- Gannan Medical University, Ganzhou, Jiangxi, China
Background: In recent years, platelet-rich plasma (PRP) injections for osteoarthritis (OA) have been widely promoted in clinical practice, but their effectiveness is controversial. Therefore, we conducted a meta-analysis of relevant randomized controlled trials (RCTs) to determine the efficacy and safety of PRP injections for the treatment of OA.
Methods: We searched databases including Embase, Web of Science, Medline, PubMed, and the Cochrane Library for relevant studies. Two researchers (YQX and CG) performed literature screening, baseline data extraction, literature quality assessment, and heterogeneity analysis of RCTs from the retrieved studies. Based on the magnitude of heterogeneity I2, random-effects or fixed-effects models were selected for the meta-analysis.
Results: We included 24 RCTs comprising 1344 patients with OA who met the inclusion criteria, with the main types of morbidity being knee osteoarthritis (KOA), hip osteoarthritis (HOA), ankle osteoarthritis (AOA), and temporomandibular joint osteoarthritis (TMJOA). Our results indicate that PRP injections were effective in improving Visual Analog Scale (VAS) pain scores in patients with KOA, HOA, and AOA compared to controls (AOA, MD = −1.15, CI = 95% [−1.74, −0.56], I2 = 40%, P < 0.05; KOA, MD = −1.03, CI = 95% [−1.16, −0.9], I2 = 87%, P < 0.05; TMJOA, MD = −1.35, CI = 95% [−1.74, −0.97], I2 = 92%, P < 0.05) but showed no significant efficacy in patients with HOA (MD = −0.27, CI = 95% [−0.8, 0.26], I2 = 56%, P>0.05). Compared to controls, PRP injections were effective in improving Knee Injury and Osteoarthritis Outcome Score (KOOS), including the patient's pain symptoms, activities of daily living (ADL), and adhesion symptomatology, but not for that of sports function (KOOS-pain, MD = 2.77, CI = 95% [0, 5.53], I2 = 0%, P < 0.05; KOOS-symptoms, MD = 3.73, CI = 95% [0.76, 6.71], I2 = 0%, P < 0.05; KOOS-ADL, MD = 3.61, CI = 95% [0.79, 6.43], I2 = 0%, P < 0.05; KOOS-QOL, MD = 4.66, CI = 95% [0.98, 8.35], I2 = 29%, P < 0.05, KOOS-sport, MD = 0.48, CI = 95% [−3.02, 3.98], I2 = 0%, P > 0.05). PRP injections were effective in improving Western Ontario and McMaster Universities Arthritis Index (WOMAC) scores, including pain, stiffness, and functional joint motion, in patients with OA compared with the control group (WOMAC-pain, MD = −1.08, CI = 95% [−1.62, −0.53], I2 = 87%, P < 0.05; WOMAC-stiffness, MD = −1.17, CI = 88% [−1.72, −0.63], I2 = 87%, P < 0.05; WOMAC-function, MD = −1.12, CI = 95% [−1.65, −0.58], I2 = 87%, P < 0.05). In addition, subgroup analysis showed that leukocyte-poor (LP) PRP injections were more effective than leukocyte-rich (LR) PRP injections in improving pain symptoms in patients with OA (VAS, LR-PRP, MD = −0.81, CI = 95% [−1.65, −0.03], I2 = 83%, P = 0.06 > 0.05; LP-PRP, MD = −1.62, CI = 95% [−2.36, −0.88], I2 = 92%, P < 0.05). A subgroup analysis based on injection sites showed that no statistical difference in efficacy between intra-articular (IA) combined with intra-osseous (IO) simultaneous PRP injections. IA PRP injections only improved VAS pain scores in patients with OA (IA+IO PRP injections, MD = −0.74, CI =95% [−1.29, −0.18], I2 = 61%, P < 0.05; IA PRP injections, MD = −1.43, CI = 95% [−2.18, −0.68], I2 = 87%, P < 0.05, test for subgroup differences, P > 0.05, I2 = 52.7%).
Conclusion: PRP injection therapy can safely and effectively improve functional activity in patients with OA and produce positive analgesic effects in patients with KOA, TMJOA, and AOA. However, PRP injection therapy did not significantly reduce pain symptoms in patients with HOA. In addition, the analgesic effect of LP-PRP was greater than that of LR-PRP.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/, identifier: CRD42022362066.
1. Introduction
Osteoarthritis (OA) is a degenerative disease that affects the entire joint and is characterized by progressive cartilage degeneration, synovial inflammation, bone formation, and subchondral sclerosis (1). Clinical features include local inflammation, pain, stiffness, and limited joint movement (2). Currently, OA affects ~3.3–3.6% of the global population and causes moderate-to-severe disability in 43 million people, severely affecting patients' quality of life and increasing the burden on families, making it the 11th most debilitating disease in the world (3). As the population ages and obesity rates rise, the incidence of OA is further increasing (4, 5).
Current OA treatments focus on disease prevention and early treatment (6). Common OA treatments include physical therapy (7), oral medications (8), and intra-articular (IA) injections (9). IA injections, including local anesthetics (10), hyaluronic acid (HA), and corticosteroids (CCS) (11, 12), are often an option for patients with early-to mid-stage OA who fail to achieve satisfactory results after various other non-surgical treatments. However, studies have shown that these pharmacological treatments provide only temporary benefits and are often accompanied by side effects such as stiffness and swelling at the injection site (13). Platelet-rich plasma (PRP) is a more recent treatment that has attracted attention because of its high regenerative capacity, ease of extraction and preparation, low rejection rate, and few adverse effects (14, 15).
PRP is a highly concentrated autologous blood product of platelets and other active substances (16) and may play an important role in autologous cell therapy in various regenerative medicine programs. PRP therapy promotes chondrocyte proliferation and cartilage matrix formation and inhibits the expression of inflammatory factors (17, 18). Inflammation and inflammatory responses are thought to be key factors that cause and accelerate OA development (19). Thus, theoretically, PRP therapy may reduce inflammation during OA treatment.
In recent years, the clinical application of IA PRP injections for OA has been widely promoted; however, the effectiveness of this treatment remains controversial. Many new randomized controlled trials (RCTs) of PRP injection for OA have been published. We conducted a systematic review and meta-analysis of these randomized trials to analyze the efficacy and safety of PRP for OA, with the aim of providing an evidence-based basis for the clinical application of PRP injections for OA treatment.
2. Methods
2.1. Protocol and registration
Our systematic review was designed and implemented based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (20). The study was registered in Prospero (CRD42022362066).
2.2. Retrieval strategy
In the initial screening, two researchers (XYQ and CG) independently searched Embase, Web of Science, Medline, PubMed, and Cochrane Library databases and collected all studies from the date of database creation to March 2023. The main search terms were “osteoarthritis,” “osteoarthrosis,” “osteoarthritides,” and “platelet-rich plasma.” In addition, we manually searched for other relevant literature, such as studies included in some systematic reviews and meta-analyses, to broaden the search for eligible articles. For instance, the following search strategy was used for PubMed: ((“Osteoarthritis”[Mesh]) OR (((((((Osteoarthritides) OR (Osteoarthrosis)) OR (Osteoarthroses)) OR (Degenerative Arthritides[Title/Abstract])) OR (Degenerative Arthritis[Title/Abstract])) OR (Arthrosis[Title/Abstract])) OR (Arthroses[Title/Abstract]))) AND ((“Platelet-Rich Plasma”[Mesh]) OR ((Plasma, Platelet-Rich) OR (Platelet Rich Plasma))). A similar search strategy was used for the other databases. Full details of the search strategy for all databases can be found in Data Sheet 1.
2.3. Inclusion and exclusion criteria
The inclusion and exclusion criteria for literature screening were predetermined to allow for a more rigorous process. Inclusion criteria included (1) patients diagnosed with OA by clinical examination; (2) trial group received PRP injection treatment intervention; (3) control group received HA injection treatment or saline injection treatment; (4) outcome indicators of functional activity and analgesic effects were assessed by relevant scales, such as the Visual Analog Scale (VAS), Western Ontario and McMaster Universities Arthritis Index (WOMAC), Knee Injury and Osteoarthritis Outcome Score (KOOS), and International Knee documentation Committee (IKDC); (5) the experimental design was a RCT; (6) the language was restricted to English.
The exclusion criteria were as follows: (1) animal experiments; (2) IA injection of other drugs, such as Procaine and Lidocaine, over the previous 1 year; (3) full-text content not available; and (4) missing or duplicate experimental data.
2.4. Study selection
We imported all retrieved studies into the document management software Endnote 20 and removed duplicate studies. Two researchers (YX and CG) read the title and abstract of each study simultaneously and screened the studies based on previously developed criteria. For further screening, two reviewers downloaded and read the full texts, removed articles that did not meet the inclusion criteria, and discussed them to confirm their eligibility. If there was a disagreement about the screening process for a particular study, a consensus was reached through advice provided by the principal investigator (WL).
2.5. Data extraction
Two reviewers (YX and CG) independently extracted the following data from the included literature: first author of the study, year of publication, sample size, patient age, sex, disease type, disease duration, interventions, and outcome indicators of the trial. Specific therapeutic parameters, including the treatment site, dosage, and number of injections, were also recorded in detail for the intervention method. In addition, when the two reviewers (YX and CG) encountered unclear or complicated extraction of complete literature, the original authors were contacted to obtain complete experimental data. After three consecutive emails, the study was considered missing data if no response was received from the original author.
2.6. Quality assessment and risk of bias assessment
A quality assessment of the literature was completed independently by two researchers (YX and CG), followed by a discussion to produce consistent results. A risk of bias assessment was conducted using the Cochrane Risk of Bias tool (Review Manager 5.40). Items were assessed in seven areas: blinding of participants and personnel, blinding of outcome assessments, allocation concealment, random sequence generation, incomplete outcome data, selective reporting, and other biases. Risk of bias was graded as high, low, or unclear (21). The risk of bias for each study was documented by mapping the risk of bias assessment.
Heterogeneity between studies was statistically analyzed using RevMan 5.40. The size of heterogeneity was expressed as I2; high heterogeneity was judged when I2 ≥ 75%, moderate heterogeneity when 75% > I2 ≥ 50%, low heterogeneity when 50% > I2 ≥ 25%, and no heterogeneity when I2 = 0% (22).
The quality of evidence for the outcome indicators was assessed using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system, which examines areas such as study limitations, intermittency, inconsistency, and imprecision of results (23). The results were assessed by grading the evidence for the outcome indicators as “high,” “moderate,” “low,” or “very low,” and the strength of the recommendations was divided into two levels: “strong” and “weak” (24).
2.7. Statistical analysis
For statistical and analytical purposes, the extracted study data were entered into RevMan 5.40 software. This meta-analysis was performed using either a fixed-effects or a random-effects model, depending on the size of heterogeneity. When I2 ≥ 50%, a random effects model was used; when I2 < 50%, a fixed effects model was used (25). We used 95% confidence intervals (CI) and mean differences (MD) to measure the effect size. The effect sizes of different units of the same outcome index were measured using the standardized mean difference.
3. Results
3.1. Results of the literature search
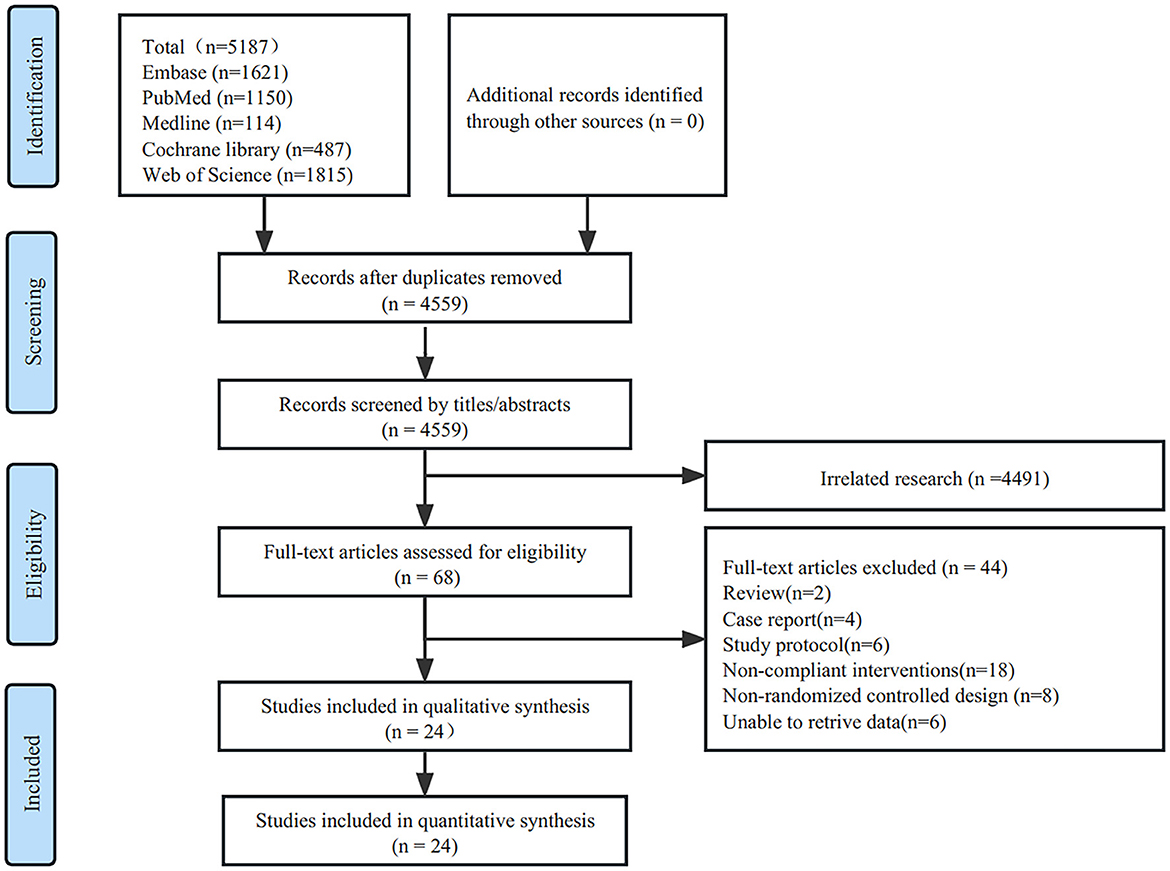
The initial search resulted in 5,187 studies obtained from five databases. After screening for duplicate studies using the software deletion function, 4,559 studies remained. Two reviewers (YQX and CG) read the titles and abstracts of these studies and screened 4,491 studies that were not relevant to the topic. The remaining 68 studies were read, leaving 44 excluded studies, including two reviews, four case reports, six study protocols, 18 studies with non-compliant interventions, 8 non-RCT studies, and 6 studies with no data. Twenty–four eligible studies were included in the final analysis (26–49) (Figure 1).
3.2. Characteristics of included studies
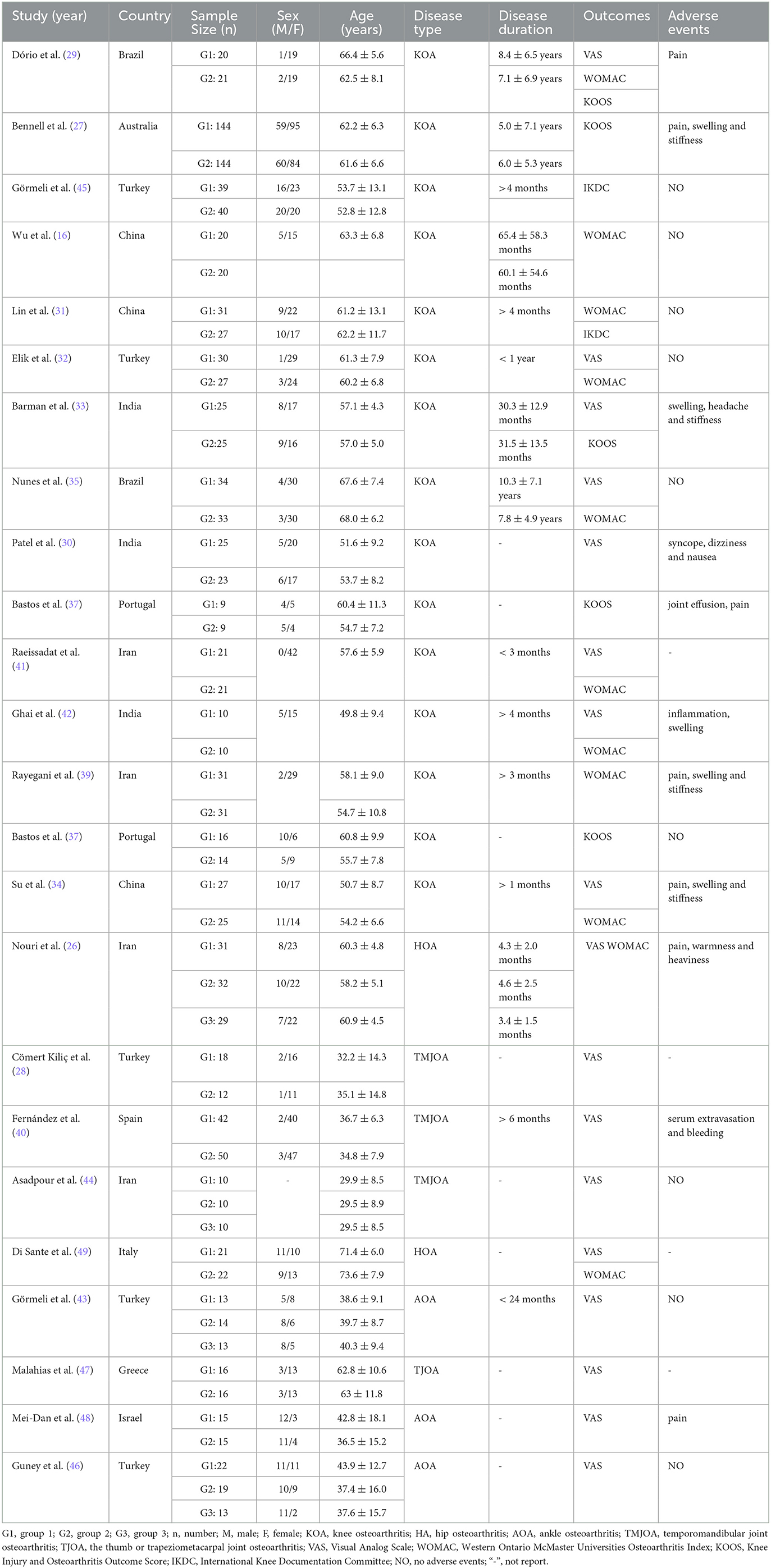
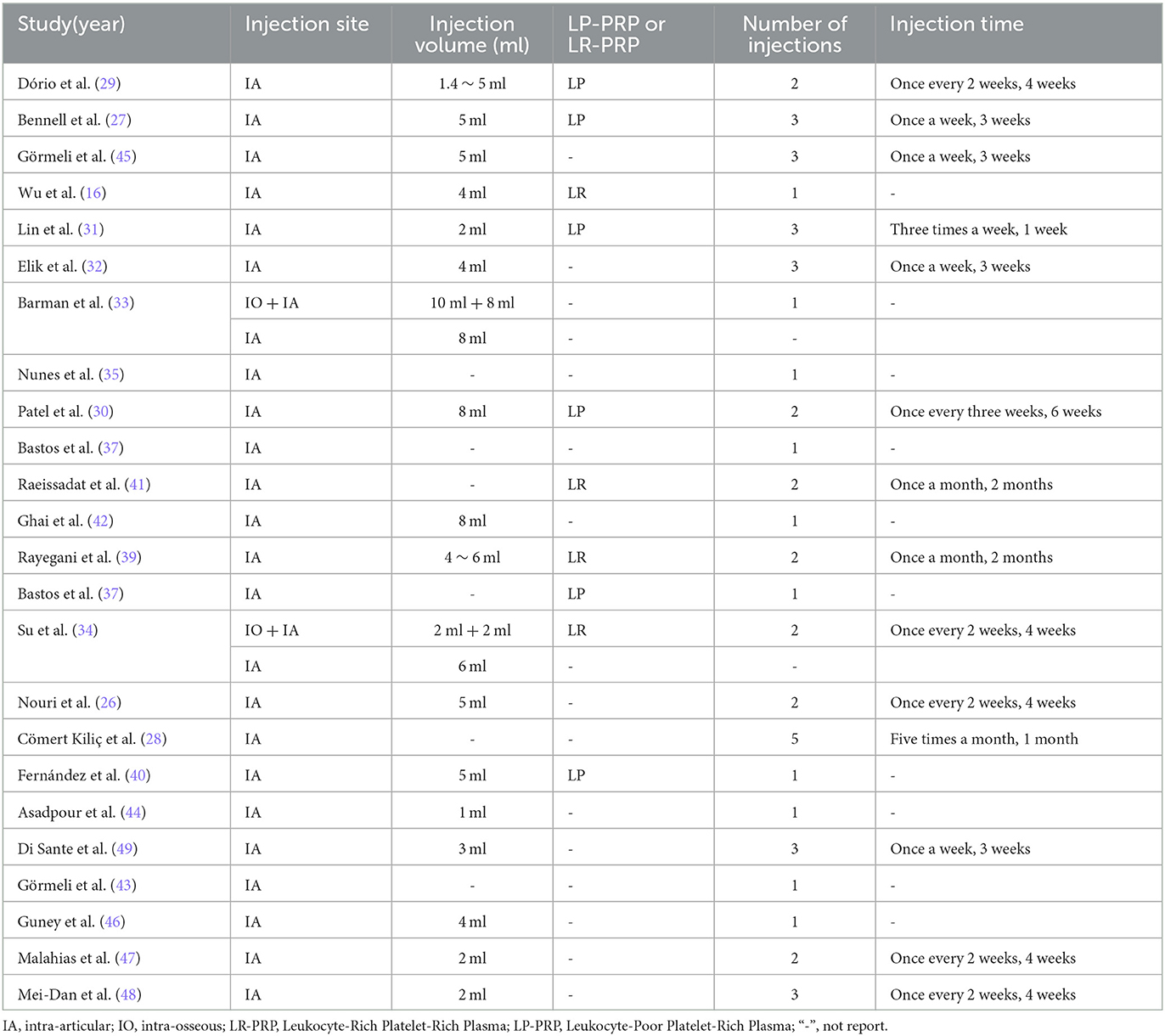
Baseline data and experimental intervention parameters were extracted from the 24 included RCTs, summarized in Tables 1, 2, respectively. A total of 1344 patients with OA were eligible across the 24 studies, 712 of whom received PRP injections, and 683 who only received HA or saline as controls (some KOA patients have received treatment in both knees). The intervention characteristics of PRP treatment, such as injection site, injection dose, presence of leukocytes, number of injections, and frequency of injections, are shown in Table 2. In our meta-analysis, 10 studies with a total of 211 patients received one PRP injection, and the remaining 14 studies with a total of 501 patients received two or more PRP injections.
3.3. Results of the quality assessment
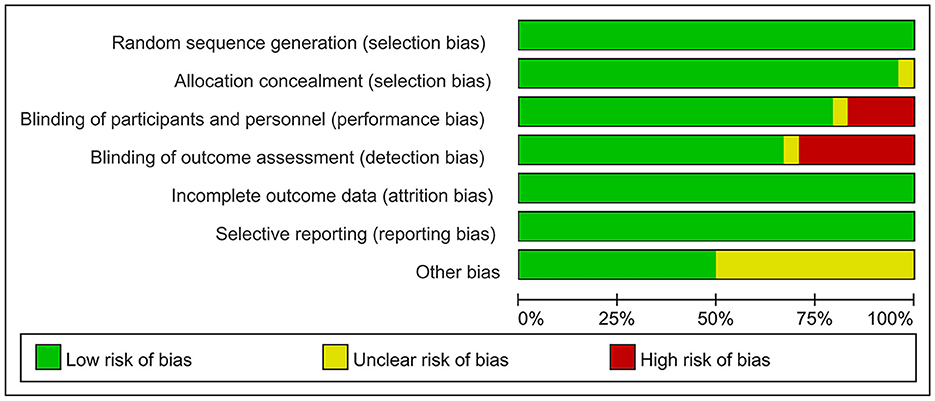
The risk of bias was assessed using the Cochrane Risk of Bias Assessment Tool (RevMan 5.40) for the 24 included RCTs. In 4 of the 24 studies, investigators did not use reasonable blinding of participants and staff, resulting in a high risk of performance bias. In another 7 of the 24 studies, investigators did not use reasonable blinding when assessing outcome indicators, resulting in a high risk of detection bias. Other studies have shown a low or unclear risk in all risk–bias assessments. Overall, the included RCTs had a low risk of bias (Figures 2, 3).
We assessed the level of evidence for the main outcome indicators (VAS, IKDC, KOOS, and WOMAC) in the included studies by using GRADE. The quality of evidence for IKDC and WOMAC was assessed as moderate owing to high heterogeneity (I2 > 80%), with serious inconsistencies between studies. The quality of evidence for the VAS and KOOS was rated as high because no serious risk or downgrading factors were identified in any of the projects. Overall, the GRADE recommendation rating was “strong” for the four primary outcome indicators (Table 3).
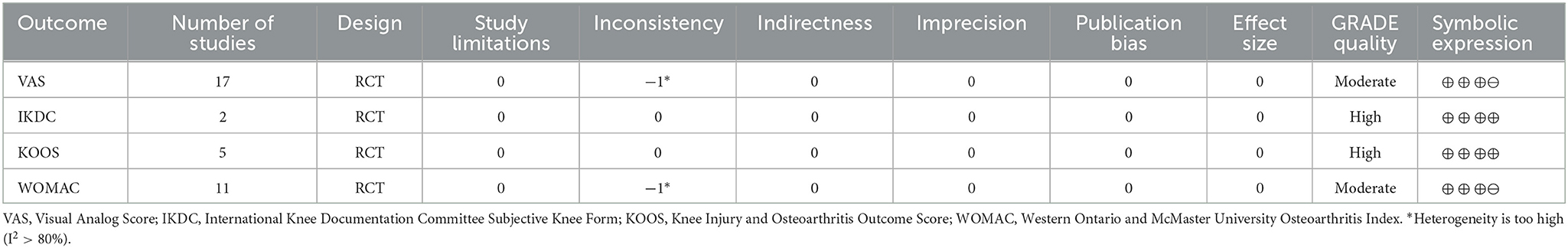
Table 3. Grading of recommendations assessment, development, and evaluation (GRADE) quality of evidence.
3.4. Results of statistical analysis
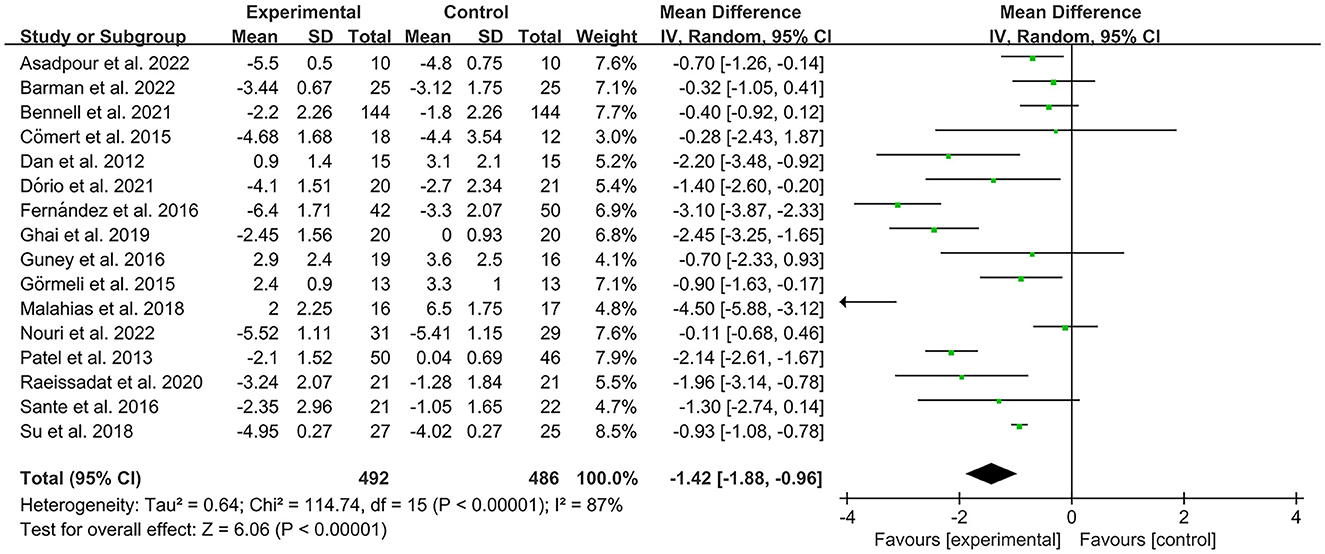
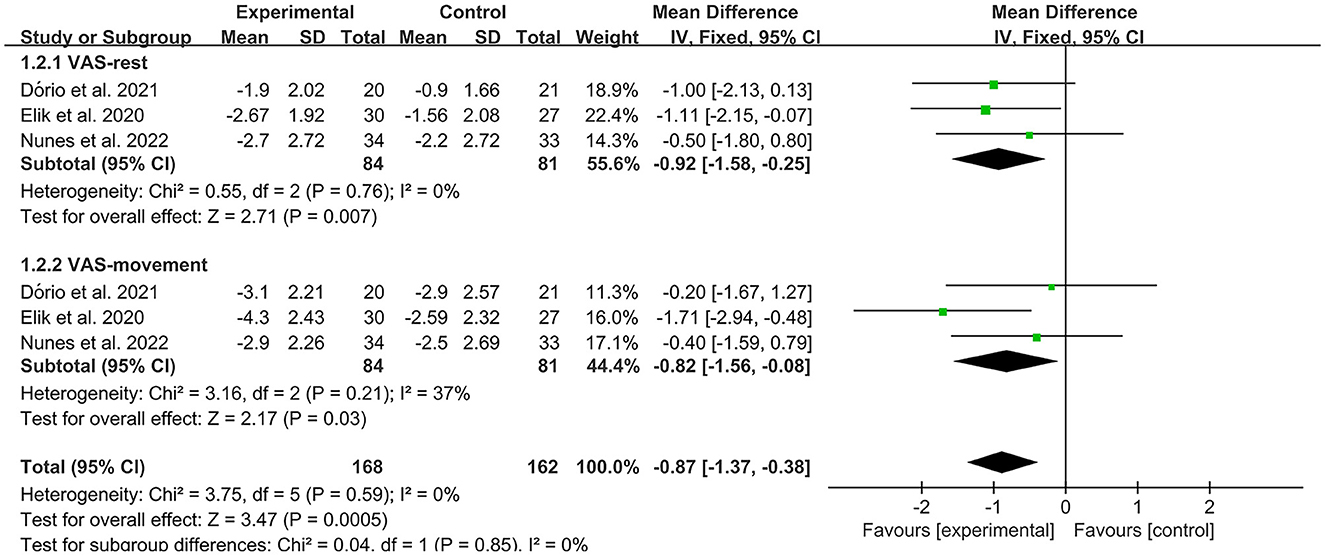
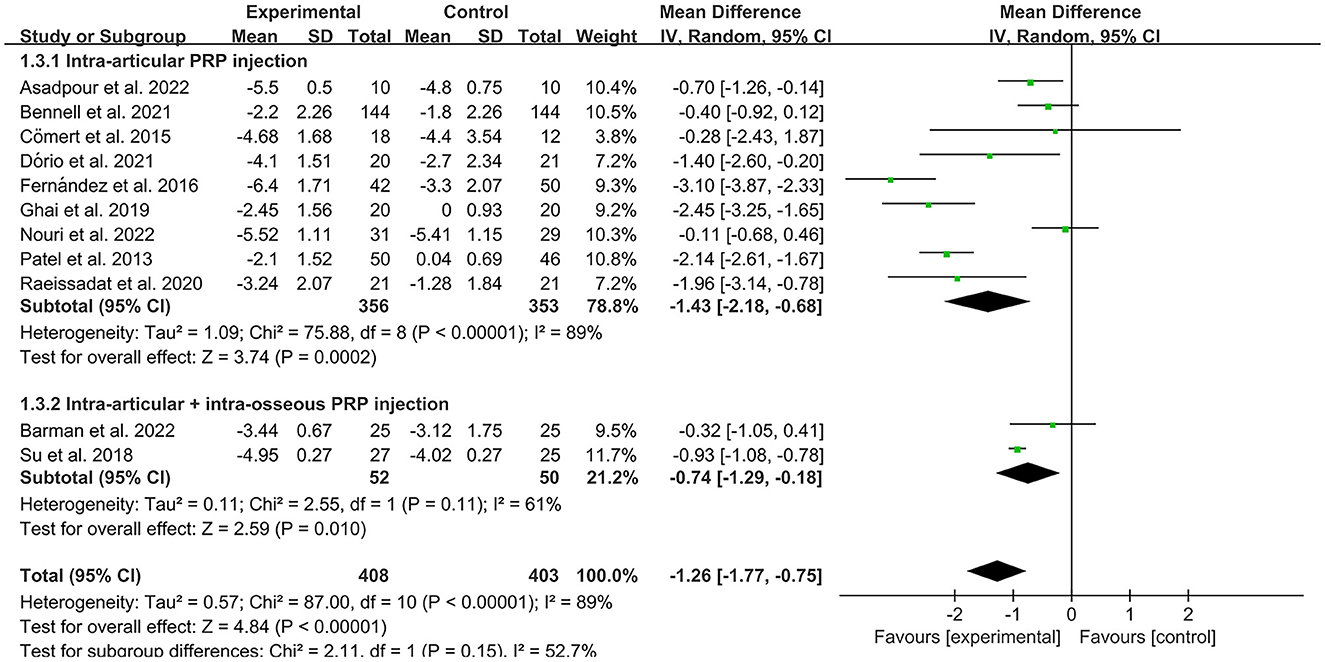
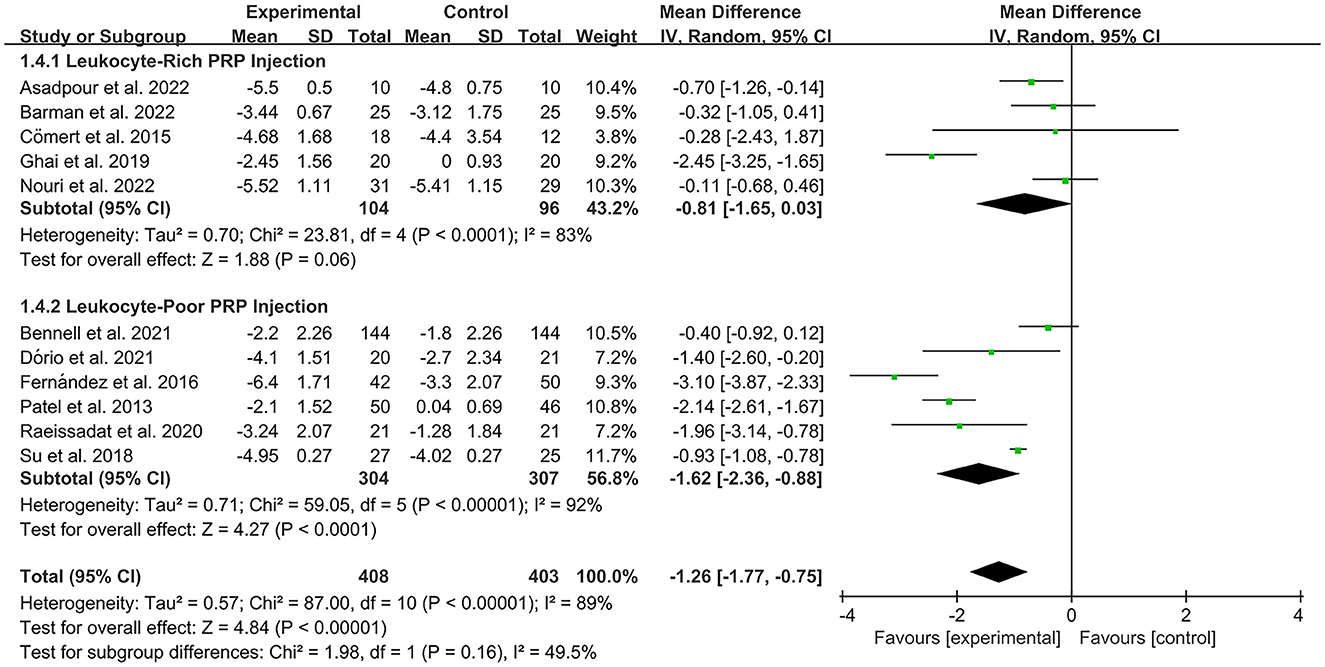
The VAS was used by investigators in 17 studies (26, 28–30, 32–35, 40–42, 44–49) to assess pain intensity. In these studies, 966 subjects participated in PRP injection therapy trials, divided into experimental and control groups. The I2 value was 87%. Therefore, a random-effects model was used. Data analysis of the forest plot showed that the experimental group with PRP injection as an intervention had a significant improvement in VAS OA patients' pain scores compared to the control group (MD = −1.42, CI = 95% [−1.88, −0.96], I2 = 87%, P < 0.05; Figure 4). A subgroup analysis showed that the pain efficacy of PRP in patients with OA was synchronized in both the rest and movement states, and pain relief was consistent in both states (VAS-rest, MD = −0.92, CI = 95% [−1.58, −0.25], I2 = 89%, P < 0.05; VAS-movement, MD = −0.82, CI = 95% [−1.56, −0.08], test for subgroup differences, P>0.05, I2= 0%; Figure 5). Additional subgroup analyses based on injection sites showed no statistical difference in efficacy between IA combined with intra-osseous (IO) simultaneous PRP injections and IA PRP injections in improving VAS scores in patients with OA (IA+IO PRP injections, MD= −0.74, CI = 95% [−1.29, −0.18], I2 = 61%, P < 0.05; IA PRP injections, MD= −1.43, CI = 95% [−2.18, −0.68], I2 = 87%, P < 0.05, test for subgroup differences, P > 0.05, I2 = 52.7%; Figure 6). A subgroup analysis based on leukocyte concentration showed that leukocyte-rich (LR) PRP injections were not considered effective for patients' pain symptoms. In contrast, leukocyte-poor (LP) PRP injections were effective in relieving pain (VAS, LR-PRP, MD = −0.81, CI = 95% [−1.65, −0.03], I2 = 83%, P = 0.06 > 0.05; LP-PRP, MD = −1.62, CI = 95% [−2.36, −0.88], I2 = 92%, P < 0.05; test for subgroup differences, P>0.05, I2 = 11.6%; Figure 7).
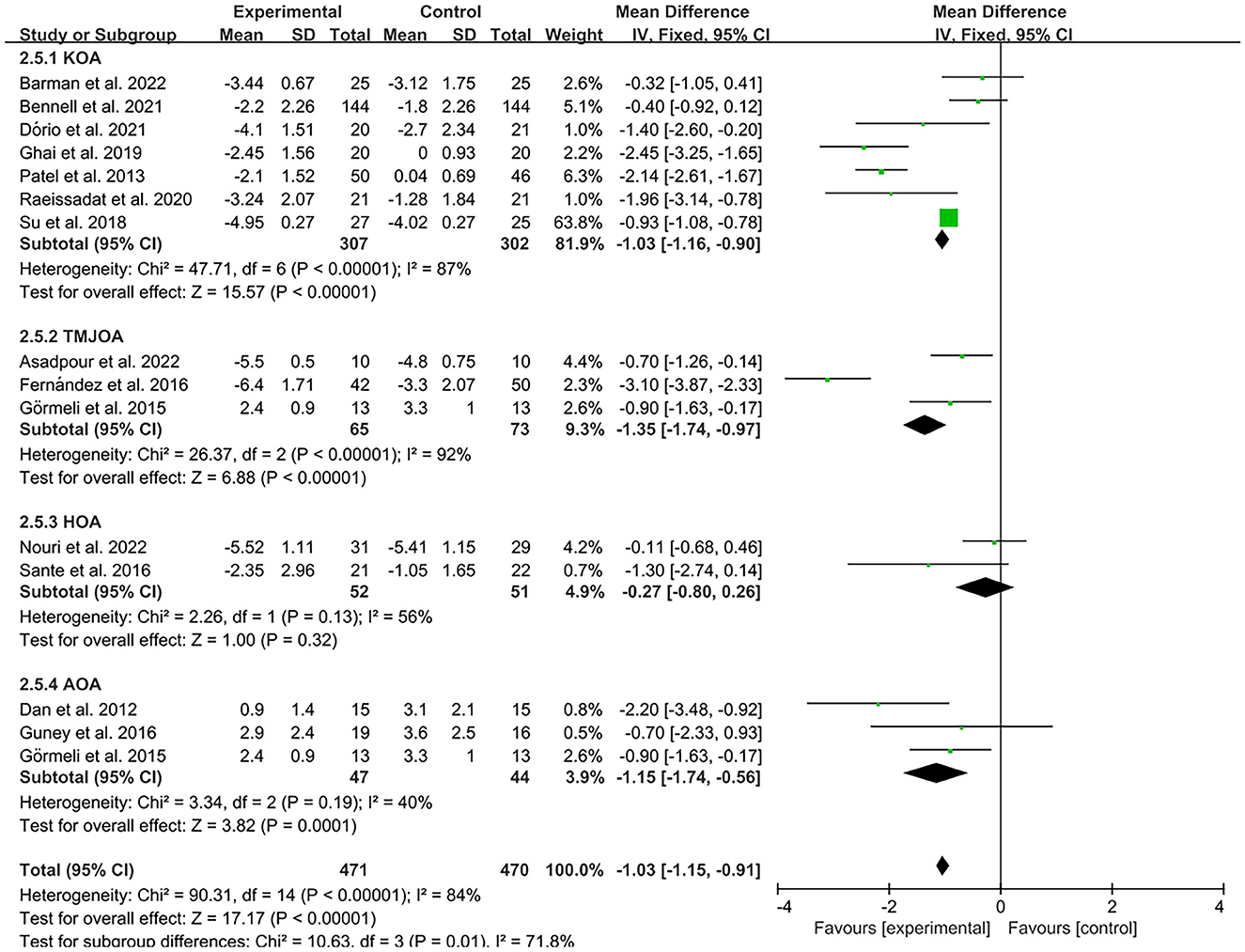
The results of the forest plot data analysis based on PRP treatments of different joint sites indicate that PRP injection therapy was effective in reducing VAS pain scores in patients with ankle osteoarthritis (AOA), knee osteoarthritis (KOA) and temporomandibular joint osteoarthritis (TMJOA) compared to controls (AOA, MD = −1.15, CI = 95% [−1.74, −0.56], I2 = 40%, P < 0.05; KOA, MD = −1.03, CI = 95% [−1.16, −0.9], I2 = 87%, P < 0.05; TMJOA, MD = −1.35, CI = 95% [−1.74, −0.97], I2 = 92%, P < 0.05; Figure 8). However, there was no statistically significant difference between the experimental and control groups in VAS pain scores of hip osteoarthritis (HOA, MD = −0.27, CI = 95% [−0.8, 0.26], I2 = 56%, P > 0.05; Figure 8).
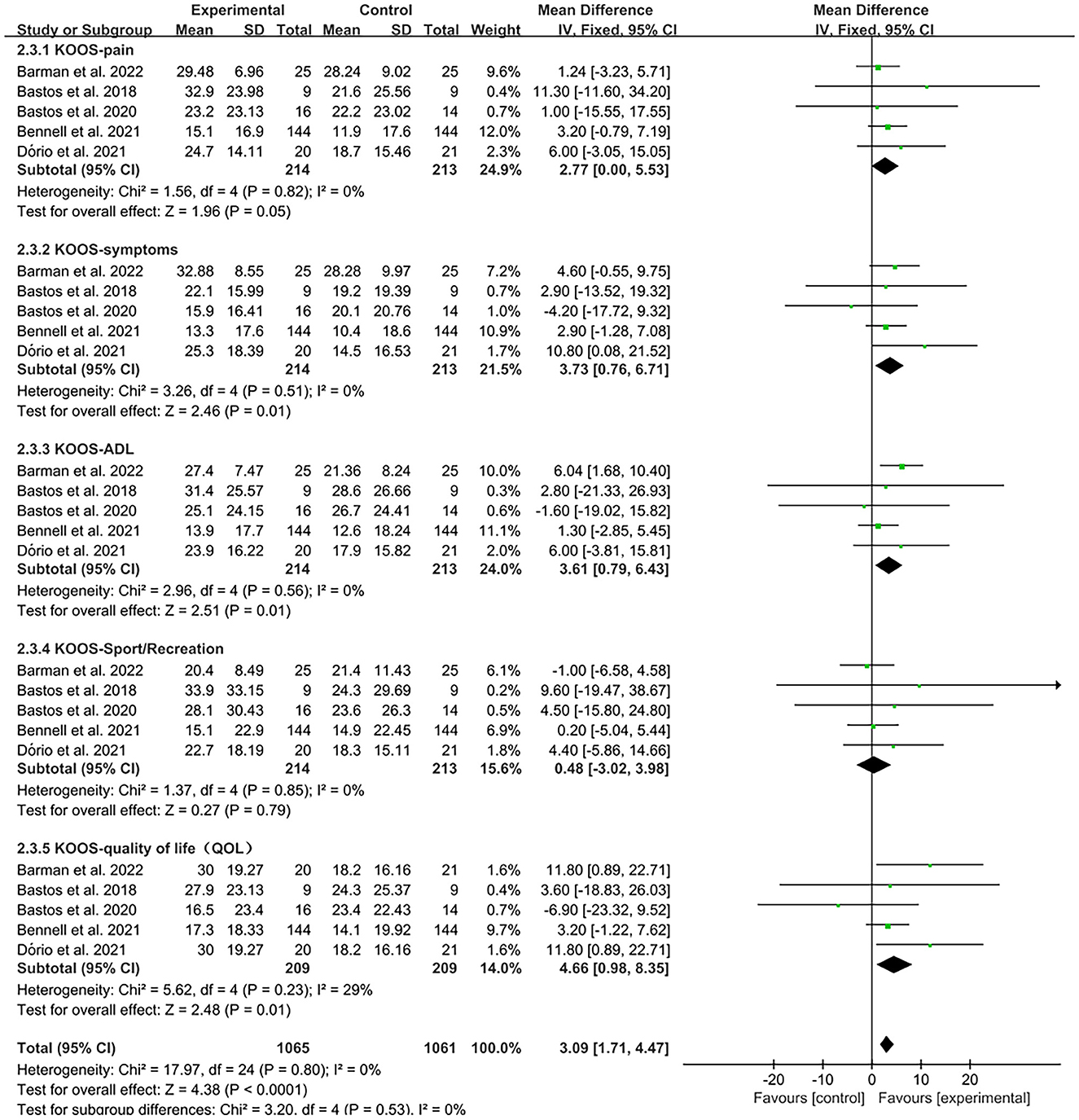
The KOOS was used by investigators in five studies (27, 29, 33, 37, 38) to assess treatment effects. In these studies, 427 subjects participated in PRP injection treatment trials, divided into experimental and control groups. The I2 value was calculated to be 0%. Therefore, we used a fixed effects model in this study. Forest plots with KOOS as an outcome indicator showed that the experimental group had significantly improved pain scores, symptom scores, activities of daily living scale (ADL) scores, and Quality of Life scores (QOL) in patients with OA compared with the control group, but no significant improvement was observed in the sport scores (KOOS-pain, MD = 2.77, CI = 95% [0, 5.53], I2 = 0%, P < 0.05; KOOS-symptoms, MD = 3.73, CI = 95% [0.76, 6.71], I2 = 0%, P < 0.05; KOOS-ADL, MD = 3.61, CI = 95% [0.79, 6.43], I2 = 0%, P < 0.05; KOOS-QOL, MD = 4.66, CI = 95% [0.98, 8.35], I2 = 29%, P < 0.05; KOOS-sport, MD = 0.48, CI = 95% [−3.02, 3.98], I2 = 0%, P > 0.05; Figure 9).
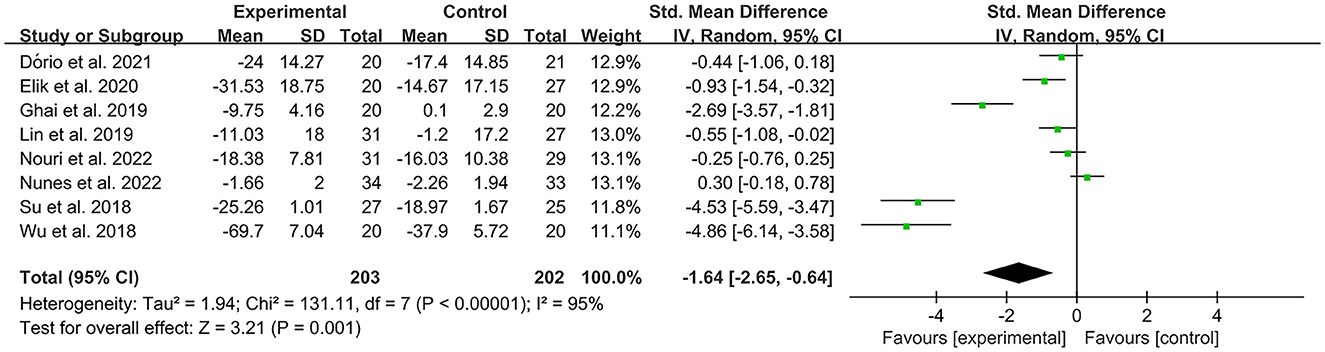
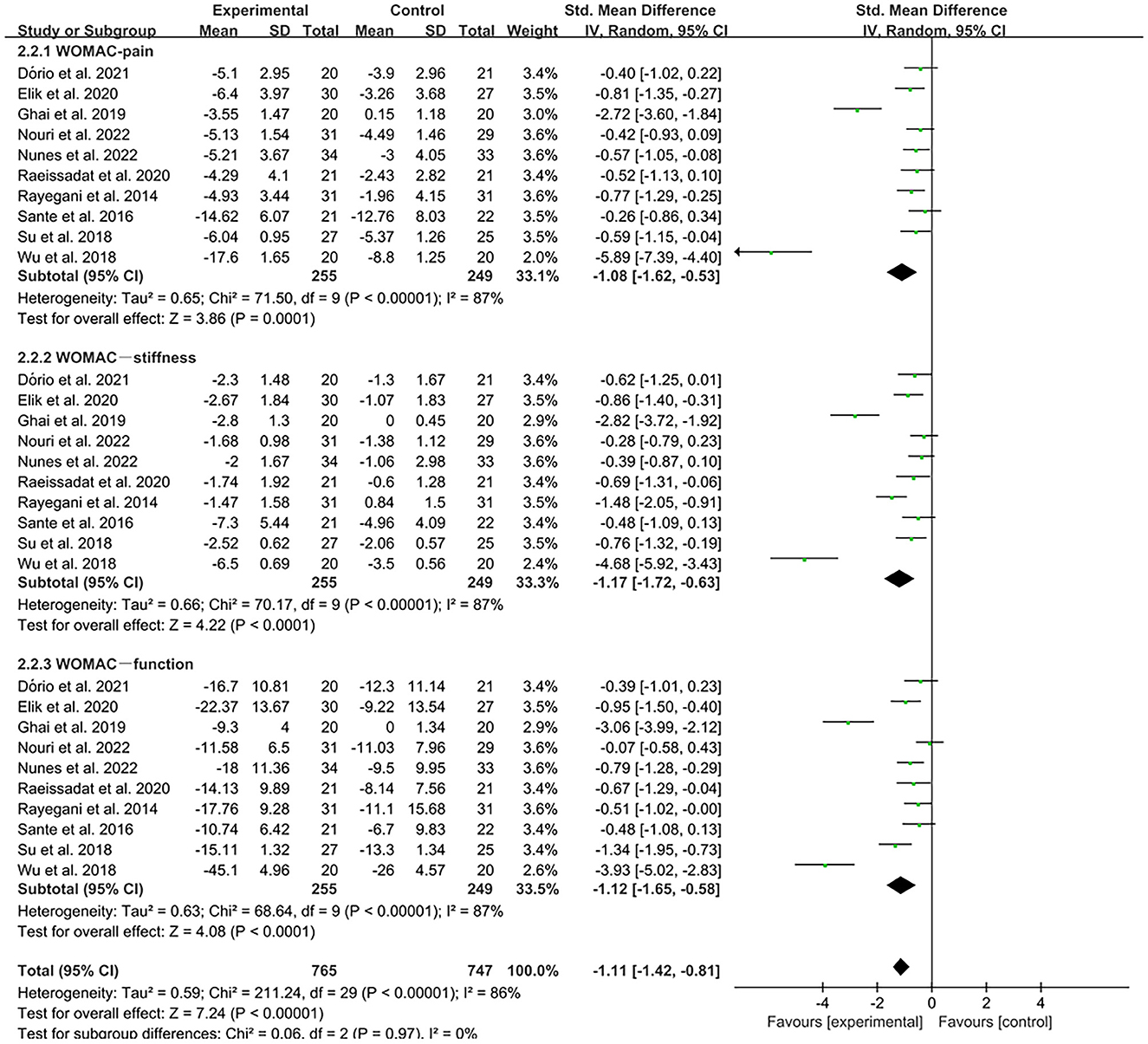
The WOMAC was used by investigators in 11 studies (26, 29, 31, 32, 34–36, 39, 41, 42, 49) to assess joint functional activity. In these studies, 574 subjects participated in PRP injection treatment trials, divided into experimental and control groups. The I2 value was 95%. Therefore, a random-effects model was used. The forest plots with WOMAC as an outcome indicator showed that WOMAC scores of patients with OA improved in the experimental group but not the control group (MD = −1.64, CI = 95% [−2.65, −0.64], I2 = 95%, P < 0.05; Figure 10). In the experimental group, patients showed significant improvements in joint pain, joint stiffness, and functional activity (WOMAC-pain, MD = −1.08, CI = 95% [−1.62, −0.53], I2 = 87%, P < 0.05; WOMAC-stiffness, MD = −1.17, CI = 88% [−1.72, −0.63], I2 = 87%, P < 0.05; WOMAC- function, MD = −1.12, CI = 95% [−1.65, −0.58], I2 = 87%, P < 0.05; Figure 11).
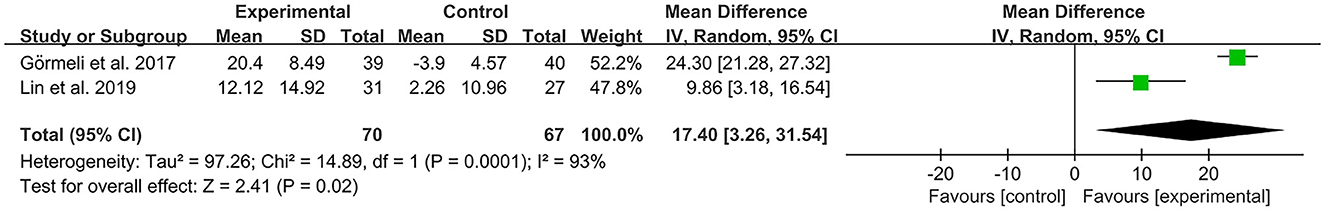
The IKDC was used by investigators in two studies (31, 43) to assess the functional activity of the joints. In these studies, 137 subjects participated in PRP injection treatment trials and were divided into experimental and control groups. The I2 value was 93%. Therefore, a random-effects model was used. The results of the forest plot data analysis with IKDC as an outcome indicator showed that the experimental group receiving PRP demonstrated improved OA patient IKDC scores when compared to the control group (MD = 17.4, CI = 95% [3.26, 31.54], I2 = 93%, P < 0.05; Figure 12).
3.5. Adverse event reporting results
Of the 24 studies included, 11 reported adverse reactions after PRP injections in patients with OA; however, most symptoms were mild and transient, such as pain and swelling at the injection site. Of these 11 studies, 2 showed more adverse reactions in the PRP injection group, with patients presenting with syncope, dizziness, headache, nausea, gastritis, sweating, and tachycardia, in addition to pain and stiffness (26, 30). Only one study reported intense knee pain in two patients, and all adverse events reported were resolved without any sequelae (38). Based on the results reported in all studies, a few patients withdrew from the experimental studies because they experienced excessive adverse effects (34).
4. Discussion
We analyzed PRP treatment of OA and also discussed the intervention parameters, such as PRP injection sites and leukocyte concentration contained in the injection solution, hoping to provide reference suggestions for the optimal treatment protocol of clinical PRP.
Currently, PRP is widely used for wound healing and tissue regeneration in orthopedics, dentistry, and plastic surgery. It has been shown to promote angiogenesis, cell proliferation, and collagen synthesis to repair tendons, ligaments, cartilage, and other avascular damaged tissues with low self-healing capacity; to act as an anti-inflammatory and analgesic; and to improve motor function (50–52). How PRP exerts its efficacy in the treatment of OA is not fully understood, but numerous studies have shown that it may be related to the presence of various growth factors and inflammatory cells. Platelets contain a large number of α particles, which include a variety of bioactive factors: transforming growth factor (TGF)-β, platelet-let-derived growth factor (PDGF), vascular endothelial growth factor (EGF), hepatocyte growth factor, epidermal growth factor, fibroblast growth factor, and insulin-like growth factor (53, 54). In OA treatment, within 10 min after the injection of PRP activated by exogenous activators, platelets aggregate and coagulate in the joint cavity, and within 1 h nearly 95% of α particles secrete large amounts of cytokines and growth factors (55). After the platelets are activated, these bioactive factors are released into the bloodstream and play an important role in tissue repair and regeneration. TGF-β and PDGF are important bioactive factors that promote tissue repair (56). Interleukin (IL)-1β interferes with the normal metabolic activity of chondrocytes, inhibits normal chondrocyte differentiation and induces chondrocyte apoptosis, while TGF-β inhibits the interference of IL-1β with chondrocytes and prevents chondrocyte apoptosis (57). TGF-β further activates activinreceptor-like kinases-5 (ALK-5), which regulates cartilage terminal differentiation through the Smad signaling pathway, has a role in promoting chondrocyte proliferation and differentiation, and induces bone marrow mesenchymal stem cells (BMSCs) to differentiate into chondrocytes, regulate the proliferation of other growth factors, and inhibit the expression of some inflammatory factors. PDGF promotes osteoblast proliferation and chemotaxis, enhances collagen synthesis, and stimulates fibroblast proliferation and chemotaxis (58). PRP contains various plasma proteins that activate fibrinogen to form fibrin scaffolds, induce chondrocyte proliferation and differentiation, and promote cartilage damage repair (59).
Most current studies on PRP have used IA injections, with some investigators studying injection sites to determine optimal injection locations. For example, subchondral IO PRP injections have become a popular research topic. The subchondral bone, the bony component of the distal end of the calcified cartilage, is located below the mineralized zone of the articular cartilage and forms the articular cartilage-subchondral bone complex unit with the cartilage. IO PRP injections, which target the subchondral bone to promote recovery of the subchondral bone, articular cartilage, and synovium, are effective in treating severe degenerative lesions (60). In the treatment of KOA, IO injection of PRP directly reaches the subchondral bone, maintains the tissue and BMSCs in the PRP matrix, promotes subchondral bone repair, and modulates the inflammatory environment, thus slowing the progression of KOA, and possibly even having a direct impact on stopping the progression of KOA (61). Several studies have shown that IO PRP injections are effective in improving the symptoms of OA (60, 62), and two of the included studies used “IA+IO” (intra-articular combined with intra-osseous) injections. Interestingly, according to our subgroup analysis, we found no statistical difference between the “IA+IO” group and the IA group improving VAS pain scores in patients with OA, probably due to the small sample size (n < 100) and the fact that we only analyzed differences in pain. Further comparative studies are needed to collect data from more studies for functional improvement as well as disease progression.
Further, many types of PRP injections are currently available. Dohan Ehrenfest et al. (63) first proposed to classify PRP products into the following four categories based on their leukocyte and fibrin content: (1) leukocyte-poor or pure platelet-rich plasma (LP-PRP) containing high concentrations of platelets with little or no leukocytes; (2) leucocyte- and platelet-rich plasma (LR-PRP) with a high concentration of platelets and a large number of leukocytes; (3) leucocyte-poor or pure platelet-rich fibrin (PPRF) with rich circulating fibrin with little or no leukocytes; and (4) leucocyte- and platelet-rich fibrin (L-PRF), rich in circulating fibrin and a large number of leukocytes. Two low-density fibrin formulations, LP-PRP and LR-PRP, are often used in injectable therapy for osteoarthritic disease. P-PRF and L-PRF tend to be gel-like because of their high fibrin content. The role of leukocytes in PRP is currently controversial, and some studies have suggested that LR-PRP and L-PRF exert anti-inflammatory and antioxidant effects, promote chondrocyte proliferation, inhibit matrix calcification, and have good prospects for the treatment of OA (18). However, a large body of high-quality evidence has recently emerged to support the use of LP-PRP in OA treatment (54, 64). Based on our subgroup analysis, it cannot yet be assumed that LR-PRP injections are effective in treating pain; therefore, we analyzed the characteristics of the leukocytes themselves. Because leukocytes may produce matrix metalloproteinase-1 (MMP-1) and inflammatory cytokines that are detrimental to joint inflammation and pain (65), injections of LR-PRP cause a more severe acute inflammatory response and increased synovial cell death (66–68), in addition to an increased risk of local adverse effects, pain, and swelling (69). Therefore, in clinical applications, LP-PRP treatment is more effective in improving pain symptoms in OA patients.
In addition, among the subjects included in the current study, only patients with HOA did not show significant efficacy after PRP injection compared to the control group. This may be due to a number of reasons. First, the hip joint itself differs from the other three joints in that it has a deeper joint cavity, fewer blood vessels in the joint cavity, and is prone to femoral head necrosis, thus making it more difficult to perform PRP injection therapy, resulting in the inability of PRP to fully function in the hip cavity. However, it has been argued that the current inclusion of insufficient RCTs with small sample sizes (n < 100) may result in statistical errors. To justify this result, multicenter RCTs of PRP injections for HOA with a larger sample size are required.
5. Limitations
This meta-analysis has several limitations. First, we included only two RCTs in which the subjects were patients with HOA, and the small sample size (n < 100) tended to bias the assessment of treatment effects, thus affecting the results of the analysis. Second, there were too few functional activity assessment indicators for patients with OA; the main outcome indicators were focused on patients with KOA, and the assessment results were not yet fully representative of the degree of functional activity improvement in OA patients. Third, although our study showed a positive therapeutic effect of LP-PRP injection therapy in OA patients, no follow-up records were reported to further confirm the long-term effect of PRP injection. Finally, there were differences in PRP injection concentrations and injection doses between RCTs; however, the current data do not allow for a subgroup analysis of these intervention parameters. Therefore, more multicenter, follow-up, double-blind, RCTs should be conducted in the future to allow for longitudinal and cross-sectional comparisons under different PRP intervention parameters to explore the optimal treatment protocol for PRP injection and improve the clinical efficacy of PRP injections in patients with OA.
6. Conclusion
This meta-analysis indicated that PRP injections were effective in reducing pain symptoms in patients with KOA, TMJ, and AOA but did not show significant efficacy in patients with HOA. Compared to LR-PRP, LP-PRP injection therapy was more effective in improving pain symptoms in OA patients. In addition, PRP injection therapy can effectively improve the functional activity of OA patients and has a high level of safety for clinical applications.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
Conceived, designed, and drafted the manuscript: YX, CG, and XP. Critical revision: XP, XL, XS, and XT. Data collection and analysis: YX and CG. Supervised the work: WL and YW. Final approval of the article: WL, YW, and YL. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Science and Technology Research Project of Jiangxi Provincial Education (GJJ190801) to WL.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2023.1204144/full#supplementary-material
References
1. Charlier E, Deroyer C, Ciregia F, Malaise O, Neuville S, Plener Z, et al. Chondrocyte dedifferentiation and osteoarthritis (OA). Biochem Pharmacol. (2019) 165:49–65. doi: 10.1016/j.bcp.2019.02.036
2. Pereira D, Ramos E, Branco J. Osteoarthritis. Acta Med Port. (2015) 28:5477. doi: 10.20344/amp.5477
4. Wang T, He C. Pro-inflammatory cytokines: the link between obesity and osteoarthritis. Cytokine Growth Factor Rev. (2018) 44:38–50. doi: 10.1016/j.cytogfr.2018.10.002
5. Prieto-Alhambra D, Judge A, Javaid MK, Cooper C, Diez-Perez A, Arden NK. Incidence and risk factors for clinically diagnosed knee, hip and hand osteoarthritis: influences of age, gender and osteoarthritis affecting other joints. Ann Rheum Dis. (2014) 73:1659–64. doi: 10.1136/annrheumdis-2013-203355
6. Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet. (2019) 393:1745–59. doi: 10.1016/S0140-6736(19)30417-9
7. Deyle GD, Allen CS, Allison SC, Gill NW, Hando BR, Petersen EJ, et al. Physical therapy versus glucocorticoid injection for osteoarthritis of the knee. N Engl J Med. (2020) 382:1420–9. doi: 10.1056/NEJMoa1905877
8. Zeng C, Wei J, Persson MSM, Sarmanova A, Doherty M, Xie D, et al. Relative efficacy and safety of topical non-steroidal anti-inflammatory drugs for osteoarthritis: a systematic review and network meta-analysis of randomised controlled trials and observational studies. Br J Sports Med. (2018) 52:642–50. doi: 10.1136/bjsports-2017-098043
9. Martel-Pelletier J, Barr AJ, Cicuttini FM, Conaghan PG, Cooper C, Goldring MB, et al. Osteoarthritis. Nat Rev Dis Primers. (2016) 2:16072. doi: 10.1038/nrdp.2016.72
10. Paskins Z, Bromley K, Lewis M, Hughes G, Hughes E, Hennings S, et al. Clinical effectiveness of one ultrasound guided intra-articular corticosteroid and local anaesthetic injection in addition to advice and education for hip osteoarthritis (HIT trial): single blind, parallel group, three arm, randomised controlled trial. BMJ. (2022) 377:e068446. doi: 10.1136/bmj-2021-068446
11. He W-W, Kuang M-J, Zhao J, Sun L, Lu B, Wang Y, et al. Efficacy and safety of intraarticular hyaluronic acid and corticosteroid for knee osteoarthritis: a meta-analysis. Int J Surg. (2017) 39:87. doi: 10.1016/j.ijsu.2017.01.087
12. Webner D, Huang Y, Hummer CD. Intraarticular hyaluronic acid preparations for knee osteoarthritis: are some better than others? Cartilage. (2021) 13:1619S−36S. doi: 10.1177/19476035211017320
13. Felson DT. Intra-articular corticosteroids and knee osteoarthritis: interpreting different meta-analyses. JAMA. (2016) 316:2607–8. doi: 10.1001/jama.2016.17227
14. Verma R, Kumar S, Garg P, Verma YK. Platelet-rich plasma: a comparative and economical therapy for wound healing and tissue regeneration. Cell Tissue Bank. (2022). doi: 10.1007/s10561-022-10039-z
15. Linertová R, Del Pino-Sedeño T, Pérez L-G, Aragón-Sánchez J, Andia-Ortiz I, Trujillo-Martín M, et al. Cost-effectiveness of platelet-rich plasma for diabetic foot ulcer in Spain. Int J Low Extrem Wounds. (2021) 20:119–27. doi: 10.1177/1534734620903239
16. Wu PIK, Diaz R, Borg-Stein J. Platelet-rich plasma. Phys Med Rehabil Clin N Am. (2016) 27:825–53. doi: 10.1016/j.pmr.2016.06.002
17. van Buul GM, Koevoet WLM, Kops N, Bos PK, Verhaar JAN, Weinans H, et al. Platelet-rich plasma releasate inhibits inflammatory processes in osteoarthritic chondrocytes. Am J Sports Med. (2011) 39:2362–70. doi: 10.1177/0363546511419278
18. Everts P, Onishi K, Jayaram P, Lana JF, Mautner K. Platelet-rich plasma: new performance understandings and therapeutic considerations in 2020. Int J Mol Sci. (2020) 21:794. doi: 10.3390/ijms21207794
19. Glyn-Jones S, Palmer AJR, Agricola R, Price AJ, Vincent TL, Weinans H, et al. Osteoarthritis. Lancet. (2015) 386:376–87. doi: 10.1016/S0140-6736(14)60802-3
20. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. (2009) 339:b2700. doi: 10.1136/bmj.b2700
21. Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. (2019) 366:l4898. doi: 10.1136/bmj.l4898
22. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
23. Goldet G, Howick J. Understanding GRADE: an introduction. J Evid Based Med. (2013) 6:50–4. doi: 10.1111/jebm.12018
24. Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. (2011) 64:383–94. doi: 10.1016/j.jclinepi.2010.04.026
25. Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, et al. Updated guidance for trusted systematic reviews: a new edition of the cochrane handbook for systematic reviews of interventions. Cochrane Database Syst Rev. (2019) 10:ED000142. doi: 10.1002/14651858.ED000142
26. Nouri F, Babaee M, Peydayesh P, Esmaily H, Raeissadat SA. Comparison between the effects of ultrasound guided intra-articular injections of platelet-rich plasma (PRP), high molecular weight hyaluronic acid, and their combination in hip osteoarthritis: a randomized clinical trial. BMC Musculoskelet Disord. (2022) 23:856. doi: 10.1186/s12891-022-05787-8
27. Bennell KL, Paterson KL, Metcalf BR, Duong V, Eyles J, Kasza J, et al. Effect of intra-articular platelet-rich plasma vs placebo injection on pain and medial tibial cartilage volume in patients with knee osteoarthritis: the restore randomized clinical trial. JAMA. (2021) 326:2021–30. doi: 10.1001/jama.2021.19415
28. Cömert Kiliç S, Güngörmüş M, Sümbüllü MA. Is arthrocentesis plus platelet-rich plasma superior to arthrocentesis alone in the treatment of temporomandibular joint osteoarthritis? A randomized clinical trial. J Oral Maxillofac Surg. (2015) 73:1473–83. doi: 10.1016/j.joms.2015.02.026
29. Dório M, Pereira RMR, Luz AGB, Deveza LA, de Oliveira RM, Fuller R. Efficacy of platelet-rich plasma and plasma for symptomatic treatment of knee osteoarthritis: a double-blinded placebo-controlled randomized clinical trial. BMC Musculoskelet Disord. (2021) 22:822. doi: 10.1186/s12891-021-04706-7
30. Patel S, Dhillon MS, Aggarwal S, Marwaha N, Jain A. Treatment with platelet-rich plasma is more effective than placebo for knee osteoarthritis: a prospective, double-blind, randomized trial. Am J Sports Med. (2013) 41:356–64. doi: 10.1177/0363546512471299
31. Lin K-Y, Yang C-C, Hsu C-J, Yeh M-L, Renn J-H. Intra-articular injection of platelet-rich plasma is superior to hyaluronic acid or saline solution in the treatment of mild to moderate knee osteoarthritis: a randomized, double-blind, triple-parallel, placebo-controlled clinical trial. Arthroscopy. (2019) 35:106–17. doi: 10.1016/j.arthro.2018.06.035
32. Elik H, Dogu B, Yilmaz F, Begoglu FA, Kuran B. The efficiency of platelet-rich plasma treatment in patients with knee osteoarthritis. J Back Musculoskelet Rehabil. (2020) 33:127–38. doi: 10.3233/BMR-181374
33. Barman A, Prakash S, Sahoo J, Mukherjee S, Maiti R, Roy SS. Single intra-articular injection with or without intra-osseous injections of platelet-rich plasma in the treatment of osteoarthritis knee: a single-blind, randomized clinical trial. Injury. (2022) 53:1247–53. doi: 10.1016/j.injury.2022.01.012
34. Su K, Bai Y, Wang J, Zhang H, Liu H, Ma S. Comparison of hyaluronic acid and PRP intra-articular injection with combined intra-articular and intraosseous PRP injections to treat patients with knee osteoarthritis. Clin Rheumatol. (2018) 37:1341–50. doi: 10.1007/s10067-018-3985-6
35. Nunes-Tamashiro JC, Natour J, Ramuth FM, Toffolo SR, Mendes JG, Rosenfeld A, et al. Intra-articular injection with platelet-rich plasma compared to triamcinolone hexacetonide or saline solution in knee osteoarthritis: a double blinded randomized controlled trial with one year follow-up. Clin Rehabil. (2022) 36:900–15. doi: 10.1177/02692155221090407
36. Wu Y-T, Hsu K-C, Li T-Y, Chang C-K, Chen L-C. Effects of platelet-rich plasma on pain and muscle strength in patients with knee osteoarthritis. Am J Phys Med Rehabil. (2018) 97:248–54. doi: 10.1097/PHM.0000000000000874
37. Bastos R, Mathias M, Andrade R, Amaral RJFC, Schott V, Balduino A, et al. Intra-articular injection of culture-expanded mesenchymal stem cells with or without addition of platelet-rich plasma is effective in decreasing pain and symptoms in knee osteoarthritis: a controlled, double-blind clinical trial. Knee Surg Sports Traumatol Arthrosc. (2020) 28:1989–99. doi: 10.1007/s00167-019-05732-8
38. Bastos R, Mathias M, Andrade R, Bastos R, Balduino A, Schott V, et al. Intra-articular injections of expanded mesenchymal stem cells with and without addition of platelet-rich plasma are safe and effective for knee osteoarthritis. Knee Surg Sports Traumatol Arthrosc. (2018) 26:3342–50. doi: 10.1007/s00167-018-4883-9
39. Rayegani SM, Raeissadat SA, Taheri MS, Babaee M, Bahrami MH, Eliaspour D, et al. Does intra articular platelet rich plasma injection improve function, pain and quality of life in patients with osteoarthritis of the knee? A randomized clinical trial. Orthop Rev. (2014) 6:5405. doi: 10.4081/or.2014.5405
40. Fernández Sanromán J, Fernández Ferro M, Costas López A, Arenaz Bua J, López A. Does injection of plasma rich in growth factors after temporomandibular joint arthroscopy improve outcomes in patients with Wilkes stage IV internal derangement? A randomized prospective clinical study. Int J Oral Maxillofac Surg. (2016) 45:828–35. doi: 10.1016/j.ijom.2016.01.018
41. Raeissadat SA, Ghorbani E, Sanei Taheri M, Soleimani R, Rayegani SM, Babaee M, et al. MRI changes after platelet rich plasma injection in knee osteoarthritis (randomized clinical trial). J Pain Res. (2020) 13:65–73. doi: 10.2147/JPR.S204788
42. Ghai B, Gupta V, Jain A, Goel N, Chouhan D, Batra YK. Effectiveness of platelet rich plasma in pain management of osteoarthritis knee: double blind, randomized comparative study. Braz J Anesthesiol. (2019) 69:439–47. doi: 10.1016/j.bjane.2019.06.005
43. Görmeli G, Görmeli CA, Ataoglu B, Çolak C, Aslantürk O, Ertem K. Multiple PRP injections are more effective than single injections and hyaluronic acid in knees with early osteoarthritis: a randomized, double-blind, placebo-controlled trial. Knee Surg Sports Traumatol Arthrosc. (2017) 25:958–65. doi: 10.1007/s00167-015-3705-6
44. Asadpour N, Shooshtari Z, Kazemian M, Gholami M, Vatanparast N, Samieirad S. Combined platelet-rich plasma and hyaluronic acid can reduce pain in patients undergoing arthrocentesis for temporomandibular joint osteoarthritis. J Oral Maxillofac Surg. (2022) 80:1474–85. doi: 10.1016/j.joms.2022.05.002
45. Görmeli G, Karakaplan M, Görmeli CA, Sarikaya B, Elmali N, Ersoy Y. Clinical effects of platelet-rich plasma and hyaluronic acid as an additional therapy for talar osteochondral lesions treated with microfracture surgery: a prospective randomized clinical trial. Foot Ankle Int. (2015) 36:891–900. doi: 10.1177/1071100715578435
46. Guney A, Yurdakul E, Karaman I, Bilal O, Kafadar IH, Oner M. Medium-term outcomes of mosaicplasty versus arthroscopic microfracture with or without platelet-rich plasma in the treatment of osteochondral lesions of the talus. Knee Surg Sports Traumatol Arthrosc. (2016) 24:1293–8. doi: 10.1007/s00167-015-3834-y
47. Malahias M-A, Roumeliotis L, Nikolaou VS, Chronopoulos E, Sourlas I, Babis GC. Platelet-rich plasma versus corticosteroid intra-articular injections for the treatment of trapeziometacarpal arthritis: a prospective randomized controlled clinical trial. Cartilage. (2021) 12:51–61. doi: 10.1177/1947603518805230
48. Mei-Dan O, Carmont MR, Laver L, Mann G, Maffulli N, Nyska M. Platelet-rich plasma or hyaluronate in the management of osteochondral lesions of the talus. Am J Sports Med. (2012) 40:534–41. doi: 10.1177/0363546511431238
49. Di Sante L, Villani C, Santilli V, Valeo M, Bologna E, Imparato L, et al. Intra-articular hyaluronic acid vs platelet-rich plasma in the treatment of hip osteoarthritis. Med Ultrason. (2016) 18:463–8. doi: 10.11152/mu-874
50. Cartron AM, Shaikh G. Platelet-rich plasma: cure-all at what cost? Dermatol Surg. (2021) 47:323. doi: 10.1097/DSS.0000000000002357
51. Tuknayat A, Bhalla M, Thami GP. Platelet-rich plasma is a promising therapy for melasma. J Cosmet Dermatol. (2021) 20:2431–6. doi: 10.1111/jocd.14229
52. Chen W-H, Lo W-C, Lee J-J, Su C-H, Lin C-T, Liu H-Y, et al. Tissue-engineered intervertebral disc and chondrogenesis using human nucleus pulposus regulated through TGF-beta1 in platelet-rich plasma. J Cell Physiol. (2006) 209:744–54. doi: 10.1002/jcp.20765
53. Dhillon MS, Patel S, Bansal T. Improvising PRP for use in osteoarthritis knee- upcoming trends and futuristic view. J Clin Orthop Trauma. (2019) 10:32–5. doi: 10.1016/j.jcot.2018.10.005
54. Cook CS, Smith PA. Clinical Update: Why PRP should be your first choice for injection therapy in treating osteoarthritis of the knee. Curr Rev Musculoskelet Med. (2018) 11:583–92. doi: 10.1007/s12178-018-9524-x
55. Bhatia R, Chopra G. Efficacy of platelet rich plasma via lumbar epidural route in chronic prolapsed intervertebral disc patients-a pilot study. J Clin Diagn Res. (2016) 10:UC05–UC7. doi: 10.7860/JCDR/2016/21863.8482
56. Khatab S, van Buul GM, Kops N, Bastiaansen-Jenniskens YM, Bos PK, Verhaar JA, et al. Intra-articular injections of platelet-rich plasma releasate reduce pain and synovial inflammation in a mouse model of osteoarthritis. Am J Sports Med. (2018) 46:977–86. doi: 10.1177/0363546517750635
57. Xie X, Zhang C, Tuan RS. Biology of platelet-rich plasma and its clinical application in cartilage repair. Arthritis Res Ther. (2014) 16:204. doi: 10.1186/ar4493
58. Setiawan IGNY, Suyasa IK, Astawa P, Dusak IWS, Kawiyana IKS, Aryana IGNW. Recombinant platelet derived growth factor-BB and hyaluronic acid effect in rat osteoarthritis models. J Orthop. (2019) 16:230–3. doi: 10.1016/j.jor.2019.02.028
59. Xie X, Wang Y, Zhao C, Guo S, Liu S, Jia W, et al. Comparative evaluation of MSCs from bone marrow and adipose tissue seeded in PRP-derived scaffold for cartilage regeneration. Biomaterials. (2012) 33:7008–18. doi: 10.1016/j.biomaterials.2012.06.058
60. Sánchez M, Delgado D, Sánchez P, Muiños-López E, Paiva B, Granero-Moltó F, et al. Combination of intra-articular and intraosseous injections of platelet rich plasma for severe knee osteoarthritis: a pilot study. Biomed Res Int. (2016) 2016:4868613. doi: 10.1155/2016/4868613
61. Sánchez M, Fiz N, Guadilla J, Padilla S, Anitua E, Sánchez P, et al. Intraosseous infiltration of platelet-rich plasma for severe knee osteoarthritis. Arthrosc Tech. (2014) 3:e713–7. doi: 10.1016/j.eats.2014.09.006
62. Lychagin A, Lipina M, Garkavi A, Islaieh O, Timashev P, Ashmore K, et al. Intraosseous injections of platelet rich plasma for knee bone marrow lesions treatment: one year follow-up. Int Orthop. (2021) 45:355–63. doi: 10.1007/s00264-020-04546-5
63. Dohan Ehrenfest DM, Rasmusson L, Albrektsson T. Classification of platelet concentrates: from pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF). Trends Biotechnol. (2009) 27:158–67. doi: 10.1016/j.tibtech.2008.11.009
64. Gilat R, Haunschild ED, Knapik DM, Evuarherhe A, Parvaresh KC, Cole BJ. Hyaluronic acid and platelet-rich plasma for the management of knee osteoarthritis. Int Orthop. (2021) 45:345–54. doi: 10.1007/s00264-020-04801-9
65. Di Nicola V. Degenerative osteoarthritis a reversible chronic disease. Regen Ther. (2020) 15:149–60. doi: 10.1016/j.reth.2020.07.007
66. Milants C, Bruyère O, Kaux J-F. Responders to platelet-rich plasma in osteoarthritis: a technical analysis. Biomed Res Int. (2017) 2017:7538604. doi: 10.1155/2017/7538604
67. Collins T, Alexander D, Barkatali B. Platelet-rich plasma: a narrative review. EFORT Open Rev. (2021) 6:225–35. doi: 10.1302/2058-5241.6.200017
68. Tischer T, Bode G, Buhs M, Marquass B, Nehrer S, Vogt S, et al. Platelet-rich plasma (PRP) as therapy for cartilage, tendon and muscle damage - German working group position statement. J Exp Orthop. (2020) 7:64. doi: 10.1186/s40634-020-00282-2
Keywords: platelet-rich plasma, osteoarthritis, systematic review, meta-analysis, randomized controlled trials
Citation: Xiong Y, Gong C, Peng X, Liu X, Su X, Tao X, Li Y, Wen Y and Li W (2023) Efficacy and safety of platelet-rich plasma injections for the treatment of osteoarthritis: a systematic review and meta-analysis of randomized controlled trials. Front. Med. 10:1204144. doi: 10.3389/fmed.2023.1204144
Received: 12 April 2023; Accepted: 12 June 2023;
Published: 27 June 2023.
Edited by:
Yi-Li Zheng, Shanghai University of Sport, ChinaReviewed by:
Ibsen Bellini Coimbra, State University of Campinas, BrazilPlamen Todorov Todorov, Plovdiv Medical University, Bulgaria
Copyright © 2023 Xiong, Gong, Peng, Liu, Su, Tao, Li, Wen and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying Li, MjUyMjA5ODcwQHFxLmNvbQ==; Youliang Wen, MTAzNzExNDg0NUBxcS5jb20=; Wei Li, MjgyNzg4MTgzQHFxLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Yongqing Xiong
Yongqing Xiong Cheng Gong
Cheng Gong Xumiao Peng
Xumiao Peng Xianlei Liu
Xianlei Liu Youliang Wen
Youliang Wen