- 1Goethe University Frankfurt, University Hospital, Medical Clinic 1, Frankfurt, Germany
- 2Department of Internal Medicine I, University Hospital of Bonn, Bonn, Germany
- 3Department of Internal Medicine B, University Clinic Münster, Münster, Germany
Background: Inflammatory bowel diseases (IBDs) are often associated with altered liver function tests (LFTs). There is little data on the relationship between abnormal LFT and IBD. Our study aimed to evaluate the prevalence and etiology of elevated LFT in patients with ulcerative colitis (UC) and to determine whether there is an association with clinical and demographic parameters.
Methods: The clinical records of the Gastroenterology Outpatients Clinic at a single center were reviewed and screened for patients with UC from 2005 to 2014. In total, 263 patients were included. Patients with Crohn’s disease (CD), colitis indeterminate, and colitis of other origins were excluded. Abnormal LFT and liver injuries were analyzed.
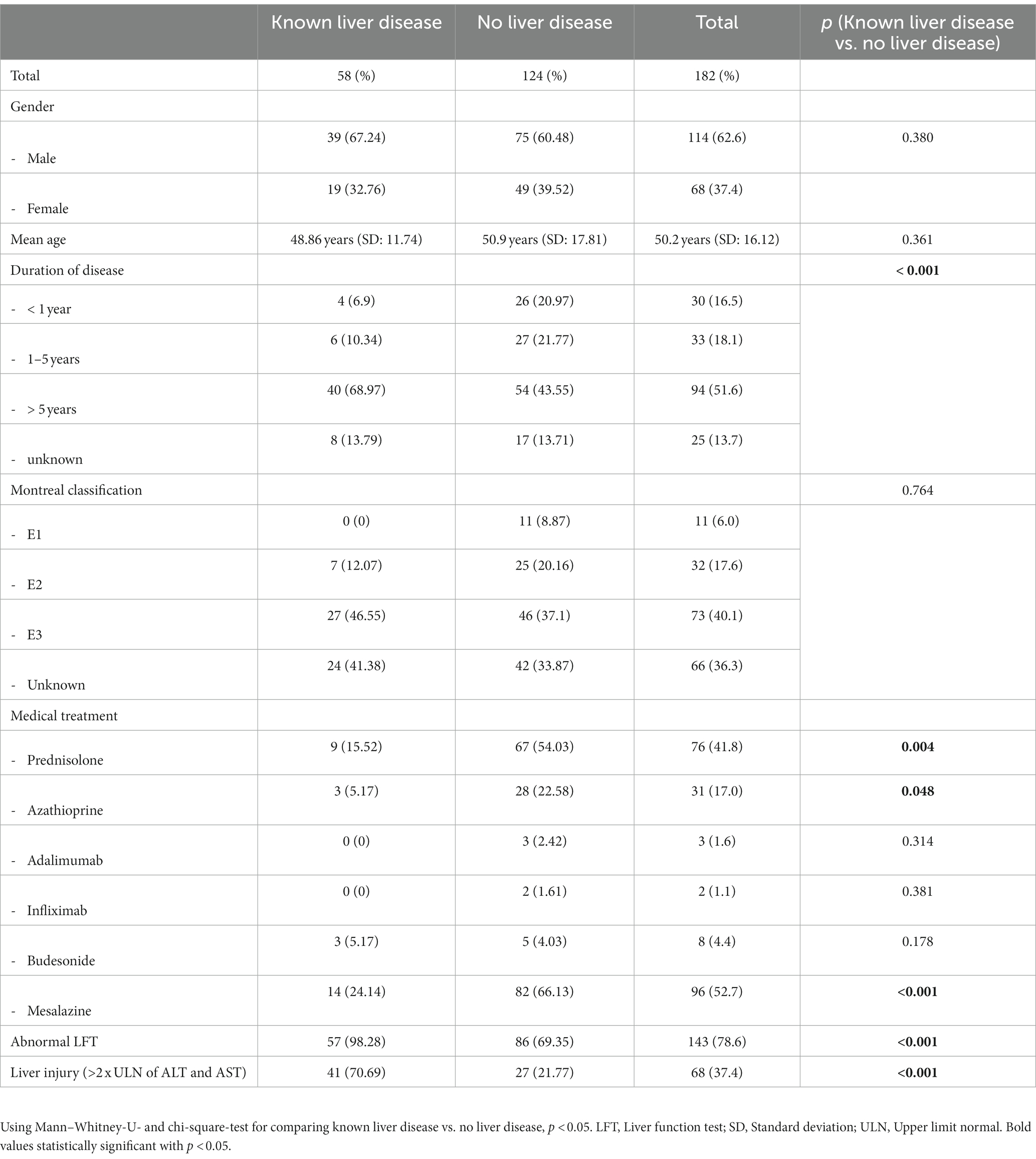
Results: A cohort of 182 patients was analyzed (114 males, 68 females; mean age = 50.2 ± 16.1 years). 58 patients had already been diagnosed with a hepatobiliary disorder. Patients with a known hepatobiliary disorder suffered from UC for a significantly longer duration. Elevated LFT in patients without known hepatobiliary disorders was 69.4%. Liver injury was found in 21.8%. A transient increase in abnormal LFT was shown in 59 patients (68.6%), a persistent increase was found in 27 patients (31.4%). Treatment with thiopurines was a risk factor for persistent elevated LFT (p = 0.029), steroids had a protective impact (p = 0.037).
Conclusion: This study clearly highlights the importance of screening for hepatobiliary disorders and abnormal LFT in patients with UC, as the prevalence of hepatobiliary disorders and abnormal LFT is detected very often among this patient group.
Introduction
Extra-intestinal manifestations in patients with an inflammatory bowel disease (IBD), including hepatobiliary manifestations, are found in more than one-third of patients with IBD (1). Elevated liver function tests (LFTs) in IBD patients have been described in 11–49% of different studies (1–4). Chronic liver disease has even been reported in about 5% of adults with IBD (2). Primary sclerosing cholangitis (PSC) is the most common hepatobiliary manifestation, especially in patients with UC (5–7). Other hepatobiliary disorders, such as steatosis, autoimmune hepatitis (AIH), or primary biliary cholangitis (PBC), are less frequent (6, 7). In particular, the association between UC and PSC is relevant due to the increased risk of developing colorectal cancer and cholangiocarcinoma in very young patients (7–11).
The pathomechanisms behind the development of hepatobiliary disorders in patients with IBD are thought to be mainly immune mediated, but multifactorial genesis is probable. Aside from the altered immune response, drug side effects may also induce hepatobiliary diseases in patients with IBD (3, 12, 13). Besides corticosteroids and aminosalicylates, there are an increasing number of biologicals that can be used for the treatment of UC, for example, the monoclonal antibody Vedolizumab which is used to treat UC and other inflammatory conditions in the gastrointestinal tract (14). Biologicals and mesalazine in particular are potentially hepatotoxic, a consideration that should be taken into account before starting IBD treatment (7, 8). Especially, the tumor-necrosis factor-alpha inhibitor infliximab is well known for a drug-induced liver injury (DILI) (15). Due to these reasons and the suspected high rate of hepatobiliary disorders in patients with IBD, screening for these abnormalities is essential (2).
This study summarizes the most frequent hepatobiliary manifestations and the prevalence of LFT abnormalities in patients with UC followed up in our hands. Furthermore, we analyzed the risk factors or predictors of hepatobiliary disease or abnormalities in LFT in patients with UC.
Materials and methods
This study was based on a retrospective screening of the clinical records of the Gastroenterology Outpatients Clinic at the Department of Internal Medicine I, University of Bonn. Patients with UC were screened in the time period from 2005 until 2014. The diagnosis of UC was determined according to standard clinical, radiological, endoscopic, and histological criteria.2.
In total, we included 263 patients. Patients with CD as well as indeterminate colitis and colitis of other origins were excluded. Additionally, we excluded patients who did not undergo follow-up. We considered a follow-up at three different points of times: (1) The patient appeared in the Gastroenterology Unit (outpatient and inpatient treatment) for the first time. (2) The second follow-up was during the monitoring period, when the liver enzymes were at a maximum. (3) The patient appeared in the Gastroenterology Unit for the last time in that monitoring period.
We used the Montreal Classification of the disease extent of UC to define disease features (1, 16).
An abnormality of LFT was defined as an increase in transaminases [alanine aminotransferase, ALT (=GPT); aspartate aminotransferase, AST (=GOT)], total bilirubin, gamma-glutamyl transpeptidase (GGT), or alkaline phosphatase (AP). We defined liver injury as an increase in ALT and AST of twice the upper limit normal (ULN).
Based on the relevant guidelines, the following methods for diagnosing NASH, alcohol-related liver disease, PBC, PSC, and AIH were used:
Nonalcoholic steatohepatitis was diagnosed if there was no anamnesis of alcohol abuse, a secondary steatosis was ruled out and if there was either an imaging or histological evidence of liver steatosis.
Alcohol related liver damage was diagnosed if there was an anamnesis of alcohol abuse and secondary steatosis was ruled out.
Primary biliary cholangitis was diagnosed if GGT and/or AP were repeatedly elevated, and anti-mitochondriale antibodies (AMA) or typical antinuclear antibodies (ANA) were positive.
Primary sclerosing cholangitis was diagnosed if GGT and/or AP were repeatedly elevated and after performing a MRCP or histological evidence of a PSC. Secondary causes of sclerosing cholangitis had to be excluded.
Autoimmune hepatitis was diagnosed if transaminases were repeatedly elevated, typical autoantibodies were positive and/or if there was histological evidence of an AIH. Viral hepatitis had to be ruled out.
Statistical analysis
Continuous variables were described as means and standard deviations (SDs) and were compared using the Mann–Whitney U test. Categorical variables were shown as numbers with percentages and were compared using the χ2 test (chi-squared test). To compare potentially interacting covariates, a multivariate Cox regression model was used. All tests were two-tailed, and the level of significance was 5%. Statistical analysis was performed with SPSS (IBM SPSS Statistics, version 27).
Ethical considerations
The ethical committee of the University of Bonn approved the study and the principle of a pseudonymized documentation (EK 254/14). All the principles outlined in the Declaration of Helsinki of 1975 have been followed in this retrospective study.
Results
General characteristics
Our study cohort included 263 patients. Patients with CD, indeterminate colitis, colitis of other origin, or no follow-up were excluded (81 patients; 30.8% of the entire cohort). A total of 182 patients with UC (69.2% of the entire cohort) were considered for analysis.
The study included 58 patients with UC and an already diagnosed hepatobiliary disorder and 124 patients without a known hepatobiliary disorder. A total of 114 (62.6%) male and 68 (37.4%) female patients were analyzed. The mean age of the cohort was 50.2 years, with a SD of 16.12 years (Table 1). The median duration of follow-up was 35 months.
Patients with abnormal LFT
In our cohort, the prevalence of elevated LFT in patients without known hepatobiliary disorders was 86 (69.4%). Liver injury, defined as an increase in LFT (ALT, AST) of more than 2-fold ULN (2XULN), was found in 27 (21.8%) patients.
The results showed a significant correlation between the presence of hepatobiliary disorder, abnormal LFT, and liver injury and the time since diagnosis of UC. Patients with known hepatobiliary disorders had a significantly higher rate of abnormal LFT and liver injury (p < 0.001) and a significantly longer duration of UC (p < 0.001). Importantly, there was no significant relationship between the presence of hepatobiliary disorders and the extent of UC at the initial diagnosis.
Furthermore, patients without known hepatobiliary disorders received significantly more often prednisolone, azathioprin, and mesalazine compared to patients with an already known hepatobiliary disorder (prednisolone: p = 0.004, 54.0 vs. 15.5%; azathioprine: p = 0.048, 22.6 vs. 5.2%; and mesalazine: p < 0.001, 66.1 vs. 24.1%; Table 1).
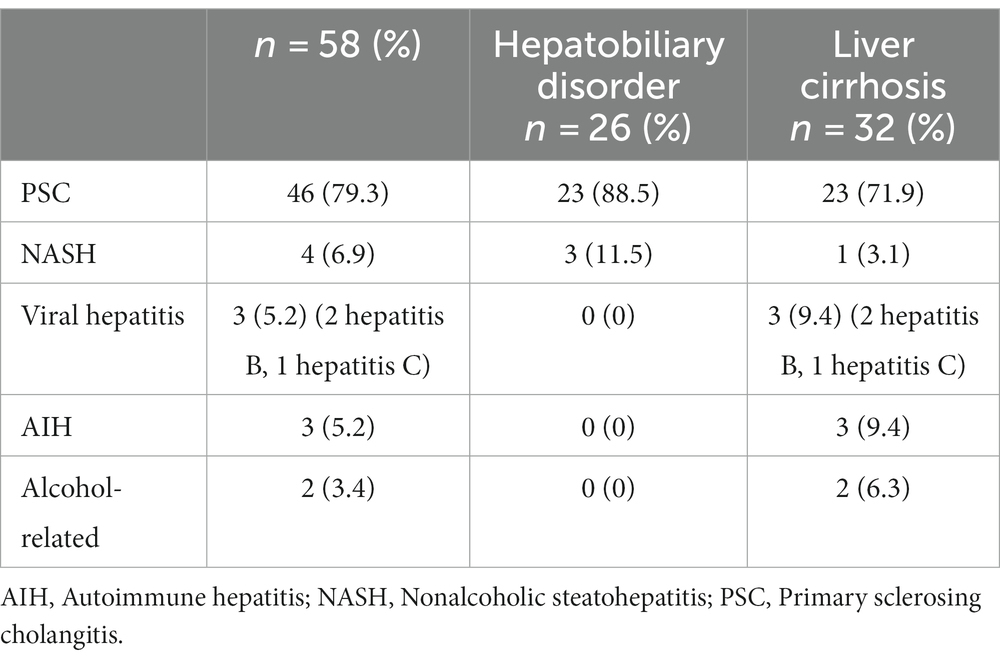
In patients with a diagnosed chronic liver disease, liver cirrhosis was already described in 55% of cases, of which 72% was due to PSC. The main hepatobiliary manifestation in our cohort was PSC (in total, in 79.3% of patients with UC and a chronic liver disease; Table 2). Table 2 shows the etiology of liver diseases in patients with a chronic liver disease.
To further investigate the predictors and risk factors of increased LFT, we focused on patients without known hepatobiliary disorders (Table 3). Specifically, we compared patients with abnormal LFT, liver injury, and normal LFT. There was no significant difference associated with abnormal LFT, liver injury, normal LFT, and gender (p = 0.27), nor was there any significant difference in duration of disease (p = 0.31) or extent of disease (p = 0.96). In addition, we found no significant difference associated with increased LFT (abnormal LFT and liver injury) or medical treatment (Table 3).
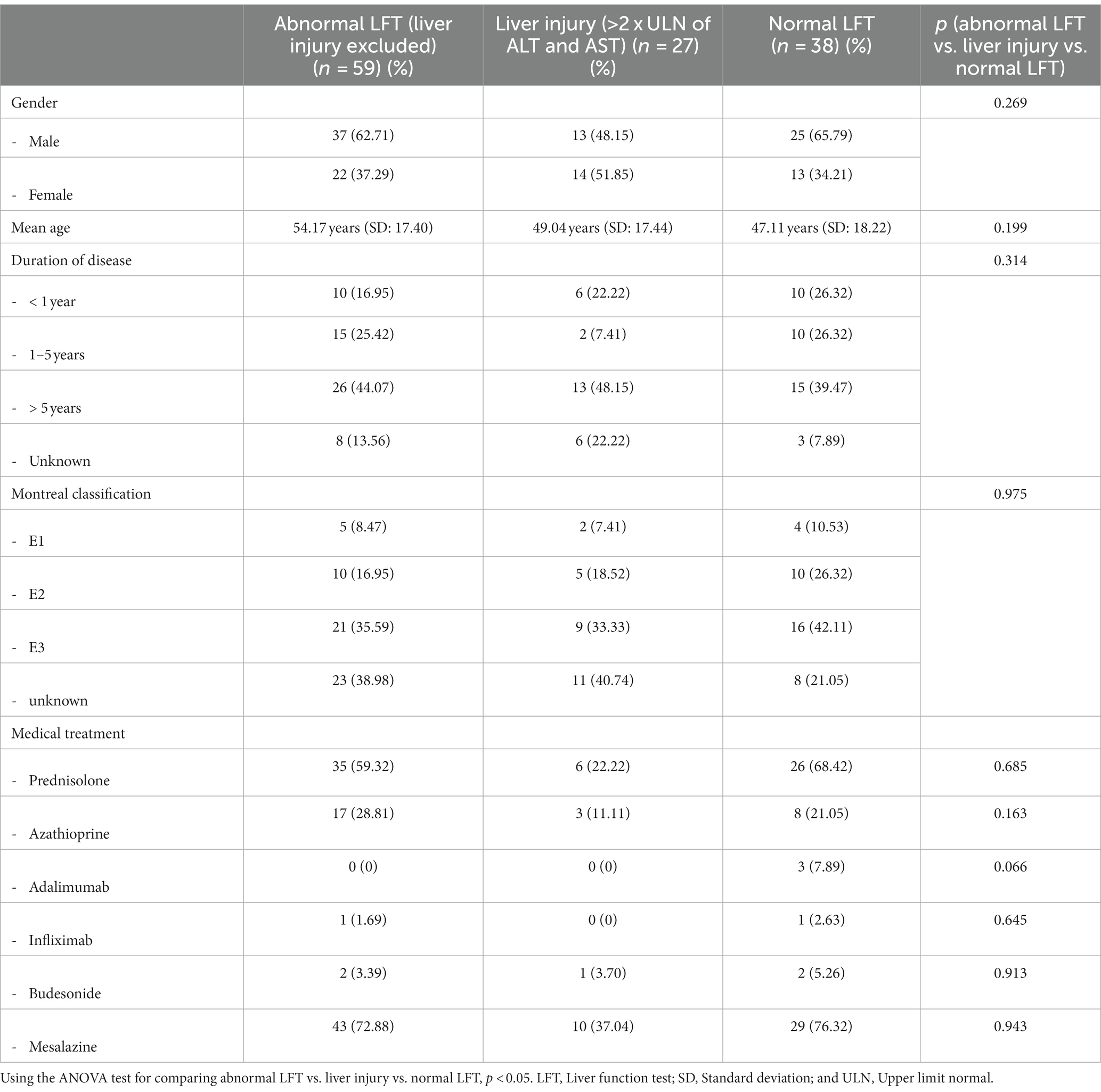
Table 3. Analysis of risk factors and predictors for any abnormal liver function tests in patients without known liver diseases.
Furthermore, we differentiated between transient and persistent increase in LFT (Table 4). We defined a persistent increase as an increase with every follow-up appointment or an increase during the monitoring period plus an increase at the end, at least for one LFT. The rest was defined as a transient increase. A transient increase in abnormal LFT was found in 59 patients (68.6%). A persistent increase was observed in 27 patients (31.4%). Gender, age, duration, and extent of disease did not show any significance. However, taking prednisolone had a protective impact (p = 0.037), while azathioprine was a risk factor for persistent elevated LFT (p = 0.029; Table 4).
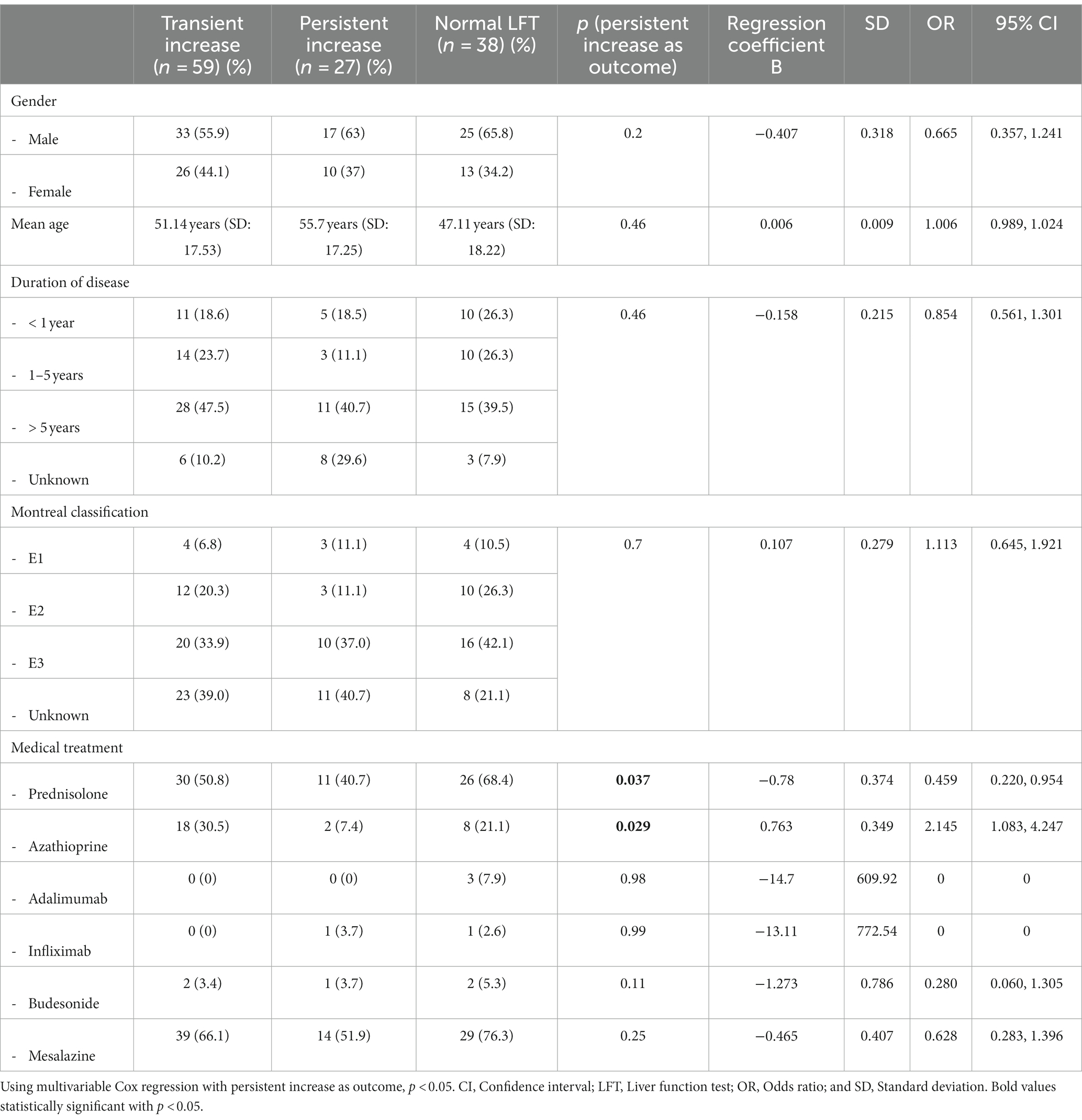
Table 4. Analysis of risk factors and predictors for any abnormal LFT with a transient or persistent increase.
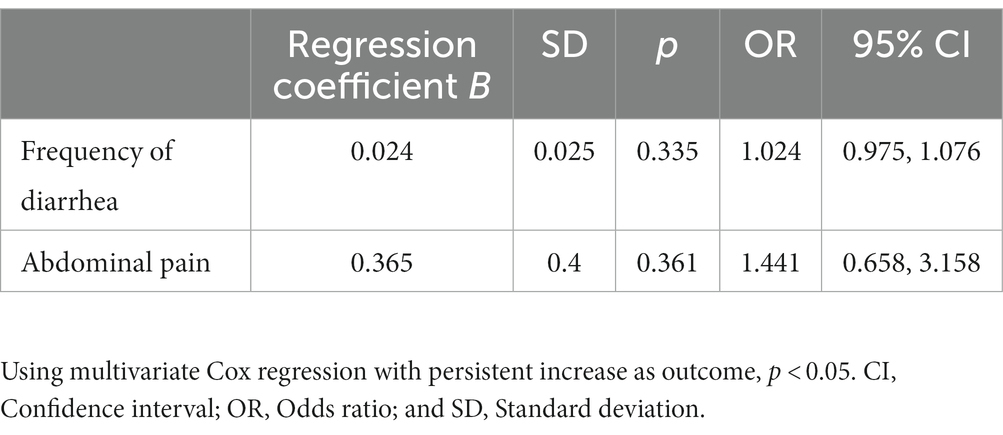
Additionally, we analyzed clinical symptoms, such as the frequency of diarrhea and abdominal pain that may have an influence on persistent elevated LFT. These symptoms are often described by patients with an acute flare-up of UC. In our cohort, there was no significant difference between clinical symptoms and the persistent increase in LFT (Table 5). Here, we also conducted a multivariate analysis using the Cox regression with persistent liver dysfunction as the outcome (Table 5).
Screening algorithm
Since there is a high rate of abnormal LFT and hepatobiliary disorders in patients with UC, screening for a liver dysfunction is essential and should be conducted regularly. We recommend using a special screening algorithm for every patient diagnosed with UC. We designed an algorithm for patients being treated in an outpatient clinic (Figure 1). Mazza et al. (17) designed an algorithm for patients with an IBD having abnormal LFT. We recommend screening for abnormal LFT in every patient with UC every 12 months when the initial assessment is inconspicuous. If there is abnormal LFT, hepatocellular and cholestasis patterns should be differentiated. Triggers, especially hepatotoxicity caused by UC medication, should be evaluated. An imaging procedure is also important. When laboratory assessment, anamneses, and imaging procedures do not offer a clear picture of the disease, we recommend a liver biopsy. A summary of this stepwise approach is shown in Figure 1.
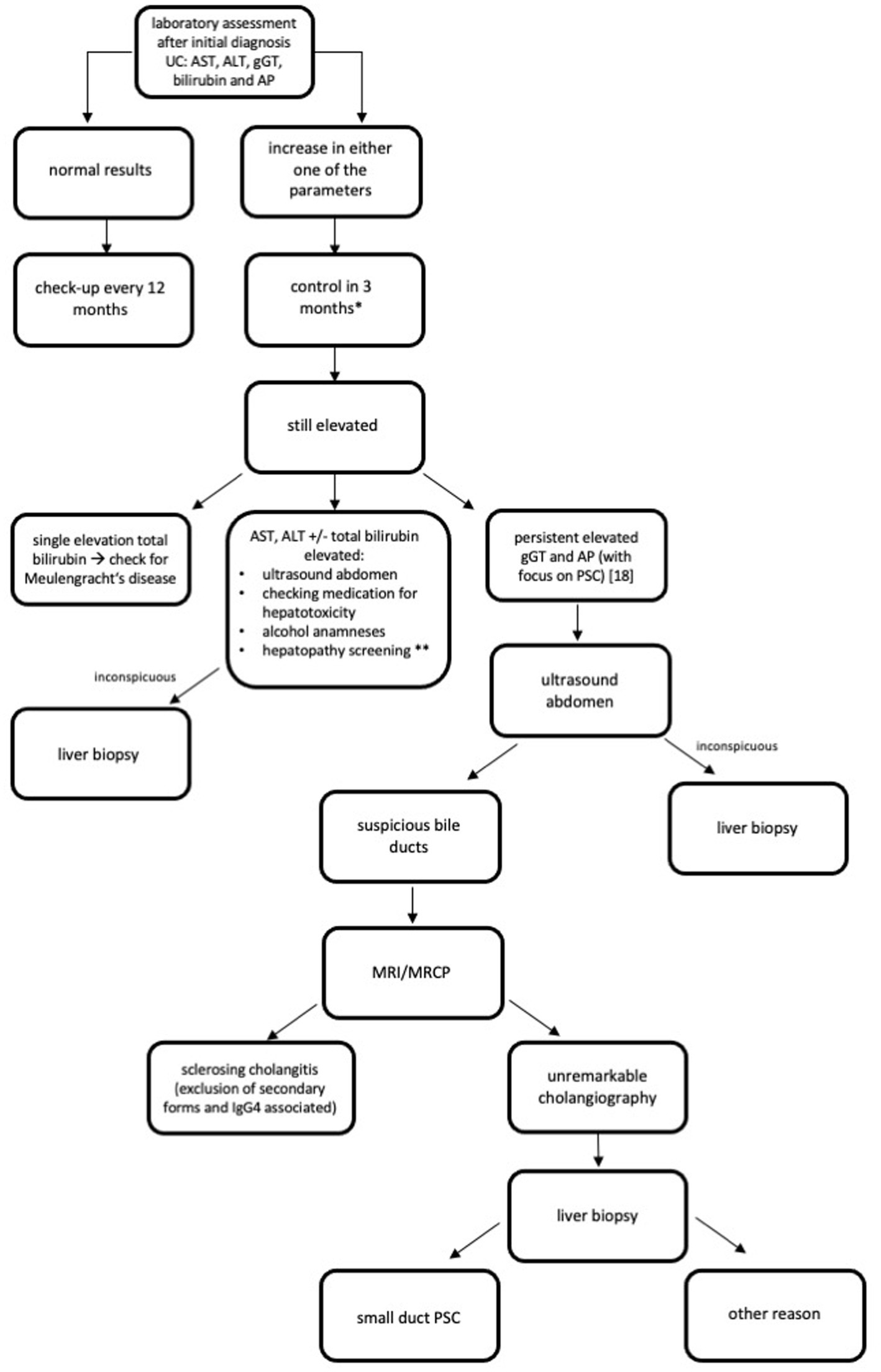
Figure 1. Recommended algorithm for screening of liver diseases after initial diagnosis CU (33). AIH, Autoimmune hepatitis; ALT, Alanine aminotransferase; AP, Alkaline phosphatase; AST, Aspartate aminotransferase; gGT, Gamma-glutamyl transpeptidase; HEV, Hepatitis E; HIV, Human immunodeficiency virus; HSV, Herpes simplex virus; MRCP, Magnetic resonance cholangiopancreatography; MRI, Magnet resonance imaging; NASH, Nonalcoholic steatohepatitis; PBC, Primary biliary cholangitis; PSC, Primary sclerosing cholangitis; UC, Ulcerative Colitis; ULN, Upper limit normal. *if liver synthesis parameter are affected and transaminases > 2xULN or clinical symptoms as jaundice and confusion appear, please check earlier and think of hospitalization. **Hepatopathy screening: viral hepatitis: hepatitis A, B, and C (perhaps investigate for further infective causes, e.g., HEV, HIV, and HSV); AIH, PSC, and PBC; Hereditary hepatitis as Wilson’s disease, alpha-1-antitrypsin-deficiency, and hemochromatosis; and NASH.
Discussion
Several studies have described elevated LFT in patients with an IBD (2, 18, 19). The prevalence of elevated LFT can be different in these studies depending on the criteria used to define abnormal LFT.
In our cohort, 98.3% with UC and an already diagnosed hepatobiliary disorder and 70.7% with a liver injury had abnormal LFT. Abnormal LFT is usually caused by liver dysfunction. However, increased LFT was found in 69.4% with no existing hepatobiliary disorder and 21.8% with liver injuries (Table 1). This underlines the importance of screening for elevated LFT regularly in patients with UC, even if there is no known proven predisposition for a liver dysfunction. This supports the hypothesis that UC and the resulting inflammation could be factors in the onset of liver damage.
Patients with a known hepatobiliary disorder had suffered UC for significantly longer than patients without (p < 0.001; Table 1). A multicenter study by Riegler et al. (19) showed that diffuse liver damage without a history of liver disease in patients with IBD (also CD and UC) is not rare. Damage was shown to correlate with the duration of the disease, to the activity index of the disease, and treatment with steroids and mesalazine (19). Therefore, we assume the longer patients suffer from UC, the higher the risk for liver damage. Multiple reasons for this hypothesis may be considered. For example, medical treatment, altered immune responses in patients with UC, duration of inflammation, and age at initial diagnosis are all factors that could increase the risk of liver damage. But further prospective studies are needed for confirmation.
In contrast to study of Riegler et al. (19), our analyses did not show any correlations between abnormal LFT or liver injury in patients without a hepatobiliary disorder and the duration of the disease or special medical treatments (Table 3). We explain this difference by highlighting that there was missing information in our data set and that Riegler et al. (19) used different investigation methods in their analysis. Due to missing data, we considered only laboratory parameters for our analysis. However, given the prevalence of elevated LFT in our cohort, we still consider regular testing to be essential. However, further prospective studies are needed to confirm this recommendation.
The most common hepatobiliary manifestation is PSC. Steatosis, AIH, or PBC are less common (6, 7). In our study, 58 patients already had an existing hepatobiliary disorder. In addition, PSC was the most frequent hepatobiliary disorder in our cohort as well. In 23 of the 46 patients with PSC, PSC-associated liver cirrhosis had already been diagnosed. Nonalcoholic steatohepatitis was found in a low proportion. And a viral hepatitis was found in a neglectable number (Table 2). Among patients with UC, about 4–7% develop PSC (20, 21). The difference between prior results and our data might be explained by the specialization of Bonn’s Gastroenterology Unit, which focuses on hepatologic diseases and not on patients with an IBD.
In our study, the prevalence of elevated LFT in patients without a known hepatobiliary disorder was higher than in other studies (69.4 vs. 11–49%; Table 1) (2, 18, 19). An analysis by Schrumpf et al. (22) described hepatobiliary complications in 3–15%. One reason for the different results might be, again, the specialization of Bonn’s Gastroenterology Unit. Other reasons may be the difference in the definition of abnormal LFT among different studies, as well as the different diagnostic methods used. We defined abnormal LFT as an increase in transaminases, total bilirubin, GGT, or AP. In 69.4% of patients, elevated LFT was measured at least once at some time during the follow-up period.
In our cohort, transient elevations in LFT (abnormal LFT and liver injury) were detected in 68.6%. Persistent elevations were found in 31.4% of the patients (Table 4). A study by Cappello et al. (3) showed transient elevations in LFT returning to normal levels within 3 months in 62.9% of patients. Persistent elevations were detected in 37.1% (3). The results of a large Swedish follow-up study were similar to the results of the study by Cappello et al. (23). In our study, the average time period of the follow-up was 35 months. Perhaps due to the longer follow-up period, more cases of abnormal LFT returning to normal could be found. The cause of transient abnormal LFT could not be evaluated in our study due to missing data.
In our study, azathioprine was a risk factor for persistent elevated LFT, while prednisolone had a protective impact on LFT (Table 4). Thiopurines are known to contribute to liver toxicity in a dose-dependent or dose-independent manner. Many IBD therapies, e.g., infliximab may cause liver toxicity, so DILI is a relevant issue for patients with IBD. Thiopurines, methotrexate, and infliximab are mostly associated with DILI (15, 17, 24, 25). Steroids (in our study, prednisolone) had a protective impact. Steroids are used in IBD treatment and the management of hepatobiliary disorders in IBD (e.g., in patients with AIH or IgG4-related cholangitis) (26). The protective impact of prednisolone could be explained by a suppression of immune activation contributing to further immune-mediated complications. However, as steroids could also cause hepatic complications, such as the reactivation of HBV and steatohepatitis (26), a careful use is still essential. Additionally, the protective effect of prednisone might reflect a not yet diagnosed autoimmune hepatitis partially treated. For future investigation, an AIH score could consider the response to therapy.
These results again underline the importance of regularly monitoring liver function, especially when patients are undergoing certain medical treatments.
Additionally, we assessed clinical symptoms, such as the frequency of diarrhea and abdominal pain. In our cohort, we could not find a correlation between these clinical symptoms and a persistent increase in LFT (Table 5). This could lead to the assumption that clinical parameters do not have an influence on LFT. Due to the subjectivity of these parameters, we assume that they could cause a mismatch and do not reflect the activity index in UC patients. Endoscopic scores, such as the Mayo Clinic Endoscopic Subscore (MAYO Score) and the UC Endoscopic Index of Severity (UCEIS) score, or the fecal calprotectin (fCP) are valuable parameters for determining the activity index in patients with UC (27–32). Due to missing data in our cohort, we could not take these into account.
The most important limitation of our study is its retrospective design. Due to the specialization of Bonn’s Gastroenterology Unit, a selection bias may be another important limitation as hepatobiliary disorders may be overrepresented. Moreover, there were some missing data that could have affected the analysis.
Since in our study, LFT was frequently found in patients with IBD without clear causality, this study clearly highlights the importance of screening for hepatobiliary disorders and abnormal LFT in patients with IBD, as well as careful selection of treatment. Especially laboratory assessments that should be conducted regularly for each patient. We recommend laboratory assessments for ALT, AST, GGT, AP, and bilirubin every 3–6 months. Additionally, further examinations, such as ultrasound of the abdomen and magnetic resonance imaging (MRI), are important for the screening of hepatologic changes. But in our opinion, our study serves as an upfront reference and basis for further prospective investigations.
We found a correlation between the duration of UC and the occurrence of a hepatobiliary disease. Patients with a longer duration of UC had a hepatobiliary disorder significantly more often. Therefore, we recommend that patients with a longer duration of UC be screened for hepatologic changes more often.
We hypothesize that screening for hepatobiliary disorders is necessary for patients with UC; therefore, we recommend using the special screening algorithm designed by us after the initial diagnosis of UC (Figure 1).
Since hepatobiliary disorders are often found in patients with UC, prospective studies evaluating the risk factors and predictors of hepatobiliary disorders are needed for patients with IBD. Finding potential risk factors and predictors could enhance preventive measures and enhance care for patients with IBD.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the ethical committee of the University of Bonn approved the study and the principle of a pseudonymized documentation (EK 254/14). All the principles outlined in the Declaration of Helsinki of 1975 have been followed in this retrospective study. The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from a by-product of routine care or industry. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
KS: Conzeptualization, Data curation, Formal analysis, Visualization, Writing - original draft. SA: Data curation, Writing – original draft. WG: Formal Analysis, Writing – review & editing. DH: Writing – review & editing. US: Writing – review & editing. BT: Writing – review & editing. CS: Writing – review & editing. RV: Writing – review & editing. IB: Conceptualization, Writing – review & editing. JT: Conceptualization, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ott, C , and Scholmerich, J . Extraintestinal manifestations and complications in IBD. Nat Rev Gastroenterol Hepatol. (2013) 10:585–95. doi: 10.1038/nrgastro.2013.117
2. Mendes, FD , Levy, C , Enders, FB , Loftus, EV , Angulo, P , and Lindor, KD . Abnormal hepatic biochemistries in patients with inflammatory bowel disease. Am J Gastroenterol. (2007) 102:344–50. doi: 10.1111/j.1572-0241.2006.00947.x
3. Cappello, M , Randazzo, C , Bravatà, I , Licata, A , Peralta, S , Craxì, A, et al. Liver function test abnormalities in patients with inflammatory bowel diseases: a hospital-based survey. Clin Med Insights Gastroenterol. (2014) 7:CGast.S13125. doi: 10.4137/CGast.S13125
4. Núñez, FP , Quera, R , Bay, C , Castro, F , and Mezzano, G . Drug-induced liver injury used in the treatment of inflammatory bowel disease. J Crohns Colitis. (2022) 16:1168–76. doi: 10.1093/ecco-jcc/jjac013
5. Smith, MP , and Loe, RH . Sclerosing cholangitis. Review of recent case reports and associated diseases and four new cases. Am J Surg. (1965) 110:519–26. doi: 10.1016/0002-9610(65)90018-8
6. Yarur, AJ , Czul, F , and Levy, C . Hepatobiliary manifestations of inflammatory bowel disease. Inflamm Bowel Dis. (2014) 20:1655–67. doi: 10.1097/MIB.0000000000000065
7. Fousekis, FS , Theopistos, VI , Katsanos, KH , Tsianos, EV , and Christodoulou, DK . Hepatobiliary manifestations and complications in inflammatory bowel disease: a review. Gastroenterol Res. (2018) 11:83–94. doi: 10.14740/gr990w
8. Losurdo, G , Brescia, IV , Lillo, C , Mezzapesa, M , Barone, M , Principi, M, et al. Liver involvement in inflammatory bowel disease: what should the clinician know? World J Hepatol. (2021) 13:1534–51. doi: 10.4254/wjh.v13.i11.1534
9. Zheng, HH , and Jiang, XL . Increased risk of colorectal neoplasia in patients with primary sclerosing cholangitis and inflammatory bowel disease: a meta-analysis of 16 observational studies. Eur J Gastroenterol Hepatol. (2016) 28:383–90. doi: 10.1097/MEG.0000000000000576
10. Ngu, JH , Gearry, RB , Wright, AJ , and Stedman, CAM . Inflammatory bowel disease is associated with poor outcomes of patients with primary sclerosing cholangitis. Clin Gastroenterol Hepatol. (2011) 9:1092–7. doi: 10.1016/j.cgh.2011.08.027
11. Navaneethan, U , Venkatesh, PGK , Lashner, BA , Shen, B , and Kiran, RP . The impact of ulcerative colitis on the long-term outcome of patients with primary sclerosing cholangitis. Aliment Pharmacol Ther. (2012) 35:1045–53. doi: 10.1111/j.1365-2036.2012.05063.x
12. Navaneethan, U , and Shen, B . Hepatopancreatobiliary manifestations and complications associated with inflammatory bowel disease. Inflamm Bowel Dis. (2010) 16:1598–619. doi: 10.1002/ibd.21219
13. Björnsson, ES , Gu, J , Kleiner, DE , Chalasani, N , Hayashi, PH , and Hoofnagle, JH . Azathioprine and 6-Mercaptopurine-induced liver injury. J Clin Gastroenterol. (2017) 51:63–9. doi: 10.1097/MCG.0000000000000568
14. Li, ACW , Dong, C , Tay, ST , Ananthakrishnan, A , and Ma, KSK . Vedolizumab for acute gastrointestinal graft-versus-host disease: a systematic review and meta-analysis. Front Immunol. (2022) 13:1025350. doi: 10.3389/fimmu.2022.1025350
15. Björnsson, HK , Gudbjornsson, B , and Björnsson, ES . Infliximab-induced liver injury: clinical phenotypes, autoimmunity and the role of corticosteroid treatment. J Hepatol. (2022) 76:86–92. doi: 10.1016/j.jhep.2021.08.024
16. Satsangi, J , Silverberg, MS , Vermeire, S , and Colombel, JF . The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. (2006) 55:749–53. doi: 10.1136/gut.2005.082909
17. Mazza, S , Soro, S , Verga, MC , Elvo, B , Ferretti, F , Cereatti, F, et al. Liver-side of inflammatory bowel diseases: hepatobiliary and drug-induced disorders. World J Hepatol. (2021) 13:1828–49. doi: 10.4254/wjh.v13.i12.1828
18. Heikius, B , Niemelä, S , Lehtola, J , Karttunen, T , and Lähde, S . Hepatobiliary and coexisting pancreatic duct abnormalities in patients with inflammatory bowel disease. Scand J Gastroenterol. (1997) 32:153–61. doi: 10.3109/00365529709000186
19. Riegler, G , D’Incà, R , Sturniolo, GC , Corrao, G , Del Vecchio, BC , Di Leo, V, et al. Hepatobiliary alterations in patients with inflammatory bowel disease: a multicenter study. Scand J Gastroenterol. (1998) 33:93–8.
20. Rojas-Feria, M , Castro, M , Suárez, E , Ampuero, J , and Romero-Gómez, M . Hepatobiliary manifestations in inflammatory bowel disease: the gut, the drugs and the liver. World J Gastroenterol. (2013) 19:7327–40. doi: 10.3748/wjg.v19.i42.7327
21. Broomé, U , and Bergquist, A . Primary sclerosing cholangitis, inflammatory bowel disease, and colon cancer. Semin Liver Dis. (2006) 26:031–41. doi: 10.1055/s-2006-933561
22. Schrumpf, E , Fausa, O , Elgjo, K , and Kolmannskog, F . Hepatobiliary complications of inflammatory bowel disease. Semin Liver Dis. (1988) 8:201–9. doi: 10.1055/s-2008-1040541
23. Broomé, U , Glaumann, H , Hellers, G , Nilsson, B , Sörstad, J , and Hultcrantz, R . Liver disease in ulcerative colitis: an epidemiological and follow up study in the county of Stockholm. Gut. (1994) 35:84–9. doi: 10.1136/gut.35.1.84
24. Tran-Minh, ML , Sousa, P , Maillet, M , Allez, M , and Gornet, JM . Hepatic complications induced by immunosuppressants and biologics in inflammatory bowel disease. World J Hepatol. (2017) 9:613. doi: 10.4254/wjh.v9.i13.613
25. Bastida, G , Nos, P , Aguas, M , Beltrán, B , Rubín, Á , Dasí, F, et al. Incidence, risk factors and clinical course of thiopurine-induced liver injury in patients with inflammatory bowel disease. Aliment Pharmacol Ther. (2005) 22:775–82. doi: 10.1111/j.1365-2036.2005.02636.x
26. Yaccob, A , and Mari, A . Practical clinical approach to the evaluation of hepatobiliary disorders in inflammatory bowel disease. Frontline Gastroenterol. (2019) 10:309–15. doi: 10.1136/flgastro-2018-101037
27. Travis, SPL , Schnell, D , Krzeski, P , Abreu, MT , Altman, DG , Colombel, JF, et al. Developing an instrument to assess the endoscopic severity of ulcerative colitis: the ulcerative colitis endoscopic index of severity (UCEIS). Gut. (2012) 61:535–42. doi: 10.1136/gutjnl-2011-300486
28. Schroeder, KW , Tremaine, WJ , and Ilstrup, DM . Coated Oral 5-Aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. N Engl J Med. (1987) 317:1625–9. doi: 10.1056/NEJM198712243172603
29. Roseth, AG , Aadland, E , and Grzyb, K . Normalization of faecal calprotectin: a predictor of mucosal healing in patients with inflammatory bowel disease. Scand J Gastroenterol. (2004) 39:1017–20. doi: 10.1080/00365520410007971
30. Schoepfer, AM , Beglinger, C , Straumann, A , Trummler, M , Renzulli, P , and Seibold, F . Ulcerative colitis:correlation of the Rachmilewitz endoscopic activity index with fecal calpro- tectin, clinical activity, C-reactive protein, and blood leukocytes. Inflamm Bowel Dis. (2009) 15:1851–8. doi: 10.1002/ibd.20986
31. Mao, R , Xiao, Y , Gao, X , Chen, BL , He, Y , Yang, L, et al. Fecal calprotectin in predicting relapse of inflammatory bowel diseases: a meta-analysis of prospective studies. Inflamm Bowel Dis. (2012) 18:1894–9. doi: 10.1002/ibd.22861
32. Lin, JF , Chen, JM , Zuo, JH , Yu, A , Xiao, ZJ , Deng, FH, et al. Meta-analysis: fecal calprotectin for assessment of inflammatory bowel disease activity. Inflamm Bowel Dis. (2014) 20:1407–15. doi: 10.1097/MIB.0000000000000057
33. Schramm, CS (2017). Practice guideline autoimmune liver diseases. AWMF. Available at: https://www.awmf.org/uploads/tx_szleitlinien/021-027l_S2k_Autoimmune_Lebererkrankungen_2017-11.pdf
Glossary
Keywords: inflammatory bowel disease, ulcerative colitis, hepatobiliary disorder, liver function test (LFT), risk factor
Citation: Stratmann K, Aydolmus S, Gu W, Heling D, Spengler U, Terjung B, Strassburg CP, Vollenberg R, Blumenstein I and Trebicka J (2024) Hepatobiliary manifestations in patients with ulcerative colitis: a retrospective analysis. Front. Med. 10:1273797. doi: 10.3389/fmed.2023.1273797
Edited by:
Pavel Strnad, University Hospital RWTH Aachen, GermanyReviewed by:
Gabriel Mezzano Puentes, Clínica Universidad de Los Andes, ChileKevin Sheng-Kai Ma, Harvard University, United States
Copyright © 2024 Stratmann, Aydolmus, Gu, Heling, Spengler, Terjung, Strassburg, Vollenberg, Blumenstein and Trebicka. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Katharina Stratmann, Sy5TdHJhdG1hbm5AbWVkLnVuaS1mcmFua2Z1cnQuZGU=
†These authors have contributed equally to this work
‡These authors share first authorship
 Katharina Stratmann
Katharina Stratmann Songül Aydolmus2†‡
Songül Aydolmus2†‡ Wenyi Gu
Wenyi Gu Richard Vollenberg
Richard Vollenberg Irina Blumenstein
Irina Blumenstein