- 1Division of Rheumatology, Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Republic of Korea
- 2Division of Gastroenterology, Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Republic of Korea
- 3Department of Statistics and Actuarial Science, Soongsil University, Seoul, Republic of Korea
- 4Department of Neurology, Inha University Hospital, Incheon, Republic of Korea
Objective: Immune-mediated inflammatory disease (IMID) is associated with an increased risk of mortality. It is unclear whether the higher mortality is attributable to the IMIDs themselves or to the higher prevalence of comorbidities in IMIDs. We aimed to investigate whether IMIDs per se confer a higher risk of mortality.
Methods: From the Korean National Health Insurance Service-National Sample Cohort database, this population-based cohort study included 25,736 patients newly diagnosed with IMIDs between January 2007 and December 2017, and 128,680 individuals without IMIDs who were matched for age, sex, income, hypertension, type 2 diabetes, dyslipidemia, and the Charlson comorbidity index. All individuals were retrospectively observed through December 31, 2019. The outcomes included all-cause and cause-specific mortalities. Adjustments for age, sex, and comorbidities were performed using multivariable Cox proportional hazard regression analyses, and adjusted hazard ratios (aHRs) with 95% confidence intervals (CIs) for the outcomes were estimated.
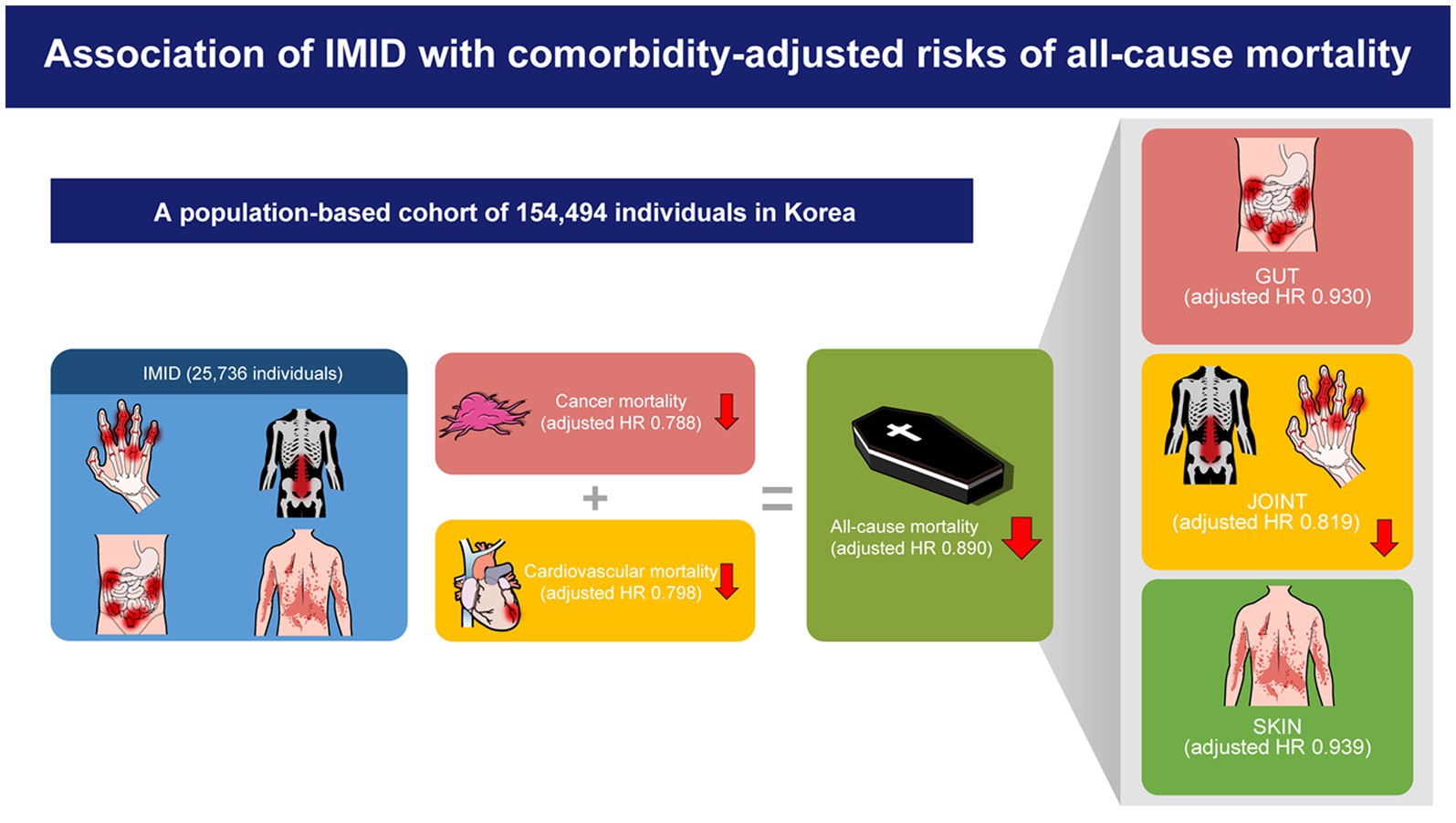
Results: The adjusted risk of all-cause mortality was significantly lower in patients with IMIDs than that in those without (aHR, 0.890; 95% CI, 0.841–0.942). Regarding cause-specific mortality, cancer-specific (aHR, 0.788; 95% CI, 0.712–0.872) and cardiovascular disease-specific (aHR, 0.798; 95% CI, 0.701–0.908) mortalities were the two causes of death that showed significantly lower risks in patients with IMIDs. A similar trend was observed when organ based IMIDs were analyzed separately (i.e., gut, joint, and skin IMIDs).
Conclusion: After adjusting for comorbidities, IMIDs were associated with a lower risk of all-cause mortality compared to those without IMIDs. This was attributable to the lower risks of cancer-and cardiovascular disease-specific mortalities.
Introduction
Immune-mediated inflammatory diseases (IMIDs) are a heterogeneous group of diseases characterized by chronic inflammation and damage to various organs (1–3). These include Crohn’s disease (CD) and ulcerative colitis (UC), which affect the gut; rheumatoid arthritis (RA) and ankylosing spondylitis (AS), which affect the joints; and psoriasis (PsO), which affects the skin (4–20).
A higher mortality rate has been consistently reported in patients with RA, AS, and PsO than that among the general population (21–23). Patients with RA have a 1.4-to 2-fold high mortality rate (21); those with AS have a 1.8-fold high mortality rate (22); patients with PsO have a 1.2-fold high risk of mortality than the rates among the general population (23). Less consistent results have been reported regarding mortality in patients with inflammatory bowel disease (IBD) (24–26). A population-based study from Canada reported 1.5-fold and 1.2-fold high mortality in patients with CD and UC, respectively, compared with the rates in the general population (24). In contrast, studies from the United States (25) and South Korea (26) reported a higher mortality trend among patients with CD and lower mortality among patients with UC than among the general population.
Although there are several mortality studies on IMIDs, the results were mostly adjusted for age and sex but not for comorbidities such as hypertension, type 2 diabetes, dyslipidemia, and cardiovascular disease. Moreover, patients with IMIDs are more prone to comorbidities than the general population (3, 27–32). Therefore, comorbidities should be adjusted to evaluate whether IMIDs are associated with the risk of mortality among the general population. In this study, we conducted a nationwide population-based study to estimate the age-, sex-and comorbidity-adjusted risks of all-cause and cause-specific mortalities according to the presence of IMIDs.
Patients and methods
Data source
Data from the Korean National Health Insurance Service-National Sample Cohort (NHIS-NSC) database were used in this study. The NHIS provides comprehensive data that includes demographics, socioeconomic status, medical treatments and procedures, disease diagnoses according to the International Classification of Diseases – Tenth Revision (ICD-10), and rare intractable disease (RID) registration information (33). In the Korean RID system, a diagnosis is based on the uniform diagnostic criteria provided by the NHI and is carefully reviewed by the corresponding healthcare institution and the NHI before registration. The profile of the data source has been described previously (34). The NHIS-NSC is a large-scale, population-based cohort comprising a representative sample of approximately 2% of the general Korean population.
Study population
From the NHIS-NSC database, we identified 25,736 patients who were newly diagnosed with IMIDs between January 1, 2007, and December 31, 2017 (IMID group). CD was defined as ICD-10 code K50 with RID code V130, UC was defined as ICD-10 code K51 with RID code V131, RA was defined as ICD-10 code M05 or M06 with prescriptions for disease-modifying anti-rheumatic drugs, AS was defined as ICD-10 code M45 with RID code V140, and PsO was defined as ICD-10 code L40 (35, 36). The control group (N = 128,680) was selected at a ratio of 1:5, matched for age, sex, income, hypertension, type 2 diabetes, dyslipidemia, and Charlson comorbidity index (CCI), respectively, with the IMID group. All individuals were followed up through December 31, 2019, for the occurrence of mortality (Figure 1).
Figure 1. Flowchart of the study process. aMatched by age, sex, income, hypertension, type 2 diabetes, dyslipidemia, and Charlson comorbidity index. NHIS-NSC, National Health Insurance Service-National Sample Cohort; IMIDs, immune-mediated inflammatory diseases.
Mortality data
Data on mortality and causes of death were available for all individuals included in the study population. The cause of death was identified according to the Korean Standard Classification of Disease and Cause of Death, based on ICD-10 codes (37). We classified the causes of death into cancer (C00-97), cardiovascular disease (I00-99), infectious disease (J00-06, J09-18, J20-22, and J85-86), renal disease (N00-08 and N10-19), endocrine disease (E00-90), and digestive system disease (K00-93). Cancer-specific mortality was further divided into the top five cancers with the highest mortality in South Korea (lung, liver, stomach, pancreatic, and colorectal cancers) and others (38).
Comorbidities
Presences of comorbidities were determined based on their existence prior to the diagnosis of IMIDs, and were defined as follows: hypertension was defined as ICD-10 codes I10–I13 and I15 with prescriptions for antihypertensive agents; type 2 diabetes was defined as ICD-10 codes E11–14, and at least one annual claim of a prescription of anti-diabetic medications; and dyslipidemia was defined as ICD-10 code E78 with prescriptions for lipid-lowering agents (35). CCI, which is a sum of weighted scores that are assigned to 17 comorbidities according to their relative risk of 1-year mortality, was calculated (39) and included as a covariate.
Statistical analysis
Continuous variables were expressed as mean ± standard deviation, and categorical variables were expressed as numbers (%). Continuous variables were compared using an independent Student’s t-test, and categorical variables were compared using the χ2 test. All-cause and cause-specific mortality rates were expressed as the number of deaths per 1,000 person-years. All-cause and cause-specific standardized mortality ratio (SMR) were estimated using 2010 mortality statistics in Korea. Cause-specific hazard models based on multivariable Cox proportional hazard models adjusted for age, sex, income, hypertension, type 2 diabetes, dyslipidemia, and CCI were used to estimate the hazard ratios (HRs) and 95% confidence intervals (CI) for all-cause mortality and cause-specific mortality, respectively. In cause-specific hazard models, the competing risk events are treated as censored observations (40). This method is advantageous in that it directly quantifies the HRs among individuals who are actually at risk of developing the event of interest (41, 42). We first estimated the HRs and 95% CIs of all IMIDs as a whole compared with the controls and then estimated the HRs and 95% CIs of each organ-based IMID (gut IMIDs [CD or UC], joint IMIDs [RA or AS], and skin IMID [PsO]) compared with the controls. Subgroup analyses were performed according to age and sex. All p values were two sided, and a p value <0.05 was considered statistically significant. All the statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA).
Results
Baseline characteristics
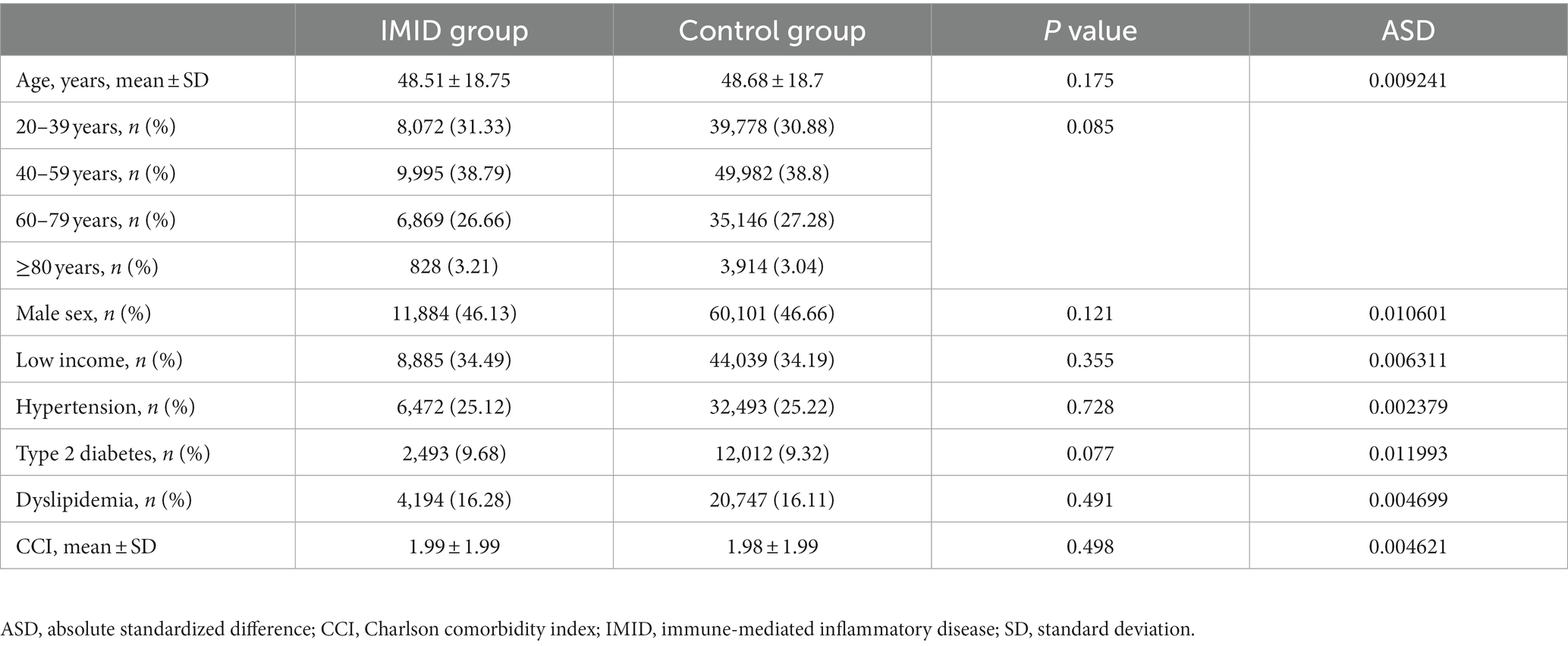
The baseline characteristics of the 25,764 patients with IMIDs and a comparison with those of the 1:5 matched control group (128,820) are shown in Table 1. The mean age of the patients with IMIDs was 48.5 ± 18.8 years, and 11,884 (46.1%) patients were male. One third of the patients (8,885; 34.5%) had a low income (lower 25 percentile). The proportion of patients with hypertension, type 2 diabetes, and dyslipidemia was 25.1, 9.7, and 16.3%, respectively. The mean CCI was 1.99 ± 1.99. The characteristics were well balanced through the matching process between the IMID and control groups, with absolute standardized differences below 0.1 for all covariates.
Mortality rate and risk of mortality according to the presence of IMIDs
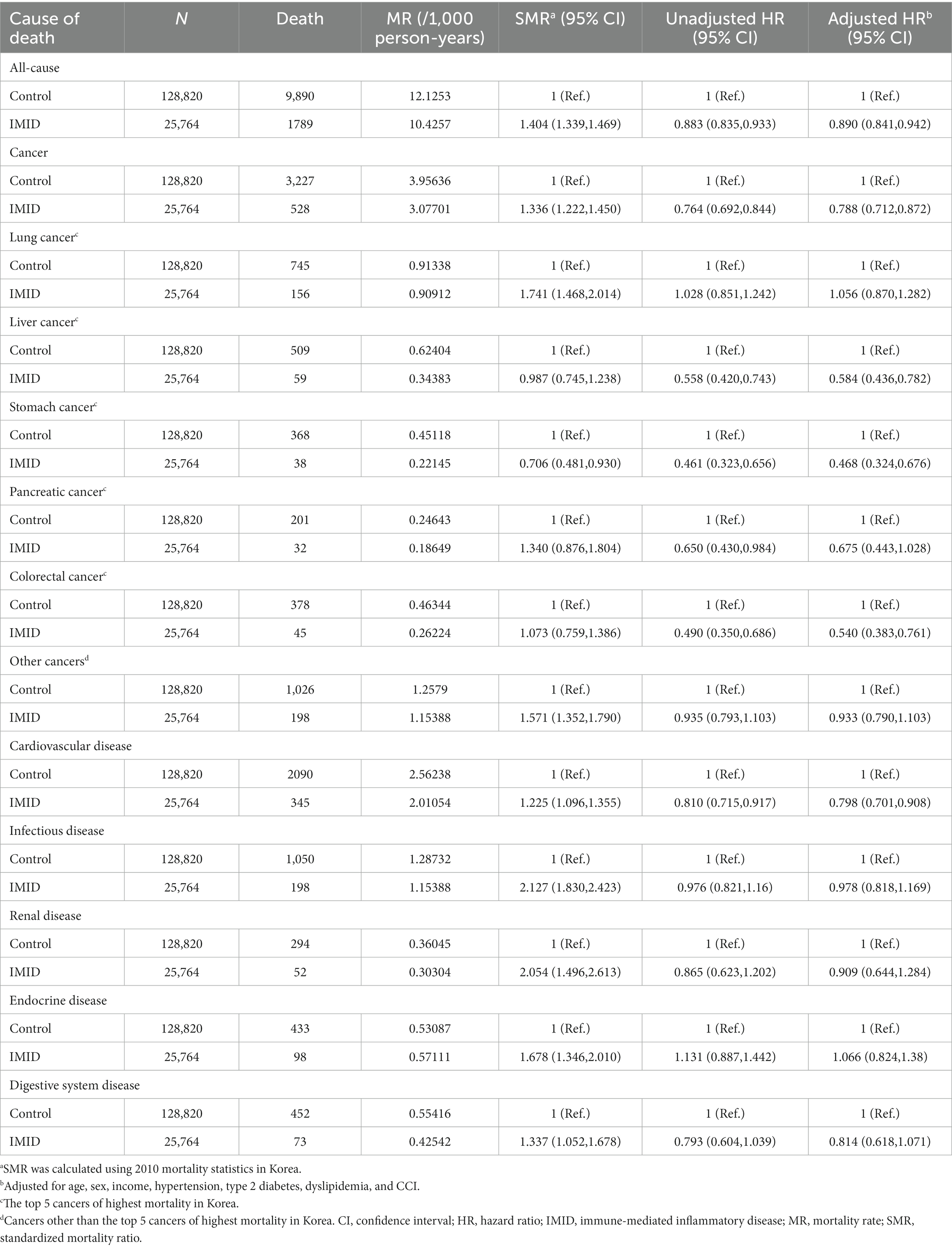
The all-cause mortality rates of the IMID and control group were 10.43 and 12.13 per 1,000 person-years, respectively. The all-cause and cause-specific SMR were higher in patients with IMIDs than in unmatched general population (all-cause SMR, 1.404; cancer-specific SMR, 1.336; cardiovascular disease-specific SMR, 1.225; infectious disease-specific SMR, 2.127; renal disease-specific SMR, 2.054; endocrine disease-specific SMR, 1.678; and digestive system disease-specific SMR, 1.337). However, the results of the multivariable Cox model summarized in Table 2 revealed that, compared with matched controls, patients with IMIDs had a significantly reduced risk of all-cause mortality (adjusted hazard ratio [aHR], 0.890; 95% CI, 0.841–0.942). With regard to cause-specific mortality, patients with IMIDs had a significantly lower risk of cancer-specific mortality (aHR, 0.788; 95% CI, 0.712–0.872) and cardiovascular disease-specific mortality (aHR, 0.798; 95% CI, 0.701–0.908) than that in controls. In contrast, the risks of infectious disease-specific, renal disease-specific, endocrine disease-specific, and digestive system disease-specific mortality did not show significant differences between the IMID and control groups. When cancer-specific mortality was further classified according to the specific cancer type, patients with IMIDs had a significantly reduced risk of liver cancer-specific (aHR, 0.584; 95% CI, 0.436–0.782), stomach cancer-specific (aHR, 0.468; 95% CI, 0.324–0.676), and colorectal cancer-specific mortality (aHR, 0.540; 95% CI, 0.383–0.761), but not lung cancer-specific, pancreatic cancer-specific, or other cancer-specific mortalities.
As we included patients newly diagnosed with IMIDs between 2007 and 2017, the disease duration of IMIDs may vary among patients at the end of follow up (2019). To address the variable disease duration among patients, we stratified the patients into those who were newly diagnosed with IMIDs in 2012–2017, and those who were newly diagnosed with IMIDs in 2007–2011, and performed Cox models in each group. The results are shown in Tables 3, 4. In patients who were newly diagnosed with IMIDs in 2012–2017, similar results were observed as in the total study population (reduced risk of all-cause mortality [aHR, 0.765; 95% CI, 0.701–0.834], cancer-specific mortality [aHR, 0.693; 95% CI, 0.595–0.806], and cardiovascular disease-specific mortality [aHR, 0.646; 95% CI, 0.527–0.792]) (Table 3). On the other hand, in patients who were newly diagnosed with IMIDs in 2007–2011, the reduced risk of all-cause mortality (aHR, 1.007; 95% CI, 0.934–1.086), cancer-specific mortality (aHR, 0.882; 95% CI, 0.768–1.012), and cardiovascular disease-specific mortality (aHR, 0.934; 95% CI, 0.789–1.105) was not observed (Table 4).
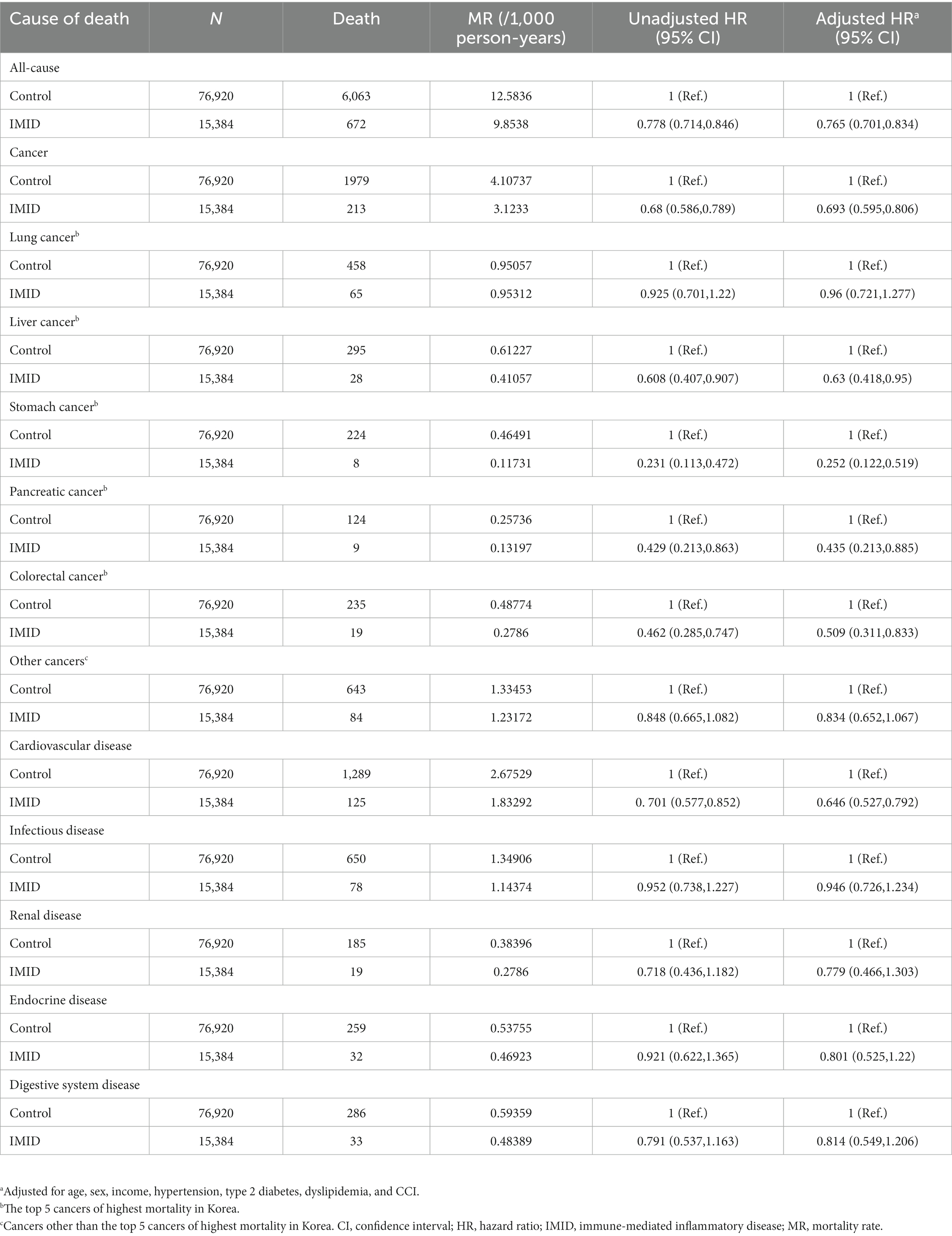
Table 3. Mortality rate and hazard ratios for death according to the presence of IMIDs (newly diagnosed in 2012–2017).
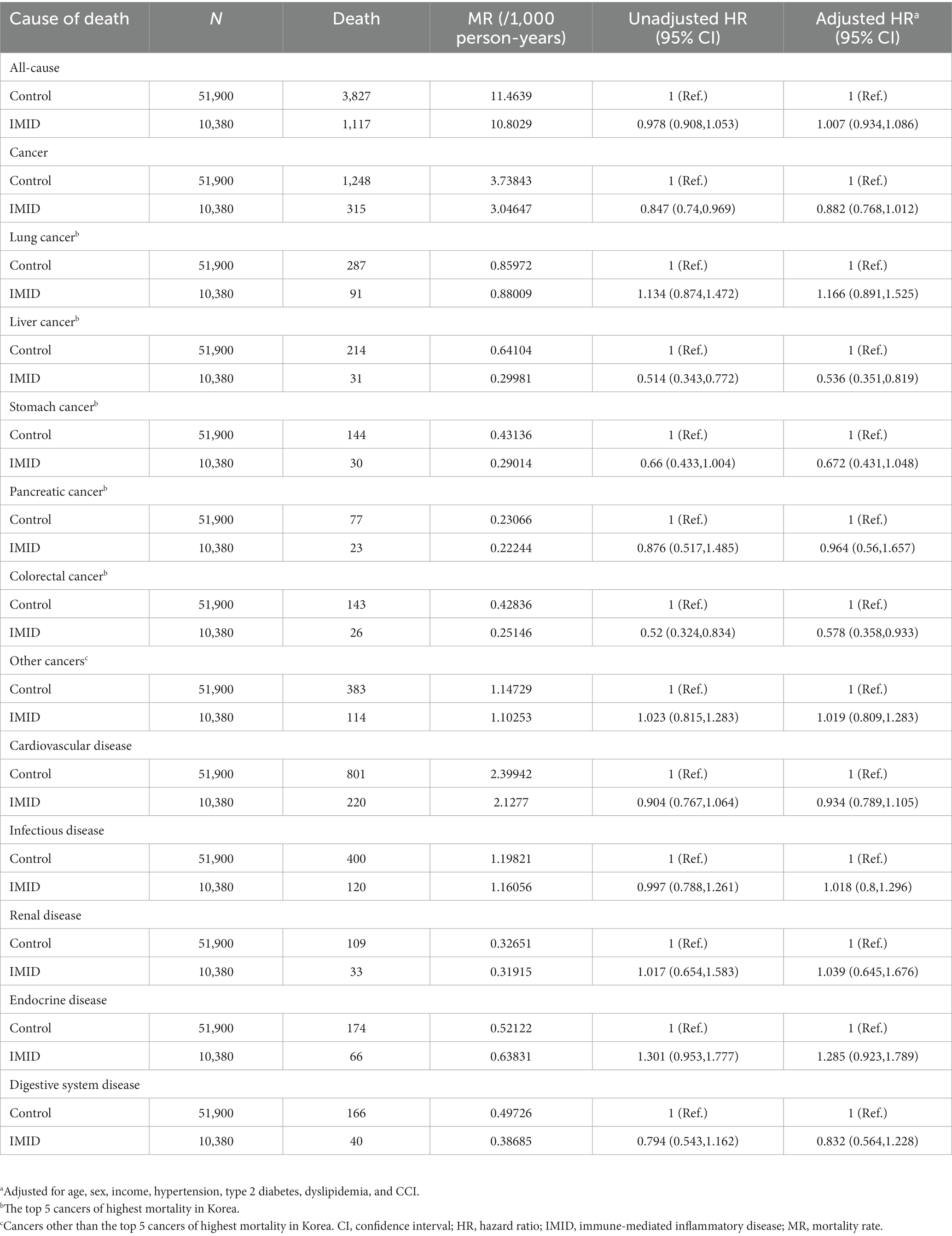
Table 4. Mortality rate and hazard ratios for death according to the presence of IMIDs (newly diagnosed in 2007–2011).
Mortality rate and risk of mortality according to the presence of organ-based IMIDs
The all-cause mortality rates of patients with gut, joint, and skin IMIDs were 5.39, 11.17, and 10.32 per 1,000 person-years, respectively. In the multivariable Cox model (Table 5), patients with joint IMIDs had a significantly lower risk of all-cause mortality (aHR, 0.819; 95% CI, 0.747–0.898) than the controls. Regarding cause-specific mortality, patients with joint IMIDs had a significantly lower risk of cancer-specific mortality (aHR, 0.512; 95% CI, 0.428–0.612), those with skin IMID had a significantly low risk of cardiovascular disease-specific mortality (aHR, 0.790; 95% CI, 0.668–0.935), those with joint IMIDs had a significantly high risk of infectious disease-specific mortality (aHR, 1.397; 95% CI, 1.059–1.843), those with skin IMID had a significantly low risk of infectious disease-specific mortality (aHR, 0.778; 95% CI, 0.613–0.988), and those with gut IMIDs had a significantly higher risk of disease-specific mortality in the digestive system (aHR, 5.407; 95% CI, 1.481–19.740) than the controls. In addition, the risk of renal disease-specific mortality and endocrine disease-specific mortality did not show significant differences between patients with organ-based IMIDs and controls.
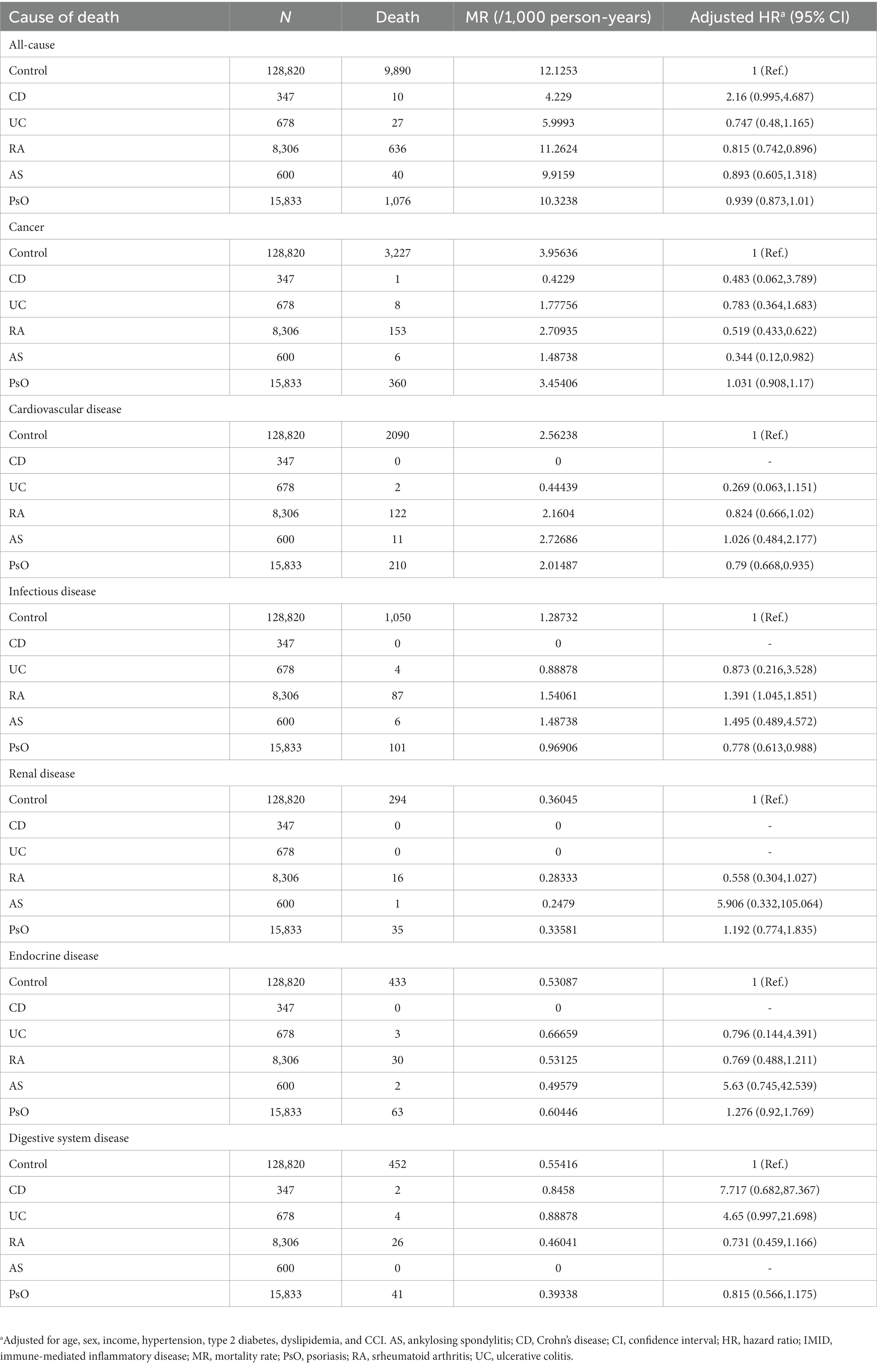
When organ-based IMIDs were further subdivided into individual IMIDs (Table 6), the trends were similar between CD and UC (i.e., gut IMIDs) and between RA and AS (i.e., joint IMIDs), except for the risk of all-cause mortality in CD and UC in which CD showed a trend of high risk of mortality (aHR, 2.160; 95% CI, 0.995–4.687), whereas UC showed a trend of low risk of mortality (aHR, 0.747; 95% CI, 0.480–1.165).
Subgroup analysis
The results of subgroup analyses for the risk of all-cause mortality and cause-specific mortality are shown in Figure 2. There were no significant differences in the aHRs for all-cause mortality and cause-specific mortality between the subgroups of patients divided by age (<65 years vs. ≥ 65 years) and sex (male vs. female).
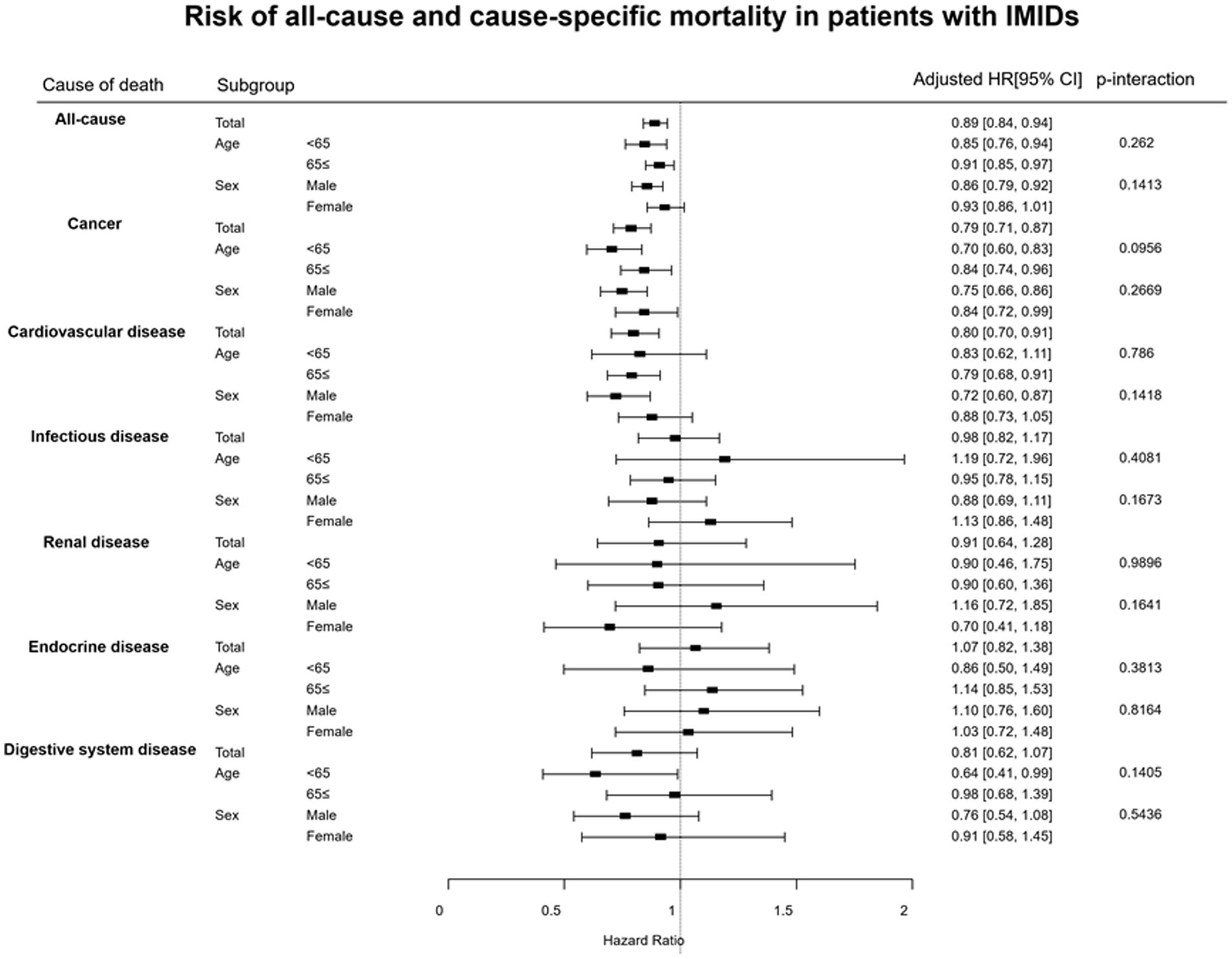
Figure 2. Subgroup analyses results for the risks of all-cause and cause-specific mortality in patients with IMIDs. CI, confidence interval; HR, hazard ratio; IMIDs, immune-mediated inflammatory diseases.
Discussion
In this population-based cohort study in which patients with IMIDs and controls were matched for age, sex, and comorbidities, we observed that the presence of IMIDs was not associated with a high risk of all-cause mortality and cause-specific mortality. Rather, the presence of IMIDs was associated with a significantly low risk of all-cause, cancer-, and cardiovascular disease-specific mortality. Our findings provide important information that IMIDs themselves do not confer an additional risk of mortality when the influence of other comorbidities is minimized. To our best knowledge, this is the first study to compare mortality risk between patients with and without IMIDs after rigorous adjustment for comorbidities using matching and multivariable analyses.
In contrast to previous studies that compared mortality between patients with IMIDs and controls and reported a high risk of all-cause mortality in patients with IMIDs (21–24), we observed a low risk of all-cause mortality (aHR, 0.815) in patients with IMIDs that that in controls. However, it is important to note that the risk of all-cause mortality had not been fully adjusted for comorbidities in previous studies (21–24); therefore, the association between IMIDs and the risk of all-cause mortality could not be clearly stated. The higher risk of all-cause mortality in patients with IMIDs observed in previous studies could potentially be attributable to the differences in comorbidities between those with and without IMIDs. Indeed, patients with IMIDs have comorbidities more commonly than the general population (27–30). This study, in which the risk of mortality was adjusted for age, sex, and comorbidities, suggests that IMIDs per se confer a low risk of all-cause mortality. Meanwhile, the all-cause and cause-specific SMR were > 1 in our study, which means that when the influence of comorbidities is not adjusted, patients with IMIDs have higher mortality than the general population. Taken together, these emphasize that comorbidities are the main mediators of increased mortality in patients with IMIDs, whereas IMIDs themselves are not.
When patients were stratified into those who were newly diagnosed with IMIDs in 2012–2017 and those who were newly diagnosed with IMIDs in 2007–2011, and analyzed separately, the reduced risk of all-cause mortality, cancer-specific mortality, and cardiovascular disease-specific mortality was attenuated in the latter group (i.e., those who have longer disease duration at the end of follow up) and lost statistical significance. As the disease duration of IMIDs extends, more patients would likely develop comorbidities related to increased mortality, which might lead to the attenuation of mortality risk reduction that was observed in those with shorter disease duration. Although different results were observed based on the disease duration, it is important to note that IMIDs per se were not associated with additional risk of mortality following the adjustment for comorbidities at diagnosis, regardless of disease duration. Although direct statistical comparison of demographics and follow-up period cannot be made between our study and previous studies that reported higher mortality in patients with IMIDs (24, 43–48), there are some numerical differences to be noted (Supplementary Table S1). Patients with CD in our study were younger (mean age 29.07 years), more commonly male (64.27%), and followed for a longer period (mean 6.81 years) than those in previous studies [age, mean 42.8 years (43) and 72.13% of the patients older than 60 years (24); proportion of male, 41.3% (43) and 43.1% (24); and follow-up period, mean 3.62 years (43) and mean 4.67 years (24)]. Patients with UC in our study were younger (mean age 43.58 years), more commonly male (61.8%), and followed for a longer period (mean 6.64 years) than those in previous studies [age, mean 48.59 years (43) and 81.89% of the patients older than 60 years (24); proportion of male, 51.1% (43) and 56.7% (24); and follow-up period, mean 3.75 years (43) and mean 4.38 years (24)]. Patients with PsO in our study were younger (mean age 46.6 years), more commonly male (54.76%), and followed for a longer period (mean 6.58 years) than those in previous study (age, mean 52.19 years; proportion of male, 48.57%; and follow-up period, mean 3.43 years) (44). Patients with AS in our study were younger (mean age 41.52 years) and more commonly male (73.33%) than those in previous studies [age, mean 49.3 years (45) and mean 48.5 years (46); proportion of male, 65.5% (45) and 57.0% (46)]. The follow-up period (mean 6.72 years) of patients with AS in our study was similar to one study (mean 6.05 years) (45), but shorter than another study (mean 8.0 years) (46). Patients with RA in our study were younger (mean age 53.86 years) than those in previous studies [age, mean 58.5 years (47) and 58.0 years (48)]. The proportion of male (25.55%) in our study was similar to previous studies [20.1% (47) and 26.9% (48)]. The follow-up period (mean 6.8 years) of patients with RA in our study was longer than one study (mean 5 years) (47), but shorter than another study (mean 14.2 years) (48). Nonetheless, when the effect of comorbidities was not adjusted, we similarly observed higher mortality (i.e., SMR > 1) in patients with IMIDs as in the previous studies (24, 43–48).
Interestingly, regarding cause-specific mortality, a lower risk of mortality was observed in cancer-specific mortality (aHR, 0.788; the aHR was lowest for stomach cancer-specific mortality [0.468], followed by colorectal cancer [0.540] and liver cancer [0.584]) and cardiovascular disease-specific mortality (aHR, 0.798), but not for other cause-specific mortalities. Notably, cancer and cardiovascular disease are the two causes of death in which early detection is especially important for reducing mortality (49–53). Moreover, as the patients with IMIDs regularly visit the hospital for the management of their diseases, there is a higher chance of early detection of other serious diseases apart from the IMIDs. Hence, earlier detection of serious diseases, particularly cancer or cardiovascular disease, in patients with IMIDs through regular hospital visits is a possible explanation for the lower risk of cancer-specific and cardiovascular disease-specific mortality and, ultimately, the all-cause mortality observed in this group of patients.
Chronic inflammation, the hallmark of IMIDs, plays a major role in the pathogenesis of endothelial dysfunction, which leads to accelerated atherosclerosis (54). In particular, tumor necrosis factor (TNF)-α, the key pro-inflammatory cytokine of IMIDs, upregulates arginase expression in the endothelial cells leading to impairment of nitric oxide-mediated vasodilation, and consequently to endothelial dysfunction (55–59). Furthermore, vascular endothelial growth factor (VEGF), a critical mediator of inflammation in IMIDs, is a pro-angiogenic factor that alters microvascular network contributing to the development and acceleration of atherosclerosis (60, 61). These could all contribute to higher cardiovascular risk in patients with IMIDs (54). Nevertheless, TNF-α inhibitors, which are commonly used for the treatment of IMIDs, decrease systemic inflammation and VEGF production, and thus have potential efficacy on endothelial dysfunction (56, 59, 62). Hence, the use of TNF-α inhibitors could also be a possible explanation for the lower risk of cardiovascular disease-specific mortality in patients with IMIDs.
Similar to that observed when all IMIDs were analyzed as a whole, in the analysis where each organ based IMID was separately analyzed, joint IMIDs were significantly associated with a lower risk of all-cause mortality (aHR, 0.819). Furthermore, although statistical significance was not reached, gut IMIDs (aHR, 0.930; 95% CI, 0.635–1.364) and skin IMID (aHR, 0.939; 95% CI, 0.873–1.010) showed a trend of a lower risk of all-cause mortality. Similar results were also observed in the association of organ-based IMIDs with cancer-specific mortality and cardiovascular disease-specific mortality: joint IMIDs were associated with a low risk of cancer-specific mortality (aHR, 0.512), and gut IMIDs tended to have a low risk of cancer-specific mortality (aHR, 0.730; 95% CI, 0.357–1.494). In addition, skin IMID was significantly associated with a low risk of cardiovascular disease-specific mortality (aHR, 0.790), and gut IMIDs (aHR, 0.268; 95% CI, 0.063–1.150) and joint IMIDs (aHR, 0.837; 95% CI, 0.682–1.028) tended to have a lower risk of cardiovascular disease-specific mortality. Importantly, none of the organ-based IMIDs had a higher risk of all-cause, cancer-specific, and cardiovascular disease-specific mortality.
Regarding other cause-specific mortalities, joint IMIDs were associated with a high risk of infectious disease-specific mortality (aHR, 1.397). When joint IMIDs were further subdivided, RA, but not AS, was significantly associated with a high risk of infectious-disease-specific mortality (Table 6). Among the IMIDs, RA has the widest range of biological agents with different modes of action or small molecule inhibitors available for treatment (tumor necrosis factor inhibitors, interleukin-6 receptor inhibitors, co-stimulation inhibitors, anti-B cells, and Janus kinase inhibitors) (63). Of these, interleukin-6 receptor inhibitors, used only in RA, pose a higher risk of serious infection than tumor necrosis factor inhibitors (64), commonly used throughout all IMIDs (63, 65–67). This could explain the higher risk of infectious disease-specific mortality observed only in RA.
Another interesting finding was the association between gut IMIDs and a high risk of digestive system disease-specific mortality (aHR, 5.407). Similarly, a hospital-based cohort study from South Korea also reported a high risk of digestive system disease-specific mortality in patients with IBD assessed by age-and sex-adjusted standardized mortality ratios (26). Our findings add to the previous knowledge that even after adjusting for comorbidities, digestive system disease is an important cause of death in patients with gut IMIDs. This implies the importance of optimal disease management in patients with gut IMIDs for better mortality outcomes.
This study had some limitations. First, we lacked data on the disease activity of each IMID, and we were unable to analyze whether the disease activity of each IMID was associated with the risk of mortality. In addition, we also lacked data on the duration of comorbidities and were unable to match this covariate. Second, data on alcohol consumption and smoking status are unavailable in the NHIS-NSC; thus, the possibility of confounding by these unmeasured covariates exists. In addition, data on the dose and duration of corticosteroid and immunosuppressive drugs are also unavailable in the NHIS-NSC, and we were unable to evaluate the mortality rates according to the use of these drugs. Third, we were unable to analyze psychological disease-specific mortality (i.e., suicide). However, given that the risk of all-cause mortality was lower in patients with IMIDs, we presume that psychological distress, as a cause of death, may not significantly increase overall mortality risk. Despite these limitations, using a large-scale, population-based cohort representative of the general Korean population, we were able to strictly adjust for comorbidities between patients with IMIDs and controls by conducting matching and multivariable analyses. This enabled us to investigate the impact of IMIDs, excluding the influence of comorbidities, on the risk of all-cause and cause-specific mortality.
In conclusion, IMIDs were associated with a reduced risk of all-cause mortality after adjusting for comorbidities. This was attributable to the low risks of cancer-specific and cardiovascular disease-specific mortalities, suggesting that earlier detection of cancer and cardiovascular diseases in patients with IMIDs through regular hospital visits could have led to a lower risk of all-cause mortality.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
This study was approved by the institutional review board (IRB) of Gangnam Severance Hospital (IRB No:3-2020-0269). In addition, owing to the retrospective nature of this study, the requirement for obtaining informed consent was waived and this decision approved by the IRB of Gangnam Severance Hospital.
Author contributions
OK, SL, JC, and KH contributed to conception and design of the study. OK, SL, JC, KH, YK, and RK organized the database. KH performed the statistical analysis. OK, SL, and JC wrote the first draft of the manuscript. OK, SL, JC, KH, YK, RK, M-CP, J-HK, YY, and HP reviewed and edited sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Acknowledgments
The authors would like to thank MID (Medical Illustration & Design), a part of the Medical Research Support Services of Yonsei University College of Medicine, for providing excellent support with medical illustration, and Editage (www.editage.co.kr) for English language editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2023.1185300/full#supplementary-material
References
1. Schett, G, McInnes, IB, and Neurath, MF. Reframing immune-mediated inflammatory diseases through signature cytokine hubs. N Engl J Med. (2021) 385:628–39. doi: 10.1056/NEJMra1909094
2. Jun, YK, Yoon, H, Koh, SJ, Kim, AH, Kim, KW, Park, JW, et al. Concomitant ankylosing spondylitis can increase the risk of biologics or small molecule therapies to control inflammatory bowel disease. Intest Res. (2022) 21:244–51. doi: 10.5217/ir.2022.00057
3. Khanna, S. Management of Clostridioides difficile infection in patients with inflammatory bowel disease. Intest Res. (2021) 19:265–74. doi: 10.5217/ir.2020.00045
4. Park, SH. Update on the epidemiology of inflammatory bowel disease in Asia: where are we now? Intest Res. (2022) 20:159–64. doi: 10.5217/ir.2021.00115
5. Kim, H, and Sung, Y-K. Epidemiology of rheumatoid arthritis in Korea. J Rheumatic Dis. (2021) 28:60–7. doi: 10.4078/jrd.2021.28.2.60
6. Inman, RD. Axial Spondyloarthritis: current advances, Future Challenges. J Rheumatic Dis. (2021) 28:55–9. doi: 10.4078/jrd.2021.28.2.55
7. Nestle, FO, Kaplan, DH, and Barker, J. Psoriasis. N Engl J Med. (2009) 361:496–509. doi: 10.1056/NEJMra0804595
8. Cho, CW, You, MW, Oh, CH, Lee, CK, and Moon, SK. Long-term disease course of Crohn's disease: changes in disease location, phenotype, activities, and predictive factors. Gut Liver. (2022) 16:157–70. doi: 10.5009/gnl210118
9. Park, SB, Yoon, JY, and Cha, JM. What are the different phenotypes of inflammatory bowel disease in Asia? Gut Liver. (2022) 16:676–85. doi: 10.5009/gnl210385
10. Park, J, and Cheon, JH. Incidence and prevalence of inflammatory bowel disease across Asia. Yonsei Med J. (2021) 62:99–108. doi: 10.3349/ymj.2021.62.2.99
11. Koh, SJ, Hong, SN, Park, SK, Ye, BD, Kim, KO, Shin, JE, et al. Korean clinical practice guidelines on biologics for moderate to severe Crohn's disease. Intest Res. (2022) 21:43–60. doi: 10.5217/ir.2022.00029
12. Na, SY, Choi, CH, Song, EM, Bang, KB, Park, SH, Kim, ES, et al. Korean clinical practice guidelines on biologics and small molecules for moderate-to-severe ulcerative colitis. Intest Res. (2022) 21:61–87. doi: 10.5217/ir.2022.00007
13. Seo, MR, Kim, G, Moon, KW, Sung, YK, Yoo, JJ, Yoon, CH, et al. Quality indicators for evaluating the health Care of Patients with rheumatoid arthritis: a Korean expert consensus. J Korean Med Sci. (2021) 36:e109. doi: 10.3346/jkms.2021.36.e109
14. Shin, SY, Kim, Y, Kim, WS, Moon, JM, Lee, KM, Jung, SA, et al. Compositional changes in fecal microbiota associated with clinical phenotypes and prognosis in Korean patients with inflammatory bowel disease. Intest Res. (2022) 21:148–60. doi: 10.5217/ir.2021.00168
15. Kim, HA, Lee, E, Park, SY, Lee, SS, and Shin, K. Clinical characteristics of patients with psoriatic spondylitis versus those with ankylosing spondylitis: features at baseline before biologic therapy. J Korean Med Sci. (2022) 37:e253. doi: 10.3346/jkms.2022.37.e253
16. Ahn, SM, Oh, JS, Heo, HM, Hong, S, Lee, CK, Yoo, B, et al. Predictive factors for rheumatoid arthritis flare after switching from intravenous to subcutaneous formulation of tocilizumab in real-world practice. J Korean Med Sci. (2022) 37:e138. doi: 10.3346/jkms.2022.37.e138
17. Song, YJ, Cho, SK, Kim, H, Kim, HW, Nam, E, Bae, SC, et al. Risk of tuberculosis development in patients with rheumatoid arthritis receiving targeted therapy: a prospective single center cohort study. J Korean Med Sci. (2021) 36:e70. doi: 10.3346/jkms.2021.36.e70
18. Choi, MG, Ye, BD, Yang, SK, Shim, TS, Jo, KW, and Park, SH. The risk of tuberculosis in patients with inflammatory bowel disease treated with Vedolizumab or Ustekinumab in Korea. J Korean Med Sci. (2022) 37:e107. doi: 10.3346/jkms.2022.37.e107
19. Seo, GH, and Jung, SH. The comparative risk of serious adverse events with Tofacitinib and TNF inhibitors in patients with ulcerative colitis: the Korean experience as revealed by a National Database. J Korean Med Sci. (2022) 37:e123. doi: 10.3346/jkms.2022.37.e123
20. Lee, JW, Song, EM, Jung, SA, Jung, SH, Kim, KW, Koh, SJ, et al. Clinical course of COVID-19 in patients with inflammatory bowel disease in Korea: a KASID multicenter study. J Korean Med Sci. (2021) 36:e336. doi: 10.3346/jkms.2021.36.e336
21. Lee, Y-K, and Bae, S-C. Mortality in Korean patients with rheumatoid arthritis. J Rheumatic Dis. (2021) 28:113–8. doi: 10.4078/jrd.2021.28.3.113
22. Lee, YH, and Song, GG. Overall and sex-specific mortality in psoriatic arthritis and ankylosing spondylitis: a meta-analysis. J Rheumatic Dis. (2018) 25:197–202. doi: 10.4078/jrd.2018.25.3.197
23. Dhana, A, Yen, H, Yen, H, and Cho, E. All-cause and cause-specific mortality in psoriasis: a systematic review and meta-analysis. J Am Acad Dermatol. (2019) 80:1332–43. doi: 10.1016/j.jaad.2018.12.037
24. Bitton, A, Vutcovici, M, Sewitch, M, Suissa, S, and Brassard, P. Mortality trends in Crohn's disease and ulcerative colitis: a population-based study in Québec, Canada. Insflamm Bowel Dis. (2016) 22:416–23. doi: 10.1097/mib.0000000000000608
25. Aniwan, S, Harmsen, WS, Tremaine, WJ, Kane, SV, and Loftus, EV Jr. Overall and cause-specific mortality of inflammatory bowel disease in Olmsted County, Minnesota, from 1970 through 2016. Mayo Clin Proc. (2018) 93:1415–22. doi: 10.1016/j.mayocp.2018.03.004
26. Lee, HS, Choe, J, Kim, SO, Lee, SH, Lee, HJ, Seo, H, et al. Overall and cause-specific mortality in Korean patients with inflammatory bowel disease: a hospital-based cohort study. J Gastroenterol Hepatol. (2017) 32:782–8. doi: 10.1111/jgh.13596
27. Argollo, M, Gilardi, D, Peyrin-Biroulet, C, Chabot, JF, Peyrin-Biroulet, L, and Danese, S. Comorbidities in inflammatory bowel disease: a call for action. Lancet Gastroenterol Hepatol. (2019) 4:643–54. doi: 10.1016/s2468-1253(19)30173-6
28. Dougados, M, Soubrier, M, Antunez, A, Balint, P, Balsa, A, Buch, MH, et al. Prevalence of comorbidities in rheumatoid arthritis and evaluation of their monitoring: results of an international, cross-sectional study (COMORA). Ann Rheum Dis. (2014) 73:62–8. doi: 10.1136/annrheumdis-2013-204223
29. Redeker, I, Callhoff, J, Hoffmann, F, Marschall, U, Haibel, H, Sieper, J, et al. The prevalence and impact of comorbidities on patients with axial spondyloarthritis: results from a nationwide population-based study. Arthritis Res Ther. (2020) 22:210. doi: 10.1186/s13075-020-02301-0
30. Yamazaki, F. Psoriasis: Comorbidities. J Dermatol. (2021) 48:732–40. doi: 10.1111/1346-8138.15840
31. Hayashi, R, Ueno, Y, Tanaka, S, Onishi, K, Takasago, T, Wakai, M, et al. Clinical characteristics of inflammatory bowel disease patients with immunoglobulin a nephropathy. Intest Res. (2021) 19:430–7. doi: 10.5217/ir.2020.00067
32. Liu, J, Gao, X, Chen, Y, Mei, Q, Zhu, L, Qian, J, et al. Incidence and risk factors for venous thrombosis among patients with inflammatory bowel disease in China: a multicenter retrospective study. Intest Res. (2021) 19:313–22. doi: 10.5217/ir.2020.00017
33. Kim, JA, Yoon, S, Kim, LY, and Kim, DS. Towards actualizing the value potential of Korea health insurance review and assessment (HIRA) data as a resource for Health Research: strengths, limitations, applications, and strategies for optimal use of HIRA data. J Korean Med Sci. (2017) 32:718–28. doi: 10.3346/jkms.2017.32.5.718
34. Lim, WH, Choi, EK, Han, KD, Rhee, TM, Lee, HJ, Lee, SR, et al. Proteinuria detected by urine dipstick test as a risk factor for atrial fibrillation: a Nationwide population-based study. Sci Rep. (2017) 7:6324. doi: 10.1038/s41598-017-06579-0
35. Kwon, OC, Han, K, Chun, J, Kim, R, Hong, SW, Kim, JH, et al. Effects of immune-mediated inflammatory diseases on cardiovascular diseases in patients with type 2 diabetes: a nationwide population-based study. Sci Rep. (2022) 12:11548. doi: 10.1038/s41598-022-15436-8
36. Kim, SY, Servi, A, Polinski, JM, Mogun, H, Weinblatt, ME, Katz, JN, et al. Validation of rheumatoid arthritis diagnoses in health care utilization data. Arthritis Res Ther. (2011) 13:R32. doi: 10.1186/ar3260
37. Chung, GE, Jeong, SM, Cho, EJ, Yoo, JJ, Cho, Y, Lee, KN, et al. Association of fatty liver index with all-cause and disease-specific mortality: a nationwide cohort study. Metabolism. (2022) 133:155222. doi: 10.1016/j.metabol.2022.155222
38. Statistics Korea. (2020). Casuses of Death Statistics. Available at: http://kostat.go.kr/assist/synap/preview/skin/miri.html?fn=dbcc61637198371108175934&rs=/assist/synap/preview (Accessed May 24, 2023).
39. Sundararajan, V, Henderson, T, Perry, C, Muggivan, A, Quan, H, and Ghali, WA. New ICD-10 version of the Charlson comorbidity index predicted in-hospital mortality. J Clin Epidemiol. (2004) 57:1288–94. doi: 10.1016/j.jclinepi.2004.03.012
40. Noordzij, M, Leffondré, K, van Stralen, KJ, Zoccali, C, Dekker, FW, and Jager, KJ. When do we need competing risks methods for survival analysis in nephrology? Nephrol Dial Transplant. (2013) 28:2670–7. doi: 10.1093/ndt/gft355
41. Lau, B, Cole, SR, and Gange, SJ. Competing risk regression models for epidemiologic data. Am J Epidemiol. (2009) 170:244–56. doi: 10.1093/aje/kwp107
42. Andersen, PK, Geskus, RB, de Witte, T, and Putter, H. Competing risks in epidemiology: possibilities and pitfalls. Int J Epidemiol. (2012) 41:861–70. doi: 10.1093/ije/dyr213
43. Card, T, Hubbard, R, and Logan, RF. Mortality in inflammatory bowel disease: a population-based cohort study. Gastroenterology. (2003) 125:1583–90. doi: 10.1053/j.gastro.2003.09.029
44. Abuabara, K, Azfar, RS, Shin, DB, Neimann, AL, Troxel, AB, and Gelfand, JM. Cause-specific mortality in patients with severe psoriasis: a population-based cohort study in the U.K. Br J Dermatol. (2010) 163:586–92. doi: 10.1111/j.1365-2133.2010.09941.x
45. Exarchou, S, Lie, E, Lindström, U, Askling, J, Forsblad-d'Elia, H, Turesson, C, et al. Mortality in ankylosing spondylitis: results from a nationwide population-based study. Ann Rheum Dis. (2016) 75:1466–72. doi: 10.1136/annrheumdis-2015-207688
46. Kelty, E, Ognjenovic, M, Raymond, WD, Inderjeeth, CA, Keen, HI, Preen, DB, et al. Mortality rates in patients with ankylosing spondylitis with and without Extraarticular manifestations and comorbidities: a retrospective cohort study. J Rheumatol. (2022) 49:688–93. doi: 10.3899/jrheum.210909
47. Lee, EE, Shin, A, Lee, J, Lee, JH, Ha, YJ, Lee, YJ, et al. All-cause and cause-specific mortality of patients with rheumatoid arthritis in Korea: a nation-wide population-based study. Joint Bone Spine. (2022) 89:105269. doi: 10.1016/j.jbspin.2021.105269
48. Gabriel, SE, Crowson, CS, Kremers, HM, Doran, MF, Turesson, C, O'Fallon, WM, et al. Survival in rheumatoid arthritis: a population-based analysis of trends over 40 years. Arthritis Rheum. (2003) 48:54–8. doi: 10.1002/art.10705
49. Cainap, C, Pop, LA, Balacescu, O, and Cainap, SS. Early diagnosis and screening in lung cancer. Am J Cancer Res. (2020) 10:1993–2009. doi: 10.3390/biology10090864
50. Pasechnikov, V, Chukov, S, Fedorov, E, Kikuste, I, and Leja, M. Gastric cancer: prevention, screening and early diagnosis. World J Gastroenterol. (2014) 20:13842–62. doi: 10.3748/wjg.v20.i38.13842
51. Bresalier, RS, Grady, WM, Markowitz, SD, Nielsen, HJ, Batra, SK, and Lampe, PD. Biomarkers for early detection of colorectal Cancer: the early detection research network, a framework for clinical translation. Cancer Epidemiol Biomark Prev. (2020) 29:2431–40. doi: 10.1158/1055-9965.Epi-20-0234
52. Capotosto, L, Massoni, F, De Sio, S, Ricci, S, and Vitarelli, A. Early diagnosis of cardiovascular diseases in workers: role of standard and advanced echocardiography. Biomed Res Int. (2018) 2018:7354691–15. doi: 10.1155/2018/7354691
53. Groenewegen, A, Zwartkruis, VW, Rienstra, M, Hollander, M, Koffijberg, H, Cramer, MJM, et al. Improving early diagnosis of cardiovascular disease in patients with type 2 diabetes and COPD: protocol of the RED-CVD cluster randomised diagnostic trial. BMJ Open. (2021) 11:e046330. doi: 10.1136/bmjopen-2020-046330
54. Murdaca, G, Colombo, BM, Cagnati, P, Gulli, R, Spanò, F, and Puppo, F. Endothelial dysfunction in rheumatic autoimmune diseases. Atherosclerosis. (2012) 224:309–17. doi: 10.1016/j.atherosclerosis.2012.05.013
55. Murdaca, G, Colombo, BM, Cagnati, P, Gulli, R, Spanò, F, and Puppo, F. Update upon efficacy and safety of TNF-α inhibitors. Expert Opin Drug Saf. (2012) 11:1–5. doi: 10.1517/14740338.2012.630388
56. Murdaca, G, Colombo, BM, and Puppo, F. Adalimumab for the treatment of immune-mediated diseases: an update on old and recent indications. Drugs Today (Barc). (2011) 47:277–88. doi: 10.1358/dot.2011.47.4.1576692
57. Murdaca, G, Colombo, BM, and Puppo, F. Anti-TNF-alpha inhibitors: a new therapeutic approach for inflammatory immune-mediated diseases: an update upon efficacy and adverse events. Int J Immunopathol Pharmacol. (2009) 22:557–65. doi: 10.1177/039463200902200301
58. Murdaca, G, Colombo, BM, Barabino, G, Caiti, M, Cagnati, P, and Puppo, F. Anti-tumor necrosis factor-α treatment with infliximab for disseminated granuloma annulare. Am J Clin Dermatol. (2010) 11:437–9. doi: 10.2165/11311040-000000000-00000
59. Murdaca, G, Spanò, F, Miglino, M, and Puppo, F. Effects of TNF-α inhibitors upon the mechanisms of action of VEGF. Immunotherapy. (2013) 5:113–5. doi: 10.2217/imt.12.151
60. Ciprandi, G, Murdaca, G, Colombo, BM, De Amici, M, and Marseglia, GL. Serum vascular endothelial growth factor in allergic rhinitis and systemic lupus erythematosus. Hum Immunol. (2008) 69:510–2. doi: 10.1016/j.humimm.2008.05.010
61. Colombo, BM, Cacciapaglia, F, Puntoni, M, Murdaca, G, Rossi, E, Rodriguez, G, et al. Traditional and non traditional risk factors in accelerated atherosclerosis in systemic lupus erythematosus: role of vascular endothelial growth factor (VEGATS study). Autoimmun Rev. (2009) 8:309–15. doi: 10.1016/j.autrev.2008.10.002
62. Murdaca, G, Spanò, F, Cagnati, P, and Puppo, F. Free radicals and endothelial dysfunction: potential positive effects of TNF-α inhibitors. Redox Rep. (2013) 18:95–9. doi: 10.1179/1351000213y.0000000046
63. Smolen, JS, Landewé, RBM, Bijlsma, JWJ, Burmester, GR, Dougados, M, Kerschbaumer, A, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis. (2020) 79:685–99. doi: 10.1136/annrheumdis-2019-216655
64. Riley, TR, and George, MD. Risk for infections with glucocorticoids and DMARDs in patients with rheumatoid arthritis. RMD Open. (2021) 7:e001235. doi: 10.1136/rmdopen-2020-001235
65. Ward, MM, Deodhar, A, Gensler, LS, Dubreuil, M, Yu, D, Khan, MA, et al. 2019 update of the American College of Rheumatology/spondylitis Association of America/Spondyloarthritis research and treatment network recommendations for the treatment of ankylosing spondylitis and nonradiographic axial Spondyloarthritis. Arthritis Rheumatol. (2019) 71:1599–613. doi: 10.1002/art.41042
66. Lamb, CA, Kennedy, NA, Raine, T, Hendy, PA, Smith, PJ, Limdi, JK, et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. (2019) 68:s1–s106. doi: 10.1136/gutjnl-2019-318484
Keywords: immune-mediated inflammatory diseases, risk, comorbidities, all-cause mortality, cause-specific mortality
Citation: Kwon OC, Lee SY, Chun J, Han K, Kim Y, Kim R, Park M-C, Kim J-H, Youn YH and Park H (2023) Risk of all-cause and cause-specific mortality associated with immune-mediated inflammatory diseases in Korea. Front. Med. 10:1185300. doi: 10.3389/fmed.2023.1185300
Edited by:
Ching-Mao Chang, Taipei Veterans General Hospital, TaiwanReviewed by:
Peiqi Wang, University of Washington, United StatesGiuseppe Murdaca, University of Genoa, Italy
Monica Vázquez-Del Mercado, University of Guadalajara, Mexico
Copyright © 2023 Kwon, Lee, Chun, Han, Kim, Kim, Park, Kim, Youn and Park. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jaeyoung Chun, Y2h1bmptZEB5dWhzLmFj; Kyungdo Han, aGtkOTE3QG5hdmVyLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Oh Chan Kwon
Oh Chan Kwon See Young Lee
See Young Lee Jaeyoung Chun
Jaeyoung Chun Kyungdo Han
Kyungdo Han Yuna Kim
Yuna Kim Ryul Kim
Ryul Kim Min-Chan Park
Min-Chan Park Jie-Hyun Kim
Jie-Hyun Kim Young Hoon Youn
Young Hoon Youn Hyojin Park
Hyojin Park on behalf of Gastroenterology
on behalf of Gastroenterology Neurology and Rheumatology National Data Science Research (GUARANTEE) Group
Neurology and Rheumatology National Data Science Research (GUARANTEE) Group