaluate the effectiveness of repeated community-wide screening over 3 years, as compared with standard passive case detection alone, for reducing the prevalence of tuberculosis. By the conclusion of the study, adult tuberculosis prevalence was 44% lower and prevalence of tuberculosis infection (positive interferon gamma release assay) among children aged 6 to 14 years in the actively screened clusters was 50% lower than in the control clusters (30, 31). This trial suggests that if all people with tuberculosis disease are found and treated, then it is possible to drastically reduce community transmission (32). However, not all active case finding interventions have found a similarly dramatic effect and the high-uptake of community-wide tuberculosis screening required may be hard to replicate in all high-incidence settings (20, 33). However, multiple implementation studies are underway seeking to identify the most pragmatic strategies in a wider array of real-life settings, which are discussed further below.
Treating latent tuberculosis infection
Latent tuberculosis infection (LTBI) is a state of persistent immune response to stimulation by Mycobacterium tuberculosis antigens, without evidence of clinically manifest disease (14). Individuals with LTBI have an increased lifetime risk of subsequent progression to active tuberculosis, although this risk is highly variable and concentrated in the period soon after infection or re-infection (10, 34). Globally, the prevalence of LTBI has been estimated to be ∼25% (95% uncertainty interval 20–26%), based upon historical cross-sectional surveys and tuberculosis prevalence estimates (35). However, substantial variation occurs between different populations on account of the differing risks of transmission. The tests used to diagnose LTBI [tuberculin skin test (TST) and interferon gamma release assays (IGRAs)] have significant limitations in their accuracy and ability to predict subsequent incident disease (36, 37). Diagnostic accuracy was poorer among studies from high-incidence settings (38) and no reference standard exists to assess accuracy for LTBI detection. In general, both tests are associated with a similar risk of future tuberculosis disease progression when positive, although even in the absence of tuberculosis preventive treatment (TPT) most people with a positive result never develop disease. The estimated positive predictive value of future TB disease for a positive TST is 2.75% and for a positive Quantiferon Gold is 2.46% (38).
Latent tuberculosis infection can be treated using 1 to 9 months of oral antibiotics (14), which reduce the incidence of tuberculosis by approximately two thirds compared to placebo. However, all available regimens may cause side effects and require careful clinical monitoring, which is a particular disincentive in people who are otherwise well. Consequently, the decision to treat individuals with LTBI requires careful evaluation of the risks of preventive treatment against the benefits to the affected individuals and their communities. This presents an ethical dilemma since the short-term individual benefit from reduced tuberculosis risk may be far less than the long-term community benefit of tuberculosis elimination, while the individual carries all the risk associated with preventive treatment. WHO currently recommends screening of identified high-risk populations, such as people living with HIV, recent household contacts and other groups, such as people initiating immunosuppressive treatment considered to be at high risk of tuberculosis disease progression (14). Whether additional groups can be identified who will benefit from LTBI-linked ACF in high-incidence settings remains under evaluation (10, 39). Three studies in the Asia-Pacific are currently underway to investigate this evidence gap including a randomized controlled trial and “real-life” implementation study, explored below. While evidence for its general implementation is not yet conclusive, combined systematic TB screening and LTBI detection provides an opportunity to strengthen the overall characterization of TB disease (especially early and asymptomatic disease) and transmission within communities.
Current and future studies combining population-wide active case finding and prevention
A recent modeling study estimated the effects of population-wide ACF in the Marshall Islands (40). The analysis emphasized the crucial role of including LTBI screening and TPT as part of the intervention, in order to achieve significant and sustained reductions in tuberculosis burden. The findings also suggested that by implementing these strategies repeatedly, local tuberculosis pre-elimination could be achieved using existing tools alone. Additionally, the estimated number of severe adverse events associated with preventive treatment—a weekly dose of rifapentine and isoniazid for 12-weeks (3HP regimen)—was minimal compared to the significant reductions in tuberculosis deaths and disease episodes resulting from the interventions. The study highlighted another significant advantage of incorporating LTBI screening in ACF programs, as such approaches provide direct measures of LTBI prevalence in addition to those of active tuberculosis prevalence, which increases the richness of the dataset and allows measurement of transmission impact over time. The combination of these two indicators enables a more accurate characterization of the risk of future tuberculosis disease following exposure and infection, a factor that is essential for refining modeling projections and identifying the tuberculosis control approaches that will achieve the greatest reductions in tuberculosis burden.
The ACT5 trial is a randomized controlled trial currently assessing the effectiveness of universal testing and treatment for LTBI, together with ACF for tuberculosis disease, on the population prevalence of tuberculosis disease and ongoing community transmission in a high incidence setting in Vietnam. The overall goal of this trial is to acquire evidence that will underpin a transformation in the global approach to tuberculosis elimination in low and middle-income countries with a high burden of tuberculosis. Results of the trial will be available in 2026 (trial registration number ACTRN12622000115730).
Another example aiming to assess the impact of combining ACF and TPT at population level is the PEARL [Pathway to the Elimination of Antibiotic Resistant and Latent Tuberculosis (and also Leprosy) in the Pacific] study conducted in South Tarawa, Kiribati (39, 41). In this program, ACF is delivered using the best available combination of technologies (symptoms screening, mobile digital chest radiograph with computer-aided detection and sputum Xpert MTB/RIF Ultra® on all who can expectorate) along with TPT (using short course regimens) for those who are TST positive. The impact of this ambitious public health intervention on case notifications will be compared to case notification rates before the intervention and trends in the rest of country where the intervention was not delivered. In addition, TB transmission impact will be assessed by comparing TST positivity in primary school aged children before and after the intervention. As with the Vietnam studies, a broader goal is to provide practical examples of how this active population-based approach can be effectively scaled in geographically diverse high-incidence locations, especially in remote Pacific settings. These and similar studies should provide insights into the value-add of combining TB prevention with community-wide active TB case-finding efforts. Given the complexity of such activities, these studies are essential to assess the feasibility of population-wide screening for infection and disease in resource-limited settings.
Challenges of combining active tuberculosis case finding and prevention
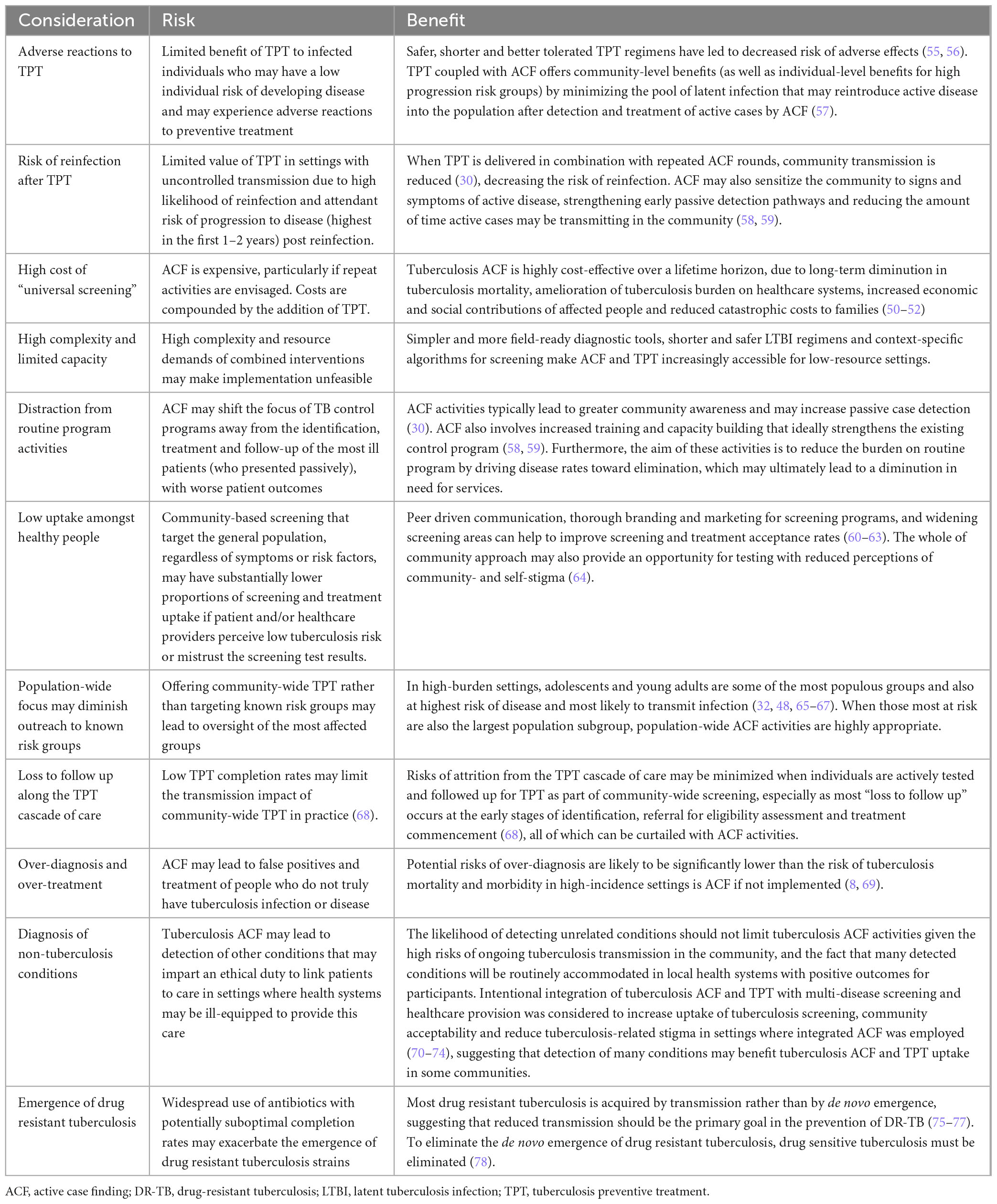
Challenges facing the expansion of screening and treatment for LTBI in a community-wide fashion include the relatively high prevalence of remote past infection for which LTBI treatment will have reduced benefit, ethical concerns regarding the administration of a potentially toxic medication to someone for whom the individual benefit may be limited and the highly variable risk of future re-infection against which past LTBI treatment will offer no protection. Studies are ongoing to explore the feasibility, effectiveness and acceptability of large-scale screening and treatment for LTBI as a part of community-wide tuberculosis elimination efforts. Further considerations of the risks and benefits of combining ACT and TPT are highlighted in Table 1. Careful consideration must be given to contexts where dual active and latent tuberculosis screening and treatment is appropriate, but potential risks of over-diagnosis should be weighed against the higher risk of disease and death in high-incidence settings.
Progressing the tuberculosis elimination agenda
The current slow progress toward ending tuberculosis in high-incidence settings implies that more needs to be done to actively “move the dial” toward this goal (42). Several observations about the epidemiology of the global epidemic and the adequacy of existing healthcare provision are relevant:
1. Prevalence surveys have demonstrated that many people with bacteriologically confirmed pulmonary tuberculosis do not have overt symptoms and hence are unlikely to seek care or be diagnosed using a passive case finding approach (26, 43–45). They can only be found through active screening.
2. People who do have symptoms of tuberculosis (cough, fever or weight loss) are most likely to attend primary healthcare providers (46, 47), but these are common symptoms in persons without tuberculosis and primary health services in many low-middle income countries are fragmentary and poorly equipped to diagnose tuberculosis.
3. In settings with a high-incidence of TB, most people with tuberculosis are not in an identifiable high-risk group (32). In these settings everyone is at risk, all the time. Hence, targeted case finding or limited treatment of latent tuberculosis infection is unlikely to make a major contribution toward ending the epidemic (48).
4. There is evidence that re-infection with tuberculosis occurs commonly in high-incidence settings and that previous tuberculosis infection or disease does not protect against subsequent reinfection. In fact, people who have had tuberculosis in the past are more likely to become diseased again if reinfected (49). Hence, the benefit of treatment for tuberculosis infection for a person who lives in a high tuberculosis incidence setting is likely to be limited and transient unless simultaneous efforts are made to eliminate community transmission at the same time in order to substantially reduce that person’s risk of re-exposure and, hence, reinfection.
It follows that, to reduce the incidence of new cases of tuberculosis in high incidence settings and make substantial progress toward ending tuberculosis, it is necessary to drastically reduce the number of persons with untreated (and usually undiagnosed) tuberculosis who sustain transmission within communities (7). Since these people will not present for care and are not identifiable by specific risk factors in high tuberculosis incidence settings, it is necessary to screen (test) everyone for tuberculosis using a symptom-agnostic test (either radiology or a molecular test on sputum) as the front-line screening test. It is also essential that those who are diagnosed with tuberculosis as a result of this screening are linked to effective care, given appropriate treatment and are adequately supported to complete their treatment.
The role of mass treatment of tuberculosis infection in this approach remains to be fully elucidated. In theory, it should expedite the ending of endemic transmission, when linked to screening for active disease, by averting development of tuberculosis in those who were recently infected. Several studies to clarify the role of mass treatment of tuberculosis infection in tuberculosis elimination are currently underway. Community-wide screening for tuberculosis disease, with or without mass treatment of tuberculosis infection, is a major logistical and financial challenge for tuberculosis programs, Ministries of Health and funders. Importantly, these activities may be cost saving by reducing the load of passive cases on the health system when considered over a longer time horizon. More work is required to establish the best way to implement these programs at scale. As tuberculosis is a slowly evolving condition, some people who were recently infected at the time of screening may only develop tuberculosis over the next 1 to 2 years and longer. There is also a conversion window of 6–8 weeks for TST and IGRA test to register recent TB infection and sensitivity of these tests is suboptimal. In addition, even comprehensive population-wide screening is likely to miss a sizable part of the population, which may include those at highest risk of LTBI and tuberculosis disease. This means that screening interventions may have to be repeated, until the disease prevalence has fallen to a low enough level so that ongoing endemic transmission can be prevented through more targeted means.
Conclusion
Effective tuberculosis elimination strategies have challenges and are daunting to implement, but with 23.8 million avoidable tuberculosis deaths predicted to occur globally by 2035 if current downward trends in tuberculosis incidence are not accelerated (50), the cost of inaction is high. Bold strategies are required to “move the dial” towards elimination, which would not only save lives lost to tuberculosis, but will also reduce pressure on fragile healthcare systems. Despite the need for considerable up-front investment, strategies that effectively reduce community transmission and make real progress toward tuberculosis elimination will ultimately save money, if a sufficient time horizon is adopted for the analysis (51–54). Recent transmission insights, technological advances, funding commitments and increased international political will, create new opportunities to bring tuberculosis elimination within our grasp. It is therefore prudent to reflect whether tried and tested active case finding strategies combined with modern advances can be leveraged to replicate and even accelerate historical achievements in tuberculosis control.
Author contributions
MC: Conceptualization, Writing – original draft, Writing – review and editing. T-AN: Conceptualization, Writing – original draft, Writing – review and editing. BL: Writing – original draft. JH: Writing – original draft. RR: Writing – original draft. JT: Writing – review and editing. GF: Writing – original draft, Writing – review and editing. GM: Writing – original draft. BM: Conceptualization, Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Golub JE, Mohan CI, Comstock GW, Chaisson RE. Active case finding of tuberculosis: historical perspective and future prospects. Int J Tuberc Lung Dis. (2015) 9:1183–203.
2. Oommen A. Review of mass radiography services; a report by the Joint Tuberculosis Council. Tubercle. (1964) 45:255–66. doi: 10.1016/S0041-3879(64)80016-7
3. Wallace JM. Changes in the pattern of respiratory tuberculosis in an urban community following a mass radiography campaign. Tubercle. (1964) 45:7–16. doi: 10.1016/s0041-3879(64)80081-7
4. Comstock GW, Ferebee SH, Hammes LM. A controlled trial of community-wide isoniazid prophylaxis in Alaska. Am Rev Respir Dis. (1967) 95:935–43. doi: 10.1164/arrd.1967.95.6.935
6. Raviglione M, Marais B, Floyd K, Lönnroth K, Getahun H, Migliori GB, et al. Scaling up interventions to achieve global tuberculosis control: progress and new developments. Lancet Lond Engl. (2012) 379:1902–13. doi: 10.1016/S0140-6736(12)60727-2
7. Emery JC, Dodd PJ, Banu S, Frascella B, Garden FL, Horton KC, et al. Estimating the Contribution of Subclinical Tuberculosis Disease to Transmission – An Individual Patient Data Analysis from Prevalence Surveys [Internet]. Infectious Diseases (except HIV/AIDS). (2022). Available online at: http://medrxiv.org/lookup/doi/10.1101/2022.06.09.22276188 (Accessed August 30, 2022)
8. Ho J, Fox GJ, Marais BJ. Passive case finding for tuberculosis is not enough. Int J Mycobacteriol. (2016) 5:374–8. doi: 10.1016/j.ijmyco.2016.09.023
9. World Health Organization [WHO]. Global Tuberculosis Report 2022. Report No.: CC BY-NC-SA 3.0 IGO. Geneva: WHO (2022).
10. Trauer JM, Moyo N, Tay EL, Dale K, Ragonnet R, McBryde ES, et al. Risk of active tuberculosis in the five years following infection. 15%? Chest. (2016) 149:516–25. doi: 10.1016/j.chest.2015.11.017
11. Cardona PJ. A dynamic reinfection hypothesis of latent tuberculosis infection. Infection. (2009) 37:80–6. doi: 10.1007/s15010-008-8087-y
12. Drain PK, Bajema KL, Dowdy D, Dheda K, Naidoo K, Schumacher SG, et al. Incipient and subclinical tuberculosis: a clinical review of early stages and progression of infection. Clin Microbiol Rev. (2018) 31:e00021–18. doi: 10.1128/CMR.00021-18
13. Menzies NA, Wolf E, Connors D, Bellerose M, Sbarra AN, Cohen T, et al. Progression from latent infection to active disease in dynamic tuberculosis transmission models: a systematic review of the validity of modelling assumptions. Lancet Infect Dis. (2018) 18:e228–38. doi: 10.1016/S1473-3099(18)30134-8
14. World Health Organization [WHO]. Latent Tuberculosis Infection: Updated and Consolidated Guidelines for Programmatic Management. Report No.: CC BY-NC-SA 3.0 IGO. Geneva: WHO (2018).
15. Rangaka MX, Cavalcante SC, Marais BJ, Thim S, Martinson NA, Swaminathan S, et al. Controlling the seedbeds of tuberculosis: diagnosis and treatment of tuberculosis infection. Lancet. (2015) 386:2344–53. doi: 10.1016/S0140-6736(15)00323-2
16. Teo AKJ, Singh SR, Prem K, Hsu LY, Yi S. Duration and determinants of delayed tuberculosis diagnosis and treatment in high-burden countries: a mixed-methods systematic review and meta-analysis. Respir Res. (2021) 22:251. doi: 10.1186/s12931-021-01841-6
17. World Health Organization [WHO]. Systematic Screening for Active Tuberculosis: Principles and Recommendations. Geneva: WHO (2013).
18. Fair E, Miller C, Ottmani S, Fox G, Hopewell P. Tuberculosis contact investigation in low-and middle-income countries: standardized definitions and indicators. Int J Tuberc Lung Dis. (2015) 19:269–72. doi: 10.5588/ijtld.14.0512
19. National Tuberculosis Controllers Association, Centers for Disease Control and Prevention [CDC]. Guidelines for the investigation of contacts of persons with infectious tuberculosis. Recommendations from the National Tuberculosis Controllers Association and CDC. MMWR Recomm Rep. (2005) 54:1–47.
20. Ayles H, Muyoyeta M, Du Toit E, Schaap A, Floyd S, Simwinga M, et al. Effect of household and community interventions on the burden of tuberculosis in southern Africa: the ZAMSTAR community-randomised trial. Lancet. (2013) 382:1183–94. doi: 10.1016/S0140-6736(13)61131-9
21. Fox GJ, Nhung NV, Sy DN, Hoa NLP, Anh LTN, Anh NT, et al. Household-contact investigation for detection of tuberculosis in Vietnam. N Engl J Med. (2018) 378:221–9. doi: 10.1056/NEJMoa1700209
22. Shah NS, Yuen CM, Heo M, Tolman AW, Becerra MC. Yield of contact investigations in households of patients with drug-resistant tuberculosis: systematic review and meta-analysis. Clin Infect Dis. (2014) 58:381–91. doi: 10.1093/cid/cit643
23. World Health Organization. Guidelines for Intensified Tuberculosis Case-Finding and Isoniazid Preventive Therapy for People Living with HIV in Resource-Constrained Settings. Geneva: WHO (2011).
24. Martinez L, Warren JL, Harries AD, Croda J, Espinal MA, Olarte RAL, et al. Global, regional, and national estimates of tuberculosis incidence and case detection among incarcerated individuals from 2000 to 2019: a systematic analysis. Lancet Public Health. (2023) 8:e511–9. doi: 10.1016/S2468-2667(23)00097-X
25. Sohn H, Sweeney S, Mudzengi D, Creswell J, Menzies NA, Fox GJ, et al. Determining the value of TB active case-finding: current evidence and methodological considerations. Int J Tuberc Lung Dis. (2021) 25:171–81. doi: 10.5588/ijtld.20.0565
26. Frascella B, Richards AS, Sossen B, Emery JC, Odone A, Law I, et al. Subclinical tuberculosis disease—a review and analysis of prevalence surveys to inform definitions, burden, associations, and screening methodology. Clin Infect Dis. (2021) 73:e830–41. doi: 10.1093/cid/ciaa1402
27. Williams CM, Abdulwhhab M, Birring SS, De Kock E, Garton NJ, Townsend E, et al. Exhaled Mycobacterium tuberculosis output and detection of subclinical disease by face-mask sampling: prospective observational studies. Lancet Infect Dis. (2020) 20:607–17. doi: 10.1016/S1473-3099(19)30707-8
28. Horsburgh CR, O’Donnell M, Chamblee S, Moreland JL, Johnson J, Marsh BJ, et al. Revisiting rates of reactivation tuberculosis: a population-based approach. Am J Respir Crit Care Med. (2010) 182:420–5. doi: 10.1164/rccm.200909-1355OC
29. Marks GB, Bai J, Simpson SE, Sullivan EA, Stewart GJ. Incidence of tuberculosis among a cohort of tuberculin-positive refugees in Australia: reappraising the estimates of risk. Am J Respir Crit Care Med. (2000) 162:1851–4. doi: 10.1164/ajrccm.162.5.2004154
30. Marks GB, Nguyen NV, Nguyen PTB, Nguyen TA, Nguyen HB, Tran KH, et al. Community-wide screening for tuberculosis in a high-prevalence setting. N Engl J Med. (2019) 381:1347–57. doi: 10.1056/NEJMoa1902129
31. Marks GB, Ho J, Nguyen PTB, Nguyen TA, Boi KL, Tran KH, et al. A direct measure of tuberculosis incidence — effect of community screening. N Engl J Med. (2022) 386:1380–2. doi: 10.1056/NEJMc2114176
32. Marks GB, Horsburgh CR, Fox GJ, Nguyen TA. Epidemiological approach to ending tuberculosis in high-burden countries. Lancet. (2022) 400:1750–2. doi: 10.1016/S0140-6736(22)01433-7
33. Burke RM, Nliwasa M, Feasey HRA, Chaisson LH, Golub JE, Naufal F, et al. Community-based active case-finding interventions for tuberculosis: a systematic review. Lancet Public Health. (2021) 6:e283–99. doi: 10.1016/S2468-2667(21)00033-5
34. Getahun H, Matteelli A, Chaisson RE, Raviglione M. Latent Mycobacterium tuberculosis infection. N Engl J Med. (2015) 372:2127–35. doi: 10.1056/NEJMra1405427
35. Houben RMGJ, Dodd PJ. The global burden of latent tuberculosis infection: a re-estimation using mathematical modelling. PLoS Med. (2016) 13:e1002152. doi: 10.1371/journal.pmed.1002152
36. Sharma SK, Vashishtha R, Chauhan LS, Sreenivas V, Seth D. Comparison of TST and IGRA in diagnosis of latent tuberculosis infection in a high TB-Burden setting. PLoS One. (2017) 12:e0169539. doi: 10.1371/journal.pone.0169539
37. Campbell JR, Winters N, Menzies D. Absolute risk of tuberculosis among untreated populations with a positive tuberculin skin test or interferon-gamma release assay result: systematic review and meta-analysis. BMJ. (2020) 368:m549. doi: 10.1136/bmj.m549
38. Hamada Y, Gupta RK, Quartagno M, Izzard A, Acuna-Villaorduna C, Altet N, et al. Predictive performance of interferon-gamma release assays and the tuberculin skin test for incident tuberculosis: an individual participant data meta-analysis. Eclinicalmedicine. (2023) 56:101815. doi: 10.1016/j.eclinm.2022.101815
39. Coleman M, Hill J, Timeon E, Tonganibeia A, Eromanga B, Islam T, et al. Population-wide active case finding and prevention for tuberculosis and leprosy elimination in Kiribati: the PEARL study protocol. BMJ Open. (2022) 12:e055295. doi: 10.1136/bmjopen-2021-055295
40. Ragonnet R, Williams BM, Largen A, Nasa J, Jack T, Langinlur MK, et al. Estimating the long-term effects of mass screening for latent and active tuberculosis in the Marshall Islands. Int J Epidemiol. (2022) 51:1433–45. doi: 10.1093/ije/dyac045
41. Coleman M, Hill J, Timeon E, Rimon E, Bauro T, Ioteba N, et al. Effectiveness of population-wide screening and mass drug administration for leprosy control in Kiribati: the COMBINE protocol. BMJ Open. (2023) 13:e065369. doi: 10.1136/bmjopen-2022-065369
42. Marais B, Zumla A. Advancing global tuberculosis control after the UNGA-HLM. Lancet. (2018) 392:1096–7. doi: 10.1016/S0140-6736(18)32361-4
43. Hoa NB, Cobelens FGJ, Sy DN, Nhung NV, Borgdorff MW, Tiemersma EW. Yield of interview screening and chest X-ray abnormalities in a tuberculosis prevalence survey. Int J Tuberc Lung Dis. (2012) 16:762–7. doi: 10.5588/ijtld.11.0581
44. Kendall EA, Shrestha S, Dowdy DW. The epidemiological importance of subclinical tuberculosis. A critical reappraisal. Am J Respir Crit Care Med. (2021) 203:168–74. doi: 10.1164/rccm.202006-2394PP
45. Araujo S, Freitas LO, Goulart LR, Goulart IMB. Molecular evidence for the aerial route of infection of Mycobacterium leprae and the role of asymptomatic carriers in the persistence of leprosy. Clin Infect Dis. (2016) 63:1412–20. doi: 10.1093/cid/ciw570
46. Ottmani S-E, Scherpbier R, Pio A, Chaulet P, Aït Khaled N, Blanc L, et al. Practical Approach to Lung Health (PAL): A Primary Health Care Strategy for the Integrated Management of Respiratory Conditions in People Five Years of Age and Over. Report No.: WHO/HTM/TB/2005.351. Geneva: World Health Organization (2005).
47. Banda H, Robinson R, Thomson R, Squire SB, Mortimer K. The ‘Practical Approach to Lung Health’ in sub-Saharan Africa: a systematic review. Int J Tuberc Lung Dis. (2016) 20:552–9. doi: 10.5588/ijtld.15.0613
48. McCreesh N, White RG. An explanation for the low proportion of tuberculosis that results from transmission between household and known social contacts. Sci Rep. (2018) 8:5382. doi: 10.1038/s41598-018-23797-2
49. Erkens C, Tekeli B, van Soolingen D, Schimmel H, Verver S. Recurrent tuberculosis in the Netherlands–a 24-year follow-up study, 1993 to 2016. Eurosurveillance. (2022) 27:2100183. doi: 10.2807/1560-7917.ES.2022.27.12.2100183
50. Silva S, Arinaminpathy N, Atun R, Goosby E, Reid M. Economic impact of tuberculosis mortality in 120 countries and the cost of not achieving the Sustainable Development Goals tuberculosis targets: a full-income analysis. Lancet Glob Health. (2021) 9:e1372–9. doi: 10.1016/S2214-109X(21)00299-0
51. Vo LNQ, Forse RJ, Codlin AJ, Dang HM, Van Truong V, Nguyen LH, et al. Socio-protective effects of active case finding on catastrophic costs from tuberculosis in Ho Chi Minh City, Viet Nam: a longitudinal patient cost survey. BMC Health Serv Res. (2021) 21:1051. doi: 10.1186/s12913-021-06984-2
52. Gurung SC, Dixit K, Rai B, Caws M, Paudel PR, Dhital R, et al. The role of active case finding in reducing patient incurred catastrophic costs for tuberculosis in Nepal. Infect Dis Poverty. (2019) 8:99. doi: 10.1186/s40249-019-0603-z
53. Bohlbro AS, Hvingelby VS, Rudolf F, Wejse C, Patsche CB. Active case-finding of tuberculosis in general populations and at-risk groups: a systematic review and meta-analysis. Eur Respir J. (2021) 58:2100090. doi: 10.1183/13993003.00090-2021
54. Azman AS, Golub JE, Dowdy DW. How much is tuberculosis screening worth? Estimating the value of active case finding for tuberculosis in South Africa, China, and India. BMC Med. (2014) 12:216. doi: 10.1186/s12916-014-0216-0
55. Abidi S, Achar J, Assao Neino MM, Bang D, Benedetti A, Brode S, et al. Standardised shorter regimens versus individualised longer regimens for rifampin- or multidrug-resistant tuberculosis. Eur Respir J. (2020) 55:1901467. doi: 10.1183/13993003.01467-2019
56. Nunn AJ, Phillips PPJ, Meredith SK, Chiang CY, Conradie F, Dalai D, et al. A trial of a shorter regimen for rifampin-resistant tuberculosis. N Engl J Med. (2019) 380:1201–13. doi: 10.1056/NEJMoa1811867
57. Churchyard GJ, Swindells S. Controlling latent TB tuberculosis infection in high-burden countries: a neglected strategy to end TB. PLoS Med. (2019) 16:e1002787. doi: 10.1371/journal.pmed.1002787
58. Galea JT, Puma D, Tzelios C, Valdivia H, Millones AK, Jiménez J, et al. A structured community engagement strategy to support uptake of TB active case-finding. Public Health Action. (2022) 12:18–23. doi: 10.5588/pha.21.0059
59. Dam TA, Forse RJ, Tran PMT, Vo LNQ, Codlin AJ, Nguyen LP, et al. What makes community health worker models for tuberculosis active case finding work? A cross-sectional study of TB REACH projects to identify success factors for increasing case notifications. Hum Resour Health. (2022) 20:25. doi: 10.1186/s12960-022-00708-1
60. Van Wyk SS, Medley N, Young T, Oliver S. Repairing boundaries along pathways to tuberculosis case detection: a qualitative synthesis of intervention designs. Health Res Policy Syst. (2022) 20:7. doi: 10.1186/s12961-021-00811-0
61. Tuot S, Teo AKJ, Cazabon D, Sok S, Ung M, Ly S, et al. Acceptability of active case finding with a seed-and-recruit model to improve tuberculosis case detection and linkage to treatment in Cambodia: a qualitative study. PLoS One. (2019) 14:e0210919. doi: 10.1371/journal.pone.0210919
62. Kerrigan D, West N, Tudor C, Hanrahan CF, Lebina L, Msandiwa R, et al. Improving active case finding for tuberculosis in South Africa: informing innovative implementation approaches in the context of the Kharitode trial through formative research. Health Res Policy Syst. (2017) 15:42. doi: 10.1186/s12961-017-0206-8
63. Yassin MA, Datiko DG, Tulloch O, Markos P, Aschalew M, Shargie EB, et al. Innovative community-based approaches doubled tuberculosis case notification and improve treatment outcome in Southern Ethiopia. PLoS One. (2013) 8:e63174. doi: 10.1371/journal.pone.0063174
64. Biermann O, Tran PB, Forse RJ, Vo LNQ, Codlin AJ, Viney K, et al. Capitalizing on facilitators and addressing barriers when implementing active tuberculosis case-finding in six districts of Ho Chi Minh City, Vietnam: a qualitative study with key stakeholders. Implement Sci. (2021) 16:54. doi: 10.1186/s13012-021-01124-0
65. Andrews JR, Morrow C, Walensky RP, Wood R. Integrating social contact and environmental data in evaluating tuberculosis transmission in a South African Township. J Infect Dis. (2014) 210:597–603. doi: 10.1093/infdis/jiu138
66. Classen CN, Warren R, Richardson M, Hauman JH, Gie RP, Ellis JHP, et al. Impact of social interactions in the community on the transmission of tuberculosis in a high incidence area. Thorax. (1999) 54:136–40. doi: 10.1136/thx.54.2.136
67. Patterson B, Morrow CD, Kohls D, Deignan C, Ginsburg S, Wood R. Mapping sites of high TB transmission risk: integrating the shared air and social behaviour of TB cases and adolescents in a South African township. Sci Total Environ. (2017) 583:97–103. doi: 10.1016/j.scitotenv.2017.01.026
68. Alsdurf H, Hill PC, Matteelli A, Getahun H, Menzies D. The cascade of care in diagnosis and treatment of latent tuberculosis infection: a systematic review and meta-analysis. Lancet Infect Dis. (2016) 16:1269–78. doi: 10.1016/S1473-3099(16)30216-X
69. Dowdy DW, Basu S, Andrews JR. Is passive diagnosis enough?: the impact of subclinical disease on diagnostic strategies for tuberculosis. Am J Respir Crit Care Med. (2013) 187:543–51. doi: 10.1164/rccm.201207-1217OC
70. Katende B, Bresser M, Kamele M, Chere L, Tlahali M, Erhardt RM, et al. Impact of a multi-disease integrated screening and diagnostic model for COVID-19, TB, and HIV in Lesotho. PLoS Glob Public Health. (2023) 3:e0001488. doi: 10.1371/journal.pgph.0001488
71. Semitala FC, Chaisson LH, Dowdy DW, Armstrong DT, Opira B, Aman K, et al. Tuberculosis screening improves preventive therapy uptake (TB SCRIPT) trial among people living with HIV in Uganda: a study protocol of an individual randomized controlled trial. Trials. (2022) 23:399. doi: 10.1186/s13063-022-06371-0
72. Basir MS, Habib SS, Zaidi SMA, Khowaja S, Hussain H, Ferrand RA, et al. Operationalization of bi-directional screening for tuberculosis and diabetes in private sector healthcare clinics in Karachi, Pakistan. BMC Health Serv Res. (2019) 19:147. doi: 10.1186/s12913-019-3975-7
73. Zawedde-Muyanja S, Nakanwagi A, Dongo JP, Sekadde MP, Nyinoburyo R, Ssentongo G, et al. Decentralisation of child tuberculosis services increases case finding and uptake of preventive therapy in Uganda. Int J Tuberc Lung Dis. (2018) 22:1314–21. doi: 10.5588/ijtld.18.0025
74. Chang W, Chamie G, Mwai D, Clark TD, Thirumurthy H, Charlebois ED, et al. Implementation and operational research: cost and efficiency of a hybrid mobile multidisease testing approach with high HIV testing coverage in East Africa. J Acquir Immune Defic Syndr. (2016) 73:e39–45. doi: 10.1097/QAI.0000000000001141
75. Grandjean L, Gilman RH, Martin L, Soto E, Castro B, Lopez S, et al. Transmission of multidrug-resistant and drug-susceptible tuberculosis within households: a Prospective Cohort Study. PLoS Med. (2015) 12:e1001843. doi: 10.1371/journal.pmed.1001843
76. Kendall EA, Fofana MO, Dowdy DW. The burden of transmitted multi-drug resistance among epidemics of tuberculosis: a transmission model. Lancet Respir Med. (2015) 3:963–72. doi: 10.1596/978-1-4648-0524-0_ch11
77. Shah NS, Auld SC, Brust JCM, Mathema B, Ismail N, Moodley P, et al. Transmission of extensively drug-resistant tuberculosis in South Africa. N Engl J Med. (2017) 376:243–53. doi: 10.1056/NEJMoa1604544
Keywords: Mycobacterium tuberculosis, TB, latent infection, TPT, LTBI, active case finding, systematic screening, prevention
Citation: Coleman M, Nguyen T-A, Luu BK, Hill J, Ragonnet R, Trauer JM, Fox GJ, Marks GB and Marais BJ (2023) Finding and treating both tuberculosis disease and latent infection during population-wide active case finding for tuberculosis elimination. Front. Med. 10:1275140. doi: 10.3389/fmed.2023.1275140
Received: 09 August 2023; Accepted: 02 October 2023;
Published: 16 October 2023.
Edited by:
Miguel Santin, Bellvitge University Hospital, SpainReviewed by:
Nicole Kruh-Garcia, Colorado State University, United StatesCopyright © 2023 Coleman, Nguyen, Luu, Hill, Ragonnet, Trauer, Fox, Marks and Marais. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mikaela Coleman, bWlrYWVsYS5jb2xlbWFuQHN5ZG5leS5lZHUuYXU=; Thu-Anh Nguyen, dGh1YW5oLm5ndXllbkBzeWRuZXkuZWR1LmF1
†These authors have contributed equally to this work and share first authorship
 Mikaela Coleman
Mikaela Coleman Thu-Anh Nguyen
Thu-Anh Nguyen Boi Khanh Luu3
Boi Khanh Luu3 Romain Ragonnet
Romain Ragonnet Ben J. Marais
Ben J. Marais