
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
OPINION article
Front. Med. , 30 August 2023
Sec. Healthcare Professions Education
Volume 10 - 2023 | https://doi.org/10.3389/fmed.2023.1226475
Surgical simulation is become increasingly used for surgical training (1), as it provides trainees a controlled learning environment in a safe place for both the patient and the student (2). In most countries, simulation is now a mandatory teaching modality during the residency to acquire surgical skills. At the same time, awareness of climate change (3, 4) is challenging us to find more sustainable educational solutions (4). However, the concept of an environmentally friendly learning is not well established in the literature. When possible, surgical simulation should avoid the use of animals (5) for ethical and sustainable reasons. However, to date, it is not possible to train all technical skills in synthetic or virtual simulators (6), and animal models may still need to be used. As an example, the porcine model allows training on entire organs [e.g.: heart (7), eyes (8)], to train for complex procedures [e.g.: liver transplantation (9), craniostenosis treatment (10), endoscopic submucosal dissection (11), and to design (12)] or to train for the use of medical devices mainly in the field of robotic surgery (10, 13, 14). Given 1/difficulty of accessing food resources around the world (15), and 2/the environmental impact of pig production (16), we need to question the relevance of using such models for surgical learning.
For instance, in the field of Otolaryngology-head and neck surgery (OHNS) only a few simulators are available to train neck and pharyngolaryngeal surgery (17). Some procedures may be learned with low fidelity reusable synthetic simulators, such as percutaneous tracheotomy (18). However, for more complex procedures, few simulation-based teaching solutions are available apart from human cadaver training (17), due to the difficulty of replicating the physical properties of soft tissues (6). A neck surgery simulator must therefore be able to replicate the anatomy and its planes, to be dissectible (19), and to provide haptic sensations close to those encountered in humans (20). The porcine model meets most of these expectations. When used alive (21), it allows the simulation of bleeding, which makes it particularly interesting for learning vascular dissection. In our experience, the use of a dead porcine model provides satisfactory anatomical and haptic fidelity for surgical simulation of the central neck compartment, while diminishing the environmental costs, handling, paperwork, and ethical issues of using live animals. It is thus possible to simulate most surgical procedures on the upper airway, such as tracheotomy (21–23), cricothyroidotomy (24), laryngotracheoplasty (23), total laryngectomy (21) or even some endolaryngeal surgical procedures (25).
In our opinion, when the use of an animal model for surgical simulation is strictly necessary, every effort should be made to optimize the use of the animal so that the greatest number of trainees benefit while limiting the environmental impact of surgical education.
Ethical considerations in animal research are based on the “3 R” principles (26):
• The Replacement principle consists in avoiding animal use when it's possible. In the case of pharyngolaryngeal surgery simulation, there is to date no other alternative model (Figure 1).
• The Reduction principle emphasizes the need to reduce animal numbers by optimizing the experimental design.
• The Refinement principle emphasizes the methods to minimize animals suffering and improve wellbeing before and during the experiments.
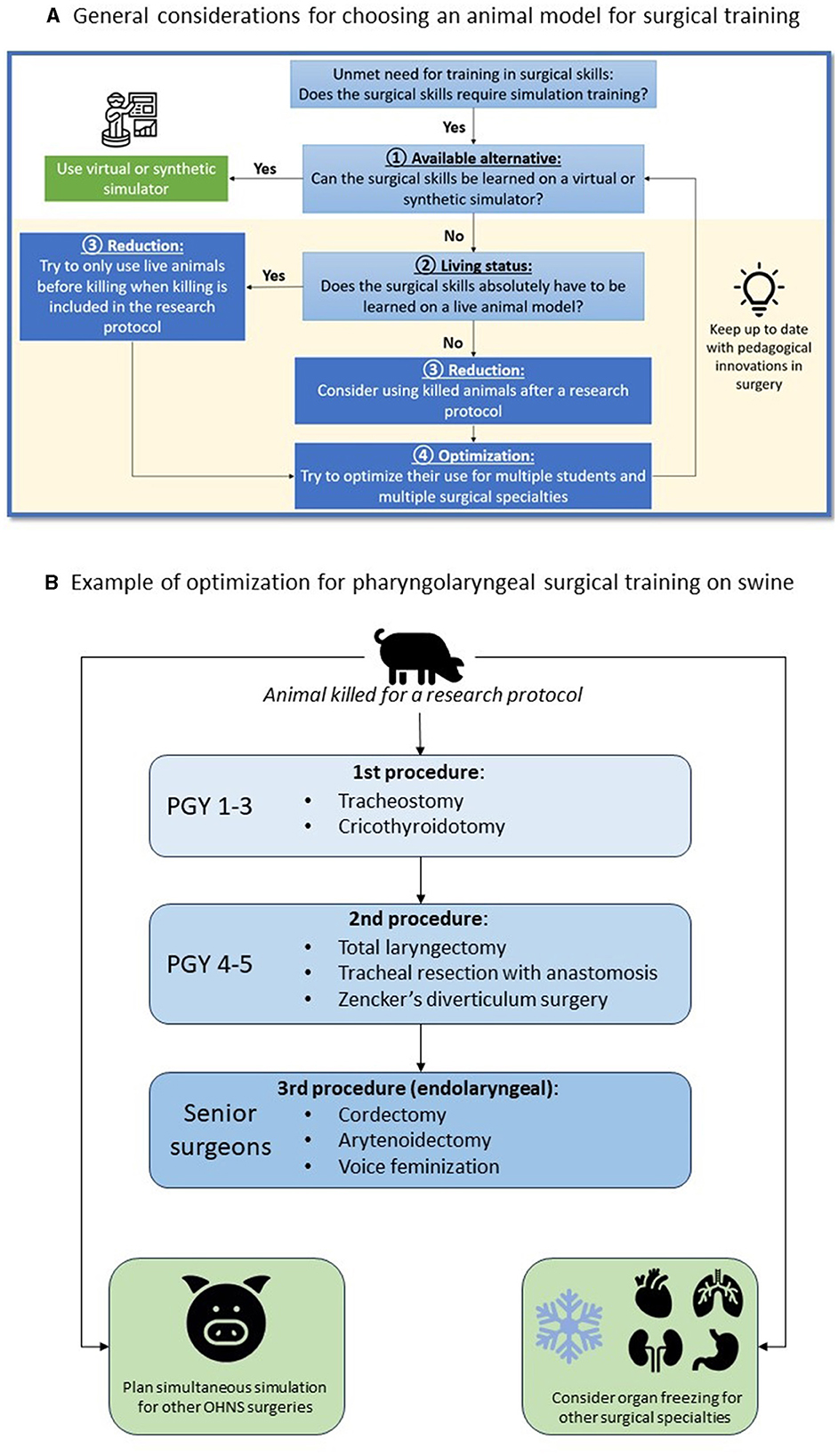
Figure 1. Strategy to reduce and optimize (A) the use of animal models in surgical simulation and example with the use of a porcine model for OHNS surgical simulation (B).
The use of dead animals from animal research when killing is included in the protocol, responds to the Reduction and Refinement principles. Institutional approval was obtained from the French ministry of higher education, research and innovation (No. APAFIS#26921-202008181721597, approval 2020-066), which has the jurisdiction to provide animal ethics approval.
We propose to use the example of pig's median compartment neck surgery to illustrate the procedures that can be trained and tips to optimize its use for education in OHNS and other surgical specialties (Figure 1). First, we advocate to use only dead animals who were killed for a research protocol. If the animal's body is available in the morning, the entire simulation sequence can be conducted. In other situations, the body can be stored outdoors for around 12 h, or frozen for a future use. In the latter case, defrosting takes around 24 h. Ideally, the simulation session has been planned in advance with different surgical specialties in order to make the best use of the available resources. The order of procedures and the rotation of learners of different level must be defined before the simulation session.
While the relative dimensions and anatomy of the pharyngolaryngeal-tracheal axis of the pig are similar to humans, some differences should be known and teach before performing procedures (22, 27). This information is provided during the briefing preceding simulation-based learning. It aims at saving time for trainees and allows to complete all the procedures during the simulation session.
Once the pig has been used alive for research or teaching purposes, and when killing is included in the protocol, it is possible to make use of the animal's body for the simulation in OHNS (Figure 1). The pig should be placed in a supine position to provide cervical extension. Since the larynx is larger in its vertical dimension in pigs than in humans, learners need to palpate and mark on the skin the landmarks of the hyoid bone, thyroid and cricoid cartilage before starting the procedure. A vertical midline incision is preferred to remain in the central compartment. We propose here to describe a surgical simulation session for the sequential learning of tracheotomy, total laryngectomy, and endolaryngeal procedures, to allow three couple of learners of different levels to train on the same animal.
For the simulation of neck surgery, we propose a pair made up of one young (postgraduate year 1 or 2, PGY1-2) and one experienced (PGY3-5) resident. First, it is necessary to recall the anatomical differences concerning the procedures to be performed. The PGY1-2 resident will start the simulation by performing a tracheotomy, assisted by PGY3-5. Then, the PGY3-5 resident will perform a total laryngectomy, assisted by PGY1-2. All the steps of a laryngectomy in humans can be performed on the dead pig. To study the oncology principle of resection, a tumor can be simulated using povidone-iodine gel with submucosal injection before entering the pharynx, to assess the surgical margins. The removed larynx will then be used to study endo-laryngeal techniques (cordectomy, arytenoidectomy, or even feminization techniques) during the same simulation session, or later by freezing the larynx. The endolaryngeal surgical simulation allows performing cordectomy, arytenoidectomy, or even feminization techniques. When the simulation is used as a summative evaluation for residents, the teacher will supervise the simulations by giving the different surgical steps without providing spontaneous help. In this way, the procedure can be scored based on the “O-SCORE” scale (27, 28), which allows for the assessment of the resident's ability to perform the procedure independently and safely. At the end of the simulation session, the teacher will debrief the session in the presence of all residents, and the animal body may be used for simulation in other surgical specialties.
According to our experience in three French academic centers, teaching and learning neck surgery on dead pigs provides many advantages. First of all, pig is the only simulator—excepting the human cadaver—allowing performing complete neck procedures, “from skin to skin,” with haptic sensations imitating those of a living tissue. This advantage is always perceived within 12 to 24 h after the killing, bringing a possibility of saving pigs (reduction principle) by coupling with other research manipulations. The main disadvantage is the absence of active bleeding, but massive hemorrhage is uncommon during such surgical procedures in humans. The use of live pigs for tracheostomy (21, 23, 29) or laryngectomy (21) has proved its content [i.e., experts' assessment of the suitability of the pig as a teaching tool (30)] validity. This validity was assessed by sending questionnaires to the experts, asking them to judge whether all the surgical steps could be performed on the model. In our opinion, content validity does not depend on whether the pig is alive or dead to simulate pharyngolaryngeal surgery. Furthermore, some teams already used ex vivo porcine skin, larynx, and trachea to simulate tracheostomy (31), cricothyroidotomy (22, 32–34) or partial laryngectomy (35, 36). Using the whole body of a dead pig, as we propose, allows reproducing all the dissection steps.
Secondly, the porcine model provides an anatomy close to that of humans in the medial neck compartment. The anatomy differs in the lateral compartments due to arterial and venous vascularization and lymphatic drainage. Thus, contrary to Alcalá Rueda et al. (22), we do not recommend the use of the pig as a training model for neck lateral dissection procedures. Moreover, the optimized use of the same pig allows performing emergency procedures (tracheostomy, cricothyroidotomy), neck and laryngeal surgery, so that students of different levels can train during the same simulation session.
Finally, other OHNS procedures can be performed in a delayed manner by freezing the pig's head, and other surgical specialties can train from the same animal (Figure 1). This optimization allows for a thoughtful use of the porcine model, as animal dissection is a current ethical issue (3, 8) and may have an environmental impact (16). The use of a previously killed animal for a research protocol requires coordination between research and teaching teams in order to make the best possible use of the animal. We advocate avoiding the use of additional animals for education only, to limit the environmental impact of surgical education.
Our feeling is that the use of a dead pig allows to simulate many surgical procedures, without the need to kill an animal only for the purpose of surgical training. This would make it possible to combine quality surgical training with sustainable objectives. To verify this, it will be necessary to prove that the dead pig—as well as the live one—achieves content validity and that it can also prove its ability to help students progress in surgical skills (content validity). Finally, the question of the environmental impact of surgical simulation must be raised. Studies comparing the environmental impact of different learning methods need to be undertaken.
When designing a curriculum for simulation-based surgical training, teachers should consider both ethical, and environmental aspects. We took the example of the dead porcine model which seems to be a reliable simulator to train midline neck procedures by providing haptic sensation and by its anatomical resemblance to humans. The same animal can be used for several OHNS procedures and by other surgical specialties to responds to the reduction and refinement principles that are essential for ethical and sustainable purposes.
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
This work was supported by the Montpellier Université d'Excellence (MUSE) Soutien à la formation en 2021 program.
The authors acknowledge the staff of the School of Surgery of Nancy-Lorraine and of the Laboratory Animal of Nîmes, France, for their technical support.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Rosendal AA, Sloth SB, Rölfing JD, Bie M, Jensen RD. Technical, non-technical, or both? A scoping review of skills in simulation-based surgical training. J Surg Educ. (2023) 80:731–49. doi: 10.1016/j.jsurg.2023.02.011
2. Sadideen H, Hamaoui K, Saadeddin M, Kneebone R. Simulators and the simulation environment: getting the balance right in simulation-based surgical education. Int J Surg Lond Engl. (2012) 10:458–62. doi: 10.1016/j.ijsu.2012.08.010
3. Carsuzaa F, Fieux M, Bartier S, Fath L, Alexandru M, Legré M, et al. Perception of environmental issues in the head-and-neck surgery room: a preliminary study. Eur Ann Otorhinolaryngol Head Neck Dis. (2023). doi: 10.1016/j.anorl.2023.06.003
4. Pietrabissa A, Sylla P. Green surgery: time to make a choice. Surg Endosc. (2023). doi: 10.1007/s00464-023-10229-0
5. Vacharathit V, Walsh RM, Utech J, Asfaw SH. Action in healthcare sustainability is a surgical imperative: this is a novel way to do it. J Surg Educ. (2022) 79:275–8. doi: 10.1016/j.jsurg.2021.09.002
6. Gala SG, Crandall ML. Global collaboration to modernize advanced trauma life support training. J Surg Educ. (2019) 76:487–96. doi: 10.1016/j.jsurg.2018.08.011
7. Ratinam R, Quayle M, Crock J, Lazarus M, Fogg Q, McMenamin P. Challenges in creating dissectible anatomical 3D prints for surgical teaching. J Anat. (2019) 234:419–37. doi: 10.1111/joa.12934
8. Toto F, Torre T, Pozzoli A, Biroova S, Ferrari E, Demertzis S. Cardiac surgery simulation: step-by-step Nicks procedure in a preclinical model. Multimed Man Cardiothorac Surg MMCTS. (2023) 10:2023. doi: 10.1510/mmcts.2023.036
9. Tothova JD, Gatto C, Giurgola L, Romano MR, Ferrara M. 25 Simulation of eye surgery in porcine eye globes and evaluation of retinal cytotoxicity. BMJ Open Ophthalmol. (2022) 7:A11. doi: 10.1136/bmjophth-2022-EEBA.25
10. Yoshimoto S, Soyama A, Fukumoto M, Hara T, Hidaka M, Torai S, et al. Preliminary observations of an ex vivo normothermic whole blood machine perfusion in an experimental liver transplant porcine model. Transplant Proc. (2023) 55:106. doi: 10.1016/j.transproceed.2023.03.067
11. Lei B, Sun T, Ma H, Li B, Yang B. Application and accuracy of craniomaxillofacial plastic surgery robot in congenital craniosynostosis surgery. J Craniofac Surg. (2023) 34:1371–5. doi: 10.1097/SCS.0000000000009283
12. Huang J, Du BR, Qiao WG, Huang SL, Xue LF, Deng L, et al. Endoscopic submucosal dissection training: evaluation of an ex vivo training model with continuous perfusion (ETM-CP) for hands-on teaching and training in China. Surg Endosc. (2023) 37:4774–83. doi: 10.1007/s00464-023-09940-9
13. Burger L, Sharan L, Karl R, Wang C, Karck M, De Simone R, et al. Comparative evaluation of three commercially available markerless depth sensors for close-range use in surgical simulation. Int J Comput Assist Radiol Surg. (2023) 18:1109–18. doi: 10.1007/s11548-023-02887-1
14. Hashemi N, Svendsen MBS, Bjerrum F, Rasmussen S, Tolsgaard MG, Friis ML. Acquisition and usage of robotic surgical data for machine learning analysis. Surg Endosc. (2023) 37:6588–601. doi: 10.1007/s00464-023-10214-7
15. Raison N, Poulsen J, Abe T, Aydin A, Ahmed K, Dasgupta P. An evaluation of live porcine simulation training for robotic surgery. J Robot Surg. (2021) 15:429–34. doi: 10.1007/s11701-020-01113-3
16. Wheeler T, von Braun J. Climate change impacts on global food security. Science. (2013) 341:508–13. doi: 10.1126/science.1239402
17. Andretta I, Hickmann FMW, Remus A, Franceschi CH, Mariani AB, Orso C, et al. Environmental impacts of pig and poultry production: insights from a systematic review. Front Vet Sci. (2021) 8:750733. doi: 10.3389/fvets.2021.750733
18. Favier V, Ayad T, Blanc F, Fakhry N, Andersen SAW. Use of simulation-based training of surgical technical skills among ENTs: an international YO-IFOS survey. Eur Arch Oto-Rhino-Laryngol. (2021) 278:5043–50. doi: 10.1007/s00405-021-06846-x
19. Favier V, Kimmoun A, Gatin A, Gallet P. Percutaneous tracheostomy simulation training for ENT physicians in the treatment of COVID-19-positive patients. Eur Ann Otorhinolaryngol Head Neck Dis. (2020) 137:333–8. doi: 10.1016/j.anorl.2020.06.002
20. Alessa MA, Kwak SH, Lee YW, Kang ML, Sung HJ, Ahn SH, et al. Porcine as a training module for head and neck microvascular reconstruction. JoVE (J Vis Exp). (2018) 139:58104. doi: 10.3791/58104-v
21. Favier V, Subsol G, Duraes M, Captier G, Gallet P. Haptic fidelity: the game changer in surgical simulators for the next decade? Front Oncol. (2021) 11:713343. doi: 10.3389/fonc.2021.713343
22. Alcalá Rueda I, Villacampa Aubá JM, Encinas Vicente A, Gabernet MB, Guerrero CC, Reparaz CCC, et al. A live porcine model for surgical training in tracheostomy, neck dissection, and total laryngectomy. Eur Arch Oto-Rhino-Laryngol. (2021) 278:3081–90. doi: 10.1007/s00405-021-06613-y
23. Gustafson ML, Hensley B, Dotson M, Broce M, Tager A. Comparison of manikin versus porcine trachea models when teaching emergent cricothyroidotomy among emergency medicine residents. AEM Educ Train. (2019) 3:280–5. doi: 10.1002/aet2.10333
24. Deonarain AR, Harrison RV, Gordon KA, Wolter NE, Looi T, Estrada M, et al. Live porcine model for surgical training in tracheostomy and open-airway surgery. Laryngoscope. (2020) 130:2063–8. doi: 10.1002/lary.28309
25. Añez Simón C, Serrano Gonzalvo V, Carrillo Luna LH, Farré Nebot V, Holgado Pascual CM, Grupo de investigación ANESTARRACO (IISPV). Results of a surgical cricothyrotomy workshop with a pig trachea model. Rev Esp Anestesiol Reanim. (2019) 66:129–36. doi: 10.1016/j.redare.2018.09.014
26. Nasser Kotby M, Wahba HA, Kamal E, El-Makhzangy AMN, Bahaa N. Animal model for training and improvement of the surgical skills in endolaryngeal microsurgery. J Voice. (2012) 26:351–7. doi: 10.1016/j.jvoice.2011.04.002
27. Cheng PC, Cho TY, Hsu WL, Lo WC, Wang CT, Cheng PW, et al. Training residents to perform tracheotomy using a live swine model. Ear Nose Throat J. (2019) 98:E87–91. doi: 10.1177/0145561319840835
28. Gofton WT, Dudek NL, Wood TJ, Balaa F, Hamstra SJ. The ottawa surgical competency operating room evaluation (O-SCORE): a tool to assess surgical competence. Acad Med J Assoc Am Med Coll. (2012) 87:1401–7. doi: 10.1097/ACM.0b013e3182677805
29. Smith AJ. Guidelines for planning and conducting high-quality research and testing on animals. Lab Anim Res. (2020) 36:21. doi: 10.1186/s42826-020-00054-0
30. MacEwan MJ, Dudek NL, Wood TJ, Gofton WT. Continued validation of the O-SCORE (Ottawa Surgical Competency Operating Room Evaluation): Use in the simulated environment. Teach Learn Med. (2016) 28:72–9. doi: 10.1080/10401334.2015.1107483
31. Alsalamah A, Campo R, Tanos V, Grimbizis G, Van Belle Y, Hood K, et al. Face and content validity of the virtual reality simulator ‘ScanTrainer®'. Gynecol Surg. (2017) 14:18. doi: 10.1186/s10397-017-1020-6
32. Sacco Botto F, Ingrassia PL, Donato P, Garzaro M, Aluffi P, Gentilli S, et al. Manufacture of a multi-purpose low-cost animal bench-model for teaching tracheostomy. JoVE (J Vis Exp). (2019) 147:e59396. doi: 10.3791/59396
33. Cho J, Kang GH, Kim EC, Oh YM, Choi HJ, Im TH, et al. Comparison of manikin versus porcine models in cricothyrotomy procedure training. Emerg Med J EMJ. (2008) 25:732–4. doi: 10.1136/emj.2008.059014
34. Iverson K, Riojas R, Sharon D, Hall AB. Objective comparison of animal training versus artificial simulation for initial cricothyroidotomy training. Am Surg. (2015) 81:515–8. doi: 10.1177/000313481508100535
35. Pandian V, Leeper WR, Jones C, Pugh K, Yenokyan G, Bowyer M, et al. Comparison of surgical cricothyroidotomy training: a randomized controlled trial of a swine model versus an animated robotic manikin model. Trauma Surg Acute Care Open. (2020) 5:e000431. doi: 10.1136/tsaco-2019-000431
Keywords: surgical simulation, animal model, reduction principle, sustainability, pig, Otolaryngology-head and neck surgery postgraduate degree
Citation: Payen C, Gallet P, Lechien JR and Favier V (2023) Teachers should apply the principle of reduction for more sustainable surgical simulation practice: the example of training pharyngolaryngeal surgery in a porcine model. Front. Med. 10:1226475. doi: 10.3389/fmed.2023.1226475
Received: 21 May 2023; Accepted: 11 August 2023;
Published: 30 August 2023.
Edited by:
Arnaud Alves, Université de Caen Normandie, FranceReviewed by:
Hosein Daneshpour, Tampere University of Applied Sciences, FinlandCopyright © 2023 Payen, Gallet, Lechien and Favier. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Valentin Favier, dmFsZW50aW5fZmF2aWVyQGhvdG1haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.