
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Med. , 05 December 2023
Sec. Intensive Care Medicine and Anesthesiology
Volume 10 - 2023 | https://doi.org/10.3389/fmed.2023.1271664
Background: Patients often experience shivering after spinal anesthesia. In recent years, more and more studies have compared the efficacy and side effects of intravenous butorphanol and tramadol in the treatment of shivering after spinal anesthesia. Therefore, we conducted a MATE analysis and systematic review to compare the efficacy and side effects of butorphanol vs. tramadol in the treatment of shivering after spinal anesthesia.
Methods: PubMed, Cochrane Library, and Embase databases were searched for randomized controlled trials (RCTs) from inception to 30 December 2022, comparing the effects of butorphanol vs. tramadol for the control of shivering after spinal anesthesia. Data assessment and collection were analyzed using the Review Manager 5.4 software.
Results: Five randomized controlled trials involving 302 adult patients were included in this meta-analysis. The results showed that butorphanol has a shorter time to cease shivering (standardized mean difference (SMD) = −0.53; 95% confidence interval (CI) [−0.89, −0.17], P = 0.004, I2 = 0%), a higher rate of cessation of shivering within 1 min after administering the study drugs (relative risk (RR), 1.69; 95% CI [1.15,2.48], P = 0.008, I2 = 0%), and higher incidences of sedation (RR, 2.98; 95% CI [2.11, 4.21], P < 0.00001, I2 = 0%), compared with tramadol.
Conclusion: In the treatment of shivering after spinal anesthesia, butorphanol has a shorter onset time and a higher rate of cessation of shivering within 1 min after the study drugs were administered than tramadol. Therefore, butorphanol is superior to tramadol in the treatment of shivering after spinal anesthesia.
Spinal anesthesia is widely used in lower abdominal or lower limb surgeries. Shivering is a common complication of spinal anesthesia, with an incidence of approximately 70% (1, 2). Neuraxial (spinal and epidural) anesthesia inhibits vasoconstriction and produces vasodilation, which leads to a rapid loss of heat by redistribution from the core to the periphery. Therefore, the threshold of shivering is reduced (3, 4). There are many reasons for a drop in core temperature, including mental stress, the cold environment of operating rooms, and cold infusion fluids (5, 6). Shivering interferes with the monitoring of vital signs such as pulse rate, blood pressure (BP), and electrocardiography (ECG) (4, 7, 8). It may cause increased wound pain and delayed wound healing, which can prolong the patient's hospital stay (9, 10) and increase intraocular pressure and intracranial pressure (11, 12). Most importantly, it can increase oxygen consumption by 200–600%, and at the same time, increase the production of carbon dioxide linearly (13), leading to hypoxemia. In patients with coronary artery disease, shivering could further compromise myocardial function (14). Therefore, we must promptly control post-anesthetic shivering.
Currently, there are pharmacological and non-pharmacological methods for controlling shivering. The non-pharmacological methods are performed by external heating, such as the use of heaters, blankets, and infusion of warm fluids (15–17). However, some recent studies have shown that non-pharmacological methods, including forced air and warmed fluid, have no significant effect on preventing shivering (18, 19). Therefore, pharmacotherapy is still the main method to control shivering at present.
In the past, tramadol has been widely used in the treatment of shivering after spinal anesthesia (20, 21). It mainly inhibits the synaptic reuptake of norepinephrine and 5-HT (hydroxytryptamine), increasing their concentration outside the neurons, thereby increasing the activity of 5-HT and norepinephrine, and then regulates the monoamine downward inhibitory pathway, resulting in an anti-shivering effect (22–24). However, the main side effects of tramadol are nausea and vomiting (25, 26), which make the patient quite uncomfortable. Currently, some studies have shown that butorphanol has anti-corrosion effects (27). Butorphanol is an opioid receptor agonist-antagonist; it exerts analgesic and anti-shivering effects by activating the K receptor while also partially antagonizing the μ opioid receptor. As a result, it has a minimal impact on respiration and circulation, with only minor adverse reactions (27–29).
The purpose of this study is to conduct a systematic review and meta-analysis to compare the effective rate, the time of onset of action, and the side effects of butorphanol vs. tramadol in the treatment of shivering after spinal anesthesia.
This systematic review is based on the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines for the preparation of the statement of the review. The authors registered the protocol in the International Prospective Register of Systematic Reviews (Registration Number: CRD42022349679; available at https://www.crd.york.ac.uk/prospero/#myprospero).
A systematic literature search was conducted on the PubMed, Cochrane Library, and Embase databases. Each database was searched by two investigators separately. The databases were searched from inception to 30 December 2022. The subject headings used for searching included tramadol, butorphanol, spinal anesthesia, shivering, and combinations of these without limits. Furthermore, the researchers scanned the references to relative articles to find further studies. An example illustration of the search strategy used for PubMed is shown in Appendices 1 and 2 (Supplementary material).
The inclusion criteria for selecting studies were as follows: (1) patients who underwent surgery under spinal anesthesia; (2) the experimental group was given intravenous butorphanol injection, and the control group was given intravenous tramadol; (3) the study was a randomized controlled trial (RCT); (4) patients of American Society of Anesthesiologists Physical Status (ASA) I–III; (5) adult patients.
The exclusion criteria were as follows: (1) retrospective studies, non-randomized and/or randomized literature with incorrect methods; (2) animal studies; (3) case reports. The intensity of shivering was graded on the following scale (30, 31): 0 = no shivering, 1 = no visible muscular activity, but one or more of piloerection, peripheral vasoconstriction, or peripheral (other causes excluded); 2 = visible muscular activity confined to one muscle group; 3 = visible muscular activity in more than one muscle group; 4 = violent muscular activity involving the entire body.
After the studies were selected, two investigators (J-XW and X-CL) independently determined the following primary outcomes and secondary outcomes of shivering treatment (Table 1).
Primary outcomes:
1. Effective rate of shivering treatment: the number of patients who stopped shivering after treatment.
2. Time to cease shivering: the time from administration of intravenous butorphanol or tramadol to the end of the shivering.
3. The rate of cessation of shivering within a certain period of time after giving study drugs: the number of patients who stopped shivering within a certain period of time after taking the study drugs.
4. Recurrent rate of shivering: the number of patients with a recurrence of chills after treatment.
Secondary outcomes:
1. The incidence of nausea.
2. The incidence of vomiting.
3. The incidence of sedation.
First, the titles and abstracts of all retrieved articles were screened, and the full text of the articles that could be included was screened independently by two reviewers (J-XW and S-SZ). The discrepancies were resolved through discussion.
Two investigators (J-XW and Y-QL) independently assessed the risk of bias of the included RCTs based on the Cochrane Manual v5.0.2.10 (37). Each of the following risk-of-bias items was classified as “high risk of bias,” “uncertain risk of bias,” or “low risk of bias”: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other biases. Disputes were resolved by discussion, and if necessary, a third researcher helped make a decision.
Review Manager 5.4 (Cochrane Collaboration, Copenhagen, Denmark) was used to perform all the statistical analyses. For dichotomous data, the Mantel-Haenszel (M-H) method was used to calculate the risk ratio (RR) with a 95% confidence interval (CI). The standardized mean difference was used for continuous variables. The heterogeneity analysis was conducted using the chi-square test, and the heterogeneity of the included studies was assessed using I2. When the I2-values were < 40%, 40–60%, and >60%, the heterogeneity levels corresponded to low, medium, and high (38), respectively. Due to the anticipated heterogeneity in this study, a random-effects model was employed. Sensitivity analyses were performed by removing one study at a time and combining the other studies to assess whether a single study significantly affected the pooled results as well as to find the potential causes of the heterogeneity.
The research flowchart is shown in Figure 1. We have identified five randomized controlled trials, including 302 patients (152 received butorphanol and 150 received tramadol).
In the five selected studies, two (32, 33) compared butorphanol with tramadol, one (35) compared butorphanol with tramadol and clonidine, one (34) compared butorphanol with tramadol and ondansetron, and the remaining one (36) compared butorphanol with tramadol and dexmedetomidine. However, this meta-analysis only compares butorphanol and tramadol, ignoring clonidine, ondansetron, and dexmedetomidine.
The characteristics of the randomized controlled trials (RCTs) are listed in Table 1.
The summary risk of bias for the five selected studies is quite low, as shown in Figure 2.
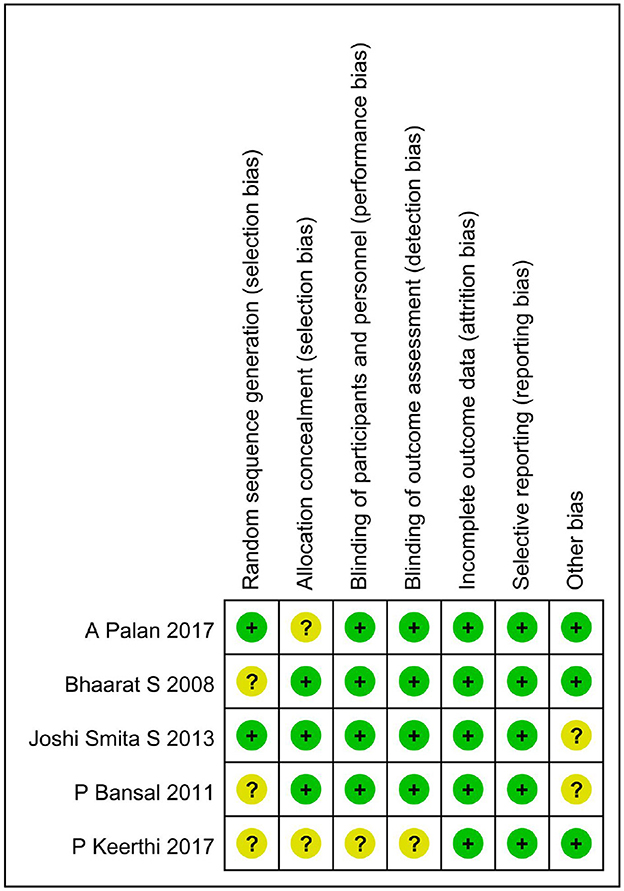
Figure 2. The risk of bias assessment of the included studies. +, low risk of bias; ?, unclear risk of bias; –, high risk of bias.
A total of 5 studies (32–36) involving 302 patients directly compared the effective rate of butorphanol and tramadol for shivering after spinal anesthesia, and all data were available for collection (Figure 3). The effective rate of shivering treatment decreased from 97% in the butorphanol group to 93% in the tramadol group (RR, 1.01; 95% CI [0.98, 1.05], P = 0.48, I2 = 0%). The result showed no significant difference between the butorphanol group and the tramadol group.
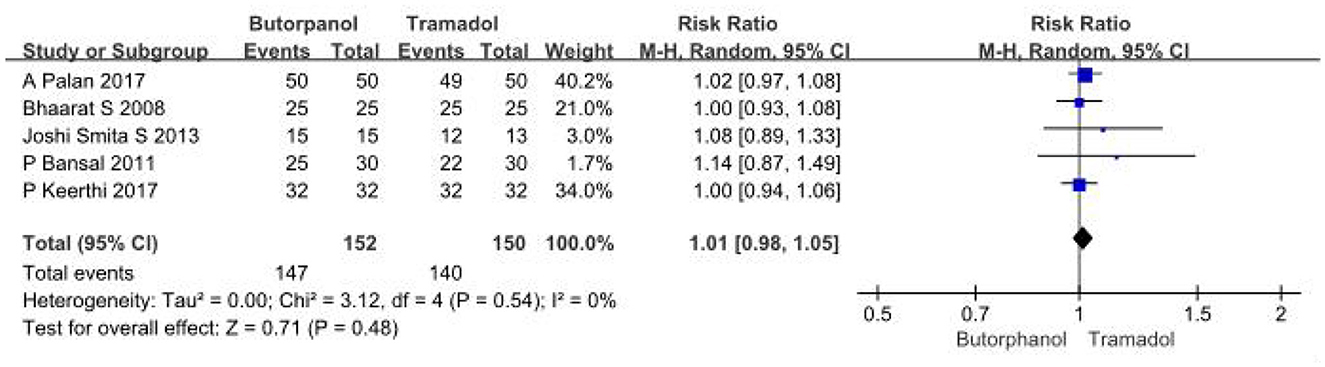
Figure 3. Forest plots of the effective rate of shivering treatment comparing butorphanol with tramadol. CI, confidence interval; M-H, Mantel-Haenszel.
A total of 2 studies (35, 36) reported the time to cease shivering, and data from 2 studies with 124 patients were available for collection (Figure 4). The result showed that butorphanol was associated with a shorter time to stop shivering than tramadol (SMD = −0.53; 95% CI [−0.89, −0.17], P = 0.004, I2 = 0%).
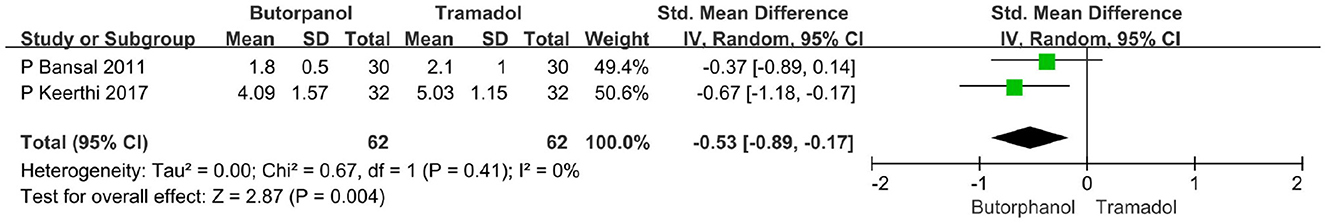
Figure 4. Forest plots of time to cease shivering in minutes comparing butorphanol with tramadol. CI, confidence interval; SMD, standardized mean difference.
A total of two studies (32, 34) reported the rate of cessation of shivering within 1 min after giving the study drugs. Data from two studies with 128 patients were available for collection (Figure 5A). The rate of cessation of shivering within 1 min after giving the study drugs decreased from 60.00% in the butorphanol group to 35% in the tramadol group (RR, 1.69; 95% CI [1.15, 2.48]; P = 0.008, I2 = 0%).
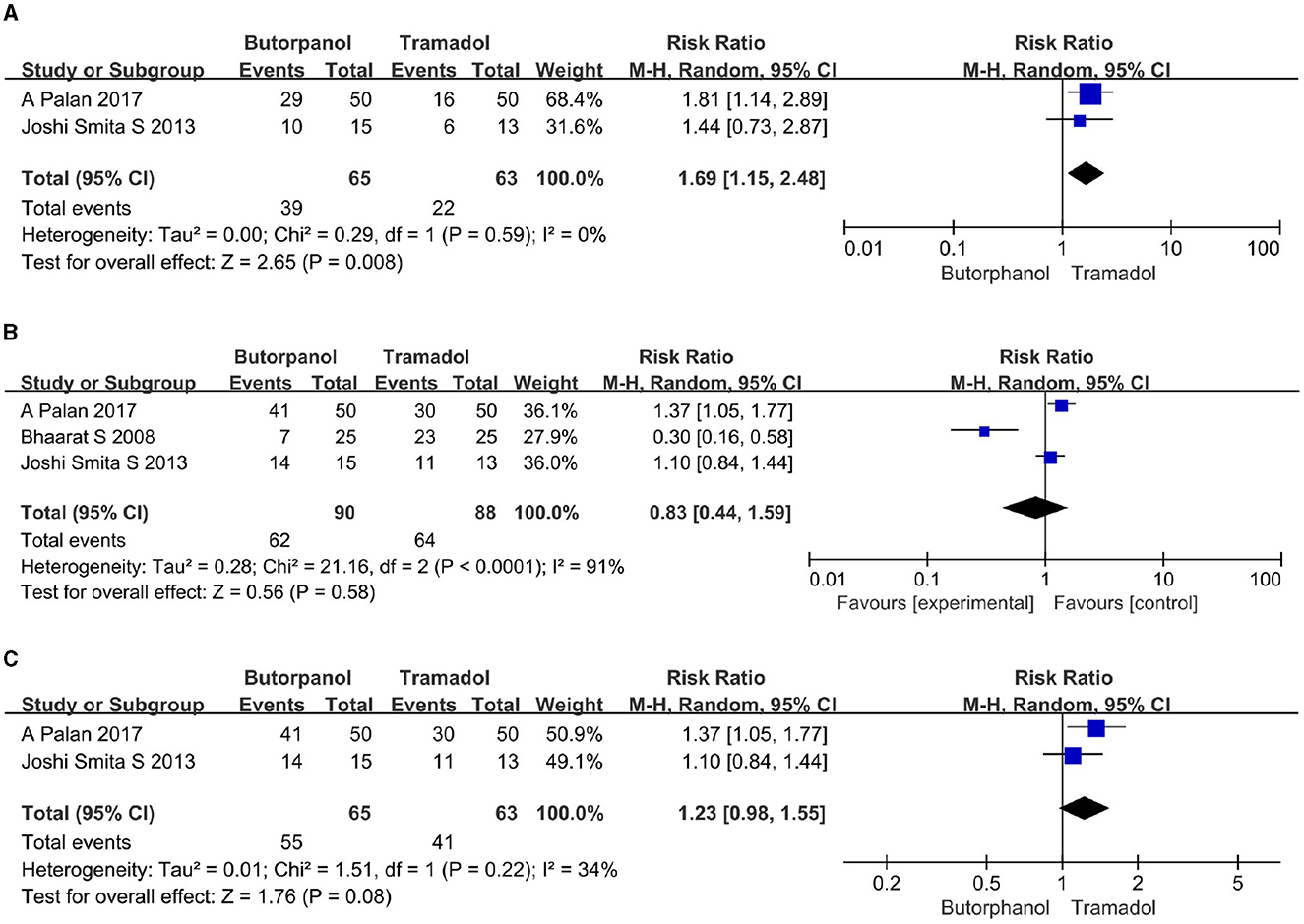
Figure 5. (A) Forest plots of time taken to stop shivering within 1 min comparing butorphanol with tramadol. (B) Forest plots of time taken to stop shivering within 3 min comparing butorphanol with tramadol. (C) Forest plots of time taken to stop shivering within 3 min after sensitivity analysis comparing butorphanol with tramadol. CI, confidence interval; M-H, Mantel-Haenszel.
A total of three studies (32–34) reported the rate of cessation of shivering within 3 min after giving the study drugs. Data from 3 studies with 178 patients were available for collection. The rate of cessation of shivering within 3 min after giving the study drugs increased from 69% in the butorphanol group to 73% in the tramadol group (Figure 5B), but there was not enough for a statistically significant difference (RR, 0.83; 95% CI [0.44, 1.59]; P = 0.58, I2 = 91%). Sensitivity analysis showed that when the study of Maheshwari et al. (33) was removed (Figure 5C), the heterogeneity was significantly reduced (RR, 1.23; 95% CI [0.98, 1.55]; P = 0.08, I2 = 34%). This shows that the study conducted by Maheshwari et al. (33) is the primary source of heterogeneity, and the result is unstable.
A total of five studies (32–36) reported the recurrent rate of shivering. Data from five studies with 302 patients were available for collection (Figure 6). The recurrent rate of shivering decreased from 14% in the butorphanol group to 13% in the tramadol group (RR, 1.26; 95% CI [0.53, 2.99], P = 0.59, I2 = 45%). There was no significant difference between the butorphanol group and the tramadol group.
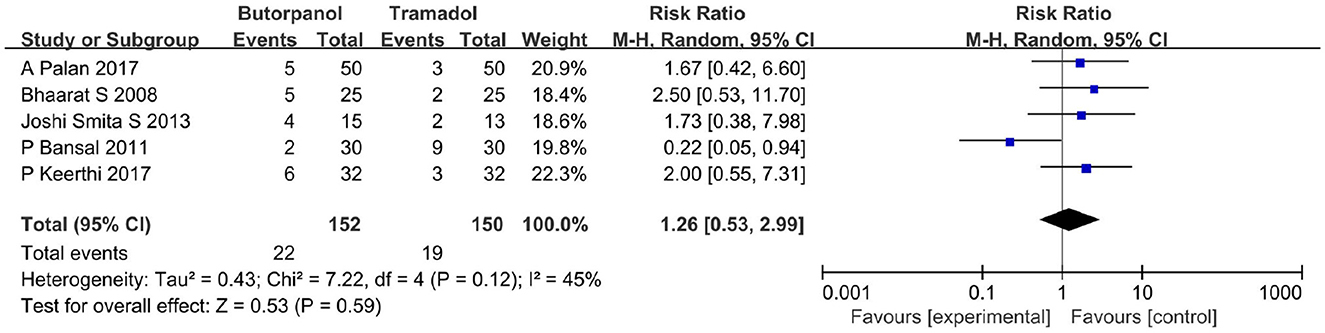
Figure 6. Forest plots of recurrent rates of shivering comparing butorphanol with tramadol. CI, confidence interval; M-H, Mantel-Haenszel.
A total of five studies (32–36) reported the incidence of nausea. Data from five studies with 302 patients were available for collection (Figure 7). The incidence of nausea increased from 8% in the butorphanol group to 21% in the tramadol group (RR, 0.33; 95% CI [0.09, 1.28], P = 0.11, I2 = 67%). The result did not reveal a significant difference between the butorphanol group and the tramadol group. The sensitivity analysis was conducted, and it was determined that the pooled analysis result remained stable and the heterogeneity was still high.

Figure 7. Forest plots of the incidence of nausea comparing butorphanol with tramadol. CI, confidence interval; M-H, Mantel-Haenszel.
A total of five studies (32–36) reported the incidence of vomiting. Data from five studies with 302 patients were available for collection (Figure 8). The incidence of vomiting increased from 4.61% in the butorphanol group to 9% in the tramadol group (RR, 0.68; 95% CI [0.28, 1.69], P = 0.41, I2 = 0%). There was no significant difference between the butorphanol group and the tramadol group.
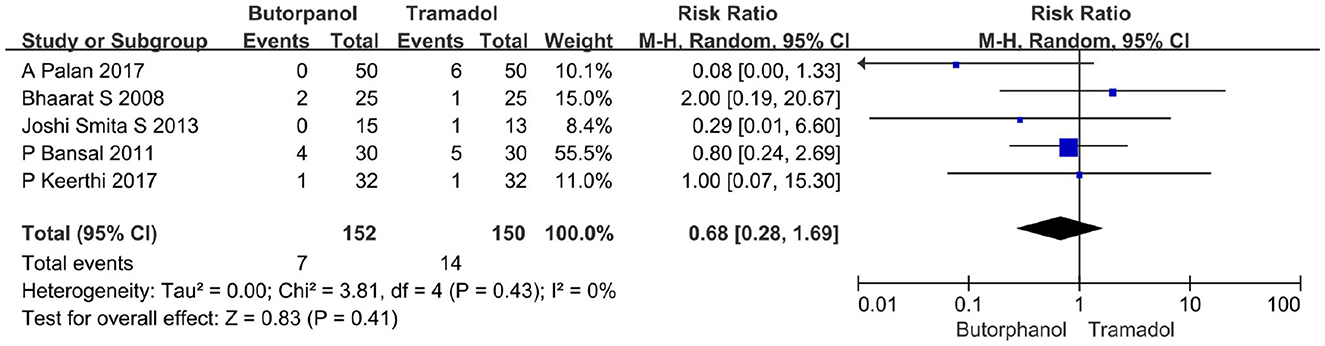
Figure 8. Forest plots of the incidence of vomiting comparing butorphanol with tramadol. CI, confidence interval; M-H, Mantel-Haenszel.
A total of five studies (32–36) reported the incidence of sedation. We defined it as being lethargic and responding to speech or physical stimuli. Data from 5 studies with 302 patients were available for collection (Figure 9). The incidence of sedation decreased from 59% in the butorphanol group to 18% in the tramadol group (RR, 2.98; 95% CI [2.11, 4.21], P < 0.00001, I2 = 0%).

Figure 9. Forest plots of the incidence of sedation comparing butorphanol with tramadol. CI, confidence interval; M-H, Mantel-Haenszel.
In this systematic review and meta-analysis, we compare the efficacy of intravenous butorphanol vs. tramadol for the treatment of chills after spinal anesthesia. Compared with tramadol, butorphanol is associated with a shorter onset time of action, a higher rate of cessation of shivering within 1 min after administering the study drugs, and higher incidences of sedation. Therefore, butorphanol is superior to tramadol in the treatment of chills after spinal anesthesia.
In this meta-analysis, butorphanol has a higher rate of cessation of shivering within 3 min after the study drugs were administered and a lower incidence of nausea than tramadol. However, these outcomes are highly heterogeneous, which may be related to the following factors: (1) The inclusion criteria for the degree of shivering were different among the included studies; two studies (32, 36) used the wrench scale of shivering (30, 31) to grade the intensity of shivering from 0 to 4, and only patients who developed either 2 or 4 shivering were considered for treatment. In two studies (27, 29), the intensity of shivering was scored on a scale of 0–3 (39), and only patients who developed chills of grade 2 or 3 during the perioperative period were treated on an intention-to-treat basis. (2) One study (33) did not mention the type and dosage of local anesthetics for spinal anesthesia, and the dosage of local anesthetics for spinal anesthesia was different among the remaining studies. (3) The type and duration of surgeries were different.
In our study, we found that the butorphanol group (97%) and the tramadol group (93%) were equally effective in controlling shivering, but there was no significant difference (P > 0.05). Maheshwari et al. (33) also found similar results that 100% of patients in the butorphanol group and 100% of patients in the tramadol group were relieved of shivering. This suggests that both butorphanol and tramadol can effectively treat shivering after spinal anesthesia; there is no statistically significant difference in the effective rate of shivering treatment. We also found that the time to cease shivering was significantly shorter with the butorphanol group than with the tramadol group (P = 0.004). These findings were similar to those of other investigators like Bansal and Jain (35) and Keerthi and Kamath (36), Bansal and Jain (35) observed that butorphanol (1.8 ± 0.5 min) acted faster than tramadol (2.1 ± 1.0 min) to cease shivering after spinal anesthesia, and Keerthi and Kamath (32) found that the time to cease shivering was quite less with butorphanol (4.09 ± 1.57 min) than with tramadol (5.03 ± 1.15 min). Therefore, the time to cease shivering in the butorphanol group is shorter than that in the tramadol group. In the present study, we found that the recurrent rate of shivering was higher in the butorphanol group (14%) than in the tramadol group (13%), but the difference was not statistically significant. Keerthi and Kamath (36) observed a higher rate of recurrence with butorphanol (18%) compared with tramadol (9%). In this study, all patients who received the study drugs had a reappearance of shivering after 20 min of treatment, which suggested that additional anti-shivering drugs were required after 20 min of treatment with butorphanol or tramadol.
In our study, the incidence of nausea was significantly lower with butorphanol (8%) than with tramadol (23%). The results were comparable with studies done by Joshi et al. (34). Four patients (31%) in the tramadol group had nausea as compared to none in the butorphanol group. Contrary to our results, Maheshwari et al. (33) found a higher incidence of nausea with butorphanol (28%) compared with tramadol (20%). This may be due to the small sample size of the trial by Maheshwari et al. Therefore, randomized controlled trials with larger samples are needed for further validation. We also found that butorphanol (59%) had a higher incidence of sedation as compared to tramadol (18%), and this was comparable to observations made by Bansal and Jain (35). This suggests that butorphanol has a higher incidence of sedation in the treatment of shivering after spinal anesthesia compared to tramadol, which is due to the sedative effect of butorphanol by activating K receptors (27, 29). Since operations were performed under spinal anesthesia, the higher incidence of sedation was not only conducive to intraoperative management but also conducive to the surgeon's intraoperative operation and could provide good comfort for the patient. In addition, Keerthi and Kamath (36) observed that at 5 min after the administration of the drug, all cases in the butorphanol group stopped shivering, while 50% of cases in the tramadol group still had grade 1 shivering. There was a significant decrease in shivering grades in the butorphanol group. However, there are few relevant studies at present, and a large sample study is needed in the future.
Most importantly, butorphanol and tramadol were safe for the treatment of chills because there were no patients with excessive sedation (excessive sedation was defined as unresponsiveness to sound or tactile stimuli) in all the included studies. Although one study (36) reported that there were two cases of respiratory depression in the butorphanol group, none of them had hypoxemia, probably because all the patients were supplemented with oxygen at the onset of shivering.
There are a few limitations to our meta-analysis. First, due to the different anesthetic drugs, the patient's physical condition in the American Society of ASA was different; there was significant heterogeneity. Second, many details about the sedation level of each patient at the same time point could not be extracted due to the inconsistency of the scale of evaluating sedation and the different times of evaluating sedation. Third, the sample size is relatively small, proportional to the burden of this perioperative problem. In addition, we have not conducted a dose-response study for a single drug, which can describe its anti-shivering properties and the corresponding increase in side effects. In the future, we can do some studies in this area or compare the efficacy of combination drugs to control chills.
In general, compared with tramadol, we found that butorphanol has a shorter onset time and, at the same time, a higher rate of cessation of shivering within 1 min after giving study drugs. Therefore, butorphanol is superior to tramadol in the treatment of shivering after spinal anesthesia.
J-XW: Conceptualization, Data curation, Investigation, Methodology, Writing—original draft, Writing—review & editing. X-CL: Conceptualization, Data curation, Investigation, Methodology, Writing—original draft, Formal analysis, Project administration. S-SZ: Formal analysis, Resources, Software, Supervision, Writing—review & editing. Y-QL: Conceptualization, Data curation, Formal analysis, Funding acquisition, Project administration, Resources, Supervision, Writing—review & editing. F-JW: Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Software, Visualization, Writing—original draft, Writing—review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2023.1271664/full#supplementary-material
1. Gholinataj A, Baradari AG, Najafi S, Kiabi FH. Comparison of intravenous ketamine with intrathecal meperidine in prevention of post-anesthetic shivering after spinal anesthesia for lower limb orthopedic surgeries: a double-blind randomized clinical trial. Ethiop J Health Sci. (2021) 31:1207–14. doi: 10.4314/ejhs.v31i6.16
2. Shen QH, Li HF, Zhou X, Lu Y, Yuan XZ. 5-HT3 receptor antagonists for the prevention of perioperative shivering undergoing spinal anaesthesia: a systematic review and meta-analysis of randomised controlled trials. BMJ Open. (2020) 10:e038293. doi: 10.1136/bmjopen-2020-038293
3. Luggya TS, Kabuye RN, Mijumbi C, Tindimwebwa JB, Kintu A. Prevalence, associated factors and treatment of post spinal shivering in a Sub-Saharan tertiary hospital: a prospective observational study. BMC Anesthesiol. (2016) 16:100. doi: 10.1186/s12871-016-0268-0
4. Tubog TD, Bramble RS. Ondansetron for shivering after spinal anesthesia in cesarean delivery: a systematic review and meta-analysis. J Perianesth Nurs. (2022) 37:105–13. doi: 10.1016/j.jopan.2021.05.007
5. Botros JM, Mahmoud AMS, Ragab SG, Ahmed MAA, Roushdy HMS, Yassin HM, et al. Comparative study between Dexmedetomidine and Ondansteron for prevention of post spinal shivering. A randomized controlled trial. BMC Anesthesiol. (2018) 18:179. doi: 10.1186/s12871-018-0640-3
6. Azizian Z, Hesami Z, Mansouri P, Ebrahimpour A, Attar B, Chalangari R. Skin complications of orthopedic procedures and devices. Iran J Public Health. (2018) 47:1937–44.
7. Kaur H, Kaur S, Gupta KK, Singh A. Comparative evaluation of the intravenous dexmedetomidine and nalbuphine for treatment of post spinal shivering-a randomized prospective trial. Asian J Anesthesiol. (2022) 60:146–54. doi: 10.6859/aja.202212_60(4).0004
8. Moeen SM, Moeen AM. Intrathecal dexamethasone vs. meperidine for prevention of shivering during transurethral prostatectomy: a randomized controlled trial. Acta Anaesthesiol Scand. (2017) 61:749–57. doi: 10.1111/aas.12920
9. Kleimeyer JP, Harris AHS, Sanford J, Maloney WJ, Kadry B, Bishop JA. Incidence and risk factors for postoperative hypothermia after orthopaedic surgery. J Am Acad Orthop Surg. (2018) 26:e497–503. doi: 10.5435/JAAOS-D-16-00742
10. Bajwa SJ, Gupta S, Kaur J, Singh A, Parmar S. Reduction in the incidence of shivering with perioperative dexmedetomidine: a randomized prospective study. J Anaesthesiol Clin Pharmacol. (2012) 28:86–91. doi: 10.4103/0970-9185.92452
11. Lema GF, Gebremedhn EG, Gebregzi AH, Desta YT, Kassa AA. Efficacy of intravenous tramadol and low-dose ketamine in the prevention of post-spinal anesthesia shivering following cesarean section: a double-blinded, randomized control trial. Int J Womens Health. (2017) 9:681–8. doi: 10.2147/IJWH.S139655
12. De Witte J, Sessler DI. Perioperative shivering: physiology and pharmacology. Anesthesiology. (2002) 96:467–84. doi: 10.1097/00000542-200202000-00036
13. Nallam SR, Cherukuru K, Sateesh G. Efficacy of intravenous ondansetron for prevention of postspinal shivering during lower segment cesarean section: a double-blinded randomized trial. Anesth Essays Res. (2017) 11:508–13. doi: 10.4103/aer.AER_26_17
14. Dal D, Kose A, Honca M, Akinci SB, Basgul E, Aypar U. Efficacy of prophylactic ketamine in preventing postoperative shivering. Br J Anaesth. (2005) 95:189–92. doi: 10.1093/bja/aei148
15. Panneer M, Murugaiyan P, Rao SV. A comparative study of intravenous dexmedetomidine and intravenous clonidine for postspinal shivering in patients undergoing lower limb orthopedic surgeries. Anesth Essays Res. (2017) 11:151–4. doi: 10.4103/0259-1162.183157
16. Jun JH, Chung MH, Jun IJ, Kim Y, Kim H, Kim JH, et al. Efficacy of forced-air warming and warmed intravenous fluid for prevention of hypothermia and shivering during caesarean delivery under spinal anaesthesia: a randomised controlled trial. Eur J Anaesthesiol. (2019) 36:442–8. doi: 10.1097/EJA.0000000000000990
17. Moheb M, Rezaei M, Azizi-Fini I, Atoof F, Saadati MA. Comparison of the effect of forced-air warming and warmed intravenous fluid on the comfort and prevention of shivering after spinal anesthesia in patients undergoing orthopedic surgery. J Perianesth Nurs. (2022) 37:865–71. doi: 10.1016/j.jopan.2022.01.010
18. Thiel B, Mooijer BC, Kolff-Gart AS, Kerklaan BM, Poolman RW, de Haan P, et al. Is preoperative forced-air warming effective in the prevention of hypothermia in orthopedic surgical patients? A randomized controlled trial. J Clin Anesth. (2020) 61:109633. doi: 10.1016/j.jclinane.2019.109633
19. Alderson P, Campbell G, Smith AF, Warttig S, Nicholson A, Lewis SR. Thermal insulation for preventing inadvertent perioperative hypothermia. Cochrane Database Syst Rev. (2014) (6):CD009908. doi: 10.1002/14651858.CD009908.pub2
20. Nain P, Kundra S, Singh T, Singh MR, Kapoor R, Singh A. Comparative evaluation of oral tramadol and gabapentin for prophylaxis of post-spinal shivering. Indian J Anaesth. (2021) 65:S5–11. doi: 10.4103/ija.IJA_979_20
21. Mathews S, Al Mulla A, Varghese PK, Radim K, Mumtaz S. Postanaesthetic shivering–a new look at tramadol. Anaesthesia. (2002) 57:394–8. doi: 10.1046/j.1365-2044.2002.2457_3.x
22. Nirala DK, Prakash J, Ram B, Kumar V, Bhattacharya PK, Priye S. Randomized double-blinded comparative study of intravenous nalbuphine and tramadol for the treatment of postspinal anesthesia shivering. Anesth Essays Res. (2020) 14:510–4. doi: 10.4103/aer.AER_95_20
23. Gemechu AD, Gebremedhin TD, Andebiku AA, Solomon F, Sorsa A. The effect of ketamine versus tramadol on prophylactic post-spinal shivering in those patients undergoing orthopedic surgery: a prospective cohort study design 2020. BMC Anesthesiol. (2022) 22:361. doi: 10.1186/s12871-022-01906-z
24. Tudimilla S, Suryawanshi C, SaravanKumar K. A comparative evaluation of nalbuphine and tramadol for the control of post-spinal anaesthesia shivering. Cureus. (2021) 13:e20481. doi: 10.7759/cureus.20481
25. Wang J, Wang Z, Liu J, Wang N. Intravenous dexmedetomidine versus tramadol for treatment of shivering after spinal anesthesia: a meta-analysis of randomized controlled trials. BMC Anesthesiol. (2020) 20:104. doi: 10.1186/s12871-020-01020-y
26. Shukla U, Malhotra K, Prabhakar T. A comparative study of the effect of clonidine and tramadol on post-spinal anaesthesia shivering. Indian J Anaesth. (2011) 55:242–6. doi: 10.4103/0019-5049.82666
27. Commiskey S, Fan LW, Ho IK, Rockhold RW. Butorphanol: effects of a prototypical agonist-antagonist analgesic on kappa-opioid receptors. J Pharmacol Sci. (2005) 98:109–16. doi: 10.1254/jphs.CRJ05001X
28. Yang L, Sun DF, Wu Y, Han J, Liu RC, Wang LJ. Intranasal administration of butorphanol benefits old patients undergoing H-uvulopalatopharyngoplasty: a randomized trial. BMC Anesthesiol. (2015) 15:20. doi: 10.1186/1471-2253-15-20
29. Lv S, Sun D, Li J, Yang L, Sun Z, Feng Y. Anesthetic effect of different doses of butorphanol in patients undergoing gastroscopy and colonoscopy. BMC Surg. (2021) 21:266. doi: 10.1186/s12893-021-01262-8
30. Amsalu H, Zemedkun A, Regasa T, Adamu Y. Evidence-based guideline on prevention and management of shivering after spinal anesthesia in resource-limited settings: review article. Int J Gen Med. (2022) 15:6985–98. doi: 10.2147/IJGM.S370439
31. Crossley AW, Mahajan RP. The intensity of postoperative shivering is unrelated to axillary temperature. Anaesthesia. (1994) 49:205–7. doi: 10.1111/j.1365-2044.1994.tb03422.x
32. Palan A, Agrawal NK. Control of intraoperative shivering under spinal anaesthesia–A prospective randomized comparative study of butorphanol with tramadol. J Krishna Instit Med Sci Univ. (2017) 6:57–65.
33. Maheshwari BS, Shah SK, Chadha IA. Tramadol and butrophanol for control of shivering: randomised double blind comparative study. J Anaesthesiol Clin Pharmacol. (2008) 24:343–6.
34. Joshi SS, Adit A, Arun G, Shidhaye RV. Comparison of intravenous butorphanol, ondansetron and tramadol for control of shivering during regional anesthesia: a prospective, randomized double-blind study. Anaesthesia Pain Intensive Care. (2013) 17:33–9.
35. Bansal P, Jain G. Control of shivering with clonidine, butorphanol, and tramadol under spinal anesthesia: a comparative study. Local Reg Anesth. (2011) 4:29–34. doi: 10.2147/LRA.S15366
36. Keerthi P, Kamath SS. Comparative study of dexmedetomidine, butorphanol and tramadol for post-spinal anesthesia shivering. Res J Pharm Biol Chem Sci. (2017) 18:1801–9.
37. Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. (2019) 10:ED000142. doi: 10.1002/14651858.ED000142
38. Zhu X, Yang M, Mu J, Wang Z, Zhang L, Wang H, et al. The effect of general anesthesia vs. regional anesthesia on postoperative delirium-a systematic review and meta-analysis. Front Med. (2022) 9:844371. doi: 10.3389/fmed.2022.844371
Keywords: butorphanol, tramadol, spinal anesthesia, shivering, meta-analysis
Citation: Wan J-X, Li X-C, Zeng S-S, Li Y-Q and Wang F-J (2023) Comparison of intravenous butorphanol vs. tramadol for post-spinal anesthesia shivering: a meta-analysis and systematic review. Front. Med. 10:1271664. doi: 10.3389/fmed.2023.1271664
Received: 02 August 2023; Accepted: 13 November 2023;
Published: 05 December 2023.
Edited by:
Wan-Jie Gu, Jinan University, ChinaReviewed by:
Behzad Nazemroaya, Isfahan University of Medical Sciences, IranCopyright © 2023 Wan, Li, Zeng, Li and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fang-Jun Wang, d2ZqbHh5MDA2QG5zbWMuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.