- 1Department of Anesthesiology, The Affiliated Shunde Hospital of Jinan University, Foshan, China
- 2Department of Anesthesiology, The Seventh Affiliated Hospital of Southern Medical University, Foshan, China
Objective: Propofol-opioids are the most common drug combination and can reduce the dose of propofol and the incidence of adverse events in painless artificial abortion. We hypothesized that butorphanol may reduce the median effective dose (ED50) of propofol, propofol injection pain, and postoperative uterine contraction pain.
Methods: This was a randomized, double-blind, controlled study. A total of 54 female patients, who had ASA I or II, aged 18–49 years, undergoing painless artificial abortion, were randomly assigned into two groups, namely, Group P (propofol) and Group PB (propofol plus 10 μg/kg butorphanol). According to the pre-experiment, the initial dose of propofol for the P and PB groups was 3 and 2.5 mg/kg, respectively, with a dose gradient of 0.25 mg/kg. The ED50 of propofol was analyzed using probit regression analysis. The total propofol dose consumed, recovery time, and anesthesia-related adverse events were also recorded.
Results: There were 25 and 29 patients in the P and PB groups, respectively. The ED50 (95% CI) of propofol for artificial abortion were 2.477 (2.186–2.737) and 1.555 (1.173–1.846) mg/kg in the P and PB groups, respectively. The total propofol dose consumed was (150.7 ± 21.7) mg and (110.4 ± 28.2) mg in the P and PB groups, respectively (P < 0.001). Compared with the P group, injection-site pain (76 vs. 20.7%) and uterine contraction pain (72 vs. 6.9%) in the PB group had a significant decrease (P < 0.001).
Conclusion: Combination of propofol with 10 μg/kg butorphanol reduced the ED50 of propofol and decreased the incidence of propofol injection-site pain and postoperative uterine contraction pain during painless artificial abortion compared with propofol alone.
Clinical trial registration: https://www.chictr.org.cn/showproj.html?proj=166610, identifier: ChiCTR2200059795.
1 Introduction
Artificial abortion is a relatively fast procedure and can be completed within 3 to 5 min. However, cervical dilation and uterine suction cause intense pain, and some patients exhibit involuntary limb movements, which may increase the risk of uterine perforation (1). Therefore, artificial abortion frequently requires general anesthesia to eliminate the patient's physical discomfort during the procedure.
Propofol, a sedative-hypnotic drug with a rapid onset of action, has been widely used in outpatient surgery or examination anesthesia. However, propofol can cause adverse reactions such as respiratory and circulatory depression, increasing the risk of side effects in high-risk patients (2, 3). When compared to propofol alone, propofol combined with low-dose opioids can provide effective analgesia while lowering the propofol dose (4, 5). Therefore, propofol-opioid combinations can minimize the adverse reactions of high-dose propofol. Butorphanol, a synthetic opioid, exerts analgesic and sedative effects via kappa receptor agonist activity. The most common related adverse reactions to butorphanol include nausea, vomiting, and dizziness, which are also dose-dependent (6, 7). Butorphanol has recently been widely used in outpatient surgery due to its advantages of sedative and analgesic effects with a lower degree of respiratory depression (8, 9).
Sedative and analgesic drugs are the most common drug combinations for painless artificial abortions. However, light or deep anesthesia may cause severe adverse events. Thus, it is necessary to discuss the optimal dose of propofol. We will apply propofol/propofol combined with 10 μg/kg butorphanol in painless artificial abortion to assess the median effective dose (ED50) of the propofol in inhibiting cervical dilatation. It is expected to provide a reference for the safety and rational use of the drug in painless artificial abortions without relevant research.
2 Materials and methods
2.1 Study design and patients
This was a randomized, double-blind, controlled study. The study was approved by the Medical Ethics Committee of the Affiliated Shunde Hospital of Jinan University (number: JDSY-LL-2022005, dated 10 April 2022) and was also registered at www.chictr.org.cn (number: ChiCTR2200059795, dated 11 May 2022; date of the first patient enrollment, 12 May 2022).
2.2 Inclusion and exclusion criteria
The inclusion criteria for this study are as follows: elective artificial abortion; American Society of Anesthesiologists (ASA) class I or II; clinically confirmed early pregnancy by color Doppler ultrasound (<12 weeks); age between 18 and 49 years; and body mass index (BMI) between 18 and 30 kg/m2.
The exclusion criteria for this study include refusal to participate; ASA class III or higher; allergy to drug-related substances; severe liver, kidney, cardiopulmonary, or central nervous system dysfunction; a procedure time exceeding 10 min; and long-term use of sedative or analgesic medications.
2.3 Anesthesia management and surgical procedure
Enrolled patients were randomly assigned into one of the two groups: Group P (propofol) and Group PB (propofol plus 10 μg/kg butorphanol). Patients in group P and group PB received intravenous propofol (Nhwa Pharma Corporation, 20 ml: 0.2 g, lot number: BB220308) for sedation. Patients in group PB received intravenous 10 μg/kg of butorphanol (Jiangsu Hengrui Medicine Co., China, 1 ml:1 mg, diluted to 10 ml with normal saline, lot number: 220129BP) at least 5 min before intravenous propofol; patients in group P received intravenous an equal volume of normal saline. The maximal consumption of butorphanol was 1 mg.
Patients fasted for more than 8 h, and drinking was forbidden for at least 2 h. After entering the operating room, venous access was obtained at the dorsum of the left hand using an intravenous infusion needle with a diameter of 0.6 mm. The oxygen was administered at a flow rate of 3–5 L/min via a nasal straw. The electrocardiogram (ECG), non-invasive blood pressure (NIBP), and peripheral capillary oxygen saturation (SpO2) were measured.
According to reports, cervical dilation was the most painful part of the procedure (10). Cervical dilatation with a cervical dilating rod was defined as “Ineffective” if the patient has body movement and it affects the gynecologist's operation. Therefore, the propofol dosage was increased for the next patient. Otherwise, it was defined as “Effective,” and the propofol dosage was decreased for the next patient. According to the pre-experiment, the initial dose of propofol for the P and PB groups was 3 and 2.5 mg/kg, respectively, with a dose gradient of 0.25 mg/kg. After an “Effective” sedation, propofol dosage was decreased by 0.25 mg/kg for the next patient. However, if the sedation was “Ineffective,” the propofol dose was increased by 0.25 mg/kg for the next patient.
All patients were transferred to the post-anesthesia care unit (PACU) following the procedure until their consciousness was regained. Continuous monitoring of ECG, NIBP, and SpO2 was performed at 5-min intervals for a minimum duration of 30 min. The criteria for transfer out of the PACU encompassed stable vital signs, independent ambulation, and the absence of evident adverse reactions.
2.4 Outcome assessments
The primary outcome measure was ED50 of the propofol.
The secondary scales were mean arterial pressure (MAP), heart rate (HR), and SpO2 after entering the operating room (T1) and after intravenous administration of propofol (T2). Initial propofol dosage, total propofol dosage, procedure duration, and recovery time were recorded. Adverse events included respiratory depression (SpO2 < 90%), hypotension, bradycardia, injection-site pain, uterine contraction pain, postoperative nausea and vomiting (PONV), and dizziness.
Injection-site pain was defined as pain in the backhand or ipsilateral arm escape reflex. The recovery time was between the last propofol injection and the eye-opening on command.
The adverse events were handled as follows: hypotension, defined as a 20% reduction in MAP compared to baseline or <60 mmHg, was treated with intravenous ephedrine at a dosage of 6–12 mg. Bradycardia, indicated by an HR lower than 50 beats/min, was addressed with intravenous atropine at a dosage of 0.25–1 mg. Respiratory depression, characterized by SpO2 below 90%, was managed by maintaining ventilation with either a mask or a laryngeal mask. PONV was treated with intravenous tropisetron at a dosage of 2 mg. Uterine contraction pain, assessed using the visual analog scale (VAS) ranging from 0 (painless) to 10 (severe pain), score ≥ 4 intravenous sufentanil at a dosage of 3–5 μg.
2.5 Blinding method
The assignments for randomization were generated by computers, and subsequently, group information was concealed within an opaque envelope. The same experienced gynecologist and anesthesiologist performed all surgical procedures and anesthesia. The butorphanol was diluted with normal saline to a volume of 10 ml, which appeared colorless and odorless. The 10-ml transparent syringe without any label (butorphanol or normal saline) was placed in a tray together with propofol for the recruited patient. The distribution of drugs was the responsibility of an independent researcher.
2.6 Statistical analysis
Patients' sample sizes were calculated using Dixon's up-down method (11). The approach required at least seven crossovers (Effective to Ineffective) for statistical analysis.
All statistical analyses were performed using SPSS version 20.0 (Inc., Chicago, IL, United States). The data were presented as means ± standard deviations (SD), median [interquartile ranges, IQR], or many patients (n), depending on the distribution of the data. Normally distributed continuous variables were compared using the Student's t-test, while the Mann-Whitney U test was used for non-normally distributed continuous variables. Categorical variables were compared using the chi-square or Fisher's exact probability test. The ED50 of propofol and its 95% confidence interval (CI) were analyzed using probit regression analysis. P < 0.05 indicated a statistically significant difference.
3 Results
3.1 Patient characteristics
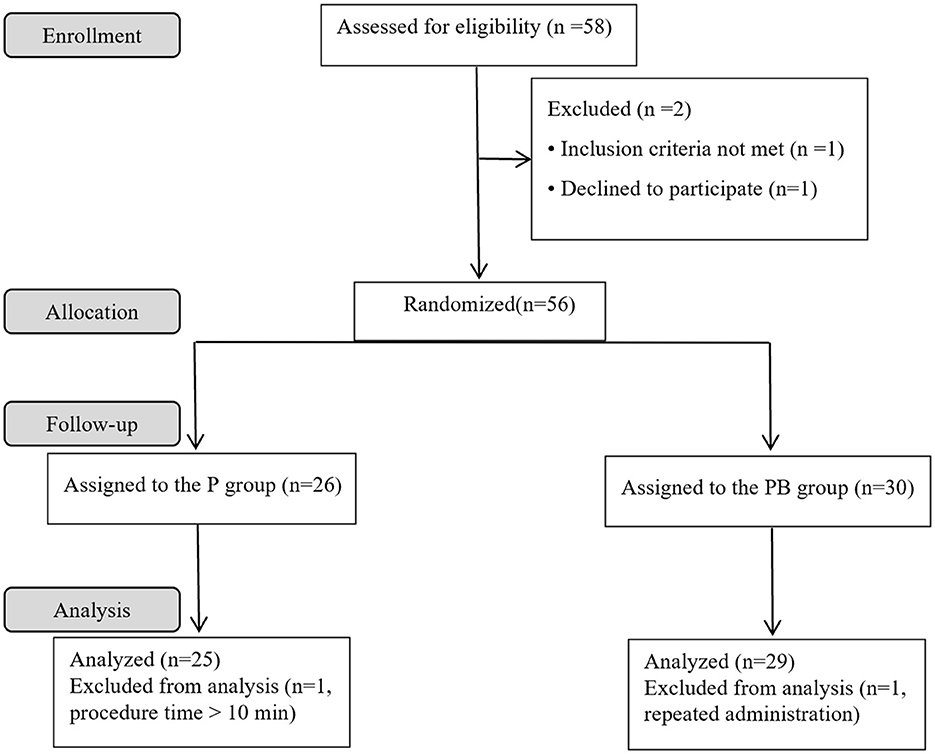
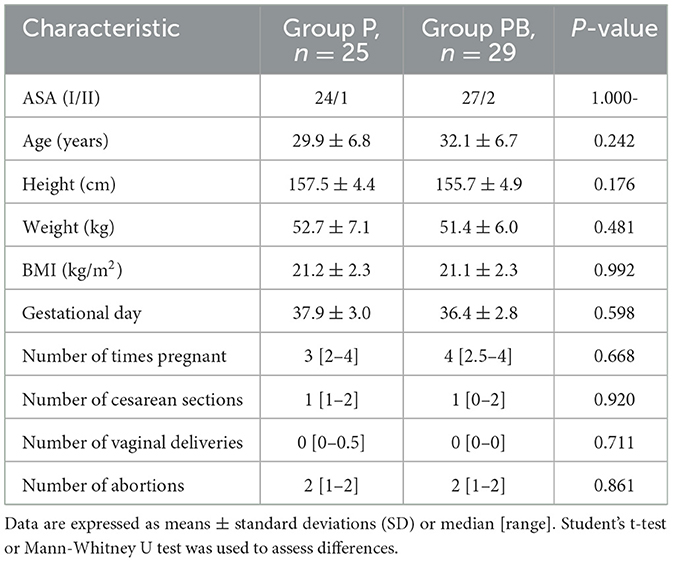
A total of 58 female patients were enrolled in the present study. Four patients were excluded, and 54 patients completed the study successfully. Figure 1 depicts the study flowchart. Table 1 demonstrates patients' characteristic data for all patients. There were no statistically significant differences (P > 0.05) between the two groups in terms of ASA, age, height, weight, BMI, gestational day, number of times pregnant, number of cesarean sections, number of vaginal deliveries, and number of abortions.
3.2 ED50 of propofol
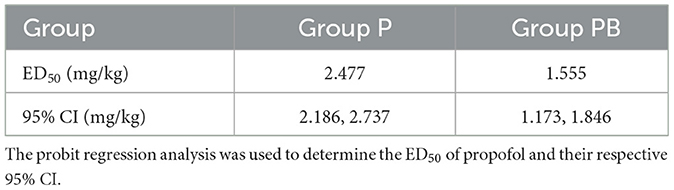
After seven “Effective/Ineffective” crossovers, the sample size was achieved using the up-and-down method. There were 25 and 29 patients in the P and PB groups, respectively (Figure 2). There were 12 and 13 patients who were ineffective and given propofol as rescue therapy in the P and PB groups, respectively. The ED50 (95% CI) of propofol for artificial abortion were 2.477 (2.186–2.737) and 1.555 (1.173–1.846) mg/kg in the P and PB groups, respectively (Table 2). Compared with the P group, the ED50 of the propofol in the PB group decreased by 37.2%.
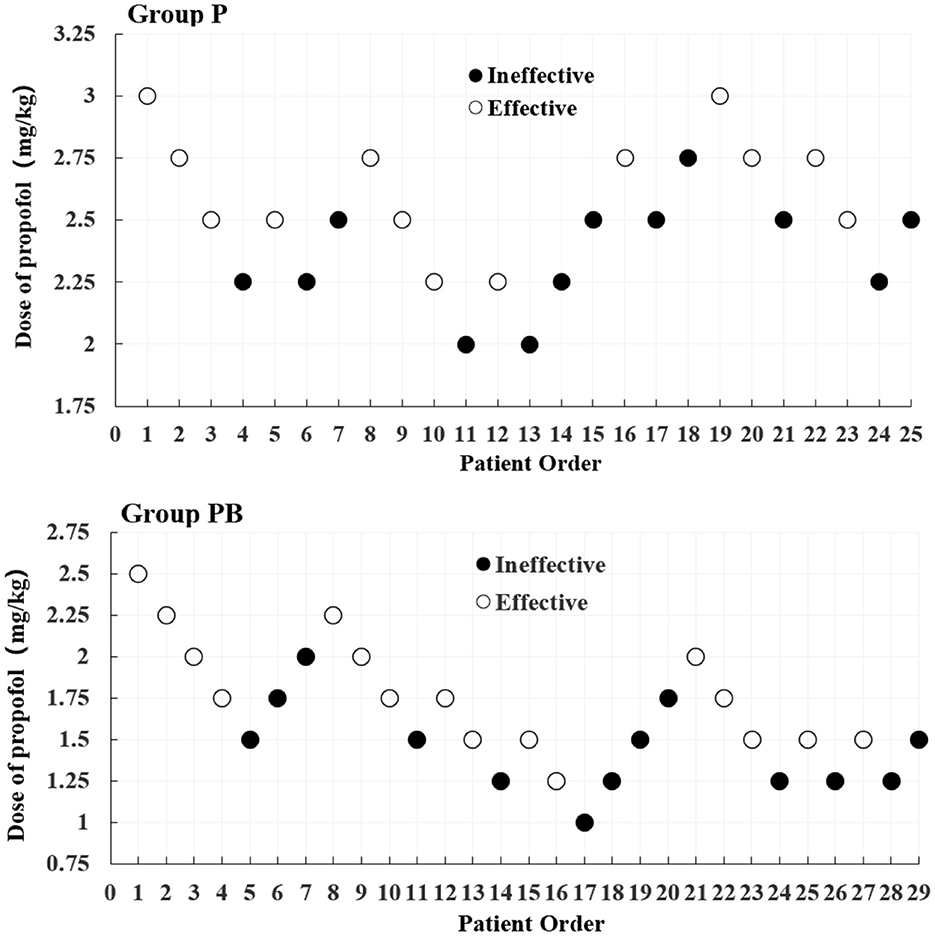
Figure 2. Dixon's up-down method plots for two groups. The white and black dots represent the “Effective” and “Ineffective” patient orders, respectively.
3.3 Perioperative outcomes
Table 3 displays the perioperative outcomes. The initial and total dosage of propofol consumed in the P group was significantly higher than that in the PB group (131.5 ± 22.2 vs. 84.9 ± 23.6 mg, 150.7 ± 21.7 vs. 110.4 ± 28.2 mg, P = 0.000, respectively). The procedure duration (3.9 ± 1.1 vs. 3.6 ± 0.9 min, P = 0.237) and recovery time (5.6 ± 1.8 vs. 6.8 ± 1.7 min, P = 0.176) were not significantly different between the two groups.
3.4 Hemodynamic changes at two different time points
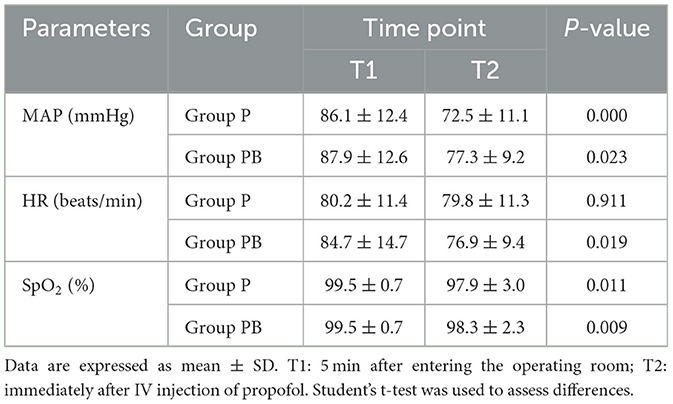
HR, MAP, and SpO2 levels at two different time points decreased to a certain extent (P < 0.05) after intravenous propofol in the two groups. In the P group, the MAP experienced an average drop of 15.7%, whereas the PB group achieved only a 12% drop (Table 4).
3.5 Anesthesia-related adverse events
There were no statistically significant differences (P > 0.05) between the two groups in anesthesia-related adverse events of SpO2 < 90% (8 vs. 3.4%), hypotension (28 vs. 17.2%), bradycardia (4 vs. 0%), dizziness (4 vs. 10.3%), and PONV (4 vs. 7.9%). Compared with the P group, we discovered a significant decrease in both injection-site pain (76 vs. 20.7%) and uterine contraction pain (72 vs. 6.9%) in the PB group; however, this pain was mostly mild (Table 5).
4 Discussion
Among the many methods for determining ED50, Dixon's up-and-down method is rapid and easy and can draw solid conclusions with a relatively small sample size (11). The minimum effective dose can achieve the appropriate depth of anesthesia while reducing drug dosage and the incidence of adverse events. In our study, the trial was terminated when the 25th and 29th female patients reached seven crossover points in the two groups, respectively. The results depict that the ED50 of propofol was 2.477 and 1.555 mg/kg, respectively, using probit regression analysis.
All patients were operated on by an experienced gynecologist with ten years of experience to avoid surgical skills affecting the research results. The operation time was controlled within 10 min. When conducting an up-and-down clinical trial, it is important to provide rigorous safeguards. Otherwise, insufficient drug administration may result in serious adverse events if some patients are particularly sensitive to pain stimulation. Propofol, combined with opioids, is the most common drug for intravenous anesthesia in outpatient gynecological procedures. Combined with opioids, they can reduce sedative-hypnotic drug requirements during surgical abortion (12). The analgesic effect of butorphanol is approximately 3.5–7 times that of morphine (13). It has a stronger analgesic effect on women than on men (14), especially suitable for visceral pain (15). When compared with other opioids such as fentanyl or sufentanil, butorphanol presents specific advantages in surgical abortion. Butorphanol, like other opioids, causes adverse reactions such as respiratory depression, postoperative nausea and vomiting, awakening delay, dizziness, etc. Therefore, the selection of an appropriate dosage is particularly important. A dose of butorphanol at 9.07 μg/kg was deemed appropriate for sedating during gastrointestinal endoscopy procedures (9). Accordingly, 10 μg/kg butorphanol was chosen for this study. Some studies show that when propofol was combined with 0.2 μg/kg sufentanil or 0.2 mg/kg nalbuphine in hysteroscopy, the ED50 of propofol was 1.651 and 1.658 mg/kg, respectively (16, 17). These findings are consistent with our results. Our results showed that the ED50 of propofol in group PB decreased by 37.2% (2.477 vs. 1.555 mg/kg) compared with group P. Although propofol combined with opioids is a preferred option during procedural sedation, taking into account the differences of individuals across geographic regions and clinical settings, propofol anesthesia alone is still a superior alternative, and the findings of this study can serve as valuable references for painless artificial abortion.
Propofol, an intravenous sedative-hypnotic with rapid onset, deep sedative efficacy, and rapid recovery time, has been widely used in outpatient surgery, including in children and the elderly. Painless artificial abortion is one of the most common outpatient surgeries under procedural sedation, and most patients receive propofol. However, its clinical application is limited by injection-site pain, respiratory depression, and hemodynamic instability (18–20). Our research results also exhibited that HR, MAP, and SpO2 declined after intravenous propofol administration. The latest research reveals that the incidence of propofol-induced injection pain was 66.3% (21). The most effective intervention to relieve propofol injection pain was pretreatment with opioids, lidocaine, or 5-HT3 receptor antagonists (22–24), which can increase comfort during anesthesia and improve patient satisfaction. Uterine contraction pain is a common complication after abortion surgery (1), and opioids are one of the most effective methods to reduce postoperative uterine contractions (25, 26). Our research discovered that propofol-induced injection pain decreased from 76 to 20.7% and postoperative uterine contraction pain decreased from 72 to 6.9% after pre-treatment with butorphanol. We can observe that pretreatment with 10 μg/kg butorphanol for at least 5 min can significantly reduce the incidence of propofol-induced injection pain and postoperative uterine contraction pain.
Hypotension is one of the most common adverse reactions during propofol sedation. If propofol is used alone, 35% of patients experience one or more hypotensions during colonoscopy (3). Moreover, studies have demonstrated that 55% of patients who received 3 μg/ml of propofol via target-controlled infusion (TCI) experienced hypotension (27). Our results revealed that the incidence of hypotension in both groups was 28 and 17.2%, respectively. It can be seen from the result that the incidence of hypotension can be reduced to some extent by butorphanol pretreatment; this may be related to the reduced usage of propofol. There is no need to treat with vasoactive medication, and the patient can recover relatively quickly. The average recovery time was approximately 6 min (5.6 vs. 6.8 min, p > 0.05), and there was also no statistically significant difference in the incidence of dizziness (4 vs. 10.3%) or PONV (4 vs. 7.9%) after waking up within 30 min but not receiving any medication between the two groups. These results demonstrate that propofol combined with 10 μg/kg butorphanol is effective and safe for painless artificial abortion.
The present study has several limitations. First, the patient's age, pregnancy history, production history, and other factors may affect propofol dosage and adverse reactions. Further research is necessary to determine if there will be differences in the effective dose of propofol that inhibits cervical dilation. Second, the effect of other doses of butorphanol on the effective dose of propofol and the incidence of adverse reactions remain unexplored. Third, due to the combination of propofol and opioids is the most frequently used drug combination in procedural sedation, the applicability of the ED50 of propofol (propofol used alone) in clinical practice may be limited.
5 Conclusion
The combination of propofol with 10 μg/kg butorphanol reduced the ED50 of propofol and decreased the incidence of propofol injection-site pain and postoperative uterine contraction pain during painless artificial abortion compared with propofol alone.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Medical Ethics Committee of the Affiliated Shunde Hospital of Jinan University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
ZZ conceived and designed the study. YZ wrote the manuscript. YM contributed to data collection. JH performed the data analysis and interpretation. XL contributed to the blind design. All authors read and approved the final manuscript.
Funding
Medical Scientific Research Project of Foshan Municipal Health Bureau, Number: 20230823A010458; Foshan Self-funded Science and Technology Innovation Project, Number: 2220001003819; Key Medical Talents Training Project of Shunde District; Medical Science and Technology Research Foundation of Guangdong Province, Number: A2022515.
Acknowledgments
We thank the Home for Researchers editorial team (www.home-for-researchers.com) for the language editing service.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Renner RM, Jensen JT, Nichols MD, Edelman AB. Pain control in first-trimester surgical abortion: a systematic review of randomized controlled trials. Contraception. (2010) 81:372–88. doi: 10.1016/j.contraception.2009.12.008
2. Hemphill S, McMenamin L, Bellamy MC, Hopkins PM. Propofol infusion syndrome: a structured literature review and analysis of published case reports. Br J Anaesth. (2019) 122:448–59. doi: 10.1016/j.bja.2018.12.025
3. Sneyd JR, Absalom AR, Barends CRM, Jones JB. Hypotension during propofol sedation for colonoscopy: a retrospective exploratory analysis and meta-analysis. Br J Anaesth. (2022) 128:610–22. doi: 10.1016/j.bja.2021.10.044
4. Wang LL, Guan ZY, Wang CM, Zhang YW, Zhang J, Zhao P. A comparative study on the efficacy and safety of propofol combined with different doses of alfentanil in gastroscopy: a randomized controlled trial. J Anesth. (2023) 37:201–9. doi: 10.1007/s00540-022-03145-5
5. Tang L, Ye C, Wang N, Chen C, Chen S, Gao S, et al. The median effective doses of propofol combined with two different doses of nalbuphine for adult patients during painless gastroscopy. Front Pharmacol. (2022) 13:1014486. doi: 10.3389/fphar.2022.1014486
6. Lv S, Sun D, Li J, Yang L, Sun Z, Feng Y. Anesthetic effect of different doses of butorphanol in patients undergoing gastroscopy and colonoscopy. BMC Surg. (2021) 21:266. doi: 10.1186/s12893-021-01262-8
7. Dinges HC, Otto S, Stay DK, Baumlein S, Waldmann S, Kranke P, et al. Effect rates of opioids in equianalgesic doses via intravenous patient-controlled analgesia: a systematic review and network meta-analysis. Anesth Analg. (2019) 129:1153–62. doi: 10.1213/ANE.0000000000003887
8. Ding X, Cui H, Ma P, Chen X, Sun Y, Qu M, et al. Efficacy of dexmedetomidine versus midazolam when combined with butorphanol for sedation and analgesia during burn dressing changes: A randomized clinical trial. Front Pharmacol. (2022) 13:965441. doi: 10.3389/fphar.2022.965441
9. Zhu X, Chen L, Zheng S, Pan L. Comparison of ED95 of Butorphanol and Sufentanil for gastrointestinal endoscopy sedation: a randomized controlled trial. BMC Anesthesiol. (2020) 20:101. doi: 10.1186/s12871-020-01027-5
10. Briery CM, Veillon EW, Klauser CK, Martin RW, Magann EF, Chauhan SP, et al. Women with preterm premature rupture of the membranes do not benefit from weekly progesterone. Am J Obstet Gynecol. (2011) 204:54e1–5. doi: 10.1016/j.ajog.2010.08.022
11. Dixon WJ. Staircase bioassay: the up-and-down method. Neurosci Biobehav Rev. (1991) 15:47–50. doi: 10.1016/S0149-7634(05)80090-9
12. Gao W, Sha B, Zhao Y, Fan Z, Liu L, Shen X. Comparison of simultaneous and sequential administration of fentanyl-propofol for surgical abortion: a randomized single-blinded controlled trial. Artif Cells Nanomed Biotechnol. (2017) 45:1045–50. doi: 10.1080/21691401.2016.1239106
13. Commiskey S, Fan LW, Ho IK, Rockhold RW. Butorphanol: effects of a prototypical agonist-antagonist analgesic on kappa-opioid receptors. J Pharmacol Sci. (2005) 98:109–16. doi: 10.1254/jphs.CRJ05001X
14. Gear RW, Miaskowski C, Gordon NC, Paul SM, Heller PH, Levine JD. Kappa-opioids produce significantly greater analgesia in women than in men. Nat Med. (1996) 2:1248–50. doi: 10.1038/nm1196-1248
15. Riviere PJ. Peripheral kappa-opioid agonists for visceral pain. Br J Pharmacol. (2004) 141:1331–4. doi: 10.1038/sj.bjp.0705763
16. Chen C, Tang W, Ye W, Zhong W, Li Y. ED50 of propofol combined with nalbuphine on the sedative effect in painless hysteroscopy. Pain Ther. (2021) 10:1235–43. doi: 10.1007/s40122-021-00280-x
17. Yu J, Xiang B, Song Y, Chen H, Li Y, Liu C. ED50 of propofol in combination with low-dose sufentanil for intravenous anaesthesia in hysteroscopy. Basic Clin Pharmacol Toxicol. (2019) 125:460–5. doi: 10.1111/bcpt.13280
18. Fang Y, Xu Y, Cao S, Sun X, Zhang H, Jing Q, et al. Incidence and risk factors for hypoxia in deep sedation of propofol for artificial abortion patients. Front Med. (2022) 9:763275. doi: 10.3389/fmed.2022.763275
19. Guan X, Jiao Z, Gong X, Cao H, Liu S, Lan H, et al. Efficacy of pre-treatment with remimazolam on prevention of propofol-induced injection pain in patients undergoing abortion or curettage: a prospective, double-blinded, randomized and placebo-controlled clinical trial. Drug Des Devel Ther. (2021) 15:4551–8. doi: 10.2147/DDDT.S334100
20. Bryson HM, Fulton BR, Faulds D. Propofol. An update of its use in anaesthesia and conscious sedation. Drugs. (1995) 50:513–59. doi: 10.2165/00003495-199550030-00008
21. Bakhtiari E, Mousavi SH, Gharavi Fard M. Pharmacological control of pain during propofol injection: a systematic review and meta-analysis. Expert Rev Clin Pharmacol. (2021) 14:889–99. doi: 10.1080/17512433.2021.1919084
22. Zhang L, Bao Y, Shi D. Comparing the pain of propofol via different combinations of fentanyl, sufentanil or remifentanil in gastrointestinal endoscopy. Acta Cir Bras. (2014) 29:675–80. doi: 10.1590/S0102-8650201400160008
23. Euasobhon P, Dej-Arkom S, Siriussawakul A, Muangman S, Sriraj W, Pattanittum P, et al. Lidocaine for reducing propofol-induced pain on induction of anaesthesia in adults. Cochrane Database Syst Rev. (2016) 2:CD007874. doi: 10.1002/14651858.CD007874.pub2
24. Wang W, Zhou L, Wu LX, Wang T, Zhang CB, Sun L. 5-HT3 Receptor antagonists for propofol injection pain: a meta-analysis of randomized controlled trials. Clin Drug Investig. (2016) 36:243–53. doi: 10.1007/s40261-016-0375-1
25. Deussen AR, Ashwood P, Martis R, Stewart F, Grzeskowiak LE. Relief of pain due to uterine cramping/involution after birth. Cochrane Database Syst Rev. (2020) 10:CD004908. doi: 10.1002/14651858.CD004908.pub3
26. Wang YN, Xu MJ, Feng Y, Chen XZ, Yao F, Shen MK, et al. The efficacy and safety of oxycodone in treating the uterine contraction pain after negative pressure aspiration: a randomized, compared, multicenter clinical study. Medicine. (2022) 101:e30048. doi: 10.1097/MD.0000000000030048
27. Sekiguchi R, Kinoshita M, Kawanishi R, Kakuta N, Sakai Y, Tanaka K. Comparison of hemodynamics during induction of general anesthesia with remimazolam and target-controlled propofol in middle-aged and elderly patients: a single-center, randomized, controlled trial. BMC Anesthesiol. (2023) 23:14. doi: 10.1186/s12871-023-01974-9
Keywords: propofol, butorphanol, median effective dose, painless abortion, up-and-down method
Citation: Zheng Y, Huang J, Mai Y, Li X and Zhang Z (2023) The median effective dose of propofol combined with butorphanol during artificial abortion: a randomized controlled trial. Front. Med. 10:1226495. doi: 10.3389/fmed.2023.1226495
Received: 26 June 2023; Accepted: 07 November 2023;
Published: 27 November 2023.
Edited by:
Somchai Amornyotin, Mahidol University, ThailandCopyright © 2023 Zheng, Huang, Mai, Li and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhongqi Zhang, anh6enExMUAxNjMuY29t
†These authors have contributed equally to this work
 Yuling Zheng1†
Yuling Zheng1† Zhongqi Zhang
Zhongqi Zhang