- 1Department of General Surgery, The Third People’s Hospital of Dalian, Dalian Medical University, Dalian, China
- 2Department of Central Laboratory, The Third People’s Hospital of Dalian, Dalian Medical University, Dalian, China
- 3Liaoning Province Key Laboratory of Corneal and Ocular Surface Diseases Research, The Third People’s Hospital of Dalian, Dalian Medical University, Dalian, China
Functional bowel disorder (FBD) is a common gastrointestinal disease syndrome characterized by dysmotility and secretion without known organic lesions. The pathogenesis of FBD is still unclear. In recent years, with the rise of neurogastroenterology, it has initially revealed its close relationship with the “brain-gut axis.” Transcranial magnetic stimulation (TMS) is a technique for detecting and treating the nervous system, that is characterized by non-invasiveness and painlessness. TMS plays an important role in the diagnosis and treatment of diseases, and provides a new method for the treatment of FBD. In this paper, we summarized and analyzed the research progress of using TMS therapy applied to patients with irritable bowel syndrome and functional constipation by domestic and foreign scholars in recent years by means of literature search, and found that TMS therapy could improve the intestinal discomfort and accompanying mental symptoms in patients with FBD.
1. Introduction
Functional bowel disorder (FBD) is one of the common diseases of the digestive system, which is characterized by intestinal sensory, secretion, and motor dysfunction, and is a group of intestinal diseases with recurrent episodes without organic lesions (1). The pathogenesis of FBD is not fully understood and is thought to be the result of complex interactions between physiology, psychology, social influences, genetic susceptibility, and early life experiences (2). In recent years, with the development of neurogastroenterology, researchers have conducted in-depth studies of the “brain-gut axis” using brain imaging techniques and have confirmed the influence of central brain activity on the pathophysiological changes in FBD (3). The activity of the cerebral cortex affects the motility of the gastrointestinal tract through afferent and efferent pathways and is regulated by nerves. As a non-invasive, painless, safe, and reliable technique, TMS has achieved remarkable clinical results, and has now become one of the hot spots in the treatment of FBD (4). This article reviews the classification of FBD, brain imaging of FBD under different stimulation conditions, and the effect of TMS on FBD.
2. Functional bowel disease and classification
FBD is one of the most common diseases in the clinical practice of gastroenterology, accounting for more than 40% of the patients visiting the gastroenterology department (5). FBD refers to a disease in which symptoms such as bloating, abdominal pain, diarrhea, and constipation appear in the digestive system, but without organic lesions. In the 2016 release of Rome IV: Functional Gastrointestinal Disorders, FBD includes six disorders: irritable bowel syndrome (IBS), functional constipation, functional diarrhea, bloating/abdominal distention, nonspecific functional enteropathy, and opioid-induced constipation (OIC) (6, 7). Rome IV revised the diagnosis of the first five diseases, with opioid-induced constipation as a new disease. From the diagnostic criteria established by the Rome Commission, we can see that the clinical manifestations of the various subtypes of functional enteropathy are a combination of one or more symptoms, i.e., the symptoms of each type can be converted to each other and there are obvious repetitions (8). In view of this, the experts of the Rome IV Committee proposed a conceptual framework for FBD, namely that FBD has the same pathophysiological mechanisms and that different diseases can be seen as a continuous spectrum of diseases with varying frequency, intensity, and severity of symptoms (9).
3. “Brain-gut axis” and brain imaging of functional bowel disease
With the publication of the Rome IV, the “brain-gut axis” is considered to be the basis of the pathophysiological changes in FBD, and there is increasing evidence to support that the evolution of functional enteropathy occurs in three stages: stage 1—gastrointestinal motility disorder; stage 2—visceral hypersensitivity; and stage 3—bidirectional brain-gut axis dysfunction (3, 10, 11). The brain-gut axis is a bidirectional regulatory axis linking the brain to the gastrointestinal tract through a neuro-immune-endocrine network (12). The central, autonomic, and enteric nervous systems are the main pathways of the brain-gut axis (13). In this model perspective, emotions, thoughts, and perceptions affect gastrointestinal sensation, secretion, motility, immune regulation, mucosal inflammation, and permeability (14). Conversely, altered or disturbed gastrointestinal function can also affect conscious perception and behavior in the brain (2). This bidirectional signal can lead to dysregulation of the autonomic nervous system, which may play an important role in the pathophysiology of irritable bowel syndrome (15). However, it is not feasible to directly assess brain-gut axis function in humans. Therefore, researchers are currently focusing on brain stimulation and brain imaging techniques to better understand the cortical functions associated with intestinal function and functional dyspepsia (16). Recent advances in functional brain imaging techniques and their availability as research tools in clinical studies have facilitated the study of FBD, especially regarding the role of the central nervous system and the brain-gut axis in their pathophysiology. There is new evidence that the central nervous system in patients with IBS may process noxious visceral stimuli abnormally. Changes in brain structure and function are observed on magnetic resonance imaging both before and after repetitive transcranial magnetic stimulation (rTMS) (17). Although the results varied, they all involved the anterior cingulate cortex and prefrontal cortical areas (18). Increased regional cerebral blood flow in the anterior cingulate cortex and insular cortex activates the hippocampus, thalamus, and hypothalamus in response to visceral stimulation (19). The frequency and pattern of rTMS also change the resting state activity of neurons at the stimulation site and adjacent sites, and the magnitude and pattern of changes in neuronal activity depend on the frequency and pattern of rTMS (20). It follows that the use of brain stimulation and brain imaging can provide a better understanding of the relationship between gut motility and the cerebral cortex. By looking at the results presented by brain imaging and then using brain stimulation to modulate the movement of the gut, the researchers have provided a theoretical basis for TMS to treat FBD.
4. Transcranial magnetic stimulation and its effect on functional bowel disease
4.1. TMS in the medical field
The stimulation methods of brain neurons are mainly divided into electrical stimulation and magnetic stimulation (21). The electrical stimulation of brain neurons acts on the cerebral cortex with a weak polarized direct current and affects the membrane potential of the nerve cells under the electrode site by the action of an electric field, causing depolarization or hyperpolarization of the resting membrane potential. The altered polarization of the membrane is the main mechanism for the different effects following electrical stimulation. However, the operation of direct current stimulation is cumbersome and traumatic, which will cause obvious discomfort to the patient. The existence of the skull will lead to a large attenuation of the stimulation current. Therefore, it is relatively difficult to use direct current stimulation to stimulate deep tissues. Compared to electrical stimulation, TMS is a non-invasive brain stimulation technique that generates a short, rapidly changing magnetic field to induce an electric current in the brain, which has a strong penetrating ability in the brain and fundamentally solves the problem that electrical stimulation cannot access deep stimulation (22). In general, single-pulse and double-pulse TMS are used to explore brain function, while rTMS is used to induce changes in brain activity that can persist beyond the stimulation period (23). The excitability of the stimulated area, as well as the intensity and frequency of the stimulation, determines the changes in brain activity.
Functional magnetic resonance imaging (fMRI) studies have shown that the effects of rTMS induce changes in distal brain regions, with low frequency (<1 HZ) rTMS producing inhibitory effects and high frequency (>5 HZ) rTMS producing excitatory effects (24). The persistent effects after rTMS are not only manifested in motor cortical areas but also in occipital, prefrontal, parietal, and cerebellar sites, a phenomenon that makes it possible to apply rTMS to modulate excitability and inhibition in the corresponding cortical areas (25). Although TMS has only been in development for a few decades, it has demonstrated great therapeutic potential in several clinical areas. It has also shown great possibilities in the study of brain function. In terms of diagnostic, TMS is less costly and more reliable, while in terms of therapeutic, TMS is safer, more convenient, and more acceptable to patients than electrical stimulation. TMS has been widely used in medical treatment for epilepsy (26), Parkinson’s (27), depression (28), neuropathic pain (29), and stroke (30), but there are few studies on TMS applied to FBD, which is also a relatively novel treatment.
TMS has been studied in patients and healthy volunteers around the world for more than 25 years, and the results support the safety and reliability of TMS by evaluating its adverse effects through meta-analysis (31). The most common side effect of TMS is headache or neck pain, which is usually transient and either relieves spontaneously or with over-the-counter pain medication. A rare and serious potential adverse effect of TMS is seizure induction, but fewer than 20 cases of TMS-induced seizures have been reported in recent years, with an induction rate of less than 1 in 1,000. In individuals susceptible to epilepsy, the risk is higher, but the incidence of triggering remains relatively low, at about 1%–2% (32). In general, the risk of serious adverse reactions is low as long as safety guidelines and recommendations are followed. Rosa summarized and analyzed thousands of cases treated with rTMS, only 6 cases had transient seizures. The adverse effects are usually closely related to the extent of the patient’s condition, the concomitant underlying disease, tolerance, and frequency of stimulation. The side effects are minimal and not statistically significant compared to the clinical outcome, so rTMS is considered a safe and non-invasive technique (33).
4.2. The therapeutic effect of TMS on functional bowel disease
Although the Rome Committee classified FBD into 6 different subtypes, the clinical manifestations of different classifications have similar and repetitive phenomena, and may have the same pathophysiology (5). Constipation, abdominal pain, diarrhea, abdominal discomfort, and anxiety, depression, and mental distress caused by recurrent episodes of FBD are the main symptoms that plague patients (34). TMS has only been used in the treatment of FBD for just over a decade but has yielded exciting results for patients with FBD (22). Because research on TMS applied to FBD is just beginning, researchers at home and abroad have mainly investigated the improvement effect of TMS on constipation and IBS. In the current study, researchers found that TMS significantly improved bowel movements in patients with functional constipation and also significantly improved anxiety and depression associated with the chronic condition (35). The main clinical manifestation of IBS patients is abdominal pain, which is also significantly improved after TMS treatment (36).
4.2.1. TMS improves functional constipation
Functional constipation is one of the common types of FBD. Recurrent episodes of persistent constipation have a huge impact on the physical and mental health of patients, and over time, most patients experience anxiety and depression. Chronic anxiety and depression can affect the hypothalamus, plant nerves and inhibit peripheral autonomic nerves, triggering aggravation of constipation and thus increasing the burden of treatment. TMS therapy not only improves the patient’s constipation symptoms but also significantly reduces the patient’s mental state, which is beneficial to the recovery of the disease (37). Patients with functional constipation using TMS of motor evoked potentials (MEP) showed a significant decrease in MEP wave amplitude (reduced number of firing neurons) and a general increase in latency (slowed nerve conduction velocity). Impaired conduction of the cortico-lumbosacral-anorectal nerve pathway in patients with functional constipation may be responsible for the significant reduction in MEP wave amplitude. Scholars have applied fMRI and PET studies to reveal that the occurrence of these disorders is associated with abnormalities in the central cortical or subcortical pathways of the brain. TMS motor evoked potential (TMS-MEP) assessed the status of the cortico-spinal-anorectal motor nerve efferent pathway, providing information on the efferent pathway of the brain-gut axis (38).
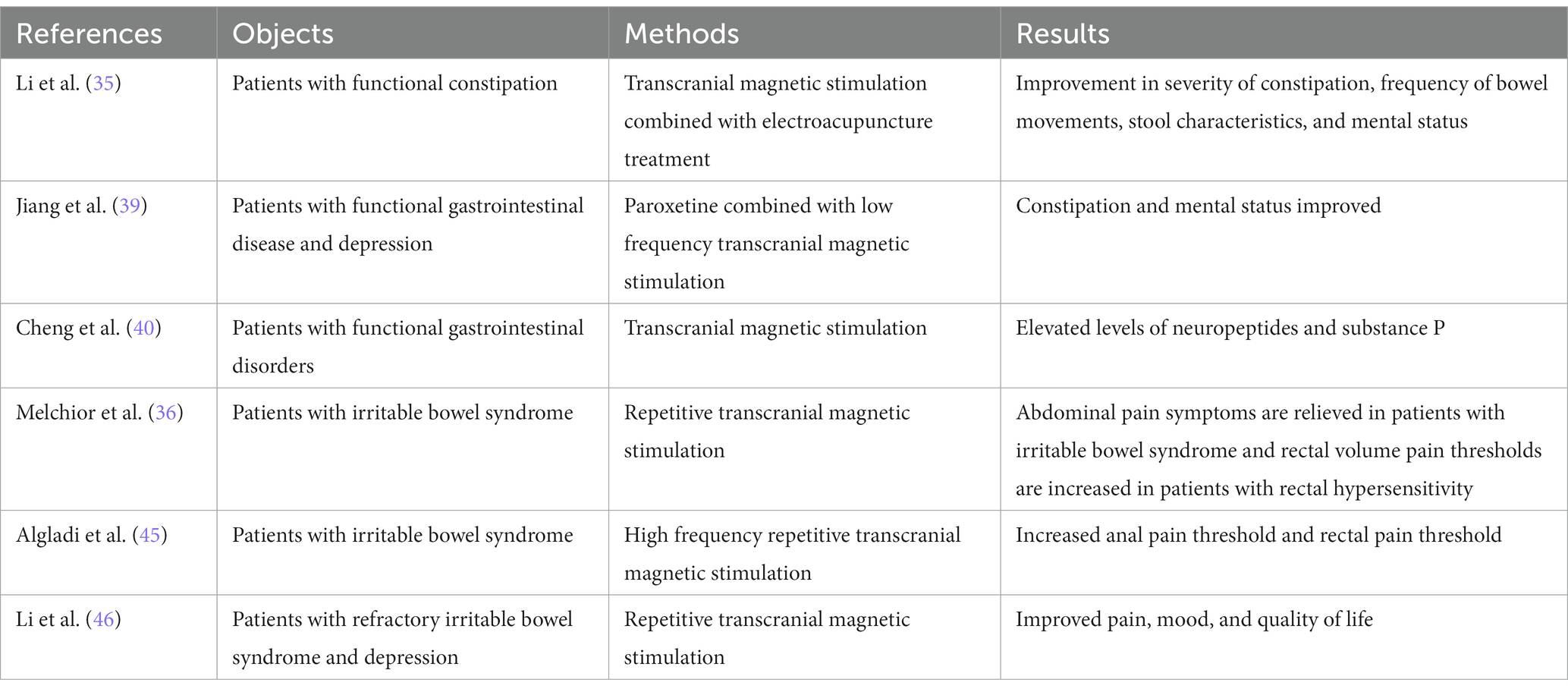
In the clinical efficacy observation of patients with functional constipation treated with TMS combined with electroacupuncture, Li et al. (35) found that after 4 weeks of TMS treatment, the severity (CAS score decreased from 7.31 ± 2.66 to 4.08 ± 1.04), frequency (SBM number increased from 2.62 ± 0.65 to 5.15 ± 1.14, CSBM number increased from 1.31 ± 0.85 to 3.31 ± 1.44), stool character (BSS score increased from 2.92 ± 1.26 to 4.08 ± 0.76) and mental status (SDS score decreased from 58.62 ± 8.53 to 42.77 ± 4.15 and SAS score decreased from 56.46 ± 4.88 to 37.08 ± 6.50) were improved. After using paroxetine combined with low-frequency TMS to treat depression with functional gastrointestinal disease for 4 weeks, Jiang et al. found that the Hamilton depression scale (HAMD) score in the control group decreased from 21.7 ± 4.0 to 15.3 ± 4.1 in 34 cases, the Hamilton anxiety scale (HAMA) score decreased from 17.5 ± 3.8 to 12.9 ± 4.4, the constipation history impression scale (CGI-S) score decreased from 3.7 ± 0.8 to 2.9 ± 0.6. Only a small amount of dizziness and headache or blood pressure fluctuations (±≥10 mmHg) occurred in both groups during treatment, and the incidence of side effects was not statistically significant (39). The use of TMS in patients with functional gastrointestinal disorders may facilitate the recovery of the patient’s disease and mental status. Cheng et al. (40) used TMS to treat patients with functional gastrointestinal diseases for 4 weeks and found that the levels of neuropeptides (94.82 ± 18.53 to 118.53 ± 22.29) and substance P (35.16 ± 8.42 to 47.65 ± 11.06) in the treatment group were significantly improved.
TMS treatment effectively improves gastrointestinal motility, mediates emotions, and relieves symptoms by regulating the brain-gut axis, providing a safe, non-invasive, and effective treatment for the clinical treatment of functional gastrointestinal diseases. Long-term constipation will have a negative impact on the patient’s mental condition. More than half of the patients with constipation are accompanied by severe anxiety or depression. In the long term, in a state of high tension, anxiety can cause brain and intestinal axis dysfunction, which in turn aggravates the constipation condition, forming a vicious circle. TMS not only regulates the brain-gut axis disorder in patients with functional constipation but also significantly improves the patient’s anxiety and depression, which is more conducive to the treatment of constipation (35).
4.2.2. Effectiveness of TMS for irritable bowel syndrome
The Rome Commission has identified abdominal pain as a necessary condition for the diagnosis of IBS, and abdominal pain is often the main cause of distress in IBS patients (34). Although the pathogenesis of IBS is not cleared, at least half of the patients with IBS have visceral hypersensitivity, which is associated with abnormal processing of visceral injurious signals within the central nervous system. Research confirms that IBS is associated with changes in structural brain activity involved in pain processing (41). The application of repetitive low-frequency TMS increases the rectal pain threshold in patients with IBS, thereby reducing their abdominal pain symptoms. TMS applied to the motor cortex also induces analgesic effects in patients with chronic pain syndrome (42). Several neuroimaging studies have shown that TMS in the motor cortex causes not only local changes in brain activity but also bilateral changes in some distal cortical and subcortical areas, including some involved in pain processing. The analgesic effect of rTMS may be caused mainly by alterations in the central pain modulation system (43).
In Melchior’s experiment, it was found that while rTMS had only a slight improvement in pain threshold (VAS score decreased from 3.8 ± 2.2 to 3.0 ± 2.3), maximum tolerance and rectal compliance in IBS patients compared to healthy controls, in IBS patients with rectal hypersensitivity (pressure pain threshold ≤ for 40 mmHg), rTMS significantly increased rectal volume pain threshold and decreased pain threshold (VAS score decreased from 4.0 ± 2.4 to 3.2 ± 2.2). All patients showed a significant improvement in the sensation of abdominal pain. The failure to achieve the expected results may be related to the small sample size (only 16 patients), the different staging of rTMS, and the fact that most rTMS patients have a history of taking painkillers [painkillers can reduce rectal sensitivity (44)] (36). However, the therapeutic effect of rTMS in patients with rectal hypersensitivity is exciting. Algladi et al. (45) found that high-frequency cortical rTMS could modulate experimentally induced anorectal pain after using TMS in IBS patients. Anal pain thresholds were increased immediately, 30 and 60 min after application of 10 Hz rTMS, but anal sphincter sensation was barely affected. Similarly, increased rectal pain thresholds were found immediately, 30, and 60 min after the use of 10 Hz rTMS, with the difference that rectal sensory thresholds were increased 60 min after the use of 10 Hz rTMS. Somatic effects are enhanced with increasing duration and intensity of rTMS. Activation of the motor cortex may play a role in modulating affective factors of pain, and downward stimulation of the brainstem would block pain transmission. Li et al. (46) found that stimulation of the dorsolateral prefrontal cortex (DLPFC) significantly improved pain, mood, and quality of life in patients with refractory IBS with depression after using rTMS. This may be due to stimulation of the dorsolateral prefrontal cortex resulting in altered metabolism in the distribution area.
IBS is a chronic pain condition, and the use of active pain management and placebo therapy is not effective. Pain-relieving therapy is mostly treated with opioids, which will cause adverse reactions such as constipation over time. The placebo may have a biological analgesic response, causing adverse effects that may be more pronounced than the pain relief. TMS has been preliminarily shown to be effective for pain relief in IBS and may provide a safe, effective, and well-tolerated alternative therapy for IBS patients in the near future instead of conventional drugs (see Table 1).
5. Conclusion and future perspectives
As one of the common diseases of the gastrointestinal tract, the specific pathogenesis of FBD has not been clarified, which brings a heavy burden to patients physically, mentally, and economically. In recent years, with the development of neurogastroenterology, the brain-gut axis has gradually come into view and has attracted the widespread attention of researchers, and more and more studies have shown that the brain-gut axis may be the pathophysiological alteration of functional enteropathy. As a non-invasive, painless, safe, and reliable new technology, TMS can be used both for the detection of brain function and the localization of the cerebral cortex, providing new ideas for people to study the mechanism of disease occurrence and pathophysiology, and as an emerging treatment modality, providing new means for clinical treatment. In just a few decades, TMS has achieved remarkable results in clinical practice. TMS has been widely used in the treatment of psychiatric disorders and pain relief in China and abroad, but few scholars have studied its application to gastrointestinal disorders. Although only a few researchers in China and abroad have used TMS for the treatment of FBD, patients have shown significant improvement after a period of treatment with no associated adverse effects. Future research efforts on TMS for FBD on a larger scale are needed to explore the underlying mechanisms in greater depth. It is believed that with the continuous development of TMS technology and the progress of clinical medical research, TMS will be better, more closely, and more widely integrated with clinical treatment, providing more possibilities for clinical treatment plans.
Author contributions
GL and BJ reviewed the relevant literature and wrote the manuscript. ZF designed the structure and revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (81701965); Natural Science Foundation of Liaoning Province (20180550116 and 2019-MS-069).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kovacic, K. Current concepts in functional gastrointestinal disorders. Curr Opin Pediatr. (2015) 27:619–24. doi: 10.1097/MOP.0000000000000262
2. Carabotti, M, Scirocco, A, Maselli, MA, and Severi, C. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol. (2015) 28:203–9.
3. Black, CJ, Drossman, DA, Talley, NJ, Ruddy, J, and Ford, AC. Functional gastrointestinal disorders: advances in understanding and management. Lancet. (2020) 396:1664–74. doi: 10.1016/S0140-6736(20)32115-2
4. Aizawa, Y, Morishita, J, Kano, M, Kanazawa, M, and Fukudo, S. Modification of rectal function and emotion by repetitive transcranial magnetic stimulation in humans. Neurosci Res. (2021) 168:54. doi: 10.1016/j.neures.2021.05.013
5. Aboubakr, A, and Cohen, MS. Functional bowel disease. Clin Geriatr Med. (2021) 37:119–29. doi: 10.1016/j.cger.2020.08.009
6. Marx, M, Maye, H, Abdelrahman, K, Hessler, R, Moschouri, E, Aslan, N, et al. Functional gastrointestinal disorders: update on the Rome IV criteria. Rev Med Suisse. (2018) 14:1512–6. doi: 10.53738/REVMED.2018.14.616.1512
7. Tack, J, and Drossman, DA. What’s new in Rome IV? Neurogastroenterol Motil. (2017) 29:e13053. doi: 10.1111/nmo.13053
8. Sperber, AD, Bangdiwala, SI, Drossman, DA, Ghoshal, UC, Simren, M, Tack, J, et al. Worldwide prevalence and burden of functional gastrointestinal disorders, results of Rome foundation global study. Gastroenterology. (2021) 160:99–114.e3. doi: 10.1053/j.gastro.2020.04.014
9. Talley, NJ. What causes functional gastrointestinal disorders? A proposed disease model. Am J Gastroenterol. (2020) 115:41–8. doi: 10.14309/ajg.0000000000000485
10. Wiley, JW, and Chang, L. Functional bowel disorders. Gastroenterology. (2018) 155:1–4. doi: 10.1053/j.gastro.2018.02.014
11. Xiao, L, Liu, Q, Luo, M, and Xiong, L. Gut microbiota-derived metabolites in irritable bowel syndrome. Front Cell Infect Microbiol. (2021) 11:729346. doi: 10.3389/fcimb.2021.729346
12. Mayer, EA, Nance, K, and Chen, S. The gut-brain axis. Annu Rev Med. (2022) 73:439. doi: 10.1146/annurev-med-042320-014032
13. Gracie, DJ, Hamlin, PJ, and Ford, AC. The influence of the brain-gut axis in inflammatory bowel disease and possible implications for treatment. Lancet Gastroenterol Hepatol. (2019) 4:632–42. doi: 10.1016/S2468-1253(19)30089-5
14. Ancona, A, Petito, C, Iavarone, I, Petito, V, Galasso, L, Leonetti, A, et al. The gut-brain axis in irritable bowel syndrome and inflammatory bowel disease. Dig Liver Dis. (2021) 53:298–305. doi: 10.1016/j.dld.2020.11.026
15. Hillestad, EMR, van der Meeren, A, Nagaraja, BH, Bjørsvik, BR, Haleem, N, Benitez-Paez, A, et al. Gut bless you: the microbiota-gut-brain axis in irritable bowel syndrome. World J Gastroenterol. (2022) 28:412–31. doi: 10.3748/wjg.v28.i4.412
16. Giron, MC, and Mazzi, U. Molecular imaging of microbiota-gut-brain axis: searching for the right targeted probe for the right target and disease. Nucl Med Biol. (2021) 92:72. doi: 10.1016/j.nucmedbio.2020.11.002
17. Taib, S, Arbus, C, Sauvaget, A, Sporer, M, Schmitt, L, and Yrondi, A. How does repetitive transcranial magnetic stimulation influence the brain in depressive disorders?: a review of neuroimaging magnetic resonance imaging studies. J ECT. (2018) 34:79–86. doi: 10.1097/YCT.0000000000000477
18. van der Werf, YD, Sanz-Arigita, EJ, Menning, S, and van den Heuvel, OA. Modulating spontaneous brain activity using repetitive transcranial magnetic stimulation. BMC Neurosci. (2010) 11:1–6. doi: 10.1186/1471-2202-11-145
19. Naliboff, BD, Derbyshire, SW, Munakata, J, Berman, S, Mandelkern, M, Chang, L, et al. Cerebral activation in patients with irritable bowel syndrome and control subjects during rectosigmoid stimulation. Psychosom Med. (2001) 63:365–75. doi: 10.1097/00006842-200105000-00006
20. Speer, AM, Kimbrell, TA, Wassermann, EM, D Repella, J, Willis, MW, Herscovitch, P, et al. Opposite effects of high and low frequency rTMS on regional brain activity in depressed patients. Biol Psychiatry. (2000) 48:1133–41. doi: 10.1016/S0006-3223(00)01065-9
21. Sasegbon, A, Cheng, I, Zhang, M, and Hamdy, S. Advances in the use of neuromodulation for neurogenic dysphagia: mechanisms and therapeutic application of pharyngeal electrical stimulation, transcranial magnetic stimulation, and transcranial direct current stimulation. Am J Speech Lang Pathol. (2020) 29:1044–64. doi: 10.1044/2020_AJSLP-19-00073
22. Burke, MJ, Fried, PJ, and Pascual-Leone, A. Transcranial magnetic stimulation: neurophysiological and clinical applications. Handb Clin Neurol. (2019) 163:73–92. doi: 10.1016/B978-0-12-804281-6.00005-7
23. Klomjai, W, Katz, R, and Lackmy-Vallee, A. Basic principles of transcranial magnetic stimulation (TMS) and repetitive tms (rTMS). Ann Phys Rehabil Med. (2015) 58:208–13. doi: 10.1016/j.rehab.2015.05.005
24. Wang, J, Deng, XP, Wu, YY, Li, XL, Feng, ZJ, Wang, HX, et al. High-frequency rTMS of the motor cortex modulates cerebellar and widespread activity as revealed by svm. Front Neurosci. (2020) 14:186. doi: 10.3389/fnins.2020.00186
25. Kosslyn, SM, Pascual-Leone, A, Felician, O, Camposano, S, Keenan, JP, Ganis, G, et al. The role of area 17 in visual imagery: convergent evidence from pet and rTMS. Science. (1999) 284:167–70. doi: 10.1126/science.284.5411.167
26. Carrette, S, Boon, P, Dekeyser, C, Klooster, DCW, Carrette, E, Meurs, A, et al. Repetitive transcranial magnetic stimulation for the treatment of refractory epilepsy. Expert Rev Neurother. (2016) 16:1093–110. doi: 10.1080/14737175.2016.1197119
27. Chung, CL, Mak, MK, and Hallett, M. Transcranial magnetic stimulation promotes gait training in parkinson disease. Ann Neurol. (2020) 88:933–45. doi: 10.1002/ana.25881
28. McClintock, SM, Reti, IM, Carpenter, LL, McDonald, WM, Dubin, M, Taylor, SF, et al. Consensus recommendations for the clinical application of repetitive transcranial magnetic stimulation (rTMS) in the treatment of depression. J Clin Psychiatry. (2018) 79:35–48. doi: 10.4088/JCP.16cs10905
29. Walter, A, Denier, N, Hund, M, and Suenderhauf, C. Repetitive transcranial magnetic stimulation as treatment for neuropathic pain in patients with spinal cord injury. J Neurosurg Sci. (2020) 64:404–5. doi: 10.23736/S0390-5616.19.04716-7
30. Zhang, C, Zheng, X, Lu, R, Yun, W, Yun, H, and Zhou, X. Repetitive transcranial magnetic stimulation in combination with neuromuscular electrical stimulation for treatment of post-stroke dysphagia. J Int Med Res. (2019) 47:662–72. doi: 10.1177/0300060518807340
31. Janicak, PG, O’Reardon, JP, Sampson, SM, Hussain, MM, Lisanby, SH, Rado, JT, et al. Transcranial magnetic stimulation in the treatment of major depressive disorder: a comprehensive summary of safety experience from acute exposure, extended exposure, and during reintroduction treatment. J Clin Psychiatry. (2008) 69:222–32. doi: 10.4088/JCP.v69n0208
32. Bae, EH, Schrader, LM, Machii, K, Alonso-Alonso, M, Riviello, JJ Jr, Pascual-Leone, A, et al. Safety and tolerability of repetitive transcranial magnetic stimulation in patients with epilepsy: a review of the literature. Epilepsy Behav. (2007) 10:521–8. doi: 10.1016/j.yebeh.2007.03.004
33. Taylor, R, Galvez, V, and Loo, C. Transcranial magnetic stimulation (TMS) safety: a practical guide for psychiatrists. Australas Psychiatry. (2018) 26:189–92. doi: 10.1177/1039856217748249
34. Lacy, BE, and Patel, NK. Rome criteria and a diagnostic approach to irritable bowel syndrome. J Clin Med. (2017) 6:99. doi: 10.3390/jcm6110099
35. Li, X, Yu, W, Su, J, Zhou, X, Gan, Y, Xu, J, et al. Clinical efficacy of transcranial magnetic stimulation combined with electroacupuncture in the treatment of patients with functional constipation. Electron J Clin Med Lit. (2019) 8:64–6. doi: 10.3390/jcm8010064
36. Melchior, C, Gourcerol, G, Chastan, N, Verin, E, Menard, JF, Ducrotte, P, et al. Effect of transcranial magnetic stimulation on rectal sensitivity in irritable bowel syndrome: a randomized, placebo-controlled pilot study. Color Dis. (2014) 16:O104–11. doi: 10.1111/codi.12450
37. Li, X, Feng, R, Wu, H, Zhang, L, Zhao, L, Dai, N, et al. Psychological characteristics and gonogo research of patients with functional constipation. Medicine. (2016) 95:e5685. doi: 10.1097/MD.0000000000005685
38. Ni, M, Ding, Y, Ding, S, Jin, X, and Wang, J. Study of transcranial motor-evoked potentials in the patients with functional constipation. Acta Univ Med Nanjing. (2011) 31:1674–8.
39. Jiang, B, and Zhu, Y. Efficacy of paroxetine combined with transcranial magnetic stimulation in the treatment of depression with functional gastrointestinal disorders. Chin J Phys Med Rehabil. (2020) 42:653–5. doi: 10.3760/cma.j.issn.0254-1424.2020.07.017
40. Cheng, H, Yang, C, and Sun, C. Effect of rTMS on plasma neuropeptide y and substance p levels in patients with functional dyspepsia. Chin Foreign Med Res. (2018) 16:51–2. doi: 10.14033/j.cnki.cfmr.2018.26.022
41. Enck, P, Aziz, Q, Barbara, G, Farmer, AD, Fukudo, S, Mayer, EA, et al. Irritable bowel syndrome. Nat Rev Dis Primers. (2016) 2:16014. doi: 10.1038/nrdp.2016.14
42. Nizard, J, Lefaucheur, JP, Helbert, M, de Chauvigny, E, and Nguyen, JP. Non-invasive stimulation therapies for the treatment of refractory pain. Discov Med. (2012) 14:21–31.
43. Lefaucheur, JP. The use of repetitive transcranial magnetic stimulation (rTMS) in chronic neuropathic pain. Neurophysiol Clin. (2006) 36:117–24. doi: 10.1016/j.neucli.2006.08.002
44. Coffin, B, Farmachidi, J, Rueegg, P, Bastie, A, and Bouhassira, D. Tegaserod, a 5-HT4 receptor partial agonist, decreases sensitivity to rectal distension in healthy subjects. Aliment Pharmacol Ther. (2003) 17:577–85. doi: 10.1046/j.1365-2036.2003.01449.x
45. Algladi, T, Harris, M, Whorwell, PJ, Paine, P, and Hamdy, S. Modulation of human visceral sensitivity by noninvasive magnetoelectrical neural stimulation in health and irritable bowel syndrome. Pain. (2015) 156:1348–56. doi: 10.1097/j.pain.0000000000000187
Keywords: functional bowel disorder, brain-gut axis, brain imaging, transcranial magnetic stimulation, treatment
Citation: Li G, Jin B and Fan Z (2023) Clinical application of transcranial magnetic stimulation for functional bowel disease. Front. Med. 10:1213067. doi: 10.3389/fmed.2023.1213067
Edited by:
Hao Sun, Nanjing Medical University, ChinaReviewed by:
Georgios Mikellides, University of Nicosia, CyprusCopyright © 2023 Li, Jin and Fan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhe Fan, ZmFuemhlMTk4MkBob3RtYWlsLmNvbQ==
†These authors have contributed equally to this work
 Guangyao Li
Guangyao Li Binghui Jin
Binghui Jin Zhe Fan
Zhe Fan