- 1Laboratory of Pulmonary Investigation, Institute of Biophysics Carlos Chagas Filho, Federal University of Rio de Janeiro, Rio de Janeiro, Brazil
- 2Unit of Anaesthesia and Intensive Care, San Martino Hospital (IRCCS), Genoa, Italy
Coronavirus disease (COVID-19) is caused by the severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) virus and may lead to severe respiratory failure and the need for mechanical ventilation (MV). At hospital admission, patients can present with severe hypoxemia and dyspnea requiring increasingly aggressive MV strategies according to the clinical severity: noninvasive respiratory support (NRS), MV, and the use of rescue strategies such as extracorporeal membrane oxygenation (ECMO). Among NRS strategies, new tools have been adopted for critically ill patients, with advantages and disadvantages that need to be further elucidated. Advances in the field of lung imaging have allowed better understanding of the disease, not only the pathophysiology of COVID-19 but also the consequences of ventilatory strategies. In cases of refractory hypoxemia, the use of ECMO has been advocated and knowledge on handling and how to personalize strategies have increased during the pandemic. The aims of the present review are to: (1) discuss the evidence on different devices and strategies under NRS; (2) discuss new and personalized management under MV based on the pathophysiology of COVID-19; and (3) contextualize the use of rescue strategies such as ECMO in critically ill patients with COVID-19.
1. Introduction
Coronavirus disease 2019 (COVID-19) is caused by the severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) virus and can lead to respiratory failure (1, 2). COVID-19 may manifest with different degrees of respiratory failure, up to acute respiratory distress syndrome (ARDS), named “C-ARDS” (3). Initially interpreted as viral pneumonia, its radiologic picture includes ground-glass opacities (GGOs), with large alveolar edema and consequent collapse and increase in blood volume and interstitial space. GGOs also have dilated vessels (4), with a risk of microthrombosis and endotheliitis (5). At hospital admission, patients can present with severe hypoxemia even under conventional oxygen therapy (COT) and dyspnea, which may require ventilatory support. The disease can develop heterogeneity among patients, and COVID-19 can assume different phenotypes (6). Three phenotypes have been described: L-type, characterized by low lung elastance; H-type, characterized by high lung elastance (7–9); and F-type, the final evolution of COVID-19 characterized by lung fibrosis (10–13).
However, depending on the severity of the disease, the need for supportive strategies may evolve to mechanical ventilation (MV) with the use of low tidal volumes (VT) (14, 15). If hypoxemia persists, prone position (PP) and alveolar recruitment maneuvers (ARM) can be considered (9, 12, 16–19). In addition, extracorporeal membrane oxygenation (ECMO) should be considered in the most severe cases of C-ARDS (17, 20–22). Recently, the literature has focused on individualization of ventilatory strategies, according to a broad range of patient variables (9, 10), including physiological data, lung imaging, laboratory data, biomarkers, and even omics data (10). However, some of these tools are not routine practice in many hospitals. Nevertheless, adopting personalized medicine could better implement the therapy in the patients with C-ARDS.
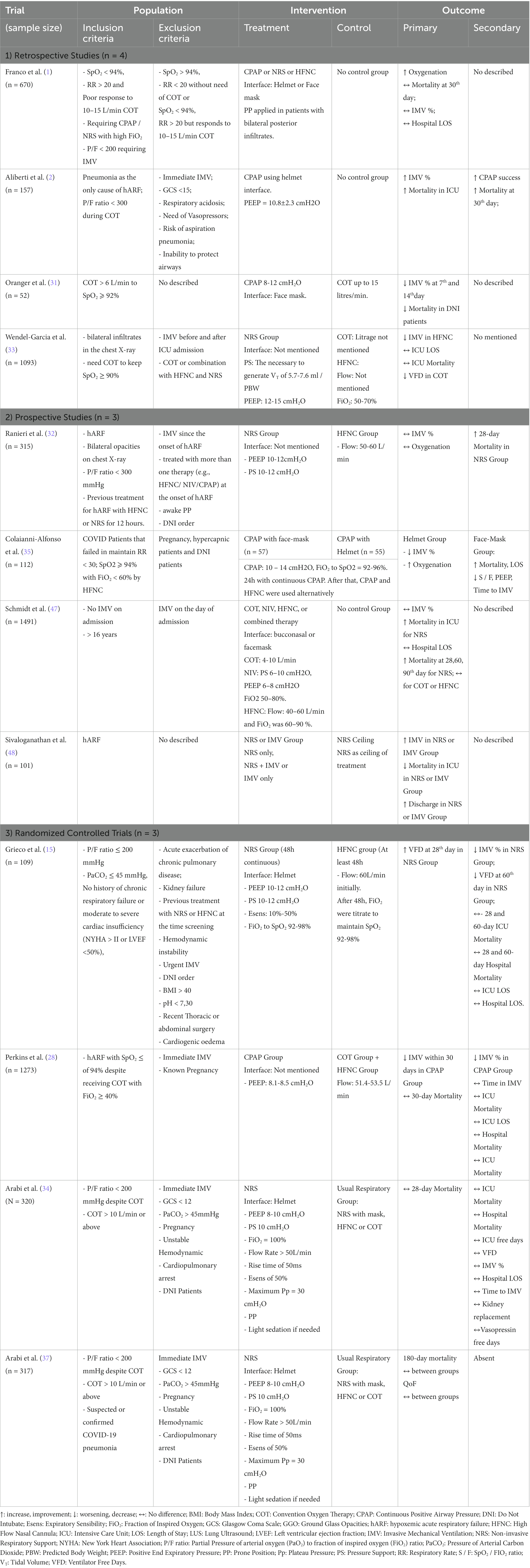
The aim of this narrative review are to: (1) discuss the evidence on different devices and strategies for noninvasive respiratory support (NRS); (2) discuss new and personalized management under MV based on the pathophysiology of COVID-19; and (3) contextualize the use of rescue strategies such as ECMO in critically ill patients with COVID-19 (Figure 1).
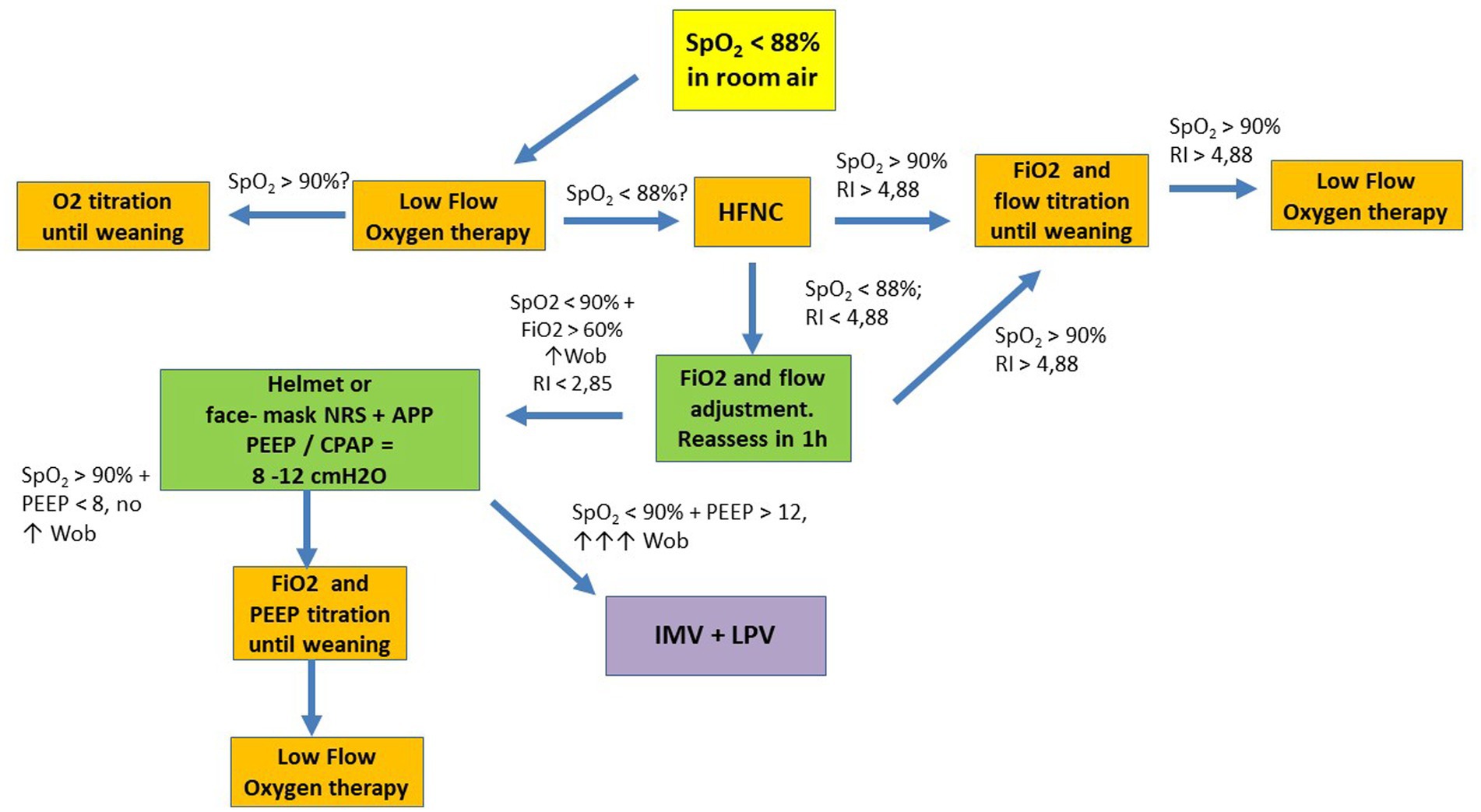
Figure 1. Suggested flowchart for noninvasive ventilatory management in COVID-19. APP, awake prone position; HFNC, high flow nasal cannula; LPV, lung protective ventilation; NRS, noninvasive respiratory support; P/F, PaO2 to FiO2 ratio; RI, ROX Index; SpO2, oxygen saturation; WOB, work of breathing; CPAP, continuous positive airway pressure; IMV, invasive mechanical ventilation; PEEP, positive end expiratory pressure.
2. Physiopathology and phenotypes
In the early stages of COVID-19, the virus targets nasal, bronchial, and pneumocytic epithelial cells. The spike protein of the virus binds to the angiotensin-converting enzyme 2 (ACE2) receptor (23), which allows the virus to enter the host cells, mainly into type II pneumocytes, where the virus starts to replicate. Subsequently, damage to endothelial cells occurs with consequent damage to the alveolar-capillary barrier, resulting in increased cell permeability (23).
The late phase is characterized by a large inflammatory cascade mediated by neutrophils and monocytes, which leads to large diffuse alveolar lesions (4, 5). In this phase, vascular lysis is often observed, with extensive destruction of the lung parenchyma and pneumocytes, alveolar collapse, and the formation of hyaline tissue (5). At the vascular level, dysregulation with stasis, microthrombi, microhemorrhages, and pulmonary embolism are commonly observed due to the high vascular permeability. The alveolar-capillary destruction caused by vascular lysis results in progressive hypoxemia and hypercapnia (4, 9, 24). At first, hyperventilation is noted. However, with progression of the inflammatory cascade, arterial partial pressure of carbon dioxide (PaCO2) levels increase and pH becomes acidic (5).
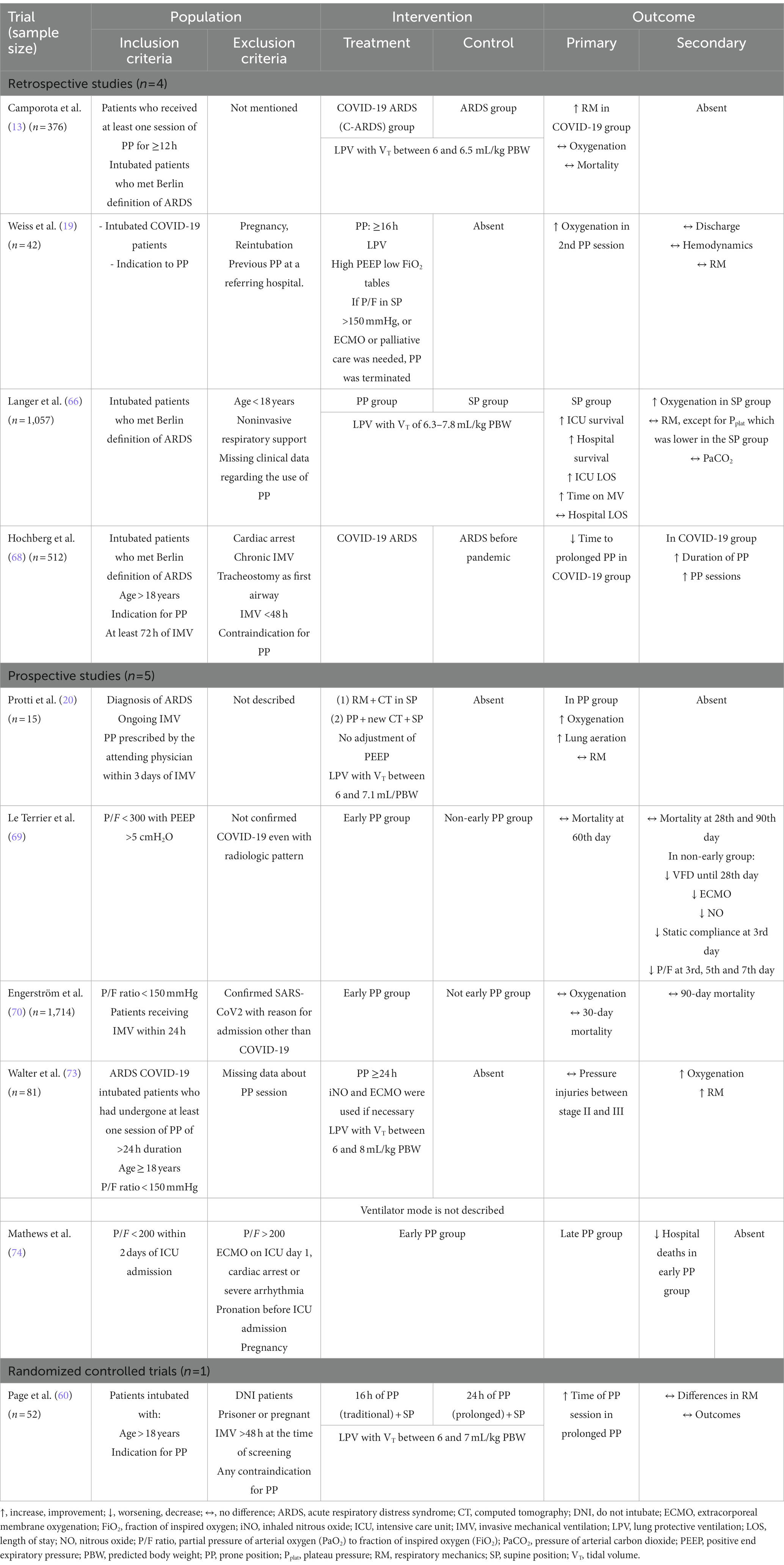
Faced with great alveolar damage, COVID-19 presents as a disease with severe hypoxemia (4, 5, 9, 24, 25). The development of the disease is characterized by the predominance of non-aerated lung tissue, mainly in the dependent regions of the lung. Under normal conditions, these regions have normal blood flow. However, in COVID-19, perfusion is observed to be antigravity, diverting to non-dependent (normally aerated) lung regions (8, 26, 27), with loss of the hypoxic vasoconstriction reflex (4, 6, 8, 27). One hypothesis is that there is a loss of response of sensitive chemoreceptors to low arterial partial pressure of oxygen (PaO2). Another possibility is dysregulation of mitochondria and the pathways involved in oxygen sensing (26). Ventilation/perfusion (V/Q) dysregulation is observed, which is initially due to the presence of hyperperfused ground-glass regions (8, 9, 24, 26, 27). In later stages, the formation of atelectasis is observed, distributed non-homogeneously. V/Q irregularity remains due to the presence of extremely non-aerated areas (8, 9, 24, 26, 28). In autopsy studies, lungs with confirmed SARS-CoV-2 infection exhibit paste within the alveolar cavity, fibrinous exudation, proliferation of type II alveolar epithelial cells and macrophages, vascular congestion of the alveolar septum, and vascular thrombi (23). This points to the importance of the vascular bed in the development of COVID-19 pneumonia (Figure 2).
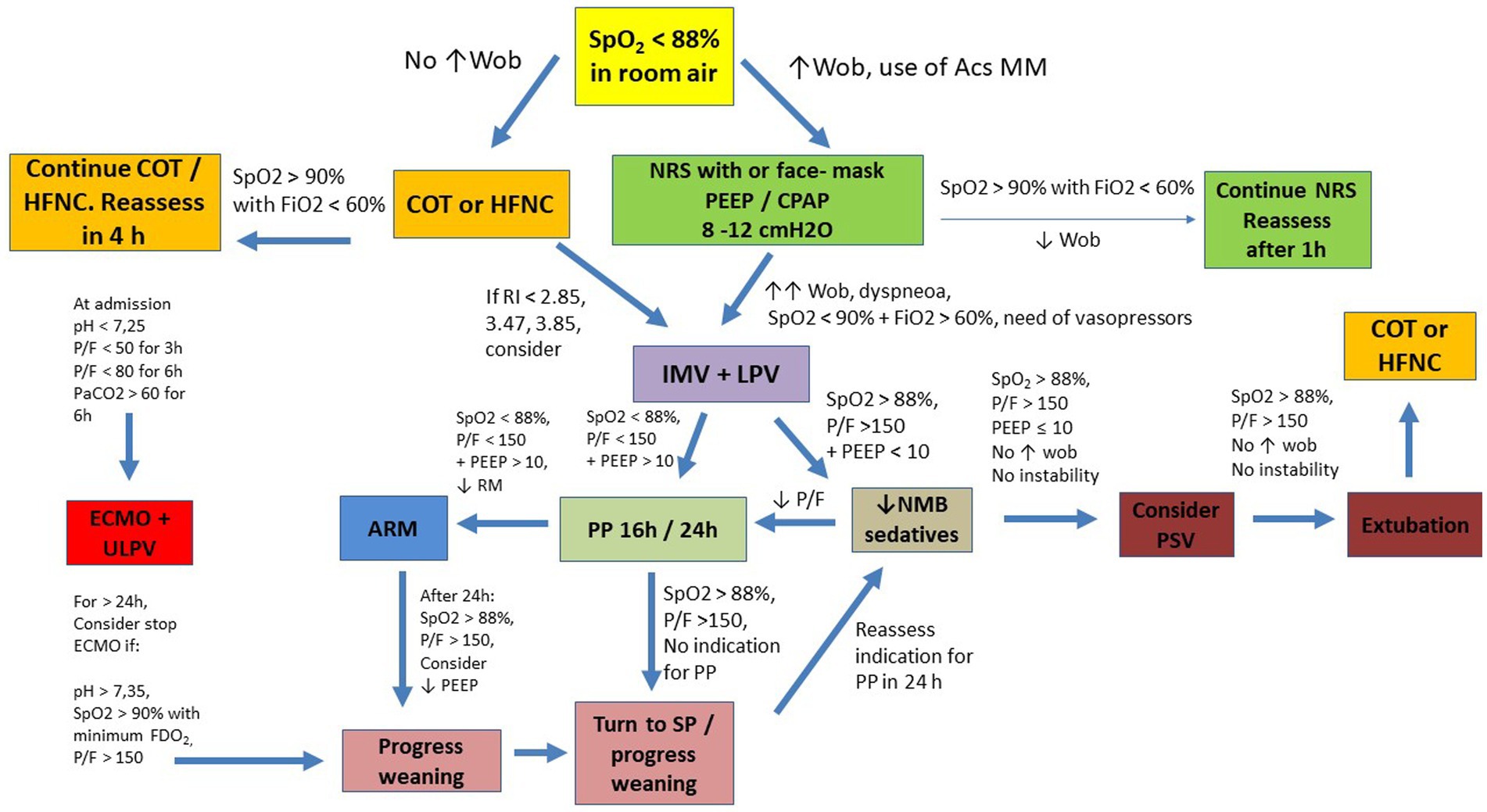
Figure 2. Suggested flowchart for ventilatory management in COVID-19. Acs MM: Accessories Muscles; ARM, alveolar recruitment maneuver; COT, conventional oxygen therapy; ECMO, Extracorporeal Membrane Oxygenation; IMV, invasive mechanical ventilation; HFNC, high flow nasal cannula; LPV, lung protective ventilation; NMB, neuromuscular blocker; NRS, noninvasive respiratory support; P/F, PaO2 to FiO2 ratio; PP, prone position; PSV, pressure support ventilation; RI, ROX Index; SP, supine position; SpO2, oxygen saturation; ULPV, ultra lung protective ventilation; WOB, work of breathing; CPAP, continuous positive airway pressure; PaO2, arterial partial pressure of oxygen; FiO2, fraction of inspired oxygen; FDO2, fraction of oxygen in the sweep gas stream; PEEP, positive end-expiratory pressure.
Some authors divide the histopathology of COVID-19 into three phases that resemble pulmonary ARDS due to the presence of diffuse alveolar damage; (1) the acute/early phase, characterized by intra-alveolar edema and interstitial widening, with peak hyaline membrane formation, both diffuse and focal, which occurs between 4 and 5 days after the initial insult; (2) the organizer stage, also called myeloproliferative, characterized by intense cell proliferation of fibroblasts and hyperplasia of type II epithelial cells; (3) the late/fibrous stage with honeycombing (8, 23, 26).
Three COVID-19 phenotypes can be established to broaden understanding of the pathophysiology (4, 8, 9, 24–27), although the literature does not recommend this (9).
2.1. COVID-19 phenotypes
2.1.1. The L phenotype
The L phenotype occurs in mild to moderate cases, mainly in the early stages. It is classified as low respiratory system elastance, low ventilation-to-perfusion ratio, low lung weight, low lung recruitability (25). It is the longest surviving phenotype (25). It is characterized by hyperperfused subpleural focal GGOs maintaining lung areas that are normally aerated (4, 8, 9, 24–27). Increased perfusion can lead to capillary collapse and hypercoagulability/microthrombosis, leading to deviation of blood flow to the non-dependent regions of the lung (very aerated) and resulting in loss of the hypoxic vasoconstriction reflex (4). Thus, an increase in poorly perfused dependent areas is observed. This situation decreases the V/Q ratio. The clinical picture includes severe hypoxemia with satisfactory ventilatory mechanics (“happy hypoxemia”) (4, 5). Low lung weight is observed, and compliance of the respiratory system is normal or minimally reduced. Therefore, the percentage of poorly aerated tissue is low, as is recruitability. Zubieta-Calleja et al. (23) suggest that this happy hypoxemia may be due to a reduced ventilatory drive, commonly found in this phenotype. Given the dissociation between the extent of hypoxemia and normal compliance, two explanations have been proposed to characterize the severe hypoxemia. The first is the focality of the lung lesion, as demonstrated by the ground-glass pattern. This partially reduces ventilation without affecting elastic recoil. Because there is great lung perfusion, low V/Q areas are diffusely distributed throughout the lung from ventral to dorsal and cranial to caudal (4, 5, 8, 9, 24–27). Pulmonary involvement is low at this stage, therefore the patient’s ventilatory work is still normal. A second explanation is that gas exchange abnormalities arise primarily from vascularly mediated injury, which is not observed at this stage (25). Gattinoni et al. (6) suggest that hypoxemia is due to perfusion irregularity and that vasoplegia is also responsible for low PaO2. In addition to diverting blood flow, ventilation is directed toward non-dependent regions, which allows the creation of dead space areas (5). In conjunction with low V/Q lung units, this phenotype is considered to have wasted ventilation, which does not substantially affect oxygenation (8). Patients with this phenotype may be candidates for NRS or high flow nasal oxygen to correct hypoxemia (8, 29). This phenotype can also be found in promptly intubated patients.
As the disease progresses, the L phenotype may progress to the H phenotype, characterized by low lung compliance. One of the signs of transition between phenotypes is the need for a high fraction of inspired oxygen (FiO2) and increased ventilatory drive (8, 25). Increases in inspiratory efforts are directly associated with worsening of inflammation and, in turn, with increased VT and increased vascular permeability with the formation of alveolar edema. This is one of the mechanisms of patient self-inflicted lung injury (P-SILI) (discussed in Section “Patient self-inflicted lung injury in NRS”). In addition, NRS should be considered with utmost caution in patients with L phenotype, because progression from type L to type H phenotype can be also caused by mechanisms of inflammatory amplification overlapping the host inflammatory response phase (25). Over time, alveolar edema increases and lung volume decreases, reducing the lung area available for gas exchange (8, 9, 24–27). Thus, the inspiratory volumes generated for a given inspiratory pressure decrease, resulting in dyspnea. At this time, transition from the L to the H phenotype is expected (8, 25).
2.1.2. The H phenotype
The H phenotype represents evolution of the L phenotype and is found in critically ill patients (8, 9, 24–27). In this phase, there is amplification of the inflammatory response, allowing greater cellular permeability and formation of alveolar edema (25). As a result of the decrease in gas volume during the evolution of the L phenotype, an increase in lung weight is observed due to the presence of irregularly distributed consolidated areas, predominantly in the dependent regions. This leads to an increase in lung elastance and, in turn, a decrease in lung compliance, with the development of a restrictive pattern of ventilation. Alveolar units of low V/Q ratio are increased (8, 9, 24–27). In this phenotype, however, this happens due to increased lung consolidation, unlike the L phenotype, where the explanation rested on GGOs (8). By increasing pulmonary edema and the pressure exerted on the lung parenchyma, the cardiac output perfusing non-aerated lung areas contributes to the formation of a right-to-left shunt (6). As in the L phenotype, wasted ventilation persists to a great extent (8). The H phenotype has a high capacity for alveolar recruitment (Section “Alveolar recruitment maneuvers and PEEP titration”).
2.1.3. The F phenotype
Faced with the ventilatory dysfunctions found in the two previous phenotypes, Tonelli et al. (25) considered the final pathway of COVID-19 to be the development of pulmonary fibrosis, namely the F phenotype (8, 25, 30).
The evolution of the L to H phenotype is mediated by an intense inflammatory cascade (24). During its evolution, there is activation of multiple aberrant inflammatory pathways that unbalance the relationship between pro-fibrotic and anti-fibrotic mediators (25). In the F phenotype, fibroproliferation occurs so that the lung resembles a patchwork quilt. This causes the alveolar units to have different lung elasticities, with different capacities for volumetric accommodation (8, 9, 24–27).
During spontaneous ventilation, some alveolar units may be more distensible than others, generating high transpulmonary pressures with a high risk of lung injury and pneumocyte rupture (8, 9, 24–27). Furthermore, the pulmonary fibrotic pattern found in this phenotype reduces carbon dioxide diffusing capacity, leading to hypercapnia (8, 25). Rescue therapies such as alveolar recruitment and PP are not very effective because there is a high density of collagen, which is not easily distensible.
3. Noninvasive respiratory support
At hospital admission, patients with COVID-19 may present with low PaO2 and dyspnea. Both can be explained by silent hypoxemia and the presence of non-ventilated areas, as shown by computed tomography, with important ventilation-perfusion inequalities (2, 31). NRS is able to correct hypoxemia, reduce the work of breathing, and improve poor ventilated areas (28), and, even in some scenarios, endotracheal intubation can be avoided (31).
3.1. The choice of NRS interface
Overall, two strategies have been adopted: continuous positive airway pressure (CPAP) and BILEVEL, i.e., pressure support (PS) +: positive end expiratory pressure (PEEP) with the use of two interfaces: facemask and helmet (1, 2, 15, 28, 31, 32). Both interfaces improve oxygenation and reduce aerosolization compared with a high flow nasal cannula (HFNC) and COT.
Patients with COVID-19 may require prolonged NRS therapies (due to low oxygenation on admission) to avoid MV. In this case, there might be a need to apply high PS of up to 12 cmH2O (15, 29, 32), PEEP values between 8 and 12 cmH2O (2, 15, 29, 32), reaching up to 15 cmH2O (33) and as little air leakage as possible. The helmet is one of the best interfaces to promote patient comfort (1, 2, 15, 34). It is also associated with reduced intubation rates and better correction of hypoxemia (35). However, the helmet is associated with greater rebreathing of CO2, requiring intensive monitoring (36). Furthermore, a randomized clinical trial by Arabi et al. (34) divided 320 patients into two groups: half used a helmet and the other half received COT. No differences in mortality were observed after 28 days, but helmet mortality was observed at day 180. Compared with COT, no differences in mortality were observed (37).
3.2. Parameter adjustments during NRS
The best comfort level can be achieved by adjusting ventilation parameters, such as PS and PEEP levels. The PS level is associated with the generation of VT necessary to ensure adequate aeration; the PEEP level is responsible for ensuring oxygenation. A retrospective study reported the use of CPAP in 46 patients with PEEP ranging between 8 and 12 cmH2O. The PEEP level was adjusted according to clinical tolerance, air leakage, and peripheral saturation of oxygen (SpO2) (31). Only nine patients were intubated between days 7 and 14, and the authors recommended the use of CPAP to avoid intubation. Similar results were found in another study that compared HFNC with CPAP in 151 patients (98% with helmet) and NRS in 72 patients (15 with a helmet and 57 with a facemask) (1). One hundred sixty-three patients received HFNC. For the first two interfaces, the authors established a mean PEEP level of 10.2 cmH2O during CPAP and 9.5 cmH2O in NRS. Although all the interfaces were shown to improve oxygenation, there was no difference in intubation rates and length of stay (1). In an important randomized clinical trial, COT (low or high flow) was compared with CPAP adjusted to a mean PEEP of 8.2 cmH2O. Only 36% of the patients in the CPAP group were intubated compared with 44% of the HFNC group. In addition, CPAP reduced mortality compared with COT (26). More recently, Colaianni-Alfonso et al. (35) studied 112 patients with moderate to severe COVID-19 who failed HFNC; the patients were divided into two groups for CPAP: one group used a facemask interface with median PEEP of 12 cmH2O and the other a helmet interface with median PEEP of 14 cmH2O. The groups remained on continuous CPAP for 24 h. It was observed that the helmet group had lower intubation rates and a more marked improvement in oxygenation compared with the facemask group, which had higher intubation rates and longer length of stay. Although these data favor the helmet, caution is required in the interpretation, because the PEEP value applied must be consistent with the clinical condition of the patient.
Adjustments other than PS and PEEP can be fine-tuned at the bedside. In 2009, a study (36) evaluated 13 patients after extubation and randomly performed three 20-min periods of NRS with three interfaces: facemask, helmet, and helmet with a 50% increase in PS and PEEP associated with a high rate of pressurization (rise time). Using the first two interfaces, PS had a mean level of 10 cmH2O, PEEP of 5 cmH2O and 0.2 s of pressurization time. In the third group, PS had a mean level of 15 cmH2O, PEEP of 8 cmH2O, and the shortest possible pressurization time, i.e., 0.05 s. The authors analyzed transdiaphragmatic pressure (Pdi), which is a surrogate of inspiratory effort through an esophageal catheter. Pdi was reduced in the helmet group with higher PS (15 cmH2O) and PEEP (8 cmH2O) and fast pressurization time (0.05 s). This highlights an important comparison between the facemask and helmet interfaces. Keeping the same cycling-off (25%), the helmet had more asynchrony events at the end of inspiration compared with the facemask, with ventilator cycling sooner or later compared with the end of the patient’s inspiratory time. In this case, the ventilator’s inspiratory time was shorter than the patient’s neural time. The authors point out that an overlap exists between the PS applied by the ventilator and the patient’s neural time. As an explanation, the authors hypothesize that cycling with the helmet seems to occur due to changes in flow caused by the mechanical characteristics of the interface and not by the characteristics of the patient. With the patient’s inspiratory time longer than that of the helmet, the interface promotes minimal reduction in ventilatory overload. Further studies on patients with COVID-19 comparing the two interfaces are necessary for a better understanding of their ventilatory repercussions.
The choice of the ideal interface, as well as fine ventilator adjustments, should aim to achieve patient-ventilator synchrony, reduce the work of breathing, especially with the helmet interface and to ensure comfort. A multicenter randomized clinical trial randomized 54 patients (mean age, 66 years) to NRS and 55 to HFNC (15). The NRS group underwent therapy for at least 48 h and used a helmet interface with PS and PEEP levels ranging between 10 and 12 cmH2O, no pressurization time (rise time), expiratory trigger between 10 and 50% to avoid double trigger, inspiratory trigger to avoid auto-triggering, and maximum inspiration time between 1 and 1.2 s. PS was titrated individually to ensure high flows to the patient. The NRS group showed lower intubation rates and more MV-free days compared with the HFNC group. This allows us to conclude that the success of NRS is based on fine ventilatory adjustments.
Typically, NIV is a therapy performed in the ICU. However, with the pandemic exceeding the capacity of available beds, this tool gained space outside the ICU. Cammarota et al. (37) conducted a systematic review with meta-analysis including 17 articles containing 3,377 patients which showed the effectiveness of NIV outside the ICU environment as an adequate tool to deal with the demand for ventilatory assistance.
3.3. NRS therapeutic targets
The literature is not concordant regarding the therapeutic objectives of NRS. Perkins et al. (28) indicated different factors as therapeutic targets, such as: SpO2 > 90%, respiratory rate ≤ 25 bpm, and a reduction in the work of breathing (26). Aliberti et al. (2) stated that NRS weaning can be performed if the patient’s SpO2 > 94% with FiO2 < 50% and PEEP ≤5 cmH2O. Arabi et al. (34) stated that application of PEEP should target SpO2 between 92 and 98%, and that the respiratory rate should be <25 bpm. More recently, Colaianni-Alfonso et al. (35) state that application of PEEP should target an SpO2 between 92 and 96%.
3.4. Predictors of NRS failure
After starting NRS, patient monitoring must be constant to assess its effectiveness or failure. Arabi et al. (34) suggest assessment every 1 to 3 h, but this may vary according to the intensive care unit (ICU). In case of therapy refractoriness, the literature indicates that MV should not be postponed. Some signs of failure mentioned in the literature are: respiratory rate > 40 bpm, respiratory acidosis with pH <7.25–7.30, use of accessory muscles, dyspnea, swallowing disturbance, SpO2 < 88–90% for more than 5 min, PaO2/FiO2 ratio < 100, persistent requirement for FiO2 > 70%, hemodynamic instability (systolic blood pressure < 90 mmHg or mean blood pressure < 65 mmHg, even with volume resuscitation), deterioration in the level of consciousness (2, 15, 34, 35). Contrary to the data, the intubation criteria in the randomized clinical study by Perkins et al. (28) are stricter. These authors compared CPAP with low and high flow oxygen therapy and considered an SpO2 ≤ 94% with an FiO2 of at least 40% to be a ventilatory risk. Robba et al. (27) suggest immediate intubation if the PaO2/FiO2 ratio does not improve and/or PaCO2 < 30 mmHg and/or respiratory rate > 28 bpm using accessory muscles for more than 3 h. More recently, the study by Colaianni-Alfonso et al. (35), who compared helmet CPAP and helmet facemask (discussed earlier), considered pH <7.35 as a criterion for intubation, in addition to all previous signs. These data indicate that there is no clear guideline for the signs of NRS failure, allowing the use of some scales to help diagnose it (Figure 1).
3.5. NRS failure prediction scales
The literature presents some scales/indices that help in the diagnosis of therapeutic failure during NRS:
• Sepsis-related Organ Failure Assessment (SOFA) (41)
• HACOR Score (heart rate, acidosis, consciousness, oxygenation, and respiratory rate) (42–45)
• Simplified Acute Physiology Score (SAPS) (46)
3.5.1. ROX index
Originally developed to assess the effectiveness of HFNC, the ROX Index has been used as a predictor of the success or failure of NRS. It consists of dividing the SpO2/FiO2 quotient by the respiratory rate. It must be calculated in periods of time not yet defined in the literature, but which may be the same as the HFNC. Values < 2.85, <3.47, and < 3.85 after 2, 6, and 12 h, respectively, have been demonstrated to be predictors of therapy failure (38). At the same cutoff points, values ≥ 4.88 indicate success of NRS (38). A recent article applied CPAP with a mean PEEP of 12 cmH2O in 112 patients with a facemask interface. All patients remained on CPAP for 24 h (39). The researchers calculated the ROX Index after 2, 6, 12, and 24 h of positive airway pressure, and values <6.64 after 24 h of therapy were associated with therapeutic failure. The cutoff periods of 2, 6, and 12 h showed low specificity and sensitivity (39). Higher cutoff points were found in an American study in 2022 (40). The researchers applied CPAP in 95 patients with an initial PEEP of 5 cmH2O. FiO2 was adjusted individually by SpO2. The ROX Index was measured after 2, 6, 12, 18, and 24 h of positive airway pressure, and values < 8.76, <9.08, <9.50, <8.58, and < 7.77, respectively, were predictors of NRS failure. However, details of the interfaces were not given (34).
3.5.2. Sepsis-related organ failure assessment
SOFA was developed in 1996 (41) to assess multiorgan failure, which is a characteristic of COVID-19 (30). It includes six domains each with scores between 1 and 4: breathing, coagulation, liver, cardiovascular, neurologic, and renal. Values > 2 indicate the presence of sepsis and, therefore, a risk of mortality. A recent prospective study (47) evaluated 1,491 patients, 158 of whom received NRS; the rest received low flow or high flow oxygen therapy. Mean PS was 8 cmH2O, mean PEEP was 7 cmH2O, and mean FiO2 was 60%. Patients on NRS had a mean SOFA score of 3. This group had higher intubation and mortality rates at 28, 60, and 90 days. Although the authors did not provide a cutoff point for NRS failure, they showed that most patients had a score > 3 in the cardiovascular domain after 24 h in the ICU. Previously, another prospective study (48) included 58 patients received NRS. Twenty-seven patients who progressed to intubation had an average of 4 points on the SOFA; the group who were not intubated had 3 points. Furthermore, the authors showed that high scores on the scale were associated with low oxygenation. Although the authors did not explain this association, within the respiratory domain of SOFA, a score of 4 indicates a PaO2/FiO2 ratio < 100, suggesting therapeutic failure. They concluded that high SOFA was related to intubation but did not provide data on mortality. These data are in agreement with previous studies (1). With the facemask and helmet interfaces, the average SOFA values were 3.3 and 4, respectively. The difference in scores between the interfaces remains unknown.
3.5.3. HACOR score
Originally developed in 2017 (42), the HACOR Score consists of five parameters easily collected at the bedside: heart rate, acidosis, consciousness, oxygenation and respiratory rate. Of these domains, four are included in the evaluation of the effectiveness of NRS, which makes this score very accurate in detecting therapeutic failure (43). The authors reported that the cutoff point for NRS failure must be ≥5. The study by Innocenti et al. (44) evaluated 135 patients who underwent CPAP with a full-face or oronasal mask. The HACOR Score, ROX Index, and SOFA scales were applied 3 days and 1 day before the start of NRS, on the day of admission, and on days 1, 2, 5, 8, and 11 after NRS. The authors did not provide information about PEEP adjustments. FiO2 was titrated to achieve an SpO2 of 94%. Thirty-five patients died (considered as a therapeutic failure) given the presence of several comorbidities. This group had a HACOR Score > 5, ROX Index <4.88, and SOFA scores ≥4.
An observational study by Guia et al. (43) evaluated the HACOR Score of 128 patients with a mean age of 61 years after performing 1 h of CPAP with a mean PEEP of 10 cmH2O. Thirty-two patients had a HACOR Score ≥ 5, and 22 failed therapy; i.e., 69% of the positive predictive value. On the other hand, 96 patients had a HACOR Score < 5 points. Of these, 83 had successful CPAP; i.e., 86% of the negative predictive value.
3.5.4. Simplified acute physiology score
Originally developed in 1993 by Le Gall et al. (46), SAPS includes cardiorespiratory and renal parameters, laboratory analysis of red and white blood series, and electrolytes. It is a larger scale than the previous ones. Values vary between 0 and 163 points. Higher scores are associated with worse prognosis. According to the original study, a SAPS of 29 points is associated with 10% of deaths, and values of 40 are correlated with 25% of deaths. Very few studies on COVID-19 included in this review used SAPS. Patients in the study by Oranger et al. (31) showed SAPS of 26 points, whereas the study by Grieco et al. (15) reported mean values of 32 points. The multicenter study by Schmidt et al. (47) reported that patients with COVID-19 on NRS had a mean SAPS of 33 points. It is reasonable to conclude that there is a correlation between SOFA, SAPS II, HACOR Score, and the ROX Index regarding the diagnosis of NRS failure.
3.6. Patient self-inflicted lung injury in NRS
At the beginning of the pandemic, the initial recommendation was early intubation to protect the lungs (3, 49). Due to the urgency caused by the pandemic, limited staff, and few noninvasive ventilatory resources to meet the demand, many patients experienced worsening respiration and consequent intubation.
With the reduction in the number of cases, noninvasive ventilatory support such as NRS and HFNC was introduced with the aim of reducing ventilatory effort and avoiding intubation (29). However, when instituting noninvasive therapy, adequate monitoring is required to avoid P-SILI (8, 27, 50). In patients with COVID-19, reduced lung compliance and heterogeneous distribution of inspired VT are observed due to the presence of areas of low V/Q ratio that are distributed irregularly throughout the lung (4, 8, 24, 25, 51). In spontaneous ventilation, during the inspiratory phase, sufficient diaphragmatic contraction is required to counteract pulmonary elastic recoil forces (8, 24, 25, 27, 50). This generates large variations in transpulmonary pressure (PL).
In the early stages of COVID-19 (L phenotype), when there are no large pulmonary consolidations, no variations in transpulmonary pressure are observed, which allows the application of NRS with greater safety (6, 25, 29). Under normal conditions of spontaneous breathing, during the inspiratory phase, pleural pressure decreases uniformly, whereas PL increases uniformly (50). In situations of increased ventilatory drive, greater inspiratory efforts are observed to generate a given VT caused by greater negative pleural pressure, increasing PL, which reflects inspiratory efforts. This allows non-homogeneous distribution of lung pressures and volumes, leading to P-SILI (8, 25, 50). Expiratory efforts can also cause P-SILI (50). During intense expiratory activity, pleural pressure increases, drastically reducing PL, with alveolar collapse occurring in most dependent lung regions and peripheral airways. Battaglini et al. (50) suggest a study of stress/strain for a better understanding of the disease. Stress is the distribution of force applied per unit of lung area, and strain evaluates the stretching of this alveolar unit and is directly proportional to stress (25, 50). During the development of COVID-19, ventilatory efforts become more vigorous, leading to regional hyperdistention, especially in non-dependent regions, and further compromising dependent regions (8, 24, 25, 50). Therefore, both stress and strain are increased. This is associated with the development of pneumothorax and pneumomediastinum (50).
3.6.1. Pendelluft phenomenon
Inspiratory pendelluft is a phenomenon found in the development of COVID-19 and contributes to the genesis of P-SILI (8, 25, 50, 52). It is defined as the disorganized distribution of gas when the inspiratory effort has not yet produced an inspiratory flow at the airway opening. It occurs due to different regional time constants or negative fluctuations in pleural pressure in patients who are breathing spontaneously. This allows irregular distribution of VT and, consequently, of PL (8, 25). In the pendelluft phenomenon, the gas moves from the non-dependent region to the dependent region, which remains under significant recruitment and hyperdistention and may release inflammatory mediators. Analyzing the transition of phenotypes is also useful to monitor P-SILI.
In the L phenotype, when lung compliance is normal or slightly reduced, a fluid-like behavior is predominant. Thus, the distribution of pleural pressure is homogeneous along the lung surface (25). With the worsening of inflammation and alveolar edema, this pulmonary phenotype can progress to type H (25). One of the signs of phenotypic transition is an increased respiratory rate (even in NRS), resulting in intense respiratory efforts (8, 25). Another sign of phenotypic transition is an increase in PEEP and an increase in FiO2 to maintain SpO2 > 90% (50). The generation of high VT values can also indicate a phenotypic transition. When positive airway pressure is applied, PL may increase with consequent production of high VT outside the protective concept, i.e., between 6 and 8 mL/kg of predicted body weight (PBW) (33, 47, 50). This increases the chances of barotrauma.
Considering the pendelluft effect, the chances of P-SILI also increase. The gold standard for detecting ventilatory effort is esophageal pressure through a catheter that rests just above the diaphragm. However, its use is still restricted to experimental studies, not yet viable at the bedside (50). Tonelli et al. (52) proposed that measuring the variation in nasal pressure (Pnos) is directly related to the variation in esophageal pressure (Pes). For this, they studied 61 patients, of which 83% tested positive for COVID-19. The authors calculated both pressures. They used a nose clip for the analysis of Pnos and asked the patients to keep their mouth closed throughout the evaluation. On the third day of NRS, the authors observed that patients who evolved to invasive MV had a mean ΔPes of 14 cmH2O and a mean ΔPnos of 6.5 cmH2O. The values for those who remained in NRS were 12 and 5.6 cmH2O, respectively. This was an early cohort study. New studies are important to confirm this information.
In addition, asynchrony events are also associated with the genesis of P-SILI; double triggering is the most common. In patients with COVID-19, the expiratory phase is marked by a significant increase in pleural pressure, reducing pleural pressure, causing collapse of most dependent lung regions and peripheral airways. Hence, P-SILI is also influenced by the pendelluft effect. This leads to alveoli with different regional time constants.
3.6.2. Squishball phenomenon
During the transition from the H to F phenotype, a severe increase in esophageal pressure is observed as the lung is assuming a pattern of fibrosis or a patchwork. There is deposition of collagen and elastin, poorly contractile proteins, therefore the chance of P-SILI and ventilator-induced lung injury (VILI) increases dangerously if the patient remains on NRS (8, 25). During the inspiratory phase, fibrotic lungs present heterogeneous behavior, because lung tissue does not have the same mechanical properties in all directions when a given transpulmonary pressure is applied (8, 25). In addition, the application of PEEP or high VT can determine hyperdistention of more distensible lung areas (34). This is called the squishball phenomenon, which increases regional stress and strain (25). Its understanding is similar to the pendelluft effect.
3.6.3. Mechanical power for monitoring P-SILI
Considering that the amount of energy to which the lung is subjected, even during assisted spontaneous breathing, can be crucial in the development of P-SILI, application of inappropriate ventilator pressure or the phenotypic evolution of the disease increase the patient’s esophageal pressure, resulting in VILI. Mechanical power can be assessed at the bedside to evaluate this phenomenon in a simple way (8) using the formula 0.098 × respiratory rate × VT × (Ppeak − 0.5∆Paw), where Ppeak is the peak pressure and Paw is the airway pressure. This index may represent a reliable estimate of the amount of energy transferred from the respiratory muscles and ventilatory assistance to the lung during assisted spontaneous breathing (8). Thus, the need for ventilatory adjustments, such as increased PS or PEEP, can be assessed.
The use of mechanical power is useful to assess pulmonary recruitability at the bedside (8). Decreased dynamic compliance is correlated with increased mechanical power and may suggest limited lung recruitability and predict the risk of local overdistention (8).
3.6.4. P-SILI and perfusion irregularities
Another factor that increases the chances of P-SILI is the irregularity of lung perfusion (4, 5, 24, 26, 50). With increased inspiratory effort, pulmonary capillaries can be compressed, increasing pulmonary resistance. This leads to increased transalveolar and transcapillary pressures recruiting previously collapsed capillaries (50). On the other hand, it leads to hyperdistention of those located in healthy areas and in ground-glass regions, which can lead to increased blood flow in injured regions and damage to the alveolar-capillary membrane (50). This predisposes the formation of interstitial and alveolar edema, increasing the risk of P-SILI (25). With this, the phenomenon of pendelblut is observed, in which traction forces applied to vessels adjacent to stress generators can generate a blood siphon effect toward areas of greater PL (8).
All these factors may lead to higher lung perfusion and predispose the formation of interstitial and/or alveolar edema and worsening lung inflammation (47). This may explain why patients intubated at a late stage are not responsive to PEEP and have low static compliance, increasing mortality (51). Despite the signs of NRS failure mentioned earlier, and considering the heterogeneous development of the disease among patients, the decision to intubate needs to be taken after discussion with a multidisciplinary team (2).
3.6.5. Early versus late intubation
The decision to intubate should be made considering the course of the disease and the patient’s clinical condition. It should be performed in cases of complete refractoriness to NRS. However, with the reduction in the number of cases, patients under NRS can be better monitored, allowing for a lower rate of intubation.
The L phenotype normally appears hypoxemic, with no change in compliance. In this case, HFNC and NRS are first-choice interventions, because the patient still benefits from the therapy (25) and orotracheal intubation can be postponed.
With evolution from the L to the H phenotype, consolidations and alveolar collapse, which need to be reopened to ensure adequate oxygenation and reduction of ventilatory work, are present (8, 25). The problem is that the patient must develop extra diaphragmatic force due to the increase in elastic recoil (25). NRS at this point starts to become contradictory because the patient increases inspired VT to overcome the elastic recoil leading to P-SILI. Robba et al. (27) stated that patients who remain on NRS for a long time may develop the H phenotype, which may result in diaphragmatic dysfunction. At this point, orotracheal intubation is recommended.
It is difficult to ventilate patients who have the F phenotype because the lungs present great heterogeneity in gas distribution, leading to the pendelluft effect (8, 25, 27). Maintaining spontaneous ventilation in this phenotype may increase the release of inflammatory mediators, and therefore intubation is recommended (8, 25).
Prolonged endotracheal intubation is associated with a worse prognosis, the need for emergency airway management (27), and increased mortality (18, 38, 47). Wendel-Garcia et al. (42) showed that compromised respiratory system mechanics during prolonged endotracheal intubation may explain the increase in mortality observed under NRS. It may also make it difficult to maintain protective ventilation and contraindicate ARM or PP due to increased areas of pulmonary consolidation and/or a radiologic pattern similar to fibrosis.
In a study by Ball et al. (41), 52 patients with a mean age of 64 years who failed helmet CPAP after a minimum of 2 h were divided into two groups: early intubation and late intubation, with a cutoff point of 2 days. After endotracheal intubation, patients underwent computed tomography imaging with two levels of PEEP: 8 and 16 cmH2O to assess ARM. The late intubated group had lower static compliance and a lower P/F ratio. Regarding ventilation distribution, the late intubated group had a higher percentage of poorly and non-aerated areas. Furthermore, this group did not respond to increased PEEP (8 to 16 cmH2O), requiring higher FiO2, indicating that these patients were not recruitable. There was no difference in mortality between the groups.
There is still a lack of studies in the literature that quantify the results of patients intubated early or late after NRS failure. The research carried out for this paper allowed the creation of Table 1.
3.6.6. Aerosol risk during NRS
At the beginning of the pandemic, there was great concern about the production of aerosols which would spread the SARS-CoV-2. The current recommendation stated that the patient should be allocated in a room with negative pressure, and undergo NIV therapy with a double branch circuit and antibacterial filter (54, 55). Whittley et al. (56) at the beginning of the pandemic, when comparing low and high flow oxygen therapy devices with NIV reported that high flow oxygen with NIV had the greatest particle dispersion capacity. After the reduction in the number of cases, the therapy became flexible to meet the demand. In the current scenario, NIV is no longer considered to be a large-scale aerosol-producing therapy (57, 58). Dell’Olio et al. (57) carried out a study that evaluated the production of aerosols in 4 regions around patients undergoing NIV with total face interface. The regions were 50, 80, 150 and 200 meters from the patients’ mouths. The results showed that only 21% of these regions were contaminated by SARS-CoV-2, indicating that NIV is a safe therapy.
Winslow et al. (58) compared COT, NRS, and HFNC in terms of virus shedding rates. Each group had 10 patients and the analysis was performed with the patient ventilating properly and with a cough stimulus. The authors concluded that NRS and HFNC have a low dispersion rate when compared to COT.
4. Prone position
Patients with COVID-19 who have an indication for MV need to be protectively ventilated to prevent VILI. For this, a plateau pressure (Pplat) <30 cmH2O, driving pressure (ΔP) <15 cmH2O, and VT between 6 and 8 mL/kg of PBW are recommended (59). However, within the pathophysiology of COVID-19, the patient may have poorly or non-ventilated lung areas, mainly in the basal and dorsal regions, in contrast to great aeration in the ventral regions, leading to hyperinflation (8). This is called pulmonary heterogeneity and may lead to low respiratory compliance. As a result, there is intrapulmonary shunt formation, mismatching the V/Q ratio (60). Thus, some patients may not respond to lung protective ventilation (LPV), requiring rescue maneuvers, such as PP (19, 20).
Recent studies have shown that COVID-19 has features of ARDS (61), allowing the Surviving Sepsis Campaign panel to recommend that the treatment of COVID-19 be similar to that of ARDS (12).
4.1. Effects of PP
PP is a non-pharmacologic strategy widely adopted in moderate/severe cases of ARDS with inadequate gas exchange (i.e., PaO2/FiO2 ratio < 150, with FiO2 > 60%) even with PEEP optimized within the concept of LPV. In ARDS, PP redistributes air volume from ventral to dorsal areas, promoting lung homogeneity (19, 20) because lung ventilation is dependent on gravity (20). PP also reduces regional lung stress/tension by displacing non-ventilated areas ventrally (20, 62, 63). Recruitment of the dorsal region of the lung is observed with subsequent increase in regional oxygenation and de-recruitment of the ventral region, leading to a decrease of the hyperinflated tissue (63, 64). In this case, a reduction of the dorsal shunt is observed, improving oxygenation (19, 20). Grasselli et al. (62) state that oxygenation can improve between 60 and 80%.
4.2. Ventilatory mechanics versus oxygenation
Final PaO2 is a weighted average of the PaO2 of blood flowing from different lung units. This means that the number of atelectatic units in the dependent lung regions is proportional to the severity of hypoxemia (63). In a supine position, with an angle of 0°, approximately 60% of the total lung mass is dependent. In COVID-19, perfusion irregularity promotes greater perfusion in these regions, leading to a decrease in the V/Q ratio (20, 62). During PP, however, only 40% are in the dependent position; i.e., fewer lung units are hyperperfused, resulting in better oxygenation (63). The consequence, in terms of ventilatory mechanics to the PP, is a decrease in total compliance of the chest wall, due to the functional stiffening of the anterior chest wall (63, 64). Thus, an improvement in lung compliance values and a more homogeneous V/Q distribution are expected (64). This also reduces VILI, resulting in improved parameters of ventilatory mechanics (49).
4.3. Patients eligible for prone position
The correct indication for PP is directly correlated with the duration of the disease and the patient’s clinical status. The L phenotype is characterized by moderate to severe hypoxemia, even with normal lung compliance (6). This phenotype is considered unresponsive to PP, and the observed improvement in oxygenation is due to the redistribution of blood flow from dorsal to ventral areas, without any alveolar recruitment, as seen in ARDS (42). This, PP in this phenotype does not bring great benefits, because this phenotype has no or little recruitment capacity. However, better aeration of dorsal regions is noted, reducing the chances of atelectrauma (64). Furthermore, COVID-19 is progressive, evolving to the H phenotype, which is more recruitable (6, 25). In this phenotype, there may be a worsening of lung compliance, without any relationship with the conduct. It is at this point that PP becomes more indicated. There is also an improvement in oxygenation, but at the expense of directing blood flow to dorsal regions with alveolar recruitment between patients (62). The ventilatory difficulty of the F phenotype contraindicates PP, because the benefits will be few. This is due to organizing pulmonary fibrosis (25, 27, 63). At this time, protective ventilation is prioritized (25).
The study by Fossali et al. (64) provides information relevant to the topic. The authors studied 21 patients with a mean age of 67 years. They performed chest computed tomography in a supine position and PP. Afterward, within the ICU, the authors performed electric impedance tomography (EIT) to verify distribution and ventilation and perfusion. All were protectively ventilated, without adjustments, in pressure regulated volume-controlled mode with PEEP maintained at 10 cmH2O. The authors described that there was no difference in the compliance of the respiratory system in both decubitus positions. The authors hypothesizes that in supine position, there may be alveolar units subject to cyclic openings and closings, which would be reduced in PP. In addition, another possible reason is that there was a decrease in lung elastance associated with increased chest wall rigidity. In addition, there was recruitment of dorsal regions, with perfusion improvement in these regions and de-recruitment of ventral regions. This allowed reduction in barotrauma and atelectrauma, reduction of areas with dead space, reducing the number of alveolar units with low V/Q, which improved V/Q matching. This dorsal de-recruitment is called spongelung (65) and is characterized by a reduction in dorsal pulmonary tension and ventral hyperdistention. The authors also point out that there was a reduction in the dead space/shunt ratio in PP and that this is also a marker of lung protection. However, the patients included in this study had been ill for an average of 8 days. Considering that COVID-19 is a progressive disease, it can be inferred that the patients were in phenotype transition to H and F, when the PP has few benefits.
The retrospective study by Langer et al. (66) divided 1,057 patients ventilating protectively into two groups (PP and supine position) with a mean age of 63 years. The average time to perform the first PP was 2 days. The authors observed that there was no difference in oxygenation and ventilatory mechanics between the groups. This can be explained by the high compliance at baseline. Therefore, the effect of PP may not work solely by recruitability, but through the redistribution of pulmonary blood flow.
Weiss et al. (19) studied 42 patients with a mean age of 59 years, but with significant obesity (body mass index (BMI) > 34 kg/m2), also under LPV. The researchers performed three PP sessions. In contrast to the article mentioned earlier, there was improvement in oxygenation after the first PP session, but a similar effect was not observed during the second and third PP sessions. This can be attributed to disease progression.
Recently, the COVID-19 Veneto ICU Network research group developed the PROVENT-C19 Registry, a large multicenter protocol specifically for patients with COVID-19 with the aim of describing the population that most benefits from PP (67). On admission, anthropometric data, data on comorbidities, and the type of ventilatory support used before EIT will be collected. The outcomes to be analyzed include differences in gas exchange and the PaO2/FiO2 ratio and ventilatory parameters before and after PP, prone duration, and ICU and hospital mortality. Considering the expected large population of this study, there will be an important improvement in clinical practice.
4.4. Duration of PP
The recommended duration of PP is at least 16 h (61, 68, 69). However, some studies have reported durations longer than 16 h of PP with different outcomes. The prospective study by Engerström et al. (70) evaluated 1,714 patients with a mean age of 64 years. The mean time between intubation and first PP session was 20.4 h. No association between early PP and survival was observed. Protti et al. (20) studied 15 patients with a mean age of 69 years and a mean BMI of 29 kg/m2. Patients were intubated within 2 days and were placed in PP within 3 days. There was a reduction in the volume of non-aerated gas and hyperventilated areas, indicating a lower possibility of VILI and an increase in respiratory compliance. An important point in this study is that the patients did not experience delayed intubation. This certainly has effects on the outcomes.
Encouraging results were also found in the study by Page et al. (60). The authors studied 52 obese patients (BMI >32 kg/m2) with a mean age of 62 years. They were randomized between conventional prone (16 h) and extended prone (24 h). There was no change in respiratory mechanics, but patients who remained prone longer had more ventilator-free days.
A longer time in the prone position was reported by Rezoagli et al. (71). The standard PP group lasted for 16 h, while extended PP consisted of 40 h. Although the extended PP group was younger than standard PP group, extended PP was feasible and was able to reduce the workload of health professionals. Taking into account the oppressive condition during pandemic, the reduction in workload is an important issue to consider. Furthermore, no benefits or harm in terms of gas exchange or respiratory mechanics were found when extended PP was compared to the standard PP group.
On returning to supine position, some patients may experience a decrease and loss of oxygenation gain (59, 72), further favoring extended PP. Recently, the retrospective study by Okin et al. (72) compared 267 patients with a mean age of 62 years who were subjected to 16 h and 24 h of PP in terms of mortality; 157 patients underwent extended PP (>24 h) and 110 underwent conventional PP (up to 16 h). The authors observed that mortality at 30 and 90 days was lower in the extended PP group. In addition, the study highlights that extended PP is safe, because it reduces the number of supine sessions that are associated with alveolar de-recruitment, increased atelectasis, and VILI, contributing to mortality. It also reduces the amount of neuromuscular blockers, reducing diaphragmatic dysfunction (72).
Thus, there is no limit on the number of PP sessions as long as they are recommended. For example, Walter et al. (73) reported that some patients underwent PP 22 times. The same study also suggests that PP should be interrupted when the FiO2 requirement is ≤60%, when the PaO2/FiO2 ratio is >150, and when the PEEP is ≤12 cmH2O.
4.5. Early or late PP?
Delaying PP is associated with higher mortality. The study by Mathews et al. (74) included 2,338 patients; 702 were placed in PP within 2 days of MV and the other 1,636 within 2 days of MV with a P/F ratio < 200. The authors observed that the early PP group had greater chance of developing shock and use of corticosteroids. However, the risk of death was lower. COVID-19 is a heterogeneous disease, therefore it is not possible to define a suitable time to implement PP. One suggestion is to use the same reasoning used to determine the need to transition from NRS to intubation: the worsening of compliance and the need for high FiO2 fractions to maintain adequate SpO2. In this case, it is possible to infer a change from the L to the H phenotype, which has a greater possibility of recruitment, benefiting from PP (25, 63).
With regard to the objectives of prone decubitus, associated articles describe the physiologic and ventilatory changes of the position. However, when analyzing the effects of PP, the increase in survival must be considered. Directing the therapeutic target only to improve oxygenation can be a scientific limitation. To guide the understanding of this topic, Table 2 contains a summary of the included studies and outcomes found.
5. Alveolar recruitment maneuvers and PEEP titration
At the beginning of the pandemic, there were doubts whether the pulmonary presentation of COVID-19 was similar to that of ARDS (51). A common factor is the difficulty in setting an ideal PEEP, although guidelines recommend the use of PEEP >10 cmH2O due to the large non-aerated area observed in COVID-19 (12). However, in some patients, oxygenation does not normalize, resulting in worse respiratory mechanics (i.e., ΔP >15 cmH2O; Pplat > 30 cmH2O), even during PP sessions.
5.1. Recruitability assessment
The use of PEEP tables, widely used for ARDS, is an easy alternative to titrate PEEP and sustain ARM (75–78). However, this strategy fails to optimize oxygenation; the PEEP response in patients with COVID-19 is highly heterogeneous due to the facts mentioned earlier (79–82). In this context, some studies chose the recruitment to inflation (R/I) ratio developed by Chen et al. (78) to assess the potential for recruitability in patients with ARDS. It ranges from 0 to 2. R/I < 0.5 indicates low potential for recruitability, increasing the risk of pulmonary overdistension without any benefit. R/I > 0.5 indicates high recruitability (79, 82, 83). After assessing recruitability, the choice of PEEP is based on ARM with decremental PEEP titration. Some studies have used only decremental PEEP titration (8, 78). Briefly, this strategy consists of gradually increasing the airway opening pressure up to 45 cmH2O and then performing PEEP titration (in steps of 2–3 cmH2O), maintaining the stability of hemodynamic and airway ΔP and allowing PL to increase (80–83).
5.2. Effects of PEEP in oxygenation and perfusion
The issue of heterogeneity of oxygenation targets is a topic of discussion. Zerbib et al. (80) states that an SpO2 between 88 and 92% is satisfactory. Ball et al. (8) suggested that the best PEEP is the one in which PaO2 remains >60 mmHg. These two studies were less rigid about oxygenation, in contrast to previous studies dealing with ARM and PEEP titration. Randomized studies are needed to confirm whether these oxygenation targets are suitable for COVID-19.
5.3. Effects of PEEP with the L phenotype
For lungs with low recruitability (L-type phenotype; i.e., high static compliance), low levels of PEEP are sufficient to optimize PaO2 and reduce hyperdistended areas, Pplat and airway ΔP.
When high PEEP is applied to the L-type phenotype, it is expected to increase lung volume and reduce lung heterogeneity, at the cost of increased overinflated areas compared with low PEEP (8). Usually, airway pressure increases followed by impairment in respiratory system compliance. In the L phenotype, high PEEP values are not recommended, because this is a poorly recruitable phenotype (6, 8, 25). Increasing PEEP in this phenotype contributes to worsening lung compliance.
Pan et al. (76) studied 12 patients who were protectively ventilated; mean age was 59 years and the mean R/I ratio was 0.21, indicating low pulmonary recruitability. They showed that after applying high PEEP using the PEEP table (>15 cmH2O), Pplat remained high, with a low response in oxygenation. In addition, the authors reported that this patient profile may not respond to high PEEP in the supine position, but that recruitability seems to increase after PP. It can be inferred that this gain is due to displacement of poorly ventilated areas. This reinforces the fact that the PEEP table has partial applicability and seems to suggest that ARM should be performed together with PP.
5.4. Effects of PEEP in the H phenotype
When PEEP is applied incases with the H phenotype, the response is an improvement in lung compliance, with a reduction in Pplat and in poorly ventilated or non-ventilated areas, reducing intrapulmonary shunt (8, 25).
Protti et al. (20) studied 40 patients with early COVID-19 in the supine position and performed ARM plus decremental PEEP at three levels: 15, 10, and 5 cmH2O. With PEEP of 15 cmH2O, oxygenation improved in 36% of patients, but respiratory compliance improved in only 11%. There was also a reduction in non-ventilated areas and an increase in hyperventilated areas. Furthermore, two different responses were observed as PEEP increased. With an increase in PEEP from 5 to 10 cmH2O, recruitment was predominantly dorsal, reducing non-aerated tissue, with an improvement in the PaO2/FiO2 ratio and an increase in respiratory compliance. However, when PEEP was increased from 10 to 15 cmH2O, the recruitment obtained previously overlapped with the appearance of hyperventilated areas, predominantly ventral, and a decline in respiratory compliance. Furthermore, the improvement in oxygenation at high PEEP cannot be explained by recruitability, but rather by the improvement in left ventricular function, which decreases cardiac output (50).
–Ball et al. (8) studied a group of 42 recruitable and non-recruitable patients with a mean age of 63 years using LPV. The authors evaluated lung mechanics and oxygenation at two PEEP levels (8 and 16 cmH2O). The first group benefited from high PEEP by reducing the percentage of non-aerated lung units. However, only the non-recruitable group had a reduction in poorly aerated areas. Both groups showed improved oxygenation via increased hyperaerated areas, with consequent worsening of respiratory compliance. In practical terms, this led to an increase in ΔP, Pplat, mechanical power, variables associated with VILI. The authors explained that the improvement in the P/F ratio should be interpreted as redistribution of the V′/Q′ ratio, prioritizing areas with low ventilation, and not as recruitment, even in so-called recruitable patients.
5.5. ARM and obese patients
Some of the studies discussed in this review analyzed obese patients, represented by BMI >30 kg/m2 (63, 64). Obese patients have a high recruitment potential and can tolerate high PEEP values, as long as the Pplat remains up to 30 cmH2O. Usually, studies have pointed out two main reasons for the need for high PEEP in this population: (1) decreased PL (79); and (2) predominantly ventral ventilation with a tendency to dorsal alveolar collapse under low PEEP. This scenario can be prone to VILI due to low static compliance. After application of PEEP, the studies have highlighted decreased airway ΔP and dead space, with improvement in static lung compliance, PL, the PaO2/FiO2 ratio, and redistribution of pulmonary blood flow with subsequent reduction of intrapulmonary shunt (81).
Highly specialized centers have introduced EIT to expand understanding of the effect of PEEP levels (75, 81). EIT consists of placing a belt with electrodes between the fourth and fifth ribs to verify the ventilatory distribution (whether predominantly dorsal or ventral) in real time and macroscopically assess the effect of PEEP. The use of EIT during ARM and PEEP titration may guarantee the most adequate value for the patient, which may be two values below or above the values suggested by the PEEP table (75, 81). EIT shows the percentage of well-ventilated, poorly ventilated, collapsed, and hyperinflated areas; the latter two are of interest to the professional at the bedside to avoid VILI (49, 59).
5.6. The balance between oxygenation and ventilatory mechanics
Oxygenation is a therapeutic target, as is the assessment of ventilatory mechanics. Both need to be evaluated together and systematically. This review recommends that the search for the ideal P/F ratio, as well as optimal SpO2/PaO2 values, can lead to dangerous maneuvers of alveolar recruitment, exceeding protection limits, with the risk of P-SILI.
Beloncle et al. (10) studied 25 patients with COVID-19, 16 of whom were considered highly recruitable and 9 were considered poorly recruitable. Two PEEPs were applied: 5 and 15 cmH2O. At high PEEP, the recruitable group showed the same mean compliance for both PEEP levels. However, oxygenation in the recruitable group was higher than in the non-recruitable group. Ball et al. (11) studied 42 patients, 32 non-recruitable and 10 recruitable. The researchers applied two levels of PEEP (8 and 16 cmH2O). All patients then underwent computed tomography. They found that there was no percentage difference in recruitable areas despite the increase in PaO2. Therefore, it can be concluded that the compliance of the respiratory system can mitigate oxygenation. The articles of this topic were organized in Table 3, to direct the understanding of ARM.
6. Extracorporeal membrane oxygenation
The administration of low VT in severely collapsed lungs results in increases in CO2 levels (i.e., >45 mmHg) leading to the development of respiratory acidosis and extremely severe hypoxemia (84). Patients with extensive alveolar consolidations are likely to be refractory to the PP and ARM maneuver with decremental PEEP (84, 85). Analysis of lung mechanics demonstrates Pplat and ΔP above protective limits (30 and 15 cmH2O, respectively), and pH less than 7.35 (85, 86). This clinical picture could benefit from ECMO.
ECMO is a potentially life-saving strategy recommended in patients who are extremely hypoxemic and acidotic, with the aim of clearing CO2 levels and allowing the lungs to reduce activity, allowing the ECMO to perform gas exchange. Due to its high complexity, use of ECMO is recommended only in specialized centers and by dedicated staff (87). The studies in this review were based on the ELSO (Extracorporeal Life Support Organization) and EOLIA (ECMO to Rescue Lung Injury in Severe ARDS) definitions to define patients eligible or not for therapy. Among so many recommendations, we highlight: (1) PaO2/FiO2 ratio < 50 mmHg over 3 h; (2) PaO2/FiO2 ratio < 80 mmHg over 6 h; (3) arterial blood gas pH <7.25 and PaCO2 > 60 mmHg over 6 h (Figure 2).
There are different ventilatory strategies during ECMO. The randomized clinical trial by McNamee et al. (87) studied 412 patients with severe hypoxemia and < 48 h of intubation and randomized them into two groups: (1) ECMO + LPV and (2) LPV only. The first group showed a reduction in Pplat and ΔP and more ventilator-free days, indicating improved lung protection compared with the second group. No difference in mortality was found.
6.1. Time to start ECMO
Considering the inclusion criteria for ECMO, it is pertinent to consider its rapid start after detection of the disorder. There is no consensus in the literature about when to start therapy, because this depends on the availability of equipment and trained staff (85). Even so, the prospective study Mustafa et al. (88) studied 160 patients with a mean age of 49 years. The researchers divided them into two groups: (1) ECMO + LPV; (2) Only MVA. The first group progressed to ECMO within 3.8 days. ECMO + LPV was associated with 68% survival, whereas LPV only was associated with 26% survival. Karagiannidis et al. (89) stated that ECMO should start within 3 days because it is associated with longer patient survival. The multicenter study by Lorusso et al. (90) analyzed 1,215 patients ventilated with a VT < 3 mL/PBW and concluded that age > 60 years and a time longer than 4 days between the start of MV and the start of ECMO was associated with higher mortality.
Recently, Hajage et al. (91) studied 2,858 patients; 269 (mean age, 53 years) received ECMO within 14 days of hospitalization. Patients were intubated within 1 day of hospitalization, the average time to start ECMO was 6 days, and 89 and 97% of patients received PP and neuromuscular blockers, respectively, before ECMO. All patients were ventilated ultraprotectively, i.e., VT < 4 mL/kg. It was observed that eligible patients had poor ventilatory mechanics, with a mean ΔP of 18 and a mean Pplat of 30 cmH2O. The results showed that there was a significant improvement in ΔP to 12 cmH2O and Pplat to 18 cmH2O within 48 h of ECMO. These results are encouraging and reinforce the recommendations for successful ECMO: young age, few days of MV, and few comorbidities.
ECMO is a high-cost strategy that requires highly trained staff (85, 87), which may limit its widespread application and/or late start, when the patient may not benefit from the therapy.
6.2. Eligible patients
Preliminary prospective results from Kon et al. (92) highlighted important issues. The authors studied 27 obese patients with a mean age of 40 years. The authors chose to include only functional independent patients without comorbidities in the study. Before being eligible for ECMO, patients were LPV with a mean PEEP of 14 cmH2O and FiO2 > 90%. The primary endpoint of the study was survival during hospitalization and lung recovery (defined by the authors as ECMO weaning). They reported that 11 patients fully recovered on ECMO, and 13 were still on ECMO. The recovered group was successfully decannulated. All patients were tracheostomized with a median time of 24 h, allowing for lower rates of sedation and neuromuscular blockers, in addition to reducing the possibility of nosocomial infections, in contrast to other studies that reported at least 2 days from intubation to ECMO. All patients were ventilated in volume-controlled ventilation mode with 5 mL/kg PBW, with a mean PEEP of 10 cmH2O. The patients had median low compliance (22 mL/cmH2O) and ΔP ranging from 14 to 18 cmH2O. In addition, patients had a mean PaCO2 of 80 mmHg, pH <7.25, and mean serum lactate levels of 2.45 mmol/L. The primary endpoint of the study was 90-day mortality. All these factors were associated with mortality, which was 38.8%. Therefore, the main success factor for ECMO is young age, indicating the need for correct selection of patients (89).
The study by Schmidt et al. (21) included 83 patients, 30 of whom died. Forty-eight patients survived and were discharged from the ICU. The average age was 48 years. Interestingly, the surviving group had higher mean d-dimer values than the group who died. Moreover, 88% of the patients were ventilated in airway pressure release ventilation (APRV) mode, known to ensure alveolar stability and allow for greater pressurization with reduced occurrence of VILI (93). This ventilation mode was not used in almost all of the articles cited that opted for volume-controlled ventilation or pressure-controlled ventilation. Mortality was 31%. There was no information about the association of APRV and the effects of ECMO. But given the purpose of the APRV, it is possible to infer that the association would behave as double lung protection. Other studies associating ECMO and APRV are needed to confirm the positive relationship between them.
6.3. PP on ECMO
Although there are few studies reporting the use of PP in ECMO, recent evidence points to a good response from the combined therapies. Garcia et al. (94) studied 25 patients with COVID-19 that required V/V ECMO. 14 were placed on PP at least once for 16 h on average. All of them were protectively ventilated. In terms of lung mechanics, there were no statistical differences between PP and non-PP patients. However, there was an improvement in oxygenation in the PP group. Massart et al. (95) evaluated 517 patients with a mean age of 55 years on ECMO; 364 were prone during therapy and 153 were not prone. All were protectively ventilated. Lower mortality rates were observed in the PP group. There was no statistical difference between lung compliance and gas exchange values. As with PP, the outcome that should guide clinical practice is mortality. Only randomized studies will be able to confirm if the improvement in oxygenation is due to ECMO or PP or to joint therapy.
6.4. Side effects of ECMO
Despite its beneficial effects, the articles cited here highlight that ECMO presents a high risk of bleeding requiring anticoagulation, and many patients progress to hemodialysis (53, 85–88, 94–98) These facts, added to the fibrotic evolution of COVID, increase the chances of mortality and therapeutic failure with ECMO. However, these factors may have less impact on young patients and/or those with few or no comorbidities. The positive and negative outcomes of the ECMO studies are shown in Table 4.
6.5. ECMO in non COVID-ARDS versus COVID-ARDS patients
Some studies compared ECMO in non-COVID-ARDS patients and COVID-ARDS patients. Although similar results were gathered about oxygenation (99, 100), the treatment time and complications were different. Chandel et al. (99) analyzed 9,271 patients who required ECMO between 2017 and 2021. Authors showed that COVID patients remained longer on ECMO when compared to non-COVID patients (19.6 days versus 10 days). Additionally, COVID patients had higher rates of developing kidney failure, requiring hemodialysis. Furthermore, COVID patients remained longer on mechanical ventilation before starting ECMO. This condition may lead to increased diaphragmatic dysfunction and mortality in COVID compared with non-COVID group. Other complications also observed in the COVID group included pneumothorax and intracranial hypertension. This can be explained by the high inflammatory cascade due to COVID.
Similar results were found in the retrospective study by Dave et al. (100). The authors studied 89 patients who used V/V ECMO, divided in two groups: 35 COVID patients and 54 non-COVID patients. COVID patients had higher in-hospital mortality rates (49% versus 24%), longer ECMO and mechanical ventilation time before ECMO (654 h versus 394 h; 3 versus 1 day, respectively) than non-COVID patients.
Conclusion
This narrative review with a literature search strategy concludes that NRS, PP, ARM with decremental PEEP, and ECMO are therapeutic strategies that should only be applied in strictly selected patients. Noninvasive ventilatory support should be the therapy of choice with the aim of improving hypoxemia and ventilatory work. If no improvement is seen, orotracheal intubation should be instituted with a protective strategy. In cases of inefficient gas exchange, i.e., P/F ratio < 150, PP and ARMs can be performed provided that the patient has recruitability potential. ECMO should only be instituted in patients who, on MV for a short time, have inefficient gas exchange. However, ECMO needs a trained team, and its use is recommended only in highly specialized centers.
Author contributions
LR, CR, DB, PP, PR, and PS wrote the manuscript and revised the final version. All authors read and approved the final version of the manuscript.
Funding
The Brazilian Council for Scientific and Technological Development (CNPq) funded research projects and scholarships for students, Rio de Janeiro State Research Foundation (FAPERJ) funded research projects and scholarships for students, Coordination for the Improvement of Higher Education Personnel (CAPES) funded publication costs and scholarships for students, and the National Institute of Science and Technology for Regenerative Medicine/CNPq funded research projects.
Acknowledgments
The authors would like to thank Lorna O’Brien (authorserv.com) for editing assistance.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Franco, C, Facciolongo, N, Tonelli, R, Dongilli, R, Vianello, A, Pisani, L, et al. Feasibility and clinical impact of out-of-ICU noninvasive respiratory support in patients with COVID-19-related pneumonia. Eur Respir J. (2020) 56:2002130. doi: 10.1183/13993003.02130-2020
2. Aliberti, S, Radovanovic, D, Billi, F, Sotgiu, G, Costanzo, M, Pilocane, T, et al. Helmet CPAP treatment in patients with COVID-19 pneumonia: a multicentre cohort study. Eur Respir J. (2020) 56:2001935. doi: 10.1183/13993003.01935-2020
3. Marini, JJ, and Gattinoni, L. Management of COVID-19 respiratory distress. JAMA. (2020) 323:2329. doi: 10.1001/jama.2020.6825
4. Swenson, KE, Ruoss, SJ, and Swenson, ER. The pathophysiology and dangers of silent hypoxemia in COVID-19 lung injury. Ann Am Thorac Soc. (2021) 18:1098–105. doi: 10.1513/AnnalsATS.202011-1376CME
5. Zubieta-Calleja, G, and Zubieta-DeUrioste, N. Pneumolysis and “silent hypoxemia” in COVID-19. Ind J Clin Biochem. (2021) 36:112–6. doi: 10.1007/s12291-020-00935-0
6. Gattinoni, L, Chiumello, D, Caironi, P, Busana, M, Romitti, F, Brazzi, L, et al. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med. (2020) 46:1099–102. doi: 10.1007/s00134-020-06033-2
7. Zubieta-Calleja, GR, Zubieta-DeUrioste, N, de Jesús, MF, Sanchez, MGR, Campoverdi, AF, Rocco, PRM, et al. Morphological and functional findings in COVID-19 lung disease as compared to pneumonia, ARDS, and high-altitude pulmonary edema. Respir Physiol Neurobiol. (2023) 309:104000. doi: 10.1016/j.resp.2022.104000
8. Pelosi, P, Tonelli, R, Torregiani, C, Baratella, E, Confalonieri, M, Battaglini, D, et al. Different methods to improve the monitoring of noninvasive respiratory support of patients with severe pneumonia/ARDS due to COVID-19: an update. J Clin Med. (2022) 11:1704. doi: 10.3390/jcm11061704
9. Robba, C, Battaglini, D, Ball, L, Patroniti, N, Loconte, M, Brunetti, I, et al. Distinct phenotypes require distinct respiratory management strategies in severe COVID-19. Respir Physiol Neurobiol. (2020) 279:103455. doi: 10.1016/j.resp.2020.103455
10. Beloncle, FM, Pavlovsky, B, Desprez, C, Fage, N, Olivier, PY, Asfar, P, et al. Recruitability and effect of PEEP in SARS-Cov-2-associated acute respiratory distress syndrome. Ann Intensive Care. (2020) 10:55. doi: 10.1186/s13613-020-00675-7
11. Ball, L, Robba, C, Maiello, L, Herrmann, J, Gerard, SE, Xin, Y, et al. Computed tomography assessment of PEEP-induced alveolar recruitment in patients with severe COVID-19 pneumonia. Crit Care. (2021) 25:81. doi: 10.1186/s13054-021-03477-w
12. Rossi, S, Palumbo, MM, Sverzellati, N, Busana, M, Malchiodi, L, Bresciani, P, et al. Mechanisms of oxygenation responses to proning and recruitment in COVID-19 pneumonia. Intensive Care Med. (2022) 48:56–66. doi: 10.1007/s00134-021-06562-4
13. Camporota, L, Sanderson, B, Chiumello, D, Terzi, N, Argaud, L, Rimmelé, T, et al. Prone position in coronavirus disease 2019 and noncoronavirus disease 2019 acute respiratory distress syndrome: an international multicenter observational comparative study. Crit Care Med. (2022) 50:633–43. doi: 10.1097/CCM.0000000000005354
14. Alhazzani, W, Møller, MH, Arabi, YM, Loeb, M, Gong, MN, Fan, E, et al. Surviving Sepsis campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Intensive Care Med. (2020) 46:854–87. doi: 10.1007/s00134-020-06022-5
15. Grieco, DL, Menga, LS, Cesarano, M, Rosà, T, Spadaro, S, Bitondo, MM, et al. Effect of helmet noninvasive ventilation vs high-flow nasal oxygen on days free of respiratory support in patients with COVID-19 and moderate to severe hypoxemic respiratory failure: the HENIVOT randomized clinical trial. JAMA. (2021) 325:1731–43. doi: 10.1001/jama.2021.4682
16. Chiumello, D, Bonifazi, M, Pozzi, T, Formenti, P, Papa, GFS, Zuanetti, G, et al. Positive end-expiratory pressure in COVID-19 acute respiratory distress syndrome: the heterogeneous effects. Crit Care. (2021) 25:431. doi: 10.1186/s13054-021-03839-4
17. Chiumello, D, Chiodaroli, E, Coppola, S, Cappio Borlino, S, Granata, C, Pitimada, M, et al. Awake prone position reduces work of breathing in patients with COVID-19 ARDS supported by CPAP. Ann Intensive Care. (2021) 11:179. doi: 10.1186/s13613-021-00967-6
18. Robba, C, Ball, L, Battaglini, D, Cardim, D, Moncalvo, E, Brunetti, I, et al. Early effects of ventilatory rescue therapies on systemic and cerebral oxygenation in mechanically ventilated COVID-19 patients with acute respiratory distress syndrome: a prospective observational study. Crit Care. (2021) 25:111. doi: 10.1186/s13054-021-03537-1
19. Weiss, TT, Cerda, F, Scott, JB, Kaur, R, Sungurlu, S, Mirza, SH, et al. Prone positioning for patients intubated for severe acute respiratory distress syndrome (ARDS) secondary to COVID-19: a retrospective observational cohort study. Br J Anaesth. (2021) 126:48–55. doi: 10.1016/j.bja.2020.09.042
20. Protti, A, Santini, A, Pennati, F, Chiurazzi, C, Ferrari, M, Iapichino, GE, et al. Lung response to prone positioning in mechanically-ventilated patients with COVID-19. Crit Care. (2022) 26:127. doi: 10.1186/s13054-022-03996-0
21. Schmidt, M, Hajage, D, Lebreton, G, Monsel, A, Voiriot, G, Levy, D, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome associated with COVID-19: a retrospective cohort study. Lancet Respir Med. (2020) 8:1121–31. doi: 10.1016/s2213-2600(20)30328-3
22. Schmidt, M, Langouet, E, Hajage, D, James, SA, Chommeloux, J, Bréchot, N, et al. Evolving outcomes of extracorporeal membrane oxygenation support for severe COVID-19 ARDS in Sorbonne hospitals, Paris. Crit Care. (2021) 25:355. doi: 10.1186/s13054-021-03780-6
23. Angeles Montero-Fernandez, M, and Pardo-Garcia, R. Histopathology features of the lung in COVID-19 patients. Diagn Histopathol. (2021) 27:123–7. doi: 10.1016/j.mpdhp.2020.11.009
24. Osuchowski, MF, Winkler, MS, Skirecki, T, Cajander, S, Shankar-Hari, M, Lachmann, G, et al. The COVID-19 puzzle: deciphering pathophysiology and phenotypes of a new disease entity. Lancet Respir Med. (2021) 9:622–42. doi: 10.1016/S2213-2600(21)00218-6
25. Tonelli, R, Marchioni, A, Tabbì, L, Fantini, R, Busani, S, Castaniere, I, et al. Spontaneous breathing and evolving phenotypes of lung damage in patients with COVID-19: review of current evidence and forecast of a new scenario. J Clin Med. (2021) 10:975. doi: 10.3390/jcm10050975
26. Ball, L, Silva, PL, Giacobbe, DR, Bassetti, M, Zubieta-Calleja, GR, Rocco, PRM, et al. Understanding the pathophysiology of typical acute respiratory distress syndrome and severe COVID-19. Expert Rev Respir Med. (2022) 16:437–46. doi: 10.1080/17476348.2022.2057300
27. Robba, C, Battaglini, D, Ball, L, Pelosi, P, and Rocco, PRM. Ten things you need to know about intensive care unit management of mechanically ventilated patients with COVID-19. Expert Rev Respir Med. (2021) 15:1293–302. doi: 10.1080/17476348.2021.1906226
28. Perkins, GD, Ji, C, Connolly, BA, Couper, K, Lall, R, and Baillie, JK. Effect of noninvasive respiratory strategies on intubation or mortality among patients with acute hypoxemic respiratory failure and COVID-19: the RECOVERY-RS randomized clinical trial. JAMA. (2022) 327:546–58. doi: 10.1001/jama.2022.5279
29. Parke, RL, and McGuinness, SP. Pressures delivered by nasal high flow oxygen during all phases of the respiratory cycle. Respir Care. (2013) 58:1621–4. doi: 10.4187/respcare.02358
30. Robba, C, Battaglini, D, Pelosi, P, and Rocco, PRM. Multiple organ dysfunction in SARS-CoV-2: MODS-CoV-2. Expert Rev Respir Med. (2020) 14:865–8. doi: 10.1080/17476348.2020.1778470
31. Oranger, M, Gonzalez-Bermejo, J, Dacosta-Noble, P, Llontop, C, Guerder, A, Trosini-Desert, V, et al. Continuous positive airway pressure to avoid intubation in SARS-CoV-2 pneumonia: a two-period retrospective case-control study. Eur Respir J. (2020) 56:2001692. doi: 10.1183/13993003.01692-2020
32. Ranieri, VM, Tonetti, T, Navalesi, P, Nava, S, Antonelli, M, Pesenti, A, et al. High-flow nasal oxygen for severe hypoxemia: oxygenation response and outcome in patients with COVID-19. Am J Respir Crit Care Med. (2022) 205:431–9. doi: 10.1164/rccm.202109-2163OC
33. Wendel-Garcia, PD, Mas, A, González-Isern, C, Ferrer, R, Máñez, R, Masclans, JR, et al. Non-invasive oxygenation support in acutely hypoxemic COVID-19 patients admitted to the ICU: a multicenter observational retrospective study. Crit Care. (2022) 26:37. doi: 10.1186/s13054-022-03905-5
34. Arabi, YM, Aldekhyl, S, Al Qahtani, S, Al-Dorzi, HM, Abdukahil, SA, Al Harbi, MK, et al. Effect of helmet noninvasive ventilation vs usual respiratory support on mortality among patients with acute hypoxemic respiratory failure due to COVID-19: the HELMET-COVID randomized clinical trial. JAMA. (2022) 328:1063–72. doi: 10.1001/jama.2022.15599
35. Colaianni-Alfonso, N, Montiel, GC, Vega, ML, Mazzinari, G, Alonso-Íñigo, JM, and Grieco, DL. Helmet vs facemask CPAP in COVID-19 respiratory failure. Chest. (2023) 163:341–4. doi: 10.1016/j.chest.2022.08.2221
36. Vargas, F, Thille, A, Lyazidi, A, Master, BE, Roche Campo, F, and Brochard, L. Helmet with specific settings versus facemask for noninvasive ventilation. Crit Care Med. (2009) 37:1921–8. doi: 10.1097/CCM.0b013e31819fff93
37. Arabi, YM, Al-Dorzi, HM, Aldekhyl, S, Al Qahtani, S, Abdukahil, SA, Al Qasim, E, et al. Long-term outcomes of patients with COVID-19 treated with helmet noninvasive ventilation or usual respiratory support: follow-up study of the helmet-COVID randomized clinical trial. Intensive Care Med. (2023) 49:302–12. doi: 10.1007/s00134-023-06981-5
38. Roca, O, Caralt, B, Messika, J, Samper, M, Sztrymf, B, Hernández, G, et al. An index combining respiratory rate and oxygenation to predict outcome of nasal high-flow therapy. Am J Respir Crit Care Med. (2019) 199:1368–76. doi: 10.1164/rccm.201803-0589OC
39. Colaianni-Alfonso, N, Montiel, GC, Castro-Sayat, M, Roca, O, and Grieco, DL. ROX index to predict CPAP outcome in hypoxemic respiratory failure due to COVID-19. Intensive Care Med. (2022) 48:1818–9. doi: 10.1007/s00134-022-06913-9
40. Yousuf, A, Gottlieb, DS, Aggarwal, A, Peacock, B, and Konda, S. An observational longitudinal study of the use of ROX index to predict treatment failure in patients receiving continuous positive airway pressure for COVID-19. Health Sci Rep. (2022) 5:e482. doi: 10.1002/hsr2.482
41. Vincent, JL, Moreno, R, Takala, J, Willatts, S, De Mendonça, A, Bruining, H, et al. The SOFA (Sepsis-related organ failure assessment) score to describe organ dysfunction/failure: on behalf of the working group on Sepsis-related problems of the European Society of Intensive Care Medicine. Intensive Care Med. (1996) 22:707–10. doi: 10.1007/BF01709751
42. Duan, J, Han, X, Bai, L, Zhou, L, and Huang, S. Assessment of heart rate, acidosis, consciousness, oxygenation, and respiratory rate to predict noninvasive ventilation failure in hypoxemic patients. Intensive Care Med. (2017) 43:192–9. doi: 10.1007/s00134-016-4601-3
43. Guia, MF, Boléo-Tomé, JP, Imitazione, P, Polistina, GE, Alves, C, Ishikawa, O, et al. Usefulness of the HACOR score in predicting success of CPAP in COVID-19-related hypoxemia. Respir Med. (2021) 187:106550. doi: 10.1016/j.rmed.2021.106550
44. Innocenti, F, Lazzari, C, Paolucci, E, De Paris, A, Lagomarsini, A, Guerra, F, et al. Role of prognostic scores in predicting in-hospital mortality and failure of non-invasive ventilation in adults with COVID-19. Intern Emerg Med. (2022) 17:2367–77. doi: 10.1007/s11739-022-03058-x
45. Mannarino, MR, Bianconi, V, Cosentini, E, Figorilli, F, Natali, C, Cellini, G, et al. The HACOR score predicts worse in-hospital prognosis in patients hospitalized with COVID-19. J Clin Med. (2022) 11:3509. doi: 10.3390/jcm11123509
46. Le Gall, JR. A new simplified acute physiology score (SAPS II) based on a European/north American multicenter study. JAMA. (1993) 270:2957–63. doi: 10.1001/jama.1993.03510240069035
47. Schmidt, M, Demoule, A, Hajage, D, Pham, T, and Combes, A. COVID-ICU group, for the REVA network, COVID-ICU investigators. Benefits and risks of noninvasive oxygenation strategy in COVID-19: a multicenter, prospective cohort study (COVID-ICU) in 137 hospitals. Crit Care. (2021) 25:421. doi: 10.1186/s13054-021-03784-2
48. Sivaloganathan, AA, Nasim-Mohi, M, Brown, MM, Abdul, N, Jackson, A, Fletcher, SV, et al. Noninvasive ventilation for COVID-19-associated acute hypoxaemic respiratory failure: experience from a single Centre. Br J Anaesth. (2020) 125:e368–71. doi: 10.1016/j.bja.2020.07.008
49. Matta, A, Chaudhary, S, Bryan Lo, K, DeJoy, R, Gul, F, Torres, R, et al. Timing of intubation and its implications on outcomes in critically ill patients with coronavirus disease 2019 infection. Crit Care Explor. (2020) 2:e0262. doi: 10.1097/CCE.0000000000000262
50. Battaglini, D, Robba, C, Ball, L, Silva, PL, Cruz, FF, Pelosi, P, et al. Noninvasive respiratory support and patient self-inflicted lung injury in COVID-19: a narrative review. Br J Anaesth. (2021) 127:353–64. doi: 10.1016/j.bja.2021.05.024
51. Gattinoni, L, Gattarello, S, Steinberg, I, Busana, M, Palermo, P, Lazzari, S, et al. COVID-19 pneumonia: pathophysiology and management. Eur Respir Rev. (2021) 30:210138. doi: 10.1183/16000617.0138-2021
52. Tonelli, R, Cortegiani, A, Marchioni, A, Fantini, R, Tabbì, L, Castaniere, I, et al. Nasal pressure swings as the measure of inspiratory effort in spontaneously breathing patients with de novo acute respiratory failure. Crit Care. (2022) 26:70. doi: 10.1186/s13054-022-03938-w
53. Musso, G, Taliano, C, Molinaro, F, Fonti, C, Veliaj, D, Torti, D, et al. Early prolonged prone position in noninvasively ventilated patients with SARS-CoV-2-related moderate-to-severe hypoxemic respiratory failure: clinical outcomes and mechanisms for treatment response in the PRO-NIV study. Crit Care. (2022) 26:118. doi: 10.1186/s13054-022-03937-x
54. World Health Organization. Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected. Interim Guid Pediatr Med Rodz. (2020) 16:9–26. doi: 10.15557/PiMR.2020.0003
55. Patout, M, Fresnel, E, Lujan, M, Rabec, C, Carlucci, A, Razakamanantsoa, L, et al. Recommended approaches to minimize aerosol dispersion of SARS-CoV-2 during noninvasive ventilatory support can cause ventilator performance deterioration. Chest. (2021) 160:175–86. doi: 10.1016/j.chest.2021.02.047
56. Whittle, JS, Pavlov, I, Sacchetti, AD, Atwood, C, and Rosenberg, MS. Respiratory support for adult patients with COVID-19. JACEP Open. (2020) 1:95–101. doi: 10.1002/emp2.12071
57. Dell’Olio, A, Vocale, C, Primavera, A, Pisani, L, Altavilla, S, Roncarati, G, et al. Environmental contamination by SARS-CoV-2 during noninvasive ventilation in COVID-19. Respir Care. (2023) 68:1–7. doi: 10.4187/respcare.10323
58. Winslow, RL, Zhou, J, Windle, EF, Nur, I, Lall, R, Ji, C, et al. SARS-CoV-2 environmental contamination from hospitalised patients with COVID-19 receiving aerosol-generating procedures. Thorax. (2022) 77:259–67. doi: 10.1136/thoraxjnl-2021-218035
59. Battaglini, D, Pelosi, P, and Rocco, RMP. Prone positioning in COVID-19 ARDS: more pros than cons. J Bras Pneumol. (2022) 48:e20220065. doi: 10.36416/1806-3756/e20220065
60. Page, DB, Vijaykumar, K, Russell, DW, Gandotra, S, Chiles, JW, Whitson, MR, et al. Prolonged prone positioning for COVID-19–induced acute respiratory distress syndrome: a randomized pilot clinical trial. Ann Am Thorac Soc. (2022) 19:685–7. doi: 10.1513/AnnalsATS.202104-498RL
61. Guérin, C, Reignier, J, Richard, JC, Beuret, P, Gacouin, A, Boulain, T, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. (2013) 368:2159–68. doi: 10.1056/NEJMoa1214103
62. Grasselli, G, Tonetti, T, Protti, A, Langer, T, Girardis, M, Bellani, G, et al. Pathophysiology of COVID-19-associated acute respiratory distress syndrome: a multicentre prospective observational study. Lancet Respir Med. (2020) 8:1201–8. doi: 10.1016/S2213-2600(20)30370-2
63. Gattinoni, L, Camporota, L, and Marini, JJ. Prone position and COVID-19: mechanisms and effects. Crit Care Med. (2022) 50:873–5. doi: 10.1097/CCM.0000000000005486
64. Fossali, T, Pavlovsky, B, Ottolina, D, Colombo, R, Basile, MC, Castelli, A, et al. Effects of prone position on lung recruitment and ventilation-perfusion matching in patients with COVID-19 acute respiratory distress syndrome: a combined CT scan/electrical impedance tomography study. Crit Care Med. (2022) 50:723–32. doi: 10.1097/CCM.0000000000005450
65. Gattinoni, L, Pesenti, A, and Carlesso, E. Body position changes redistribute lung computed-tomographic density in patients with acute respiratory failure: impact and clinical fallout through the following 20 years. Intensive Care Med. (2013) 39:1909–15. doi: 10.1007/s00134-013-3066-x
66. Langer, T, Brioni, M, Guzzardella, A, Carlesso, E, Cabrini, L, Castelli, G, et al. Prone position in intubated, mechanically ventilated patients with COVID-19: a multi-centric study of more than 1000 patients. Crit Care. (2021) 25:128. doi: 10.1186/s13054-021-03552-2
67. De Rosa, S, Sella, N, Rezoagli, E, Lorenzoni, G, Gregori, D, Bellani, G, et al. The PROVENT-C19 registry: a study protocol for international multicenter SIAARTI registry on the use of prone positioning in mechanically ventilated patients with COVID-19 ARDS. PLoS One. (2022) 17:e0276261. doi: 10.1371/journal.pone.0276261
68. Hochberg, CH, Psoter, KJ, Sahetya, SK, Nolley, EP, Hossen, S, Checkley, W, et al. Comparing prone positioning use in COVID-19 versus historic acute respiratory distress syndrome. Crit Care Explor. (2022) 4:e0695. doi: 10.1097/CCE.0000000000000695
69. Le Terrier, C, Sigaud, F, Lebbah, S, Desmedt, L, Hajage, D, Guérin, C, et al. Early prone positioning in acute respiratory distress syndrome related to COVID-19: a propensity score analysis from the multicentric cohort COVID-ICU network—the ProneCOVID study. Crit Care. (2022) 26:71. doi: 10.21203/rs.3.rs-895727/v1
70. Engerström, L, Thermaenius, J, Mårtensson, J, Oldner, A, Petersson, J, Kåhlin, J, et al. Prevalence and impact of early prone position on 30-day mortality in mechanically ventilated patients with COVID-19: a nationwide cohort study. Crit Care. (2022) 26:264. doi: 10.1186/s13054-022-04122-w
71. Rezoagli, E, Mariani, I, Rona, R, Foti, G, and Bellani, G. Difference between prolonged versus standard duration of prone position in COVID-19 patients: a retrospective study. Minerva Anestesiol. (2021) 87:15864. doi: 10.23736/S0375-9393.21.15864-X
72. Okin, D, Huang, CY, Alba, GA, Jesudasen, SJ, Dandawate, NA, Gavralidis, A, et al. Prolonged prone position ventilation is associated with reduced mortality in intubated COVID-19 patients. Chest. (2023) 163:533–42. doi: 10.1016/j.chest.2022.10.034
73. Walter, T, Zucman, N, Mullaert, J, Thiry, I, Gernez, C, Roux, D, et al. Extended prone positioning duration for COVID-19-related ARDS: benefits and detriments. Crit Care. (2022) 26:208. doi: 10.1186/s13054-022-04081-2
74. Mathews, KS, Soh, H, Shaefi, S, Wang, W, Bose, S, Coca, S, et al. Prone positioning and survival in mechanically ventilated patients with coronavirus disease 2019–related respiratory failure. Crit Care Med. (2021) 49:1026–37. doi: 10.1097/CCM.0000000000004938
75. Sella, N, Zarantonello, F, Andreatta, G, Gagliardi, V, Boscolo, A, and Navalesi, P. Positive end-expiratory pressure titration in COVID-19 acute respiratory failure: electrical impedance tomography vs. PEEP/FiO2 tables. Crit Care. (2020) 24:540. doi: 10.1186/s13054-020-03242-5
76. Pan, C, Chen, L, Lu, C, Zhang, W, Xia, JA, Sklar, MC, et al. Lung recruitability in COVID-19–associated acute respiratory distress syndrome: a single-center observational study. Am J Respir Crit Care Med. (2020) 201:1294–7. doi: 10.1164/rccm.202003-0527LE
77. Somhorst, P, van der Zee, P, Endeman, H, and Gommers, D. PEEP-FiO2 table versus EIT to titrate PEEP in mechanically ventilated patients with COVID-19-related ARDS. Crit Care. (2022) 26:272. doi: 10.1186/s13054-022-04135-5
78. Chen, L, Del Sorbo, L, Grieco, DL, Junhasavasdikul, D, Rittayamai, N, Soliman, I, et al. Potential for lung recruitment estimated by the recruitment-to-inflation ratio in acute respiratory distress syndrome. A clinical trial. Am J Respir Crit Care Med. (2020) 201:178–87. doi: 10.1164/rccm.201902-0334OC
79. Perier, F, Tuffet, S, Maraffi, T, Alcala, G, Victor, M, Haudebourg, AF, et al. Effect of positive end-expiratory pressure and proning on ventilation and perfusion in COVID-19 acute respiratory distress syndrome. Am J Respir Crit Care Med. (2020) 202:1713–7. doi: 10.1164/rccm.202008-3058LE
80. Zerbib, Y, Lambour, A, Maizel, J, Kontar, L, De Cagny, B, Soupison, T, et al. Respiratory effects of lung recruitment maneuvers depend on the recruitment-to-inflation ratio in patients with COVID-19-related acute respiratory distress syndrome. Crit Care. (2022) 26:12. doi: 10.1186/s13054-021-03876-z
81. van der Zee, P, Somhorst, P, Endeman, H, and Gommers, D. Electrical impedance tomography for positive end-expiratory pressure titration in COVID-19–related acute respiratory distress syndrome. Am J Respir Crit Care Med. (2020) 202:280–4. doi: 10.1164/rccm.202003-0816LE
82. Protti, A, Santini, A, Pennati, F, Chiurazzi, C, Cressoni, M, Ferrari, M, et al. Lung response to a higher positive end-expiratory pressure in mechanically ventilated patients with COVID-19. Chest. (2022) 161:979–88. doi: 10.1016/j.chest.2021.10.012
83. Schulz, L, Stewart, A, O’Regan, W, McCanny, P, Austin, D, Hallback, M, et al. Capnodynamic monitoring of lung volume and blood flow in response to increased positive end-expiratory pressure in moderate to severe COVID-19 pneumonia: an observational study. Crit Care. (2022) 26:232. doi: 10.1186/s13054-022-04110-0
84. Bonny, V, Janiak, V, Spadaro, S, Pinna, A, Demoule, A, and Dres, M. Effect of PEEP decremental on respiratory mechanics, gas exchange, pulmonary regional ventilation and hemodynamics in patients with SARS-Cov-2 associated acute respiratory distress syndrome. Crit Care. (2020) 24:596. doi: 10.1186/s13054-020-03311-9
85. Yang, X, Cai, S, Luo, Y, Zhu, F, Hu, M, Zhao, Y, et al. Extracorporeal membrane oxygenation for coronavirus disease 2019-induced acute respiratory distress syndrome: a multicenter descriptive study. Crit Care Med. (2020) 48:1289–95. doi: 10.1097/CCM.0000000000004447
86. Rabie, AA, Azzam, MH, Al-Fares, AA, Abdelbary, A, Mufti, HN, Hassan, IF, et al. Implementation of new ECMO centers during the COVID-19 pandemic: experience and results from the Middle East and India. Intensive Care Med. (2021) 47:887–95. doi: 10.1007/s00134-021-06451-w
87. McNamee, JJ, Gillies, MA, Barrett, NA, Perkins, GD, Tunnicliffe, W, Young, D, et al. Effect of lower tidal volume ventilation facilitated by extracorporeal carbon dioxide removal vs standard care ventilation on 90-day mortality in patients with acute hypoxemic respiratory failure: the REST randomized clinical trial. JAMA. (2021) 326:1013–23. doi: 10.1001/jama.2021.13374
88. Mustafa, AK, Joshi, DJ, Alexander, PJ, Tabachnick, DR, Cross, CA, Jweied, EE, et al. Comparative propensity matched outcomes in severe COVID-19 respiratory failure—extracorporeal membrane oxygenation or maximum ventilation alone. Ann Surg. (2021) 274:e388–94. doi: 10.1097/SLA.0000000000005187
89. Karagiannidis, C, Bein, T, and Welte, T. ECMO during the COVID-19 pandemic: moving from rescue therapy to more reasonable indications. Eur Respir J. (2022) 59:2103262. doi: 10.1183/13993003.03262-2021
90. Lorusso, R, De Piero, ME, Mariani, S, Di Mauro, M, Folliguet, T, Taccone, FS, et al. In-hospital and 6-month outcomes in patients with COVID-19 supported with extracorporeal membrane oxygenation (EuroECMO-COVID): a multicentre, prospective observational study. Lancet Respir Med. (2023) 11:151–62. doi: 10.1016/s2213-2600(22)00403-9
91. Hajage, D, Combes, A, Guervilly, C, Lebreton, G, Mercat, A, Pavot, A, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome associated with COVID-19: an emulated target trial analysis. Am J Respir Crit Care Med. (2022) 206:281–94. doi: 10.1164/rccm.202205-0906ED
92. Kon, ZN, Smith, DE, Chang, SH, Goldenberg, RM, Angel, LF, Carillo, JA, et al. Extracorporeal membrane oxygenation support in severe COVID-19. Ann Thorac Surg. (2021) 111:537–43. doi: 10.1016/j.athoracsur.2020.07.002
93. Trejnowska, E, Drobiński, D, Knapik, P, Wajda-Pokrontka, M, Szułdrzyński, K, Staromłyński, J, et al. Extracorporeal membrane oxygenation for severe COVID-19-associated acute respiratory distress syndrome in Poland: a multicenter cohort study. Crit Care. (2022) 26:97. doi: 10.1186/s13054-022-03959-5
94. Garcia, B, Cousin, N, Bourel, C, Jourdain, M, Poissy, J, Duburcq, T, et al. Prone positioning under VV-ECMO in SARS-CoV-2-induced acute respiratory distress syndrome. Crit Care. (2020) 24:428. doi: 10.1186/s13054-020-03162-4
95. Massart, N, Guervilly, C, Mansour, A, Porto, A, Flécher, E, Esvan, M, et al. Impact of prone position in COVID-19 patients on extracorporeal membrane oxygenation. Crit Care Med. (2023) 51:36–46. doi: 10.1097/CCM.0000000000005714
96. Lebreton, G, Schmidt, M, Ponnaiah, M, Folliguet, T, Para, M, Guihaire, J, et al. Extracorporeal membrane oxygenation network organisation and clinical outcomes during the COVID-19 pandemic in greater Paris, France: a multicentre cohort study. Lancet Respir Med. (2021) 9:851–62. doi: 10.1016/s2213-2600(21)00096-5
97. Herrmann, J, Lotz, C, Karagiannidis, C, Weber-Carstens, S, Kluge, S, Putensen, C, et al. Key characteristics impacting survival of COVID-19 extracorporeal membrane oxygenation. Crit Care. (2022) 26:190. doi: 10.1186/s13054-022-04053-6
98. Whebell, S, Zhang, J, Lewis, R, Berry, M, Ledot, S, Retter, A, et al. Survival benefit of extracorporeal membrane oxygenation in severe COVID-19: a multi-Centre-matched cohort study. Intensive Care Med. (2022) 48:467–78. doi: 10.1007/s00134-022-06645-w
99. Chandel, A, Puri, N, Damuth, E, Potestio, C, Peterson, LKN, Ledane, J, et al. Extracorporeal membrane oxygenation for COVID-19: comparison of outcomes to non-COVID-19–related viral acute respiratory distress syndrome from the extracorporeal life support organization registry. Crit Care Explor. (2023) 5:e0861. doi: 10.1097/CCE.0000000000000861
Keywords: COVID-19, noninvasive respiratory support, invasive mechanical ventilation, prone position, recruitment maneuvers, extracorporeal membrane oxygenation
Citation: Rodrigues de Moraes L, Robba C, Battaglini D, Pelosi P, Rocco PRM and Silva PL (2023) New and personalized ventilatory strategies in patients with COVID-19. Front. Med. 10:1194773. doi: 10.3389/fmed.2023.1194773
Edited by:
Oriol Roca, Vall d'Hebron University Hospital, SpainReviewed by:
Tommaso Tonetti, University of Bologna, ItalyRoberto Rona, San Gerardo Hospital, Italy
Copyright © 2023 Rodrigues de Moraes, Robba, Battaglini, Pelosi, Rocco and Silva. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pedro Leme Silva, cGVkcm9sZW1lQGJpb2YudWZyai5icg==
 Lucas Rodrigues de Moraes
Lucas Rodrigues de Moraes Chiara Robba
Chiara Robba Denise Battaglini
Denise Battaglini Paolo Pelosi
Paolo Pelosi Patricia R. M. Rocco
Patricia R. M. Rocco Pedro Leme Silva
Pedro Leme Silva