- 1Division of Gastroenterology, Hepatology and Nutrition, Department of Internal Medicine, College of Medicine, University of Florida, Gainesville, FL, United States
- 2Division of Geriatrics, Department of Medicine, College of Medicine, University of Florida, Gainesville, FL, United States
- 3Department of Pathology, Immunology and Laboratory Medicine, College of Medicine, University of Florida, Gainesville, FL, United States
- 4Department of Community Health and Family Medicine, College of Medicine, University of Florida, Gainesville, FL, United States
A 72-year-old man visited cardiology for exertional chest pain, lightheadedness, and fatigue. Six years prior, he was surgically treated for cutaneous malignant melanoma of the lower back. After a negative cardiac work-up, primary care diagnosed severe iron deficiency anemia. Emergent upper and lower gastrointestinal (GI) endoscopy revealed simultaneous melanoma metastases to the stomach and colon with discrete macroscopic features. Metastatic disease, including brain, lung, and bone, was discovered on imaging. Treatment included immunotherapy with nivolumab and stereotactic radiosurgery of the brain metastases, and our patient has remained in continued remission even after 2 years. Melanoma with GI tract (GIT) metastasis has a poor prognosis and rarely presents symptomatically or with synchronous gastric and colonic lesions. This case illustrates the importance of early primary care involvement to expedite work-up for multifocal GI metastases in patients with a remote melanoma history presenting with symptoms related to iron deficiency anemia (IDA).
Introduction
Cutaneous melanoma is among the most common malignancies to metastasize to the GIT (1), despite the GIT being the second least common location for melanoma metastasis (2). They most commonly metastasize to the GIT from extremities (15–57%), followed by the trunk (13–54%) and head/neck (5–33%) (3). GIT metastases are usually asymptomatic, with only 1–5% of melanoma patients having clinically apparent GI involvement (3) and up to 43.5% having GIT metastasis at autopsy (4). It can be difficult to determine if a GIT melanoma is metastatic or primary when there is no synchronous cutaneous primary. Metastatic GIT melanoma can present decades later as recurrence (4, 5) or from a spontaneously regressed primary (6, 7).
Primary GI-mucosal melanomas are typically esophageal or anorectal (8), not gastric or colonic (9), and are usually aggressive with a worse prognosis than melanoma metastatic to the GIT (4). The small bowel is the most common location for GIT metastasis (2, 3, 6, 10), and the stomach or colon is less common (2, 10). A retrospective cohort describing 55% of secondary GIT melanomas as “multifocal” probably noted a greater frequency in the upper GIT because it included small bowel (1). Here, we describe a rare case of melanoma presenting with angina from symptomatic anemia and with simultaneous gastric and colonic metastases.
Case presentation
A 72-year-old man with atrial fibrillation on apixaban presented in September 2021 (Table 1) to his cardiologist with exertional chest pressure, postural lightheadedness, and fatigue for weeks. An echocardiogram and stress test were unrevealing. Cardiac catheterization showed mild, non-obstructive coronary artery disease. Cardiology lab results showed severe microcytic anemia (hemoglobin 7.0 G/DL) and primary care lab results showed iron deficiency (ferritin 7.6 NG/ML), although there was no overt GI hemorrhage. Apixaban was held, and an emergent upper and lower endoscopy was ordered (11).
Relevant past medical history includes a cutaneous melanoma of the lower back that was diagnosed in 2015 by punch biopsy (Clark Level IV, Breslow Depth 2.25 mm, mitotic index approximately 1 mitosis/mm2, no significant tumor regression, epidermal ulceration, microvascular invasion, or satellite micro-metastases), which was at clinical stage IIA (T3a N0 M0) (Supplementary Figure S1). A wide local excision had negative margins, and a sentinel lymph node biopsy was negative. The pathological stage was also IIA and further staging work-up was therefore not performed based on the early stage of this typical superficial spreader. Adjuvant therapies were experimental at the time and not prescribed. Bi-annual skin and periodic ophthalmologic exams remained unremarkable. Screening colonoscopies were up to date (12), and a 25-mm tubular adenoma had been removed 4 years prior.
In October 2021, esophagogastroduodenoscopy and colonoscopy identified a gastric body polyp (Figure 1, Supplementary Figure S2), a partially obstructing ascending colon mass (Figure 2), and hepatic flexure polyp (Figure 3, Supplementary Figure S3). All three biopsies diagnosed metastatic melanoma (Supplementary Figures S4–S6). PET-CT found lung and right humeral head metastases, but none of the GI metastases were detectable. Abdomen/pelvis CT with contrast was non-contributory, but a chest CT demonstrated bilateral solid pulmonary nodules up to 2.5 cm, a 2.1 cm right humeral head lytic lesion, and a few left supraclavicular lymph nodes up to 1.0 cm, corresponding to PET-CT. A brain MRI showed left caudate and cerebellar metastases. Serum lactate dehydrogenase (LDH) was normal, but GI metastases harbored BRAF V600E, PTEN, and TERT mutations. The pathological stage was IV [rTX, N2b, M1d(0)] based on the American Joint Committee on Cancer (AJCC) 8th Edition staging system (13).
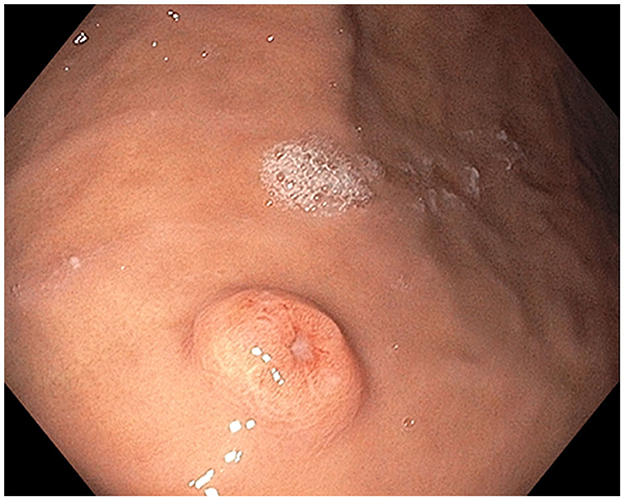
Figure 1. Stomach polyp: Single non-melanotic, 12 mm in diameter and 2.5 mm in height, sessile umbilicated polyp (equivalent to a Paris-1s classification of colonic polyps) found on the gastric body.
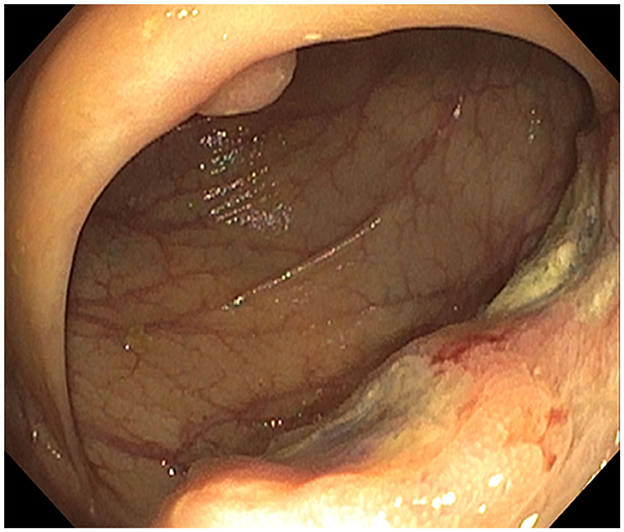
Figure 2. Ascending colon mass: infiltrative and ulcerated non-melanotic, partially obstructing 4-cm mass involving one-half of the lumen circumferences.
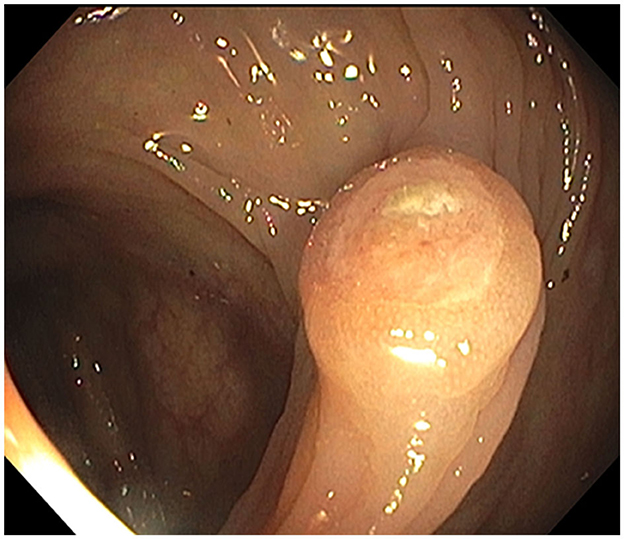
Figure 3. Hepatic flexure polyp: endoscopic appearance of the non-melanotic, 12 mm in diameter and 3 mm in height, sessile umbilicated polyp (Paris-1s classification of colonic polyps).
The patient's symptomatic anemia was successfully treated with two iron infusions. He underwent stereotactic radiosurgery for the brain metastases without complications. Twelve cycles of immunotherapy with nivolumab (480 mg monthly infused over 30 min) were completed in September 2022. Nivolumab-induced vitiligo developed at cycle 9 and guttate psoriasis on the chest, arms, and legs at cycle 11, the latter of which resolved with 0.1% triamcinolone cream twice daily. Shortly after cycle 12, he developed oral candidiasis, and preexisting seborrheic keratoses became inflamed, both thought to be nivolumab-induced. The former was treated with oral nystatin, and the latter with either a topical steroid or cryotherapy. Immunotherapy response was monitored with monthly circulating tumor DNA (ctDNA) levels and PET-CTs every 3 months. The patient went into remission after 3 months of immunotherapy and remains in continued remission as of July 2023. His surveillance has included brain MRI and whole-body PET-CT at baseline and every 3 months, along with ctDNA testing that was reduced from monthly after 6 months of disease-free surveillance.
Discussion
Our patient's cutaneous melanoma 6 years prior is congruent with the interim average and median reported times from primary cutaneous melanoma treatment to find a GI metastasis, or 3.65 years (10) and 10 years (14), respectively. However, the locations of his metastases were very unusual. We found no reports of simultaneous upper and lower GIT lesions in metastatic GIT cases and only two reports in primary GIT cases: gastric/ileocecal (15) and esophageal/ileal (16). While our patient did have several risk factors for multiple primary GI-mucosal melanomas, including older age at diagnosis of first melanoma (66 years old), male sex, and white race (17), these were unlikely to occur in the stomach or colon (8, 9), and proposed criteria for other intestinal locations require a solitary lesion (6).
Prompt diagnosis is important in symptomatic GIT metastasis because of life-threatening complications (18), which were prevented in this case. In addition to IDA, patients can present with abdominal pain, dysphagia, small bowel obstruction, and/or perforation (1, 4, 19). Endoscopy is superior to radiography for diagnosing GIT melanoma and its complications (19), and capsule endoscopy may be necessary (5). GIT melanoma appears as either pigmented or amelanotic ulcerated polypoid lesions (1, 4, 19).
GIT metastasis is a poor prognostic marker (5-year survival 14%, median survival 12.5 months) (3), and autopsy often reveals multiple organ metastases (95%) (2). GI metastases are not typically the cause of death, which is usually respiratory failure from lung metastasis (2). LDH elevation is a negative predictor of survival in the AJCC staging system (13) but is not melanoma-specific.
BRAF, PTEN, and TERT mutations were present in his melanoma metastases. Half of cutaneous melanomas have BRAF mutations, and the most common V600E mutation is associated with decreased overall survival in advanced melanoma (20). BRAF mutation testing is required for resectable or unresectable melanoma stage III or stage IV, highly recommended for stage IIC high-risk resected disease, and not recommended for stage I or stages IIA–IIB (21). His overall survival was decreased with PTEN and TERT mutations, both of which often coexist with BRAF mutations (22, 23). Interestingly, however, the vitiligo he experienced during his immunotherapy has been associated with progression-free and overall survival that is significantly increased (24).
Conclusion
Melanoma often progresses to metastatic disease but can be challenging to quickly diagnose in the absence of a cutaneous lesion. GIT metastasis has a particularly poor prognosis. Synchronous gastric and colonic melanomas are extremely rare but important to consider in patients with IDA and a remote history of melanoma because of their poor prognosis and potential to cause an abdominal emergency. The patient's metastatic melanoma presented as angina, showing the importance of early primary care involvement to rapidly diagnose the cause of symptomatic IDA. The expedited upper and lower endoscopy and multidisciplinary involvement prevented abdominal complications and began timely treatment. Our patient has remained in continued remission even after 2 years.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the studies involving humans because we contacted our Institutional Review Board, and their approval is not required for case reports. We contacted our ethics committee, and their approval is also not required for de-identified case reports with all protected health information is removed and with written consent from the patient who has reviewed the report. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
FO: Conceptualization, Funding acquisition, Investigation, Project administration, Software, Writing—original draft, Writing—review & editing. MA: Conceptualization, Data curation, Funding acquisition, Investigation, Writing—review & editing. HK: Data curation, Funding acquisition, Investigation, Methodology, Writing—original draft, Writing—review & editing. DG: Data curation, Funding acquisition, Investigation, Software, Writing—review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The co-authors would like to thank each of their affiliated departments for sharing the financial cost of the article processing charges.
Acknowledgments
The authors thank Ariel Pomputius, MLIS, for contributing to the literature search during manuscript preparation and Art Watson for assisting with the quality of endoscopy photographs.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2023.1268973/full#supplementary-material
Supplementary Figure S1. (A) Skin, mid-right back, punch biopsy: non-encapsulated well circumscribed tumoral nodule (H&E, original magnification 40X). (B) Malignant melanoma composed of a monomorphic population of epithelioid cells with melanin pigment (H&E, original magnification 400X).
Supplementary Figure S2. (A, B) Stomach polyp: Additional close-up, endoscopic views of single non-melanotic, 12 mm in diameter and 2.5 mm in height, sessile umbilicated polyp (equivalent to a Paris-1s classification of colonic polyps) found on the gastric body.
Supplementary Figure S3. Hepatic flexure polyp: Additional close-up view showing endoscopic appearance of the 12 mm in diameter and 3 mm in height non-melanotic sessile umbilicated polyp (Paris-1s classification of colonic polyps).
Supplementary Figure S4. Stomach polyp: Melanoma cells undermining gastric epithelium (H&E, original magnification 200X).
Supplementary Figure S5. Ascending colon mass: Tumoral cells (arrows) flanked by colonic crypts (H&E, original magnification 200X).
Supplementary Figure S6. D. Hepatic flexure polyp: Melanoma cells stain strongly for melanocytic marker SOX-10 (SOX-10 immunohistochemical stain, original magnification 20X).
References
1. Gilg MM, Gröchenig HP, Schlemmer A, Eherer A, Högenauer C, Langner C. Secondary tumors of the GI tract: origin, histology, and endoscopic findings. Gastrointest Endosc. (2018) 88:151–158.e1. doi: 10.1016/j.gie.2018.02.019
2. Patel JK, Didolkar MS, Pickren JW. Metastatic pattern of malignant melanoma. Am J Surg. (1978) 135:807–10. doi: 10.1016/0002-9610(78)90171-X
3. Asad-Ur-Rahman F, Abbass A, Majeed U, Navaneethan U. Melanoma metastasizing to the small intestine: A case report illustrating symptomatic and asymptomatic involvement. Cureus. (2016) 8:e608. doi: 10.7759/cureus.608
4. Liang KV, Sanderson SO, Nowakowski GS, Arora AS. Metastatic malignant melanoma of the gastrointestinal tract. Mayo Clin Proc. (2006) 81:511–6. doi: 10.4065/81.4.511
5. Ahmed M, Ardor GD, Hanna H, Alhaj AM, Nassar A. Two unique cases of metastatic malignant melanoma of the gastrointestinal tract. Int J Surg Case Rep. (2023) 103:107907. doi: 10.1016/j.ijscr.2023.107907
6. Patti R, Cacciatori M, Guercio G, Territo V, Di Vita G. Intestinal melanoma: A broad spectrum of clinical presentation. Int J Surg Case Rep. (2012) 3:395–8. doi: 10.1016/j.ijscr.2012.03.033
7. Khalid U, Saleem T, Imam AM, Khan MR. Pathogenesis, diagnosis and management of primary melanoma of the colon. World J Surg Oncol. (2011) 9:14. doi: 10.1186/1477-7819-9-14
8. Qiu Y, Jiao H, Huang W, Chen Z, Yang Q, Wu S, et al. 18F-FDG PET/CT imaging of primary malignant melanoma of rectum with liver metastases mimicking rectum cancer: case report and literature review. Am J Nucl Med Mol Imaging. (2022) 12:188–94.
9. Xie J, Dai G, Wu Y. Primary colonic melanoma: a rare entity. World J Surg Oncol. (2022) 20:256. doi: 10.1186/s12957-022-02721-z
10. Wysocki WM, Komorowski AL, Darasz Z. Gastrointestinal metastases from malignant melanoma: report of a case. Surg Today. (2004) 34:542–6. doi: 10.1007/s00595-004-2741-5
11. Ko CW, Siddique SM, Patel A, Harris A, Sultan S, Altayar O, et al. AGA clinical practice guidelines on the gastrointestinal evaluation of iron deficiency anemia. Gastroenterology. (2020) 159:1085–94. doi: 10.1053/j.gastro.2020.06.046
12. Shaukat A, Kahi CJ, Burke CA, Rabeneck L, Sauer BG, Rex DK, et al. Clinical guidelines: colorectal cancer screening 2021. Am J Gastroenterol. (2021) 116:458–79. doi: 10.14309/ajg.0000000000001122
13. Keung EZ, Gershenwald JE. The eighth edition American Joint Committee on Cancer (AJCC) melanoma staging system: Implications for melanoma treatment and care. Expert Rev Anticancer Ther. (2018) 18:775–84. doi: 10.1080/14737140.2018.1489246
14. Patel V, Nasri E, Kresak JL, Feely M. Metastatic malignancy to the upper gastrointestinal tract encountered on endoscopic biopsy: a clinicopathologic study. Mod Pathol. (2017) 30:157–210. doi: 10.1038/modpathol.2016.24
15. Kostromitsky DN, Dobrodeev AY, Afanasyev SG, Li NA, Vasilyev NV, Podyablonsky AS. Metastatic multiple primary gastrointestinal melanoma: A case report. Sib J Oncol. (2020) 19:155–60. doi: 10.21294/1814-4861-2020-19-6-155-160
16. Sazon RJV, Cinco RUC. A case report of a synchronous gastrointestinal melanoma: A multidisciplinary approach. Am J Gastroenterol. (2021) 116:S884. doi: 10.14309/01.ajg.0000781684.80342.ba
17. Nosrati A, Wesley YY, McGuire J, Griffin A, de Souza JR, Singh R, et al. Outcomes and risk factors in patients with multiple primary melanomas. J Invest Dermatol. (2019) 139:195–201. doi: 10.1016/j.jid.2018.07.009
18. Mantas D, Damaskos C, Garmpis N, Dimitroulis D, Garmpi A, Gogas H. Abdominal emergencies in patients with stage IV melanoma: The role of surgery: A single-centre experience. Anticancer Res. (2018) 38:3713–8. doi: 10.21873/anticanres.12650
19. Nelson RS, Lanza F. Malignant melanoma metastatic to the upper gastrointestinal tract: endoscopic and radiologic correlations, form and evolution of lesions, and value of directed biopsy in diagnosis. Gastrointest Endosc. (1978) 24:156–8. doi: 10.1016/S0016-5107(78)73493-0
20. Tanda ET, Vanni I, Boutros A, Andreotti V, Bruno W, Ghiorzo P, et al. Current state of target treatment in BRAF mutated melanoma. Front Mol Biosci. (2020) 14:154. doi: 10.3389/fmolb.2020.00154
21. Michielin O, Van Akkooi AC, Ascierto PA, Dummer R, Keilholz U. Cutaneous melanoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. (2019) 30:1884–901. doi: 10.1093/annonc/mdz411
22. Cabrita R, Mitra S, Sanna A, Ekedahl H, Lövgren K, Olsson H, et al. The role of PTEN loss in immune escape, melanoma prognosis and therapy response. Cancers. (2020) 12:742. doi: 10.3390/cancers12030742
23. Bianco PD, Stagni C, Giunco S, Fabozzi A, Elefanti L, Pellegrini S, et al. TERT promoter mutations differently correlate with the clinical outcome of MAPK inhibitor-treated melanoma patients. Cancers. (2020) 12:946. doi: 10.3390/cancers12040946
Keywords: melanoma, gastrointestinal, metastasis, gastric, colonic, iron deficiency anemia, multifocal, BRAF
Citation: Amaris MA, Kallas HE, Gonzalo DH and Orlando FA (2023) Gastric and colonic metastases of malignant melanoma diagnosed during endoscopic evaluation of symptomatic anemia presenting as angina: a case report. Front. Med. 10:1268973. doi: 10.3389/fmed.2023.1268973
Received: 28 July 2023; Accepted: 29 September 2023;
Published: 02 November 2023.
Edited by:
Barry Hall, Connolly Hospital Blanchardstown, IrelandReviewed by:
Neil O'Morain, St. Vincent's University Hospital, IrelandOrlaith Kelly, Connolly Hospital Blanchardstown, Ireland
Copyright © 2023 Amaris, Kallas, Gonzalo and Orlando. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Frank A. Orlando, Zm9ybGFuZG9AdWZsLmVkdQ==
 Manuel A. Amaris
Manuel A. Amaris Henrique E. Kallas2
Henrique E. Kallas2 Frank A. Orlando
Frank A. Orlando