
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med., 15 November 2023
Sec. Rheumatology
Volume 10 - 2023 | https://doi.org/10.3389/fmed.2023.1257045
This article is part of the Research TopicModern Treatment of Autoinflammatory DiseasesView all 8 articles
 Ekaterina Alexeeva1,2
Ekaterina Alexeeva1,2 Meiri Shingarova1,2
Meiri Shingarova1,2 Tatyana Dvoryakovskaya1,2
Tatyana Dvoryakovskaya1,2 Olga Lomakina1
Olga Lomakina1 Anna Fetisova1
Anna Fetisova1 Ksenia Isaeva1
Ksenia Isaeva1 Aleksandra Chomakhidze1
Aleksandra Chomakhidze1 Kristina Chibisova1
Kristina Chibisova1 Elizaveta Krekhova1
Elizaveta Krekhova1 Aleksandra Kozodaeva2
Aleksandra Kozodaeva2 Kirill Savostyanov3
Kirill Savostyanov3 Aleksandr Pushkov3
Aleksandr Pushkov3 Ilya Zhanin3
Ilya Zhanin3 Dmitry Demyanov3
Dmitry Demyanov3 Evgeny Suspitsin4,5
Evgeny Suspitsin4,5 Konstantin Belozerov6
Konstantin Belozerov6 Mikhail Kostik6*
Mikhail Kostik6*Introduction: The blockade of interleukine-1 (anakinra and canakinumab) is a well-known highly effective tool for monogenic autoinflammatory diseases (AIDs), such as familial Mediterranean fever, tumor necrosis factor receptor-associated periodic syndrome, hyperimmunoglobulinaemia D syndrome, and cryopyrin-associated periodic syndrome, but this treatment has not been assessed for patients with undifferentiated AIDs (uAIDs). Our study aimed to assess the safety and efficacy of canakinumab for patients with uAIDs.
Methods: Information on 32 patients with uAIDs was retrospectively collected and analyzed. Next-generation sequencing and Federici criteria were used for the exclusion of the known monogenic AID.
Results: The median age of the first episode was 2.5 years (IQR: 1.3; 5.5), that of the disease diagnosis was 5.7 years (IQR: 2.5;12.7), and that of diagnostic delay was 1.1 years (IQR: 0.4; 6.1). Patients had variations in the following genes: IL10, NLRP12, STAT2, C8B, LPIN2, NLRC4, PSMB8, PRF1, CARD14, IFIH1, LYST, NFAT5, PLCG2, COPA, IL23R, STXBP2, IL36RN, JAK1, DDX58, LACC1, LRBA, TNFRSF11A, PTHR1, STAT4, TNFRSF1B, TNFAIP3, TREX1, and SLC7A7. The main clinical features were fever (100%), rash (91%; maculopapular predominantly), joint involvement (72%), splenomegaly (66%), hepatomegaly (59%), lymphadenopathy (50%), myalgia (28%), heart involvement (31%), intestinal involvement (19%); eye involvement (9%), pleuritis (16%), ascites (6%), deafness, hydrocephalia (3%), and failure to thrive (25%). Initial treatment before canakinumab consisted of non-biologic therapies: non-steroidal anti-inflammatory drugs (NSAID) (91%), corticosteroids (88%), methotrexate (38%), intravenous immunoglobulin (IVIG) (34%), cyclosporine A (25%), colchicine (6%) cyclophosphamide (6%), sulfasalazine (3%), mycophenolate mofetil (3%), hydroxychloroquine (3%), and biologic drugs: tocilizumab (62%), sarilumab, etanercept, adalimumab, rituximab, and infliximab (all 3%). Canakinumab induced complete remission in 27 patients (84%) and partial remission in one patient (3%). Two patients (6%) were primary non-responders, and two patients (6%) further developed secondary inefficacy. All patients with partial efficacy or inefficacy were switched to tocilizumab (n = 4) and sarilumab (n = 1). The total duration of canakinumab treatment was 3.6 (0.1; 8.7) years. During the study, there were no reported Serious Adverse Events (SAEs). The patients experienced non-frequent mild respiratory infections at a rate that is similar as before canakinumab is administered. Additionally, one patient developed leucopenia, but it was not necessary to stop canakinumab for this patient.
Conclusion: The treatment of patients with uAIDs using canakinumab was safe and effective. Further randomized clinical trials are required to confirm the efficacy and safety.
Autoinflammatory diseases (AIDs) represent a group of disorders characterized by recurrent episodes of seemingly unprovoked systemic inflammation predominantly mediated by the innate immune system's cells, without the high-titer autoantibodies or antigen-specific T cells that are usually detected in classic autoimmunity (1). Interleukine-1 hyperproduction is a key point in the pathogenesis of AIDs, and interleukine-1 blockade is a successful treatment for known monogenic AIDs, such as familial Mediterranean fever (FMF), cryopyrin-associated periodic syndrome (CAPS), tumor necrosis factor-associated periodic syndrome (TRAPS), and mevalonate kinase deficiency (MKD) syndrome (2–5). More than 50% of patients with AIDs do not have an exact molecular diagnosis (6). These patients exhibit episodes of fever and systemic inflammation, but they do not have the known abovementioned monogenic AIDs; hence, they belong to the family of undifferentiated AIDs (uAIDs) (7). Patients with uAIDs may experience arthralgia, arthritis, myalgia, abdominal pain, skin and mucosal involvement, and fatigue. Due to multiple genetic variants operating in the innate system regulation found in patients with uAIDs, differences are suspected in the pathogenesis of these diseases (8).
A previous study investigating the efficacy of anakinra (an IL-1 receptor antagonist) on uAIDs revealed its efficacy in patients with uAIDs, but the data on canakinumab are scarce and limited to case reports (7, 9). Our study aimed to evaluate the safety and efficacy of canakinumab (anti-IL-1β agent) for patients with uAIDs.
We included information about 32 patients: 13 (41%) boys and 19 (59%) girls with a median age of 2.5 (0.08; 16.7) years and with uAIDs. The patients were recruited from two tertiary centers in the retrospective cohort study. The study included patients who received the first dose of canakinumab from 2013 to 2022.
- Patients with uAIDs were included in this study. The term uAIDs includes children who exhibit clear features of an AID but do not fit a specific diagnosis due to either a non-diagnostic phenotype or negative genetic tests that are typical for known AIDs (9). We applied Federici criteria to exclude well-defined monogenic AIDs (10).
- Patients with NGS (with or without variations in genes, operating in autoinflammatory conditions), except for known diseases (FMF, MKD, CAPS, and TRAPS), were included.
- All included patients had received at least one dose of canakinumab.
Genomic DNA was isolated from whole blood samples using a DNA Blood Mini Kit (QIAGEN, Germany), on a QIAcube automated station (QIAGEN, Germany) according to the manufacturer's protocol. The DNA quality and quantity were evaluated spectrophotometrically on a NanoPhotometer N60 (Implen, Germany) and using a Qubit dsDNA HS Assay Kit for a Qubit 3.0 fluorometer (Invitrogen, USA). Genomic libraries were prepared using the KAPA library preparation kit (Rosche USA). Sequencing was performed using custom NGS panels, containing 14 and 83 genes. Additionally, clinical or whole exome sequencing was performed for three patients. Enrichment was carried out using SeqCapEZ technology (Roche, USA). The total size of the panel was 50 and 200 kbp, while the average reading depth was 100×. The number of reads with 50× depth was more than 99% in all target areas. Miseq (Illumina, USA) was used as a sequencing platform. Bioinformatic analysis was carried out according to the recommendations of GATK Best Practices (https://gatk.broadinstitute.org/).
All detected genome missense variants with a frequency of <5% according to the international base gnomAD version 2.1.1 (http://gnomad.broadinstitute.org), which were absent from the HGMD prof database (version 2020.1., June 2020), underwent bioinformatic analysis. Consequently, we separated variants with pathogenicity confirmed by at least three out of four bioinformatic resources: SIFT (damaging), PolyPhen-1 (probably damaging), PolyPhen-2 (probably damaging), and Mutation Tester (disease-causing). The conserved novel missense and splicing mutations were analyzed using the bioinformatic software Alamut Visual Plus (version 1.5.1).
Patients with known rheumatic conditions, infections, malignancies, confirmed primary immunodeficiency syndromes, known monogenic AIDs (FMF, TRAPS, MKD, CAPS), or other known causes of periodic fever unrelated to AIDs were excluded from the study. Patients with PFAPA syndrome were also not included in the study.
For every patient, we evaluated the following parameters:
- Demography: We evaluated the onset age, age of genetic testing, gender, family history, diagnosis delay, and initial diagnosis.
- Clinical characteristics: We evaluated the fever duration and number of fever episodes per year, the presence of rash and its characteristics, the presence of lymphadenopathy, hepatomegaly, splenomegaly, arthritis/arthralgia, myalgia, eye, ear, and CNS involvement. For patients with persistent fever, the number of fever episodes was established as 12 per year.
- Laboratory findings: We evaluated the C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), white blood cells (WBC), platelets, and the hemoglobin level during the acute phase of the disease.
- Data of observed genetic variants classified according to the ACMG criteria: We classified the data into pathogenic, likely pathogenic, variant of unknown significance, likely benign, and benign (11).
- Treatment before canakinumab administration and their efficacy, time since disease onset, and time between the diagnosis and canakinumab administration were evaluated.
- Characteristics of canakinumab treatment: We evaluated the characteristics of canakinumab treatment, including initial doses and frequency.
- Outcomes of canakinumab treatment: We evaluated the outcomes, including remission (complete, partial, or no remission) and time before remission. In cases of failed efficacy, the type of inefficacy (primary or secondary) was evaluated, as well as the switching to other biologic drugs. The duration of canakinumab treatment was also evaluated.
- Complete remission indicates the absence of clinical [physician global assessment (PGA) = 0/2] and laboratory features (normal CRP) of the disease without corticosteroid treatment. Partial remission indicates significant clinical and laboratory improvement according to the attending physician's opinion or clinical remission with remaining laboratory activity.
The primary aim was to assess the efficacy of canakinumab by the type of response, and its corresponding primary endpoint would be the number/percentage of patients achieving complete and/or partial response alongside their definitions.
- Complete response was defined as clinical and serological remission, as well as normal CRP.
- Partial response was defined clinically as a change in the Likert category to a category below and/or serologically as a 50% reduction in CRP but not in the normal range (0–5 mg/l) (7, 12).
- No response included patients who failed to meet the criteria for either remission or partial response (as above), despite canakinumab.
The secondary aims were paired comparisons of (i) markers of systemic inflammation before and after the treatment (ESR, CRP, WBC, PLT, and hemoglobin); (ii) concomitant treatment changes, including steroids-sparing effect; and (iii) the safety profile.
The data on adverse events were collected during routine follow-up visits, by asking parents or looking at patients' medical papers.
The sample size was not calculated. The software XLSTAT Version 2016.02.28451, Addinsoft, France (release 10.0, StatSoft Corporation, Tulsa, OK, USA), was used for data analyzes. The descriptive statistics were reported in medians and interquartile ranges (IQRs) for continuous variables and in absolute frequencies and percentages for categorical variables. A comparison of quantitative variables recorded before and after therapy was carried out using the Wilcoxon criterion, and a similar comparison of categorical variables was carried out using the McNemar criterion. Survival analysis of each group, with each being flare-free as the event of interest, was conducted by employing the Kaplan–Meier method. The log-rank test was performed to compare the survival curves. The differences were considered statistically significant at a p-value of < 0.05.
The median age of disease onset was 2.5 (1.3; 5.5) years, that of disease diagnosis was 5.7 (2.5; 12.7) years, and that of diagnostic delay was 1.1 (0.4; 6.1) years. The main clinical features were fever (100 %) and rash (91%), which included maculopapular rash predominantly (53.3%), polymorphic rash (16.7%), erythematous rash (10%), coarse-spotted and finely-spotted rash (7%), and papular and urticaria rash (3%). Joint involvement occurred in 72% of the patients had polyarticular (54%) and oligoarticular (46%) courses. Musculoskeletal manifestations were mainly represented by arthralgia (96%), arthritis (83%), reduced range of motion (79%), and myalgia (28%). Splenomegaly was noted in 66% of the patients, hepatomegaly in 59%, and lymphadenopathy in 50% of the patients. Serositis was detected in 17 patients, with pericardial effusion in 10 (31%) of them, followed by pleural (n = 5; 16%) and abdominal effusion (n = 2; 6%).
Four patients (9%) had eye involvement, including conjunctivitis (n = 2; 6%) and congenital cataract (n = 1; 3%). Six patients (19%) had gastrointestinal involvement, and eight patients (25%) experienced the failure to thrive. One patient (3%) had sensorineural hearing loss, and one patient (3%) had hydrocephalus. The highest parameters of laboratory activity at the disease onset were as follows: ESR 47 mm/h (30; 58) and CRP 75.6 mg/l (42.5; 119.4), white blood cells 18.5 (13; 21.4) × 109/l, with neutrophilia 12.6 (17; 52.8) × 109/l, thrombocytosis 449 (320.5; 529) × 109/l, and anemia: hemoglobin 93 (85; 102) g/l. The data are presented in Table 1.
Next-generation sequencing was performed in all patients, and no genetic variants were found only in two (6%) patients. One patient underwent clinical exome sequencing, and two patients underwent whole-exome sequencing. NGS was performed using a panel of 15 genes in six patients (18%) and using a panel of 83 genes in 24 patients (73%). Clinical exome sequencing was performed in one patient (3%), and whole-exome sequencing was performed in two patients (6%). Heterozygous genetic variants were found in the remaining 31 patients (97%) in the following genes: IL10, NLRP12, STAT2, C8B, LPIN2, NLRC4, PSMB8, PRF1, CARD14, IFIH1, LYST, NFAT5, PLCG2, COPA, IL23R, STXBP2, IL36RN, JAK1, DDX58, LACC1, LRBA, TNFRSF11A, PTHR1, STAT4, TNFRSF1B, TNFAIP3, and TREX1 и SLC7A7. According to the ACMG criteria, pathogenic variants were found in the genes C8B (p.R428X) and TREX1 (p.Y360C), likely pathogenic variants were found in the gene NFAT5 (p.A622V), likely benign variants were found in the genes PSMB8 (p.G8R), STAT2 (p.G825C), TNFRSF1B (p.P205A) and IL23R (p.G149R), and variants of unknown significance were found in the genes IL10 (p.G15R), PTHR1 (p.R150C), and STAT4 (p.E128V). Variants with unknown significance were the most frequent, accounting for 35 out of 45 variants (77.8%) among the total identified variants. In our study, patients neither met the Federici criteria nor did they have variants in the NLRP3, TNFRSF1A, MVK, and MEFV genes according to the NGS data. When applying the Federici criteria, the majority of patients (n = 29, 91%) from our study were not clinically classified into any of the main syndromes of periodic fever. Table 2 lists the genetic mutations detected for each patient.
The initial treatment before canakinumab consisted of non-biologic drugs: non-steroidal anti-inflammatory drugs (NSAID) (91%), corticosteroids (85%), methotrexate (38%), intravenous immunoglobulin (IVIG) (34%), cyclosporine A (24%), colchicine (6%), cyclophosphamide (6%), sulfasalazine (3%), and mycophenolate mofetil (3%), and biologic drugs: tocilizumab (62%), sarilumab, etanercept, adalimumab, rituximab, and infliximab (all by 3%). Before the year 2022, we had no access to anakinra. The median time from the disease onset to canakinumab administration was 2.5 (1.3; 5.5) years. Thirteen patients (41%) received canakinumab as the first-line biologic treatment, and 19 patients (59%) received it as the second-line biologic treatment. In the majority of patients, 26 (81%), the initial dose of canakinumab was 4 mg/kg every 4 weeks, in 5 patients (16%), it was 2 mg/kg every 4 weeks, and in one patient (3%), it was 8 mg/kg every 4 weeks. The detailed patients' characteristics are presented in Table 3.
Complete remission was achieved in 27 (84%) patients. Two patients (6%) displayed secondary inefficacy in 3 months, while two others displayed primary inefficacy. One patient had a partial response to canakinumab. In all five patients, canakinumab was switched to other drugs: tocilizumab (n = 3; 60%), anakinra (n = 1; 20%), and tofacitinib (n = 1; 20%). In two patients, it was switched to tocilizumab, and complete remission was achieved. The remaining two patients who were switched to anakinra and tofacitinib required a switch to sarilumab administration, which induced remission. One patient (Pt 4) died after switching to tocilizumab. This patient had early onset of symptoms (at 1.5 years), with rash, pericarditis, bilateral interstitial lung disease, and MAS. Unfortunately, the patient failed to respond to high-dose intravenous and oral glucocorticosteroids and IVIG. Canakinumab was prescribed due to MAS with multiorgan failure. The patient partially responded to the first dose (which resulted in decreased fever, rash, arthritis, and laboratory activity) but developed a new flare after an attempt to taper corticosteroid treatment and was switched to tocilizumab. The patient again had a partial response but died due to the exacerbation of the underlying disease, protract MAS, multiorgan failure, and infection complication.
An analysis of the effectiveness depending on the time of initiation of therapy with canakinumab was carried out. Early administration (≤ 12 months from onset) of canakinumab was carried out in 11 out of 32 patients (35%). Consequently, therapy was effective in 9 out of 11 patients (82%), one patient (9%) had primary inefficiency, and one patient (9%) had secondary inefficiency. Late administration of canakinumab (>12 months from onset) was carried out in 21 out of 32 patients (65%). Consequently, therapy was effective in 18 out of 21 patients (85%), one patient had primary inefficiency (5%), one patient developed secondary inefficiency (5%), and one patient had a partial response (5%).
As the first line of therapy, canakinumab was administered early to 5 out of 11 patients (46%) and late to 7 out of 21 patients (33%). It was prescribed as the second line of therapy in 6 out of 11 patients (54%) in the group with early initiation and in 14 out of 21 patients (67%) in the group with late initiation.
Therapy survival was evaluated in 11 out of 32 patients (35%) with early initiation of canakinumab treatment and 21 out of 31 patients (65%) with late initiation of canakinumab treatment. The flare-free survival in the group of patients with early administration was 96.1 months, and that in the group with late administration was 96.2 months (p = 0.908; Figure 1A).
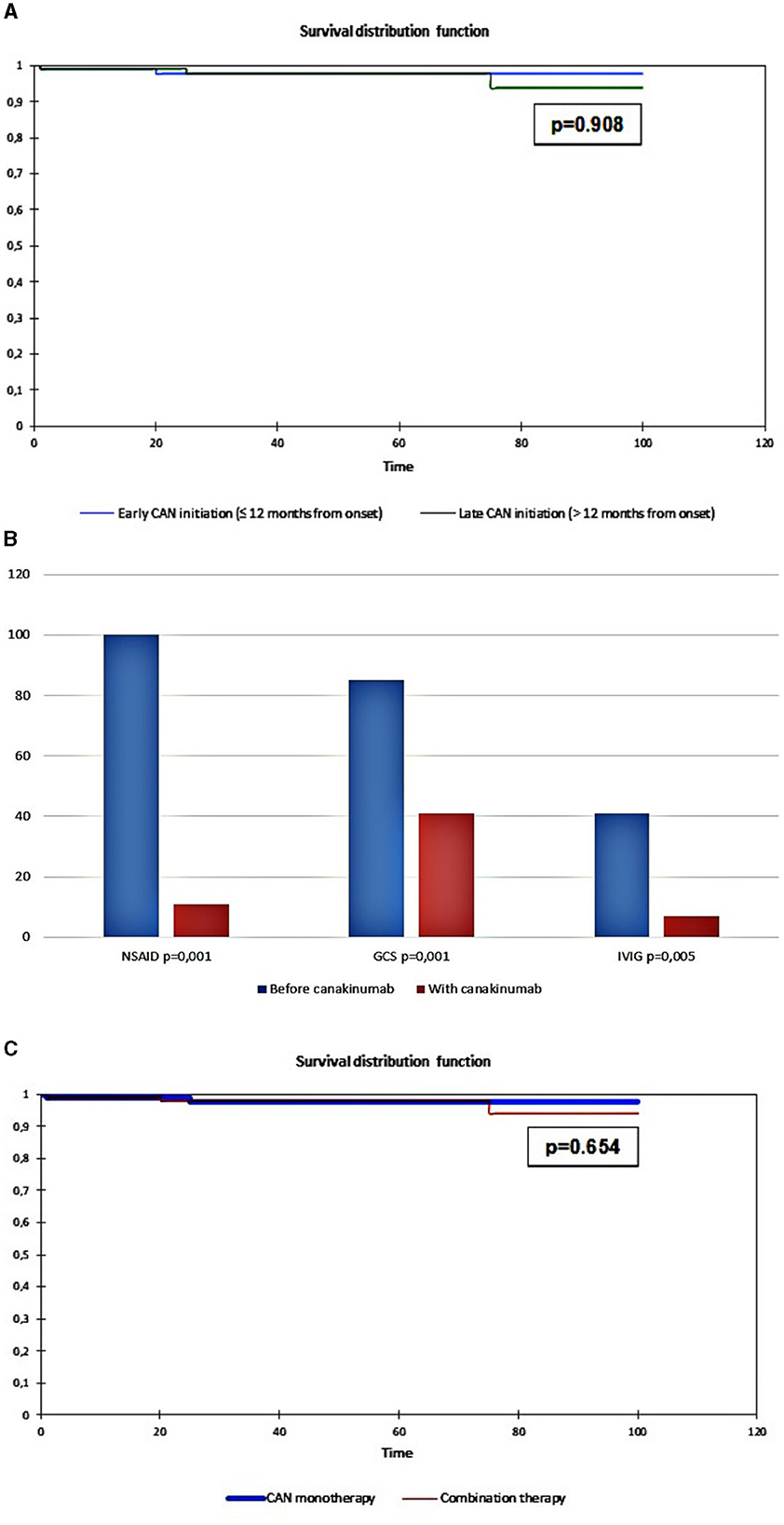
Figure 1. (A) Cumulative probability of being flare-free in uAID patients depending on early canakinumab administration (≤12 months from the onset, blue line) and late canakinumab administration (>12 months from the onset, green line); (B) Dynamics of concomitant treatment during the canakinumab trial; (C) Cumulative probability of being flare-free in uAID patients depending on the canakinumab monotherapy (canakinumab only, blue line) and the combination of canakinumab with non-biologic DMARDs (combination therapy, red line).
i) Dynamics of the markers of systemic inflammation
During the 12-month canakinumab trial, ESR decreased significantly from 53 (23; 57) mm/h to 3 (2; 7) mm/h (p < 0.001), CRP decreased from 73.5 (46; 103) mg/l to 0.7 (0.3; 1.9) mg/l (p < 0.001), WBC decreased from 18.6 × 109/l (12.9; 21.8) to 6.6 (5.6;8.9) × 109/l (p < 0.001), neutrophil count decreased from 11.6 × 109/l (17.4; 11.6) to 3.1 (2.2; 3.7) × 109/l, (p < 0.001), platelets decreased from 439 × 109/l (307; 560) to 268 (226; 341) × 109/l (p = 0.003), and hemoglobin increased from 96 g/l (89; 102) to 124 (119; 134) g/l (p < 0.001).
ii) Changes in the concomitant treatment
All patients in our study received concomitant treatment. At the onset, 29 patients (91%) received NSAID, 28 patients (88%) received systemic corticosteroids, 12 patients (38%) received methotrexate, 11 patients (34%) received IVIG, 8 patients (25%) received cyclosporine A, 2 patients (6%) received cyclophosphamide, 2 patients (6%) received colchicine, and 1 patient (3%) received sulfasalazine, mycophenolate mofetil, and hydroxychloroquine.
Among those who responded to canakinumab (n = 27), corticosteroids were successfully discontinued in 12 patients (44%, p = 0.001), NSAID was discontinued in all patients (100%, p = 0.001), and IVIG was discontinued in 9 patients (82%, p = 0.005). All other non-biologic medications were also discontinued. Data are shown in Figure 1B.
Flare-free survival (Figure 1C) was 97.1 months for 17 out of 32 (53%) patients treated with canakinumab monotherapy, and it was 96.1 months for 15 out of 32 (47%) patients treated with combination therapy (canakinumab + non-biologic DMARDS) (Log-rank test, p = 0.654).
iii) Safety profile
Regarding the safety profile, no serious adverse events were observed during canakinumab treatment. Five patients developed mild acute respiratory infections, with a similar rate to what was already detected before canakinumab initiation. One case of leukopenia was also reported, but treatment discontinuation was not required.
Canakinumab showed efficacy in patients with uAID, which was realized in the control of clinical and laboratory features of inflammation as well as in the reduction of concomitant immune-suppressive treatment. We used inclusion criteria for these groups, similar to what was published earlier (9). Canakinumab showed efficacy in AIDs in an earlier study, where it was administered off-label (64). It was successfully prescribed in several distinct AIDs, including DIRA, Majeed syndrome, PAPA syndrome, DADA2, Blau syndrome, systemic juvenile idiopathic arthritis (sJIA), MAS, and fibrodysplasia ossificans progressiva (65–74). Among polygenic multifactorial AIDs, canakinumab showed efficacy in pediatric and adult Behçet syndrome, being predominantly effective in eye disease (75, 76). There are several case reports of using canakinumab in idiopathic relapsed pericarditis, but the Italian experience suggests the superiority of anakinra over canakinumab, attributed to the main role of IL-1a over IL-1β (77, 78).
We used the results for NGS to exclude patients with well-defined monogenic AIDs because some such patients might present with a non-classical phenotype. Broad molecular testing of patients with uAIDs with NGS panels may provide a diagnosis. In 50 patients with uAIDs, 100 genetic variants were found (2 per patient, range 0–6), a quarter of which were in genes. These were related to autoinflammation, and only two patients had variants, allowing us to make a definitive diagnosis (mevalonate kinase deficiency syndrome). Two more patients had variations in the PLCG2 gene, consistent with the clinical phenotype APLAID and PLAID, respectively.
During the canakinumab trial, the majority of our patients (84%) achieved remission. The presence of concomitant non-biologic DMARDs did not influence the cumulative probability of being flare-free. Many previous studies have shown no efficacy of non-biologic DMARDs in patients with uAIDs (7, 79). We did not observe any difference between early and late canakinumab administration, as well as between the first-line and second-line biologic therapy.
Similar to our results with the anti-IL1β blocking agent, a complete response to anakinra (interleukin-1 receptor antagonist) was observed in 5 out of 10 patients (50%) and a partial response in 1 out of 10 patients (10%), while 4 out of 10 patients (40%) were non-responders (9, 80). In the biggest pediatric case series of 22 patients with uAIDs, 8 out of 22 patients (36%) achieved clinical and serological remission, 8 out of 22 patients (36%) had a partial response, and 6 out of 22 patients (28%) were non-responders. A total of 16 out of 22 patients (72%) responded to anakinra. More than half the patients (12/22; 55%) required anakinra dosing up-titration to control their disease activity. This pattern of up-titration is typical for IL-1 blocking agents in the treatment of AIDs (7, 79). Eighteen out of 20 patients (90%) had a normal CRP level within 3 months, and 17 out of 18 patients (94%) had a normal CRP level within 6 months.
In the biggest review of pediatric patients with uAIDs, named syndrome of undifferentiated recurrent fever (SURF), IL-1 antagonists (mainly anakinra) used in 46 patients were effective in 74% of cases, superior to other non-biologic treatment options (79).
Anakinra was effective in 9 out of 11 adult patients with uAIDs within 4–6 weeks after starting the treatment. One patient discontinued therapy due to being an incomplete responder and another due to a severe injection-site reaction (9). Previous treatment with systemic corticosteroids failed in 5 of 11 patients; 6 out of 11 patients had partial control and 3 out of 11 patients had side effects, associated with the chronic use of systemic corticosteroids. Ten patients had 16 courses of ineffective non-biologic DMARDS, and four patients had adverse events. Treatment with anakinra not only controlled inflammation but also allowed for the tapering of corticosteroids in responsive patients who were still alive. It also led to a decrease in deceased patients, as well as non-biologic DMARDs in all responders. Non-responders were successfully switched to the IL-6 receptor antagonist, tocilizumab (9).
Anakinra showed efficacy in controlling inflammation in 8 out of 9 adult patients (89%) with uAIDs and in 5 out of 6 patients (83%) with VEXAS syndrome, who had previously failed to respond to systemic corticosteroid treatment (81).
In one patient from our cohort, canakinumab was chosen for the treatment of both uAIDs and MAS, due to the lack of access to anakinra. Data about canakinumab efficacy in MAS is controversial and limited to several studies (65, 74, 82). In real practice, physicians usually choose anakinra as the first-line biologic treatment for MAS (83). In the randomized clinical trial, the frequency of MAS was two times lower than that in placebo. A recent case series showed the efficacy of a high dose of canakinumab in controlling MAS in sJIA (65).
Previous data similar to ours showed that IL-1 blockers, which are theoretically a universal drug for the treatment of patients with AIDs, were not effective in all patients.
The pathogenesis of known autoinflammatory diseases consists in the abnormal activation of inflammatory processes; this leads to hyperproduction of IL-1ß or IL-18, which, in turn, stimulates the production of inflammatory cytokines, such as IL-6 and TNF-α, and promotes the activation of immune cells, such as macrophages and T-cells (84). Thus, IL-6 inhibitors with the ineffectiveness of IL-1 inhibitors can be considered potential methods of treatment for AIDs (85).
The treatment of non-responders is a challenging problem, requiring the use of other medications, such as colchicine, IL-6, Janus-kinase inhibitors, or their combination (79, 86). The question of whom an IL-1 blocking agent should be prescribed to and when it is still open and requires the development of a treat-to-target strategy due to the different response rates in the literature (87).
The “new-old” drug colchicine has shown its efficacy in FMF and is currently being repositioned for use in the treatment of other AIDs, such as adult-onset Still disease (AOSD) and uAIDs, with a 96% maximal efficacy rate (9, 84, 85). Ten of 11 undiagnosed patients with a strong suspicion of autoinflammatory conditions and formal diagnosis in three of them responded well (91%) to empirically prescribed colchicine, IL-1, and IL-6 blocking without Serious Adverse Events (SAEs) (88).
Tocilizumab, an IL-6 blocking biologic, showed efficacy in sJIA (89–91) and is currently being repositioned for use in the treatment of AIDs, but its efficacy in AID is lower than that of IL-1 blockers (92). The use of IL-6 inhibitors as a second-line therapy in patients with monogenic AIDs, such as familial Mediterranean fever and TRAPS, has been described (93, 94). Additionally, data on the effectiveness of tocilizumab in a small cohort of children with a refractory course of undifferentiated AIDs has been previously published (95). In some patients, tocilizumab has been observed to work well, especially in patients with a sJIA-like phenotype (96). In our cohort, 21 out of 32 patients (66%) received tocilizumab as the first-line biologic treatment, but the treatment was discontinued in 20 out of 21 patients (95%) due to the following reasons: primary (n = 5, 25%) and secondary (n = 7; 35%) inefficacies, a partial response (n = 4; 20%), and severe adverse events, including leucopenia in 3 out 20 patients (15%), infusion reaction (n = 3; 15%) и, and infectious complication (n = 1; 5%). The majority of the patients (n = 19; 95%) were switched to canakinumab, and one patient (5%) was switched to sarilumab, followed by canakinumab due to the primary inefficacy of sarilumab. Tocilizumab has been shown to be more effective when employed as a second-line option, after the failure of IL-1 blockers (7, 9, 93, 94).
Janus-kinase inhibitors (tofacitinib and baricitinib) are considered a new promising option for the treatment of uAIDs, including AOSD, sJIA, and aoSD- and sJIA-like diseases (49, 86, 97). Among 37 patients with previously undiagnosed inflammatory cases, increased IFN signaling was found in 19 patients, with 10 exhibiting clinical features typical of type I interferonopathy (98). Different signaling pathways might explain the failure of IL-1 inhibitors in patients with uAIDs. It is necessary to mention JAK-STAT signaling in patients with uAIDs, especially those with clinical features of type I interferonopathy (99).
In our cohort, there were no reported SAEs. Patients had non-frequent mild respiratory infections with a rate similar to that before canakinumab; one patient developed leukopenia (3%), but it was not required to stop canakinumab. All patients also had a formal diagnosis of sJIA to qualify for biologic treatment.
The treatment of uAIDs with anakinra was associated with SAEs. The main adverse events were death (3/22, 14%), allogeneic hematopoietic stem cell transplantation (1/22, 5%), injection-site reactions (5/22, 23%), infections (8/22, 36%), and neutropenia (7/22, 32%). Ten patients (46%) had 12 SAE episodes. Three deaths occured, among which one was related to macrophage activation syndrome (n = 1), while two were due to multiorgan failure resulting from underlined conditions (n = 2). In both cases, the patients received anakinra, which was stopped before their deaths (7).
Another study reported two SAEs. Among nine responders two patients died: 81-year-old patient treated with anakinra for 3 months died from influenza and 42-year-old woman treated with anakinra for eight months died due to progression of her underlying condition (9).
Creating a worldwide network of experts (international registries) who can share their experiences will potentially be useful for overcoming some of the unmet needs of patients with AIDs (100).
The main limitations of this study were related to the retrospective study design. The lack of whole-exome sequence data of all participants might have led to the omission of some genetic variants. There were no specific universal treatments and diagnostic algorithms. The authors did not influence time and indication for either the use of biologics or the dynamics of concomitant treatment, which might influence treatment efficacy, outcomes, and adverse events. The data on side effects might also have been incomplete due to the absence of a universal electronic medical system, which would have allowed for the timely checking of all patient events and records.
Many cases of uAIDs belong to the family of IL-1-driven diseases. The treatment of patients with uAIDs using canakinumab was safe and effective; it may be recommended as a first-line biologic treatment with or without corticosteroids and non-biologic DMARDs. Molecular diagnostics may help categorize patients into a group of uAIDs, and IL-1 blockers can be considered in cases where the IL-1-mediated pathway is suspected. For non-responders, the use of different treatments, e.g., IL-6 blockade or JAK-STAT inhibitors, is required. More robust, powered, and properly designed studies are warranted to draw firm conclusions regarding the efficacy and safety of canakinumab in uAIDs.
Publicly available datasets were analyzed in this study. This data can be found here: https://www.ncbi.nlm.nih.gov/bioproject/PRJNA1012850.
The Ethics Committee of National Medical Research Center of Children's Health, Moscow, Russian Federation (protocol number 4 from 22.04.2021). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin. Written informed consent was obtained from the participant/patient(s) or their legal guardians/next of kin for the publication of this article.
EA: Writing—original draft, Conceptualization, Funding acquisition, Methodology, Project administration, Supervision, Writing—review and editing. MS: Formal analysis, Investigation, Methodology, Writing—original draft, Writing—review and editing. TD: Investigation, Methodology, Validation, Writing—review and editing. OL: Data curation, Investigation, Writing—review and editing. AF: Investigation, Methodology, Writing—review and editing. KI: Investigation, Methodology, Writing—review and editing. AC: Investigation, Methodology, Writing—review and editing. KC: Investigation, Writing—review and editing. EK: Investigation, Writing—review and editing. AK: Investigation, Writing—review and editing. KS: Writing—review and editing, Formal analysis, Investigation, Methodology, Resources. AP: Data curation, Formal analysis, Investigation, Methodology, Writing—review and editing. IZ: Formal analysis, Investigation, Methodology, Project administration, Resources, Writing—review and editing. DD: Data curation, Formal analysis, Investigation, Methodology, Software, Writing—review and editing. ES: Data curation, Formal analysis, Investigation, Methodology, Writing—review and editing. KB: Formal analysis, Investigation, Writing—review and editing. MK: Conceptualization, Methodology, Writing—original draft, Writing—review and editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The study was supported by the Russian Science Foundation (grant no. 22-45-08004).
EA received research grants from Roche, Pfizer, Centocor, Eli Lilly, AbbVie, Bristol-Myers Squibb, MSD, Sanofi, Amgen, and Novartis, speaker at the Roche, AbbVie, Bristol-Myers, Squibb, MSD, Novartis, and Pfizer bureau. TD received research grants from Roche, Pfizer, Centocor, Eli Lilly, AbbVie, Bristol-Myers Squibb, MSD, Amgen, and Novartis, speaker at the Roche, AbbVie, Bristol-Myers, Squibb, MSD, Novartis, and Pfizer bureau. KI received research grants from Roche, Novartis, and Sanofi. OL received research grants from Pfizer and Eli Lilly. EK speaker at the Novartis bureau.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Georgin-Lavialle S, Fayand A, Rodrigues F, Bachmeyer C, Savey L, Grateau G. Autoinflammatory diseases: State of the art. Presse Med. (2019) 48:e25–48. doi: 10.1016/j.lpm.2018.12.003
2. Kuemmerle-Deschner JB, Ramos E, Blank N, Roesler J, Felix SD, Jung T, et al. Canakinumab (ACZ885, a fully human IgG1 anti-IL-1β mAb) induces sustained remission in pediatric patients with cryopyrin-associated periodic syndrome (CAPS). Arthritis Res Ther. (2011) 13:R34. doi: 10.1186/ar3266
3. La Torre F, Caparello MC, Cimaz R. Canakinumab for the treatment of TNF-receptor associated periodic syndrome. Expert Rev Clin Immunol. (2017) 13:513–23. doi: 10.1080/1744666X.2017.1324783
4. Galeotti C, Meinzer U, Quartier P, Rossi-Semerano L, Bader-Meunier B, Pillet P, et al. Efficacy of interleukin-1-targeting drugs in mevalonate kinase deficiency. Rheumatology. (2012) 51:1855–9. doi: 10.1093/rheumatology/kes097
5. Özen S, Bilginer Y, Ayaz NA, Calguneri M. Anti-interleukin 1 treatment for patients with familial Mediterranean fever resistant to colchicine. J Rheumatol. (2011) 38:516–8. doi: 10.3899/jrheum.100718
6. Rusmini M, Federici S, Caroli F, Grossi A, Baldi M, Obici L, et al. Next-generation sequencing and its initial applications for molecular diagnosis of systemic auto-inflammatory diseases. Ann Rheum Dis. (2016) 75:1550–7. doi: 10.1136/annrheumdis-2015-207701
7. Garg S, Wynne K, Omoyinmi E, Eleftheriou D, Brogan P. Efficacy and safety of anakinra for undifferentiated autoinflammatory diseases in children: a retrospective case review. Rheumatol Adv Pract. (2019) 3:rkz004. doi: 10.1093/rap/rkz004
8. Ter Haar NM, Eijkelboom C, Cantarini L, Papa R, Brogan PA, Kone-Paut I, et al. Clinical characteristics and genetic analyses of 187 patients with undefined autoinflammatory diseases. Ann Rheum Dis. (2019) 78:1405–11. doi: 10.1136/annrheumdis-2018-214472
9. Harrison SR, McGonagle D, Nizam S, Jarrett S, van der Hilst J, McDermott MF, et al. Anakinra as a diagnostic challenge and treatment option for systemic autoinflammatory disorders of undefined etiology. JCI Insight. (2016) 1:e86336. doi: 10.1172/jci.insight.86336
10. Federici S, Sormani MP, Ozen S, Lachmann HJ, Amaryan G, Woo P, et al. Evidence-based provisional clinical classification criteria for autoinflammatory periodic fevers. Ann Rheum Dis. (2015) 74:799–805. doi: 10.1136/annrheumdis-2014-206580
11. Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. (2015) 17:405–24. doi: 10.1038/gim.2015.30
12. Bernstein IH. Likert scale analysis. Encyclop Soc Meas. 497–504. doi: 10.1016/b0-12-369398-5/00104-3
13. van der Linde K, Boor PP, van Bodegraven AA, de Jong DJ, Crusius JB, Naber TH, et al. A functional interleukin-10 mutation in Dutch patients with Crohn's disease. Dig Liver Dis. (2005) 37:330–5. doi: 10.1016/j.dld.2004.12.009
14. Klein W, Tromm A, Griga T, Fricke H, Folwaczny C, Hocke M, et al. The IL-10 gene is not involved in the predisposition to inflammatory bowel disease. Electrophoresis. (2000) 21:3578–82. doi: 10.1002/1522-2683(200011)21:17<3578::AID-ELPS3578>3.0.CO
15. Mendonça LO, Toledo-Barros MAM, Leal VNC, Roa MEGV, Cambuí RAG, Toledo E, et al. In-vitro NLRP3 functional test assists the diagnosis of cryopyrin-associated periodic syndrome (CAPS) patients: A Brazilian cooperation. Clin Immunol. (2022) 245:109159. doi: 10.1016/j.clim.2022.109159
16. Kostik MM, Suspitsin EN, Guseva MN, Levina AS, Kazantseva AY, Sokolenko AP, et al. Multigene sequencing reveals heterogeneity of NLRP12-related autoinflammatory disorders. Rheumatol Int. (2018) 38:887–93. doi: 10.1007/s00296-018-4002-8
17. Pati S, Sarkar S, Das E, Sherpa N, Kanti Das M, Datta S. Frightening Fever: Familial Cold Autoinflammatory Syndrome 2 (FCAS-2) with Macrophage Activation Syndrome (MAS). Indian J Pediatr. (2022) 89:1055. doi: 10.1007/s12098-022-04322-w
18. Jéru I, Le Borgne G, Cochet E, Hayrapetyan H, Duquesnoy P, Grateau G, et al. Identification and functional consequences of a recurrent NLRP12 missense mutation in periodic fever syndromes. Arthritis Rheum. (2011) 63:1459–64. doi: 10.1002/art.30241
19. Greenawalt DM, Liang WS, Saif S, Johnson J, Todorov P, Dulak A, et al. Comparative analysis of primary versus relapse/refractory DLBCL identifies shifts in mutation spectrum. Oncotarget. (2017) 8:99237–44. doi: 10.18632/oncotarget.18502
20. Vidmar L, Maver A, Drulović J, Sepčić J, Novaković I, Ristič S, et al. Multiple Sclerosis patients carry an increased burden of exceedingly rare genetic variants in the inflammasome regulatory genes. Sci Rep. (2019) 9:9171. doi: 10.1038/s41598-019-45598-x
21. Szczawińska-Popłonyk A, Popłonyk N, Niedziela M, Sowińska-Seidler A, Sztromwasser P, Jamsheer A, et al. Case report: The cardio-facio-cutaneous syndrome due to a novel germline mutation in MAP2K1: A multifaceted disease with immunodeficiency and short stature. Front Pediatr. (2022) 10:990111. doi: 10.3389/fped.2022.990111
22. Avnat E, Shapira G, Shoval S, Israel-Elgali I, Alkelai A, Shuldiner AR, et al. Comprehensive Genetic Analysis of Druze Provides Insights into Carrier Screening. Genes (Basel). (2023) 14:937. doi: 10.3390/genes14040937
23. Lu C, Xie M, Wendl MC, Wang J, McLellan MD, Leiserson MD, et al. Patterns and functional implications of rare germline variants across 12 cancer types. Nat Commun. (2015) 6:10086. doi: 10.1038/ncomms10086
24. Griffith OL, Spies NC, Anurag M, Griffith M, Luo J, Tu D, et al. The prognostic effects of somatic mutations in ER-positive breast cancer. Nat Commun. (2018) 9:3476. doi: 10.1038/s41467-018-05914-x
25. Garofalo A, Sholl L, Reardon B, Taylor-Weiner A, Amin-Mansour A, Miao D, et al. The impact of tumor profiling approaches and genomic data strategies for cancer precision medicine. Genome Med. (2016) 8:79. doi: 10.1186/s13073-016-0333-9
26. Abe K, Cox A, Takamatsu N, Velez G, Laxer RM, Tse SML, et al. Gain-of-function mutations in a member of the Src family kinases cause autoinflammatory bone disease in mice and humans. Proc Natl Acad Sci U S A. (2019) 116:11872–7. doi: 10.1073/pnas.1819825116
27. Csaki LS, Reue K. Lipins: multifunctional lipid metabolism proteins. Annu Rev Nutr. (2010) 30:257–72. doi: 10.1146/annurev.nutr.012809.104729
28. Atschekzei F, Dubrowinskaja N, Anim M, Thiele T, Witte T, Sogkas G. Identification of variants in genes associated with autoinflammatory disorders in a cohort of patients with psoriatic arthritis. RMD Open. (2022) 8:e002561. doi: 10.1136/rmdopen-2022-002561
29. Zou J, Zhang G, Li H, Zhao Z, Zhang Q, Pyykkö I, et al. Multiple genetic variants involved in both autoimmunity and autoinflammation detected in Chinese patients with sporadic Meniere's disease: a preliminary study. Front Neurol. (2023) 14:1159658. doi: 10.3389/fneur.2023.1159658
30. Popplewell LF, Encarnacion M, Bernales CQ, Sadovnick AD, Traboulsee AL, Quandt JA, et al. Genetic analysis of nucleotide-binding leucine-rich repeat (NLR) receptors in multiple sclerosis. Immunogenetics. (2020) 72:381–5. doi: 10.1007/s00251-020-01170-w
31. Trifiletti R, Lachman HM, Manusama O, Zheng D, Spalice A, Chiurazzi P, et al. Identification of ultra-rare genetic variants in pediatric acute onset neuropsychiatric syndrome (PANS) by exome and whole genome sequencing. Sci Rep. (2022) 12:11106. doi: 10.1038/s41598-022-15279-3
32. Gomes AV. Genetics of proteasome diseases. Scientifica (Cairo). (2013) 2013:637629. doi: 10.1155/2013/637629
33. Marzano AV, Ortega-Loayza AG, Heath M, Morse D, Genovese G, Cugno M. Mechanisms of inflammation in neutrophil-mediated skin diseases. Front Immunol. (2019) 10:1059. doi: 10.3389/fimmu.2019.01059
34. Cannella S, Santoro A, Bruno G, Pillon M, Mussolin L, Mangili G, et al. Germline mutations of the perforin gene are a frequent occurrence in childhood anaplastic large cell lymphoma. Cancer. (2007) 109:2566–71. doi: 10.1002/cncr.22718
35. Tesi B, Lagerstedt-Robinson K, Chiang SC, Ben Bdira E, Abboud M, Belen B, et al. Targeted high-throughput sequencing for genetic diagnostics of hemophagocytic lymphohistiocytosis. Genome Med. (2015) 7:130. doi: 10.1186/s13073-015-0244-1
36. Qin P, Zhang Q, Chen M, Fu X, Wang C, Wang Z, et al. Variant analysis of CARD14 in a Chinese Han population with psoriasis vulgaris and generalized pustular psoriasis. J Invest Dermatol. (2014) 134:2994–6. doi: 10.1038/jid.2014.269
37. Israel L, Mellett M. Clinical and genetic heterogeneity of CARD14 mutations in psoriatic skin disease. Front Immunol. (2018) 9:2239. doi: 10.3389/fimmu.2018.02239
38. Triki M, Rinaldi G, Planque M, Broekaert D, Winkelkotte AM, Maier CR, et al. mTOR signaling and SREBP activity increase FADS2 expression and can activate sapienate biosynthesis. Cell Rep. (2020) 31:107806. doi: 10.1016/j.celrep.2020.107806
39. Almlöf JC, Nystedt S, Leonard D, Eloranta ML, Grosso G, Sjöwall C, et al. Whole-genome sequencing identifies complex contributions to genetic risk by variants in genes causing monogenic systemic lupus erythematosus. Hum Genet. (2019) 138:141–50. doi: 10.1007/s00439-018-01966-7
40. Ittisoponpisan S, David A. Structural biology helps interpret variants of uncertain significance in genes causing endocrine and metabolic disorders. J Endocr Soc. (2018) 2:842–854. doi: 10.1210/js.2018-00077
41. Homann OR, Misura K, Lamas E, Sandrock RW, Nelson P, McDonough SI, et al. Whole-genome sequencing in multiplex families with psychoses reveals mutations in the SHANK2 and SMARCA1 genes segregating with illness. Mol Psychiatry. (2016) 21:1690–5. doi: 10.1038/mp.2016.24
42. Reinhold WC, Varma S, Sousa F, Sunshine M, Abaan OD, Davis SR, et al. NCI-60 whole exome sequencing and pharmacological CellMiner analyses. PLoS ONE. (2014) 9:e101670. doi: 10.1371/journal.pone.0101670
43. Onodera K, Arimura Y, Isshiki H, Kawakami K, Nagaishi K, Yamashita K, et al. Low-frequency IL23R coding variant associated with crohn's disease susceptibility in Japanese subjects identified by personal genomics analysis. PLoS ONE. (2015) 10:e0137801. doi: 10.1371/journal.pone.0137801
44. Nasser KK, Shinawi T. Genotype-protein phenotype characterization of NOD2 and IL23R missense variants associated with inflammatory bowel disease: A paradigm from molecular modelling, dynamics, and docking simulations. Front Med (Lausanne). (2023) 9:1090120. doi: 10.3389/fmed.2022.1090120
45. Leonenko G, Richards AL, Walters JT, Pocklington A, Chambert K, Al Eissa MM, et al. Mutation intolerant genes and targets of FMRP are enriched for nonsynonymous alleles in schizophrenia. Am J Med Genet B Neuropsychiatr Genet. (2017) 174:724–31. doi: 10.1002/ajmg.b.32560
46. Aldrian D, Vogel GF, Frey TK, Ayyildiz Civan H, Aksu AÜ, Avitzur Y, et al. Congenital diarrhea and cholestatic liver disease: phenotypic spectrum associated with MYO5B mutations. J Clin Med. (2021) 10:481. doi: 10.3390/jcm10030481
47. Rajan N, Sinclair N, Nakai H, Shimomura Y, Natarajan S, A. tale of two sisters: identical IL36RN mutations and discordant phenotypes. Br J Dermatol. (2016) 174:417–20. doi: 10.1111/bjd.14003
48. Sugiura K. Role of interleukin 36 in generalised pustular psoriasis and beyond. Dermatol Ther (Heidelb). (2022) 12:315–28. doi: 10.1007/s13555-021-00677-8
49. Raupov R, Suspitsin E, Belozerov K, Gabrusskaya T, Kostik M. IFIH1 and DDX58 gene variants in pediatric rheumatic diseases. World J Clin Pediatr. (2023) 12:107–114. doi: 10.5409/wjcp.v12.i3.107
50. Rabionet R, Remesal A, Mensa-Vilaró A, Murías S, Alcobendas R, González-Roca E, et al. Biallelic loss-of-function LACC1/FAMIN mutations presenting as rheumatoid factor-negative polyarticular juvenile idiopathic arthritis. Sci Rep. (2019) 9:4579. doi: 10.1038/s41598-019-40874-2
51. Al-Mayouf SM, Yateem M, Al-Dusery H, Monies D, Wakil S, AlShiakh M, et al. New or vanishing frontiers: LACC1-associated juvenile arthritis. Int J Pediatr Adolesc Med. (2021) 8:44–7. doi: 10.1016/j.ijpam.2020.11.005
52. Scales M, Chubb D, Dobbins SE, Johnson DC, Li N, Sternberg MJ, et al. Search for rare protein altering variants influencing susceptibility to multiple myeloma. Oncotarget. (2017) 8:36203–36210. doi: 10.18632/oncotarget.15874
53. Tessoulin B, Moreau-Aubry A, Descamps G, Gomez-Bougie P, Maïga S, Gaignard A, et al. Whole-exon sequencing of human myeloma cell lines shows mutations related to myeloma patients at relapse with major hits in the DNA regulation and repair pathways. J Hematol Oncol. (2018) 11:137. doi: 10.1186/s13045-018-0679-0
54. Kiel MJ, Sahasrabuddhe AA, Rolland DCM, Velusamy T, Chung F, Schaller M, et al. Genomic analyses reveal recurrent mutations in epigenetic modifiers and the JAK-STAT pathway in Sézary syndrome. Nat Commun. (2015) 6:8470. doi: 10.1038/ncomms9470
55. Alankus B, Ecker V, Vahl N, Braun M, Weichert W, Macher-Göppinger S, et al. Pathological RANK signaling in B cells drives autoimmunity and chronic lymphocytic leukemia. J Exp Med. (2021) 218:e20200517. doi: 10.1084/jem.20200517
56. Rozeman LB, Sangiorgi L, Briaire-de Bruijn IH, Mainil-Varlet P, Bertoni F, Cleton-Jansen AM, et al. Enchondromatosis (Ollier disease, Maffucci syndrome) is not caused by the PTHR1 mutation p.R150C. Hum Mutat. (2004) 24:466–73. doi: 10.1002/humu.20095
57. Couvineau A, Wouters V, Bertrand G, Rouyer C, Gérard B, Boon LM, et al. PTHR1 mutations associated with Ollier disease result in receptor loss of function. Hum Mol Genet. (2008) 17:2766–75. doi: 10.1093/hmg/ddn176
58. Philips RL, Wang Y, Cheon H, Kanno Y, Gadina M, Sartorelli V, et al. The JAK-STAT pathway at 30: Much learned, much more to do. Cell. (2022) 185:3857–76. doi: 10.1016/j.cell.2022.09.023
59. Saevarsdottir S, Stefansdottir L, Sulem P, Thorleifsson G, Ferkingstad E, Rutsdottir G, et al. Multiomics analysis of rheumatoid arthritis yields sequence variants that have large effects on risk of the seropositive subset. Ann Rheum Dis. (2022) 81:1085–95. doi: 10.1136/annrheumdis-2021-221754
60. Richards AL, Leonenko G, Walters JT, Kavanagh DH, Rees EG, Evans A, et al. Exome arrays capture polygenic rare variant contributions to schizophrenia. Hum Mol Genet. (2016) 25:1001–7. doi: 10.1093/hmg/ddv620
61. Olafsdottir TA, Theodors F, Bjarnadottir K, Bjornsdottir US, Agustsdottir AB, Stefansson OA, et al. Eighty-eight variants highlight the role of T cell regulation and airway remodeling in asthma pathogenesis. Nat Commun. (2020) 11:393. doi: 10.1038/s41467-019-14144-8
62. Foddis M, Blumenau S, Holtgrewe M, Paquette K, Westra K, Alonso I, et al. TREX1 pA129fs and pY305C variants in a large multi-ethnic cohort of CADASIL-like unrelated patients. Neurobiol Aging. (2023) 123:208–15. doi: 10.1016/j.neurobiolaging.2022.11.013
63. Mönkäre S, Kuuluvainen L, Schleutker J, Bras J, Roine S, Pöyhönen M, et al. Genetic analysis reveals novel variants for vascular cognitive impairment. Acta Neurol Scand. (2022) 146:42–50. doi: 10.1111/ane.13613
64. Del Giudice E, Sota J, Orlando F, Picciano L, Cimaz R, Cantarini L, et al. Off-label use of canakinumab in pediatric rheumatology and rare diseases. Front Med. (2022) 9:998281. doi: 10.3389/fmed.2022.998281
65. Kostik MM, Isupova EA, Belozerov K, Likhacheva TS, Suspitsin EN, Raupov R, et al. Standard and increased canakinumab dosing to quiet macrophage activation syndrome in children with systemic juvenile idiopathic arthritis. Front Pediatr. (2022) 10:894846. doi: 10.3389/fped.2022.894846
66. Miettunen PM, Narendran A, Jayanthan A, Behrens EM, Cron RQ. Successful treatment of severe paediatric rheumatic disease-associated macrophage activation syndrome with interleukin-1 inhibition following conventional immunosuppressive therapy: case series with 12 patients. Rheumatology (Oxford). (2011) 50:417–9. doi: 10.1093/rheumatology/keq218
67. Ulusoy E, Karaca NE, El-Shanti H, Kilicoglu E, Aksu G, Kutukculer N. Interleukin-1 receptor antagonist deficiency with a novel mutation; late onset and successful treatment with canakinumab: a case report. J Med Case Rep. (2015) 9:145. doi: 10.1186/s13256-015-0618-4
68. Bhuyan F, de Jesus AA, Mitchell J, Leikina E, VanTries R, Herzog R, et al. Novel Majeed Syndrome-Causing LPIN2 mutations link bone inflammation to inflammatory M2 macrophages and accelerated osteoclastogenesis. Arthritis Rheumatol. (2021) 73:1021–32. doi: 10.1002/art.41624
69. Kuemmerle-Deschner JB, Welzel T, Hoertnagel K, Tsiflikas I, Hospach A, Liu X, et al. New variant in the IL1RN-gene (DIRA) associated with late-onset, CRMO-like presentation. Rheumatology (Oxford). (2020) 59:3259–63. doi: 10.1093/rheumatology/keaa119
70. Kisla Ekinci RM, Balci S, Bisgin A, Hershfield M, Atmis B, Dogruel D, et al. Renal amyloidosis in deficiency of adenosine deaminase 2: successful experience with canakinumab. Pediatrics. (2018) 142:e20180948. doi: 10.1542/peds.2018-0948
71. Herlin T, Fiirgaard B, Bjerre M, Kerndrup G, Hasle H, Bing X, et al. Efficacy of anti-IL-1 treatment in Majeed syndrome. Ann Rheum Dis. (2013) 72:410–3. doi: 10.1136/annrheumdis-2012-201818
72. Haviv R, Moshe V, De Benedetti F, Prencipe G, Rabinowicz N, Uziel Y. Is fibrodysplasia ossificans progressiva an interleukin-1 driven auto-inflammatory syndrome? Pediatr Rheumatol Online J. (2019) 17:84. doi: 10.1186/s12969-019-0386-6
73. Papatesta EM, Kossiva L, Tsolia M, Maritsi D. Persistent tenosynovitis, steroid dependency and a hyperpigmented scaly macular rash in a child with juvenile idiopathic arthritis. Cureus. (2020) 12:e11208. doi: 10.7759/cureus.11208
74. Grom AA, Ilowite NT, Pascual V, Brunner HI, Martini A, Lovell D, et al. Rate and clinical presentation of macrophage activation syndrome in patients with systemic juvenile idiopathic arthritis treated with canakinumab. Arthr Rheumatol. (2016) 68:218–28. doi: 10.1002/art.39407
75. Emmi G, Talarico R, Lopalco G, Cimaz R, Cantini F, Viapiana O, et al. Efficacy and safety profile of anti-interleukin-1 treatment in Behçet's disease: a multicenter retrospective study. Clin Rheumatol. (2016) 35:1281–6. doi: 10.1007/s10067-015-3004-0
76. Cantarini L, Talarico R, Generali E, Emmi G, Lopalco G, Costa L, et al. Safety profile of biologic agents for Behçet's disease in a multicenter observational cohort study. Int J Rheum Dis. (2017) 20:103–8. doi: 10.1111/1756-185X.12732
77. Signa S, D'Alessandro M, Consolini R, Miniaci A, Bustaffa M, Longo C, et al. Failure of anti Interleukin-1 b monoclonal antibody in the treatment of recurrent pericarditis in two children. Pediatr Rheumatol Online J. (2020) 18:51. doi: 10.1186/s12969-020-00438-5
78. Caorsi R, Insalaco A, Longo C, Martin G, Cattalini M, Consolini R, et al. IL-1 blockade in pediatric recurrent pericarditis: a multicentric retrospective study on the Italian cohort. Pediatr Rheumatol. (2019) 17:136. doi: 10.1136/annrheumdis-2019-eular.5854
79. Papa R, Penco F, Volpi S, Sutera D, Caorsi R, Gattorno M. Syndrome of undifferentiated recurrent fever (SURF): an emerging group of autoinflammatory recurrent fevers. J Clin Med. (2021) 10:1963. doi: 10.3390/jcm10091963
80. Dey M, Nagy G, Nikiphorou E. Comorbidities and extra-articular manifestations in difficult-to-treat rheumatoid arthritis: different sides of the same coin? Rheumatology (Oxford). (2023) 62:1773–9. doi: 10.1093/rheumatology/keac584
81. Delplanque M, Aouba A, Hirsch P, Fenaux P, Graveleau J, Malard F, et al. associated with myeloid neoplasm and VEXAS syndrome: two differential diagnoses of suspected adult onset Still's disease in elderly patients. J Clin Med. (2021) 10:5586. doi: 10.3390/jcm10235586
82. Schulert GS, Minoia F, Bohnsack J, Cron RQ, Hashad S, KonÉ-Paut I, et al. Effect of biologic therapy on clinical and laboratory features of macrophage activation syndrome associated with systemic juvenile idiopathic arthritis. Arthritis Care Res (Hoboken). (2018) 70:409–19. doi: 10.1002/acr.23277
83. Sönmez HE, Demir S, Bilginer Y, Özen S. Anakinra treatment in macrophage activation syndrome: a single center experience and systemic review of literature. Clin Rheumatol. (2018) 37:3329–35. doi: 10.1007/s10067-018-4095-1
84. Koga T, Kawakami A. Diagnosis and treatment of autoinflammatory diseases in adults: a clinical approach from rheumatologists. Immunol Med. (2018) 41:177–80. doi: 10.1080/25785826.2018.1524105
85. Koga T, Kawakami A. Interleukin-6 inhibition in the treatment of autoinflammatory diseases. Front Immunol. (2022) 13:956795. doi: 10.3389/fimmu.2022.956795
86. Kostik MM, Raupov RK, Suspitsin EN, Isupova EA, Gaidar EV, Gabrusskaya TV, et al. The safety and efficacy of tofacitinib in 24 cases of pediatric rheumatic diseases: single centre experience. Front Pediatr. (2022) 10:820586. doi: 10.3389/fped.2022.820586
87. Vinit C, Georgin-Lavialle S, Theodoropoulou A, Barbier C, Belot A, Mejbri M, et al. Real-life indications of interleukin-1 blocking agents in hereditary recurrent fevers: data from the JIRcohort and a Literature Review. Front Immunol. (2021) 12:744780. doi: 10.3389/fimmu.2021.744780
88. Shen JZ, Callaway K, Korf B, Rodriguez JM, Gaffo A. Empiric treatment for persistent fever from suspected autoinflammatory disease: Experience from an undiagnosed diseases program. Am J Med Sci. (2023) 366:71–5. doi: 10.1016/j.amjms.2023.04.008
89. Yokota S, Kishimoto T. Tocilizumab: molecular intervention therapy in children with systemic juvenile idiopathic arthritis. Expert Rev Clin Immunol. (2010) 6:735–43. doi: 10.1586/eci.10.41
90. De Benedetti F. Tocilizumab for systemic juvenile idiopathic arthritis. N Engl J Med. (2013) 368:1256–7. doi: 10.1056/NEJMc1301017
91. Alexeeva E, Krekhova E, Dvoryakovskaya T, Isaeva K, Chomakhidze A, Chistyakova E, et al. Efficacy and safety of canakinumab as a second line biologic after tocilizumab treatment failure in children with systemic juvenile idiopathic arthritis: A single-centre cohort study using routinely collected health data. Front Pediatr. (2023) 11:1114207. doi: 10.3389/fped.2023.1114207
92. Snegireva LS, Kostik MM, Caroli F, Ceccherini I, Gattorno M, Chasnyk VG. Failure of tocilizumab treatment in a CINCA patient: clinical and pathogenic implications. Rheumatology (Oxford). (2013) 52:1731–2. doi: 10.1093/rheumatology/ket121
93. Henes JC, Saur S, Kofler DM, Kedor C, Meisner C, Schuett M, et al. Tocilizumab for the treatment of familial mediterranean fever-a randomized, double-blind, placebo-controlled phase II study. J Clin Med. (2022) 11:5360. doi: 10.3390/jcm11185360
94. La Torre F, Muratore M, Vitale A, Moramarco F, Quarta L, Cantarini L. Canakinumab efficacy and long-term tocilizumab administration in tumor necrosis factor receptor-associated periodic syndrome (TRAPS). Rheumatol Int. (2015) 35:1943–7. doi: 10.1007/s00296-015-3305-2
95. Kuemmerle-Deschner J, Sturm D, Benseler S. Tocilizumab – an effective rescue therapy for refractory unclassified autoinflammatory diseases in children. Arthritis Rheumatol. (2019) 71.
96. Kawakami A, Endo Y, Koga T, Yoshiura KI, Migita K. Autoinflammatory disease: clinical perspectives and therapeutic strategies. Inflamm Regen. (2022) 42:37. doi: 10.1186/s41232-022-00217-7
97. Kacar M, Fitton J, Gough AK, Buch MH, McGonagle DG, Savic S. Mixed results with baricitinib in biologicalresistant adult-onset Still's disease and undifferentiated systemic autoinflammatory disease. RMD Open. (2020) 6:e001246. doi: 10.1136/rmdopen-2020-001246
98. Miyamoto T, Honda Y, Izawa K, Kanazawa N, Kadowaki S, Ohnishi H, et al. Assessment of type I interferon signatures in undifferentiated inflammatory diseases: A Japanese multicenter experience. Front Immunol. (2022) 13:905960. doi: 10.3389/fimmu.2022.905960
99. Sönmez HE, Karaaslan C, de Jesus AA, Batu ED, Anlar B, Sözeri B, et al. clinical score to guide in decision making for monogenic type I IFNopathies. Pediatr Res. (2020) 87:745–52. doi: 10.1038/s41390-019-0614-2
Keywords: autoinflammation, autoinflammatory disorders, AID, interleukin-1, canakinumab, undifferentiated autoinflammatory disorders
Citation: Alexeeva E, Shingarova M, Dvoryakovskaya T, Lomakina O, Fetisova A, Isaeva K, Chomakhidze A, Chibisova K, Krekhova E, Kozodaeva A, Savostyanov K, Pushkov A, Zhanin I, Demyanov D, Suspitsin E, Belozerov K and Kostik M (2023) Safety and efficacy of canakinumab treatment for undifferentiated autoinflammatory diseases: the data of a retrospective cohort two-centered study. Front. Med. 10:1257045. doi: 10.3389/fmed.2023.1257045
Received: 11 July 2023; Accepted: 13 September 2023;
Published: 15 November 2023.
Edited by:
Andra Rodica Balanescu, Carol Davila University of Medicine and Pharmacy, RomaniaReviewed by:
Jurgen Sota, University of Siena, ItalyCopyright © 2023 Alexeeva, Shingarova, Dvoryakovskaya, Lomakina, Fetisova, Isaeva, Chomakhidze, Chibisova, Krekhova, Kozodaeva, Savostyanov, Pushkov, Zhanin, Demyanov, Suspitsin, Belozerov and Kostik. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mikhail Kostik, a29zdC1taWtoYWlsQHlhbmRleC5ydQ==; bWlraGFpbC5rb3N0aWtAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.