- 1Department of Anesthesiology and Pain Medicine, Chosun University Hospital, Gwangju, Republic of Korea
- 2Department of Anesthesiology and Pain Medicine, School of Medicine, Chosun University, Gwangju, Republic of Korea
Introduction: There is insufficient evidence regarding the efficacy and safety of remimazolam in elderly patients. Therefore, this study evaluated the differences in the anesthesia characteristics and perioperative hemodynamic profiles of elderly patients receiving total intravenous anesthesia with remimazolam or propofol.
Methods: Eighty-four patients aged >65 years with an American Society of Anesthesiologists physical status of I–III were randomly assigned to Group R (receiving remimazolam, n = 42) or Group P (receiving propofol, n = 42). In Group R, remimazolam was initiated at a rate of 6 mg/kg/h until loss of consciousness (LOC) was achieved and maintained at 1 mg/kg/h subsequently. In Group P, 1.0–1.5 mg/kg of propofol was injected for 1 min and maintained at 100 μg/kg/min subsequently. The maintenance infusion rate was adjusted to maintain an appropriate depth of anesthesia until the end of the surgery. The primary outcome was the time to LOC. The depth of anesthesia scores and hemodynamic profiles were recorded perioperatively.
Results: The time to LOC was significantly longer in Group R (120 s) than in Group P (60 s) (p < 0.001). The time to eye-opening (Group R, 10 min; Group P, 10 min; p = 0.056), the incidence of maintenance of hemodynamic changes within 20% of the peri-anesthetic values, and treatments for hemodynamic instability did not differ significantly between the groups. The depth of anesthesia scores did not differ significantly between the groups; however, the scores were higher in Group R than those in Group P before endotracheal intubation. The hemodynamic parameters did not differ significantly at any time point. The time to extubation was longer in Group R (12 min) than that in Group P (10 min) (p = 0.007). Similarly, the time to discharge from the operating room was significantly longer in Group R (15 min) compared to Group P (12 min) (p = 0.018).
Conclusion: Remimazolam does not exhibit a comparable effect to propofol in terms of anesthesia induction and recovery. However, it demonstrates a similar effect to propofol regarding intraoperative anesthesia depth and hemodynamic profile in elderly patients undergoing remifentanil-based total intravenous anesthesia.
1. Introduction
Inhaled anesthetics, intravenous anesthetics or sedatives, and opioids are used by anesthesiologists to induce and maintain general anesthesia. Total intravenous anesthesia (TIVA) is a universal anesthesia management technique performed using intravenous anesthetics or sedatives and opioids. Propofol is a common intravenous sedative that is frequently used in TIVA. However, it can induce serious adverse effects, such as hypotension and bradycardia, delayed recovery, respiratory failure, and propofol infusion syndrome (1, 2). Therefore, there is an increasing need to identify ideal sedatives for TIVA.
Remimazolam, a benzodiazepine that acts on γ-aminobutyric acid (GABA) receptors (1, 3), has specific, unique, and favorable clinical characteristics compared with those of the currently available short-acting anesthetics and sedatives (3, 4). Owing to its structural similarity to remifentanil, it is quickly hydrolyzed by non-specific tissue esterases, resulting in its characteristic rapid onset and offset of sedation, short half-life, and predictable duration of action (1, 4). Similar to other benzodiazepines, the desired levels of sedation with a limited risk of airway obstruction can be achieved more easily with remimazolam, unlike propofol, which has a high ceiling effect (1, 5). In addition, recovery from remimazolam-induced sedation is faster than recovery from sedation induced by other benzodiazepines, and it can be hastened further with the use of flumazenil (4, 5).
Although there are physiological differences between adults and older patients, few studies have evaluated the clinical response to remimazolam in elderly patients (6–8). The time to loss of consciousness (LOC) and extubation after the administration of remimazolam did not show any relationship with age in a previous study (6). However, the time to LOC (TLOC) after the administration of remimazolam was found to be shorter, and the risk of delayed extubation was found to be higher in elderly patients than in adult patients (6, 8). Dose adjustments are not necessary for elderly patients according to manufacturers; however, the rate of administration and dosage should be adjusted carefully based on the patient’s physical condition.
Fast onset and offset of action of sedatives are important; however, minimizing hemodynamic changes is equally important. Several studies have shown that remimazolam is safer than propofol in terms of stable hemodynamic changes (1, 4, 5, 9). Compared with propofol, remimazolam has the advantage of preventing hypotension during the induction of anesthesia and similar anesthetic effects in elderly patients (10, 11). However, there is insufficient evidence regarding the efficacy and safety of remimazolam in elderly patients, and it is unknown whether remimazolam can be used safely and effectively in elderly patients at the clinical doses proposed by manufacturers.
We hypothesized that remimazolam would be as efficient and safe as propofol for the induction, maintenance, and recovery of TIVA. Thus, this study evaluated the anesthesia and hemodynamic profiles during the perioperative period of elderly patients receiving TIVA with remimazolam/remifentanil or propofol/remifentanil. The primary outcome assessed in this study was TLOC after remimazolam or propofol infusion.
2. Materials and methods
This prospective, randomized, controlled, single-blind study was approved by the Institutional Review Board of the Chosun University Hospital (Chosun 2021-08-010-001) on September 29, 2021, and prospectively registered with the Clinical Research Information Service (CRIS:1 ref.: KCT0006796) on December 2, 2021. This study was conducted in accordance with the Declaration of Helsinki of 1964 and its subsequent revisions.
2.1. Inclusions and exclusions
Patients aged >65 years with an American Society of Anesthesiologists physical status (ASA-PS) of I–III who were scheduled to undergo elective laparoscopic cholecystectomy under TIVA between March 24, 2022, and June 30, 2023, were eligible for inclusion in this study. Written informed consent was obtained from all participants or their legal surrogates after providing a thorough explanation of the purpose of the study. Patients with hemodynamic instability, renal or hepatic functional abnormalities, neuromuscular disorders, acute narrow-angle glaucoma, alcohol or drug dependence, or a history of resistance or hypersensitivity to benzodiazepines or other anesthetic drugs were excluded.
2.2. Randomizations
Eighty-four patients were randomly assigned to two groups that received either remimazolam (Group R, n = 42) or propofol (Group P, n = 42). Randomization was performed using a table of random numbers at a 1:1 allocation ratio via a website.2 The attending anesthesiologists were responsible for obtaining informed consent from the participants, assigning remimazolam or propofol according to the randomization scheme, and gathering and recording data from the participants. The participants were blinded to the group allocation; however, the attending anesthesiologists were not blinded to the group allocation because of the color difference between the study drugs. All other researchers, except for the attending anesthesiologists, participated in the statistical analysis.
2.3. Interventions
In Group R, remimazolam was administered at a rate of 6 mg/kg/h to induce anesthesia until LOC was achieved. After intubation, remimazolam was infused at 1 mg/kg/h and adjusted to a maximum infusion rate of 2 mg/kg/h to maintain an appropriate depth of anesthesia until the end of the surgery. In Group P, 1.0–1.5 mg/kg of propofol was slowly injected for 1 min to induce anesthesia. After intubation, propofol was infused at 100 μg/kg/min and adjusted to maintain an appropriate depth of anesthesia until the end of the surgery. LOC was defined as no response to shaking the patient’s shoulder every 5 s after initiating the administration of the study drug. Appropriate depth of anesthesia was defined as an entropy or bispectral index (BIS) maintained between 40 and 60.
The patients were transferred to the operating room (OR) after premedication with 0.05 mg/kg of intramuscular midazolam. Standard patient monitoring, including electrocardiography, non-invasive blood pressure, end-tidal partial pressure of carbon dioxide, and peripheral pulse oximetry, was initiated prior to the induction of anesthesia. In addition, a device was mounted for train of four (TOF) monitoring using a nerve stimulator to evaluate the degree of muscle relaxation during the surgery.
The attending anesthesiologists anesthetized the patients using TIVA based on the group allocation. After confirming LOC, endotracheal intubation was performed after injecting 0.6–0.9 mg/kg of rocuronium according to the anesthesia management protocol followed at our hospital. Remifentanil was continuously infused at a rate of 0.1–2 μg/kg/min and then maintained within this range after endotracheal intubation. The depth of anesthesia was controlled between 40 and 60 during the maintenance of anesthesia, and the hemodynamic parameters were maintained within 20% of the baseline values (before initiating remimazolam or propofol infusion). Optimal neuromuscular paralysis was maintained under 2 counts of TOF with intermittent injections of rocuronium.
Hypotension, defined as a systolic blood pressure (SBP) of <80 mmHg, was managed with intermittent bolus doses of 100 μg of phenylephrine or 10 mg of ephedrine. Hypertension, defined as an SBP of ≥150 mmHg, was managed with intermittent bolus doses of 1 mg of nicardipine, 60 mg of lidocaine, or 10 mg of esmolol. Bradycardia, defined as a heart rate of <50 beats/min, was managed with intermittent bolus doses of 0.5 mg of atropine. Tachycardia, defined as a heart rate of ≥100 beats/min, was managed with intermittent bolus doses of 10 mg of esmolol or adjustment of the infusion rate of remifentanil, propofol, or remimazolam. An air-forced blanket warmer was used to prevent intraoperative hypothermia.
The infusion of remimazolam, propofol, and remifentanil was discontinued at the end of the surgery after reversing the neuromuscular relaxation with 2 mg/kg of sugammadex. If the return of consciousness was not achieved within 10 min of discontinuing the remimazolam infusion, 0.2 mg of flumazenil was administered, and 0.1 mg of flumazenil was repeatedly administered, if necessary, at the discretion of the attending anesthesiologist. The patients were transferred to the recovery room after the complete reversal of rocuronium-induced neuromuscular paralysis and regaining consciousness.
2.4. Outcomes
The primary outcome assessed in this study was TLOC after remimazolam or propofol infusion. The depth of anesthesia score (ADS), SBP, diastolic blood pressure (DBP), mean blood pressure (MBP), and heart rate (HR) were recorded on admission to the operating room (AD_OR), before the infusion of each study drug (before; baseline value), on achieving LOC (LOC), 2 min after LOC (LOC2), after endotracheal intubation (PI), 15 min after anesthesia maintenance (A15), at the end of study drug infusion (ES), and 10 min after ES (ES10).
The time to achieve ADS 50 (TADS50), ADS at ES10, extubation time, and time to discharge from the OR were recorded. The age, sex, height, weight, body mass index (BMI), ASA-PS, comorbid diseases, duration of surgery and anesthesia, cumulative doses of infused remifentanil, use of flumazenil, incidence of maintenance of hemodynamic changes within 20% of the baseline values, and therapeutic interventions for perioperative adverse events were evaluated.
2.5. Statistical analysis
G*Power software (ver. 3.1.9.1, Heinrich-Heine-Universität Düsseldorf, Germany) was used to estimate the sample size required to evaluate the primary outcome. The effect size for TLOC was calculated as 0.7 using the mean/standard deviation of the remimazolam group (102/26.6) and the propofol group (78.7/38.4) (12). Sixty-eight patients had to be recruited to achieve 2-tailed statistical significance, α = 0.05, and a power of 80%, with an effect size of 0.7. Considering a dropout rate of approximately 20%, 84 patients were enrolled in this study.
IBM SPSS Statistics for Windows ver. 27.0 (IBM Corp., Armonk, NY, United States) was used for all statistical analyses. Student’s t-test was used to analyze continuous variables with normal distribution after the Kolmogorov–Smirnov and Shapiro–Wilk tests. The Mann–Whitney U test was used to analyze continuous variables without normal distribution. The data are presented as mean (95% confidence interval [CI] or median [interquartile range]). The nominal variables were analyzed using the Chi-square or Fisher’s exact test and are presented as numbers (percentage) of patients (n [%]). Statistical significance was set at p < 0.05.
3. Results
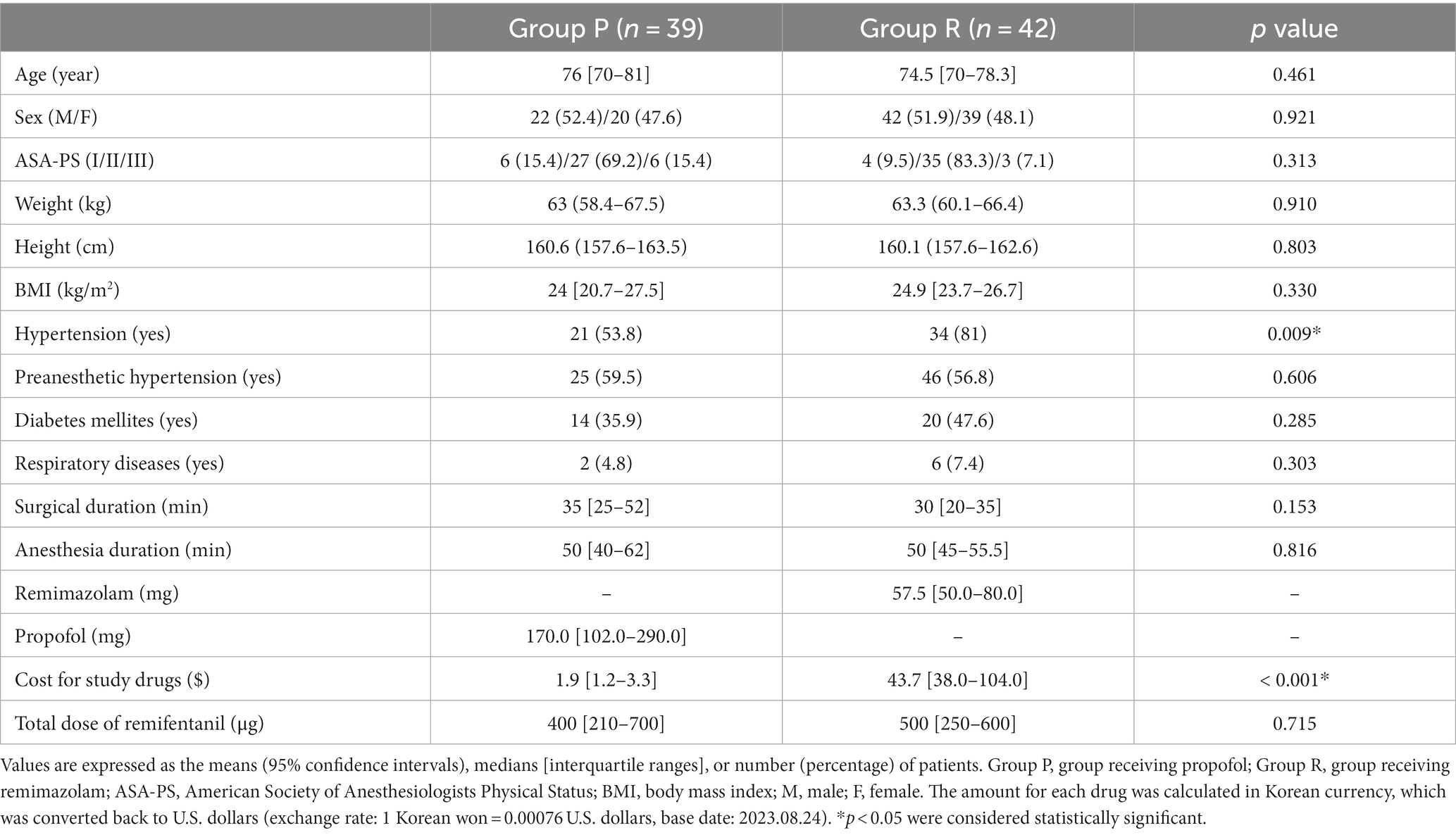
Among the 84 patients who completed this study without dropping out, three patients from Group P were excluded from the final analysis as they had an ADS of <80 before the infusion of the study drug (Figure 1). The demographic data, except for the history of hypertension, showed no statistically significant difference between the groups (p = 0.009; Table 1). Although a statistically significant difference was observed in the history of hypertension, the frequency of pre-anesthetic hypertension, defined as SBP of ≥150 mmHg before anesthesia induction, was not statistically significant (p = 0.606; Table 1). The cost difference between propofol and remimazolam exceeded 20-fold (p < 0.001; Table 1).
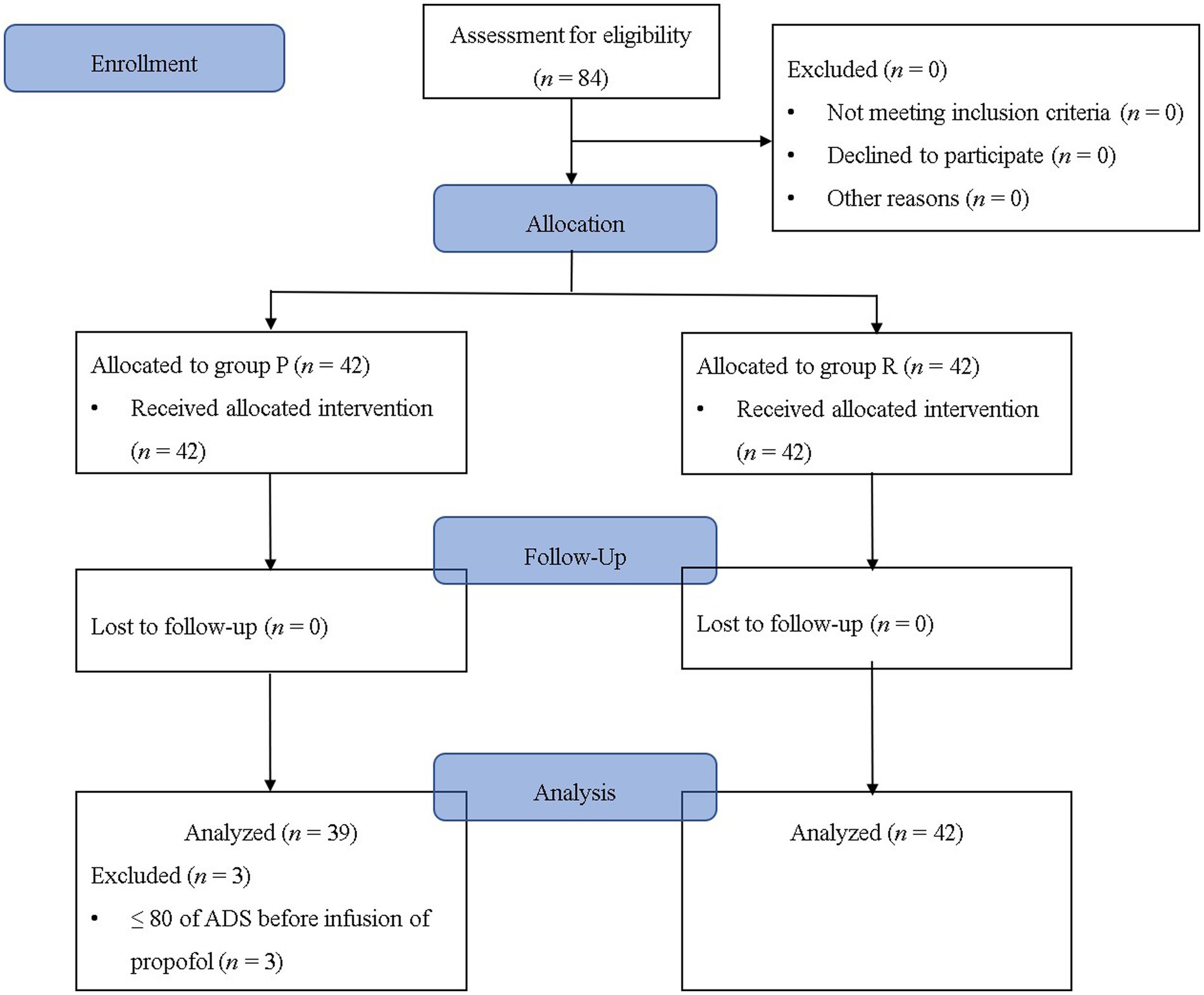
Figure 1. Flowchart of the study. Group P: group receiving propofol, Group R: group receiving remimazolam. ADS, anesthetic depth score.
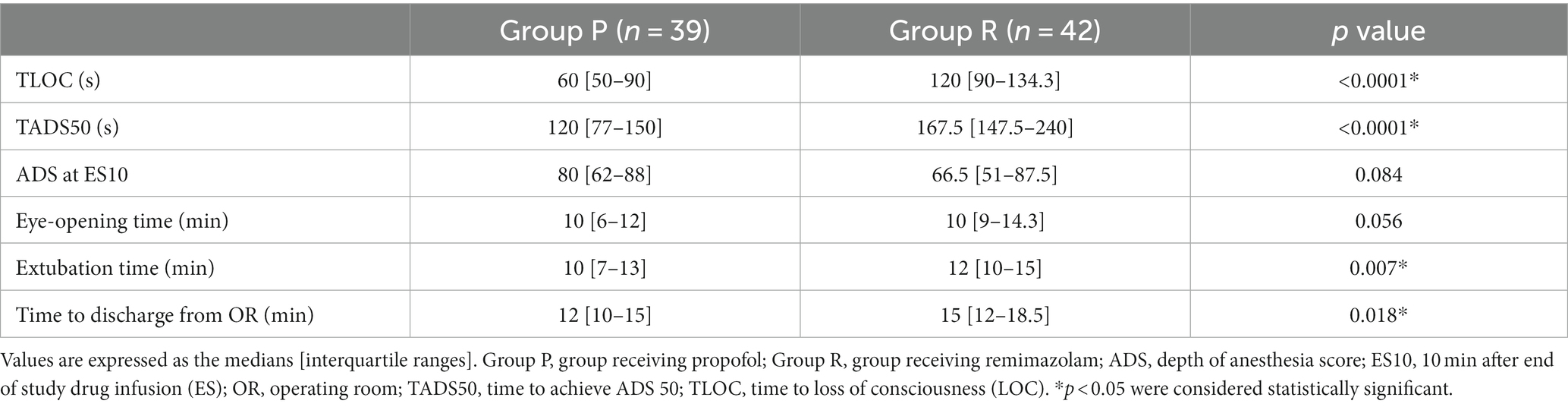
TLOC was significantly longer in Group R (120 s) than that in Group P (60 s) (p < 0.001; Table 2). TADS50 was significantly longer in Group R (167.5 s) than in Group P (120 s) (p < 0.001; Table 2). ADS at ES10 was lower in Group R (66.5) than in Group P (85.5); however, the difference was not significant (p = 0.084; Table 2). The time to eye-opening did not differ significantly between Group R (10 min) and Group P (10 min) (p = 0.056; Table 2). The time to extubation was longer in Group R (12 min) than in Group P (10 min) (p = 0.007; Table 2). The time to discharge from the operating room was significantly longer in Group R (15 min) than in Group P (12 min) (p = 0.018; Table 2).
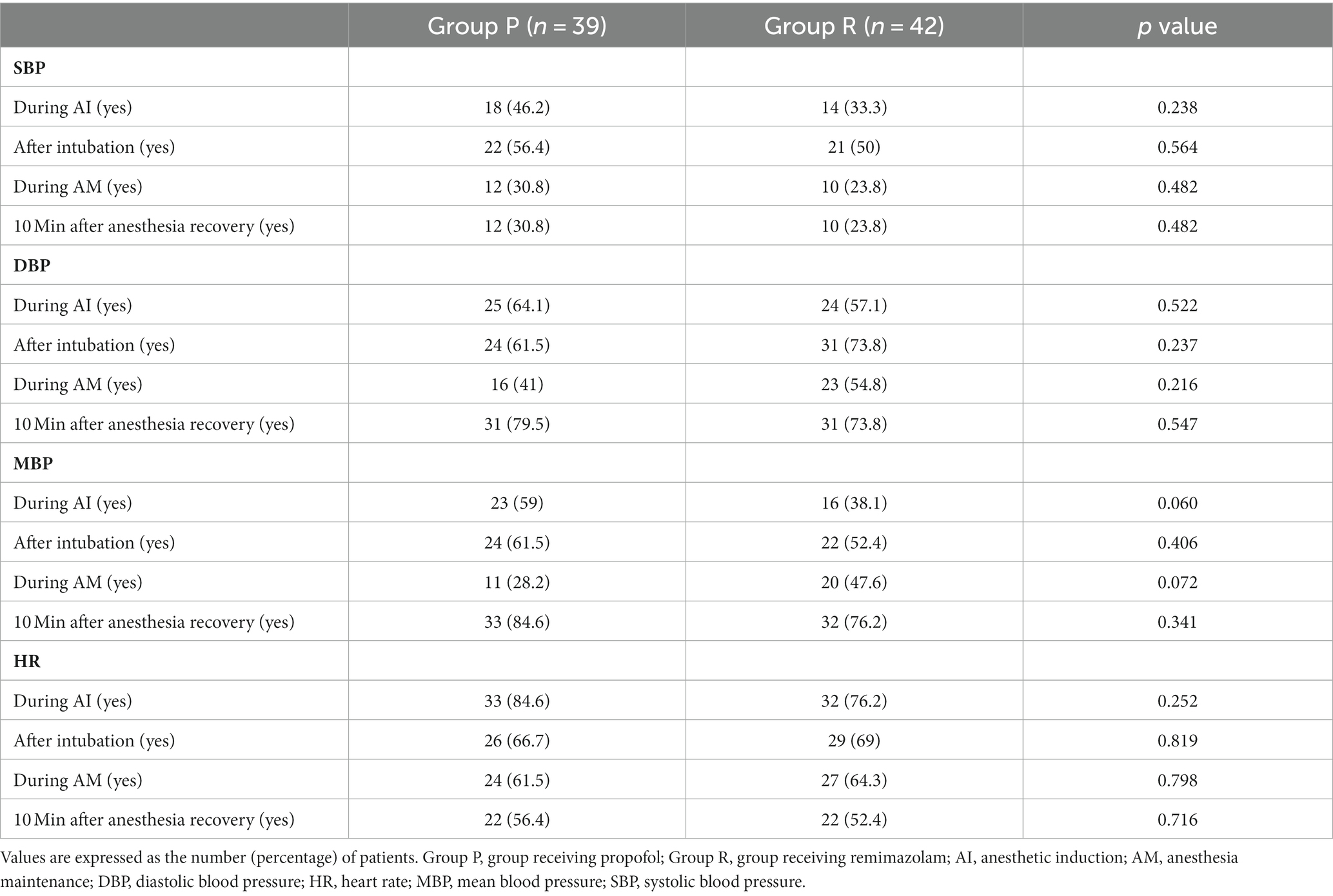
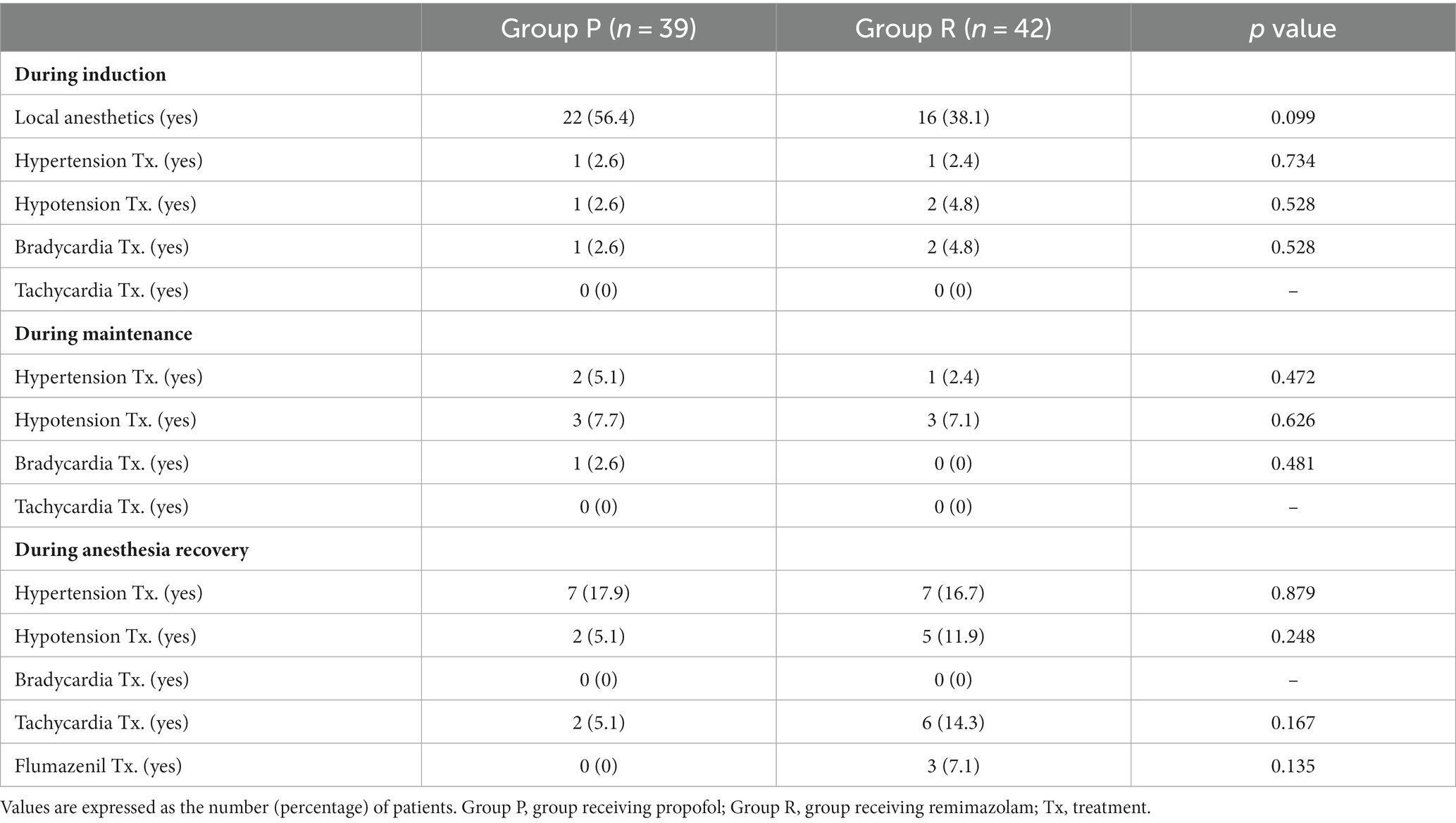
The incidence of maintenance of the hemodynamic changes within 20% of the peri-anesthetic values did not differ significantly between the groups (Table 3). Treatments for hemodynamic instability based on the study protocol did not differ significantly between the groups (Table 4).
ADS did not differ significantly between the groups; however, it was higher in Group R than in Group P before endotracheal intubation (Figure 2). The hemodynamic parameters (SBP, DBP, MBP, and HR) did not show any significant differences at any time point (Figure 3).
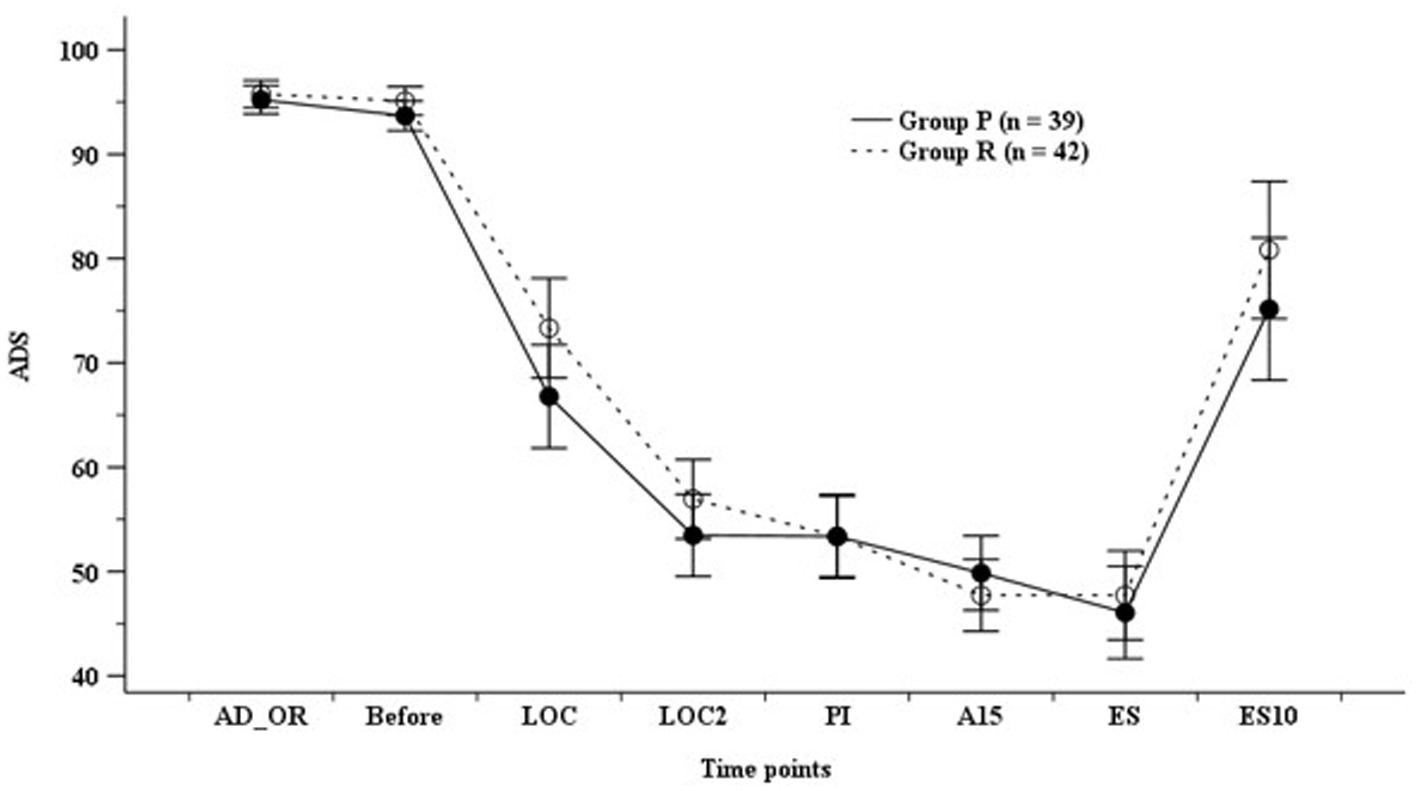
Figure 2. Anesthetic depth score (ADS). Group P: group receiving propofol, Group R: group receiving remimazolam. A15, 15 min after anesthesia induction; AD_OR, administration of operating room; ADS, anesthetic depth score; Before, before infusion of each study drug; LOC, at achieving LOC; LOC2, at 2 min after LOC; ES, at end of study drug infusion; ES10, 10 min after ES; PI, after endotracheal intubation.
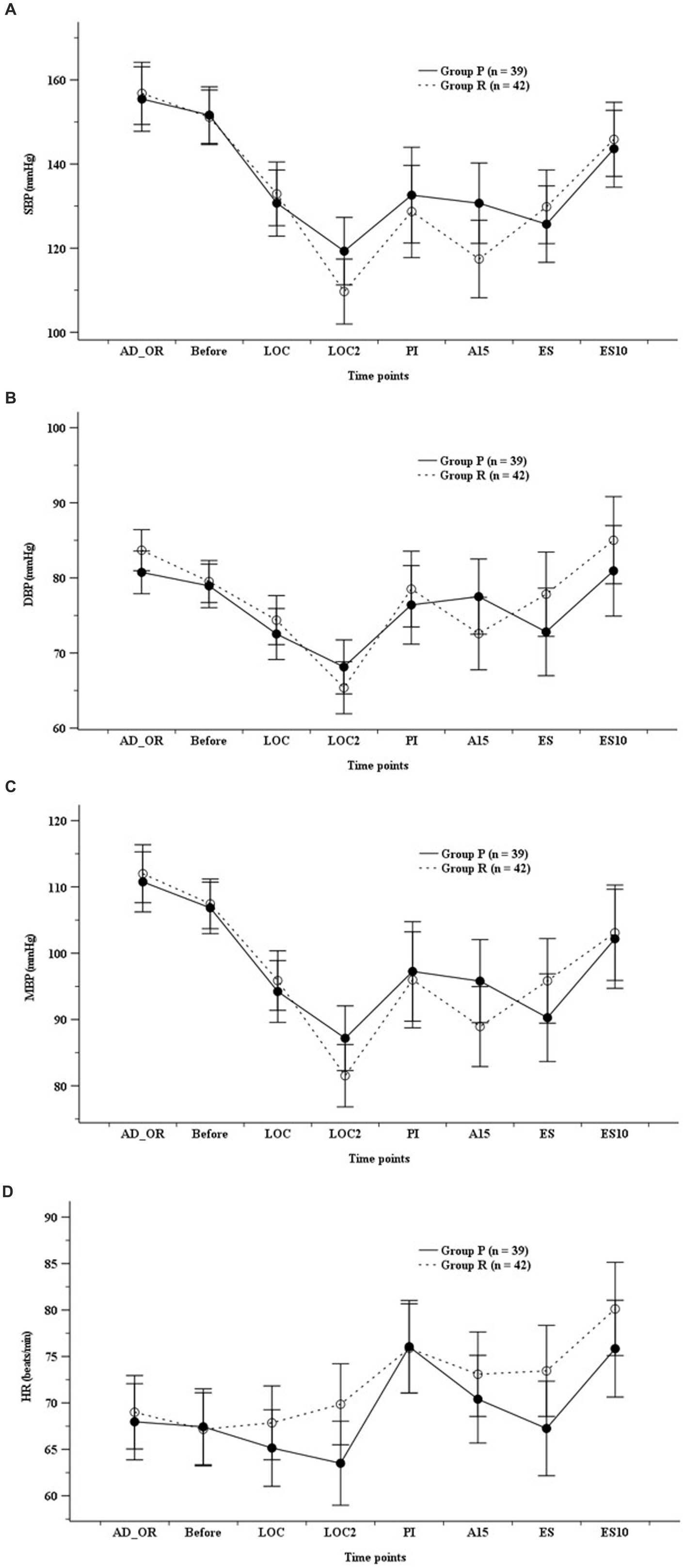
Figure 3. Hemodynamics. (A) Systolic blood pressure (SBP), (B) diastolic blood pressure (DBP), (C) mean blood pressure (MBP), (D) heart rate (HR). Group P: group receiving propofol, Group R: group receiving remimazolam. A15, 15 min after anesthesia induction; AD_OR, administration of operating room; ADS, anesthetic depth score; Before, before infusion of each study drug; LOC, at achieving LOC; LOC2, at 2 min after LOC; ES, at end of study drug infusion; ES10, 10 min after ES; PI, after endotracheal intubation.
4. Discussion
This study revealed that remimazolam was associated with longer times for LOC, extubation, and discharge from the operating room compared to propofol; however, there was no difference in the duration from the end of anesthesia to eye-opening. In addition, no significant differences were observed between remimazolam and propofol in terms of changes in the depth of anesthesia and hemodynamics.
4.1. TLOC and anesthetic depth
Several studies have been conducted comparing the efficacy and hemodynamic stability of remimazolam during anesthesia induction, maintenance, and recovery with those of propofol (12–14). These studies demonstrated that TLOC and recovery were delayed in patients receiving remimazolam compared with those receiving propofol and that remimazolam showed more stable hemodynamic changes. Remimazolam showed a 100% success rate for the induction of anesthesia with an adequate and effective depth of anesthesia at the recommended doses and was non-inferior to propofol as a sedative for general anesthesia (12, 14).
Doi et al. (12) infused remimazolam at a rate of 6 mg/kg/h until achieving LOC, followed by infusion at 1 mg/kg/h to be adjusted as appropriate or administered propofol at 2.0–2.5 mg/kg until achieving LOC followed by infusion at 4–10 mg/kg/h. They reported that TLOC was longer in the remimazolam group (102 ± 26.6 s) than in the propofol group (78.7 ± 38.4 s) and that the mean dose of remimazolam required to achieve LOC during anesthetic induction without the co-administration of remifentanil was 0.17 mg/kg. LOC was confirmed to be an average of 121.2 s in patients who received 6 mg/kg/h of remimazolam without the co-administration of remifentanil during anesthetic induction (15). A recent meta-analysis also reported that TLOC was longer in the remimazolam group than in the propofol group (mean differences = 15.49 s, 95% CI: 6.53–24.46) (13).
Nakanishi et al. (7) reported that LOC and ADS <60 were achieved at 80 s and 200 s, respectively, in elderly patients aged ≥65 years receiving remimazolam infusion (6 mg/kg/h) along with remifentanil infusion (0.25 μg/kg/min) during anesthesia induction. A recent meta-analysis reported that ADS measured using BIS after anesthetic induction was higher in the remimazolam group than in the propofol group (13, 16). Doi et al. (12) also reported that the BIS of the remimazolam group was higher (40.0–82.0) than that of the propofol group (39.0–56.3) during the anesthesia maintenance with remifentanil infusion (12). The current study also showed that TLOC and ADS 50 were higher in the group receiving remimazolam than those in the group receiving propofol during remifentanil-based TIVA; this result is consistent with the results of previous studies.
BIS and the Modified Observer Assessment of Alertness and Sedation (MOAA/S) scores have been used in previous comparative studies on the effects of remimazolam and propofol on the depth of anesthesia (17–20). However, BIS was originally developed for monitoring the depth of propofol-based anesthesia, and there is a weak correlation between benzodiazepine-induced anesthetic depth and BIS (4). Therefore, most authors use the MOAA/S score for evaluating the depth of remimazolam-induced anesthesia. Nevertheless, some studies have suggested that BIS of 60–70 is an appropriate range for remimazolam-induced depth of anesthesia (3, 12, 21–23). Thus, based on the synergy between opioids and benzodiazepines, some authors have suggested that a BIS of <60 could be achieved earlier through the use of opioids with remimazolam and initiation of opioids prior to the initiation of remimazolam (6, 24, 25). Zhao et al. (20) suggested that BIS showed a significant association with the MOAA/S score (r = 0.568) and that BIS may be used to predict the state of consciousness in patients receiving remimazolam. The current study also evaluated ADS using BIS or entropy and reported that ADS did not differ significantly despite the higher ADS during anesthesia induction. As mentioned previously, we attributed this finding to the synergistic effects of remimazolam and opioids on ADS.
4.2. Anesthetic recovery
The time for eye-opening and extubation was longer in the remimazolam group than that in the propofol group (12, 26). Doi et al. (12) reported that among patients receiving remifentanil infusion during anesthesia maintenance, the time to eye-opening and extubation was longer in the remimazolam group (14.9 min, 19.2 min) than that in the propofol group (10.3 min, 13.1 min). In contrast, Shi et al. (23) reported that among patients receiving remifentanil infusion during anesthesia maintenance and flumazenil (0.5 mg) at the end of the surgery, the time to recovery and extubation was significantly shorter in the remimazolam group than that in the propofol group. A recent meta-analysis reported no differences in the time to eye-opening and extubation between the two groups (13). In the current study, there was no significant difference in the time to eye-opening time and ADS in ES10 between the groups, but there was a significantly longer time to extubation and OR discharge in the group receiving remimazolam compared to the group receiving propofol. Although not shown in the results, except for three patients who received flumazenil in Group R (Table 4), eye-opening time was significantly longer in Group R than in Group P (p = 0.037), and ADS was lower in ES10 (p = 0.044) when analyzed.
This discrepancy can be explained by the use and difference in the dosages of flumazenil used at the end of the surgery. The use of higher doses of flumazenil hastened recovery from remimazolam (1). Other suggested risk factors associated with delayed recovery are a higher infusion rate and higher BIS after discontinuing remimazolam infusion (6). Although the recovery characteristics of remimazolam showed conflicting results, the recovery time in the group receiving remimazolam was longer than that in the group receiving propofol. However, the difference ranged from 1 to 5 min, which may not be clinically significant in daily practice (1, 12).
4.3. Hemodynamics
The greatest advantage of remimazolam is that it has more stable hemodynamic properties than propofol (14, 24, 27, 28). Most intravenous anesthetics exhibit cardiovascular depressive effects by reducing systemic vascular resistance and cardiac contractility in a dose-dependent manner (1). Propofol significantly reduces SBP and DBP in healthy patients, whereas midazolam maintains SBP and DBP. Considering that remimazolam has hemodynamic effects similar to those of midazolam, it may be associated with hemodynamic effects that are more stable than those of propofol.
Studies on continuous maintenance infusion after bolus dosing or continuous infusion with co-administration of remifentanil or sufentanil revealed that compared with propofol, remimazolam is associated with a lower incidence of bradycardia and hypotension (12, 14, 23, 27). Continuous infusion (6 mg/kg/min) of remimazolam resulted in a lower incidence of all and hypotensive adverse drug reactions (39.3, 21.3%) compared with those of propofol (61.3, 50.7%) and remifentanil at the same time (12). The bolus injection (0.3 mg/kg) of remimazolam (24%) for anesthesia induction results in a lower incidence of hypotension during anesthesia induction than bolus injections of propofol (44%) and sufentanil (24). In addition, the incidence of intraoperative hemodynamic fluctuations was lower in patients receiving remimazolam than in those receiving propofol along with opioids (14, 27). Fluctuations in MBP, HR, cardiac index, and cardiac output during anesthesia induction were lower in the remimazolam group than in the propofol group, along with opioids, without significant differences (16, 29, 30). A recent meta-analysis reported that remimazolam was associated with a significantly lower risk of post-induction hypotension than propofol in patients undergoing non-cardiac surgery (risk ratio [RR] = 0.59, 95% CI: 0.44–0.78) (13). Wu et al. (28) also reported that the remimazolam group showed better hemodynamic stability with a lower incidence of hypotension (RR = 0.43, 95% CI: 0.34–0.55) compared with that in the propofol group.
However, the current study did not show a significant difference in the hemodynamic or cardiovascular events between the remimazolam and propofol groups. Thus, further research is needed to determine the effects and mechanisms of hemodynamic changes when remimazolam and opioids are used in conjunction in elderly patients.
4.4. Limitations
The present study had some limitations. First, this study was conducted using the dose recommended for adults by pharmaceutical manufacturers (6 mg/kg/h), without using the recommended dose of remimazolam for elderly patients, based on previous studies. Although dose adjustment is not necessary for the elderly, TLOC was shorter in elderly patients than in younger patients receiving the same dose of remimazolam, and the dose of remimazolam required to achieve LOC in 95% of patients (ED95) decreased as the age increased (6, 31–33). The ED95 of remimazolam to achieve LOC without the co-administration of opioids within 5 min has been reported to decrease with age, which was suggested as 0.19–0.25 and 0.14–0.19 mg/kg in patients aged 60–80 and > 80 years, respectively (31). Another study reported that the ED95 of remimazolam to achieve LOC within 3 min without the co-administration of opioids in elderly patients was 0.25 mg/kg (95% CI, 0.20–0.29) (33). In the current study, remimazolam was continuously injected at a rate of 6 mg/kg/h until LOC was achieved, and the recalculated bolus dose with TLOC (120 s) was 0.2 mg/kg. This corresponds to the lower limit of the suggested dose in previous studies. However, it can be assumed that LOC can be achieved within 3 min because of the co-administration of remifentanil. Second, nonparametric analysis was performed in this study as most of the data were not normally distributed; therefore, it is important to be careful when interpreting and comparing the results of previous studies with the median values of this study. Third, elderly patients have various underlying diseases, which can affect the anesthesia-related efficacy and safety of remimazolam. Patients with a significantly lower BIS before anesthesia induction were excluded from this study, in consideration of this. Fourth, the small sample size may have affected the results of this study. Fifth, the impact of anesthetics on the occurrence of postoperative delirium is of significant interest to many researchers. However, this study did not assess the occurrence of postoperative delirium. Consequently, we are unable to determine whether remimazolam has a notable effect, either positive or negative, on the incidence of postoperative delirium in comparison to other sedatives. Therefore, considering the limitations of this study, further studies evaluating the efficacy and safety of remimazolam are needed.
Additionally, the attending anesthesiologist was unable to blind the research drugs because of the color difference between the study drugs. Although all researchers have tried to minimize bias in data collection from a research ethics perspective, this cannot rule out the possibility of bias in the data collected during anesthesia management. Therefore, it is necessary to be careful in interpreting the results for remimazolam, and a study of administering placebo drugs together for each drug may be necessary to analyze the effectiveness of the two drugs to minimize data collection bias.
Finally, when considering the use of remimazolam, the cost-effective aspect cannot be ignored. In fact, remimazolam costs more than 20 times higher as much as propofol. However, the authors believe that the high cost of remimazolam is inevitable, given that it is a recently developed drug and is not yet commonly covered by health insurance for all patients. Therefore, it may be somewhat unreasonable to evaluate the cost-effectiveness of remimazolam at this stage. With sufficient research on its efficacy, effectiveness, and safety, and with potential inclusion in health insurance coverage for anesthesia induction and management, there should be further discussions regarding cost-effectiveness evaluation.
5. Conclusion
In conclusion, remimazolam does not have a comparable effect to propofol on anesthesia induction and recovery in elderly patients receiving remifentanil-based TIVA. However, the times to LOC, extubation, and discharge from the operating room were longer in the remimazolam group by 1, 2, and 3 min, respectively, although these differences may not be clinically significant. Furthermore, it has a comparable effect to propofol on intraoperative anesthesia depth and hemodynamic profile.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Institutional Review Board of the Chosun University Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
JP: Conceptualization, Investigation, Methodology, Project administration, Visualization, Writing – original draft. KS: Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Visualization, Writing – original draft, Writing – review & editing. SK: Conceptualization, Investigation, Methodology, Project administration, Visualization, Writing – original draft, Data curation, Formal analysis, Funding acquisition, Resources, Software, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by a research fund from the Chosun University, 2022 (research project no. 2022-205407-01).
Acknowledgments
The authors thank the researchers (not registered in authorship) who participated in obtaining consent forms and collecting data for this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
1. Kim, SH, and Fechner, J. Remimazolam - current knowledge on a new intravenous benzodiazepine anesthetic agent. Korean J Anesthesiol. (2022) 75:307–15. doi: 10.4097/kja.22297
2. Zhang, S, Wang, J, Ran, R, Peng, Y, and Xiao, Y. Efficacy and safety of remimazolam tosylate in hysteroscopy: a randomized, single-blind, parallel controlled trial. J Clin Pharm Ther. (2022) 47:55–60. doi: 10.1111/jcpt.13525
3. Cardia, L. Remimazolam: an ultrashort-acting intravenous anesthetic suitable for general anesthesia. Minerva Anestesiol. (2021) 87:1059–63. doi: 10.23736/S0375-9393.21.16006-7
4. Kim, KM. Remimazolam: pharmacological characteristics and clinical applications in anesthesiology. Anesth Pain Med (Seoul). (2022) 17:1–11. doi: 10.17085/apm.21115
5. Goudra, BG, and Singh, PM. Remimazolam: the future of its sedative potential. Saudi J Anaesth. (2014) 8:388–91. doi: 10.4103/1658-354X.136627
6. Lohmer, LL, Schippers, F, Petersen, KU, Stoehr, T, and Schmith, VD. Time-to-event modeling for Remimazolam for the indication of induction and maintenance of general anesthesia. J Clin Pharmacol. (2020) 60:505–14. doi: 10.1002/jcph.1552
7. Nakanishi, T, Sento, Y, Kamimura, Y, Tsuji, T, Kako, E, and Sobue, K. Remimazolam for induction of anesthesia in elderly patients with severe aortic stenosis: a prospective, observational pilot study. BMC Anesthesiol. (2021) 21:306. doi: 10.1186/s12871-021-01530-3
8. Shimamoto, Y, Sanuki, M, Kurita, S, Ueki, M, Kuwahara, Y, and Matsumoto, A. Factors affecting prolonged time to extubation in patients given remimazolam. PLoS One. (2022) 17:e0268568. doi: 10.1371/journal.pone.0268568
9. Finlay, JE, and Leslie, K. Sedation/analgesia techniques for nonoperating room anesthesia: new drugs and devices. Curr Opin Anaesthesiol. (2021) 34:678–82. doi: 10.1097/ACO.0000000000001057
10. Yokose, M, Takaki, R, Mihara, T, Saigusa, Y, Yamamoto, N, Masui, K, et al. Hypotension after general anesthesia induction using remimazolam in geriatric patients: protocol for a double-blind randomized controlled trial. PLoS One. (2022) 17:e0275451. doi: 10.1371/journal.pone.0275451
11. Zhang, J, Wang, X, Zhang, Q, Wang, Z, and Zhu, S. Application effects of remimazolam and propofol on elderly patients undergoing hip replacement. BMC Anesthesiol. (2022) 22:118. doi: 10.1186/s12871-022-01641-5
12. Doi, M, Morita, K, Takeda, J, Sakamoto, A, Yamakage, M, and Suzuki, T. Efficacy and safety of remimazolam versus propofol for general anesthesia: a multicenter, single-blind, randomized, parallel-group, phase IIb/III trial. J Anesth. (2020) 34:543–53. doi: 10.1007/s00540-020-02788-6
13. Ko, CC, Hung, KC, Illias, AM, Chiu, CC, Yu, CH, Lin, CM, et al. The use of remimazolam versus propofol for induction and maintenance of general anesthesia: a systematic review and meta-analysis. Front Pharmacol. (2023) 14:1101728. doi: 10.3389/fphar.2023.1101728
14. Zhang, X, Li, S, and Liu, J. Efficacy and safety of remimazolam besylate versus propofol during hysteroscopy: single-Centre randomized controlled trial. BMC Anesthesiol. (2021) 21:156. doi: 10.1186/s12871-021-01373-y
15. Kim, KM, Bang, JY, Lee, JM, Yang, HS, Choi, BM, and Noh, GJ. Effect-site concentration of remimazolam at loss and recovery of responsiveness during general anesthesia: a simulation study. Anesth Pain Med (Seoul). (2022) 17:262–70. doi: 10.17085/apm.21121
16. Gao, J, Yang, C, Ji, Q, and Li, J. Effect of remimazolam versus propofol for the induction of general anesthesia on cerebral blood flow and oxygen saturation in elderly patients undergoing carotid endarterectomy. BMC Anesthesiol. (2023) 23:153. doi: 10.1186/s12871-023-02095-z
17. Lu, Z, Zhou, N, Li, Y, Yang, L, and Hao, W. Up-down determination of the 90% effective dose (ED90) of remimazolam besylate for anesthesia induction. Ann Palliat Med. (2022) 11:568–73. doi: 10.21037/apm-22-89
18. Sheng, XY, Liang, Y, Yang, XY, Li, LE, Ye, X, Zhao, X, et al. Safety, pharmacokinetic and pharmacodynamic properties of single ascending dose and continuous infusion of remimazolam besylate in healthy Chinese volunteers. Eur J Clin Pharmacol. (2020) 76:383–91. doi: 10.1007/s00228-019-02800-3
19. Xin, Y, Chu, T, Wang, J, and Xu, A. Sedative effect of remimazolam combined with alfentanil in colonoscopic polypectomy: a prospective, randomized, controlled clinical trial. BMC Anesthesiol. (2022) 22:262. doi: 10.1186/s12871-022-01805-3
20. Zhao, TY, Chen, D, Xu, ZX, Wang, HL, and Sun, H. Comparison of bispectral index and patient state index as measures of sedation depth during surgeries using remimazolam tosilate. BMC Anesthesiol. (2023) 23:208. doi: 10.1186/s12871-023-02172-3
21. Antonik, LJ, Goldwater, DR, Kilpatrick, GJ, Tilbrook, GS, and Borkett, KM. A placebo- and midazolam-controlled phase I single ascending-dose study evaluating the safety, pharmacokinetics, and pharmacodynamics of remimazolam (CNS 7056): part I. Safety, efficacy, and basic pharmacokinetics. Anesth Analg. (2012) 115:274–83. doi: 10.1213/ANE.0b013e31823f0c28
22. Masui, K. Remimazolam besilate, a benzodiazepine, has been approved for general anesthesia!! J Anesth. (2020) 34:479–82. doi: 10.1007/s00540-020-02755-1
23. Shi, F, Chen, Y, Li, H, Zhang, Y, and Zhao, T. Efficacy and safety of Remimazolam Tosilate versus Propofol for general anesthesia in cirrhotic patients undergoing endoscopic variceal ligation. Int J Gen Med. (2022) 15:583–91. doi: 10.2147/IJGM.S345390
24. Dai, G, Pei, L, Duan, F, Liao, M, Zhang, Y, Zhu, M, et al. Safety and efficacy of remimazolam compared with propofol in induction of general anesthesia. Minerva Anestesiol. (2021) 87:1073–9. doi: 10.23736/S0375-9393.21.15517-8
25. Zhou, J, Leonowens, C, Ivaturi, VD, Lohmer, LL, Curd, L, Ossig, J, et al. Population pharmacokinetic/pharmacodynamic modeling for remimazolam in the induction and maintenance of general anesthesia in healthy subjects and in surgical subjects. J Clin Anesth. (2020) 66:109899. doi: 10.1016/j.jclinane.2020.109899
26. Doi, M, Hirata, N, Suzuki, T, Morisaki, H, Morimatsu, H, and Sakamoto, A. Safety and efficacy of remimazolam in induction and maintenance of general anesthesia in high-risk surgical patients (ASA class III): results of a multicenter, randomized, double-blind, parallel-group comparative trial. J Anesth. (2020) 34:491–501. doi: 10.1007/s00540-020-02776-w
27. Liu, T, Lai, T, Chen, J, Lu, Y, He, F, Chen, Y, et al. Effect of remimazolam induction on hemodynamics in patients undergoing valve replacement surgery: a randomized, double-blind, controlled trial. Pharmacol Res Perspect. (2021) 9:e00851. doi: 10.1002/prp2.851
28. Wu, X, Wang, C, Gao, H, Bai, X, Zhang, Z, Chen, R, et al. Comparison of remimazolam and propofol about safety outcome indicators during general anesthesia in surgical patients: a systematic review and meta-analysis. Minerva Anestesiol. (2023) 89:553–64. doi: 10.23736/S0375-9393.23.17034-9
29. Sekiguchi, R, Kinoshita, M, Kawanishi, R, Kakuta, N, Sakai, Y, and Tanaka, K. Comparison of hemodynamics during induction of general anesthesia with remimazolam and target-controlled propofol in middle-aged and elderly patients: a single-center, randomized, controlled trial. BMC Anesthesiol. (2023) 23:14. doi: 10.1186/s12871-023-01974-9
30. Tang, F, Yi, JM, Gong, HY, Lu, ZY, Chen, J, Fang, B, et al. Remimazolam benzenesulfonate anesthesia effectiveness in cardiac surgery patients under general anesthesia. World J Clin Cases. (2021) 9:10595–603. doi: 10.12998/wjcc.v9.i34.10595
31. Chae, D, Kim, HC, Song, Y, Choi, YS, and Han, DW. Pharmacodynamic analysis of intravenous bolus remimazolam for loss of consciousness in patients undergoing general anaesthesia: a randomised, prospective, double-blind study. Br J Anaesth. (2022) 129:49–57. doi: 10.1016/j.bja.2022.02.040
32. Liu, M, Sun, Y, Zhou, L, Feng, K, Wang, T, and Feng, X. The median effective dose and bispectral index of remimazolam tosilate for anesthesia induction in elderly patients: An up-and-down sequential allocation trial. Clin Interv Aging. (2022) 17:837–43. doi: 10.2147/CIA.S364222
Keywords: efficacy, elderly patient, hemodynamics, propofol, remimazolam, safety, sedation
Citation: So KY, Park J and Kim SH (2023) Safety and efficacy of remimazolam for general anesthesia in elderly patients undergoing laparoscopic cholecystectomy: a randomized controlled trial. Front. Med. 10:1265860. doi: 10.3389/fmed.2023.1265860
Edited by:
Daniela Calina, University of Medicine and Pharmacy of Craiova, RomaniaReviewed by:
Matthew Whalin, Emory University, United StatesVikas Chauhan, Columbia University, United States
Copyright © 2023 So, Park and Kim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sang Hun Kim, a3NoMzIyM0BjaG9zdW4uYWMua3I=
 Keum Young So1,2
Keum Young So1,2 Sang Hun Kim
Sang Hun Kim