- 1Clinica Pediatrica, Azienda Ospedaliero-Universitaria, Department of Medicine and Surgery, Università di Parma, Parma, Italy
- 2Paediatrics Unit, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy
- 3Department of Medical and Surgical Sciences (DIMEC), University of Bologna, Bologna, Italy
Atopic dermatitis (AD) is a chronic inflammatory disease with a heterogeneous pathogenesis correlated with dysregulation of the immune system and a prevalence of the T2-mediated immune pathway. Recent understanding of the pathogenesis of AD has allowed the development of new drugs targeting different mechanisms and cytokines that have changed the treatment approach. The aim of this review is to update knowledge on the standard of care and recent advancements in the control of skin inflammation. In light of recent guidelines, we report on the clinical efficacy of novel treatments, with special attention to situations where biologics and small molecules are involved.
Introduction
Atopic dermatitis (AD) is a chronic relapsing inflammatory skin disease associated with dysregulation of the immune system and Th2 immune responses (1). It represents the most frequent skin disease in childhood. The multidisciplinary approach includes complex pharmacological treatments, management of allergies (2–4), and behavioral and psychological problems (5).
Treatment is based on the severity of AD scores such as the Severity ScoRing of Atopic Dermatitis (SCORAD) index (6), the Investigator’s Global Assessment (IGA) score (7), or the Eczema Area and Severity Index (EASI) (8). Regular use of emollients with any medication is the cornerstone of AD management. Most of the newer drugs will not be sufficient if emollients are not applied. However, they were not included in this review because our aim was to focus on the most commonly used and novel medications for pediatric AD skin inflammation in light of current recommendations and recent advances.
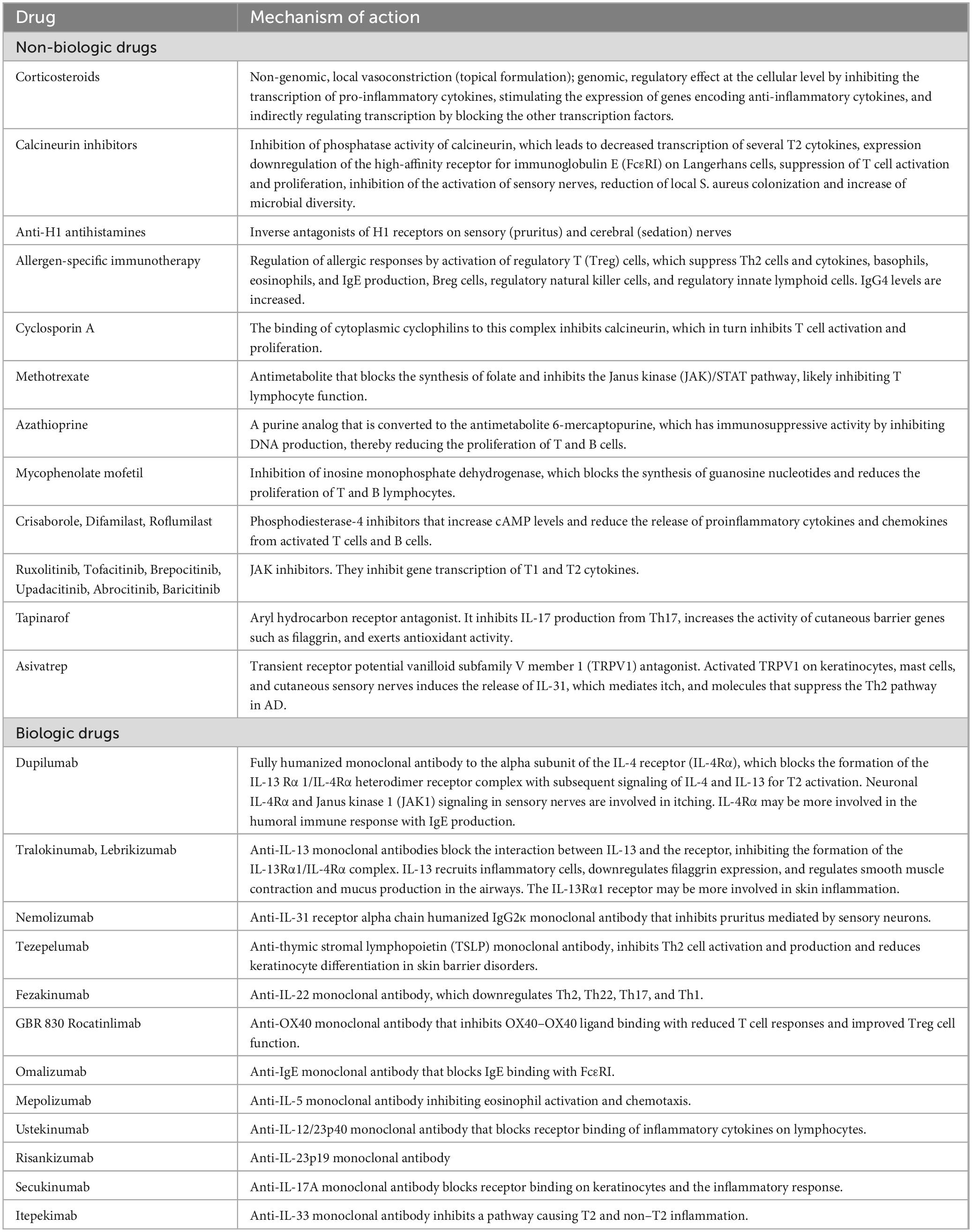
The introduction of new biologic drugs, such as dupilumab, has significantly transformed the therapeutic landscape for patients with AD. These biologics target specific molecules and pathways that are involved in the inflammatory process, providing a more precise and effective treatment approach for moderate-to-severe AD (Table 1).
Clinical trials have demonstrated the remarkable efficacy and safety of dupilumab, leading to its approval for the treatment of moderate to severe atopic dermatitis (AD) in children aged over 6 months of age. Other biologics, such as tralokinumab and lebrikizumab, have also been approved for use in adults and adolescents 12 years of age and older.
Ongoing investigations are exploring the potential of biologics targeting different cytokines and pathways implicated in the pathogenesis of AD. Clinical trials are specifically assessing the efficacy and safety of biologics that target IL-13 alone or in combination with other cytokines (Table 1). These novel agents offer potential additional treatment options for patients who do not respond adequately to existing therapies or who experience side effects (9). It is important to note, however, that these biologics are still in the clinical development phase and have not yet received regulatory approval. Limited pediatric trials have been conducted for these novel biologics, with nemolizumab being studied in adolescents (10).
In addition to biologics, small molecules are also being explored as treatment options for AD. In the context of childhood AD, inhibitors of phosphodiesterase-4 (PDE-4) and Janus Kinase (JAK) have been approved for use (Table 1).
The availability of biologic drugs has expanded the treatment options for patients with moderate-to-severe AD, particularly for those who have not achieved satisfactory results with conventional therapies or who have contraindications to systemic immunosuppressive agents. Biologics provide a more targeted and personalized approach, reducing symptoms, improving quality of life, and potentially preventing long-term complications associated with uncontrolled AD.
It is important to acknowledge that biologics have limitations. They can be costly, require regular administration through injection or infusion, and may have associated side effects. Long-term safety data are still being evaluated, and their use in specific patient populations, such as pregnant women and children, requires further investigation.
In this review, we aim to provide a comprehensive analysis of the clinical efficacy of new treatments for AD.
Materials and methods
We conducted a literature review using two electronic databases, MEDLINE (PubMed) and the Cochrane Library, to gather information on the use of anti-inflammatory and biological drugs for atopic dermatitis in children and adolescents. The search was conducted by filtering for articles published in the last 5 years (January 2017–March 2023). Only articles written in English were selected. Additional relevant articles known to the authors or identified in the references of the already selected articles were added.
Medications for AD management
Topical corticosteroids (TCSs) are a first-line option for AD (Table 1) (11–14). TCSs with low-to-moderate potency are used in mild AD, with more potency in moderate-to-severe cases (11, 12). Indeed, the majority of patients with mild-to-moderate AD are aged 0–4 years, while those with severe disease are older (Table 2) (15). Early use of adequate-potency TCSs, at the onset of the acute AD flare increases control of inflammation, regenerates the skin barrier, and reduces TCS consumption. Creams are indicated for acute or subacute lesions, ointments for chronic lesions (e.g., lichenified and xerotic lesions), and thick corneal layers (e.g., palmar/plantar regions) (16). The Fingertip Unit defines the right amount of TCS to be applied (17). The Fingertip Unit can be used to reduce parental resistance to TCSs (corticophobia). A moderate-to-high-potency TCS is applied in the acute phase, and a low-to-medium-potency is used as maintenance. In areas with higher absorption (eyelids, genitals, face, and skin folds), low-to-moderate-potency TCSs are given. Bone mineral density in children is not decreased by TCSs (18). Proactive TCS two or three times a week prevents flare relapse (19). Corticophobia has an incidence of up to 60–73% in children with AD or their parents (20). It represent a main cause of non-adherence. Healthcare professionals should prevent cortico phobia by clearly addressing the questions and fears of parents. Adequate information is often not provided (20). Wet-wrap therapy for 2–14 days is a second-line treatment with anti-inflammatory and cooling action in patients >6 months of age (21–23). Following a 5′–15′ warm bath, the skin is dried with the application of diluted (5–10%) TCSs to the skin or an internal dressing (21–23). Bandages are applied for 3–24 h, with the best results during the first week (24). Daytime dressings are not often accepted. Transiently increased cortisol levels or infections may occur.
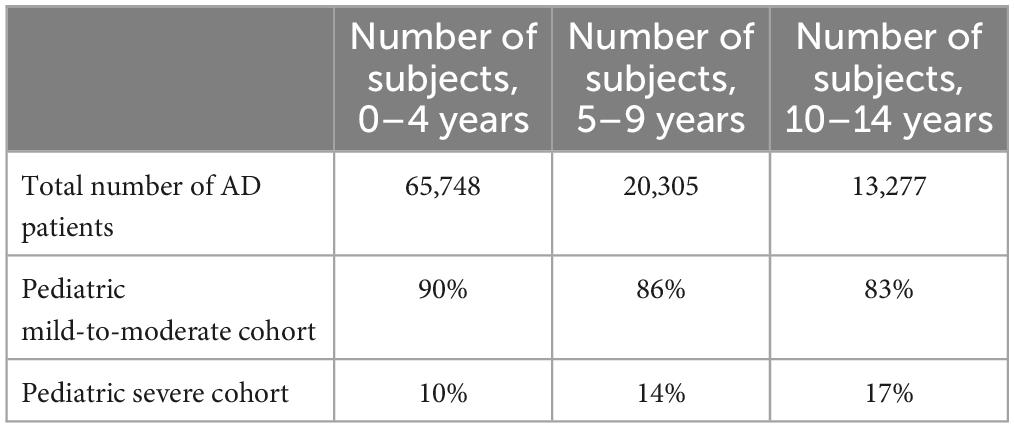
Table 2. Patient characteristics from a swedish study modified from Ortsater et al. (15).
Topical calcineurin inhibitors (TCIs) include tacrolimus (tTAC) 0.03% ointment and pimecrolimus (tPIM) 1% cream, which are both approved for AD in patients ≥2 years of age, while tTAC 0.1% is approved for those ≥16 years of age (Table 1) (25–29). TCIs quickly relieve itching and signs with sustained efficacy (30–36). tTAC treatment 2–3 times a week for up to 1 year minimizes TCS consumption and increases the number of days without acute lesions (37, 38). Both tTAC formulations are more effective than low-potency TCSs and comparable to medium-potency TCSs (12, 39). Methylprednisolone 0.1% was significantly better than tTAC 0.03% in reducing EASI, pruritus, insomnia, and costs in children (40). tTAC has greater efficacy than tPIM in children (31, 41). Local burning, prickling, itching, and erythema have been reported, especially in the first days. They are aggravated by sweating. To avoid stinging, TCSs are applied first, followed by tTAC 0.03% and then tTAC 0.1% if possible (12). Allergic contact dermatitis, rosacea-like granulomatous reactions, melanosis of the lips, and viral infections have been reported during TCI treatment (39). Pediatric studies showed a lack of systemic immunosuppression by TCIs over 5 years for tPIM (42) and 10 years for tTAC (36, 43). In 2005, the Food and Drug Administration (FDA) issued a “Black Box warning” regarding the theoretical risk of skin cancer and/or lymphoma associated with TCIs. To date, there is no evidence to support such a risk (44–46). UV protection is still recommended because the risk of photo carcinogenicity increases with long-term use of cyclosporine, a calcineurin inhibitor (47). Experts conclude that tPIM is a safe and effective “steroid-sparing” treatment (39) and that TCIs should no longer be avoided in children >3 months of age (48). Overall, TCIs are a second-line option or an alternative first option for long-term treatment in areas with elevated TCS absorption and atrophy (12, 39). tPIM is suggested for mild AD and tTAC for moderate-to-severe AD and long-term treatment.
Oral corticosteroids (Table 1) are a rescue therapy for flares or severe disease (methylprednisolone 1 mg/kg/day for 1–2 weeks). Continuous treatment leads to suppression of the hypothalamic-pituitary-adrenal axis, immunosuppression, hypertension, weight gain, osteoporosis, and growth failure in children (49, 50). Tapering is not required for use for less than 3 weeks; otherwise, the drug should be tapered in approximately 1 month (12, 51).
Anti-H1 antihistamines play a controversial role in AD, as itching is not usually linked to histamine. The evidence supporting oral anti-H1 in AD is unclear (52). However, they are included in AD guidelines (51, 53). Sedating first-generation anti-H1s is suggested when itching affects sleep (Table 1) (50, 54, 55). In 2015, the European Medicines Agency (EMA) issued a safety warning on first-generation anti-H1s in children under 2 years of age due to a low risk of QT prolongation and torsades de pointes. A Cochrane review (56) of 25 trials, comprising eight on children, showed no consistent efficacy of second-generation anti-H1s as an “add-on” therapy to topical treatment. Cetirizine and loratadine were not superior to placebo (57). The most common adverse events are sedation (even with non-sedating antihistamines) and cholinergic symptoms (58). First-generation anti-H1s may induce daytime somnolence, impairing school performance and driving (57, 59, 60). Topical antihistamines (e.g., diphenhydramine) are not recommended because of the risk of absorption with systemic toxicity and allergic contact dermatitis (61, 62).
Allergen-specific immunotherapy exerts an anti-inflammatory action since it inhibits the response to sensitizing allergens (Table 1). Trials in children with aeroallergen allergies have generally reported its efficacy. However, it should be prescribed to selected children with symptoms following exposure to the relevant allergen (53). Large, controlled studies are warranted for routine use (63, 64).
Immunosuppressive agents
Cyclosporin A (CsA), a member of the calcineurin inhibitor family (65, 66) (Table 1), is approved by the EMA for severe AD in adults and, in some countries, in patients >16 years of age. However, it is widely used in children (67, 68). When other therapies are unavailable or contraindicated, CsA is a first-line option with rapid action and a low incidence of side effects (69). European guidelines endorse CsA with a SCORAD index >50 or persistent AD (62). A dose of 2.5–5 mg/kg/day in two doses is recommended for children and adults (53). In adults (70), higher doses (5 mg/kg per day) achieve a quicker response. CsA and dupilumab proved to be more effective than methotrexate (MTX) and azathioprine (AZA) in terms of severity scores at week 16 (71). AD was significantly more reduced by CsA than by dupilumab at 1 month (72), with no difference between treatments after 4 months (73). CsA is more effective than prednisolone, UVA, and UVB (53). In children aged 2 to 16 years with severe AD, 5 mg/kg/day of CsA was effective either continuously for up to 12 months or in intermittent 12-week courses (74). Consequently, personalized dosing is an option (75). Treatment should not exceed 2 years of continuous regimen (62). Infections, nephrotoxicity, hypertension, tremor, hypertrichosis, headache, gingival hyperplasia, skin cancer, and lymphoma may develop. Nephrotoxicity is more likely when the dose is >5 mg/kg/day. Blood counts and hepatic and renal parameters, in addition to blood pressure, should be monitored (e.g., at baseline, every 4 weeks, and then every 3 months) (62, 70).
Methotrexate (MTX) (76–83), azathioprine (AZA), and mycophenolate mofetil (MMF) (Table 1), which are not approved for AD (70, 84–86), are second-line treatments in severe AD.
A paucity of data shows moderate efficacy and safety of MTX in children and adults (53, 77, 78). MTX and CsA similarly reduced SCORAD in children (79). In adults, CsA and dupilumab showed greater efficacy than MTX (71). Hepatic toxicity, pancytopenia, teratogenicity, and idiopathic pulmonary fibrosis are rare adverse events (80). Blood counts and renal and liver profiles should be monitored (81). The type III procollagen peptide should be checked if available. Folic acid supplementation (5 mg twice weekly) is useful (53, 82, 83). The response to MTX is slow (8–12 weeks) and is maintained over time. The dose is 0.2–0.5 mg/kg/week (maximum 25 mg/week) for 10–16 weeks in children. It is tapered by 2.5–5 mg/week to the lowest effective dose (39).
Azathioprine is less effective than CsA but comparable to MTX (71). The WHO stated that the side effects of AZA outweigh its benefits (86). Improvements occur within 2–3 months with an indeterminate duration (87, 88). Serum thiopurine S-methyltransferase activity allows for safer use of AZA in children (88, 89). When this is unavailable, half the standard dose (2–3 mg/kg/day) for 4–6 weeks is followed by a full dose (53, 70, 84). AZA has hepatotoxicity, myelotoxicity, and carcinogenicity. Blood counts and liver and kidney function must be checked twice monthly for 2 months, then monthly for 4 months, and then every 2 months with an increasing dose (53, 70).
Case reports and open-label studies have reported the benefits of MMF in children and adults with severe AD (90). Improvements occurred within 6–7 weeks. MMF was safer than AZA. Blood counts and renal and liver profiles should be monitored (53). The dose in children is 30–50 mg/kg/day in two doses (91).
Biologics
Atopic dermatitis is considered a type 2 (T2) disease due to the upregulation of cytokines produced by Th2 lymphocytes and T2 innate lymphoid cells (92). A role for IL-17 and IL-22 is conceivable. Even if the molecular pathophysiology is not totally understood, progress has opened the way to monoclonal antibodies targeting T2 cytokines in moderate-to-severe AD not controlled by TCSs. To date, dupilumab, tralokinumab, and lebrikizumab are marketed for adults and adolescents >12 years of age; dupilumab has been approved by the U.S. Food and Drug Administration and the European Commission for children aged >6 months of age.
Dupilumab (93–95) (Table 1) achieved IGA (0, 1), ≥75% of EASI (EASI-75), and reduced SCORAD, pruritus NRS, sleep disturbance, DLQI, and patient-oriented eczema measure (POEM) in several phase III studies in adults with AD for up to 52 weeks (96–98), even when unresponsive to CsA (99–101), and in children aged 6 months to 17 years (102–106). Laboratory monitoring was unnecessary (96–108). However, the rate of conjunctivitis was higher in the dupilumab groups than in the placebo groups at all ages (104, 106, 109). The mechanism remains elusive. Conjunctivitis is treated with corticosteroid eye drops or tacrolimus 0.03% eye ointment without interruption of dupilumab (96, 109).
Tralokinumab and lebrikizumab (Table 1) (96, 97, 110) are both approved by the European Medicines Agency (EMA) for moderate-to-severe AD in adolescents aged 12–17 years and adults. No laboratory tests are required for monitoring.
Tralokinumab. Three phase III studies have documented the efficacy and safety of tralokinumab in adults (111, 112). In a phase IIb trial in adults, the frequency of IGA (0, 1) responses was higher with increasing doses (45 < 150 < 300 mg subcutaneously twice monthly) (113, 114). Approval in adolescents is based on a 52-week phase III study (115, 116). The most common adverse events were upper respiratory tract infections and conjunctivitis, which developed in 7.5% of patients (113, 117). Reduction of both conjunctival goblet cells and mucin production may provoke conjunctivitis associated with IL-13 antagonists (118).
Lebrikizumab showed in two phase II studies in adults a significant improvement in EASI-50 at weeks 12 and 16 (111, 119). In two 16-week phase III trials enrolling adolescents and adults (120), IGA (0, 1) and EASI-75 were reached more frequently by patients in the lebrikizumab group than in the placebo group. Efficacy and safety continued after lebrikizumab withdrawal through week 52 (121). A phase III trial (122) found that TCSs did not improve the efficacy of lebrikizumab. Conjunctivitis is a common adverse event.
Novel biologics
Several anti-T2 cytokine biologics that inhibit interleukins other than IL-4 and IL-13 (Table 1) are in clinical development (9), but have not yet been licensed (53). Moreover, there are no pediatric trials on these novel biologics available, except for one on nemolizumab in adolescents (10).
Nemolizumab (123–125) (Table 1) has been used in adults with uncontrolled moderate-to-severe AD, has shown to improve pruritus, the primary outcome, sleep score, and EASI in both phase I (126) and II trials (127, 128), with efficacy up to 64 weeks (128). A dose of 30 mg subcutaneously was more effective than 10 and 90 mg every 4 weeks (127). Pruritus relief was noticeable by day 2, and sleep disturbances by day 3 (129). In a phase III trial (10), in patients aged 13 years or older, nemolizumab 60 mg/4 weeks was more effective in improving primary outcome, VAS pruritus score, and EASI and DLQI than placebo. Tolerance was good.
Fezakinumab (anti-IL-22), GBR830 (anti-OX40), itepekimab (anti-IL-33) mepolizumab (anti-IL-5), omalizumab (anti-IgE) ustekinumab (anti-IL-12/23p40), risankizumab (anti-IL-23p19), rocatinlimab (anti-OX40), secukinumab (anti-IL-17A), and tezepelumab (thymic stromal lymphopoietin) showed poor or uncertain efficacy in AD (130–147).
Small molecules
Small molecules are chemical compounds generally <0.5 kDa that require more frequent dosing and have more off-target effects when administered systemically compared to biologics. To date, phosphodiesterase-4 (PDE-4) and Janus Kinase (JAK) inhibitors have been approved for use in pediatric AD.
PDE-4 inhibitors (Table 1)
Crisaborole ointment (2% twice a day) (148) has been approved for mild-to-moderate AD by regulatory agencies in patients 3 months of age and older. It is not marketed in the European Union. Crisaborole was effective in two phase III trials (149–151). Investigator’s Static Global Assessment (ISGA) success in children aged 2–17 years with mild-to-moderate AD was achieved more frequently by crisaborole than with placebo (152). Crisaborole improves sleep disruptions (153). Crisaborole was safe, with local pain for 1–2 days being the most common adverse event. In an open-label phase IV study in infants aged 3 to < 24 months with mild-to-moderate AD, crisaborole achieved moderate ISGA success, from 20% of patients at day 8 to 30.2% at week 4, with good tolerability (154).
Difamilast is approved in some countries for AD in children >2 years of age and adults (155). In a 4-week phase III study in children aged 2–14 years with mild-to-moderate AD (156), difamilast ointment 0.3%, 1% twice daily, achieved IGA (0, 1) in 44.6 and 47.1%, respectively, with significant differences between both difamilast and placebo groups (18.1%). A phase III trial in infants aged >3 months to 2 years is ongoing.
Roflumilast cream was successful in a phase II trial in mild-to-moderate AD patients aged >12 years (157). Preliminary data from phase III trials of roflumilast cream 0.15% in adults and children aged ≥6 years are promising. A phase III trial in children aged 2–5 years is ongoing.
Topical JAK inhibitors
Ruxolitinib cream 1.5% (Table 1) is an FDA-approved selective JAK1 and JAK2 inhibitor (158) with warnings for short-term use in mild-to-moderate AD with up to 20% BSA-resistance to topical agents in adults and adolescents >12 years of age. Less than 60 g/week or 100 g/2 weeks are permitted. It is not recommended with biologics, JAK inhibitors, or immunosuppressants. Two phase III trials (159) in subjects aged ≥12 years with mild-to-moderate AD showed that ruxolitinib significantly reduced itching within 12 h and skin thickening (159). Ruxolitinib cream 1.5% and 0.75% (160) twice daily for 8 weeks significantly achieved IGA (0, 1) and reduced pruritus (p < 0.0001). Adverse events were nasopharyngitis, burning, and pruritus at the application site, but not systemic. Plasma concentrations (161) were higher at >40% BSA, but below levels of bone marrow suppression. A phase I study in children aged 2–17 years (162) showed that one patient developed neutropenia and discontinued ruxolitinib. A phase III trial in children aged ≥2 years to < 12 years is ongoing. Overall, topical ruxolitinib is useful before systemic therapy and for proactive therapy. Trials on safety are needed.
Tofacitinib Oral Tofacitinib, which targets JAK1, JAK2, and JAK3 (163), has been approved for several inflammatory diseases. A phase 2a study showed that tofacitinib 2% ointment significantly improved pruritus and signs in adults with mild-to-moderate AD (164).
Oral JAK inhibitors (Table 1)
Upadacitinib, abrocitinib, and baricitinib mainly inhibit JAK1, JAK1, and JAK1-JAK, respectively. They are approved for adolescents >12 years of age and adults with moderate-to-severe AD when drugs, including biologics, are unhelpful or contraindicated. In Europe, only adults can receive abrocitinib. Oral JAK inhibitors have been associated with cancer, major cardiovascular problems, serious infections, venous thromboembolism, and mortality (165). The EMA (166) recommended its use only when alternatives are not available in patients >65 years of age, at risk for cardiovascular disease or cancer, smokers, and with caution in patients with other risk factors for blood clots in the lungs and venous thromboembolism. Doses are reduced when possible. Patients (53) are screened for HIV, viral hepatitis B and C, and tuberculosis and receive a chest radiograph at baseline. Blood count, renal, liver, and lipid profiles, in addition to creatinine phosphokinase, are checked at baseline, at week 4, and then every 3 months.
Upadacitinib. Three 16-week phase III trials showed significant efficacy of oral upadacitinib 15 mg and 30 mg in adults and adolescents with moderate-to-severe AD (167, 168). One patient interrupted upadacitinib 30 mg due to anemia, two due to neutropenia, and one due to moderate acne (167, 168). One patient discontinued upadacitinib 15 mg due to acne (167). In a 16-week phase III trial in adults with moderate-to-severe AD (169), upadacitinib achieved a significantly greater reduction in pruritus NRS than dupilumab. Serious infections were more common in the upadacitinib group, while conjunctivitis was more common in the dupilumab group. There was one death in the upadacitinib group due to influenza-related pneumonia.
Abrocitinib. In two 12-week phase III trials in patients >12 years and older (170, 171), abrocitinib (200 mg or 100 mg) achieved significantly greater proportions of IGA (0, 1) and EASI-75 responses. In a 40-week phase III trial (172), patients >12 years of age responding to abrocitinib had less frequent flares of AD with abrocitinib 200 mg or 100 mg than with the placebo. In a 12-week phase III trial (173) in adolescents, abrocitinib 200 mg or 100 mg significantly reached IGA (0, 1) and EASI-75. Nausea, upper respiratory tract infections, acne, reduced platelet count, and headaches were reported (171, 172). In a 12-week phase III study (174), abrocitinib 100 or 200 mg and dupilumab significantly reached IGA (0, 1) and EASI-75 compared to placebo, and abrocitinib demonstrated rapid efficacy (175). However, a comparison between abrocitinib and dupilumab is lacking, and definitive conclusions cannot be drawn. A phase III study (176) showed that EASI-90 was achieved in significantly more patients with abrocitinib than with dupilumab at weeks 4 and 16. At week 2, pruritus was reduced in the abrocitinib group compared to the dupilumab group (p < 0.0001). Nausea, headaches, acne, or folliculitis were more common with abrocitinib than with dupilumab. Conjunctivitis occurred less frequently with abrocitinib than with dupilumab.
Baricitinib monotherapy or with TCSs, in 16-week phase III trials (177, 178), showed significantly greater improvement in EASI-75, IGA (0, 1), NRS, POEM, and DLQI than placebo, sustained for ≤68 weeks. Serious infections, malignancies, cardiovascular events, thromboembolic events, high blood creatine phosphokinase, and cholesterol occurred. Several trials with children are ongoing.
Aryl hydrocarbon antagonists
Tapinarof (Table 1) is approved for plaque psoriasis. Tapinarof cream 1% twice daily has shown promise in adults with AD, with folliculitis being the most common adverse event (179–181). Phase III trials are currently underway in both adult and pediatric populations.
Transient receptor potential vanilloid subfamily V member 1 (TRPV1) antagonist
Asivatrep. In an 8-week phase III study (182) in patients aged >12 years with mild-to-moderate AD, the primary outcome IGA (0, 1) was achieved by 36.0% in the asivatrep cream 1.0% group and 12.8% in the vehicle group (P < 0.001). There was a significant reduction in EASI-75, EASI-100, and VAS pruritus in the active group compared to the placebo group. Safety was good.
Conclusion
Standard therapy for AD consists of daily emollients, allergen avoidance, education, and TCSs. In some clinical situations, TCIs and oral corticosteroids are useful. Proactive treatments with TCSs or TCIs and psychological consultations may be considered. Anti-H1 antihistamines have a poor effect on itching. New anti-inflammatory drugs have paved the way for a precision medicine strategy, but only a few are approved by regulatory agencies for use in children. In recalcitrant cases, topical PHE-4 inhibitors, both for flares and proactively, are limited by efficacy, cost, and a lack of global marketing. Ruxolitinib, a topical JAK inhibitor, has been approved and successfully used for severe pruritus in young children in some countries. In severe cases, biologics are the first option since immunosuppressive drugs can have modest results and need to be monitored for adverse events. Among the biologics, dupilumab is licensed for children aged 6 months and older, while tralokinumab and lebrikizumab are approved for adolescents >12 years of age. Oral JAK inhibitors such as upadacitinib and abrocitinib have been approved in adolescents when other drugs, including biologics, have failed or are contraindicated. The main disadvantages of oral JAK inhibitors are increased cost and laboratory monitoring due to serious adverse events. So, when biologics are not successful, unavailable, or cost-prohibitive, immunosuppressive agents can be considered. CsA seems to have a better clinical profile in children, although it is only approved for patients >16 years of age.
Author contributions
CC, AG, GG, and GR performed the analysis of literature, drafted the manuscript, interpretation of the data, and the writing of the manuscript. All authors approved the version being submitted.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Thomas KS, Apfelbacher CA, Chalmers JR, Simpson E, Spuls P, Gerbens LAA, et al. Recommended core outcome instruments for health-related quality of life, long-term control, and itch intensity in atopic eczema trials: results of the HOME VII consensus meeting. Br J Dermatol. (2020) doi: 10.1111/bjd.19673 [Epub ahead of print].
2. Caffarelli C, Dondi A, Povesi Dascola C, Ricci G. Skin prick test to foods in childhood atopic eczema: pros and cons. Ital J Pediatr. (2013) 31:48. doi: 10.1186/1824-7288-39-48
3. Caffarelli C, Cavagni G, Menzies IS, Bertolini P, Atherton DJ. Elimination diet and intestinal permeability in atopic eczema: a preliminary study. Clin Exp Allergy. (1993) 23:28–31. doi: 10.1111/j.1365-2222.1993.tb02480.x
4. Ricci G, Andreozzi L, Cipriani F, Giannetti A, Gallucci M, Caffarelli C. Wheat allergy in children: a comprehensive update. Medicina. (2019) 55:400. doi: 10.3390/medicina55070400
5. Caffarelli C, Garrubba M, Greco C, Mastrorilli C, Povesi Dascola C. Asthma and food allergy in children: is there a connection or interaction? Front Pediatr. (2016) 4:34. doi: 10.3389/fped.2016.00034
6. Severity scoring of atopic dermatitis: the SCORAD index. Consensus report of the European task force on atopic dermatitis. Dermatology. (1993) 186:23–31.
7. Gooderham MJ, Bissonnette R, Grewal P, Lansang P, Papp KA, Hong CH. Approach to the assessment and management of adult patients with atopic dermatitis: a consensus document. section II: tools for assessing the severity of atopic dermatitis. J Cutan Med Surg. (2018) 22:10S–6S. doi: 10.1177/1203475418803628
8. Hanifin JM, Thurston M, Omoto M, Cherill R, Tofte SJ, Graeber M. The eczema area and severity index (EASI): assessment of reliability in atopic dermatitis. EASI Evaluator Group. Exp Dermatol. (2001) 10:11–8. doi: 10.1034/j.1600-0625.2001.100102.x
9. Facheris P, Jeffery J, Del Duca E, Guttman-Yassky E. The translational revolution in atopic dermatitis: the paradigm shift from pathogenesis to treatment. Cell Mol Immunol. (2023) doi: 10.1038/s41423-023-00992-4 [Epub ahead of print].
10. Kabashima K, Matsumura T, Komazaki H, Kawashima M. Nemolizumab-JP01 study group. Trial of nemolizumab and topical agents for atopic dermatitis with pruritus. N Engl J Med. (2020) 383:141–50. doi: 10.1056/NEJMoa1917006
11. Katoh N, Ohya Y, Ikeda M, Ebihara T, Katayama I, Saeki H, et al. Clinical practice guidelines for the management of atopic dermatitis 2018. J Dermatol. (2019) 46:1053–101. doi: 10.1111/1346-8138.15090
12. Wollenberg A, Kinberger M, Arents B, Aszodi N, Avila Valle G, Barbarot S, et al. European guideline (EuroGuiDerm) on atopic eczema - part II: non-systemic treatments and treatment recommendations for special AE patient populations. J Eur Acad Dermatol Venereol. (2022) 36:1904–26. doi: 10.1111/jdv.18429
13. Spergel JM. Immunology and treatment of atopic dermatitis. Am J Clin Dermatol. (2008) 9:233–44. doi: 10.2165/00128071-200809040-00003
14. Norris DA. Mechanisms of action of topical therapies and the rationale for combination therapy. J Am Acad Dermatol. (2005) 53:S17–25. doi: 10.1016/j.jaad.2005.04.027
15. Ortsäter G, Geale K, Dun AR, Cappelleri JC, Cha A, Romero W, et al. Clinical and economic burden of pediatric mild-to-moderate atopic dermatitis: a population-based nested case-control study in Sweden. Dermatol Ther. (2021) 11:161–72. doi: 10.1007/s13555-020-00470-z
16. Danby SG, Draelos ZD, Gold LFS, Cha A, Vlahos B, Aikman L, et al. Vehicles for atopic dermatitis therapies: more than just a placebo. J Dermatolog Treat. (2020) 16:1–14. doi: 10.1080/09546634.2020.1789050
17. Long CC, Finlay AY. The finger-tip unit–a new practical measure. Clin Exp Dermatol. (1991) 16:444–7. doi: 10.1111/j.1365-2230.1991.tb01232.x
18. van Velsen SG, Knol MJ, van Eijk RL, de Vroede MA, de Wit TC, Lam MG, et al. Bone mineral density in children with moderate to severe atopic dermatitis. J Am Acad Dermatol. (2010) 63:824–31. doi: 10.1016/j.jaad.2009.12.015
19. Berth-Jones J, Damstra RJ, Golsch S, Livden JK, Van Hooteghem O, Allegra F, et al. Twice weekly fluticasone propionate added to emollient maintenance treatment to reduce risk of relapse in atopic dermatitis: randomised, double blind, parallel group study. BMJ. (2003) 326:1367. doi: 10.1136/bmj.326.7403.1367
20. Zhao M, Liang Y, Shen C, Wang Y, Ma L, Ma X. Patient education programs in pediatric atopic dermatitis: a systematic review of randomized controlled trials and meta-analysis. Dermatol Ther. (2020) 10:449–64. doi: 10.1007/s13555-020-00365-z
21. Cadmus SD, Sebastian KR, Warren D, Hovinga CA, Croce EA, Reveles LA, et al. Efficacy and patient opinion of wet-wrap dressings using 0.1% triamcinolone acetonide ointment vs cream in the treatment of pediatric atopic dermatitis: a randomized split-body control study. Pediatr Dermatol. (2019) 36:437–41. doi: 10.1111/pde.13830
22. Mirza SA. Serum triamcinolone levels during intensive, inpatient wet dressing therapy. Clin Exp Dermatol. (2020) 45:549–54. doi: 10.1111/ced.14161
23. Witte M, Krause L, Zillikens D, Shimanovich I. Black tea dressings – a rapidly effective treatment for facial dermatitis. J Dermatolog Treat. (2019) 30:785–9. doi: 10.1080/09546634.2019.1573306
24. Huiling H, Koh MJ, Lee HY, Ang SB. Pilot study of a customized nanotextile wet garment treatment on moderate and severe atopic dermatitis: a randomized clinical trial. Pediatr Dermatol. (2020) 37:52–7. doi: 10.1111/pde.13981
25. Carr WW. Topical calcineurin inhibitors for atopic dermatitis: review and treatment recommendations. Paediatr Drugs. (2013) 15:303–10. doi: 10.1007/s40272-013-0013-9
26. Chehade A, Rao J. Topical calcineurin inhibitors. Topical calcineurin inhibitors comprehensive dermatologic drug therapy. 4th ed. London: National Eczema Society (2021). p. 549–56.
27. Nakahara T, Morimoto H, Murakami N, Furue M. Mechanistic insights into topical tacrolimus for the treatment of atopic dermatitis. Pediatr Allergy Immunol. (2018) 29:233–8. doi: 10.1111/pai.12842
28. Pereira U, Boulais N, Lebonvallet N, Pennec JP, Dorange G, Misery L. Mechanisms of the sensory effects of tacrolimus on the skin. Br J Dermatol. (2010) 163:70–7. doi: 10.1111/j.1365-2133.2010.09757.x
29. Wongpiyabovorn J, Soonthornchai W, Wilantho A, Palasuk M, Payungporn S, Sodsai P, et al. Effect of tacrolimus on skin microbiome in atopic dermatitis. Allergy. (2019) 74:1400–6. doi: 10.1111/all.13743
30. Remitz A, De Pità O, Mota A, Serra-Baldrich E, Vakirlis E, Kapp A. Position statement: topical calcineurin inhibitors in atopic dermatitis. Eur Acad Dermatol. (2018) 32:2074–82. doi: 10.1111/jdv.15272
31. Chen SL, Yan J, Wang FX. Two topical calcineurin inhibitors for the treatment of atopic dermatitis in pediatric patients: a meta-analysis of randomized clinical trials. J Dermatol Treat. (2010) 21:144–56. doi: 10.3109/09546630903401470
32. El-Batawy MM, Bosseila MA, Mashaly HM, Hafez VS. Topical calcineurin inhibitors in atopic dermatitis: a systematic review and meta-analysis. J Dermatol Sci. (2009) 54:76–87. doi: 10.1016/j.jdermsci.2009.02.002
33. Ohtsuki M, Morimoto H, Nakagawa H. Tacrolimus ointment for the treatment of adult and pediatric atopic dermatitis: review on safety and benefits. J Dermatol. (2018) 45:936–42. doi: 10.1111/1346-8138.14501
34. Siegfried EC, Jaworski JC, Kaiser JD, Hebert AA. Systematic review of published trials: long-term safety of topical corticosteroids and topical calcineurin inhibitors in pediatric patients with atopic dermatitis. BMC Pediatr. (2016) 16:75. doi: 10.1186/s12887-016-0607-9
35. Sher LG, Chang J, Patel IB, Balkrishnan R, Fleischer AB Jr. Relieving the pruritus of atopic dermatitis: a meta-analysis. Acta Derm Venereol. (2012) 92:455–61. doi: 10.2340/00015555-1360
36. Mandelin JM, Remitz A, Virtanen HM, Malmberg LP, Haahtela T, Reitamo S. A 10-year open follow-up of eczema and respiratory symptoms in patients with atopic dermatitis treated with topical tacrolimus for the first 4 years. J Dermatolog Treat. (2010) 21:167–70. doi: 10.3109/09546630903493329
37. Thaci D, Chambers C, Sidhu M, Dorsch B, Ehlken B, Fuchs S. Twice weekly treatment with tacrolimus 0.03% ointment in children with atopic dermatitis: clinical efficacy and economic impact over 12 months. J Eur Acad Dermatol Venereol. (2010) 24:1040–6. doi: 10.1111/j.1468-3083.2010.03577.x
38. Katoh N, Ohya Y, Ikeda M, Ebihara T, Katayama I, Saeki H. Japanese guidelines for atopic dermatitis 2020. Allergol Int. (2020) 69:356–69. doi: 10.1016/j.alit.2020.02.006
39. Wollenberg A, Christen-Zäch S, Taieb A, Paul C, Thyssen JP, de Bruin-Weller M. ETFAD/EADV Eczema task force 2020 position paper on diagnosis and treatment of atopic dermatitis in adults and children. J Eur Acad Dermatol Venereol. (2020) 34:2717–44. doi: 10.1111/jdv.16892
40. Bieber T, Vick K, Fölster-Holst R, Belloni-Fortina A, Städtler G, Worm M, et al. Efficacy and safety of methylprednisolone aceponate ointment 0.1% compared to tacrolimus 0.03% in children and adolescents with an acute flare of severe atopic dermatitis. Allergy. (2007) 62:184–9. doi: 10.1111/j.1398-9995.2006.01269.x
41. Paller AS, Lebwohl M, Fleischer AB Jr, Antaya R, Langley RG, Kirsner RS, et al. Tacrolimus ointment is more effective than pimecrolimus cream with a similar safety profile in the treatment of atopic dermatitis: results from 3 randomized, comparative studies. J Am Acad Dermatol. (2005) 52:810–22. doi: 10.1016/j.jaad.2004.12.038
42. Sigurgeirsson B, Boznanski A, Todd G, Vertruyen A, Schuttelaar ML, Zhu X, et al. Safety and efficacy of pimecrolimus in atopic dermatitis: a 5-year randomized trial. Pediatrics. (2015) 135:597–606. doi: 10.1542/peds.2014-1990
43. Reitamo S, Rustin M, Harper J, Kalimo K, Rubins A, Cambazard F, et al. A 4-year follow-up study of atopic dermatitis therapy with 0.1% tacrolimus ointment in children and adult patients. Br J Dermatol. (2008) 159:942–51. doi: 10.1111/j.1365-2133.2008.08747.x
44. Paller AS, Fölster-Holst R, Chen SC, Diepgen TL, Elmets C, Margolis DJ, et al. No evidence of increased cancer incidence in children using topical tacrolimus for atopic dermatitis. J Am Acad Dermatol. (2020) 83:375–81. doi: 10.1016/j.jaad.2020.03.075
45. Radovic TC, Kostovic K, Ceovic R, Mokos ZB. Topical calcineurin inhibitors and malignancy risk. Int J Cancer Manag. (2017) 10:e6173. doi: 10.5812/ijcm.6173
46. Asgari MM, Tsai AL, Avalos L, Sokil M, Quesenberry CP Jr. Association between topical calcineurin inhibitor use and keratinocyte carcinoma risk among adults with atopic dermatitis. JAMA Dermatol. (2020) 156:1066–73. doi: 10.1001/jamadermatol.2020.2240
47. Czarnecka-Operacz M, Jenerowicz D. Topical calcineurin inhibitors in the treatment of atopic dermatitis – an update on safety issues. J Dtsch Dermatol Ges. (2012) 10:167–72. doi: 10.1111/j.1610-0387.2011.07791.x
48. Luger T, Augustin M, Lambert J, Paul C, Pincelli C, Torrelo A. Unmet medical needs in the treatment of atopic dermatitis in infants: an expert consensus on safety and efficacy of pimecrolimus. Pediatr Allergy Immunol. (2021) 3:414–24. doi: 10.1111/pai.13422
49. Drucker AM, Eyerich K, de Bruin-Weller MS, Thyssen JP, Spuls PI, Irvine AD, et al. Use of systemic corticosteroids for atopic dermatitis: International Eczema council consensus statement. Br J Dermatol. (2018) 178:768–75. doi: 10.1111/bjd.15928
50. Sidbury R, Davis DM, Cohen DE, Cordoro KM, Berger TG, Bergman JN. American Academy of Dermatology, et al. Guidelines of care for the management of atopic dermatitis: section 3. Management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. (2014) 71:327–49. doi: 10.1016/j.jaad.2014.03.030
51. Chan TC, Wu NL, Wong LS, Cho YT, Yang CY, Yu Y, et al. Taiwanese dermatological association consensus for the management of atopic dermatitis: a 2020 update. J Formos Med Assoc. (2021) 120:429–42. doi: 10.1016/j.jfma.2020.06.008
52. Galli E, Neri I, Ricci G, Baldo E, Barone M, Belloni Fortina A, et al. Consensus conference on clinical management of pediatric atopic dermatitis. Ital J Pediatr. (2016) 42:26. doi: 10.1186/s13052-016-0229-8
53. Wollenberg A, Kinberger M, Arents B, Aszodi N, Avila Valle G, Barbarot S, et al. European guideline (EuroGuiDerm) on atopic eczema - part I: non-systemic treatments and treatment recommendations for special AE patient populations. J Eur Acad Dermatol Venereol. (2022) 36:1409–31. doi: 10.1111/jdv.18345
54. Nowicki RJ, Trzeciak M, Kaczmarski M, Wilkowska A, Czarnecka-Operacz M, Kowalewski C, et al. Interdisciplinary diagnostic and therapeutic recommendations of the polish dermatological society, polish society of allergology, polish pediatric society and polish society of family medicine. Part II. Systemic treatment and new therapeutic methods. Postepy Dermatol Alergol. (2020) 37:129–34. doi: 10.5114/ada.2020.94829
55. Wollenberg A, Oranje A, Deleuran M, Simon D, Szalai Z, Kunz B, et al. ETFAD/EADV Eczema task force 2015 position paper on diagnosis and treatment of atopic dermatitis in adult and paediatric patients. J Eur Acad Dermatol Venereol. (2016) 30:729–47. doi: 10.1111/jdv.13599
56. Matterne U, Böhmer MM, Weisshaar E, Jupiter A, Carter B, Apfelbacher C. Oral H1 antihistamines as ‘add-on’ therapy to topical treatment for eczema. Cochrane Database Syst Rev. (2019) 1:CD012167. doi: 10.1002/14651858.CD012167.pub2
57. Kay GG. The effects of antihistamines on cognition and performance. J Allergy Clin Immunol. (2000) 105:S622–7. doi: 10.1067/mai.2000.106153
58. Lyons JJ, Milner JD, Stone KD. Atopic dermatitis in children: clinical features, pathophysiology, and treatment. Immunol Allergy Clin North Am. (2015) 35:161–83. doi: 10.1016/j.iac.2014.09.008
59. Yu SH, Attarian H, Zee P, Silverberg Ji. Burden of sleep and fatigue in US adults with atopic dermatitis. Dermatitis. (2016) 27:50–8. doi: 10.1097/DER.0000000000000161
60. Schad CA, Skoner DP. Antihistamines in the pediatric population: achieving optimal outcomes when treating seasonal allergic rhinitis and chronic urticaria. Allergy Asthma Proc. (2008) 29:7–13. doi: 10.2500/aap2008.29.3080
61. Eichenfield LF, Tom WL, Berger TG, Krol A, Paller AS, Schwarzenberger K, et al. Management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. (2014) 71:116–32. doi: 10.1016/j.jaad.2014.03.023
62. Wollenberg A, Barbarot S, Bieber T, Christen-Zaech S, Deleuran M, Fink-Wagner A, et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part II. J Eur Acad Dermatol Venereol. (2018) 32:850–78. doi: 10.1111/jdv.14891
63. Pajno GB, Bernardini R, Peroni D, Arasi S, Martelli A, Landi M, et al. Clinical practice recommendations for allergen-specific immunotherapy in children: the Italian consensus report. Ital J Pediatr. (2017) 43:13. doi: 10.1186/s13052-016-0315-y
64. Di Rienzo V, Cadario G, Grieco T, Galluccio AG, Caffarelli C, Liotta G, et al. Sublingual immunotherapy in mite-sensitized children with atopic dermatitis: a randomized, open, parallel-group study. Ann Allergy Asthma Immunol. (2014) 113:671–3.e1. doi: 10.1016/j.anai.2014.09.009
65. Amor KT, Ryan C, Menter A. The use of cyclosporine in dermatology: part I. J Am Acad Dermatol. (2010) 63:925–46. doi: 10.1016/j.jaad.2010.02.062
66. Amber T, Tabassum S. Cyclosporin in dermatology: a practical compendium. Dermatol Ther. (2020) 30:e13934. doi: 10.1111/dth.13934
67. Ronnstand ATM, Halling-Overgaard AS, Hamann CR, Skov L, Egeberg A, Thyssen JP. Association of atopic dermatitis with depression, anxiety, and suicidal ideation in children and adults: a systematic review and meta-analysis. J Am Acad Dermatol. (2018) 79:448–56.e30. doi: 10.1016/j.jaad.2018.03.017
68. Lansang P, Lara-Corrales I, Bergman JN, Hong CH, Joseph M, Kim VHD, et al. Approach to the assessment and management of pediatric patients with atopic dermatitis: a consensus document. Section IV: consensus statements on the assessment and management of pediatric atopic dermatitis. J Cutan Med Surg. (2019) 23:32s–9s. doi: 10.1177/1203475419882655
69. Hernández-Martín A, Noguera-Morel L, Bernardino-Cuesta B, Torrelo A, Pérez-Martin MA, Aparicio-López C, et al. Cyclosporine A for severe atopic dermatitis in children efficacy and safety in a retrospective study of 63 patients. J Eur Acad Dermatol Venereol. (2017) 31:837–42. doi: 10.1111/jdv.14066
70. Roekevisch E, Spuls PI, Kuester D, Limpens J, Schmitt J. Efficacy and safety of systemic treatments for moderate-to-severe atopic dermatitis: a systematic review. J Allergy Clin Immunol. (2014) 133:429–38. doi: 10.1016/j.jaci.2013.07.049
71. Drucker AM, Ellis AG, Bohdanowicz M, Mashayekhi S, Yiu ZZN, Rochwerg B, et al. Systemic immunomodulatory treatments for patients with atopic dermatitis: a systematic review and network meta-analysis. JAMA Dermatol. (2020) 156:1–10. doi: 10.1001/jamadermatol.2020.0796
72. Finlay AY, Khan GK. Dermatology life quality index (DLQI)—a simple practical measure for routine clinical use. Clin Exp Dermatol. (1994) 19:210–6. doi: 10.1111/j.1365-2230.1994.tb01167.x
73. Dal Bello G, Maurelli M, Schena D, Girolomoni G, Gisondi P. Drug survival of dupilumab compared to cyclosporin in moderate-to-severe atopic dermatitis patients. Dermatol Ther. (2020) 33:e13979. doi: 10.1111/pde.13781
74. Harper JI, Ahmed I, Barclay G, Lacour M, Hoeger P, Cork MJ, et al. Cyclosporin for severe childhood atopic dermatitis: short course versus continuous therapy. Br J Dermatol. (2000) 142:52–8. doi: 10.1046/j.1365-2133.2000.03241.x
75. Harper JI, Berth-Jones J, Camp RD, Dillon MJ, Finlay AY, Holden CA, et al. Cyclosporin for atopic dermatitis in children. Dermatology. (2001) 203:3–6. doi: 10.1159/000051694
76. Cronstein BN, Aune TM. Methotrexate and its mechanisms of action in inflammatory arthritis. Nat Rev Rheumatol. (2020) 16:145–54. doi: 10.1038/s41584-020-0373-9
77. Weatherhead SC, Wahie S, Reynolds NY. An open label, dose-ranging study of methotrexate for moderate-to-severe adult atopic eczema. Br J Dermatol. (2007) 156:346–51. doi: 10.1111/j.1365-2133.2006.07686.x
78. El-Khalawany MA, Hassan H, Shaaban D, Ghonaim N, Eassa B. Methotrexate versus cyclosporine in the treatment of severe atopic dermatitis in children: a multicenter experience from Egypt. Eur J Pediatr. (2013) 172:351–6. doi: 10.1007/s00431-012-1893-3
79. Dogra S, Mahajan R. Systemic methotrexate therapy for psoriasis: past,present and future. Clin Exp Dermatol. (2013) 38:573–88. doi: 10.1111/ced.12062
80. Deo M, Yung A, Hill S, Rademaker M. Methotrexate for treatment of atopic dermatitis in children and adolescents. Int J Dermatol. (2014) 53:1037–41. doi: 10.1111/ijd.12314
81. Taieb Y, Baum S, Ben Amitai D, Barzilai A, Greenberger S. The use of methotrexate for treating childhood atopic dermatitis: a multicenter retrospective study. J Dermatolog Treat. (2019) 30:240–4. doi: 10.1080/09546634.2018.1508816
82. Shah N, Alhusayen R, Walsh S, Shear NH. Methotrexate in the treatment of moderate to severe atopic dermatitis: a retrospective study. J Cutaneous Med Surg. (2018) 22:484–7. doi: 10.1177/1203475418781336
83. Chavez-Alvarez S, Herz-Ruelas M, Villarreal-Martinez A. Azathioprine: its uses in dermatology. An Bras Dermatol. (2020) 95:731–6. doi: 10.1016/j.abd.2020.05.003
84. Murphy LA, Atherton DJ. Azathioprine as a treatment for severe atopic eczema in children with a partial thiopurine methyl transferase (TPMT) deficiency. Pediatr Dermatol. (2003) 20:531–4. doi: 10.1111/j.1525-1470.2003.20617.x
85. Caufield M, Tom WL. Oral azathioprine for recalcitrant pediatric atopic dermatitis: clinical response and thiopurine monitoring. J Am Acad Dermatol. (2013) 68:29–35. doi: 10.1016/j.jaad.2012.07.001
86. Meggitt SJ, Anstey AV, Mohd Mustapa MF, Reynolds NJ, Wakelin S. British Association of Dermatologists’ guidelines for the safe and effective prescribing of azathioprine 2011. Br J Dermatol. (2011) 165:711–34. doi: 10.1111/j.1365-2133.2011.10575.x
87. Murphy LA, Atherton D. A retrospective evaluation of azathioprine in severe childhood atopic eczema, using thiopurine methyltransferase levels to exclude patients at high risk of myelosuppression. Br J Dermatol. (2002) 147:308–15. doi: 10.1046/j.1365-2133.2002.04922.x
88. Noguera-Morel L, Knopfel N, Torrelo A, Hernandez-Martın A. A retrospective study of systemic treatment of severe atopic dermatitis with azathioprine: effectiveness and tolerance in 11 pediatric patients. Actas Dermosifiliogr. (2019) 110:227–31. doi: 10.1016/j.ad.2018.06.014
89. Martel RM, Melwani P, Islas D, Penate Y, Borrego L. Safety of azathioprine therapy adjusted to thiopurine methyltransferase activity in the treatment of infantile atopic dermatitis. Report on 7 cases. Actas Dermosifiliogr. (2010) 101:415–20.
90. Phan K, Smith SD. Mycophenolate mofetil and atopic dermatitis: systematic review and meta-analysis. J Dermatolog Treat. (2020) 31:810–4. doi: 10.1080/09546634.2019.1642996
91. Dias-Polak D, Bergman R, Avitan-Hersh E. Mycophenolate mofetil therapy in adult patients with recalcitrant atopic dermatitis. J Dermatolog Treat. (2019) 30:49–51. doi: 10.1080/09546634.2018.1468068
92. Gittler JK, Shemer A, Suárez-Fariñas M, Fuentes-Duculan J, Gulewicz KJ, Wang C, et al. Progressive activation of T(H)2/T(H)22 cytokines and selective epidermal proteins characterizes acute and chronic atopic dermatitis. J Allergy Clin Immunol. (2012) 130:1344–54. doi: 10.1016/j.jaci.2012.07.012
93. Bieber T. Interleukin-13: targeting an underestimated cytokine in atopic dermatitis. Allergy. (2020) 75:54–62. doi: 10.1111/all.13954
94. Czarnowicki T, He H, Krueger JG, Guttman-Yassky E. Atopic dermatitis endotypes and implications for targeted therapeutics. J Allergy Clin Immunol. (2019) 143:1–11. doi: 10.1016/j.jaci.2018.10.032
95. Oetjen LK, Mack MR, Feng J, Whelan TM, Niu H, Guo CJ, et al. Sensory neurons co-opt classical immune signaling pathways to mediate chronic itch. Cell. (2017) 171:217–28. doi: 10.1016/j.cell.2017.08.006
96. Wollenberg A, Ariens L, Thurau S, van Luijk C, Seegraber M, de Bruin- Weller M. Conjunctivitis occurring in atopic dermatitis patients treated with dupilumab-clinical characteristics and treatment. J Allergy Clin Immunol Pract. (2018) 6:1778.e–80.e. doi: 10.1016/j.jaip.2018.01.034
97. Junttila IS. Tuning the cytokine responses: an update on interleukin (IL)-4 and IL-13 receptor complexes. Front Immunol. (2018) 9:888. doi: 10.3389/fimmu.2018.00888
98. Simpson Eric L, Bieber T, Guttman-Yassky E, Beck LA, Blauvelt A, Cork MJ, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. (2016) 375:2335–48. doi: 10.1056/NEJMoa1610020
99. Blauvelt A, de Bruin-Weller M, Gooderham M, Cather JC, Weisman J, Pariser D, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet. (2017) 389:2287–303. doi: 10.1016/S0140-6736(17)31191-1
100. de Bruin-Weller M, Thaci D, Smith C, Reich K, Cork MJ, Radin A, et al. Dupilumab with concomitant topical corticosteroid treatment in adults with atopic dermatitis with an inadequate response or intolerance to ciclosporin A or when this treatment is medically inadvisable: a placebo-controlled, randomized phase III clinical trial (LIBERTY AD CAFE). Br J Dermatol. (2018) 178:1083–101. doi: 10.1111/bjd.16156
101. Simpson E, Paller AS, Siegfried EC, Boguniewicz M, Sher L, Gooderham MJ, et al. Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis: a phase 3 randomized clinical trial. JAMA Dermatol. (2020) 156:44–56. doi: 10.1001/jamadermatol.2019.3336
102. Paller AS, Bansal A, Simpson EL, Boguniewicz M, Blauvelt A, Siegfried EC, et al. Clinically meaningful responses to dupilumab in adolescents with uncontrolled moderate-to-severe atopic dermatitis: post-hoc analyses from a randomized clinical trial. Am J Clin Dermatol. (2020) 21:119–31. doi: 10.1007/s40257-019-00478-y.of
103. Paller AS, Siegfried EC, Thaçi D, Wollenberg A, Cork MJ, Arkwright PD, et al. Efficacy and safety of dupilumab with concomitant topical corticosteroids in children 6 to 11 years old with severe atopic dermatitis: a randomized, double-blinded, placebo-controlled phase 3 trial. J Am Acad Dermatol. (2020) 83:1282–93. doi: 10.1016/j.jaad.2020.06.054
104. Paller AS, Siegfried EC, Simpson EL, Cork MJ, Lockshin B, Kosloski MP, et al. A phase 2, open-label study of single-dose dupilumab in children aged 6 months to <6 years with severe uncontrolled atopic dermatitis: pharmacokinetics, safety and efficacy. J Eur Acad Dermatol Venereol. (2021) 35:464–75. doi: 10.1111/jdv.16928
105. Paller AS, Simpson EL, Siegfried EC, Cork MJ, Wollenberg A, Arkwright PD, et al. Dupilumab in children aged 6 months to younger than 6 years with uncontrolled atopic dermatitis: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. (2022) 400:908–19. doi: 10.1016/S0140-6736(22)01539-2
106. Paller AS, Siegfried EC, Cork MJ, Wollenberg A, Arkwright PD, Gonzalez ME, et al. Laboratory safety from a randomized 16-week phase III study of dupilumab in children aged 6 months to 5 years with moderate-to-severe atopic dermatitis. Paediatr Drugs. (2023) 25:67–77. doi: 10.1007/s40272-022-00553-8
107. Thaçi D, Simpson EL, Beck LA, Bieber T, Blauvelt A, Papp K, et al. Efficacy and safety of dupilumab in adults with moderate-to-severe atopic dermatitis inadequately controlled by topical treatments: a randomised, placebo-controlled, dose-ranging phase 2b trial. Lancet. (2016) 387:40–52. doi: 10.1016/S0140-6736(15)00388-8
108. Bansal A, Simpson EL, Paller AS, Siegfried EC, Blauvelt A, de Bruin-Weller M, et al. Conjunctivitis in dupilumab clinical trials for adolescents with atopic dermatitis or Asthma. Am J Clin Dermatol. (2021) 22:101–15. doi: 10.1007/s40257-020-00577-1
109. Akinlade B, Guttman-Yassky E, de Bruin-Weller M, Simpson EL, Blauvelt A, Cork MJ, et al. Conjunctivitis in dupilumab clinical trials. Br J Dermatol. (2019) 181:459–73. doi: 10.1111/bjd.17869
110. Tripp CS, Cuff C, Campbell AL, Hendrickson BA, Voss J, Melim T, et al. RPC4046, a novel anti-interleukin-13 antibody, blocks IL-13 binding to IL-13 a1 and a2 receptors: a randomized, double-blind, placebo-controlled, dose escalation first-in-human study. Adv Ther. (2017) 34:1364-1381. doi: 10.1007/s12325-017-0525-8
111. Simpson EL, Flohr C, Eichenfield LF, Bieber T, Sofen H, Taïeb A, et al. Efficacy and safety of lebrikizumab (an anti-IL-13 monoclonal antibody) in adults with moderate-to-severe atopic dermatitis inadequately controlled by topical corticosteroids: a randomized, placebo-controlled phase II trial (TRE-BLE). J Am Acad Dermatol. (2018) 78:863.e–71.e. doi: 10.1016/j.jaad.2018.01.017
112. Silverberg JI, Toth D, Bieber T, Alexis AF, Elewski BE, Pink AE, et al. Tralokinumab plus topical corticosteroids for the treatment of moderate-to-severe atopic dermatitis: results from the double-blind, randomized, multicentre, placebo-controlled phase III ECZTRA 3 trial. Br J Dermatol. (2021) 184:450–63. doi: 10.1111/bjd.19573
113. Wollenberg A, Blauvelt A, Guttman-Yassky E, Worm M, Lynde C, Lacour J, et al. Tralokinumab for moderate-to-severe atopic dermatitis: results from two 52-week, randomized, double-blind, multicentre, placebo-controlled phase III trials (ECZTRA 1 and ECZTRA 2). Br J Dermatol. (2021) 184:437–49. doi: 10.1111/bjd.19574
114. Wollenberg A, Howell M, Guttman-Yassky E, Silverberg J, Kell C, Ranade K, et al. Treatment of atopic dermatitis with tralokinumab, an anti–IL-13 mAb. J Allergy Clin Immunol. (2019) 143:135-141. doi: 10.1016/j.jaci.2018.05.029
115. Paller A, Blauvelt A, Soong W, Imafuku S, Hong C, Schuttelaar M, et al. Efficacy and safety of tralokinumab in adolescents with moderate-to-severe atopic dermatitis: results of the phase 3 ECZTRA 6 trial. Skin. (2022) 6:s29. doi: 10.25251/skin.6.supp.s29
116. Paller A, Silverberg JB, Hong H, Cork M, Puig L, Arlert P, et al. The impact of tralokinumab on quality of life and school in patients aged 12–17 with atopic dermatitis: results from the phase 3 ECZTRA 6 trial. Skin J Cutaneous Med. (2023) 7:s139.
117. Wollenberg A, Beck LA, de Bruin Weller M, Simpson EL, Imafuku S, Boguniewicz M, et al. Conjunctivitis in adult patients with moderate-to-severe atopic dermatitis: results from five tralokinumab clinical trials. Br J Dermatol. (2022) 186:453–65. doi: 10.1111/bjd.20810
118. Hansen PM, Tollenaere MAX, Hedengran A, Heegaard S, Amoudruz P, Røpke M, et al. IL-4 and IL-13 both contribute to the homeostasis of human conjunctival goblet cells in vitro. Allergy. (2022) 77:2555–8. doi: 10.1111/all.15326
119. Guttman-Yassky E, Blauvelt A, Eichenfield LF, Paller AS, Armstrong AW, Drew J, et al. Efficacy and safety of lebrikizumab, a high-affinity interleukin 13 inhibitor, in adults with moderate to severe atopic dermatitis: a phase 2b randomized clinical trial. JAMA Dermatol. (2020) 156:411–20. doi: 10.1001/jamadermatol.2020.0079
120. Silverberg JI, Guttman-Yassky E, Thaçi D, Irvine AD, Gold LS, Blauvelt A, et al. Two phase 3 trials of lebrikizumab for moderate-to-severe atopic dermatitis. N Engl J Med. (2023) 388:1080–91. doi: 10.1056/NEJMoa2206714
121. Blauvelt A, Thyssen JP, Guttman-Yassky E, Bieber T, Serra-Baldrich E, Simpson E, et al. Efficacy and safety of lebrikizumab in moderate-to-severe atopic dermatitis: 52-week results of two randomized double-blinded placebo-controlled phase III trials. Br J Dermatol. (2023) 30:ljad022. doi: 10.1093/bjd/ljad022
122. Simpson EL, Gooderham M, Wollenberg A, Weidinger S, Armstrong A, Soung J, et al. Efficacy and safety of lebrikizumab in combination with topical corticosteroids in adolescents and adults with moderate-to-severe atopic dermatitis: a randomized clinical trial (ADhere). JAMA Dermatol. (2023) 159:182–91. doi: 10.1001/jamadermatol.2022.5534
123. Wilson SR, Thé L, Batia LM, Beattie K, Katibah GE, McClain SP, et al. The epithelial cell-derived atopic dermatitis cytokine TSLP activates neurons to induce itch. Cell. (2013) 155:285–95. doi: 10.1016/j.cell.2013.08.057
124. Sonkoly E, Muller A, Lauerma AI, Pivarcsi A, Soto H, Kemeny L, et al. IL-31: a new link between T cells and pruritus in atopic skin inflammation. J Allergy Clin Immunol. (2006) 117:411–7. doi: 10.1016/j.jaci.2005.10.033
125. Miake S, Tsuji G, Takemura M, Hashimoto-Hachiya A, Vu YH, Furue M, et al. IL-4 augments IL-31/IL-31 receptor alpha interaction leading to enhanced Ccl 17 and Ccl 22 production in dendritic cells: implications for atopic dermatitis. Int J Mol Sci. (2019) 20:4053. doi: 10.3390/ijms20164053
126. Ruzicka T, Hanifin JM, Furue M, Pulka G, Mlynarczyk I, Wollenberg A, et al. Anti–interleukin-31 receptor A antibody for atopic dermatitis. N Engl J Med. (2017) 376:826–35. doi: 10.1056/NEJMoa1606490
127. Silverberg JI, Pinter A, Pulka G, Poulin Y, Bouaziz JD, Wollenberg A, et al. Phase 2B randomized study of nemolizumab in adults with moderate-to-severe atopic dermatitis and severe pruritus. J Allergy Clin Immunol. (2020) 145:173–82. doi: 10.1016/j.jaci.2019.08.013
128. Kabashima K, Furue M, Hanifin JM, Pulka G, Wollenberg A, Galus R, et al. Nemolizumab in patients with moderate-to-severe atopic dermatitis: randomized, phase II, long-term extension study. J Allergy Clin Immunol. (2018) 142:1121.e–30.e. doi: 10.1016/j.jaci.2018.03.018
129. Silverberg JI, Pinter A, Alavi A, Lynde C, Bouaziz JD, Wollenberg A. Nemolizumab is associated with a rapid improvement in atopic dermatitis signs and symptoms: subpopulation (EASI ≥ 16) analysis of randomized phase 2B study. J Eur Acad Dermatol Venereol. (2021) 35:1562–8. doi: 10.1111/jdv.17218
130. Wang SH, Zuo YG. Thymic stromal lymphopoietin in cutaneous immune-mediated diseases. Front. Immunol. (2021) 12:698522. doi: 10.3389/fimmu.2021.698522
131. Leung DY. Clinical implications of new mechanistic insights into atopic dermatitis. Curr Opin Pediatr. (2016) 28:456–62. doi: 10.1097/MOP.0000000000000374
132. Corren J, Ambrose CS, Griffiths JM, Hellqvist Å, Lindsley AW, Llanos JP, et al. Efficacy of tezepelumab in patients with evidence of severe allergic asthma: results from the phase 3 NAVIGATOR study. Clin Exp Allergy. (2023) 53:417–28. doi: 10.1111/cea.14256
133. Simpson EL, Parnes JR, She D, Crouch S, Rees W, Mo M, et al. Tezepelumab, an anti-thymic stromal lymphopoietin monoclonal antibody, in the treatment of moderate to severe atopic dermatitis: a randomized phase 2a clinical trial. J Am Acad Dermatol. (2019) 80:1013–21. doi: 10.1016/j.jaad.2018.11.059
134. Brunner PM, Pavel AB, Khattri S, Leonard A, Malik K, Rose S, et al. Baseline IL-22 expression in patients with atopic dermatitis stratifies tissue responses to fezakinumab. J Allergy Clin Immunol. (2019) 143:142–54. doi: 10.1016/j.jaci.2018.07.028
135. Guttman-Yassky E, Brunner PM, Neumann AU, Khattri S, Pavel AB, Malik K, et al. Efficacy and safety of fezakinumab (an IL-22 monoclonal antibody) in adults with moderate-to-severe atopic dermatitis inadequately controlled by conventional treatments: a randomized, double-blind, phase 2a trial. J Am Acad Dermatol. (2018) 78:872.e–81.e. doi: 10.1016/j.jaad.2018.01.016
136. Uppal SK, Kearns DG, Chat VS, Han G, Wu JJ. Review and analysis of biologic therapies currently in phase II and phase III clinical trials for atopic dermatitis. J Dermatolog Treat. (2022) 33:626–36. doi: 10.1080/09546634.2020.1775775
137. Maxwell JR, Yadav R, Rossi RJ, Ruby CE, Weinberg AD, Aguila HL, et al. IL-18 bridges innate and adaptive immunity through IFN-gamma and the CD134 pathway. J Immunol. (2006) 177:234–45. doi: 10.4049/jimmunol.177.1.234
138. Guttman-Yassky E, Pavel AB, Zhou L, Estrada YD, Zhang N, Xu H, et al. GBR 830, an anti-OX40, improves skin gene signatures and clinical scores in patients with atopic dermatitis. J Allergy Clin Immunol. (2019) 144:482–93.e7. doi: 10.1016/j.jaci.2018.11.053
139. Nakagawa H, Iizuka H, Nemoto O, Shimabe M, Furukawa Y, Kikuta N, et al. Safety, tolerability and efficacy of repeated intravenous infusions of KHK4083, a fully human anti-OX40 monoclonal antibody, in Japanese patients with moderate to severe atopic dermatitis. J Dermatol Sci. (2020) 99:82–9. doi: 10.1016/j.jdermsci.2020.06.005
140. Giannetti A, Cipriani F, Indio V, Gallucci M, Caffarelli C, Ricci G. Influence of atopic dermatitis on cow’s milk allergy in children. Medicina. (2019) 55:460. doi: 10.3390/medicina55080460
141. Chan S, Cornelius V, Cro S, Harper JI, Lack G. Treatment effect of Omalizumab on severe pediatric atopic dermatitis: the ADAPT randomized clinical trial. JAMA Pediatr. (2019) 174:29–37. doi: 10.1001/jamapediatrics.2019.4476
142. Kang EG, Narayana PK, Pouliquen IJ, Lopez MC, Ferreira-Cornwell MC, Getsy JA. Efficacy and safety of mepolizumab administered subcutaneously for moderate to severe atopic dermatitis. Allergy. (2020) 75:950–3. doi: 10.1111/all.14050
143. Saeki H, Kabashima K, Tokura Y, Murata Y, Shiraishi A, Tamamura R, et al. Efficacy and safety of ustekinumab in Japanese patients with severe atopic dermatitis: a randomized, double-blind, placebo-controlled, phase II study. Br J Dermatol. (2017) 177:419–27. doi: 10.1111/bjd.15493
144. Khattri S, Brunner PM, Garcet S, Finney R, Cohen SR, Oliva M, et al. Efficacy and safety of ustekinumab treatment in adults with moderate-to-severe atopic dermatitis. Exp Dermatol. (2017) 26:28–35. doi: 10.1111/exd.13112
145. Ungar B, Pavel AB, Li R, Kimmel G, Nia J, Hashim P, et al. Phase 2 randomized, double-blind study of IL-17 targeting with secukinumab in atopic dermatitis. J Allergy Clin Immunol. (2021) 147:394–7. doi: 10.1016/j.jaci.2020.04.055
146. Wechsler ME, Ruddy MK, Pavord ID, Israel E, Rabe KF, Ford LB, et al. Efficacy and safety of itepekimab in patients with moderate-to-severe asthma. N Engl J Med. (2021) 385:1656–68. doi: 10.1056/NEJMoa2024257
147. Wang HH, Li YC, Huang YC. Efficacy of omalizumab in patients with atopic dermatitis: a systematic review and meta-analysis. J Allergy Clin Immunol. (2016) 138:1719.e–22.e. doi: 10.1016/j.jaci.2016.05.038
148. Zebda R, Paller AS. Phosphodiesterase 4 inhibitors. J Am Acad Dermatol. (2018) 78:S43–52. doi: 10.1016/j.jaad.2017.11.056
149. Luger TA, Hebert AA, Zaenglein AL, Silverberg JI, Tan H, Ports WC, et al. Subgroup analysis of crisaborole for mild-to-moderate atopic dermatitis in children aged 2 to < 18 years. Paediatr Drugs. (2022) 24:175–83. doi: 10.1007/s40272-021-00490-y
150. Paller AS, Tom WL, Lebwohl MG, Blumenthal RL, Boguniewicz M, Call RS, et al. Efficacy and safety of crisaborole ointment, a novel, nonsteroidal phosphodiesterase 4 (PDE4) inhibitor for the topical treatment of atopic dermatitis (AD) in children and adults. J Am Acad Dermatol. (2016) 75:494.–503. doi: 10.1016/j.jaad.2016.05.046
151. Eichenfield LF, Call RS, Forsha DW, Fowler J Jr, Hebert AA, Spellman M, et al. Long-term safety of crisaborole ointment 2% in children and adults with mild to moderate atopic dermatitis. J Am Acad Dermatol. (2017) 77:641–9.e5. doi: 10.1016/j.jaad.2017.06.010
152. Eichenfield LF, Yosipovitch G, Stein Gold LF, Kalabis M, Zang C, Vlahos B, et al. Improvement in disease severity and pruritus outcomes with crisaborole ointment, 2%, by baseline atopic dermatitis severity in children and adolescents with mild-to-moderate atopic dermatitis. Pediatr Dermatol. (2020) 37:1030–7. doi: 10.1111/pde.14328
153. Fowler J, Sugarman J, Sher L, Zang C, Werth J, Myers DE, et al. Impact of crisaborole on sleep outcomes in pediatric patients with mild-to-moderate atopic dermatitis. Dermatol Ther. (2023) 13:951–60. doi: 10.1007/s13555-023-00899-y
154. Schlessinger J, Shepard JS, Gower R, Su JC, Lynde C, Cha A, et al. Safety, effectiveness, and pharmacokinetics of crisaborole in infants aged 3 to < 24 months with mild-to-moderate atopic dermatitis: a phase iv open-label study (CrisADe CARE 1). Am J Clin Dermatol. (2020) 21:275–84. doi: 10.1007/s40257-020-00510-6
155. Saeki H, Ito K, Yokota D, Tsubouchi H. Difamilast ointment in adult patients with atopic dermatitis: a phase 3 randomized, double-blind, vehicle-controlledtrial. J Am Acad Dermatol. (2022) 86:607–14. doi: 10.1016/j.jaad.2021.10.027
156. Saeki H, Baba N, Ito K, Yokota D, Tsubouchi H. Difamilast, a selective phosphodiesterase 4 inhibitor, ointment in paediatric patients with atopic dermatitis: a phase III randomized double-blind, vehicle-controlled trial. Br J Dermatol. (2022) 186:40–9. doi: 10.1111/bjd.20655
157. Gooderham M, Kircik L, Zirwas M, Lee M, Kempers S, Draelos Z, et al. The safety and efficacy of roflumilast cream 0.15% and 0.05% in patients with atopic dermatitis: randomized, double-blind, phase 2 proof of concept study. J Drugs Dermatol. (2023) 22:139–47. doi: 10.36849/JDD.7295
158. Hu X, Li J, Fu M, Zhao X, Wang W. The JAK/STAT signaling pathway: from bench to clinic. Signal Transduct Target Ther. (2021) 6:402. doi: 10.1038/s41392-021-00791-1
159. Papp K, Szepietowski JC, Kircik L, Toth D, Eichenfield LF, Leung DYM, et al. Efficacy and safety of ruxolitinib cream for the treatment of atopic dermatitis: results from 2 phase 3, randomized, double-blind studies. J Am Acad Dermatol. (2021) 85:863–72. doi: 10.1016/j.jaad.2021.04.085
160. Hoy SM. Ruxolitinib cream 1.5%: a review in mild to moderate atopic dermatitis. Am J Clin Dermatol. (2023) 24:143–51. doi: 10.1007/s40257-022-00748-2
161. Shi JG, Chen X, McGee RF, Landman RR, Emm T, Lo Y, et al. The pharmacokinetics, pharmacodynamics, and safety of orally dosed INCB018424 phosphate in healthy volunteers. J Clin Pharmacol. (2011) 51:1644–54. doi: 10.1177/0091270010389469
162. Leung DYM, Paller AS, Zaenglein AL, Tom WL, Ong PY, Venturanza ME, et al. Safety, pharmacokinetics, and efficacy of ruxolitinib cream in children and adolescents with atopic dermatitis. Ann Allergy Asthma Immunol. (2023) 130:500.e–7.e. doi: 10.1016/j.anai.2022.12.033
163. Flanagan ME, Blumenkopf TA, Brissette WH, Brown MF, Casavant JM, Shang- Poa C, et al. Discovery of CP-690,550: a potent and selective Janus kinase (JAK) inhibitor for the treatment of autoimmune diseases and organ transplant rejection. J Med Chem. (2010) 53:8468e84. doi: 10.1021/jm1004286
164. Bissonnette R, Papp KA, Poulin Y, Gooderham M, Raman M, Mallbris L, et al. Topical tofacitinib for atopic dermatitis: a phase IIa randomized trial. Br J Dermatol. (2016) 175:902e11. doi: 10.1111/bjd.14871
165. Ytterberg SR, Bhatt DL, Mikuls TR, Koch GG, Fleischmann R, Rivas JL. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. New Engl J Med. (2022) 386:316–26. doi: 10.1056/NEJMoa2109927)
166. European Medical agency. Janus kinase inhibitors (JAKi). (2023). Available online at: https://www.ema.europa.eu/en/medicines/human/referrals/janus-kinase-inhibitors-jaki (accessed on Apr 24, 2023).
167. Guttman-Yassky E, Teixeira HD, Simpson EL, Papp KA, Pangan AL, Blauvelt A, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. (2021) 397:2151–68. doi: 10.1016/S0140-6736(21)00588-2
168. Reich K, Teixeira HD, de Bruin-Weller M, Bieber T, Soong W, Kabashima K, et al. Safety and efficacy of upadacitinib in combination with topical corticosteroids in adolescents and adults with moderate-to-severe atopic dermatitis (AD Up): results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. (2021) 397:2169–81. doi: 10.1016/S0140-6736(21)00589-4
169. Blauvelt A, Teixeira HD, Simpson EL, Costanzo A, De Bruin-Weller M, Barbarot S, et al. Efficacy and safety of upadacitinib vs dupilumab in adults with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. (2021) 157:1047–55. doi: 10.1001/jamadermatol.2021.3023
170. Silverberg JI, Simpson EL, Thyssen JP, Gooderham M, Chan G, Feeney C, et al. Efficacy and safety of abrocitinib in patients with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. (2020) 156:863–73. doi: 10.1001/jamadermatol.2020.1406
171. Simpson EL, Sinclair R, Forman S, Wollenberg A, Aschoff R, Cork M, et al. Efficacy and safety of abrocitinib in adults and adolescents with moderate-to-severe atopic dermatitis (JADE MONO-1): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet. (2020) 396:255–66. doi: 10.1016/S0140-6736(20)30732-7
172. Blauvelt A, Silverberg Ji, Lynde CW, Bieber T, Eisman S, Zdybski J, et al. Abrocitinib induction, randomized withdrawal, and retreatment in patients with moderate-to-severe atopic dermatitis: results from the JAK1 Atopic Dermatitis Efficacy and Safety (JADE) REGIMEN phase 3 trial. J Am Acad Dermatol. (2022) 86:104–12. doi: 10.1016/j.jaad.2021.05.075
173. Eichenfield LF, Flohr C, Sidbury R, Siegfried E, Szalai Z, Galus R, et al. Efficacy and safety of abrocitinib in combination with topical therapy in adolescents with moderate-to-severe atopic dermatitis: the JADE TEEN randomized clinical trial. JAMA Dermatol. (2021) 157:1165–73. doi: 10.1001/jamadermatol.2021.2830
174. Bieber T, Simpson EL, Silverberg Ji, Thaçi D, Paul C, Pink AE, et al. Abrocitinib versus Placebo or Dupilumab for atopic dermatitis. N Engl J Med. (2021) 384:1101–12. doi: 10.1056/NEJMoa2019380
175. Alexis A, de Bruin-Weller M, Weidinger S, Soong W, Barbarot S, Ionita I, et al. Rapidity of improvement in signs/symptoms of moderate-to-severe atopic dermatitis by body region with abrocitinib in the phase 3 JADE COMPARE Study. Dermatol Ther. (2022) 12:771–85. doi: 10.1007/s13555-022-00694-1
176. Reich K, Thyssen JP, Blauvelt A, Eyerich K, Soong W, Rice ZP, et al. Efficacy and safety of abrocitinib versus dupilumab in adults with moderate-to-severe atopic dermatitis: a randomised, double-blind, multicentre phase 3 trial. Lancet. (2022) 400:273–82. doi: 10.1016/S0140-6736(22)01199-0
177. Hoy SM. Baricitinib: a review in moderate to severe atopic dermatitis. Am J Clin Dermatol. (2022) 23:409–20. doi: 10.1007/s40257-022-00684-1
178. Reich K, Kabashima K, Peris K, Silverberg JI, Eichenfield LF, Bieber T. Efficacy and safety of Baricitinib combined with topical corticosteroids for treatment of moderate to severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. (2020) 156:1333–43. doi: 10.1001/jamadermatol.2020.3260
179. Bissonnette R, Saint-Cyr Proulx E, Jack C, Maari C. Tapinarof for psoriasis and atopic dermatitis: 15 years of clinical research. J Eur Acad Dermatol Venereol. (2023) 37:1168–74. doi: 10.1111/jdv.18925
180. Bissonnette R, Chen G, Bolduc C, Maari C, Lyle M, Tang L, et al. Efficacy and safety of topical WBI-1001 in the treatment of atopic dermatitis: results from a phase 2A, randomized, placebo-controlled clinical trial. Arch Dermatol. (2010) 146:446–9. doi: 10.1001/archdermatol.2010.34
181. Peppers J, Paller AS, Maeda-Chubachi T, Wu S, Robbins K, Gallagher K, et al. A phase 2, randomized dose-finding study of tapinarof (GSK2894512 cream) for the treatment of atopic dermatitis. J Am Acad Dermatol. (2019) 80:89–98.e3. doi: 10.1016/j.jaad.2018.06.047
Keywords: atopic dermatitis, biologics, small molecules, dupilumab, tralokinumab, lebrikizumab, Janus Kinase, children
Citation: Caffarelli C, Giannetti A, Giannì G and Ricci G (2023) Anti-inflammatory and biologic drugs for atopic dermatitis: a therapeutic approach in children and adolescents. Front. Med. 10:1214963. doi: 10.3389/fmed.2023.1214963
Received: 30 April 2023; Accepted: 27 July 2023;
Published: 16 August 2023.
Edited by:
Salvatore Leonardi, University of Catania, ItalyReviewed by:
Piotr K. Krajewski, Wrocław Medical University, PolandGiuseppe Fabio Parisi, University of Catania, Italy
Copyright © 2023 Caffarelli, Giannetti, Giannì and Ricci. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Carlo Caffarelli, Y2FybG8uY2FmZmFyZWxsaUB1bmlwci5pdA==
 Carlo Caffarelli
Carlo Caffarelli Arianna Giannetti2
Arianna Giannetti2 Giuliana Giannì
Giuliana Giannì Giampaolo Ricci
Giampaolo Ricci