
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Med. , 25 August 2023
Sec. Gastroenterology
Volume 10 - 2023 | https://doi.org/10.3389/fmed.2023.1201769
This article is part of the Research Topic Advances in Proctology and Colorectal Surgery View all 45 articles
Background: Postoperative ileus (POI) is one of the main complications after colorectal cancer (CRC) surgery, and there is still a lack of effective treatment. At present, the evidence for improvement of POI by invasive acupuncture (manual acupuncture and electroacupuncture, IA) is limited. This meta-analysis of randomized controlled trials (RCTs) aims to systematically review and evaluate the effect of IA in improving POI after CRC surgery.
Methods: This meta-analysis was reported according to PRISMA statement and AMSTAR guidelines. The retrieval time was from the inception to February 2023. The RCTs were screened by searching the databases (PubMed, Ovid, Embase, Cochrane Library, China National Knowledge Infrastructure, VIP Database, Sinomed Database, and WANFANG). Two independent investigators screened and extracted the data, assessed the risk of bias, and performed statistical analysis. The statistical analysis was carried out by RevMan5.3. The PROSPERO International Prospective Register of Systematic Reviews received this research for registration (CRD42023387700).
Results: Thirteen studies with 795 patients were included. In the primary outcome indicators: the IA group had shorter time to the first flauts [stand mean difference (SMD), −0.57; 95% CI, −0.73 to −0.41, p < 0.00001], shorter time to the first defecation [mean difference (MD), −4.92 h, 95% CI −8.10 to −1.74 h, p = 0.002] than the blank/sham stimulation (B/S) group. In the secondary outcome indicators: the IA group had shorter time to the first bowel motion (MD, −6.62 h, 95% CI −8.73 to −4.50 h, p < 0.00001), shorter length of hospital (SMD, −0.40, 95% CI −0.60 to −0.21, p < 0.0001) than the B/S group. In terms of the subgroup analysis: IA associated with enhanced recovery after surgery (ERAS) group had shorter time to the first flauts (MD, −6.41 h, 95% CI −9.34 to −3.49 h, p < 0.0001), shorter time to the first defacation (MD, −6.02 h, 95% CI −9.28 to −2.77 h, p = 0.0003) than ERAS group.
Conclusion: Invasive acupuncture (IA) after CRC surgery, acupuncture or electricacupuncture with a fixed number of times and duration at therapeutic acupoints, can promote the recovery of POI. IA combined with ERAS is better than simple ERAS in improving POI.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=387700, identifier CRD42023387700.
Postoperative ileus (POI) is one of the main postoperative complications of colorectal cancer (CRC) surgery, and recent research shows that its incidence rate is 13.5% (1). The recovery of POI usually takes 4 days, and the main clinical manifestations are delayed exhaust and defecation, abnormal bowel sounds, abdominal distention, nausea, and vomiting (2, 3). In addition, POI also extended the time and expenses for hospitalization (4). At present, basic treatments, such as fasting, nutritional support, maintaining water, electrolyte, and acid–base balance, are used to treat POI. Other therapies like drugs to promote gastrointestinal motility, chewing gum, and so on, are also used (5). However, the clinical efficacy of the existing treatment schemes is limited. Therefore, finding a new treatment to prevent POI has become an important issue so that patients with colorectal cancer can recover quickly during postoperative period (6).
Acupuncture is a non-drug, safe and inexpensive treatment. Some existing clinical studies show that acupuncture and related therapies can effectively improve the POI in surgical operations (7). However, external treatment were combined with invasive acupuncture (IA) in the intervention measures, such as moxibustion, acupoint application, and transcutaneous acupoint electrical stimulation (8). Therefore, we want to explore whether IA alone can improve POI after CRC surgery. And we include related randomized controlled trials (RCTs) for this meta-analysis so as to clarify its effect.
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) standards were followed while reporting this study. Supplementary Table S2 contains the PRISMA checklist. This study was also followed the guidelines of the Cochrane Handbook for Systematic Reviews of Interventions. PROSPERO International prospective register of systematic reviews was where the review procedure was registered (CRD42023387700).
The following databases were searched for this meta-analysis: PubMed, Ovid, Embase, Cochrane Library, China National Knowledge Infrastructure, VIP Database, Sinomed Database, and WANFANG Medical. It was reported in accordance with the PRISMA declaration and AMSTAR criteria. The search period spanned from beginning until February 2023. High-quality RCTs were gathered by two independent researchers. According to the search’s approach, full-text search was carried out (Supplementary Table S3).
Inclusion criteria include (1) Research type: RCTs on the preventive and therapeutic effects of IA (manual acupuncture and electroacupuncture) on POI after CRC surgery; Language is not limited. (2) Research objects: Patients with CRC undergoing IA during perioperative period; Age, gender and nationality are not limited. (3) Intervention measures: The acupuncture group received manual acupuncture (MA) or electroacupuncture (EA) during perioperative period; The blank/sham stimulation group(B/S) did not receive any treatment or stimulated non-meridian points.
Exclusion criteria include (1) Non-invasive acupuncture acupoint stimulation therapy, such as transcutaneous acupoint electrical stimulation, acupoint application, auricular point pressing beans, etc. (2) Intervention measures are IA combined with other Chinese medicine treatments, such as oral Chinese medicine decoction. (3) Non-colorectal cancer surgery patients or other intervention methods entered the research literature. (4) Literature with incomplete original text or ending index cannot be obtained. (5) Non-RCT, systematic review or comments, editorials, letters, meetings, and animal trials.
According to the effect of IA on preventing and treating POI, the main outcome indicators are: (1) Time to the first flauts; (2) Time to the first defecation; Secondary outcome indicators: (3) Time to the first bowel motion; and (4) Length of hospital.
According to the eligibility criteria above, two independent investigators preliminary screened through the title and abstract, and then the full text. The author’s name, publishing years, size of the sample, intervention methods, outcome indicators, and other data were extracted from the final screened literature. In case of disagreement, any potential disagreement shall be submitted to the correspondent for arbitration.
According to the Cochrane systematic review handbook 5.1 and its suggested risk of bias assessment technique, the caliber of the included studies was assessed. Random sequence generation, allocation concealment, blinding of trial participants and staff, blinding of outcome assessors, inadequate outcome data, selective reporting, and other biases were all examined in the research. The bias assessment’s findings were “low risk,” “high risk,” and “unclear.” Two researchers independently completed the quality evaluation. Conflicts were resolved through mediation by the corresponding author.
Utilizing the program RevMan 5.3, statistical data analysis was carried out. The Standard mean difference (SMD) or Mean difference (MD) and its 95% CI are statistically described and the effect quantity is combined. Inter-study heterogeneity was assessed using chi-square test with a test level of α = 0.1, and the degree of heterogeneity was observed based on I2 values (9). The studies with clinical and methodological homogeneity were combined, and if p ≥ 0.1 and I2 ≤ 50%, the included studies had good homogeneity and were analyzed using a fixed-effects model for meta-analysis; if p < 0.1 and I2 > 50%, it was considered that there was significant heterogeneity among the included study literature, and subgroup analysis treatment or sensitivity analysis was needed to find the source of heterogeneity; if there was no significant clinical heterogeneity, the random-effects model was selected for merging; sensitivity analysis was performed when there were large weight items to check the stability of the results; if the heterogeneity was large, meta-analysis was not performed, and only descriptive analysis was performed. If the number of literatures was sufficient, funnel plots were used to determine whether there was publication bias (10).
The GRADE evidence profiles and summary of findings table was shown in Table 1.
A total of 350 relevant literatures were obtained from the search, 176 duplicates were excluded, 137 were eliminated based on title and abstract, and then 24 were excluded based on full text, resulting in the inclusion of 13 studies that met the study requirements, all in China, including one conducted in Hong Kong. There were 395 patients in the IA group and 400 patients in the B/S group, for a total of 795 patients. There of the included studies explicitly mentioned the use of open surgery, four explicitly mentioned the use of laparoscopic surgery, and the other studies described only part of the type of surgery; four studies used enhanced recovery after surgery (ERAS); nine studies used EA, four studies used MA; one study performed acupuncture 1 day before surgery; and the remaining studies were performed postoperatively. The literature screening process was shown in Figure 1. The basic characteristics of the included studies were shown in Tables 2, 3.
(1) Random sequence generation: four papers used random number table method; two papers used computer software to generate random groupings; two papers used random zone grouping method; one paper used closed envelopes; two papers only mentioned random and did not describe the method of random sequence generation; one paper mentioned minimization; one paper did not mention random. (2) Allocation concealment: three papers used closed envelope allocation concealment; two papers mentioned allocation concealment without specific methods, and the rest did not mention allocation concealment. (3) Blinding of subjects and trial personnel: five papers mentioned blinding of subjects and investigators; one mentioned using single blinding, and the rest did not mention blinding. (4) Blinding of outcome assessors: five papers mentioned blinding of outcome index assessors, and the rest did not describe it. (5) Incomplete outcome data: one study mentioned the reason why some of the outcome indicators did not appear, but the proportion of missing outcome indicators was not enough to have a significant impact, and the rest of the studies had no missing data. (6) Selective reporting: three studies had access to the study protocol and eventually reported the expected outcome indicators, while the rest had incomplete information and it was difficult to determine whether there was a risk of selective reporting of outcomes. (7) Other bias: there was no evidence or information to determine whether there was other serious risk of bias in the included studies.
Of the 13 included publications, our quality assessment using the Cochrane Risk of Bias Assessment Tool showed that the overall quality of the total literature was moderate (Figure 2).
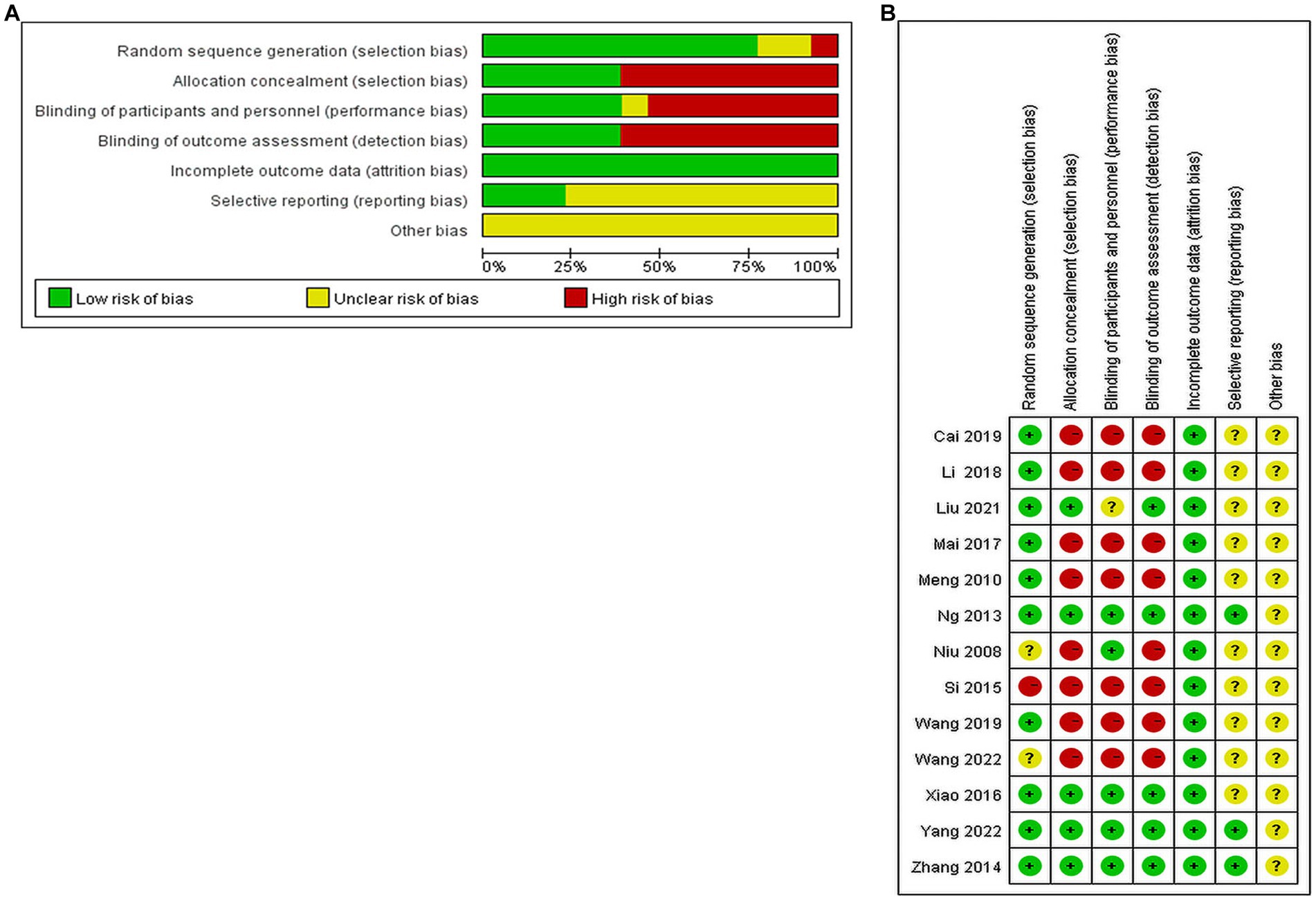
Figure 2. Percentage plot of the risk of bias of the included studies. (A) Risk of bias graph. (B) Risk of bias summary.
All studies reported the effect of IA vs. B/S on the time to first flauts after CRC surgery, involving 632 patients, 316 in the IA group and 316 in the B/S group. Heterogeneity was detected (p < 0.00001, I2 = 88%) with significant heterogeneity, and sensitivity analysis was performed and three studies were excluded before heterogeneity was detected (p = 0.53, I2 = 0%) with no significant statistical heterogeneity, low sensitivity, and good stability. The fixed-effects model combined with effect size analysis was used, and the results showed that the time to the first flauts was significantly lower in the IA group than in the B/S group [stand mean difference (SMD), −0.57; 95% CI, −0.73 to −0.41, p < 0.00001], as shown in Figure 3A.
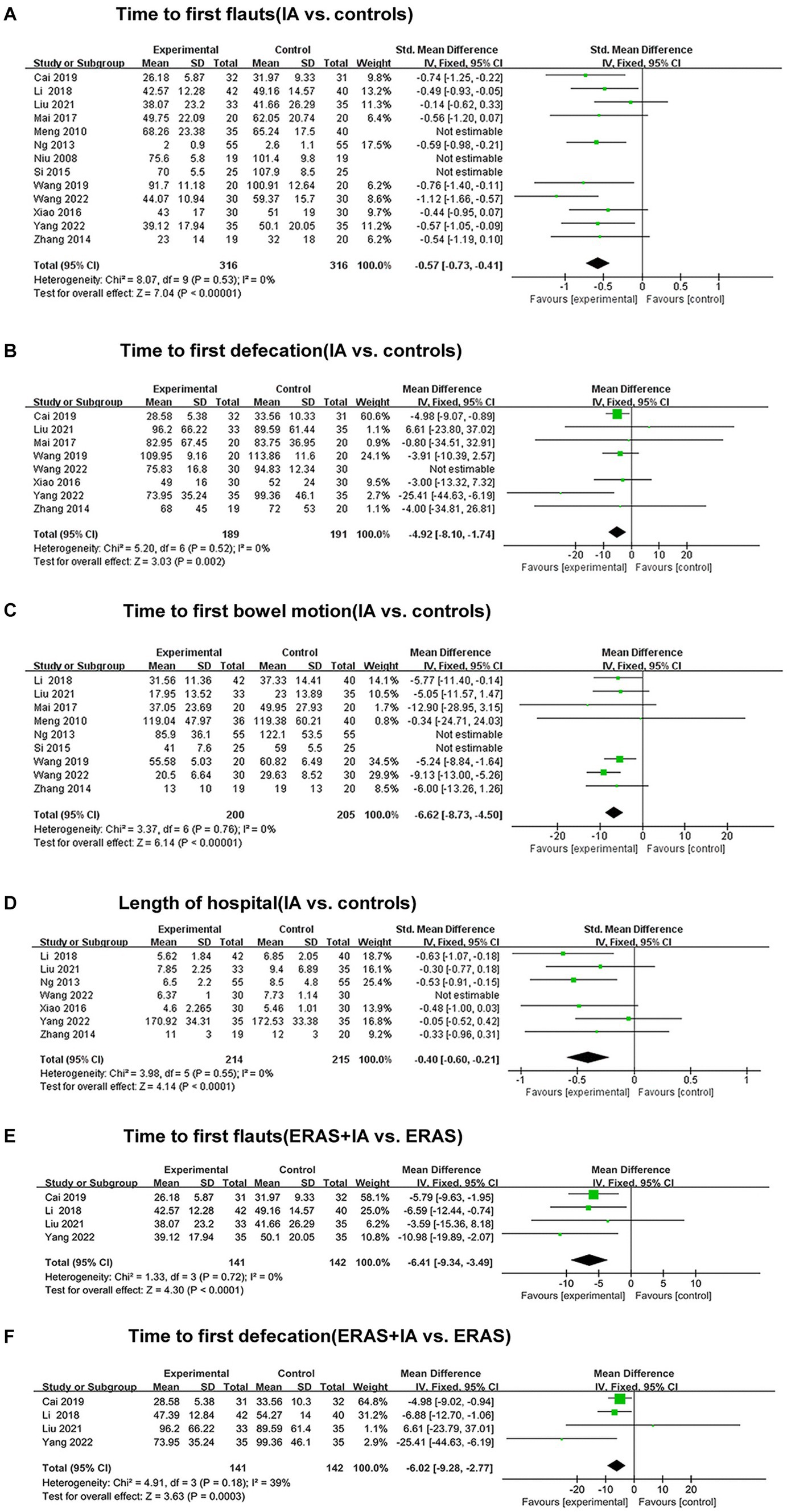
Figure 3. The forest plots. (A) Time to first flauts (IA vs. controls). (B) Time to first defecation (IA vs. controls). (C) Time to first bowel motion (IA vs. controls). (D) Length of hospital (IA vs. controls). (E) Time to first flauts (ERAS + IA vs. ERAS). (F) Time to first defecation (ERAS + IA vs. ERAS).
Eight studies reported the effect of IA vs. B/S on the time to first defecation after CRC surgery: 380 patients were involved, 189 in the IA group and 191 in the B/S group. Heterogeneity was detected (p = 0.02, I2 = 58%) with significant heterogeneity, and sensitivity analysis was performed and one study was excluded before heterogeneity was detected (p = 0.52, I2 = 0%) with no significant statistical heterogeneity, low sensitivity, and good stability, and the fixed-effects model combined with effect size analysis was used, and the results showed that the time to the first defecation was significantly lower in the IA group than in the B/S group [mean difference (MD), −4.92 h, 95% CI −8.10 to −1.74 h, p = 0.002], as shown in Figure 3B.
Nine studies reported the effect of IA vs. B/S on the first bowel motion after CRC surgery: 405 patients were involved, 200 in the IA and 205 in the B/S group. Heterogeneity was detected (p < 0.00001, I2 = 80%) with significant heterogeneity, and sensitivity analysis was performed and two studies were excluded before heterogeneity was detected (p = 0.76, I2 = 0%) with no significant statistical heterogeneity, low sensitivity, and good stability, using fixed-effects model combined with effect size analysis, the results showed that the time to the first bowel motion in the IA group was significantly lower than that of the B/S group (MD, −6.62 h, 95% CI −8.73 to −4.50 h, p < 0.00001), as shown in Figure 3C.
Seven studies reported the effect of IA vs. B/S on the number of days in hospital: 429 patients were involved, 214 in the IA and 215 in the B/S group. Heterogeneity was detected (p < 0.06, I2 = 50%) with significant heterogeneity, and sensitivity analysis was performed and one study were excluded before heterogeneity was detected (p = 0.55, I2 = 0%) with no significant statistical heterogeneity, low sensitivity, and good stability, using fixed-effects model combined with effect size analysis, and the results showed that length of hospital was significantly lower in the IA group than in the B/S group (SMD, −0.40, 95% CI −0.60 to −0.21, p < 0.0001), as shown in Figure 3D.
Four studies reported the effect of IA combined with ERAS group on the time to the first flauts: 283 patients were involved, 141 in the IA combined with ERAS group and 142 in the ERAS group. Heterogeneity was detected (p = 0.72, I2 = 0%), with no significant statistical heterogeneity, low sensitivity, and good stability, using the fixed-effects model combined with effect size analysis, which showed that the time to the first flauts was significantly lower in the IA combined with ERAS group than in the ERAS group (MD, −6.41 h, 95% CI −9.34 to −3.49 h, p < 0.0001), as shown in Figure 3E.
Four studies reported the effect of IA combined with ERAS group on the time to the first defecation: 283 patients were involved, 141 in the IA combined with ERAS group and 142 in the ERAS group. Heterogeneity was detected (p = 0.18, I2 = 39%), with no significant statistical heterogeneity, low sensitivity and good stability, using the fixed-effects model combined with effect size analysis, which showed that the time to the first defecation was significantly lower in the IA combined with ERAS group than in the ERAS group (MD, −6.02 h, 95% CI −9.28 to −2.77 h, p = 0.0003), as shown in Figure 3F.
Using Revman 5.3 software, a funnel plot was drawn for “comparing the effect of IA and B/S on the time to first flauts after CRC surgery” (Figure 4), which showed good symmetry on both sides of the central axis without significant publication bias and good reliability of the Meta-analysis results.
Acupuncture and related therapies are guided by Traditional Chinese Medicine (TCM) and are used to prevent and treat diseases by stimulating meridians and acupuncture points, and are used in treatment guidelines for cancer pain, post-operative pain, etc. (24, 25). Currently, some studies have proposed that acupuncture has an ameliorative effect on POI after abdominal surgery such as CRC (7, 26). However, acupuncture, in its broadest sense, involves acupuncture, moxibustion, acupoint application, physical and chemical therapy, and many other means. Acupuncture interventions are mixed and vary greatly among studies due to different interventions. This study was the first meta-analysis of IA for the prevention and treatment of POI after CRC surgery. In addition, ERAS can significantly reduce the incidence of post-colorectal complications and thus has been widely used in the post-surgical period (27, 28). We also compared for the first time the effect of ERAS combined with IA with that of ERAS alone for the prevention and treatment of POI. We concluded that IA can improve POI after CRC surgery, and IA combined with ERAS can significantly reduce the time to the first flauts and defecation compared with ERAS alone, which also suggests the potential of invasive needling in combination with ERAS.
A total of 24 acupoints were involved in the 13 included studies, and the four most frequently used acupoints were as follows: ST36 (92.3%, 12/13), ST37 (50%, 6/12), ST25 (50%, 6/12), and PC6 (50%, 6/12). ST36 and ST37 are located in the lower extremities, and ST25 is located in the abdomen. All the three acupoints belong to the stomach meridian. PC6 is located in the upper extremity and belongs to the pericardium meridian. In TCM, the points mentioned above can regulate the activities of abdominal organs, and some experiments suggested that these points can promote gastric or intestinal motility and help with inflammation: Lu et al. used a vagus used a rat model in which the vagus or sympathetic nerve was removed and found that EA stimulation of PC6 could promote vagal electrical activity to increase gastric motility (29). Murakami et al. used EA stimulation on ST36 and PC6 in a rat model where the animals came down with POI after undergoing Intestinal manipulation (IM) surgery and came to the conclusion that EA stimulation improved the regularity of small intestinal slow waves, accelerated intestinal transit and gastric emptying, and inhibited TNF-α levels (30). However, EA stimulation of acupoints in the abdomen and lower extremities at the same frequency may produce different therapeutic effects. Yang set a mouse model where the animals came down with POI after undergoing sham Intestinal manipulation. In this experiment, 10 hz electrical acupuncture was given to stimulate acupuncture points ST25, ST36, and ST37, and the results suggested that both ST36 and ST37 could promote intestinal motility and reduce plasma inflammatory factors TNF-α and IL6, while ST25 had no significant effect (31). The difference in therapeutic effects may be related to the different anti-inflammatory physiological mechanisms involved in the different location of points. By identifying Prokr2 sensory neurons in the abdominal fascia and deep fascia of the hind limbs in mice, Liu et al. determined that stimulation on ST36 can be more effective than that on ST25 to activate the vagal-adrenal network, which is thought to stimulate the production of substances such as catecholamines and produce anti-inflammatory effects (32). Furthermore, in a lipopolysaccharide induced systemic inflammation mouse model, Liu et al. (33) found that EA stimulation of ST25 activated sympathetic nerves connecting the spinal cord to the spleen, producing norepinephrine. In conclusion, more distinct therapeutic effects found in different acupoints, optimal treatment frequency, and active clinical trials with relevant evidence may be of significance in improving the efficacy of acupuncture against POI.
Wang, Si′s study had significant heterogeneity in two meta-analyses and Meng, Niu’s study had significant heterogeneity in one meta-analysis, respectively. Among all studies, Meng’s study was the only one that reported no significant difference in POI improvement in the IA vs. blank group, which may be the source of heterogeneity. In addition, studies with negative results may have had publication omissions that tilted the final studies included in the analysis toward positive studies, leading to publication bias. The studies by Niu, Si, and Wang either only mentioned randomization or did not mention randomization when describing the randomization method, and their selectivity bias was evaluated as unclear and high risk. The remaining studies were specific in describing their randomization methods and all were at low risk of selective bias when evaluated. The selective bias resulting from the irregular randomization method may explain the heterogeneity of the three studies. In addition, the number of acupuncture points selected, the type of acupuncture points, the frequency and intensity of electroacupuncture, and the standardization of the ERAS protocols varied among the studies included in the analysis, which could be a source of heterogeneity in outcome indicators.
Though a subgroup analysis was performed to adjust the bias, the following limitations and shortcomings will reduce the reliability of the results. The SMD was used for time to first flatus and length of hospital stay, which leaves the final combined results without a unit of measurement, so the results for these two should be viewed with caution. In addition, all the studies are from China and there was a lack of high-quality studies reported from different countries, which may lead to selection bias. A web of science-based bibliometric study on electroacupuncture suggested that 65.1% of the electroacupuncture literature was published in China in the last decade, compared to 16.4% in the second place (34). This indicates that more countries need to be involved in acupuncture-based research. Second, there was a lack of multicenter clinical studies reported in the included studies. Third, most studies ignore allocation concealment and do not mention blinding of subjects, investigators, and assessors, which may result in selection bias, implementation bias, and measurement bias in the studies included in the analysis. Last, some of the literature does not report the basis of sample size estimation, and there is a possibility of inaccurate sample size. In conclusion, future studies need to follow the STRICTA and CONSORT statements to design and conduct trials that provide more multicenter, large-sample clinical studies with standardized outcome indicators. Further validation of the effectiveness of POI for invasive treatment of CRC surgery is still needed to provide a lower bias and higher quality evidence-based medical rationale.
This study suggested that IA can improve POI of CRC. The implementations of acupuncture usually start from 1 postoperative day 1 until the ileus ending, 1–2 times a day, about 30 min minutes, and 2–10 acupoints are selected (ST36 is the most important acupoint). In addition, ERAS combined with the above IA treatment was more effective than ERAS alone in preventing POI. This meta-analysis was based on five high-quality RCTs and eight low quality RCTs with blinding defects, and further validation is still needed by including more high quality studies in the future.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
XZ and SS helped to conceive, design, analyze the data, conduct the study, and write the manuscript. JL helped to design the study and review the manuscript. XL and DZ designed, conducted, and reviewed the manuscript. YM and AH helped to design, conduct, and decide the final manuscript. All authors contributed to the article and approved the submitted version.
The authors thank Zhenzhen Gao (Guangdong University of Foreign Studies, Interpreting Studies) for polishing up the language.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2023.1201769/full#supplementary-material
1. Namba, Y, Hirata, Y, Mukai, S, Okimoto, S, Fujisaki, S, Takahashi, M, et al. Clinical indicators for the incidence of postoperative ileus after elective surgery for colorectal cancer. BMC Surg. (2021) 21:80. doi: 10.1186/s12893-021-01093-7
2. Quiroga-Centeno, AC, Jerez-Torra, KA, Martin-Mojica, PA, Castañeda-Alfonso, SA, Castillo-Sánchez, ME, Calvo-Corredor, OF, et al. Risk factors for prolonged postoperative ileus in colorectal surgery: a systematic review and meta-analysis. World J Surg. (2020) 44:1612–26. doi: 10.1007/s00268-019-05366-4
3. Stakenborg, N, Gomez-Pinilla, PJ, and Boeckxstaens, GE. Postoperative ileus: pathophysiology, current therapeutic approaches. Handb Exp Pharmacol. (2017) 239:39–57. doi: 10.1007/164_2016_108
4. Moghadamyeghaneh, Z, Hwang, GS, Hanna, MH, Phelan, M, Carmichael, JC, Mills, S, et al. Risk factors for prolonged ileus following colon surgery. Surg Endosc. (2016) 30:603–9. doi: 10.1007/s00464-015-4247-1
5. Sommer, NP, Schneider, R, Wehner, S, Wehner, S, and Kalff, JC, Vilz TO. State-of-the-art colorectal disease: postoperative ileus. Int J Color Dis. (2021) 36:2017–25. doi: 10.1007/s00384-021-03939-1
6. Shao, JK, Liu, Q, Pei, W, Wang, Y, Yang, NN, Qi, LY, et al. Electroacupuncture for postoperative ileus after laparoscopic surgery on colorectal cancer: study protocol for a randomized controlled trial. Trials. (2021) 22:610. doi: 10.1186/s13063-021-05564-3
7. Liu, Y, May, BH, Zhang, AL, Guo, X, Lu, C, Xue, CC, et al. Acupuncture and related therapies for treatment of postoperative ileus in colorectal cancer: a systematic review and meta-analysis of randomized controlled trials. Evid Based Complement Alternat Med. (2018) 2018:3178472. doi: 10.1155/2018/3178472
8. Chen, C, Guo, SN, Hao, Y, Ai, YK, and Jiang, LJ. Systematic review and meta-analysis of acupuncture and moxibustion: existing problems and countermeasures. Zhongguo Zhen Jiu. (2021) 41:1387–93. doi: 10.13703/j.0255-2930.20201207-k0002
9. Tierney, JF, Stewart, LA, Ghersi, D, Burdett, S, and Sydes, MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. (2007) 8:16. doi: 10.1186/1745-6215-8-16
10. Zhang, T. The selection of appropriate statistical models for traditional meta-analysis. Chin J Evid Based Med. (2020) 20:1477–81. doi: 10.7507/1672-2531.202008152
11. Yang, JW, Shao, JK, Wang, Y, Liu, Q, Liang, JW, Yan, SY, et al. Effect of acupuncture on postoperative ileus after laparoscopic elective colorectal surgery: a prospective, randomised, controlled trial. EClinicalMedicine. (2022) 49:101472. doi: 10.1016/j.eclinm.2022.101472
12. Wang, XZ, Wang, ZY, and Lai, YH. Clinical study on experienced ten acupoints in the treatment of postoperative gastrointestinal dysfunction of colorectal cancer. Chin J Mod Distance Educ Chin Med. (2022) 20:105–7. doi: 10.3969/j.issn.1672-2779.2022.15.040
13. Liu, XY, Huang, J, Yao, H, Yang, YR, and Zheng, RW. Effect of acupuncture on postoperative intestinal function recovery in patients with colorectal cancer. Chin J Colorectal Dis. (2021) 10:605–12. doi: 10.3877/cma.j.issn.2095-3224.2021.06.007
14. Cai, H, Han, XL, Zhou, C, Wang, G, and Gu, CL. Thumbtack needle therapy in promoting gastrointestinal function recovery of the elderly with colorectal cancer in ERAS. Geriatr Health Care. (2019) 25:760–6. doi: 10.3969/j.issn.1008-8296.2019.06.020
15. Wang, Y, and Li, TH. Study of electro-needing local and distal points on recovery of gastrointestinal function after the operation of rectum cancer. J Clin Acupunct Moxibus. (2019) 35:26–8. Chinese. doi: 10.3969/j.issn.1005-0779.2019.04.008
16. Li, DX, Yang, ZH, Qiu, FH, Li, GN, Shi, ZJ, Yang, ZT, et al. Effects of acupuncture therapy combined with nutrition support of enhanced recovery after surgery on colorectal cancer in perioperative period. Guangxi Med J. (2018) 40:872–5. doi: 10.11675/j.issn.0253-4304.2018.08.02
17. Mai, S, Meng, J, Wang, W, and Lang, S. Influence of electroacupuncture pretreatment on intestinal function in the patients of colorectal cancer surgery. Zhongguo Zhen Jiu. (2017) 37:483–7. doi: 10.13703/j.0255-2930.2017.05.008
18. Xiao, L, Zhou, B, Zhang, JZ, Cai, ZQ, Yun, T, and Zhao, GG. Clinical study of improvement of elderly patients’ bowel function after radical resection for colon cancer by lung-regulating and intestine-benefiting acupuncture method. Shandong J Tradition Chin Med. (2016) 35:701–4. doi: 10.16295/j.cnki.0257-358x.2016.08.011
19. Si, JG, and Ding, YS. Clinical study on electroacupuncture to restore gastrointestinal function after radical resection of colorectal cancer. J Pract Tradition Chin Med. (2015) 31:754–5. doi: 10.3969/j.issn.1004-2814.2015.08.050
20. Zhang, Z, Wang, C, Li, Q, Zhang, M, Zhao, H, Dong, L, et al. Electroacupuncture at ST36 accelerates the recovery of gastrointestinal motility after colorectal surgery: a randomised controlled trial. Acupunct Med. (2014) 32:223–6. doi: 10.1136/acupmed-2013-010490
21. Ng, SSM, Leung, WW, Mak, TWC, Hon, SSF, Li, JCM, Wong, CYN, et al. Electroacupuncture reduces duration of postoperative ileus after laparoscopic surgery for colorectal cancer. Gastroenterology. (2013) 144:307–313.e1. doi: 10.1053/j.gastro.2012.10.050
22. Meng, ZQ, Garcia, MK, Chiang, JS, Peng, HT, Shi, YQ, Fu, J, et al. Electro-acupuncture to prevent prolonged postoperative ileus: a randomized clinical trial. World J Gastroenterol. (2010) 16:104–11. doi: 10.3748/wjg.v16.i1.104
23. Niu, CF, and Wang, ZP. Clinical study of electroacupuncture at Zusanli, Shangjuxu and Neiguan on the recovery of intestinal peristalsis after radical resection of colorectal cancer. Chin J Gerontol. (2008) 9:924. doi: 10.3969/j.issn.1005-9202.2008.09.045
24. Mao, JJ, and Witt, CM. Acupuncture as an evidence-based treatment for cancer pain management: the joint society for integrative oncology-american society for clinical oncology guideline. J Integr Complement Med. (2023) 29:209–211. doi: 10.1089/jicm.2023.0018
25. Aldamluji, N, Burgess, A, Pogatzki-Zahn, E, Raeder, J, and Beloeil, H. PROSPECT Working Group collaborators*. PROSPECT guideline for tonsillectomy: systematic review and procedure-specific postoperative pain management recommendations. Anaesthesia. (2021) 76:947–61. doi: 10.1111/anae.15299
26. Chen, KB, Huang, Y, Jin, XL, and Chen, GF. Electroacupuncture or transcutaneous electroacupuncture for postoperative ileus after abdominal surgery: a systematic review and meta-analysis. Int J Surg. (2019) 70:93–101. doi: 10.1016/j.ijsu.2019.08.034
27. Ripollés-Melchor, J, Ramírez-Rodríguez, JM, Casans-Francés, R, Aldecoa, C, and Abad-Motos, A, Logroño-Egeaet, M, et al. Association between use of enhanced recovery after surgery protocol and postoperative complications in colorectal surgery: the postoperative outcomes within enhanced recovery after surgery protocol (POWER) study. JAMA Surg (2019) 154:725–736. doi: 10.1001/jamasurg.2019.0995
28. Ljungqvist, O, Scott, M, and Fearon, KC. Enhanced recovery after surgery: a review. JAMA Surg. (2017) 152:292–8. doi: 10.1001/jamasurg.2016.4952
29. Lu, M, Chen, C, Li, W, Yu, Z, and Xu, B. EA at PC6 promotes gastric motility: role of brainstem vagovagal neurocircuits. Evid Based Complement Alternat Med. (2019) 2019:7457485. doi: 10.1155/2019/7457485
30. Murakami, H, Li, S, Foreman, R, Yin, J, and Hirai, T. Chen JDZ ameliorating effects of electroacupuncture on dysmotility, inflammation, and pain mediated via the autonomic mechanism in a rat model of postoperative ileus. J Neurogastroenterol Motil. (2019) 25:286–99. doi: 10.5056/jnm18094
31. Yang, NN, Ye, Y, Tian, ZX, Ma, SM, Zheng, Y, Huang, J, et al. Effects of electroacupuncture on the intestinal motility and local inflammation are modulated by acupoint selection and stimulation frequency in postoperative ileus mice. Neurogastroenterol Motil. (2020) 32:e13808. doi: 10.1111/nmo.13808
32. Liu, S, Wang, Z, Su, Y, Liu, Q, Yang, W, Fu, M, et al. A neuroanatomical basis for electroacupuncture to drive the vagal-adrenal axis. Nature. (2021) 598:641–5. doi: 10.1038/s41586-021-04001-4
33. Liu, S, Wang, ZF, Su, YS, Ray, RS, Jing, XH, Wang, YQ, et al. Somatotopic organization and intensity dependence in driving distinct NPY-expressing sympathetic pathways by electroacupuncture. Neuron. (2020) 108:436–450.e7. doi: 10.1016/j.neuron.2020.07.015
Keywords: acupuncture therapy, meta-anlaysis, postoperative complications, surgical oncology, Traditional Chinese Medicine
Citation: Zhao X, Si S, Liu X, Liu J, Zhang D, Mu Y and Hou A (2023) Does invasive acupuncture improve postoperative ileus after colorectal cancer surgery? A systematic review and meta-analysis. Front. Med. 10:1201769. doi: 10.3389/fmed.2023.1201769
Received: 07 April 2023; Accepted: 15 August 2023;
Published: 25 August 2023.
Edited by:
Gaetano Gallo, Sapienza University of Rome, ItalyReviewed by:
HuangHsi Chen, Chung Shan Medical University Hospital, TaiwanCopyright © 2023 Zhao, Si, Liu, Liu, Zhang, Mu and Hou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aihua Hou, aGFoNjg3N0AxNjMuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.