- 1Department of Medicine, Division of Endocrinology, Metabolism and Diabetes, University of Colorado Anschutz Medical Campus, Aurora, CO, United States
- 2Department of Family Medicine, University of Colorado Anschutz Medical Campus, Aurora, CO, United States
- 3Department of Medicine, Division of Nephrology, University of New Mexico School of Medicine, Albuquerque, NM, United States
Purpose: The major aims were to quantify patient weight loss using various approaches adminstered by a primary care provider for at least 6 months and to unveil relevant contextual factors that could improve patient weight loss on a long-term basis.
Methods: A systematic review and meta-analysis was conducted using Cochrane Central Register of Controlled Trials, MEDLINE, EMBASE, Scopus, and Web of Science from inception to December 5, 2022. COVIDENCE systematic review software was used to identify and abstract data, as well as assess data quality and risk of bias.
Results: Seven studies included 2,187 people with obesity testing (1) anti-obesity medication (AOM), (2) AOM, intensive lifestyle counseling + meal replacements, and (3) physician training to better counsel patients on intensive lifestyle modification. Substantial heterogeneity in the outcomes was observed, as well as bias toward lack of published studies showing no effect. The random effect model estimated a treatment effect for the aggregate efficacy of primary care interventions −3.54 kg (95% CI: −5.61 kg to −1.47 kg). Interventions that included a medication component (alone or as part of a multipronged intervention) achieved a greater weight reduction by −2.94 kg (p < 0.0001). In all interventions, efficacy declined with time (reduction in weight loss by 0.53 kg per 6 months, 95% CI: 0.04–1.0 kg).
Conclusion: Weight loss interventions administered by a primary care provider can lead to modest weight loss. Weight loss is approximately doubled if anti-obesity medication is part of the treatment. Nevertheless, attenuated weight loss over time underscores the need for long-term treatment.
Systematic review registration: [https://www.crd.york.ac.uk/prospero/ CRD4202121242344], identifier (CRD42021242344).
Introduction
Obesity continues to cast an enormous human and economic toll. As of 2018, the Centers for Disease Control and Prevention estimated that 73.6% of U.S. adults are considered overweight [e.g., body mass index (BMI) ≥25 kg/m2] and 42.4% have obesity (e.g., BMI ≥30 kg/m2) (1), collectively costing the health care system approximately $1.7 T annually (2). Obesity is being increasingly recognized not only as a risk factor for disease but a disease unto itself (3). Despite this fact, only ~50% of people with a BMI of 50 kg/m2 (considered morbid or extreme obesity) have a diagnosis of obesity (4) and <1% of people with any degree of overweight or obesity are offered anything other than lifestyle advice (5). Given the paucity of certified “obesity expert” health care providers, this treatment gap can only be closed by systematically addressing the barriers to weight management in primary care.
Patient demand is high for weight management in primary care. A study by Sherson et al. (6) reported that most patients want to discuss weight loss with their physicians. Specifically, patients value physician direction with their diet, physical activity and goal setting (7). In addition, recent years have ushered in numerous and diverse options for weight management that extend signficantly beyond lifestyle advice. Medications for weight loss are being more extensively studied and demonstrating improved outcomes (8–11). Bariatric surgery provides a possible option to address weight and reverse potentially life-threatening conditions such as heart disease and diabetes in both adolescents and adults (12–15). Intensive behavioral therapy (IBT) for obesity is now a covered benefit under Medicare (16). Together, primary care practice transformation, reimbursement for obesity care and better therapies for weight management suggest new and pragmatic approaches can emerge.
Weight loss observed in clinical trials has failed to translate into real world clinical settings (5). Most of these trials have tested one or more strategies for weight loss but not the context in which the strategies will ultimately be deployed. The objective of the current systematic review and meta-analysis is to determine weight loss in randomized clinical trials specifically conducted in a primary care setting where a primary care provider adminstered the intervention for at least 6 months. Our goal was to quantify patient weight loss using various approaches tested in primary care and to unveil relevant contextual factors that could improve patient weight loss on a long-term basis.
Methods
PRISMA reporting guideline
This systematic review is reported according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement extension for network meta-analysis (17) and was conducted following an a priori—established protocol registered with PROSPERO on March 12, 2021 (CRD42021242344). Approval from the institutional review board was not required. Neither patients nor the public were involved in the preparation of this manuscript.
Selection criteria
Randomized clinical trials were included in this meta-analysis if they were conducted in a primary care setting with the primary care provider (physician, physician assistant, or nurse practitioner) administering the intervention for at least 6 months. Interventions administered exclusively by a medical assistant, health coach, dietician, or behavioral health provider were not included, even if they occurred in a primary care setting. Interventions referring patients elsewhere (e.g., to a commercial weight loss program) were also not included, even if the referral was placed by the primary care provider. The control condition may have been a placebo pill and/or some minimal amount of lifestyle intervention offered to both groups intended to resemble “usual care.” This analysis is limited to studies that included adults age ≥18 years old with a BMI ≥25 kg/m2, with or without weight-associated comorbidities, that reported absolute weight at baseline and at the end of follow-up in the intervention and control groups.
Search strategy
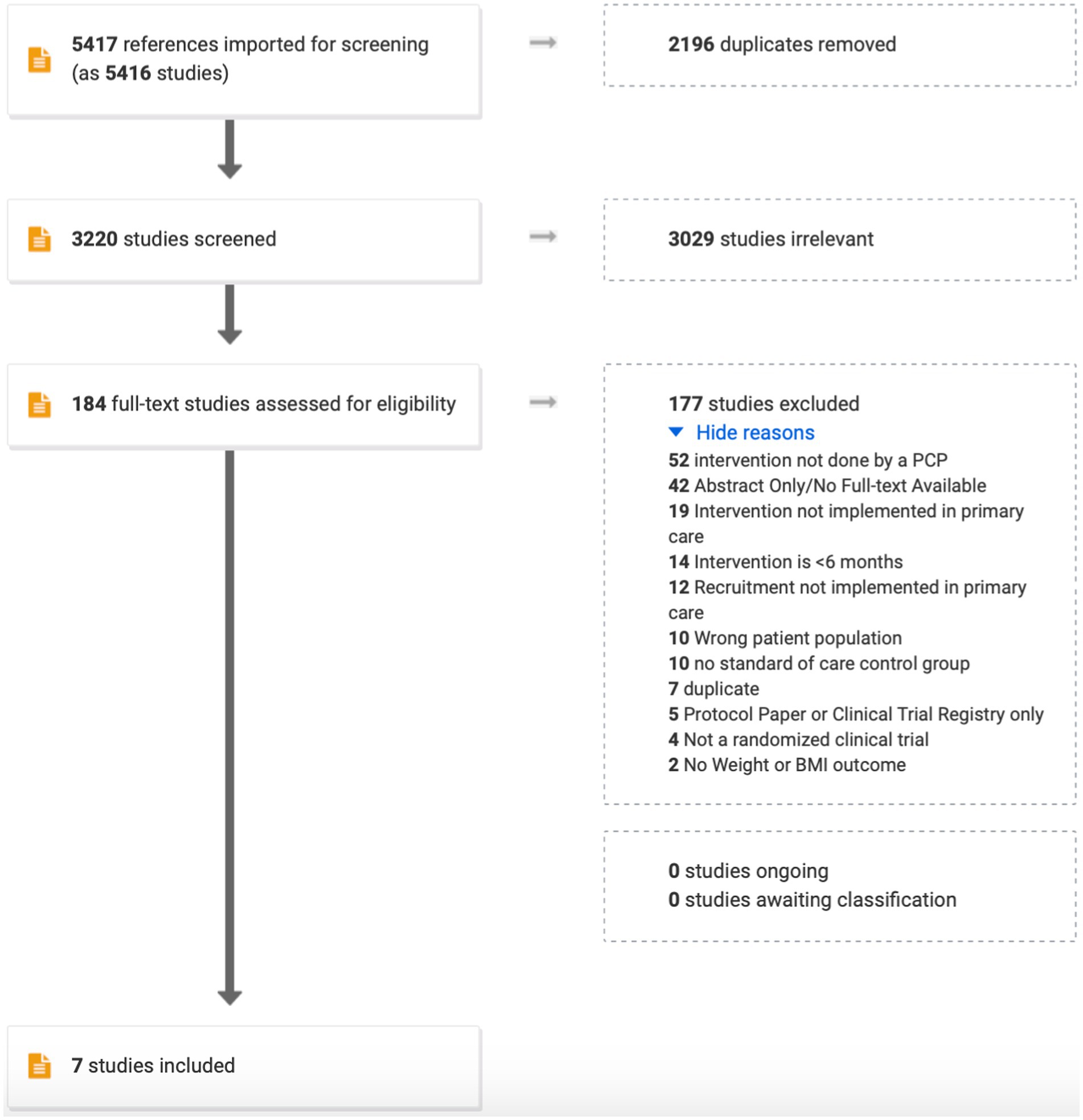
The search strategy was designed in consultation with an experienced medical librarian with input from study investigators using various databases from inception to September 5, 2022. COVIDENCE systematic review software (Veritas Health Innovation; Melbourne, Australia) was used for screening, full-text review, and data extraction (Figure 1). COVIDENCE is the primary tool used in Cochrane reviews accessing the Cochrane Central Register of Controlled Trials and databases supporting the Central Register such as MEDLINE, EMBASE, Scopus, and Web of Science. References were imported using the search terms: randomized clinical trial, obesity, overweight, weight loss, primary care, general practice, family practice, internal medicine, routine medical practice. Duplicate citations were removed. Studies advanced to full-text review as long as weight change was the primary outcome. Further exclusions were applied to ensure that only randomized controlled clinical trials conducted in a primary care setting with a primary care provider administering the intervention for ≥6 months were retained (Figure 1).
Data abstraction and quality assessment
All authors participated independently in the screening and full-text review process described above except CA, who conducted the analysis and edited the manuscript. Discrepancies were resolved by consensus. Quality assessment and risk of bias of individual studies was assessed in the context of the primary outcome using the template provided by COVIDENCE. The template stratified responses into high, low or unsure for sequence generation (the method used to generate the allocation sequence should produce comparable groups), allocation concealment (the method used to conceal the allocation sequence should not have been foreseen in advance of, or during, enrollment), blinding of participants and personnel (neither participants or personnel had knowledge of which intervention a participant received), blinding of outcomes assessment (outcome assessors had no knowledge of which intervention a participant received), incomplete outcome data (data were complete with low attrition and exclusions from the analysis), selective reporting (low probability of selective outcome reporting), and other sources of bias (no concerns about bias not addressed in the other domains in the tool).
Outcomes
All outcomes were assessed at 6 months of intervention vs. control, and again at 12, 18, or 24 months as long as the intervention vs. control conditions were ongoing according to the randomization. The primary outcome was weight change from baseline. Contextual factors of interest focused largely on strategy employed in the intervention group, but patient characteristics and control condition were also examined. When available, outcomes were abstracted using study-reported modified intention-to-treat analysis (i.e., patients exposed at least once to the intervention or control condition and had one post-randomization weight assessment).
Statistical analysis
Publication bias was assessed using contour-enhanced funnel plots (18) to detect the area of statistical significance in which studies were missing, and the statistical significance of funnel plot asymmetry was analyzed with the random effects version of the Egger test (19). To generate this plot multiple treatment effects from individual studies (change from baseline relative to control treatment at multiple time points and/or multiple interventions) were synthesized via random effects meta-analysis to provide an overall treatment effect per study. This approach was used to generate a summary forest plot from all studies considered. The fixed effects model assumes that the treatment effect is the same across all trials, while the random effects model assumes that the treatment effect varies between the trials. Statistical heterogeneity within and across trials synthesized was quantified by the I2 statistic and the between study variance (τ2), which was calculated by REstricted Maximum Likelihood (REML). The p-value of the Q test was used to test for the statistical significance of the observed heterogeneity. To explore the replicability of these findings we calculated the prediction interval (20) (i.e., the 95% confidence interval for the treatment effects in a future trial). A prediction interval that is narrow and overlapping with the confidence interval of the treatment effect, suggests that future studies are unlikely to change the conclusion we can draw from the currently available trials. Finally, a formal, multilevel meta-regression analysis was carried out to explore the hypothesis that lifestyle modifications will have a different effect than pharmacologic interventions. This hierarchical meta-regression was the primary means for synthesizing evidence for our analysis and accounts for the correlation of outcomes due to the common control group in interventions with multiple treatments assessed at multiple time-points during each study. The analyses were conducted in Microsoft R open v4.0.2 packages meta v4.13-0 (funnel plot/forest plot) and metafor v2.4-0 (Egger test, meta-regression). Data and code for all analyses are available in the Supplementary material.
Results
Quality and bias of the studies included
Based on the search terms, 5,417 studies were imported for screening. Of those, 2,196 were found to be duplicates and were removed. Of the remaining, 3,029 were omitted because of misalignment with the primary outcome (e.g., studies of lifestyle interventions to improve quality of life, not weight). Reasons for excluding 177 of the final 184 studies is shown in Figure 1. All authors participated in this process with proportionate agreement ranging from 88%–98% and random probability agreement 81%–98% (Cohen’s kappa 0.39–0.55). Quality assessment and risk of bias of individual studies was assessed in the context of the primary outcome for the following parameters: sequence generation (5/7 high; 2/7 low), allocation concealment (4/7 high; 3/7 low), blinding of participants and personnel (6/7 high; 1/7 low), blinding of outcomes assessment (3/7 high; 4/7 low), incomplete outcome data (3/7 high, 3/7 low; 1/7 unsure), selective reporting, and other sources of bias (1/7 low, 6/7 unsure).
Characteristics of the studies included
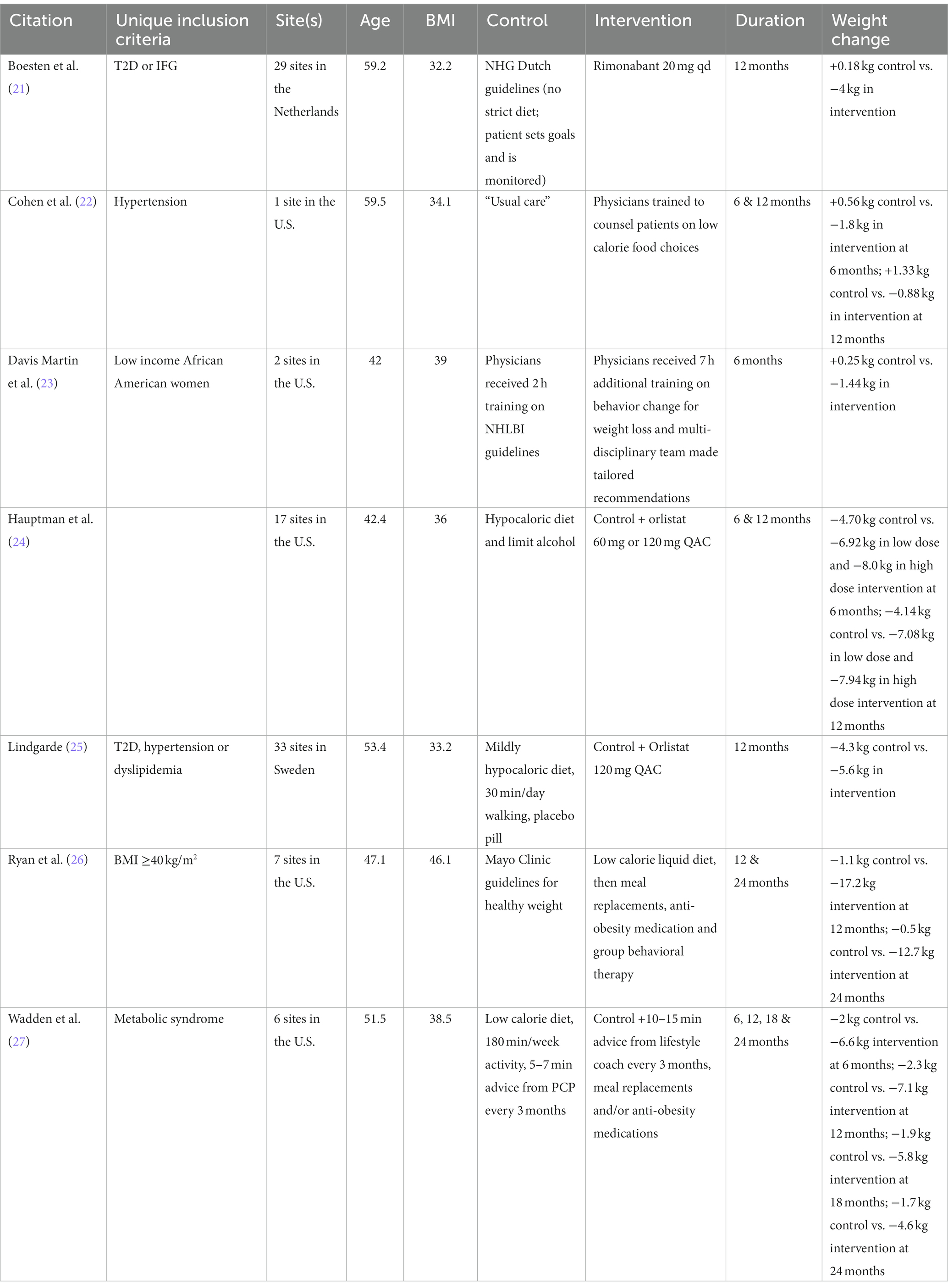
Only 7 studies met our full inclusion criteria (Table 1) (21–27). Most studies were conducted in people with obesity who also had risk factors for poor outcomes (i.e., high-risk race/ethnicity, pre-existing type 2 diabetes, prediabetes, hypertension, dyslipidemia or the metabolic syndrome). Five of the 7 studies were conducted in the U.S.—with 6 of 7 being multi-centered—in people 40–60 years old with BMI’s mostly in the 30’s. Two of the studies randomized physicians to receive training in weight management the use of which became the intervention for patients. Three studies randomized patients to anti-obesity medication vs. placebo, whereas two used anti-obesity medication in combination with intensive lifestyle counseling and meal replacements. Two studies reported on more than one active interventions: high vs. low dose of medication (24) and basic vs. enhanced lifestyle coaching (27). No study randomized patients to lifestyle modification alone in a setting where the providers had not received additional training on the topic. Control conditions frequently used their respective national or local guidelines to advise patients on healthy eating, physical activity and behavior modification. Time spent on giving this advice was minimal (5–7 min) and was intended to resemble “usual care.” Three of the studies (21, 23, 25) reported on the studied intervention at only one time-point, while the remaining studies reported on weight loss at different time points. Two of the studies (23, 26) reported outcome information on completers (completer analysis) and we could not ascertain the intention-to-treat status of the outcome in one study (22). The remaining four studies reported outcomes using modified intention-to-treat.
Meta-analysis for weight loss outcomes
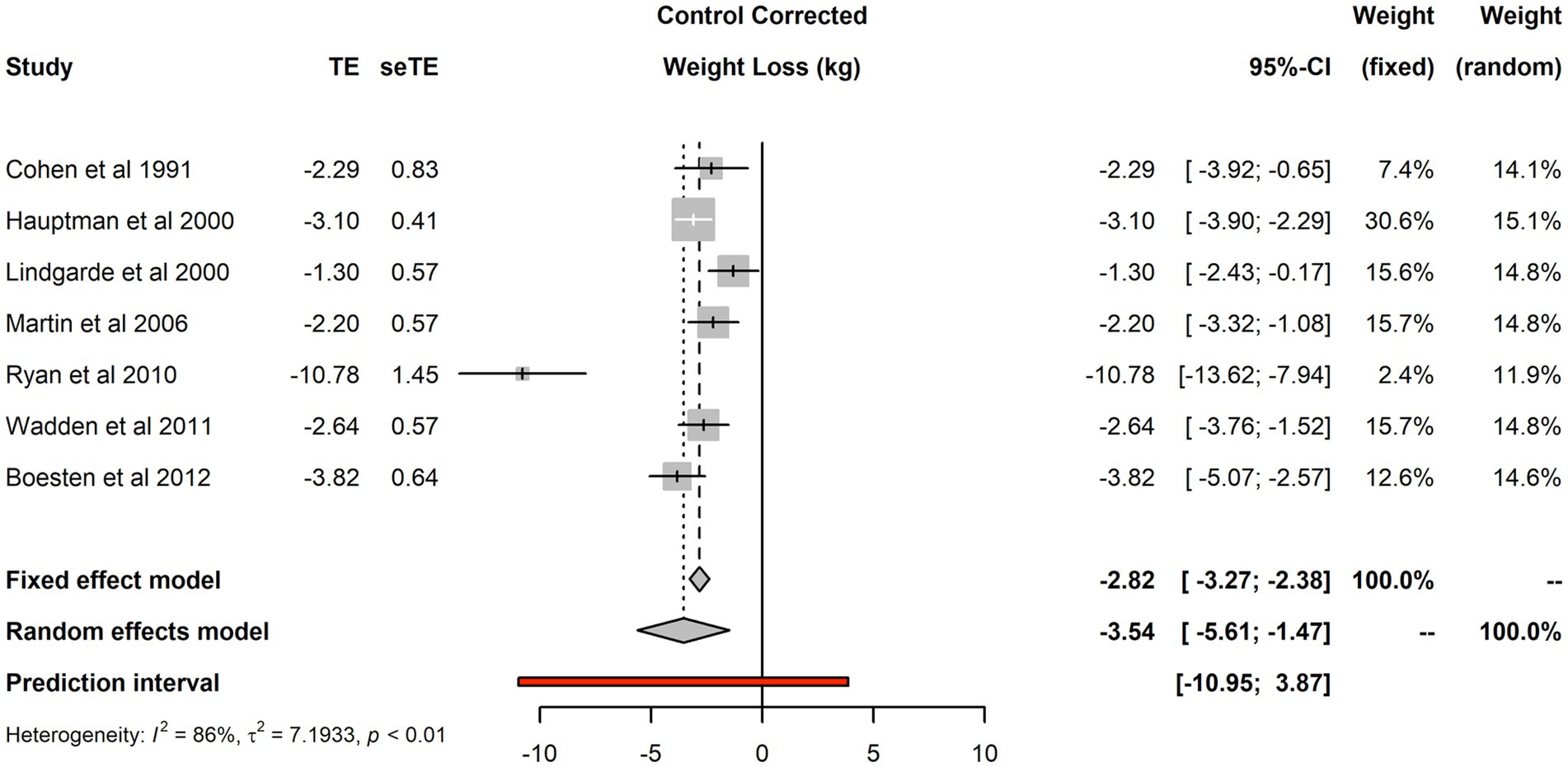
The seven studies included 2,187 individuals and reported 2,400 changes from baseline in the intervention arm and 2,453 in the control arm. Forest plot of the seven studies is shown in Figure 2. There was evidence of substantial heterogeneity (I2 was 86%, τ2 was 7.19 and p < 0.01). The fixed effect model estimated a treatment effect for the aggregate efficacy of primary care interventions −2.82 kg (95% CI: −3.27 kg to −2.38 kg), that was substantially different from the random effect estimate of −3.54 kg (95% CI: −5.61 kg to −1.47 kg). Considerable uncertainty remains about the efficacy of interventions; the 95% prediction interval for the treatment effect in future replications of such trials was rather wide: from nearly 11 kg of weight loss to nearly 4 kg of weight gain.
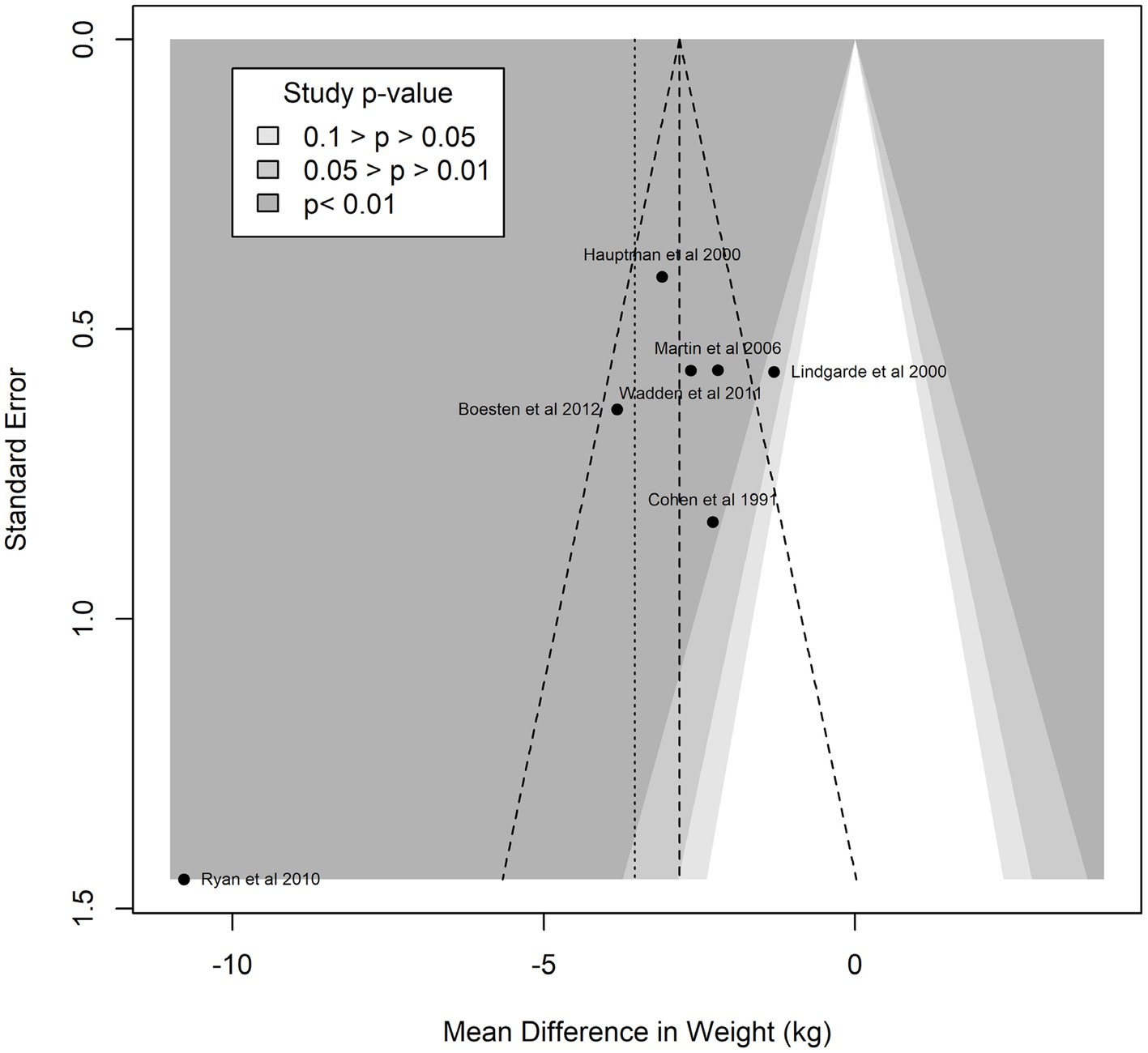
Funnel plot analyses (Figure 3) suggested the presence of publication bias: the plot itself was asymmetric, and there appeared to be missing studies in the area of non-significant studies (white area of the plot). In confirmation of these visual impressions, Egger’s test was also statistically significant (p = 0.0005). Summary treatment effects for the studies reporting multiple interventions at multiple time points is shown in Figure 4. For two of the trials reported, there was evidence of treatment heterogeneity within each study (26, 27).
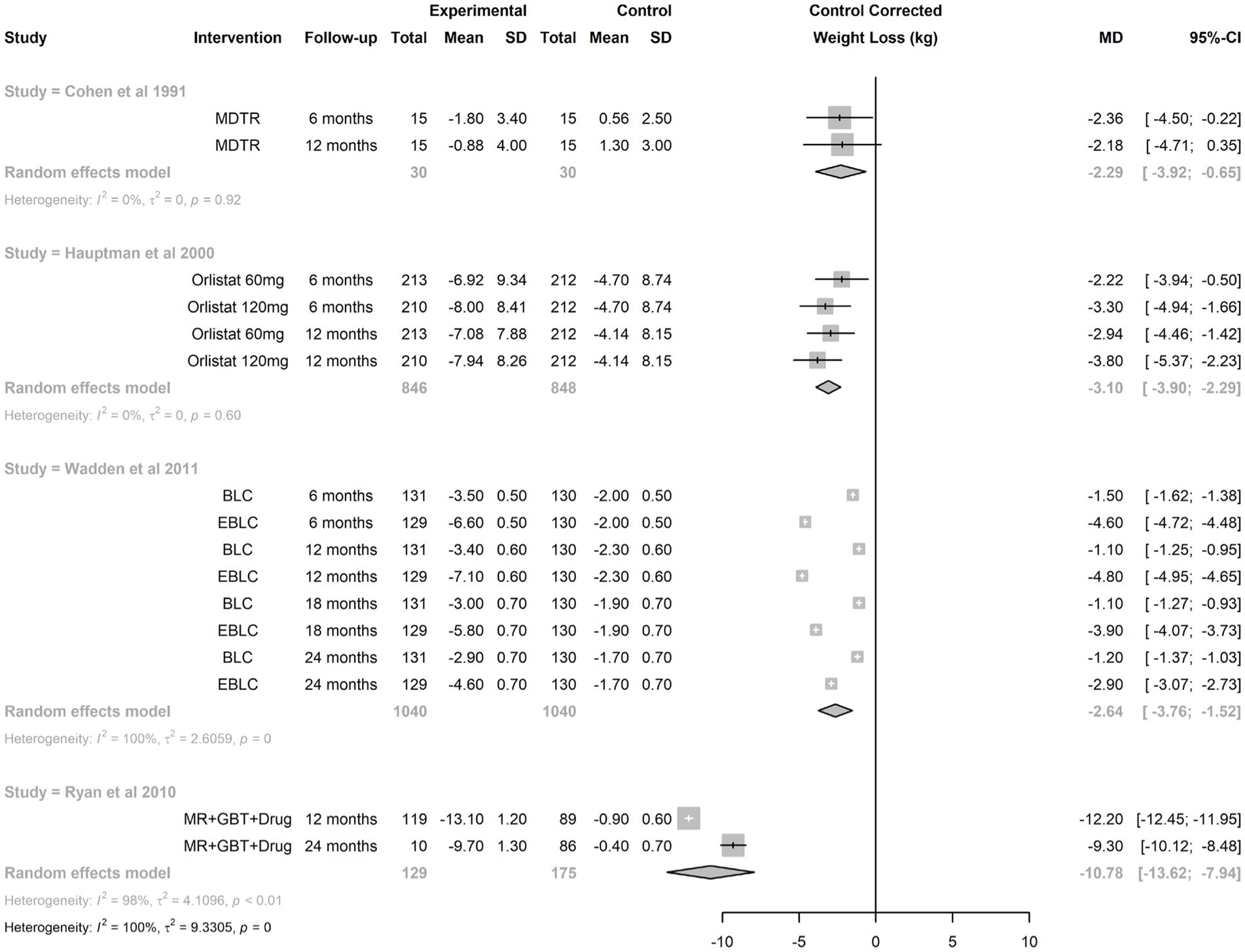
Figure 4. Synthesis of treatment effect from studies reporting multiple interventions at multiple time points. BLC, brief lifestyle coaching; EBLC, enhanced basic lifestyle coaching; GBT, group behavioral therapy; MDTR, physician training; MR, meal replacement.
Analyses of heterogeneity
In univariate multilevel modeling, using random effects at the study and follow-up (nested within study levels), there was evidence for attenuation of the treatment effect with time, i.e., a reduction in weight loss of about 0.5 kg per 6 months (p = 0.04). Interventions that included a medication component (either alone or as components of multipronged intervention) achieved a greater weight reduction by −2.94 kg (p < 0.0001). In multivariate multilevel meta-regressions that included both covariates (type of intervention and duration of the intervention), the estimated weight loss associated with a drug component was identical to that estimated by the univariate model. In these models, there was also evidence for reduction of efficacy with time (reduction in weight loss by 0.53 kg per 6 months, 95% CI: 0.04–1.0 kg, p-value = 0.04). When we analyzed intervention type by treatment time interactions, there was evidence for differential attenuation of treatment effect for interventions that included a drug component. While the loss of efficacy of interventions without a drug component was estimated to be 0.26 kg/6 months (95% CI −0.14 to 0.66 kg, p = 0.20), the efficacy of interventions with drug components attenuated at a rate of 0.44 kg/6 months, 95% CI (0.34–0.53 kg/6 months, p-value <0.001).
Discussion
Obesity—with its many comorbid conditions—has now surpassed smoking as the leading cause of preventable death in the United States (28). Despite the fact that obesity is both treatable and preventable, treating the comorbidities, rather than obesity per se has been the mainstay of therapy. Reasons for lack of weight management prioritization are extensive and complex but have included lack of clinician education on effective obesity management and processes that systematically address weight loss and weight loss maintenance long-term (6–8). There are reasons to believe, however, that the paradigm may be shifting. Clinical trials testing infrastructure for weight-prioritized visits in primary care (e.g., NCT04678752) coupled with highly effective pharmacotherapy (29, 30) may make patient weight loss of 15%–22% achievable. Major findings from the current analysis show that patients lose ~3 kg (approximately 3% body weight) when they are provided more than usual care by their primary care provider and that the amount of weight loss approximates 6 kg (roughly 6% body weight) when anti-obesity medication is part of the intervention. Nevertheless, weight regain is common underscoring the need for long-term treatment and intensification.
Most people receive their health care in primary care—not by specialists, dieticians, or health coaches—the vast majority of whom fail to maintain a normal body weight (1). Although some published data supports the use of commercial weight loss programs (31), their long-term use is uncommon. Patients want help with their weight from their primary care provider and 77% of those who receive help (vs. 33% that did not) (32) report positive behavior change and weight loss (33). The current analysis examined what has been (hence could be) done in primary care for weight management. Interventions ranged from provider use of newly reinforced knowledge in the area of behavior modification to multi-faceted approaches that included intensive behavioral therapy, anti-obesity medications and meal replacements. Wide breadth in the interventions tested led to substantial and residual heterogeneity in their treatment effect. The random effects model estimated a mean patient weight loss of −3.54 kg. Interventions that included a medication component (either alone or as components of multipronged intervention) achieved a greater weight reduction by −2.94 kg. The latter finding was unchanged when adjusting for all components of the intervention or its duration supporting the independent benefit of anti-obesity medications. Together, multi-faceted approaches appear to yield greater weight loss compared to lifestyle advice alone when administered by a primary care provider, however, larger and more internationally diverse trials are needed to confirm the magnitude of the benefit.
Weight nadir in clinical trials is consistently achieved between 6–12 months with weight regain following, despite ongoing intensive lifestyle modification (34, 35) or pharmacotherapy (36, 37). Results from the current analysis are highly aligned with those previously reported. We observed an average weight regain of 0.5 kg/6 months in the setting of ongoing active intervention. Interestingly, despite greater weight loss being achieved in trials testing anti-obesity medication, the weight regain was also greater (0.44 kg/6 months vs. 0.26 kg/6 months). The notion that rapid (vs. gradual) weight loss leads to greater weight regain has been debunked (38, 39). Hence, the greater weight regain with anti-obesity medication use may relate to the greater absolute weight loss achieved. Highly conserved evolutionary adaptations for survival are sensitive to weight loss, including lowering energy expenditure (40) and driving energy intake (41). Hence, discontinuation of anti-obesity medication after weight loss could explain the rapid weight regain. Advances in the field have made it clear that successful treatment must be long-term.
This systematic review and meta-analysis ultimately revealed few studies quantifying patient weight loss resulting from an intervention administered by a primary care provider. Those that have exhibited considerable heterogeneity and carry uncertainty around whether the results can be replicated. The included studies are collectively of moderate quality, but there appears to be a bias toward missing non-significant studies, hence this analysis may over-estimate the true effect of the interventions. We acknowledge that systematic reviews and meta-analyses have limitations on how well they can capture the complexities that exist around issues in the real world (e.g., heterogeneity in environment, human behavior, socioeconomics, etc. that were not measured), and in understanding how a specific context links with an outcome through a particular “mechanism” (42). An important strength, however, was that this analysis was not limited to trials examining behavior modification for weight loss nor did it include auxiliary personnel providing care (43). It was intended to review the full breadth of what has been (hence could be) done in routine medical practice using conventional workflow and established scope of training for primary care providers.
It is worth noting that the control conditions were intended to simulate “usual care”—citing medical guidelines or giving brief advice—widely acknowledging the lack of standard-of-care for weight management. High intra-individual variability in response to weight loss interventions (44) rightfully supports a patient-centered approach. Nevertheless, lack of consensus on recommendations for a specific approach (45) perpetuates clinical inertia. Trials included in this analysis that used established guidelines or “usual care” as the standard-of-care rendered <1 kg of weight loss (21–23, 26). Trials included in this analysis that utilized hypocaloric diets and targets for physical activity as the standard-of-care rendered 2–5 kg of weight loss (24, 25, 27) making it clear that prescribed behavior modification is essential. Granularity as to the details of the optimal behavior modification, however, remain elusive.
Conclusion
It is time to reframe the conversation such that weight management is the primary goal for treating weight-related comorbidities (46). To that end, this is the first systematic review and meta-analysis to see what has or could be done by primary care providers for weight management. Despite the highly pragmatic nature of this question, few clinical trials have pursued the answer. Results demonstrate successful patient weight loss, particularly when multiple strategies are used together. Results also show that weight regain is common, especially after using anti-obesity medication—likely because of discontinuation. These results will be used as the metric for comparison to those of an ongoing trial examining a novel process of care currently deployed in 66 primary care clinics in the University of Colorado Health Care system (NCT #04678752). It is clear that establishment of a standard-of-care is desperately needed as a first step toward treatment. Layering of additional strategies and long-term treatment must follow to promote patient weight loss and weight loss maintenance.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
LP conceived the review, screened references, and wrote the manuscript. EK, PS, and DS screened references and edited the manuscript. CA conducted the analysis and edited the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (1R18DK127003).
Conflict of interest
LP has received personal fees for speaking and/or consulting from Novo Nordisk, Sanofi, Boehringer Ingelheim, Elli Lilly, Bayer, Neurobo, Medscape, WebMD and UpToDate.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2023.1204849/full#supplementary-material
References
1. Centers for Disease Control and Prevention. Prevalence and trends of overweight and obesity in the United States. Available at:www.cdc.gov/obesity/data/adult.html. (2016). (Accessed June 21, 2022).
2. Milken Institute. Economic impact of excess weight now exceeds $17T. Available at:https://www.milkeninstitute.org/articles/economic-impact-excess-weight-now-exceeds-17-trillion-new-milken-institute-report-reveals. (2018). (Accessed June 21, 2022).
3. World Health Organization. Obesity and overweight. Available at:https://www.who.int/topics/obesity/en/. (2018). (Accessed June 21, 2022)
4. Crawford, AG, Cote, C, Couto, J, Daskiran, M, Gunnarsson, C, Haas, K, et al. Prevalence of obesity, type II diabetes mellitus, hyperlipidemia, and hypertension in the United States: findings from the GE centricity electronic medical record database. Popul Health Manag. (2010) 13:151–61. doi: 10.1089/pop.2009.0039
5. Garvey, WT, Mechanick, JI, Brett, EM, Garber, AJ, Hurley, DL, Jastreboff, AM, et al. American Association of Clinical Endocrinologists and American College of Endocrinology Comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr Pract. (2016) 22:1–203. doi: 10.4158/EP161365.GL
6. Sherson, EA, Yakes Jimenez, E, and Katalanos, N. A review of the use of the 5 A’s model for weight loss counselling: differences between physician practice and patient demand. Fam Pract. (2014) 31:389–98. doi: 10.1093/fampra/cmu020
7. Yoong, SL, Carey, M, Sanson-Fisher, R, and Grady, A. A systematic review of behavioural weight-loss interventions involving primary-care physicians in overweight and obese primary-care patients (1999–2011). Public Health Nutr. (2013) 16:2083–99. doi: 10.1017/S1368980012004375
8. Bohula, EA, Scirica, BM, Inzucchi, SE, McGuire, DK, Keech, AC, Smith, SR, et al. Effect of lorcaserin on prevention and remission of type 2 diabetes in overweight and obese patients (CAMELLIA-TIMI 61): a randomised, placebo-controlled trial. Lancet. (2018) 392:2269–79. doi: 10.1016/S0140-6736(18)32328-6
9. Bohula, EA, Wiviott, SD, McGuire, DK, Inzucchi, SE, Kuder, J, Im, K, et al. Cardiovascular safety of lorcaserin in overweight or obese patients. N Engl J Med. (2018) 379:1107–17. doi: 10.1056/NEJMoa1808721
10. Garber, AJ. Anti-obesity pharmacotherapy and the potential for preventing progression from prediabetes to type 2 diabetes. Endocr Pract. (2015) 21:634–44. doi: 10.4158/EP14460.RA
11. Svanstrom, H, Ueda, P, Melbye, M, Eliasson, B, Svensson, AM, Franzén, S, et al. Use of liraglutide and risk of major cardiovascular events: a register-based cohort study in Denmark and Sweden. Lancet Diabetes Endocrinol. (2019) 7:106–14. doi: 10.1016/S2213-8587(18)30320-6
12. Carlsson, LM, Sjoholm, K, Karlsson, C, Jacobson, P, Andersson-Assarsson, JC, Svensson, PA, et al. Long-term incidence of microvascular disease after bariatric surgery or usual care in patients with obesity, stratified by baseline glycaemic status: a post-hoc analysis of participants from the Swedish obese subjects study. Lancet Diabetes Endocrinol. (2017) 5:271–9. doi: 10.1016/S2213-8587(17)30061-X
13. Fisher, DP, Johnson, E, Haneuse, S, Arterburn, D, Coleman, KJ, O’Connor, PJ, et al. Association between bariatric surgery and macrovascular disease outcomes in patients with type 2 diabetes and severe obesity. JAMA. (2018) 320:1570–82. doi: 10.1001/jama.2018.14619
14. Inge, TH, Courcoulas, AP, Jenkins, TM, Michalsky, MP, Helmrath, MA, Brandt, ML, et al. Weight loss and health status 3 years after bariatric surgery in adolescents. N Engl J Med. (2016) 374:113–23. doi: 10.1056/NEJMoa1506699
15. Sjostrom, L, Narbro, K, Sjostrom, CD, Karason, K, Larsson, B, Wedel, H, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. (2007) 357:741–52. doi: 10.1056/NEJMoa066254
16. Department of Health and Human Services. Intensive behavioral therapy (IBT) for obesity. Available at:https://www.cms.gov/Medicare/Prevention/PrevntionGenInfo/medicare-preventive-services/MPS-QuickReferenceChart-1.html#OBESITY_IBT. (Accessed February 5, 2022).
17. Moher, D, Liberati, A, Tetzlaff, J, and Altman, DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 151:e1000097. doi: 10.1371/journal.pmed.1000097
18. Peters, JL, Sutton, AJ, Jones, DR, Abrams, KR, and Rushton, L. Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. J Clin Epidemiol. (2008) 61:991–6. doi: 10.1016/j.jclinepi.2007.11.010
19. Sterne, JAC, and Egger, M. Regression methods to detect publication and other bias in meta-analysis In: HR Rothstein, AJ Sutton, and M Borenstein, editors. Publication bias in meta-analysis: prevention, assessment, and adjustments. Chichester: Wiley (2005). 99–110.
20. Higgins, JP, Thompson, SG, and Spiegelhalter, DJ. A re-evaluation of random-effects meta-analysis. J R Stat Soc A. (2009) 172:137–59. doi: 10.1111/j.1467-985X.2008.00552.x
21. Boesten, JE, Kaper, J, Stoffers, HE, Kroon, AA, and van Schayck, OC. Rimonabant improves obesity but not the overall cardiovascular risk and quality of life; results from CARDIO-REDUSE (CArdiometabolic risk reDuctIOn by Rimonabant: the effectiveness in daily practice and its USE). Fam Pract. (2012) 29:521–7. doi: 10.1093/fampra/cms013
22. Cohen, MD, D'Amico, FJ, and Merenstein, JH. Weight reduction in obese hypertensive patients. Fam Med. (1991) 23:25–8.
23. Davis Martin, P, Rhode, PC, Dutton, GR, Redmann, SM, Ryan, DH, and Brantley, PJ. A primary care weight management intervention for low-income African-American women. Obesity. (2006) 14:1412–20. doi: 10.1038/oby.2006.160
24. Hauptman, J, Lucas, C, Boldrin, MN, Collins, H, and Segal, KR. Orlistat in the long-term treatment of obesity in primary care settings. Arch Fam Med. (2000) 9:160–7. doi: 10.1001/archfami.9.2.160
25. Lindgarde, F. The effect of orlistat on body weight and coronary heart disease risk profile in obese patients: the Swedish multimorbidity study. J Intern Med. (2000) 248:245–54. doi: 10.1046/j.1365-2796.2000.00720.x
26. Ryan, DH, Johnson, WD, Myers, VH, Prather, TL, McGlone, M, Rood, J, et al. Nonsurgical weight loss for extreme obesity in primary care settings: results of the Louisiana obese subjects study. Arch Intern Med. (2010) 170:146–54. doi: 10.1001/archinternmed.2009.508
27. Wadden, TA, Volger, S, Sarwer, DB, Vetter, ML, Tsai, AG, Berkowitz, RI, et al. A two-year randomized trial of obesity treatment in primary care practice. N Engl J Med. (2011) 365:1969–79. doi: 10.1056/NEJMoa1109220
28. Taksler, GB Life-years lost to preventable causes-of-death in the U.S.., (2014). Paper presented at: The Society of General Internal Medicine 2017 Annual Meeting; April 19–22, 2017; Washington, DC.
29. Jastreboff, AM, Aronne, LJ, Ahmad, NN, Wharton, S, Connery, L, Alves, B, et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med. (2022) 387:205–16. doi: 10.1056/NEJMoa2206038
30. Wilding, JPH, Batterham, RL, Calanna, S, Davies, M, van Gaal, LF, Lingvay, I, et al. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med. (2021) 384:989–1002. doi: 10.1056/NEJMoa2032183
31. Gudzune, KA, Doshi, RS, Mehta, AK, Chaudhry, ZW, Jacobs, DK, Vakil, RM, et al. Efficacy of commercial weight-loss programs: an updated systematic review. Ann Intern Med. (2015) 162:501–12. doi: 10.7326/M14-2238
32. Sciamanna, CN, Tate, DF, Lang, W, and Wing, RR. Who reports receiving advice to lose weight? Results from a multistate survey. Arch Intern Med. (2000) 160:2334–9. doi: 10.1001/archinte.160.15.2334
33. Rodondi, N, Humair, JP, Ghali, WA, Ruffieux, C, Stoianov, R, Seematter-Bagnoud, L, et al. Counselling overweight and obese patients in primary care: a prospective cohort study. Eur J Cardiovasc Prev Rehabil. (2006) 13:222–8. doi: 10.1097/01.hjr.0000209819.13196.a4
34. Diabetes Prevention Program Research Group. Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15 years follow-up: the diabetes prevention program outcomes study. Lancet Diabetes Endocrinol. (2015) 3:866–75. doi: 10.1016/S2213-8587(15)00291-0
35. Wing, RR, Bolin, P, Brancati, FL, Bray, GA, Clark, JM, Coday, M, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. (2013) 369:145–54. doi: 10.1056/NEJMoa1212914
36. Le Roux, CW, Astrup, A, Fujioka, K, Greenway, F, DCW, L, Van Gaal, L, et al. 3 years of liraglutide versus placebo for type 2 diabetes risk reduction and weight management in individuals with prediabetes: a randomised, double-blind trial. Lancet. (2017) 7:842–84. doi: 10.1016/S0140-6736(17)30069-7
37. Smith, SR, Weissman, NJ, Anderson, CM, Sanchez, M, Chuang, E, Stubbe, S, et al. Multicenter, placebo-controlled trial of lorcaserin for weight management. N Engl J Med. (2010) 363:245–56. doi: 10.1056/NEJMoa0909809
38. Purcell, K, Sumithran, P, Prendergast, LA, Bouniu, CJ, Delbridge, E, and Proietto, J. The effect of rate of weight loss on long-term weight management: a randomised controlled trial. Lancet Diabetes Endocrinol. (2014) 2:954–62. doi: 10.1016/S2213-8587(14)70200-1
39. Vink, RG, Roumans, NJ, Arkenbosch, LA, Mariman, EC, and van Baak, MA. The effect of rate of weight loss on long-term weight regain in adults with overweight and obesity. Obesity. (2016) 24:321–7. doi: 10.1002/oby.21346
40. Johannsen, DL, Knuth, ND, Huizenga, R, Rood, JC, Ravussin, E, and Hall, KD. Metabolic slowing with massive weight loss despite preservation of fat-free mass. J Clin Endocrinol Metab. (2012) 97:2489–96. doi: 10.1210/jc.2012-1444
41. Sumithran, P, Prendergast, LA, Delbridge, E, Purcell, K, Shulkes, A, Kriketos, A, et al. Long-term persistence of hormonal adaptations to weight loss. N Engl J Med. (2011) 365:1597–604. doi: 10.1056/NEJMoa1105816
42. Alharbi, K, Blakeman, T, van Marwijk, H, Reeves, D, and Tsang, JY. Understanding the implementation of interventions to improve the management of frailty in primary care: a rapid realist review. BMJ Open. (2022) 12:e054780. doi: 10.1136/bmjopen-2021-054780
43. Madigan, CD, Graham, HE, Sturgiss, E, Kettle, VE, Gokal, K, Biddle, G, et al. Effectiveness of weight management interventions for adults delivered in primary care: systematic review and meta-analysis of randomised controlled trials. BMJ. (2022) 377:e069719. doi: 10.1136/bmj-2021-069719
44. MacLean, PS, Rothman, AJ, Nicastro, HL, Czajkowski, SM, Agurs-Collins, T, Rice, EL, et al. The accumulating data to optimally predict obesity treatment (ADOPT) core measures project: rationale and approach. Obesity. (2018) 26:S6–S15. doi: 10.1002/oby.22154
45. Garvey, WT, Mechanick, JI, Brett, EM, Garber, AJ, Hurley, DL, Jastreboff, AM, et al. American Association of Clinical Endocrinologists and American College Of Endocrinology comprehensive clinical practice guidelines for medical care of patients with obesityexecutive summarycomplete guidelines. Endocr Pract. (2016) 22:842–84. doi: 10.4158/EP161356.ESGL
Keywords: overweight, obesity, weight management, electronic medical record (EMR), general practice
Citation: Perreault L, Kramer ES, Smith PC, Schmidt D and Argyropoulos C (2023) A closer look at weight loss interventions in primary care: a systematic review and meta-analysis. Front. Med. 10:1204849. doi: 10.3389/fmed.2023.1204849
Edited by:
Christo Karuna, Monash University, AustraliaReviewed by:
Julia Lortz, Essen University Hospital, GermanyHarm Van Marwijk, Brighton and Sussex Medical School, United Kingdom
Copyright © 2023 Perreault, Kramer, Smith, Schmidt and Argyropoulos. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Leigh Perreault, TGVpZ2gucGVycmVhdWx0QENVQW5zY2h1dHouZWR1
 Leigh Perreault
Leigh Perreault E. Seth Kramer2
E. Seth Kramer2 Peter C. Smith
Peter C. Smith