- 1Department of Pharmacy, Chi Feng Municipal Hospital, Chifeng, China
- 2Qingdao Women and Children’s Hospital, National Drug Clinical Trial Institute Office, Qingdao, China
- 3Department of Pharmacy Supplement, Chi Feng Municipal Hospital, Chifeng, China
- 4Chifeng Clinical Medicine College of Inner Mongolia Medical University, Chifeng, China
- 5Clinical Science of Stomatology, Chi Feng Municipal Hospital, Chifeng, China
Objective: Over the past few decades, the development of anti-cancer drugs in China has made outstanding achievements based on the support of national policies. To assess the progress of non-small cell lung cancer (NSCLC) drugs, we conducted a statistical analysis of clinical trials of drugs targeting NSCLC in China from 2005 to 2023.
Methods: We downloaded, screened and analysed the data from three official websites, the Centre for Drug Evaluation of China National Medical Products Administration website (NMPA), ClinicalTrials.gov and the Chinese Clinical Trial Registry (ChiCTR).
Results: From January 1, 2005 to April 15, 2023, a total of 1,357 drug clinical trials that met the standards were included, and the number of registered drug clinical trials has been increasing year by year, reaching the maximum of 199 in 2021. Among them, the maximum of 462 items (34.05%) in phase II clinical trials, followed by 333 (24.54%) in phase III clinical trials, and 139 (10.24%) in phase IV clinical trials. In all drug clinical trials, industry sponsored trials (ISTs) have 722 items (53.21%), which are higher than investigator-initiated trials (IITs). The clinical trials of chemical drugs have a maximum of 723 items (53.28%), while biopharmaceuticals have grown rapidly in the past 10 years, with a total of 374 (27.56%), and 48.19% of the drug clinical trials of combined medication. In addition, the geographical distribution of the leading units and participating units of Chinese drug clinical trials are uneven, and economic regions such as Beijing, Shanghai, Jiangsu are obviously ahead of other regions.
Conclusion: From 2005 to 2023, the clinical trials of registered drugs for the treatment of NSCLC increased rapidly. Among them, due to the development of immunotherapy, the clinical trials of biopharmaceuticals and drugs for combined medication are growing most rapidly, while the exploration of the original drugs is a little far from enough. Our research provides a direction for the future drug clinical trials of NSCLC, laying foundation for further extending the survival rate of patients with NSCLC.
1 Introduction
Lung cancer is the leading cause of cancer-related deaths worldwide. Non-small cell lung cancer (NSCLC) accounts for 85% of lung cancers, and the 5 years overall survival (OS) rate is around 15% (1, 2). There are different subtypes of NSCLC, classified as squamous cell carcinoma (SqNSCLC), adenocarcinoma, large cell carcinoma and more poorly differentiated variants (3). According to the China National Cancer Centre report, China had 4.064 million new cases of malignant tumours and 2.414 million deaths in 2016, including 828,000 new cases of lung cancer and 657,000 deaths (4).
Clinical trials are the gold standard for the evaluation of the safety and efficacy of therapeutics and the generation of evidence-based knowledge in the field of medicine (5, 6). The legitimacy of clinical trials can be ensured by informed consent, data collection of clinical trial processes, and quality assurance systems. China launched drug regulatory reforms in 2015, which include a 60 days standard approval system for clinical trial applications, expedited program (such as conditional approval), acceptance of overseas clinical trial data and the introduction of patent term extension, which have greatly facilitated the development of new drugs (7, 8). The standardized good clinical practice (GCP) standards in China enabled the scientific and reliable test results of drug clinical trials, and also protect the rights and interests of the subjects.
Surgical resection of lung tumors is the preferred therapeutic approach when early-stage disease is detected. Unfortunately, most patients present with advanced, inoperable disease because lung cancer is largely asymptomatic in its early stages (9). Therefore, drug treatment has become the best way to relieve symptoms. The advent of genetic and molecular technologies has been accompanied by dramatic changes in the treatment options for NSCLC. In particular, a large number of genomic alterations in genes that regulate cell proliferation and differentiation have been identified as driver mutations for lung cancer treatment (10–12). Several treatment modalities are being used including surgery, radiation therapy, chemotherapy, targeted therapy, laser therapy, photodynamic therapy, radiofrequency ablation, cryosurgery, electrocautery, and watchful waiting. New modalities such as immunotherapy, combination therapies and chemoprevention are being tested in clinical trials (3, 13). In immunotherapy, immune checkpoint inhibitors may be able to prolong the survival cycle and improve the prognosis of patients to some extent by modulating the immune response involving T cells to clear the tumour cells in the patient (14, 15). Various drug therapies for NSCLC are flourishing, but to date, no analytical overview of drug clinical trial for NSCLC has been undertaken.
In this study, we present an analysis of clinical trials of drugs for non-small cell lung cancer conducted in China from 2005–2023. Our study provides direction to drug trial applicants and research institutions, and provides a basis for the use of drugs and treatment plan for medical institutions.
2 Materials and methods
2.1 Data sources
Our drug clinical trial data is obtained from three official websites, namely the Centre for Drug Evaluation of China National Medical Products Administration website (NMPA),1 ClinicalTrials.gov2, and the Chinese Clinical Trial Registry (ChiCTR)3 (16). The Chinese Clinical Trial Registry was first established in 2005. Therefore, January 1, 2005 was chosen as the start date and April 15, 2023 was chosen as the cut-off date for this study. Any clinical trials of drugs outside this period were excluded.
2.2 Search strategy and selection criteria
A total of 537,178 drug clinical trials were retrieved from the three websites. In NMPA and ChiCRT, our search terms were non-small cell lung cancer, and we searched for 679 and 740 studies, respectively, in the above-mentioned websites. In ClinicalTrial.gov, we entered the keyword non-small cell lung cancer in “condition or disease” and also entered China in “country” to obtain 1,255 studies.
The inclusion criteria in this study were: (1) the drug clinical trial had to be for non-small cell lung cancer; (2) the study site was within China; (3) the trial was for drug treatment related to NSCLC; (4) one of the evaluation indicators had to be the efficacy of the drug treatment; and (5) the study was started between January 1, 2005 and April 15, 2023. Exclusion criteria were as follows: (1) studies started before 1 January 2005 and after 15 April 2023; (2) some clinical trials focused on predictive as well as prognostic markers for NSCLC; (3) inclusion criteria for drug clinical trials covering patients with solid tumours other than NSCLC. Chemotherapy alone as well as cellular therapies were not included in the study during the trial selection process.
Based on the above inclusion and exclusion criteria, we screened 1,357 clinical trials of drugs relevant to the treatment of NSCLC that were conducted in China. Figure 1 shows the full screening process for the studies.
2.3 Data extraction and definition
The following information was collected on the website: registration number of the trial, official name of the trial, year of first publication, type of drug in the trial, trial conduct status, sponsor, address of the lead unit, address of the participating unit, trial phase, type of design, randomization, and whether the protocol was co-administered. Two investigators independently downloaded drug clinical trial data from three websites, which were then identified. All discrepancies were discussed by all investigators, and data were carefully identified and screened.
2.4 Statistical analysis
We have analyzed temporal trends in specific indicators, including the number of clinical trials screened at the three sites, the status of clinical trials of drugs at various stages, the geographical distribution of trial centers, the number of clinical trials involving different types of drugs, and the distribution of clinical trials based on various sponsorships. Statistical significance of differences was evaluated using assessed by student’s t-test and one-way ANOVA followed by Tukey test (GraphPad Software, San Diego, CA), and p < 0.05 was considered statistically significant. We used R software (version 4.2.2) to collate and analyse the data.
3 Results
3.1 Time trends of clinical trials by study phase
We screened a total of 1,357 drug clinical trials. Of the total drug clinical trials, 157 were observational studies, accounting for 11.57% of the total, while the other 1,200 were interventional studies, accounting for 88.43% of the total.
Of the clinical trials reviewed, 140 were phase I, 462 were phase II, 333 trials were phase III and 139 clinical trials were phase IV, representing 10.32, 34.05, 24.54 and 10.24%, respectively (Figure 2). Of the total drug clinical trials, 72 (5.31%) included Phase I/II and 18 (1.33%) involved Phase II/III. The Food and Drug Administration (FDA) proposed the concept of exploratory investigational new drug (eIND) and in 2006 released the eIND research guide. The studies conducted under an eIND are termed Phase 0 clinical trials (17). Seventy-two (72) Phase 0 drug trials were employed accounting for 5.3% of all drug clinical trials. Overall, 121 (8.92%) observation studies or clinical trials were not staged. From the perspective of research phase distribution, highest proportion were Phase II clinical trials, which as a category had the fastest growth rate.
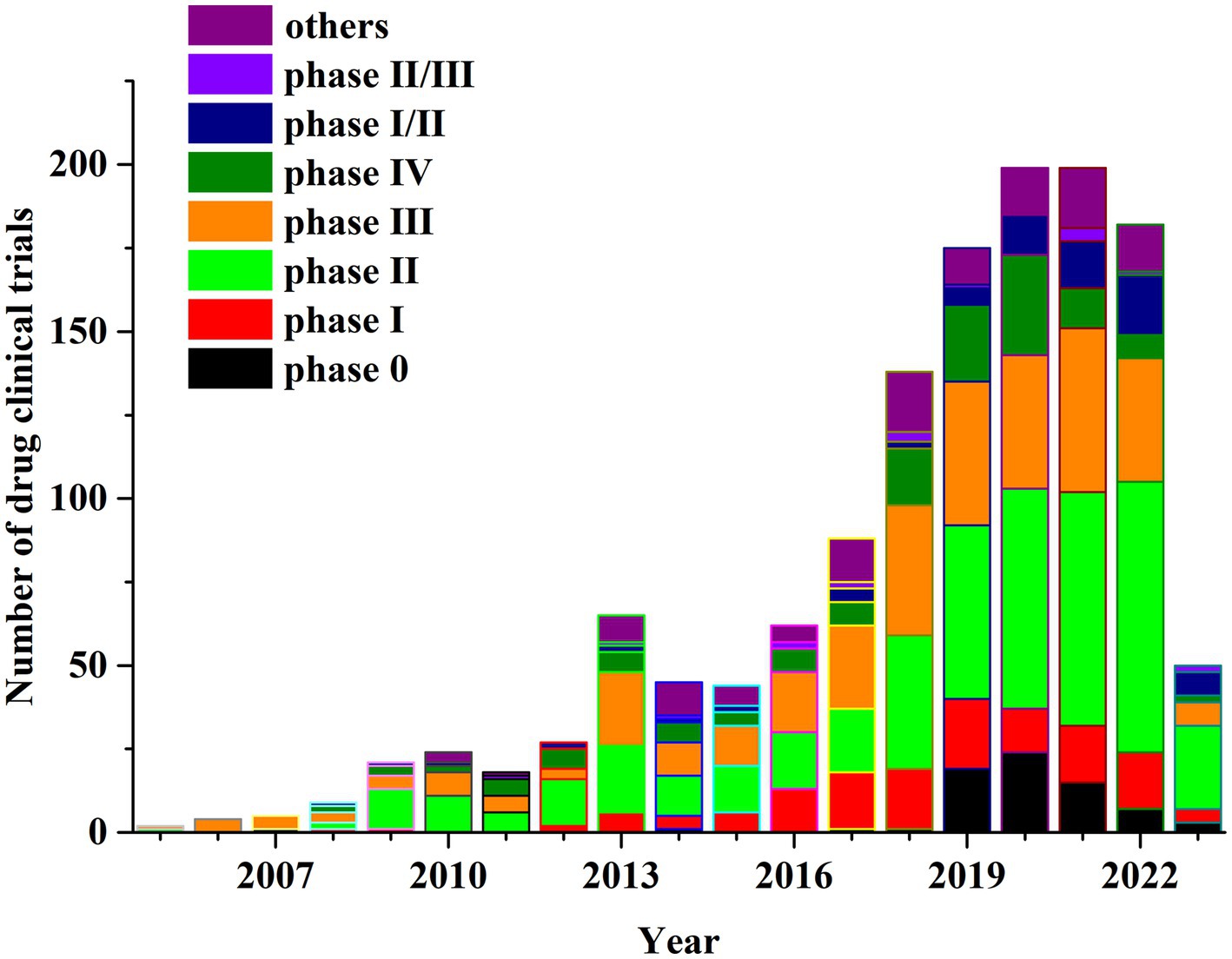
Figure 2. Annual number of clinical trials of NSCLC drugs conducted in China based on study phase from 2005 to 2023.
3.2 Distribution and time patterns of clinical trials by sponsorship
Industry sponsored trials (IST) were more prevalent than investigator-initiated trials (IIT). In NMPA, all registered drug clinical trials were ISTs; in the ChiCRT and Clinicaltrial.gov, IIT trial registration also existed. IST drug trials accounted for 53.2% (n = 722) while IIT clinical trials were 46.8% (n = 635) of the total.
NSCLC related IST clinical trials in China from 2008 to 2022 had an average annual growth rate (AARG) of 24.29%. Similarly, an IIT drug studies showed an upward trend between 2005 to 2020, with the largest annual increase of 146% in 2018 which peaked in 2020 followed by a downward trend thereafter. The AARG of IIT drug trials was 31.31% between 2005 to 2020. The total number of drug candidate studies significantly increased in 2018 and 2019 due to the rapid expansion of IST and IIT clinical trials. The IST research had two small peaks in 2019 and 2021 reflecting a new trend with corresponding growth rates of 50.00% and 28.75%, respectively. Figure 3 illustrates more detailed data on temporal trends in industry clinical trial sponsorship.
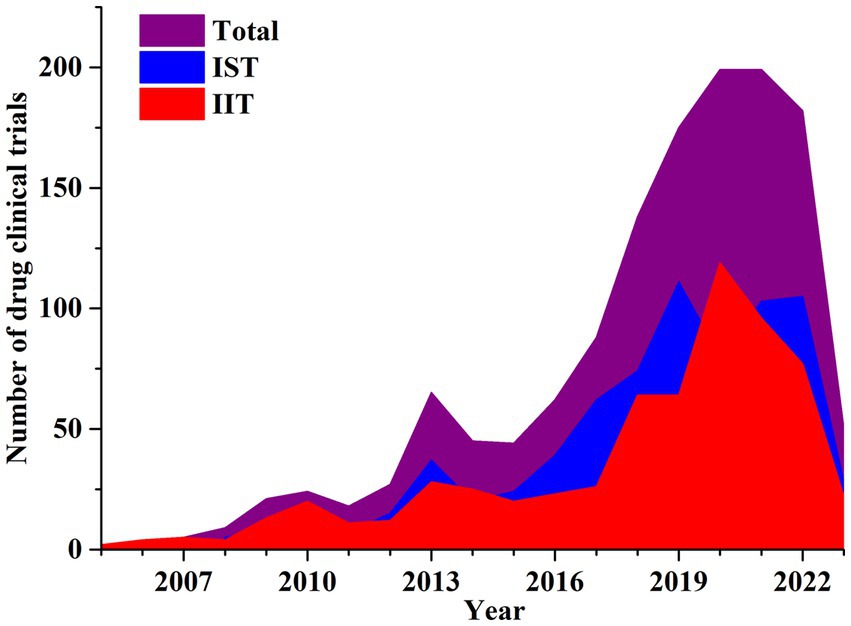
Figure 3. Annual number of clinical trials of NSCLC drugs conducted in China based on sponsorship from 2005 to 2023.
3.3 Distribution of clinical trials by drug type
Drugs incorporated into clinical trials were classified into seven categories: 53.2% (723/1357) were chemicals, 27.6% (374/1357) drugs were biopharmaceuticals, and 3.5% (48/1357) were Chinese medicines. Chemicals and biopharmaceutical testing had annual increases in clinical trials. The AARG of chemicals was 32.2% from 2005 to 2021 and 32.0% for biopharmaceuticals from 2008 to 2021; both forms peaked in 2021. Some clinical trials for NSCLC used combination drugs (654, 48.2%); whereas, 703 (51.8%) were single drug treatments. Of the combination trials, 13.6% (185/1357) included biopharmaceuticals and chemical drugs, and only 1.8% (24/1357) paired a Chinese medicine with a chemical drug. Figure 4 shows the temporal trends in these trials.
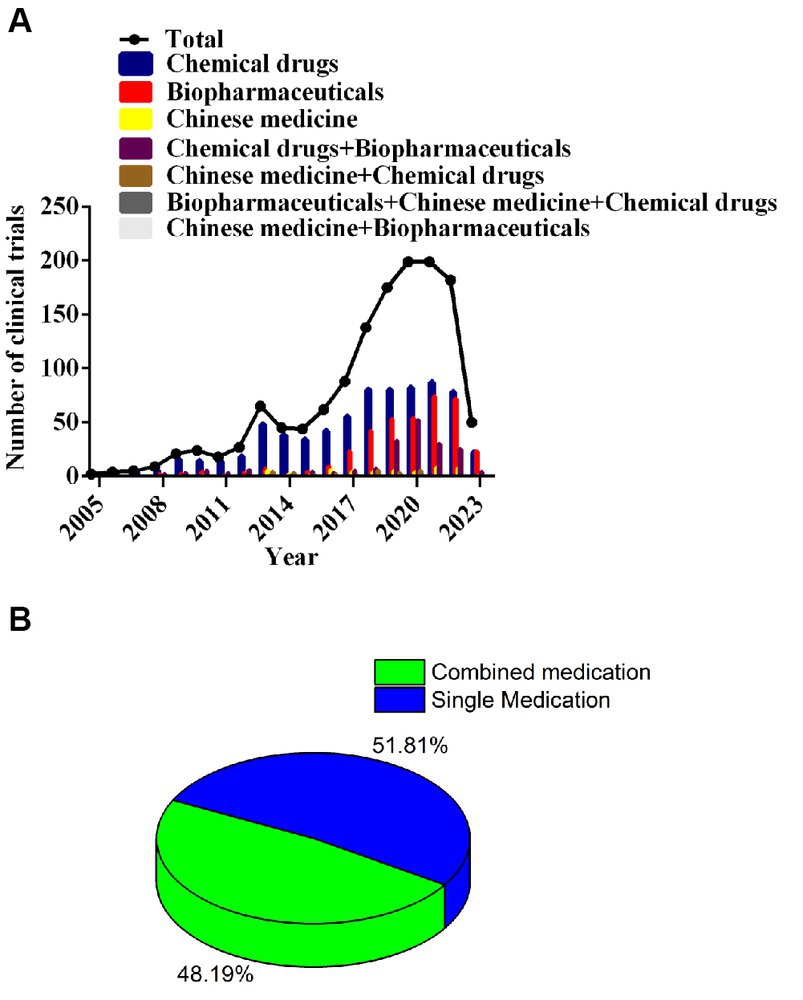
Figure 4. Annual number of clinical trials of NSCLC drugs conducted in China based on drug type from 2005 to 2023 (A); percentage of trials for combined medication versus single medication (B).
However, we classified the drugs according to the mechanism of action, categorizing the drugs as immune-therapeutics, anti-angiogenics, targeted therapeutics, chemotherapeutics, Traditional Chinese medicines, and others. Among these trials 548 were for targeted therapeutic (40.4%) and 419 were for immunotherapeutic (30.88%). The number of immunotherapeutic drug trials increased annually from 2013 to 2021, reaching a peak of 88 candidates in 2021, which declined slightly to 83 in 2022. Over the 2013 to 2022 interval, AAGR of immunotherapeutic drugs was 63.4%. Targeted therapeutics also rose annually between 2005 and 2022 with an AAGR of 29.3% (Supplementary Figure S1).
3.4 Distribution of clinical trials by recruiting status
Segmentation of clinical trials according to recruitment status revealed that 143 trials were completed (10.54%), 159 had completed recruitment (11.72%), 675 were still in the recruitment phase (49.74%), and 333 trials had not begun enrolling participants (24.54%). Notably, 47drug candidate trials were suspended or terminated (3.46%). In general, suspended or terminated drug trials were related to changes in the sponsoring company’s strategy or difficulties recruiting suitable patients, perhaps due to stringent inclusion criteria. Interestingly, a large increase in the drug trials enrolled occurred in 2018 (Figure 5).
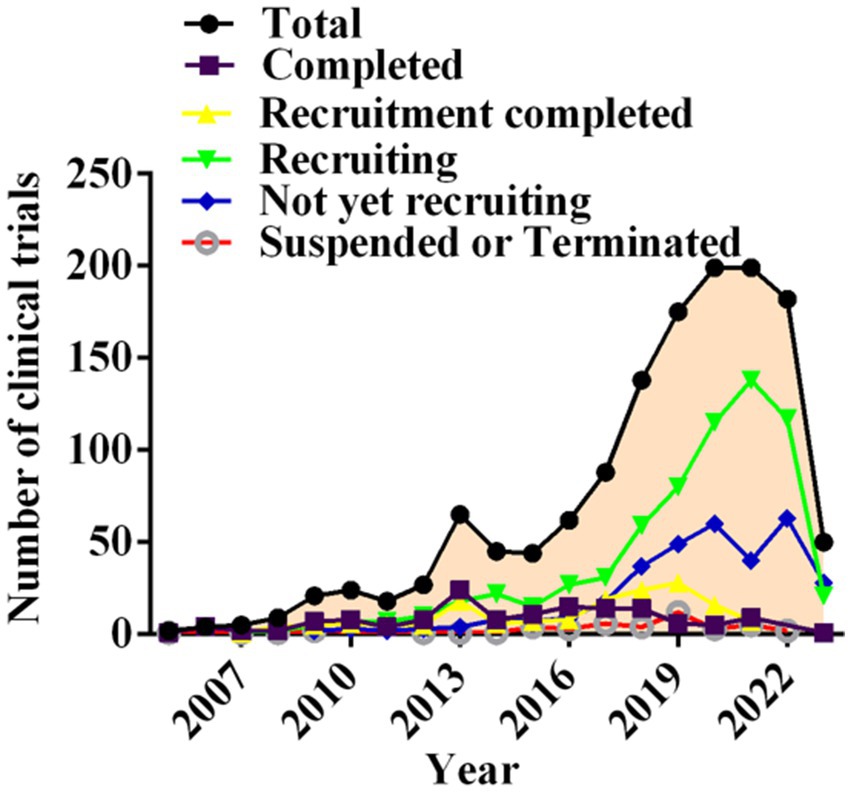
Figure 5. Annual number of clinical trials of NSCLC drugs conducted in China based on recruiting status from 2005 to 2023.
3.5 Time trends of leading clinical trial units
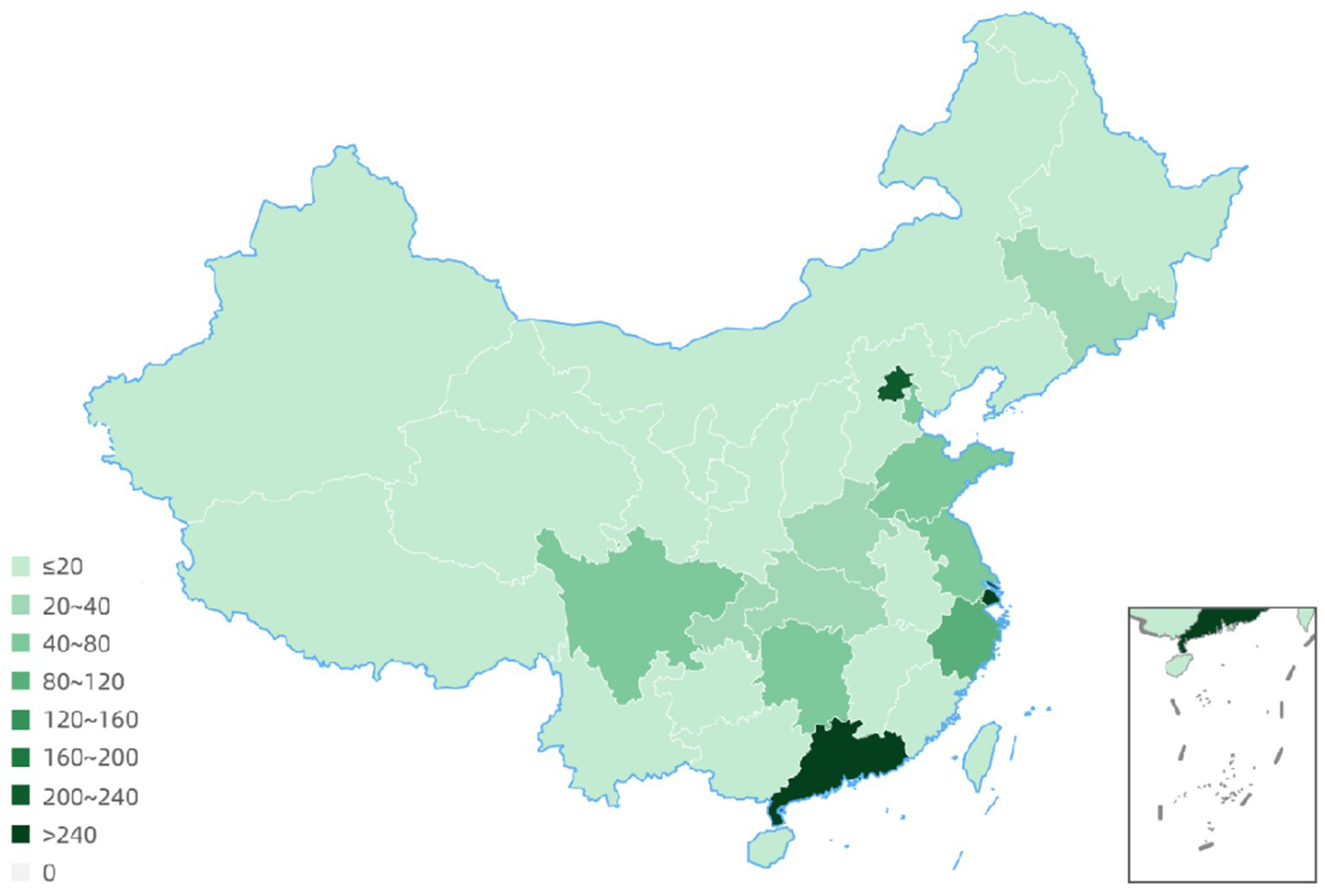
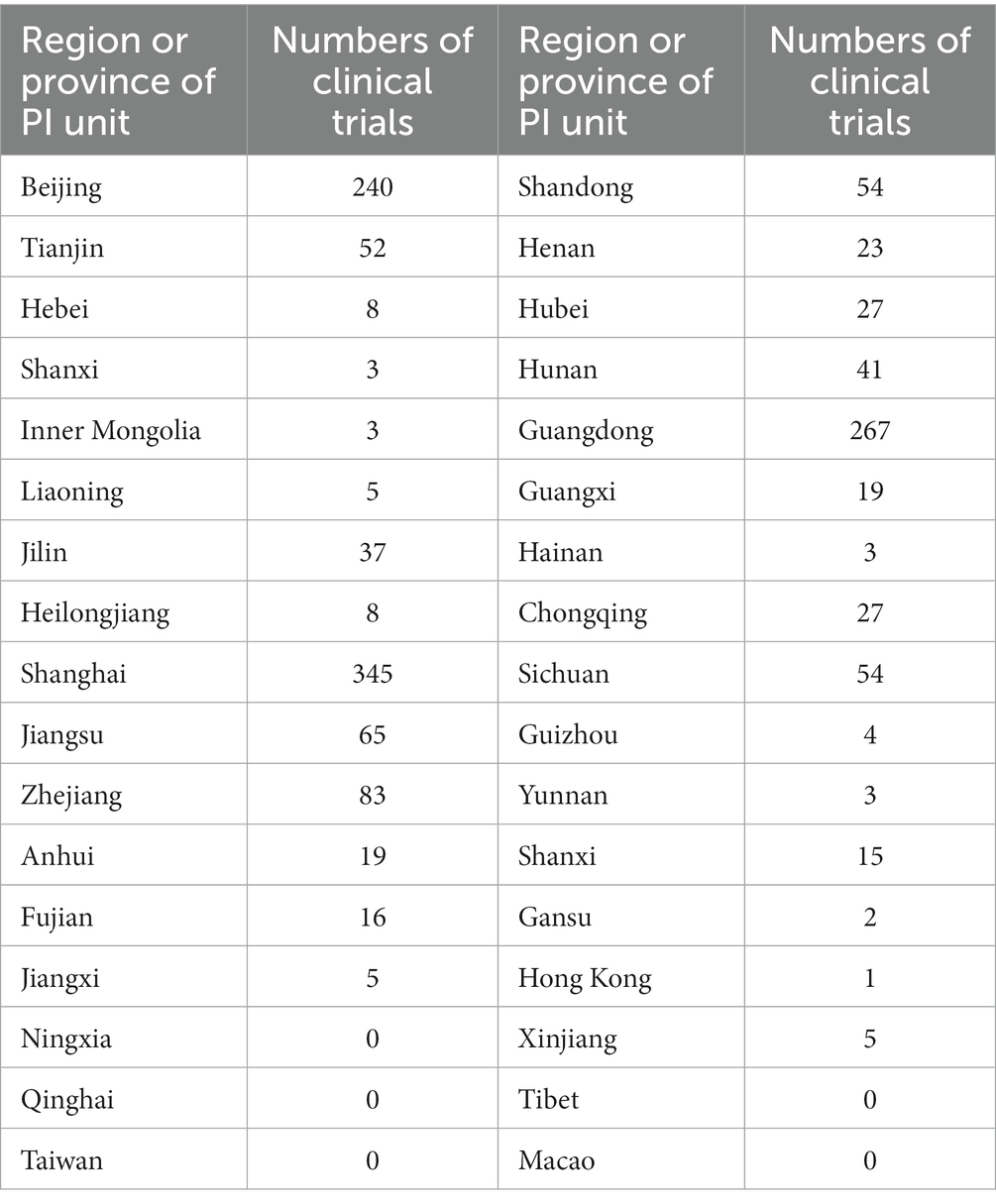
The geographical distribution of principle centers for drug clinical trials between 2005 to 2023 revealed that 54 trials had 2 to 7 leadership sites in China and 2 trials had the primary center located abroad. Details for 4 clinical trials were not available. Among all drug trials, 240 (16.7%) lead units were in Beijing; 345 (24.1%) were located in Shanghai, and 267 (18.6%) were in Guangdong province (Figure 6; Table 1).
3.6 Geographical distribution of drug clinical trials participating units
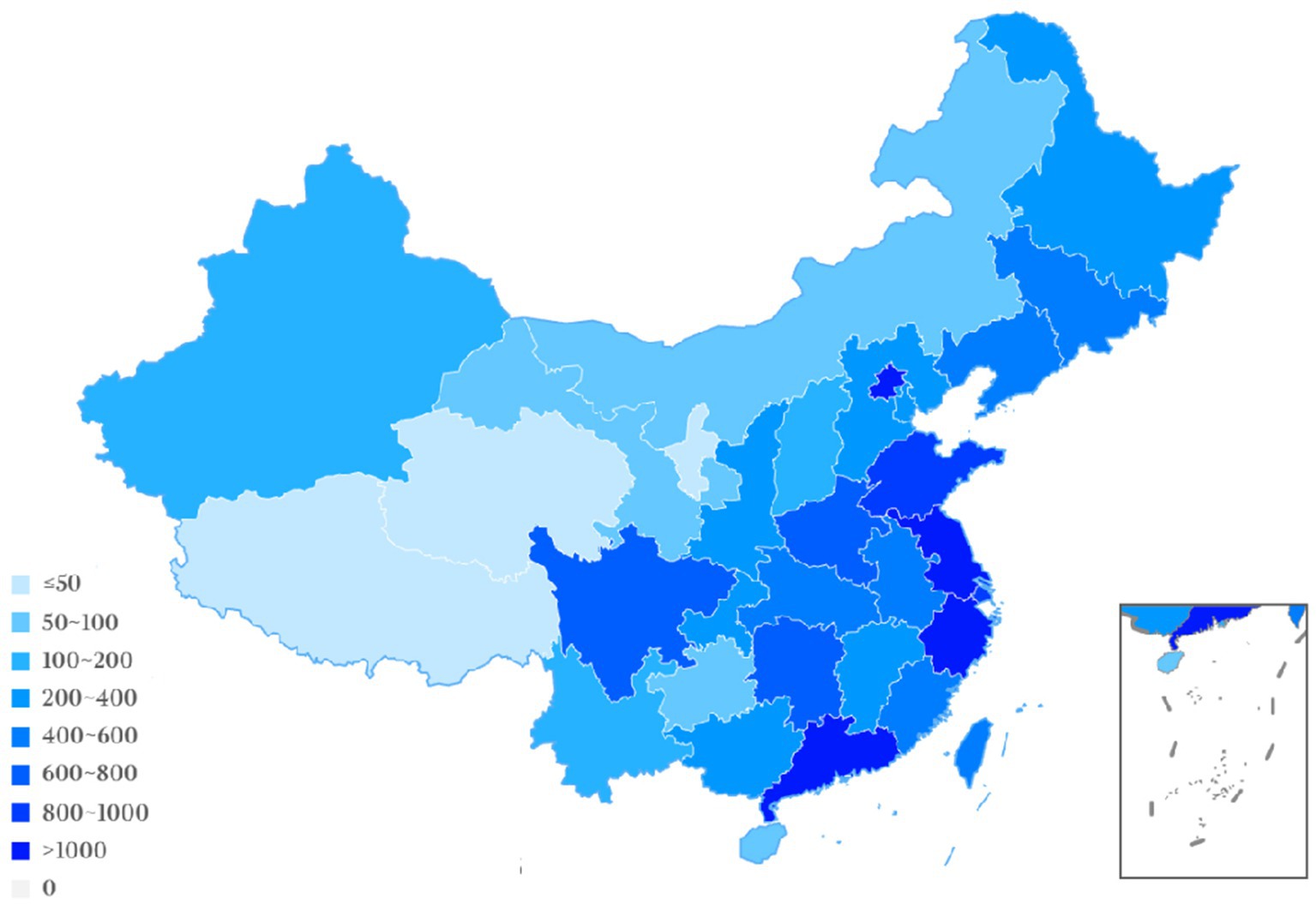
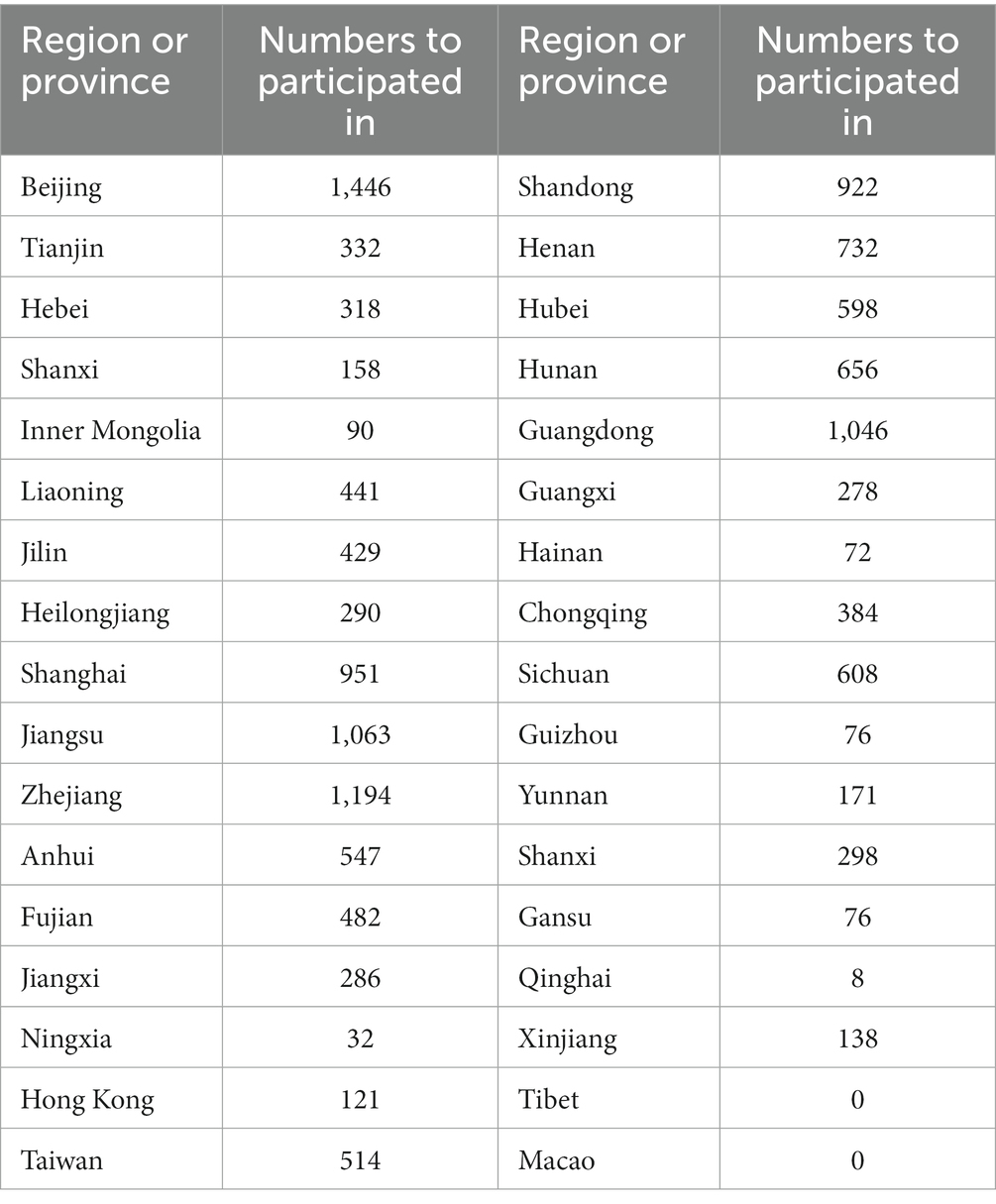
Geographic location analysis of the participating trial sites from 2005 to 2023 revealed the top five to be Beijing (n = 1,446, 9.8%), Zhejiang (n = 1,194, 8.1%), Jiangsu (n = 1,063, 7.2%), Guangdong (n = 1,046, 7.1%) and Shanghai (n = 951, 6.4%). In comparison to these locations, other outlying institution locations participating in drug clinical trials included: Tibet (n = 0, 0%), Qinghai (n = 8, 0.05%), Ningxia (n = 32, 0.2%), and Hainan (n = 72, 0.5%). The location information regarding a few drug clinical trials was unavailable. These results demonstrate the maldistribution of NSCLC drug clinical trials towards developed areas, which could present unanticipated patient selection bias. Detailed regional data are presented in Figure 7; Table 2.
3.7 Time trends of other characteristics of clinical trials
Additional analysis of the clinical trials site data revealed that 200 (14.7%) were international multi-center clinical trials, 609 (44.9%) were domestic multi-center trials, 137 (10.1%) were domestic single center studies, and the remaining clinical sites were unspecified in the trial registration. Trial experimental trial designs included 621 (45.8%) single-arm, 401 (29.6%) controlled, and 42 (3.1%) cohort studies. Patient randomization was performed in 609 (44.9%) and 748 (55.1%) were nonrandomized trials. Blinding is an important measure to control trial bias, as guided by the CDE Guidelines for Clinical Trials of Drugs. For randomised clinical trials, blinding is usually combined with randomised grouping and works throughout the trial to maximise the control of trial bias. Treatment blinding in this study was categorized as open, double-blind, single-blind. The majority of trials were open (1101), but 220 were double-blind, 3 were single-blind, 18 were retrospective research and 15 trials were prospective studies.
4 Discussion
The 2020 global cancer statistics released by the International Agency for Research on Cancer (IARC) (18), reported lung cancer as the second most common malignant tumor type, which also has the highest fatality rate in the world (19, 20). In 2020, there were 820,000 lung cancer cases and 710,000 deaths, accounting for 17.9% of the incidence of cancer in China and 23.8% of the national mortality rate (18). Histological identification confirmed that the most common subtype was NSCLC. Lung cancer develops via the activating mutations of driver genes, which include the epidermal growth factor receptor (EGFR), anaplastic, lymphoma kinase (ALK), c-ros oncogene 1 (ROS1), v-Raf murine sarcoma viral oncogene homolog, B (BRAF), and (RET) genes (21) with rearrangement at some transfection sites. These mutant genes constitute the biological pathways for targeted anti-NSCLC therapy. In the recent past, the treatment of NSCLC focused on surgical resection, radiotherapy, chemotherapy and targeted therapy. Among these, chemotherapy is mainly founded upon on highly toxic platinum drugs. Adjuvant firstline treatment consists of a doublet chemotherapy regimen derived from paclitaxel, gemcitabine, docetaxel, pemetrexed, vinorelbine or etoposide given in combination with either cisplatin or carboplatin (22). The latest Clinical Practice Guideline for Primary Lung Cancer (2022 version) released by the General Office of the National Health and Health Commission of People’s Republic of China (23), classified the NSCLC treatments into four categories: chemotherapy, anti-vascular treatment, immunotherapy and targeted therapy. Anti-vascular drugs were predominantly vascular endothelial inhibitory protein or Bevacizumab. Immunotherapy drugs were PD-1 and PD-L1 inhibitors (Table 3). In recent years, the treatment approaches to NSCLC have evolved in light of the success of immune checkpoint inhibitors (ICIs) and anti-vascular generation drugs often combined with traditional drugs of varying mechanisms.
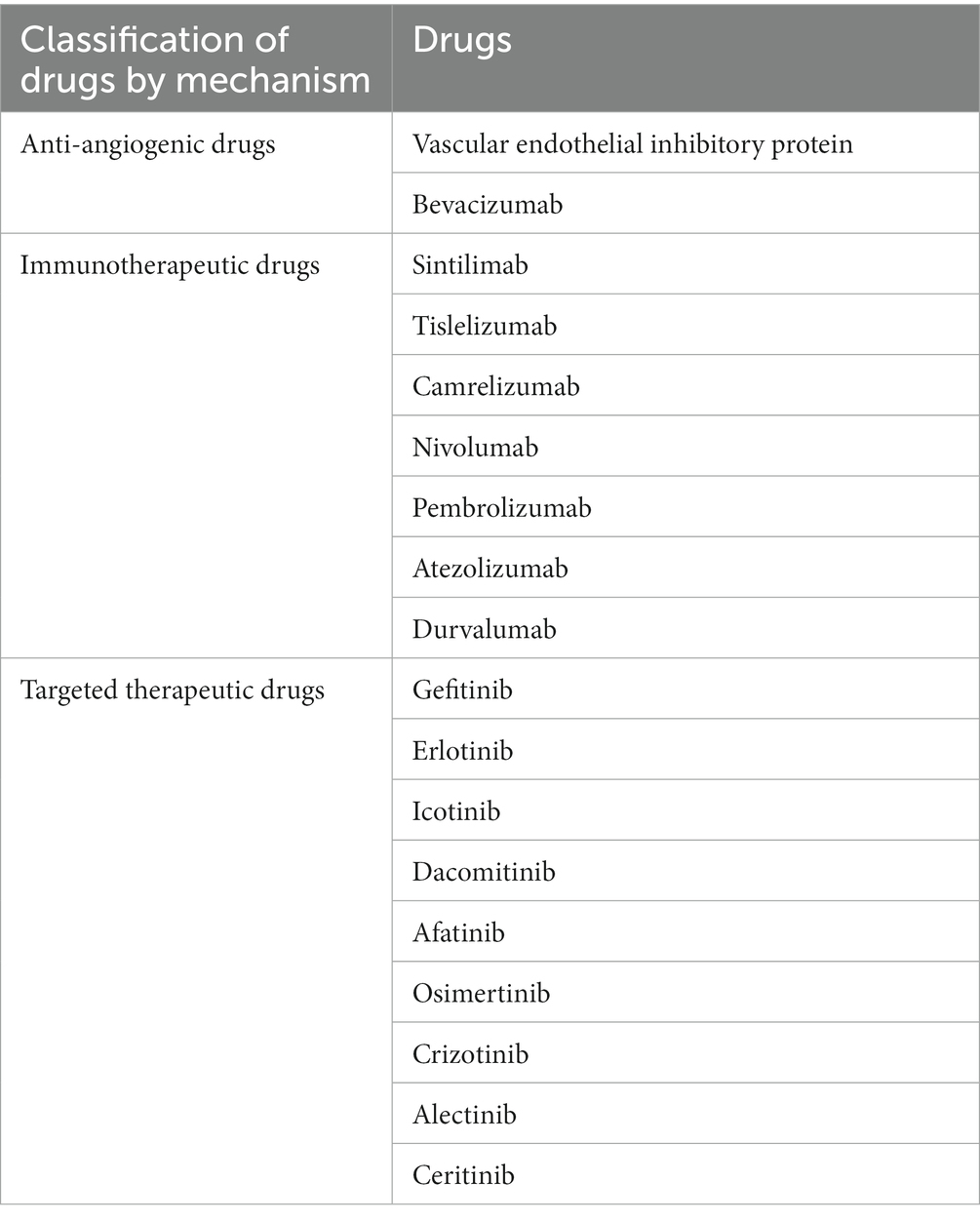
Table 3. NSCLC China guide recommended commonly used drugs (23).
Numerous reviews of clinical studies have chronicled developments in China and internationally regarding (1) new NSCLC diagnostic and prognostic biomarkers (24, 25); (2) descriptions and analyses of drug trial of combination treatment comparisons (26, 27); (3) recognition susceptible genes in lung cancer (28, 29); (4) changes of RNA and protein in the tissue of NSCLC of patients, and understanding pathways underpinning the development NSCLC (30). Despite these focused papers, no overarching and systematic study regarding development of clinical trials for NSCLC’s treatment has been considered, until this comprehensive evaluation of the numerous trends in clinical trials for NSCLC conducted in China.
Of these, chemical drugs accounted for the largest proportion (53.3%) of NSCLC treatments (Figure 4), possibly reflecting the established chemotherapy approach to cancer. Nonetheless, clinical trials evaluating biopharmaceuticals have gradually increased in the past 10 years, and by 2021, this class of drug treatment represented largest proportion tested (52.3%), reflecting increased popularity of biopharmaceuticals-based treatment approaches for NSCLC over the past 20 years. Considering all clinical trials, 123 clinical programs were bioequivalent research testing generic or analogue versions of approved drugs. Clinical trials for new drug entities were represented by immune-therapeutics (30.9%), anti-angiogenics (15.1%), targeted therapeutics (40.4%), chemotherapeutics (7.2%), a small number of Traditional Chinese medicines (5.5%) and others (1.0%). Targeted therapies account for the greatest proportion of these drugs (Supplementary Figure S1). In 2015, the Chinese government formulated new policies to boost innovative drugs through priority review status (31). In the same timeframe (2017), the Chinese government issued the “Opinions on Encouraging Medical Device Innovation to Reform the Review and Approval System” that encouraged innovative drug development (32). Collectively, these policies promoted continuously increased clinical evaluation of innovative NSCLC drugs, alone or in combination, yielding more novel and updated NSCLC treatment methods.
Despite advances in lung cancer treatment with third-generation chemotherapies and targeted therapies, prognosis for NSCLC patients remained poor. More recently inhibitory immunotherapies targeting cell programming death receptors 1(PD-1) and cell programming death ligand 1(PD-L1) have emerged as the newest treatment for NSCLC (33, 34). These therapeutics generally classified as immune checkpoint inhibitors (ICI) are monoclonal antibodies defined by three subgroups: PD-1 inhibitors (nivolumab, pembrolizumab), PD-L1 inhibitors (durvalumab, atezolizumab, and avelumab), and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) inhibitors (ipilimumab) (33, 35). The ICI therapy has markedly improved the clinical response and survival rates among patients with metastatic NSCLC. In May 2017, the US FDA approved the combination of nivolumab plus ipilimumab for the treatment of non-squamous NSCLC. On October 12, 2021, China officially listed CTLA-4 inhibitor ipilimumab and the combination of nivolumab plus ipilimumab has been approved as the hypertrophic inhibitor therapy. These events marked China’s domestic era of dual-immune inhibitors treatment era (36). Currently, dual immune checkpoint drugs, PD-(L)1 plus CTLA-4 inhibitors, has become one of the most widely prescribed treatment approaches for metastatic NSLC. Moreover, some studies suggest that neoadjuvant nivolumab in combination with ipilimumab improves the primary pathological response in early-stage NSCLC, regardless of PD-L1 expression, suggesting, that dual-ICI offered long-term survival benefits (37–39). Others suggest that the combination of the dual immune checkpoint agents was not advantageous as a neoadjuvant regimen compared to single agent treatment (40). Of note, during clinical development, some researchers terminated ICI clinical trials (41) due to the significant adverse reactions (ADRs). Immunotherapy receptor targets are distributed into many organ systems and ICIs, particularly dual-inhibitor regimens, induced potentially fatal off-target toxicities including immune-related pneumonia, myocarditis, encephalitis, and nephritis. Part of the ADRs and severity grades are listed in Table 4. These events endorsed treatment recommendation revisions, warnings, or contraindications for patients with underlying immune or autoimmune disease. Consequently, continued clinical study of nivolumab plus ipilimumab among the NSCLC patients continues, with the hope to define predictive or early biomarkers of adverse events.
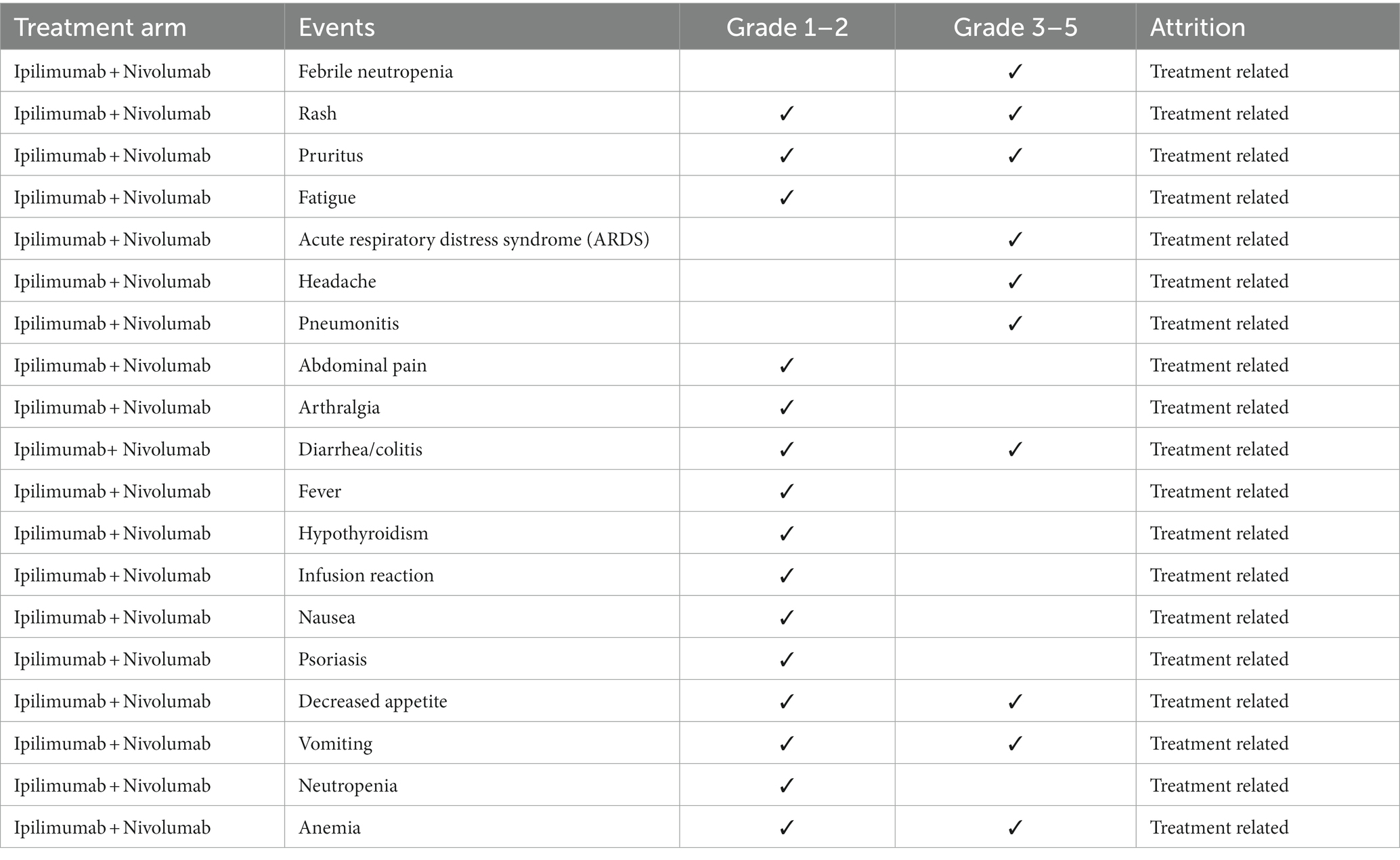
Table 4. Treatment-related adverse events (TRAEs) with possible or probable attribution to study therapies (38, 39, 41–43).
Rather than administering preformed antibodies, new clinical studies are probing the efficacy and safety of antibody-generating drugs, spawned from the evolving identification of targetable oncogenic driver alterations (44). An expanding category of oral targeted therapy options is under study for first-line use in treatment-naïve setting (45). Although oral targeting drugs have a high benefit rate in advanced NSCLC, the total survival period and the development drug resistance continues. Anti-vascular drugs reduce immunosuppression by modulating the tumor micro-environment (TME). This therapy is particularly effective when combined with other treatment NSCLC drugs (small molecular targeted therapy, chemotherapy, immunotherapy, etc.), potentially providing anti-tumor, anti-drug resistance benefits, and diminished ADR benefits (46–48). In recent decades, more than 10 anti-angiogenic therapeutics including bevacizumab, regorafenib and sorafenib have been approved for the therapy against several malignant diseases (49–51). A large number of drug clinical trials now include anti-vascular endothelial growth factor (VEGF) monoclonal antibodies such as bevacizumab, in combination drug regimens. Moreover, an increasing pre-clinical literature, suggests that targeting of the VEGF axis may not only inhibit angiogenesis, but may also trigger anti-cancer immunity (52).
Clinical trials excluded in this review studied the changes of ctDNA to track the progression of early-stage lung cancer, which may be extended to interrogate of oncogenic drivers and track expression of resistant mutations. Future clinical trials based on ctDNA-therapeutics are anticipated (53). By evaluating the dynamic changes of ctDNA values in different parts of the tumor cells may refine diagnoses and drug treatment regimens. The thrust of new clinical trials may be to individualize treatments for specific gene mutation groups. Continued exploration of NSCLC biomarkers and immune checkpoint inhibitor biology may evolve into precision medicine guidance and selection of therapeutic targets, like tyrosine kinase inhibitors (54).
Since 2015, the number of clinical trials for the treatment of NSCLC has increased rapidly, and substantial progress has been achieved. But problems remain to be resolved. First, many clinical trials are evaluating the drug or a biological equivalent. Certainly, exceptional drug development costs and the goal to optimize the use of each entity is compelling. But the shortcomings discovered in current drugs represent unmet need opportunities for new mechanistic approaches. Secondly, the drug clinical trial sites are overly concentrated in economically and academically developed centers: Beijing, Shanghai, Jiangsu, Zhejiang; however, this may induce a populational diversity bias (Figures 6, 7). Regions such as Qinghai, Gansu, and Ningxia have low participation in drug trials. Given the ethnic heterogeneity of Chinese patients nationally, the regional distribution studied is very uneven (Tables 1, 2). Thirdly, China vigorously promotes the application of traditional Chinese medicine. Yet, in the drug clinical trials registered, only 48 (3.5%) studied Chinese medicines. Although the mechanism of action of many traditional Chinese medicine products is still unclear, the therapeutic effects based on extensive experience is established. Traditional Chinese medicine has been shown to modulate tumor proliferation, cell adhesion, apoptosis, and tumor migration, impact angiogenesis and alter immune responsiveness (55–57). Mechanistic understanding underpinning Traditional Chinese medicine treatments in NSCLC need to be mined for new unanticipated opportunities. Our research provides a clear direction for the conduct of clinical trials and lays some groundwork for the development of NSCLC drugs.
This review has limitations. First, registration information provided by different official websites are inconsistent. For instance, the clinical trials of many domestic multi-centered clinical trials in ClinicalTrials.gov do not specifically identify the supporting clinical centers. Second, there is no general uniformity among the inclusion criteria and exclusion criteria in different trials, leading to interpretive challenges comparing or combining study results. Third, the data in this review were collected from 2005 onward, since prior to 2005 registration of clinical studies was not required. Fourth, searches using non -small cell lung cancer as keywords may have passed over studies unwittingly. Finally, when the registry website was first set up, there was insufficient awareness of the research information register among researchers and sponsors, and there was a discrepancy between the early data and the actual conduct.
5 Conclusion
In short, this review systematically analyzed the clinical trials and time trends registered for the treatment of NSCLC from 2005 to 2023. In the 18 years reviewed, a rapid rise in the development of biopharmaceuticals, particularly for immunotherapy, have been the most prevalent and effective, improving quality of life and survival. At the same time, our summary of ADRs to the therapy of NSCLC’s dual-immune inhibitors also further revealed the application of CTLA-4 plus PD- (L)1 in other related cancers. However, the research and development of new drug classes and mechanisms is limited. China, and the world broadly, should continue to invest time and resources to address this drug diversity shortcoming.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary material.
Author contributions
WJ, HY, LS, JW, SN, GZ, ML, JL, and RN contributed to the concept of the research question and study design. HY, SN, and WJ searched databases, analyzed data, and prepared the manuscript. JL and RN revised the article. LS and ML designed the study. ML and JW prepared figures and/or tables. GZ and ML did manuscript editing. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Natural Science Foundation Committee of Inner Mongolia (2021BS08021).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2023.1239351/full#supplementary-material
Footnotes
References
1. Goldstraw, P, Ball, D, Jett, JR, Le Chevalier, T, Lim, E, Nicholson, AG, et al. Non-small-cell lung cancer. Lancet. (2011) 378:1727–40. doi: 10.1016/S0140-6736(10)62101-0
2. Siegel, RL, Miller, KD, Wagle, NS, and Jemal, A. Cancer statistics, 2023. CA Cancer J Clin. (2023) 73:17–48. doi: 10.3322/caac.21763
3. Rafei, H, El-Bahesh, E, Finianos, A, Nassereddine, S, and Tabbara, I. Immune-based therapies for non-small cell lung cancer. Anticancer Res. (2017) 37:377–88. doi: 10.21873/anticanres.11330
4. Ran, Q, Xuting, L, Shengnan, G, and Guoqiang, L. Pharmacoeconomic evaluation of loratinib versus aletinib in the first-line treatment of mesenchymal lymphoma kinase-positive non-small cell lung cancer. Zhong Guo Yaoye. (2023) 32:115–9.
5. Akobeng, AK. Understanding randomised controlled trials. Arch Dis Child. (2005) 90:840–4. doi: 10.1136/adc.2004.058222
6. Zhou, Y, Yang, J, He, Y, Lv, Y, Wang, C, Deng, H, et al. Characteristic analysis of clinical trials for new traditional Chinese medicines in mainland China from 2013 to 2021. Front Med. (2022) 9:1008683. doi: 10.3389/fmed.2022.1008683
7. Luo, X, Yang, L, Du, X, Yang, J, Qian, F, and Yang, Y. Analysis of patent and regulatory exclusivity for novel agents in China and the United States: a cohort study of drugs approved between 2018 and 2021. Clin Pharmacol Ther. (2022) 112:335–43. doi: 10.1002/cpt.2625
8. Luo, X, Qian, F, Yang, L, Li, Y, and Yang, Y. Assessment of the breakthrough-therapy-designated drugs granted in China: a pooled analysis 2020–2022. Drug Discov Today. (2022) 27:103370. doi: 10.1016/j.drudis.2022.103370
9. Saleh, T, Cuttino, L, and Gewirtz, DA. Autophagy is not uniformly cytoprotective: a personalized medicine approach for autophagy inhibition as a therapeutic strategy in non-small cell lung cancer. Biochim Biophys Acta. (2016) 1860:2130–6. doi: 10.1016/j.bbagen.2016.06.012
10. Bergethon, K, Shaw, AT, Ou, SH, Katayama, R, Lovly, CM, McDonald, NT, et al. ROS1 rearrangements define a unique molecular class of lung cancers. J Clin Oncol. (2012) 30:863–70. doi: 10.1200/JCO.2011.35.6345
11. Kawaguchi, T, Koh, Y, Ando, M, Ito, N, Takeo, S, Adachi, H, et al. Prospective analysis of oncogenic driver mutations and environmental factors: Japan molecular epidemiology for lung cancer study. J Clin Oncol. (2016) 34:2247–57. doi: 10.1200/JCO.2015.64.2322
12. Frampton, GM, Ali, SM, Rosenzweig, M, Chmielecki, J, Lu, X, Bauer, TM, et al. Activation of MET via diverse exon 14 splicing alterations occurs in multiple tumor types and confers clinical sensitivity to MET inhibitors. Cancer Discov. (2015) 5:850–9. doi: 10.1158/2159-8290.CD-15-0285
13. Reck, M, Remon, J, and Hellmann, MD. First-line immunotherapy for non-small-cell lung cancer. J Clin Oncol. (2022) 40:586–97. doi: 10.1200/JCO.21.01497
14. Feihan, G, Rilan, B, and Jiuwei, C. Advances in the combination therapy of immune checkpoint inhibitors for lung cancer. Chin J Lung Cancer. (2020) 23:101–10. doi: 10.3779/j.issn.1009-3419.2020.02.05
15. Zhang, Q, Tang, L, Zhou, Y, He, W, and Li, W. Immune checkpoint inhibitor-associated pneumonitis in non-small cell lung cancer: current understanding in characteristics, diagnosis, and management. Front Immunol. (2021) 12:663986. doi: 10.3389/fimmu.2021.663986
16. Bo, C, Wang, T, Hou, C, Han, J, Chen, L, Zhang, H, et al. Evolution of ischemic stroke drug clinical trials in mainland China from 2005 to 2021. CNS Neurosci Ther. (2022) 28:1229–39. doi: 10.1111/cns.13867
17. Muller, PY. Comparative requirements for exploratory clinical trials—eIND, eCTA and microdosing. Adv Drug Deliv Rev. (2011) 63:511–7. doi: 10.1016/j.addr.2010.10.010
18. Sung, H, Ferlay, J, Siegel, RL, Laversanne, M, Soerjomataram, I, Jemal, A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
19. Petrella, F, Rizzo, S, Attili, I, Passaro, A, Zilli, T, Martucci, F, et al. Stage III non-small-cell lung cancer: an overview of treatment options. Curr Oncol. (2023) 30:3160–75. doi: 10.3390/curroncol30030239
20. Desai, AP, Adashek, JJ, Reuss, JE, West, HJ, and Mansfield, AS. Perioperative immune checkpoint inhibition in early-stage non-small cell lung cancer: a review. JAMA Oncol. (2023) 9:135–42. doi: 10.1001/jamaoncol.2022.5389
21. Nagano, T, Tachihara, M, and Nishimura, Y. Molecular mechanisms and targeted therapies including immunotherapy for non-small cell lung cancer. Curr Cancer Drug Targets. (2019) 19:595–630. doi: 10.2174/1568009619666181210114559
22. Ettinger, DS, Wood, DE, Aisner, DL, Akerley, W, Bauman, J, Chirieac, LR, et al. Non-small cell lung cancer, version 5.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. (2017) 15:504–35. doi: 10.6004/jnccn.2017.0050
23. China GOotNHaHCoPsRo. Clinical practice guideline for primary lung cancer (2022 version). Xiehe Med Mag. (2022) 13:549–70. doi: 10.12290/xhyxzz.2022-0352
24. Li, C, Zhang, L, Meng, G, Wang, Q, Lv, X, Zhang, J, et al. Circular RNAs: pivotal molecular regulators and novel diagnostic and prognostic biomarkers in non-small cell lung cancer. J Cancer Res Clin Oncol. (2019) 145:2875–89. doi: 10.1007/s00432-019-03045-4
25. Chen, J, Wang, R, Zhang, K, and Chen, LB. Long non-coding RNAs in non-small cell lung cancer as biomarkers and therapeutic targets. J Cell Mol Med. (2014) 18:2425–36. doi: 10.1111/jcmm.12431
26. Zhou, C, Kim, SW, Reungwetwattana, T, Zhou, J, Zhang, Y, He, J, et al. Alectinib versus crizotinib in untreated Asian patients with anaplastic lymphoma kinase-positive non-small-cell lung cancer (ALESIA): a randomised phase 3 study. Lancet Respir Med. (2019) 7:437–46. doi: 10.1016/S2213-2600(19)30053-0
27. Wu, YL, Yang, JC, Kim, DW, Lu, S, Zhou, J, Seto, T, et al. Phase II study of crizotinib in east Asian patients with ROS1-positive advanced non-small-cell lung cancer. J Clin Oncol. (2018) 36:1405–11. doi: 10.1200/JCO.2017.75.5587
28. Wang, C, Dai, J, Qin, N, Fan, J, Ma, H, Chen, C, et al. Analyses of rare predisposing variants of lung cancer in 6,004 whole genomes in Chinese. Cancer Cell. (2022) 40:1223–1239.e6. doi: 10.1016/j.ccell.2022.08.013
29. Zheng, S, Wang, X, Fu, Y, Li, B, Xu, J, Wang, H, et al. Targeted next-generation sequencing for cancer-associated gene mutation and copy number detection in 206 patients with non-small-cell lung cancer. Bioengineered. (2021) 12:791–802. doi: 10.1080/21655979.2021.1890382
30. Wang, S, Zhang, C, and Chen, R. Circ_0006220 promotes non-small cell lung cancer progression via sponging mi R-203-3p and regulating RGS17 expression. Hum Exp Toxicol. (2022) 41:096032712110628. doi: 10.1177/09603271211062854
31. The State Council. State Council’s opinions on reforming the examination and approval system for drug and medical devices 2015-08-19. Available: http://wwwgovcn/zhengce/content/2015-08/18/content_10101htm. (2015). (Accessed January 4, 2021)
32. General Office of the State Council of the People’s Republic of China. Opinions on deepening reforms on the drug review system to encourage the innovation for drug and medical equipment (in Chinese). Available at: http://wwwgovcn/zhengce/2017-10/08/content_5230105htm. (2017). (Accessed November 03, 2021)
33. Slawinski, G, Wrona, A, Dabrowska-Kugacka, A, Raczak, G, and Lewicka, E. Immune checkpoint inhibitors and cardiac toxicity in patients treated for non-small lung cancer: a review. Int J Mol Sci. (2020) 21:7195. doi: 10.3390/ijms21197195
34. Imyanitov, EN, Iyevleva, AG, and Levchenko, EV. Molecular testing and targeted therapy for non-small cell lung cancer: current status and perspectives. Crit Rev Oncol Hematol. (2021) 157:103194. doi: 10.1016/j.critrevonc.2020.103194
35. Upadhrasta, S, Elias, H, Patel, K, and Zheng, L. Managing cardiotoxicity associated with immune checkpoint inhibitors. Chronic Dis Transl Med. (2019) 5:6–14. doi: 10.1016/j.cdtm.2019.02.004
36. Gao, LL, Wang, YZ, and Yang, JB. Case introduction of PD-1 immune checkpoint inhibitors combined medication approved by FDA. Chin J New Drugs. (2019) 28:1955–63.
37. Combination immune checkpoint blockade promising in operable NSCLC. Cancer Discov. (2021) 11:1003. doi: 10.1158/2159-8290.CD-RW2021-029
38. Cascone, T, William, WN Jr, Weissferdt, A, Leung, CH, Lin, HY, Pataer, A, et al. Neoadjuvant nivolumab or nivolumab plus ipilimumab in operable non-small cell lung cancer: the phase 2 randomized NEOSTAR trial. Nat Med. (2021) 27:504–14. doi: 10.1038/s41591-020-01224-2
39. Hellmann, MD, Paz-Ares, L, Bernabe Caro, R, Zurawski, B, Kim, SW, Carcereny Costa, E, et al. Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. N Engl J Med. (2019) 381:2020–31. doi: 10.1056/NEJMoa1910231
40. Ma, T, Zhou, S, Cheng, X, Wang, Y, and Liu, Z. Progress in the study of neoadjuvant immunotherapy for non-small cell lung cancer. China Oncology. (2023) 32:780–88.
41. Reuss, JE, Anagnostou, V, Cottrell, TR, Smith, KN, Verde, F, Zahurak, M, et al. Neoadjuvant nivolumab plus ipilimumab in resectable non-small cell lung cancer. J Immunother Cancer. (2020) 8:e001282. doi: 10.1136/jitc-2020-001282
42. Cascone, T, Leung, CH, Weissferdt, A, Pataer, A, Carter, BW, Godoy, MCB, et al. Neoadjuvant chemotherapy plus nivolumab with or without ipilimumab in operable non-small cell lung cancer: the phase 2 platform NEOSTAR trial. Nat Med. (2023) 29:593–604. doi: 10.1038/s41591-022-02189-0
43. Sepesi, B, Zhou, N, William, WN Jr, Lin, HY, Leung, CH, Weissferdt, A, et al. Surgical outcomes after neoadjuvant nivolumab or nivolumab with ipilimumab in patients with non-small cell lung cancer. J Thorac Cardiovasc Surg. (2022) 164:1327–37. doi: 10.1016/j.jtcvs.2022.01.019
44. Tan, AC, and Tan, DSW. Targeted therapies for lung cancer patients with oncogenic driver molecular alterations. J Clin Oncol. (2022) 40:611–25. doi: 10.1200/JCO.21.01626
45. Tan, AC, and Pavlakis, N. Anti-angiogenic therapy in ALK rearranged non-small cell lung cancer (NSCLC). Int J Mol Sci. (2022) 23:8863. doi: 10.3390/ijms23168863
46. Han, B, Li, K, Zhou, C, and Chu, T. In advanced non-small cell lung cancer anti-vascular formation drug treatment of Chinese expert consensus. Chin Lung Cancer Mag. (2022, 2019) 22:401–12.
47. Liang, P, Ballou, B, Lv, X, Si, W, Bruchez, MP, Huang, W, et al. Monotherapy and combination therapy using anti-angiogenic nanoagents to fight cancer. Adv Mater. (2021) 33:e2005155. doi: 10.1002/adma.202005155
48. Chen, PL, Roh, W, Reuben, A, Cooper, ZA, Spencer, CN, Prieto, PA, et al. Analysis of immune signatures in longitudinal tumor samples yields insight into biomarkers of response and mechanisms of resistance to immune checkpoint blockade. Cancer Discov. (2016) 6:827–37. doi: 10.1158/2159-8290.CD-15-1545
49. Ellis, LM, and Hicklin, DJ. VEGF-targeted therapy: mechanisms of anti-tumour activity. Nat Rev Cancer. (2008) 8:579–91. doi: 10.1038/nrc2403
50. Jayson, GC, Kerbel, R, Ellis, LM, and Harris, AL. Antiangiogenic therapy in oncology: current status and future directions. Lancet. (2016) 388:518–29. doi: 10.1016/S0140-6736(15)01088-0
51. Barata, PC, and Rini, BI. Treatment of renal cell carcinoma: current status and future directions. CA Cancer J Clin. (2017) 67:507–24. doi: 10.3322/caac.21411
52. Daum, S, Hagen, H, Naismith, E, Wolf, D, and Pircher, A. The role of anti-angiogenesis in the treatment landscape of non-small cell lung cancer—new combinational approaches and strategies of neovessel inhibition. Front Cell Dev Biol. (2020) 8:610903. doi: 10.3389/fcell.2020.610903
53. Abbosh, C, Birkbak, NJ, Wilson, GA, Jamal-Hanjani, M, Constantin, T, Salari, R, et al. Phylogenetic ctDNA analysis depicts early-stage lung cancer evolution. Nature. (2017) 545:446–51. doi: 10.1038/nature22364
54. Qu, J, Jiang, M, Wang, L, Zhao, D, Qin, K, Wang, Y, et al. Mechanism and potential predictive biomarkers of immune checkpoint inhibitors in NSCLC. Biomed Pharmacother. (2020) 127:109996. doi: 10.1016/j.biopha.2020.109996
55. Wang, R, Luo, Z, Zhang, H, and Wang, T. Tanshinone IIA reverses Gefitinib-resistance in human non-small-cell lung cancer via regulation of VEGFR/Akt pathway. Onco Targets Ther. (2019) 12:9355–65. doi: 10.2147/OTT.S221228
56. Pang, L, Han, S, Jiao, Y, Jiang, S, He, X, and Li, P. Bu Fei decoction attenuates the tumor associated macrophage stimulated proliferation, migration, invasion and immunosuppression of non-small cell lung cancer, partially via IL-10 and PD-L1 regulation. Int J Oncol. (2017) 51:25–38. doi: 10.3892/ijo.2017.4014
Keywords: non-small cell lung cancer, China, drug clinical trials, registration application, statistical analysis
Citation: Jia W, Yu H, Song L, Wang J, Niu S, Zang G, Liang M, Liu J and Na R (2023) Development of clinical trials for non-small cell lung cancer drugs in China from 2005 to 2023. Front. Med. 10:1239351. doi: 10.3389/fmed.2023.1239351
Edited by:
Lina Wu, Harbin Medical University, ChinaReviewed by:
Gregory M. Lanza, Washington University in St. Louis, United StatesFang Wang, Shandong Academy of Pharmaceutical Sciences, China
Copyright © 2023 Jia, Yu, Song, Wang, Niu, Zang, Liang, Liu and Na. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinwei Liu, TDE4MDQ3NjY0MDQyQDE2My5jb20=; Risu Na, bnJzbWFpbEAxNjMuY29t
 Wanying Jia1
Wanying Jia1 Jinwei Liu
Jinwei Liu