
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med., 24 July 2023
Sec. Gastroenterology
Volume 10 - 2023 | https://doi.org/10.3389/fmed.2023.1233928
This article is part of the Research TopicTreatment and Prognostic Assessment of Liver Cirrhosis and Its ComplicationsView all 11 articles
 Chisato Saeki1,2*
Chisato Saeki1,2* Tomoya Kanai1,2
Tomoya Kanai1,2 Kaoru Ueda1
Kaoru Ueda1 Masanori Nakano1,2
Masanori Nakano1,2 Tsunekazu Oikawa1
Tsunekazu Oikawa1 Yuichi Torisu1,2
Yuichi Torisu1,2 Masayuki Saruta1
Masayuki Saruta1 Akihito Tsubota3*
Akihito Tsubota3*Aim: Insulin-like growth factor 1 (IGF-1), which is primarily produced in hepatocytes and is associated with liver functional reserve, plays a crucial role in the pathological condition of cirrhosis. This study aimed to investigate the usefulness of serum IGF-1 levels for predicting the long-term prognosis and decompensation development in patients with cirrhosis.
Methods: We retrospectively evaluated 148 patients with cirrhosis and divided them into three groups according to baseline IGF-1 levels: low (L)-, intermediate (I)-, and high (H)-IGF-1 groups. The cumulative survival rates were compared among these groups in compensated and decompensated cirrhosis, respectively. Significant and independent factors associated with mortality and decompensation development were identified using Cox proportional hazards regression analysis.
Results: The median observation period was 57.1 (41.7–63.2) months. Thirty (20.3%) patients died of liver disease-related events and 21 (22.3%) patients with compensated cirrhosis developed decompensation. Multivariate analysis identified low serum IGF-1 levels as a significant and independent factor associated with mortality (all patients: hazard ratio [HR], 0.967; p = 0.004; patients with compensated cirrhosis: HR, 0.927; p = 0.002). The cumulative survival rates were significantly lower in the L-IGF-1 group than in the H-IGF-1 and I-IGF-1 groups (all patients: p < 0.001 and = 0.009; patients with compensated cirrhosis: p = 0.012 and 0.003, respectively). However, in decompensated cirrhosis, the cumulative survival rates demonstrated no significant differences among the three groups. The cumulative decompensation incidence rates were significantly higher in the L-IGF-1 group than in the H-IGF-1 and I-IGF-1 groups (p < 0.001 and = 0.009, respectively). Low serum IGF-1 levels were significantly and independently associated with decompensation development (HR, 0.939; p < 0.001).
Conclusion: Low serum IGF-1 levels were significantly and independently associated with decompensation development and poor long-term prognosis in patients with compensated cirrhosis. Therefore, IGF-1 may be useful for predicting decompensation-related events and should be regularly monitored in the management of compensated phase.
Cirrhosis is the end stage of chronic liver disease (CLD) with different etiology, and it is a major cause of morbidity and mortality, leading to over 1.32 million (2.4%) global deaths (1, 2). It progresses from a compensated phase (in which most cases are asymptomatic) to a decompensated phase (in which variceal hemorrhage, ascites, encephalopathy, and jaundice can occur) (3). A better prognosis was observed in patients with compensated than those with decompensated cirrhosis, with median survival times of >12 years and < 2 years, respectively (3). Therefore, the management of patients with compensated cirrhosis is crucial to inhibit decompensated cirrhosis progression and thereby reduce the mortality rate. The Child–Pugh (CP) classification, as determined based on serum albumin, total bilirubin, and prothrombin time (PT) levels, ascites degree, and encephalopathy grade, is widely used to evaluate liver functional reserve (4). This classification can reflect clinical features and estimate patients’ prognoses. A better prognosis was observed in patients with CP class A (nearly corresponding to compensated cirrhosis) than those with CP class B/C (nearly corresponding to decompensated cirrhosis) (3). However, this scoring system includes subjective components, i.e., ascites and encephalopathy, and therefore may reduce clinical assessment reliability and survival prediction accuracy (5). Furthermore, it can hardly assess prognosis in patients with compensated cirrhosis (6).
Insulin-like growth factor 1 (IGF-1), which is primarily produced in hepatocytes and involved in mediating growth and metabolism, plays a crucial role in the pathological condition of CLD (7–9). Its circulating levels have been reported to correlate with liver functional reserve. They are decreased with advanced disease stage and malnutrition status, thereby exacerbating or causing insulin resistance, reactive oxygen species, inflammation, mitochondrial dysfunction, liver fibrosis, osteoporosis, and sarcopenia (7–12). A previous study revealed significantly lower 2 years survival rates in inpatients with cirrhosis with low plasma IGF-1 levels than those with high plasma IGF-1 levels (13). Furthermore, combined serum IGF-1 levels and CP scores (IGF-CP score) more accurately predict 1 year mortality than CP or model for end-stage liver disease (MELD) scores alone in patients with decompensated cirrhosis (5). Thus, circulating IGF-1 levels may be useful for estimating liver functional reserve or pathological condition and predicting prognosis in patients with cirrhosis. However, previous studies were limited to decompensated cirrhosis or short-term research duration and have not yet reported the association of IGF-1 with long-term prognosis in patients with compensated and decompensated cirrhosis, respectively.
This study aimed to investigate the usefulness of serum IGF-1 levels for predicting the long-term prognosis of patients with cirrhosis and the difference in the predictive performance of IGF-1 between patients with compensated and decompensated cirrhosis. Furthermore, we evaluated the usefulness of serum IGF-1 levels for predicting decompensation development in patients with compensated cirrhosis.
This retrospective study included 148 consecutive patients with cirrhosis who presented to the Jikei University School of Medicine (Tokyo, Japan) and Fuji City General Hospital (Shizuoka, Japan) between 2017 and 2020. Inclusion criteria were (i) diagnosis of cirrhosis caused by any etiology and (ii) available baseline serum IGF-1 measurements. Exclusion criteria were (i) preexisting malignancies, including hepatocellular carcinoma (HCC); (ii) liver transplantation history; and (iii) acute liver failure. Cirrhosis diagnosis was based on laboratory tests and imaging/endoscopic findings, such as surface nodularity, liver deformity with right lobe shrinkage and left lobe enlargement, ascites, splenomegaly, portosystemic collateral, and esophageal/gastric varices (14). Decompensated cirrhosis was diagnosed by the development of variceal hemorrhage, ascites, encephalopathy, and jaundice (15). Liver functional reserve was evaluated using the MELD score and CP classification (4, 16). The endpoint of this study was death from liver disease-related events. Patients who underwent liver transplantation for liver failure were considered as death and those who died from non-liver-related causes were considered censored cases. This study complied with the 2013 Declaration of Helsinki and was approved by the ethics committees of the Jikei University School of Medicine (approval number: 34-021) and Fuji City General Hospital (approval number: 279).
Serum albumin, total bilirubin, creatinine, sodium, Mac-2 binding protein glycosylation isomer (M2BPGi, a hepatic fibrosis marker), and PT were measured using standard laboratory methods. Serum IGF-1 levels were evaluated using an immunoradiometric assay (Fujirebio, Tokyo, Japan).
The median baseline IGF-1 levels of all patients and those with compensated and decompensated cirrhosis were 54 (interquartile range, 41–74), 61 (47–78), and 46 (32–61) ng/mL, respectively (Supplementary Figure S1). Patients were classified into three groups according to the quartiles: low (L)-IGF1all group (≤41 ng/mL), intermediate (I)-IGF1all group (41–74 ng/mL), and high (H)-IGF1all group (≥74 ng/mL) for all patients; L-IGF1com group (≤47 ng/mL), I-IGF1com group (47–78 ng/mL), and H-IGF1com group (≥78 ng/mL) for those with compensated cirrhosis; and L-IGF1deco group (≤32 ng/mL), I-IGF1deco group (32–61 ng/mL), and H-IGF1deco group (≥61 ng/mL) for those with decompensated cirrhosis.
Between-group differences were compared using the Mann–Whitney U test or the Kruskal–Wallis test followed by the Steel–Dwass post hoc test, as appropriate, for continuous variables that were presented as medians (interquartile ranges), and the chi-squared test for categorical variables that were presented as numbers (percentages). Correlations between IGF-1 and continuous variables were analyzed using Spearman’s rank correlation test. The cumulative survival rates were estimated using the Kaplan–Meier method, and the between-group differences were compared using the log-rank test and Bonferroni multiple-comparison method. Significant and independent factors associated with mortality were identified using univariate and multivariate Cox proportional hazards models. SPSS Statistics version 27 (IBM Japan, Tokyo, Japan) was used for all statistical analyses. Values of p < 0.05 were considered statistically significant.
Table 1 summarizes the baseline clinical characteristics of 148 included patients. The median age was 69 (57.0–76.0) years, and 96 (64.9%) patients were men. The median MELD score was 8.0 (7.0–11.0). The prevalence of decompensated cirrhosis was 36.5% (54/148). Serum IGF-1 levels in patients with decompensated cirrhosis were significantly lower than in those with compensated cirrhosis (median, 46 ng/mL vs. 61 ng/mL; p < 0.001; Supplementary Figure S1). Additionally, they significantly differed among CP classes (p < 0.001; Supplementary Figure S2) and were significantly higher in patients with the CP class A (median, 62 ng/mL) than in those with the CP class B (median, 43 ng/mL; p < 0.001) and class C (median, 34 ng/mL; p = 0.005) in the post hoc analysis. However, when the patients were divided into three groups according to age (<65, 65–74, and ≥75 years), serum IGF-1 levels did not significantly differ among the three groups (p = 0.738; Supplementary Figure S3).
The L-IGF1all, I-IGF1all, and H-IGF1all group distributions were 27.7% (41/148), 47.3% (70/148), and 25.0% (37/148), respectively (Table 1). Etiology, decompensated cirrhosis prevalence, M2BPGi, and liver functional reserve-related factors (CP score, MELD score, total bilirubin, albumin, and PT) significantly differed among the three groups.
The correlations between IGF-1 and liver functional reserve-related factors or M2BPGi were investigated using Spearman’s rank correlation test (Figures 1A–F). Serum IGF-1 levels significantly correlated with total bilirubin (r = −0.280), albumin (r = 0.387), PT (r = 0.481), and M2BPGi (r = −0.481) levels, CP score (r = −0.422), and MELD score (r = −0.319) (p < 0.001 for all).
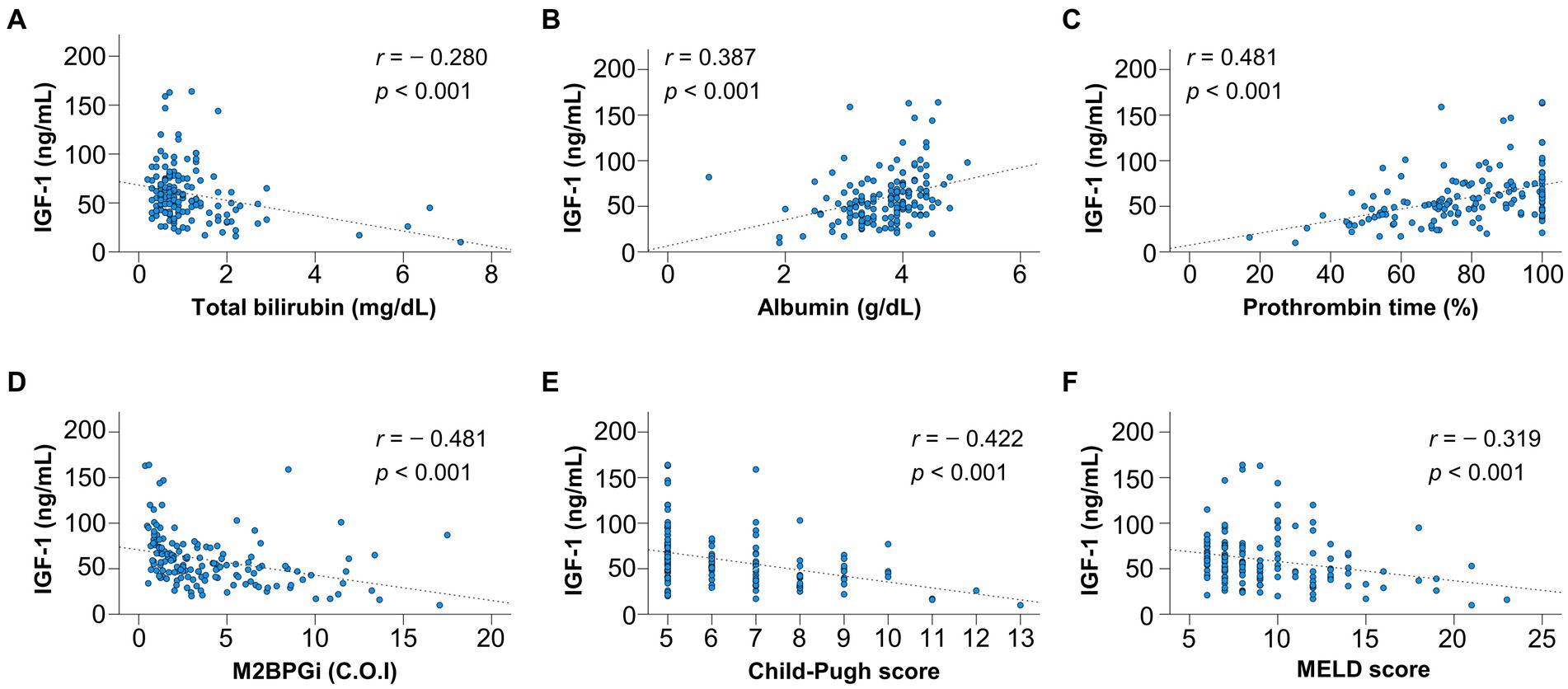
Figure 1. Correlation of insulin-like growth factor 1 (IGF-1) with liver functional reserve-related parameters and fibrosis marker. IGF-1 significantly correlated with (A) total bilirubin, (B) albumin, (C) prothrombin time, (D) Mac-2 binding protein glycosylation isomer (M2BPGi), (E) Child–Pugh score, and (F) model for end-stage liver disease (MELD) score (p < 0.001 for all).
The median observation period was 57.1 (41.7–63.2) months. During the follow-up period, 30 (20.3%) patients died of liver disease-related events, including liver failure (n = 21), rupture of esophageal varices (n = 6), liver transplantation (n = 2), and hepatocellular carcinoma (n = 1). The 1-, 3-, and 5 years cumulative survival rates were 95.1, 73.9, and 56.2% in the L-IGH1all group; 98.6, 89.5, and 81.4% in the I-IGH1all group; and 100.0, 94.3, and 94.3% in the H-IGH1all group (Figure 2). The cumulative survival rates were significantly lower in the L-IGF1all group than in the H-IGF1all and I-IGF1all groups (p < 0.001 and = 0.009, respectively). The L-IGF1all group had significantly lower cumulative survival rates than the H-IGF1all and I-IGF1all groups when nine patients who died from non-liver-related causes (L-IGF1all, n = 6; I-IGF1all, n = 2; H-IGF1all, n = 1) were considered the same as liver-related deaths (p < 0.001 for both; Supplementary Figure S4).
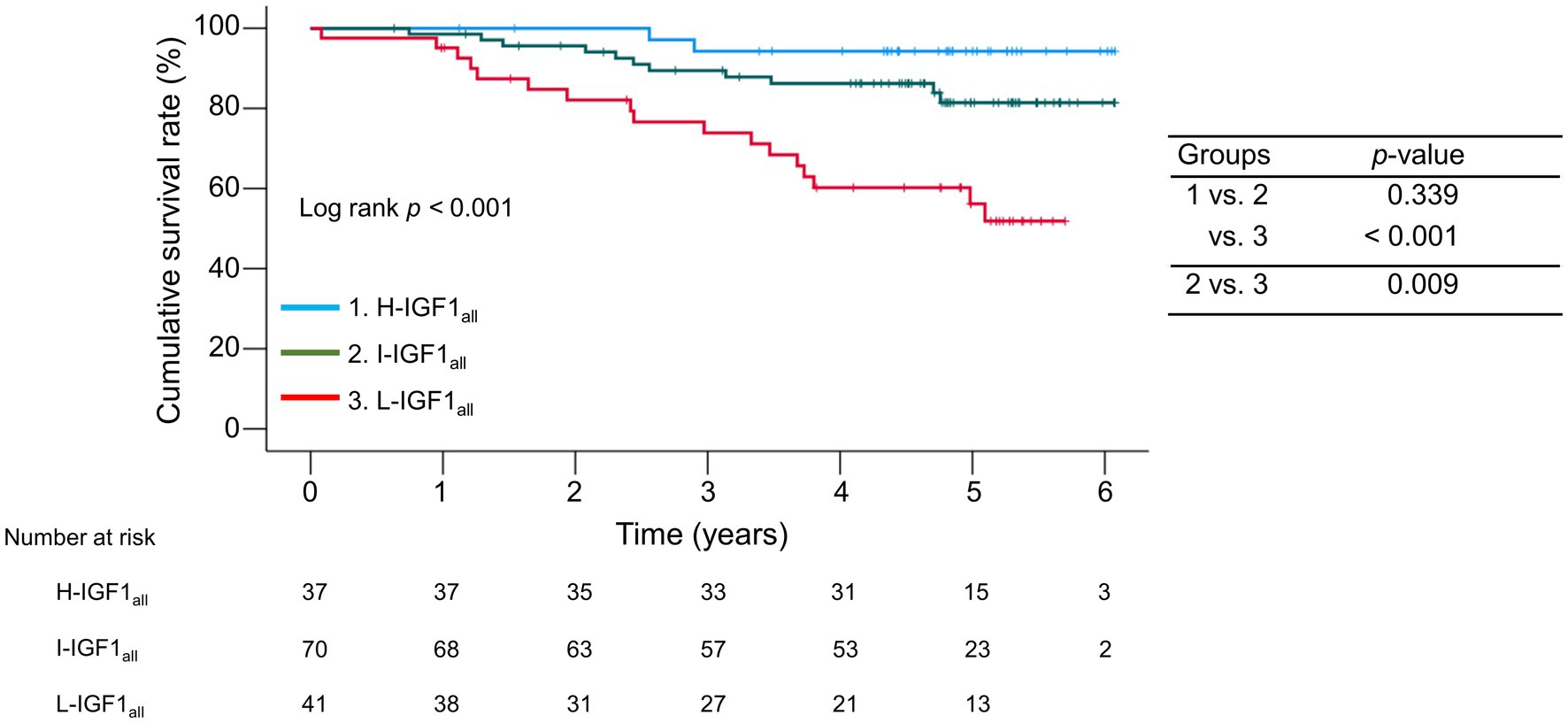
Figure 2. Comparison of the cumulative survival rates among the three groups stratified following the baseline serum levels of insulin-like growth factor 1 (IGF-1) levels. The cumulative survival rates were significantly lower in the low-IGF-1 (L-IGF1all) group than in the high-IGF-1(H-IGF1all) and intermediate-IGF-1(I-IGF1all) groups (p < 0.001 and = 0.009, respectively).
We divided the patients into the compensated and decompensated cirrhosis groups and compared the cumulative survival rates among the three IGF1 groups in each cirrhosis group (Figure 3). The 1-, 3-, and 5 years cumulative survival rates in the compensated cirrhosis group were 100.0, 86.9, and 72.4% in the L-IGF1com group; 100.0, 100, and 96.3% in the I-IGF1com group; and 100.0, 100.0, and 100.0% in the H-IGF1com group (Figure 3A). The L-IGF1com group had significantly lower cumulative survival rates than the H-IGF1com and I-IGF1com groups (p = 0.012 and 0.003, respectively). Meanwhile, the 1-, 3-, and 5 years cumulative survival rates in the decompensated cirrhosis group were 92.9, 68.1, and 34.0% in the L-IGF1deco group; 92.3, 59.1, and 47.1% in the I-IGF1deco group; and 100.0, 84.6, and 76.9% in the H-IGF1deco group (Figure 3B). These rates demonstrated no significant differences among the three groups (p = 0.177). However, the redivision from 3 to 2 groups (H-IGF1deco versus combined I-IGF1deco and L-IGF1deco) demonstrated a marginally significant difference in the cumulative survival rates between the two groups (p = 0.064; Supplementary Figure S5).
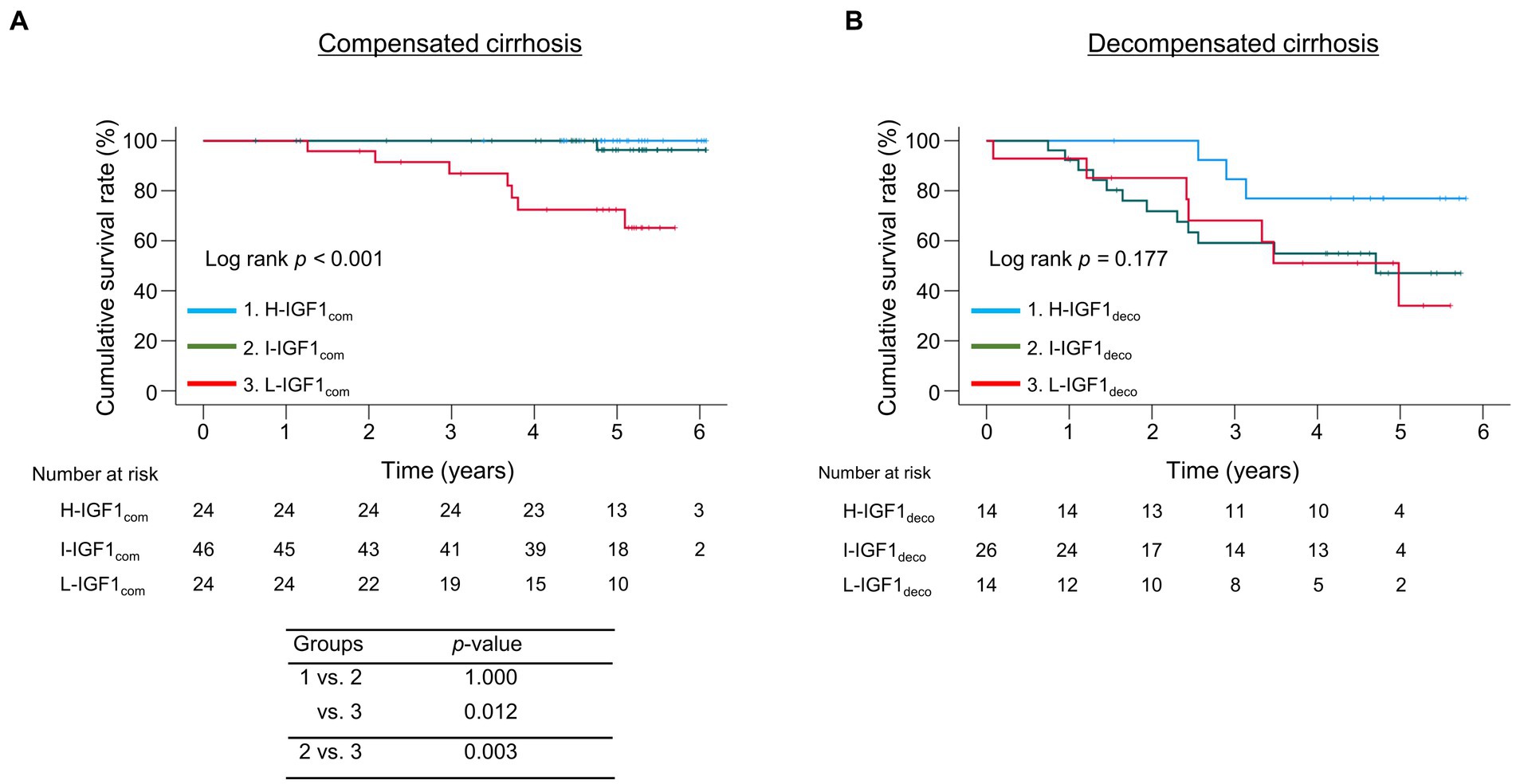
Figure 3. Comparison of the cumulative survival rates among the three groups stratified following the baseline serum levels of insulin-like growth factor 1 (IGF-1) levels in compensated or decompensated cirrhosis. (A) In compensated cirrhosis, the cumulative survival rates were significantly lower in the low-IGF-1 (L-IGF1com) group than in the high-IGF-1 (H-IGF1com) and intermediate-IGF-1 (I-IGF1com) groups (p = 0.012 and 0.003, respectively). (B) In decompensated cirrhosis, the cumulative survival rates did not significantly differ among the three groups (p = 0.177).
Univariate analysis revealed that the following variables were significantly associated with mortality: decompensated cirrhosis, CP score, MELD score, total bilirubin, albumin, sodium, PT, IGF-1, and M2BPGi in all patients (Supplementary Table S1); PT, IGF-1, and M2BPGi in those with compensated cirrhosis (Supplementary Table S2); and CP score, MELD score, total bilirubin, albumin, IGF-1, and M2BPGi in those with decompensated cirrhosis (Supplementary Table S3). Cox proportional hazards regression analysis identified the following variables as significant and independent prognostic factors: high CP score [hazard ratio (HR), 1.248; 95% confidence interval (CI), 1.014–1.538; p = 0.037], low albumin levels (HR, 0.346; 95% CI, 0.204–0.589; p < 0.001), and low IGF-1 levels (HR, 0.967; 95% CI, 0.945–0.989; p = 0.004) in all patients (Table 2); low IGF-1 levels (HR, 0.927; 95% CI, 0.884–0.972; p = 0.002) in those with compensated cirrhosis (Table 3); and low albumin levels (HR, 0.097; 95% CI, 0.038–0.245; p < 0.001) in those with decompensated cirrhosis (Table 4).
During the follow-up period, 21 (22.3%) patients with compensated cirrhosis developed decompensation. The 1-, 3-, and 5 years cumulative incidence rates of decompensation were 20.8, 38.0, and 52.8% in the L-IGH1com group; 0.0, 4.5, and 22.4% in the I-IGH1com group; and 0.0, 0.0, and 0.0% in the H-IGH1com group (Figure 4). The cumulative decompensation incidence rates were significantly higher in the L-IGH1com group than in the H-IGH1com and I-IGH1com groups (p < 0.001 and = 0.009, respectively).
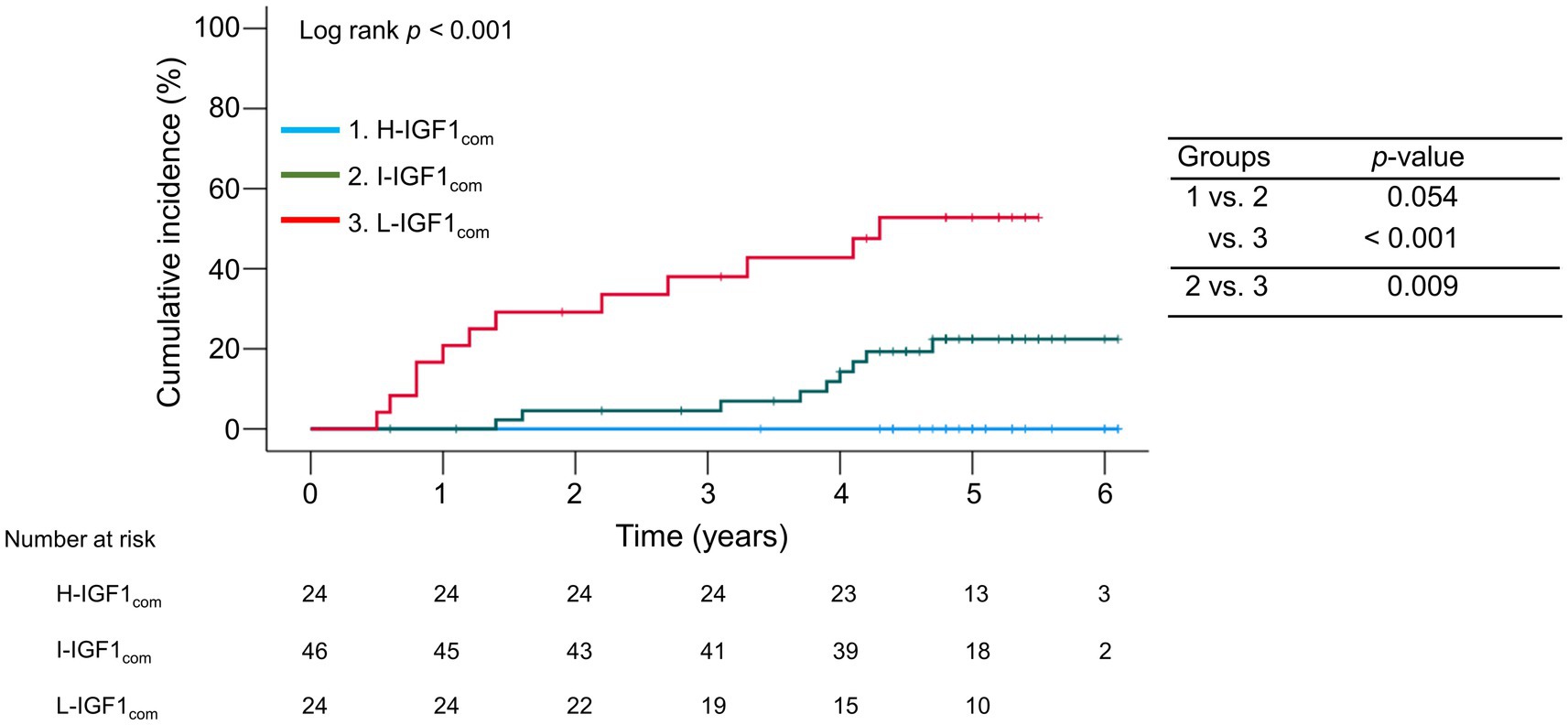
Figure 4. Comparison of the cumulative incidence rates of decompensation among the three groups stratified following the baseline serum insulin-like growth factor 1 (IGF-1) levels in compensated cirrhosis. The cumulative incidence rates of decompensation were significantly higher in the low-IGF-1 (L-IGF1com) group than in the high-IGF-1 (H-IGF1com) and intermediate-IGF-1 (I-IGF1com) groups (p < 0.001 and = 0.009, respectively).
In the univariate analysis, CP score, total bilirubin, albumin, PT, IGF-1, and M2BPGi were significantly associated with decompensation development in patients with compensated cirrhosis (Supplementary Table S4). Cox proportional hazards regression analysis identified low albumin levels (HR, 0.447; 95% CI, 0.223–0.896; p = 0.023) and low IGF-1 levels (HR, 0.939; 95% CI, 0.913–0.966; p < 0.001) as significant and independent factors associated with decompensation development (Table 5).
Most patients with compensated cirrhosis are asymptomatic and have a better prognosis compared to those with decompensated cirrhosis (3). However, various liver disease-related events develop and mortality increases evidently during liver disease progression from the compensated phase to the decompensated phase (15). Therefore, early decompensation prediction and medical countermeasures against complications are crucial in patients with compensated cirrhosis. IGF-1 can inhibit or improve hepatic inflammation and fibrosis, and therefore, may be deeply involved in the pathological CLD condition (7–9). Reportedly, circulating IGF-1 levels decrease with disease progression, and the decreased levels are associated with poor short-term prognosis in patients with cirrhosis, especially decompensated cirrhosis (5, 13). The present study is the first to report the association between serum IGF-1 levels and long-term prognosis in patients with compensated and decompensated cirrhosis, respectively. Intriguingly, serum IGF-1 levels were the only significant prognostic factor in patients with compensated cirrhosis, whereas serum albumin (but not IGF-1) levels in those with decompensated cirrhosis. Notably, deaths were not observed, as well as cases with decompensation development in the H-IGF1com group when limited to patients with compensated cirrhosis. Meanwhile, the H-IGF1deco group in patients with decompensated cirrhosis also had the highest survival rates, although marginally significant, and all H-IGF1deco patients survived during the first 2 years.
One study of patients with cirrhosis (without HCC: n = 64; CP class B/C, 73.4%) revealed that plasma IGF-1 levels (mean: 80.0, 62.7, and 32.6 ng/mL for CP class A, B, and C, respectively) were inversely correlated with the CP score, and the L-IGF1 group had lower 2 years survival rates than the H-IGF1 group (13). Another study of patients with decompensated cirrhosis (without HCC) revealed that serum IGF-1 levels (median: 70.1, 40.5, and 32.4 ng/mL for CP class A, B, and C, respectively) and IGF-CP scores were associated with 1 year mortality (5). This IGF-CP scoring system more accurately predicted 1 year mortality than the CP score alone. Another study of patients with CP class A and advanced HCC receiving antiangiogenic therapy revealed that low serum IGF-1 levels (<63.6 ng/mL) reduced the progression-free and overall survival rates (17). Our study revealed that the median IGF-1 levels were 62, 43, and 34 ng/mL for CP class A, B, and C, respectively, and 61 and 46 ng/mL for compensated and decompensated cirrhosis, respectively. The IGF-1 predictive value of poor prognosis in patients with cirrhosis (without HCC) may be approximately 40 ng/mL, considering that the cutoff IGF-1 values for the L-IGF1 groups were 41, 47, and 32 ng/mL in all patients and those with compensated and decompensated cirrhosis, respectively.
The MELD and CP scores provide prognostic information for patients with decompensated cirrhosis (18). The MELD score predicts short-term mortality in end-stage liver disease and is useful for determining organ allocation to liver transplantation candidates on transplant waiting lists (19, 20). Meanwhile, the CP score predicts differences in prognosis between patients with CP classes A and B/C (3). However, these scoring systems can hardly identify patients with poor prognoses when limited to those with compensated cirrhosis (6, 18). Therefore, circulating IGF-1 levels may be a useful predictor of long-term prognosis in the compensated phase.
The present study revealed that serum IGF-1 levels were significantly correlated with liver functional reserve-related factors, which was consistent with previous studies (10, 13, 21, 22). Serum IGF-1 levels correlated positively with albumin and PT levels and negatively with total bilirubin level, CP score, and MELD score. Reportedly, serum IGF-1 levels are substantially low in a large percentage of patients with compensated cirrhosis despite normal albumin and prealbumin levels; hence, they may be a more sensitive and earlier indicator of impaired liver function than other conventional parameters (13). As described above, patients with compensated cirrhosis must inhibit the progression to decompensation. The MELD and CP scores are widely used to estimate liver functional reserve; however, these provide little information on the risk of developing decompensation (18). The present study identified serum IGF-1 levels as the only significant factor associated with decompensation development. The L-IGF1com group had significantly higher cumulative incidence rates of decompensation than the H-IGF1com and I-IGF1com groups. Intriguingly, the H-IGF1com group demonstrated no decompensation. Therefore, serum IGF-1 levels may be a simple and useful indicator of liver functional reserve and the risk of developing decompensation.
Hepatic stellate cells (HSCs), which are activated by oxidative stress, proinflammatory cytokines, and autophagy, participate in liver regeneration and fibrosis (23). Meanwhile, cellular senescence of activated HSCs suppresses liver fibrogenesis (24). IGF-1 administration in mouse models with methionine-choline-deficient diet-induced nonalcoholic steatohepatitis and dimethylnitrosamine-induced cirrhosis ameliorated hepatic steatosis, inflammation, and fibrosis (25). Additionally, IGF-1 induced HSCs into cellular senescence in vitro and in vivo and inhibited fibrogenesis in a p53-dependent manner. Similarly, IGF-1 administration improved liver function (increased albumin, total protein, and coagulation factor levels) and reduced oxidative liver damage and fibrosis in rat models with CCl4-induced cirrhosis (26). Preoperative serum IGF-1 and IGF-binding protein-3 levels in the cirrhosis group who underwent liver transplantation were lower than those in the control group, but their postoperative levels recovered to normal (27). A pilot study of patients with cirrhosis revealed that IGF-1 administration for 4 months increased serum albumin levels and improved energy metabolism, as assessed by resting energy expenditure (28). These basic and clinical research findings indicate that IGF-1 which is largely produced in hepatocytes is closely involved in hepatic inflammation and fibrosis regulation; hence, advanced disease stage and impaired liver functional reserve reduce IGF-1 levels, which may in turn exacerbate the disease conditions and cause poor prognosis.
This study has some limitations. First, this was a retrospective, small-scale study; therefore, prospective, large-scale studies are needed to confirm our findings. Second, we were unable to assess the IGF-binding protein levels, which regulate the biological activity of IGF-1 and may be associated with liver functional reserve (8, 9, 21). Finally, serum IGF-1 levels were measured only at baseline, whereas longitudinal changes in serum IGF-1 levels were not investigated; thus, the differences in the longitudinal changes between patients with and without decompensation development or between patients with good and poor prognoses remain unclear.
In conclusion, low serum IGF-1 levels were associated with decompensation development and poor long-term prognosis in patients with compensated cirrhosis. Therefore, IGF-1 may be useful for predicting decompensation-related events and should be regularly monitored in the management of compensated cirrhosis.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by The ethics committees of the Jikei University School of Medicine (approval number: 34-021) and Fuji City General Hospital (approval number: 279). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
CS participated in the conception and design of the study. CS, TK, KU, MN, TO, and YT acquired, analyzed, and interpreted the data. CS and AT drafted the manuscript. MS and AT interpreted the data and revised the manuscript. AT substantively revised and completed the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2023.1233928/full#supplementary-material
1. Sepanlou, SG, Safiri, S, Bisignano, C, Ikuta, KS, Merat, S, and Saberifiroozi, M. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet Gastroenterol Hepatol. (2020) 5:245–66. doi: 10.1016/S2468-1253(19)30349-8
2. Huang, DQ, Terrault, NA, Tacke, F, Gluud, LL, Arrese, M, Bugianesi, E, et al. Global epidemiology of cirrhosis—aetiology, trends and predictions. Nat Rev Gastroenterol Hepatol. (2023) 20:388–98. doi: 10.1038/s41575-023-00759-2
3. D’Amico, G, Garcia-Tsao, G, and Pagliaro, L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. (2006) 44:217–31. doi: 10.1016/j.jhep.2005.10.013
4. Pugh, RN, Murray-Lyon, IM, Dawson, JL, Pietroni, MC, and Williams, R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. (1973) 60:646–9. doi: 10.1002/bjs.1800600817
5. Yao, Y, Yang, D, Huang, Y, and Dong, M. Predictive value of insulin-like growth factor 1-Child–Turcotte–Pugh score for mortality in patients with decompensated cirrhosis. Clin Chim Acta. (2020) 505:141–7. doi: 10.1016/j.cca.2020.02.031
6. Tapper, EB, Zhang, P, Garg, R, Nault, T, Leary, K, Krishnamurthy, V, et al. Body composition predicts mortality and decompensation in compensated cirrhosis patients: a prospective cohort study. JHEP Rep. (2019) 2:100061. doi: 10.1016/j.jhepr.2019.11.005
7. Takahashi, Y. The role of growth hormone and insulin-like growth factor-I in the liver. Int J Mol Sci. (2017) 18:1447. doi: 10.3390/ijms18071447
8. Adamek, A, and Kasprzak, A. Insulin-like growth factor (IGF) system in liver diseases. Int J Mol Sci. (2018) 19:1308. doi: 10.3390/ijms19051308
9. Bonefeld, K, and Møller, S. Insulin-like growth factor-I and the liver. Liver Int. (2011) 31:911–9. doi: 10.1111/j.1478-3231.2010.02428.x
10. Assy, N, Pruzansky, Y, Gaitini, D, Shen Orr, Z, Hochberg, Z, and Baruch, Y. Growth hormone-stimulated IGF-1 generation in cirrhosis reflects hepatocellular dysfunction. J Hepatol. (2008) 49:34–42. doi: 10.1016/j.jhep.2008.02.013
11. Locatelli, V, and Bianchi, VE. Effect of GH/IGF-1 on bone metabolism and osteoporsosis. Int J Endocrinol. (2014) 2014:235060. doi: 10.1155/2014/235060
12. Saeki, C, and Tsubota, A. Influencing factors and molecular pathogenesis of sarcopenia and osteosarcopenia in chronic liver disease. Life. (2021) 11:899. doi: 10.3390/life11090899
13. Caregaro, L, Alberino, F, Amodio, P, Merkel, C, Angeli, P, Plebani, M, et al. Nutritional and prognostic significance of insulin-like growth factor 1 in patients with liver cirrhosis. Nutrition. (1997) 13:v–90. doi: 10.1016/s0899-9007(96)00399-1
14. Lurie, Y, Webb, M, Cytter-Kuint, R, Shteingart, S, and Lederkremer, GZ. Non-invasive diagnosis of liver fibrosis and cirrhosis. World J Gastroenterol. (2015) 21:11567–83. doi: 10.3748/wjg.v21.i41.11567
15. European Association for the Study of the Liver. EASL clinical practice guidelines for the management of patients with decompensated cirrhosis. J Hepatol. (2018) 69:406–60. doi: 10.1016/j.jhep.2018.03.024
16. Malinchoc, M, Kamath, PS, Gordon, FD, Peine, CJ, Rank, J, and ter Borg, PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. (2000) 31:864–71. doi: 10.1053/he.2000.5852
17. Shao, YY, Huang, CC, Lin, SD, Hsu, CH, and Cheng, AL. Serum insulin-like growth factor-1 levels predict outcomes of patients with advanced hepatocellular carcinoma receiving antiangiogenic therapy. Clin Cancer Res. (2012) 18:3992–7. doi: 10.1158/1078-0432.CCR-11-2853
18. Hsu, CY, Parikh, ND, Huo, TI, and Tapper, EB. Comparison of seven noninvasive models for predicting decompensation and hospitalization in patients with cirrhosis. Dig Dis Sci. (2021) 66:4508–17. doi: 10.1007/s10620-020-06763-9
19. Wiesner, R, Edwards, E, Freeman, R, Harper, A, Kim, R, Kamath, P, et al. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology. (2003) 124:91–6. doi: 10.1053/gast.2003.50016
20. Cholongitas, E, Marelli, L, Shusang, V, Senzolo, M, Rolles, K, Patch, D, et al. A systematic review of the performance of the model for end-stage liver disease (MELD) in the setting of liver transplantation. Liver Transpl. (2006) 12:1049–61. doi: 10.1002/lt.20824
21. Wu, YL, Ye, J, Zhang, S, Zhong, J, and Xi, RP. Clinical significance of serum IGF-I, IGF-II and IGFBP-3 in liver cirrhosis. World J Gastroenterol. (2004) 10:2740–3. doi: 10.3748/wjg.v10.i18.2740
22. Ronsoni, MF, Lazzarotto, C, Fayad, L, Silva, MC, Nogueira, CL, Bazzo, ML, et al. IGF-I and IGFBP-3 serum levels in patients hospitalized for complications of liver cirrhosis. Ann Hepatol. (2013) 12:456–63. doi: 10.1016/S1665-2681(19)31009-9
23. Tsuchida, T, and Friedman, SL. Mechanisms of hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol. (2017) 14:397–411. doi: 10.1038/nrgastro.2017.38
24. Krizhanovsky, V, Yon, M, Dickins, RA, Hearn, S, Simon, J, Miething, C, et al. Senescence of activated stellate cells limits liver fibrosis. Cells. (2008) 134:657–67. doi: 10.1016/j.cell.2008.06.049
25. Nishizawa, H, Iguchi, G, Fukuoka, H, Takahashi, M, Suda, K, Bando, H, et al. IGF-I induces senescence of hepatic stellate cells and limits fibrosis in a p53-dependent manner. Sci Rep. (2016) 6:34605. doi: 10.1038/srep34605
26. Castilla-Cortazar, I, Garcia, M, Muguerza, B, Quiroga, J, Perez, R, Santidrian, S, et al. Hepatoprotective effects of insulin-like growth factor I in rats with carbon tetrachloride-induced cirrhosis. Gastroenterology. (1997) 113:1682–91. doi: 10.1053/gast.1997.v113.pm9352873
27. Castro, GR, Coelho, JC, Parolin, MB, Matias, JE, and de Freitas, AC. Insulin-like growth factor I correlates with MELD and returns to normal level after liver transplantation. Ann Transplant. (2013) 18:57–62. doi: 10.12659/AOT.883819
28. Conchillo, M, de Knegt, RJ, Payeras, M, Quiroga, J, Sangro, B, Herrero, JI, et al. Insulin-like growth factor I (IGF-I) replacement therapy increases albumin concentration in liver cirrhosis: results of a pilot randomized controlled clinical trial. J Hepatol. (2005) 43:630–6. doi: 10.1016/j.jhep.2005.03.025
Keywords: cirrhosis, insulin-like growth factor 1, liver functional reserve, prognosis, decompensation
Citation: Saeki C, Kanai T, Ueda K, Nakano M, Oikawa T, Torisu Y, Saruta M and Tsubota A (2023) Insulin-like growth factor 1 predicts decompensation and long-term prognosis in patients with compensated cirrhosis. Front. Med. 10:1233928. doi: 10.3389/fmed.2023.1233928
Received: 03 June 2023; Accepted: 11 July 2023;
Published: 24 July 2023.
Edited by:
Xingshun Qi, General Hospital of Shenyang Military Command, ChinaReviewed by:
Sheetalnath Rooge, University of Kansas Medical Center, United StatesCopyright © 2023 Saeki, Kanai, Ueda, Nakano, Oikawa, Torisu, Saruta and Tsubota. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chisato Saeki, Y2hpc2F0b0BqaWtlaS5hYy5qcA==; Akihito Tsubota, YXRzdWJvQGppa2VpLmFjLmpw
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.