- 1Department of Nuclear Medicine, Peking University First Hospital, Beijing, China
- 2Department of Pathology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan, China
- 3Department of Radiology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan, China
Background: Extranodal natural killer/T-cell lymphoma (ENKTCL) is a distinct pathological entity and accounts for ~10% of T-cell lymphomas. The histological features of ENKTCL include angiodestruction and coagulative necrosis and the association with EBV infection. ENKTCL is typically aggressive and mainly affects the nasal cavity and nasopharyngeal region. However, some patients can present with distant nodal or extranodal involvement such as the Waldeyer ring, gastrointestinal tract, genitourinary organs, lung, thyroid, skin, and testes. Compared to ENKTCL of nasal type, primary testicular ENKTCL is very rare and has a lower age of onset and faster clinical progression, with tumor cell dissemination occurring early in the disease.
Case report: Case 1: A 23-year-old man presented with 1 month of right testicular pain and swelling. Enhancement CT revealed increased density in the right testis, uneven increased enhancement, discontinuity of the local envelope, and multiple trophoblastic vessels in the arterial phase. Testicular ENKTCL was diagnosed by post-operative pathology. The patient underwent a follow-up 18F-FDG PET/CT imaging 1 month later and found elevated metabolism in the bilateral nasal, left testicular, and right inguinal lymph nodes. Unfortunately, the patient received no further treatment and died 6 months later. Case 2: A 2-year-old male child presented with an enlarged right testicle, MRI showed a mass in the right epididymis and testicular area, which showed low signal on T1WI, high signal on T2WI and DWI, and low signal on ADC. Meanwhile, CT showed soft tissue in the lower lobe of the left lung and multiple high-density nodules of varying sizes in both lungs. Based on the post-operative pathology, the lesion was diagnosed with primary testicular ENKTCL. The pulmonary lesion was diagnosed as hemophagocytic lymphohistiocytosis associated with EBV infection. The child was given SMILE chemotherapy, but pancreatitis was induced during chemotherapy, then he died 5 months later after chemotherapy.
Conclusion: Primary testicular ENKTCL is very rare in clinical practice, typically presenting as a painful testicular mass, which can mimic inflammatory lesions and cause diagnostic challenges. 18F-FDG PET/CT plays pivotal roles in the diagnosis, staging, evaluation of treatment outcomes and prognosis evaluation in patients with testicular ENKTCL, and it is helpful to assist clinical practice to better formulate individualized treatment plans.
Introduction
Lymphomas can develop anywhere in the body, both inside the lymphatic system (nodal) and outside (extranodal), accounting for about 4% of adult cancer (1). Approximately 90% of lymphomas are non-Hodgkin lymphoma, which can be divided into more than 70 disease entities based on histopathology, immunophenotypics, genetics, and clinical manifestations. According to the World Health Organization's (WHO) classification of lymphoid tumors, extranodal natural killer/T-cell lymphoma (ENKTCL) is classified as a distinct pathological entity (2). ENKTCL constitutes ~10% of T-cell lymphomas and exhibits a higher prevalence in Asia, Central, and South America compared to Western nations (3–5). The histological features of ENKTCL include angiodestruction and coagulative necrosis which are associated with EBV infection (6). ENKTCL is aggressive and mainly affects the nasal cavity and nasopharyngeal region, and this particular histopathologic type is therefore known as ENKTCL, nasal type. In some cases, patients may exhibit distant nodal or extranodal involvement, involving sites such as the Waldeyer ring, gastrointestinal tract, lung, thyroid, skin, testis, or adrenal glands (7–9). The incidence of relapse, refractory disease, and mortality associated with ENKTCL is notably elevated (10).
Here, we present two unique cases of primary testicular NK/T-cell lymphoma, one of which was characterized by bilateral nasal, left testicular, and right inguinal lymph node involvement following surgery, and the other associated with hemophagocytic lymphohistiocytosis. Both cases exhibited a dismal clinical course and prognosis. In addition, we summarized the 18F-FDG PET/CT findings of testicular NK/T-cell lymphoma from the literature in Table 1.
Case presentation
Case 1
A 23-year-old man presented with 1 month of right testicular pain and swelling. The symptoms further worsened and he came to the hospital for further examination. Physical examination revealed that the right testicle was significantly enlarged, hard, and painful to touch. Laboratory tests revealed C-reactive protein 15.39 mg/L, lactate dehydrogenase 1,050 U/L, Epstein-Barr virus (EBV) DNA 10.52 × 102 copies/ml, CA125 76.60 U/mL, CA15-3 14.06 U/mL, Alpha-fetoprotein (AFP) 4.91 ng/ml, and carcinoembryonic antigen (CEA) 7.19 ng/mL. The patient was previously healthy without testicular enlargement and had no family history of hereditary disease. The patient underwent an ultrasound, which showed an enlarged right testicle (6.2 cm × 3.5 cm × 4.7 cm) with hypoechoic parenchyma. Color Doppler showed the presence of abundant internal blood flow signals. Further enhancement computed tomography (CT) examination revealed a mass with increased density (32 HU) in the right testis (Figure 1A). The mass showed patchy increased enhancement, interrupted continuity of the local envelope, and multiple trophoblastic vessels in the arterial phase (Figure 1B). The CT attenuation values in the arterial and venous phases were 54 and 66 HU, respectively (Figures 1C, D). The initial diagnosis was considered to be seminoma by CT, but the final diagnosis of extranodal NK/T-cell lymphoma (ENKTCL) was made by pathological biopsy. The patient underwent a craniocerebral MRI examination, which revealed no abnormalities and ruled out a lesion in the nasal cavity.
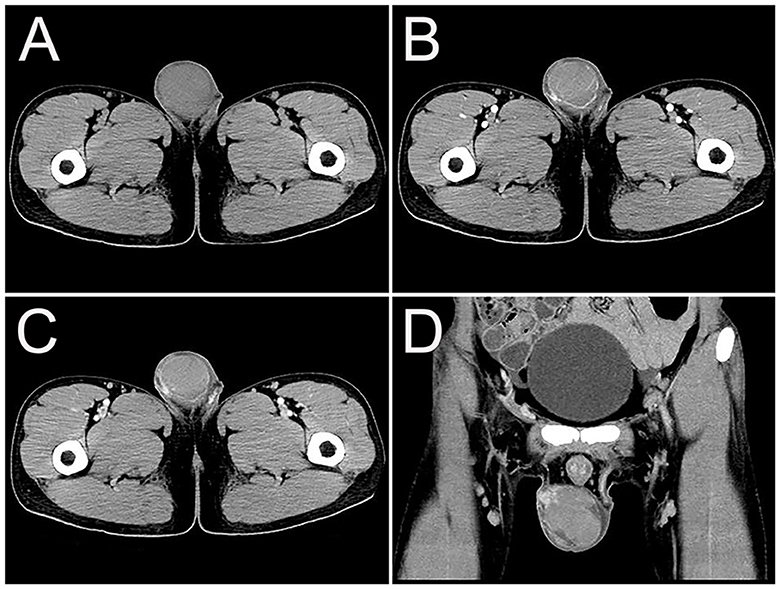
Figure 1. Computed tomography (CT) images of primary right testicular NK/T-cell lymphoma. (A) The transverse image showed a mass with increased density (32 HU) in the right testis. (B) The arterial phase transverse image showed patchy increased enhancement (54 HU), and multiple trophoblastic vessels in the tumor. (C, D) Venous phase transverse image and coronal images showed uneven moderate enhancement of tumor (66 HU), interrupted continuity of the local envelope.
The patient then underwent right orchiectomy, during which the medium-hard right testicle mass was found to be ~8.0 cm × 5.0 cm × 3.5 cm in size, with a grayish-red cut surface. Post-operative pathology revealed that the basic structure of the testis was destroyed. There were a large number of diffusely infiltrating tumor cells among the germinal tubules. The cells with abundant cytoplasm showed different sizes, large deep-stained nuclei, and easily visible nuclear schizophrenia. Coagulative necrosis was found inside with inflammatory cell infiltration around the necrotic area (Figure 2A). Immunohistochemistry showed positive expression of EBER, CD3, CD30, CD20, CD43, CD56, T-cell intracellular antigen 1 (TIA-1), granzyme B (Figures 2B–H). Ki-67 was observed to be positive in 80% of the tumor cells (Figure 2I).
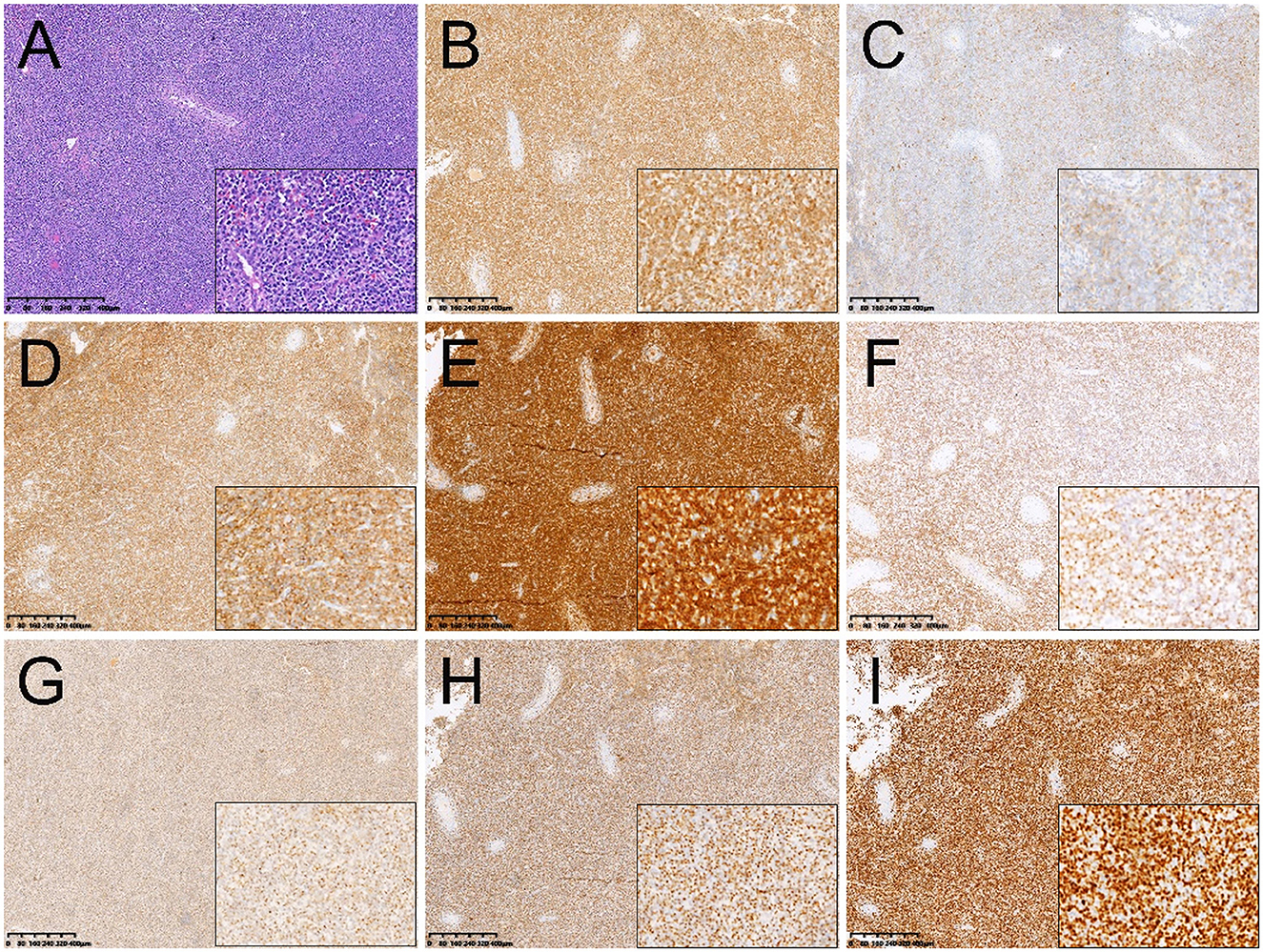
Figure 2. Histopathological and immunohistochemical images. (A) Hematoxylin-eosin (HE) staining (magnification ×40 & 200) showed a large number of diffusely infiltrating tumor cells among the germinal tubules. The cells with abundant cytoplasm showed different sizes, large deep-stained nuclei, and easily visible nuclear schizophrenia. Immunohistochemistry showed that the short spindle cells were positive for CD3 (B), CD20 (C), CD43 (D), CD56 (E), EBER (F), granzyme B (G), TIA-1 (H). Ki-67 was observed to be positive in 80% of the tumor cells (I) (magnification ×40 & 200).
The patient underwent a follow-up 18F-FDG PET/CT scan 1 month later, which revealed a missing right testis and FDG-avid circular soft tissue in the right scrotum (SUVmax = 3.9), relating to post-operative changes. However, the left testicle was enlarged at a maximum dimension of about 3.9 cm × 6.0 cm and had a significantly high FDG uptake (SUVmax = 9.7). A swelling right inguinal lymph node (1.3 cm × 2.1 cm) had an increased FDG uptake (SUVmax = 6.8). Meanwhile, a soft tissue mass with unevenly increased FDG uptake was found in the bilateral nasal cavity, inferior turbinates, and left septal sinus (SUVmax = 24.5), suggesting lymphoma involvement (Figure 3). The patient had a clinical Ann Arbor stage IV and an International Prognostic Index (IPI) score of 3. Unfortunately, the patient and his family chose to give up receiving further treatment because of the financial reasons and died 6 months later.
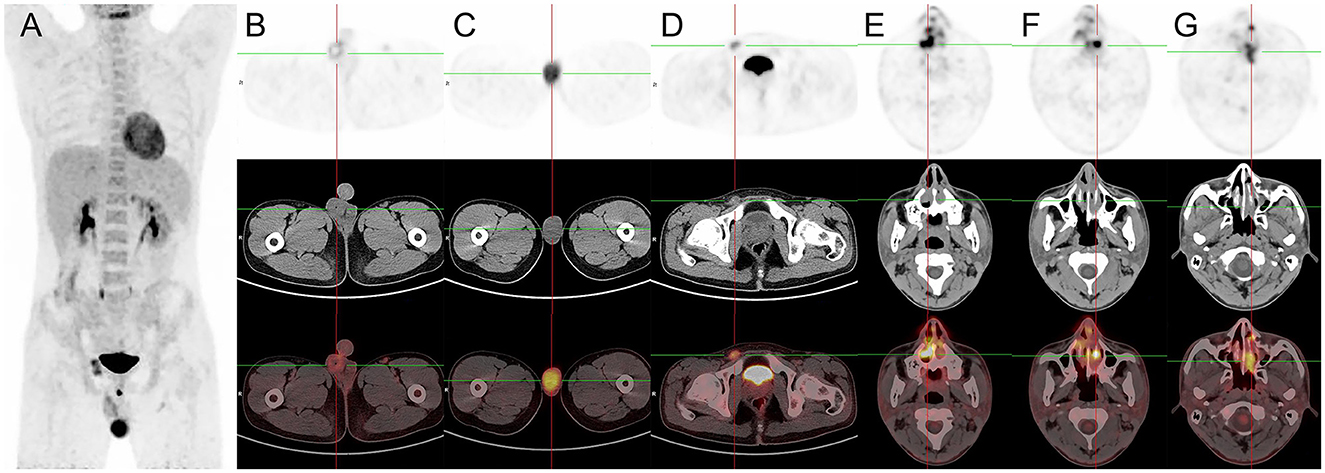
Figure 3. 18F-FDG PET/CT images of primary right testicular NK/T-cell lymphoma. (A) The anteroposterior 3-dimensional maximum intensity projection image (MIP) demonstrated elevated metabolism in the left testicular, and right inguinal lymph nodes. (B) The transverse images showed a missing right testis and FDG-avid circular soft tissue in the right scrotum (SUVmax = 3.9), relating to post-operative changes. (C) The transverse images showed the left testicle was enlarged at a maximum dimension of about 3.9 cm × 6.0 cm and had a significantly high FDG uptake (SUVmax = 9.7). (D) The transverse images showed a swelling right inguinal lymph node (1.3 cm × 2.1 cm) had an increased FDG uptake (SUVmax = 6.8). (E–G) The transverse images showed a soft tissue mass with unevenly increased FDG uptake found in the bilateral nasal cavity, inferior turbinates, and left septal sinus (SUVmax = 24.5), suggesting lymphoma involvement.
Case 2
A 2-year-old male child was found with an enlarged right testicle 10 days ago. In physical examination at the hospital, a mass of about 2 cm in diameter in the right scrotal area of the child was palpated with clear borders, pressure pain, and no obvious stalk tip into the abdominal cavity. The size of the mass did not decrease after squeezing and the transillumination test was negative. Laboratory tests revealed AFP 1.91 ng/ml, lactate dehydrogenase of 295 U/L, EBV DNA 8.48 × 103 copies/mL, positive IgG antibody to EBV capsid antigen, and positive IgG antibody to EBV nuclear antigen. The child was previously healthy with no testicular enlargement and no family history of hereditary disease. The ultrasound revealed the swelling right testicle with an internal hypoechoic zone, which did not exclude inflammatory lesions. MRI examination found that a mass-like abnormal signal was seen in the right epididymis and testicular area. The lesion at about 2.9 cm × 2.0 cm × 2.4 cm showed low signal on T1WI, high signal on T2WI and DWI, and low signal on ADC. While an abnormal signal was seen in the right epididymal area (Figures 4A–D). The shape and signal of the left testis showed no significant abnormalities. CT examination further showed multiple high-density nodules of different sizes in both lungs with the largest one at 3.0 cm × 1.8 cm in size in the lower lobe of the left lung, suggesting lung metastasis (Figures 4E, F).
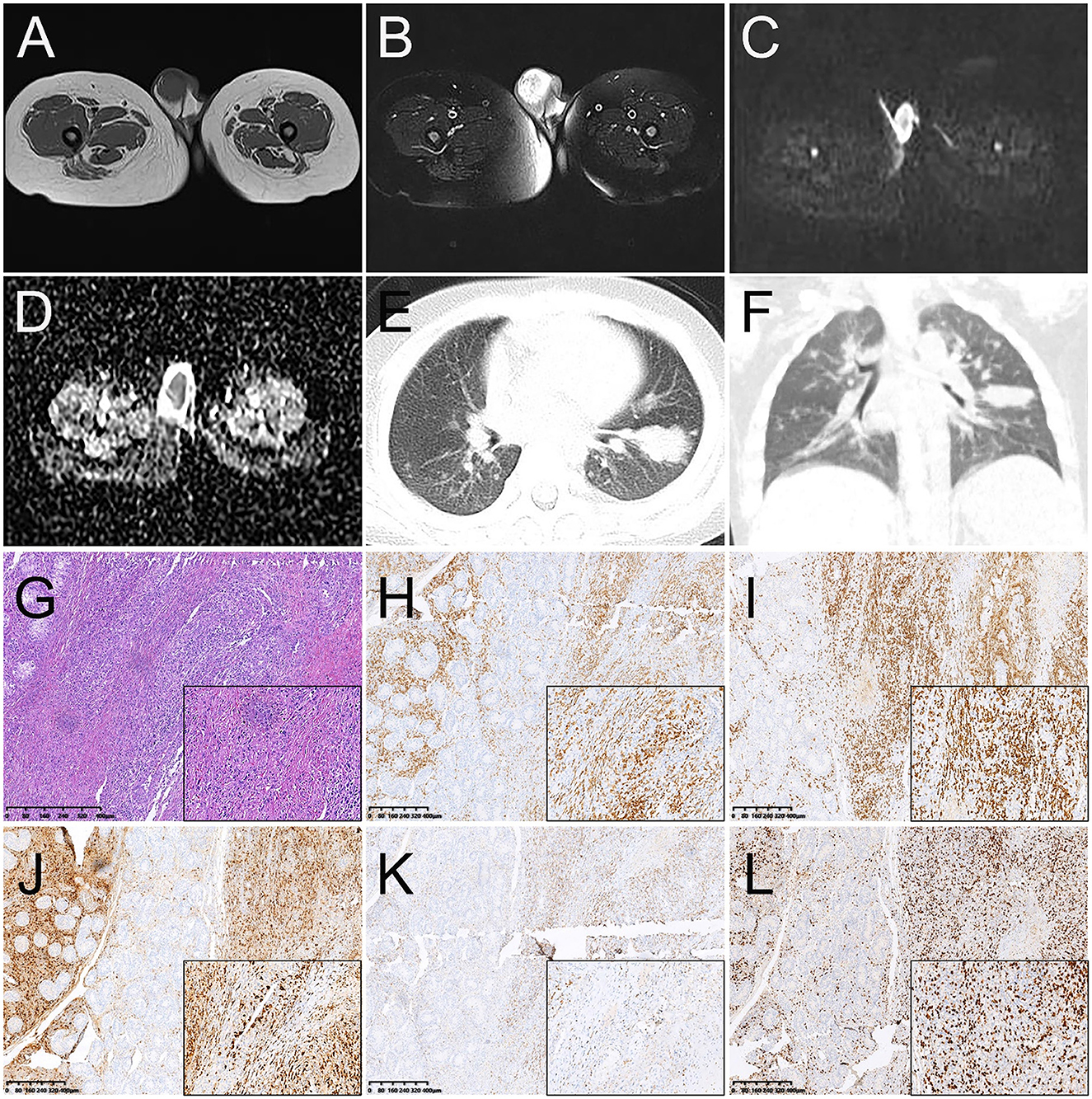
Figure 4. Magnetic resonance images (MRI) and CT images of primary right testicular NK/T-cell lymphoma (A–F). (A) T1WI transverse image shows an increase in the size of the right testicle with a heterogeneous low signal lesion. (B) T2WI transverse image shows a heterogeneous high signal lesion. The lesions showed a high signal on DWI (C) and a low signal on ADC images (D). (E, F) The transverse image and coronal images of the lung window on CT showed multiple high-density nodules in different sizes in both lungs with the largest one at 3.0 cm × 1.8 cm in size in the lower lobe of the left lung, suggesting lung metastasis. Histopathological and immunohistochemical images (G–L). (G) Hematoxylin-eosin (HE) staining (magnification ×40 & 200) showed diffuse infiltration of tumor cells into the testicular parenchyma and diffuse growth of lymph-like cells between the germinal tubules with varying sizes and nuclear schizophrenia. Immunohistochemistry showed that the short spindle cells were positive for CD3 (H), CD8 (I), granzyme B (J), and TIA-1 (K). Ki-67 was observed to be positive in 70% of the tumor cells (L) (magnification ×40 & 200).
After the consent of the child's parents, the right orchiectomy was performed. During surgery, the right testicle was found to be about 3.6 cm × 2.6 cm × 1.8 cm in size, and a grayish-red medium-hard mass was found inside the testicular parenchyma with a size of about 2.1 cm × 1.5 cm × 1.2 cm. Post-operative pathology revealed diffuse infiltration of tumor cells into the testicular parenchyma and diffuse growth of lymph-like cells between the germinal tubules with varying sizes and nuclear schizophrenia. Necrosis can also be partly seen and atrophy of the residual testicular tissue was found (Figure 4G). Immunohistochemistry showed positive expression of CK, CD3, CD8, TIA-1, CD30 (focal), EMA (focal), and granzyme B (Figures 4H–K), but the negative expression of CD20, CD5, CD4, and EBNA2. Ki67 was observed to be positive in 70% of the tumor cells (Figure 4L). In addition, an Epstein-Barr virus-encoded RNA (EBER) assay was performed and showed positive in situ hybridization for EBER. Lymphocyte immunoassay showed an increased percentage by 24.63% of NK cells (normal range, 7.0–14.0%). A cranial MRI revealed no abnormalities and ruled out a lesion in the nasal cavity. Based on the information on immunophenotype, molecular detection, and laboratory test results, the patient was diagnosed with primary testicular ENKTCL with hemophagocytic lymphohistiocytosis (HLH) associated with EBV infection in the lungs. The patient had a clinical Ann Arbor stage I and an IPI score of 3.
After the exclusion of contraindications to chemotherapy, the child was given a SMILE chemotherapy regimen (DXM + MTX + IFO + L-Asp + VP-16). During chemotherapy, the child developed abdominal pain, and elevated blood and urine amylase. Ultrasound displayed an enlarged pancreas with dilated pancreatic ducts so pancreatitis was considered and chemotherapy was suspended. The child died 5 months after leaving the hospital.
Discussion
Most lymphomas in the testicular region are disseminated from extra-testicular lymphomas. In primary testicular lymphoma (PTL), the testis is the only involved site at presentation, with no involvement of other nodes or organs (11). It is a hematologic tumor with high malignancy, accounting for 1–7% of testicular tumors, and is most likely to occur in men over 60 years of age (12). The most common pathological type of PTL is diffuse large B-cell lymphoma (DLBCL) (80–98%), while ENKTCL is rare (12, 13). Among the two subtypes of ENKTCL, the more prevalent subtype, known as the nasal type, typically involves midline structures such as the nasal cavity, nasopharynx, and sinuses. Conversely, the other subtype, which is rarer, can manifest in diverse locations such as the skin, testes, liver, gastrointestinal tract, lungs, orbits, salivary glands, and muscles (7–9, 14, 15), or may present as a disseminated form of the disease, known as NK/T cell leukemia (16).
Compared to ENKTCL of nasal type, primary testicular ENKTCL is much less reported and has a lower age of onset and rapid clinical progression. Our reports about one adolescent case and one infant case are consistent with previous reports. It is important to measure the circulating EBV DNA as a surrogate biomarker of lymphoma load (17). The child had concomitant EBV infection associated with hemophagocytic lymphohistiocytosis and his EBV-DNA load was elevated, supporting the diagnosis. In contrast to ENKTCL affecting other sites, primary testicular ENKTCL is frequently diagnosed at Ann Arbor stage I, likely due to the early detection of symptoms and timely diagnosis facilitated by the testis' accessible location at the surface of the body.
The pathological findings of primary testicular ENKTCL consist of atypical lymphoid cells, inflammatory cells, and eosinophils, that surround and infiltrate the seminiferous tubules, accompanied by angiocentricity and angiodestruction (18, 19). ENKTCL also exhibits complex molecular genetic changes. Based on terminal repeat sequence analysis, EBV is clonal in NK/T cells and exists as an episomal virus that cannot be integrated into the host genome (20–23). Lymphoma cells exhibit the typical immunophenotype of CD2+, CD56+, cytoplasmic CD3ε+, surface CD3–, and cytotoxic molecules (granzyme B, perforin, TIA1)+ (20). Expression of CD30, a cytokine receptor that induces viral infection in T and B cells, is associated with a poorer prognosis in patients with ENKTCL (15). Primary testicular NK/T-cell lymphoma tends to involve the central nervous system, nasopharynx, skin, lymph nodes, gastrointestinal tract, spleen, and contralateral testis, which may be associated with the expression of abundant CD56 molecules in these sites (24, 25). The testicular NK/T-cell lymphomas described in our study exhibit characteristic features, characterized by densely packed and widely disseminated populations of medium to large atypical lymphocytes, and are known to demonstrate EBV positivity.
Known for its aggressive clinical course, if the primary tumor is not detected in the early stage, ENKTCL may spread to many organ systems and affect many organs or systems (1). CT and MRI have been widely used to diagnose nasal ENKTL, but for non-nasal origin, CT and MRI cannot be used for staging because occult involvement of the nose and other anatomical sites cannot be detected. In this case, 18F-FDG PET/CT is performed to accurately detect lesions that are not detected in conventional imaging methods and to clarify the location and number of abnormal metabolic lesions (8, 26, 27). A study conducted by Fujiwara et al. (28) showed that 18F-FDG PET/CT was superior to conventional methods (enhanced CT, biopsies from primary sites, and bone marrow examinations) in detecting nodal lesions (100 vs. 93%), extranodal lesions (94 vs. 61%) and cutaneous lesions (100 vs. 65%). Similar findings have been found in a study made by Liu et al. (29). In their study of 39 patients with cutaneous ENKTCL, 18F-FDG PET/CT had high accuracy in the diagnosis of cutaneous and extracutaneous lesions in patients with ENKTCL, detecting 48 cutaneous and 88 extracutaneous regions while conventional methods detected only 34 cutaneous lesions and 61 extracutaneous lesions, with statistically significant differences in detection rates. Filizoglu et al. (8) reported a unique case of ENKTCL with widespread cutaneous and subcutaneous involvement. Huang et al. (30) reported a case of ENKTCL involving the left vocal cord, which showed focal FDG uptake on PET/CT. All of the above highlight the value of 18F-FDG-PET/CT in ENKTCL diagnosis and staging. Regional lymph node involvement has been reported as a rare event, even in patients with primary testicular ENKTCL patients with the widely involved disease (9). However, the first case presented with right inguinal lymph node involvement, and the possibility of lymph node involvement still needs to be considered in primary testicular ENKTCL patients. Primary testicular ENKTCL should be differentiated from seminoma, yolk sac tumor, and testicular inflammation. Testicular seminomatous cell tumor mostly occurs in patients aged 30–40 years old, often with a history of cryptorchidism. The tumor has an equal signal to normal testis on T1WI with clear margins and low signal on T2WI. After enhancement, the solid part is mildly enhanced and the fibrovascular part is highly enhanced. It may be accompanied by syringomyelia and lymph node metastasis. Yolk cystic tumors are most often seen in children, and laboratory tests tend to reveal increased AFP. Mostly equal or slightly low signal on T1WI and slightly high signal on T2WI, which may be accompanied by hemorrhage. Testicular inflammation often has symptoms of infection such as fever or pain, usually secondary to urinary tract infections, and imaging manifests as diffuse enlargement of the testicles, which might be accompanied by syringomyelia.
18F-FDG PET/CT is also valuable for the prognosis of ENKTCL and can also be used as a baseline for comparing interim and end-of-treatment scans, helping to guide the clinic to appropriate and effective treatment modalities and protocols (29, 31–39). Bai et al. (40) retrospectively analyzed the effect of SUVmax on the survival of ENKTCL and showed that SUVmax was an independent prognostic factor for overall survival (OS). A multicenter retrospective study conducted by Pak et al. (41) included 36 patients with ENKTCL to assess the prognostic value of pre-treatment PET/CT metabolic parameters, the results showed that total lesion glycolysis (TLG) was the only significant predictor of progression-free survival (PFS).
Currently, the International Prognostic Index (IPI) and the Korean Prognostic Index (KPI) are the most common prognostic models (42, 43). However, these prognostic scoring systems have been reported to have limited predictive accuracy for ENKTCL (44, 45). Li et al. (46) developed nomograms using pretreatment 18F-FDG PET/CT parameters and clinical parameters and found that PET/CT could be used as an effective tool for individualized prediction of PFS and OS in 171 ENKTCL patients. The nomograms had better predictive accuracies with a C-index of 0.729 and 0.736 for PFS and OS, respectively, compared with IPI and KPI. Guo et al. (47) found that deep learning analysis with 18F-FDG PET/CT provides an effective method for predicting the prognosis of ENKTCL patients. The identified feature prediction similarity index and maps may potentially contribute to patient stratification in treatment. Wang et al. (48) developed a PET radiomics-based model to predict PFS and OS in 110 nasal-type ENKTCL, and the R-signature constructed in the training and validation cohorts had moderate predictive ability (AUC = 0.788 and 0.473), but the performance of the radiomics model was inferior to that of the metabolism-based model.
It is common for patients with extra-nasal presentations to have more adverse clinical features (e.g., higher stage, poor performance status), and their survival rate is lower than for patients with nasal presentations (1, 49). Testicular ENKTCL is a highly aggressive malignancy, for which there is a paucity of established therapeutic regimens. Radical orchiectomy is commonly employed as the initial management strategy. Testicular ENKTCL may develop insidiously, most studies revealed survival rates of < 50% (50, 51). It is very common that patients with lesions restricted to the testis relapse within 6 months or die of widespread involvement of the skin, CNS, GI tract, and lungs within a year of the initial diagnosis (10). Kobayashi et al. (52) reported a case of testicular secondary ENKTCL in a 73-year-old man who was successfully treated with unilateral orchiectomy, DeVIC therapy, and radiotherapy to the contralateral testis. NK/T cell lymphoma patients are resistant to anthracycline-containing combination chemotherapy, with a 5-year survival rate of < 30% (23). Drénou et al. (53) suggested that this may be due to the development of multidrug resistance (MDR-1) genes. Yamaguchi et al. (54) concluded that radiotherapy of 50 Gy was required to achieve local control of ENKTCL. Unfortunately, however, radiation therapy alone is not sufficient to improve survival due to the significant number of patients who experience local and systemic recurrence after radiation therapy. The study by Ahn et al. (55) demonstrated that radiotherapy provided survival benefits to patients with only localized cutaneous involvements. The need for radiation therapy to the ipsilateral pelvis, para-aortic lymph nodes, and contralateral testes in patients with testicular ENKTCL is also uncertain. Although the occurrence of progression to the CNS frequently, prophylactic intrathecal chemotherapy to prevent CNS recurrence is often accompanied by considerable untoward effects (25). Clinical practice has recommended several combinations of radiotherapy and chemotherapy, such as concurrent, sequential, and sandwich chemoradiotherapy, as a therapeutic modality for localized ENKTCL with a low risk of treatment failure (56).
A multicenter-controlled study by Lee et al. (57) suggests that bone marrow or peripheral blood stem cell transplantation may be an effective treatment for ENKTCL. Studies of targeted drugs for ENKTCL are currently underway (58). EBV antigens might be targets for effector T cells based on the recent observation of the high efficacy of PD1 blockade (20). JAK/STAT, PDGF, aurora kinase, MYC, and NF-xB have been identified as potential therapeutic targets by Sanjay de Mei et al. (59). However, consensus on the standardized treatment of testicular ENKTCL has not been established, and in perspective, more comprehensive studies are necessary (60). In this study, we report two cases of primary testicular NK/T-cell lymphoma, one of which was characterized by bilateral nasal, left testicular, and right inguinal lymph node involvement following surgery, and the other associated with hemophagocytic lymphohistiocytosis. Both cases exhibited a dismal clinical course and prognosis.
Conclusion
In summary, primary testicular ENKTCL is very rare clinically, mostly with a combined painful testicular mass as the first symptom, and is easily misdiagnosed as an inflammatory lesion. It has a highly aggressive clinical course, and we need to raise awareness of this disease and perform pathological histology, immunohistochemistry, and EBV-related tests as early as possible to clarify the diagnosis. 18F-FDG PET/CT plays pivotal roles in the diagnosis, staging, evaluation of treatment outcomes, and prognosis evaluation in patients with testicular ENKTCL, and it is helpful to assist clinical practice for better-individualized treatment plans. Indications for 18F-FDG PET/CT in this disease typically include: finding the location of the primary site in patients with elevated EBV DNA and tumor markers of unknown cause; to better clinically stage before treatment; monitoring treatment efficacy; and evaluating the situation of tumor recurrence and metastasis after treatment. To improve the early diagnosis, 18F-FDG PET/CT should be performed to accurately detect lesions that are not detected in conventional imaging methods and clarify the location and number of abnormal metabolic lesions, and guide the puncture site for pathological biopsy. No consensus about standardized treatment has been established, and a more comprehensive study is necessary.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
This study was reviewed and approved by the Medical Ethics Committee of Peking University First Hospital. The patients and the minor's legal guardian provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) and minor(s)' legal guardian for the publication of any potentially identifiable images or data included in this article. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Author contributions
WH: manuscript draft and editing. XL and LL: imaging data collection. YZ and YG: imaging data analysis. JG: supervision. LK: writing—review and editing. All authors met the requirements for authorship for the submitted version and agreed to its submission.
Funding
This study was funded by the National Natural Science Foundation of China (82171970 and 81871385), the Beijing Science Foundation for Distinguished Young Scholars (JQ21025), and the Beijing Municipal Science & Technology Commission (Z221100007422027).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Akbar M, Clasen-Linde E, Specht L. Extranodal NK/T-cell lymphoma, nasal type, with extranasal presentation - a case report and a review of the literature. Acta Oncol. (2020) 59:1480–7. doi: 10.1080/0284186X.2020.1795250
2. Jaffe ES. The 2008 WHO classification of lymphomas: implications for clinical practice and translational research. Hematol Am Soc Hematol Educ Program. (2009)523–31. doi: 10.1182/asheducation-2009.1.523
3. Kommalapati A, Tella SH, Ganti AK, Armitage JO. Natural killer/T-cell neoplasms: analysis of incidence, patient characteristics, and survival outcomes in the United States. Clin Lymphoma Myeloma Leuk. (2018) 18:475–9. doi: 10.1016/j.clml.2018.04.009
4. Pine RR, Clark JD, Sokol JA. CD56 negative extranodal NK/T-cell lymphoma of the orbit mimicking orbital cellulitis. Orbit. (2013) 32:45–8. doi: 10.3109/01676830.2012.736603
5. Hallak B, Cairoli A, Bouayed S, Berthod G. Sinonasal relapses of a primary isolated extranodal NK/T-cell lymphoma of the testis. BMJ Case Rep. (2019) 12:e230221. doi: 10.1136/bcr-2019-230221
6. Ng S-B, Khoury JD. Epstein-Barr virus in lymphoproliferative processes: an update for the diagnostic pathologist. Adv Anat Pathol. (2009) 16:40–55. doi: 10.1097/PAP.0b013e3181916029
7. Shu P, Ling W, Zhu C, Wang J, Jiang C, Zhang W, et al. The early diagnosis of testicular natural killer/t-cell lymphoma. Hell J Nucl Med. (2016) 19:275–7. doi: 10.1967/s002449910412
8. Filizoglu N, Ozguven S, Ones T, Turoglu HT, Erdil TY. Extranodal NK/T-cell lymphoma with widespread cutaneous and subcutaneous involvement on 18 F-FDG PET/CT. Clin Nucl Med. (2022) 47:e630–1. doi: 10.1097/RLU.0000000000004205
9. Kim YB, Chang SK, Yang W-I, Hahn JS, Koom WS, Shim SJ, et al. Primary NK/T cell lymphoma of the testis. A case report and review of the literature. Acta Haematol. (2003) 109:95–100. doi: 10.1159/000068489
10. Naboush A, Farhat F, Nasser SM, Kamar FG. Bifocal presentation of primary testicular extranasal NK/T-cell lymphoma: a case report and review of the literature. Case Rep Oncol Med. (2013) 2013:267389. doi: 10.1155/2013/267389
11. Kiely JM, Massey BD, Harrison EG, Utz DC. Lymphoma of the testis. Cancer. (1970) 26:847–52. doi: 10.1002/1097-0142(197010)26:4<847::aid-cncr2820260418>3.0.co;2-6
12. Cheah CY, Wirth A, Seymour JF. Primary testicular lymphoma. Blood. (2014) 123:486–93. doi: 10.1182/blood-2013-10-530659
13. Vitolo U, Seymour JF, Martelli M, Illerhaus G, Illidge T, Zucca E, et al. Extranodal diffuse large B-cell lymphoma (DLBCL) and primary mediastinal B-cell lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. (2016) 27:v91–102. doi: 10.1093/annonc/mdw175
14. Tang P, Wang R, Su M, Shen G, Tian R. FDG PET/CT showing a primary vaginal NK/T cell lymphoma. Clin Nucl Med. (2022) 47:273–4. doi: 10.1097/RLU.0000000000003906
15. Kumar C, Jain G, Gupta A, Pramanik R, Chopra A. Extra-nasal NK/T-cell lymphoma: a rare case with a rarer presentation. Cytopathology. (2022) 33:518–21. doi: 10.1111/cyt.13095
16. Tse E, Kwong Y-L. How I treat NK/T-cell lymphomas. Blood. (2013) 121:4997–5005. doi: 10.1182/blood-2013-01-453233
17. Au W-Y, Pang A, Choy C, Chim C-S, Kwong Y-L. Quantification of circulating Epstein-Barr virus (EBV) DNA in the diagnosis and monitoring of natural killer cell and EBV-positive lymphomas in immunocompetent patients. Blood. (2004) 104:243–9. doi: 10.1182/blood-2003-12-4197
18. Tse E, Kwong Y-L. NK/T-cell lymphomas. Best Pract Res Clin Haematol. (2019) 32:253–61. doi: 10.1016/j.beha.2019.06.005
19. Tse E, Kwong Y-L. Diagnosis and management of extranodal NK/T cell lymphoma nasal type. Expert Rev Hematol. (2016) 9:861–71. doi: 10.1080/17474086.2016.1206465
20. Tse E, Kwong Y-L. The diagnosis and management of NK/T-cell lymphomas. J Hematol Oncol. (2017) 10:85. doi: 10.1186/s13045-017-0452-9
21. Barrionuevo C, Zaharia M, Martinez MT, Taxa L, Misad O, Moscol A, et al. Extranodal NK/T-cell lymphoma, nasal type: study of clinicopathologic and prognosis factors in a series of 78 cases from Peru. Appl Immunohistochem Mol Morphol. (2007) 15:38–44. doi: 10.1097/01.pai.0000205062.27174.56
22. Kuo T-T, Shih L-Y, Tsang N-M. Nasal NK/T cell lymphoma in Taiwan: a clinicopathologic study of 22 cases, with analysis of histologic subtypes, Epstein-Barr virus LMP-1 gene association, and treatment modalities. Int J Surg Pathol. (2004) 12:375–87. doi: 10.1177/106689690401200410
23. Terro K, Sharrouf L, El Cheikh J. Progress of hematopoietic stem cell transplantation and radiotherapy in the treatment of extranodal NK/T cell lymphoma. Front Oncol. (2022) 12:832428. doi: 10.3389/fonc.2022.832428
24. Tombet CA, El Houmaidi A, Aynaou M, Mhanna T, Chennoufi M, Barki A. A nasal swelling revealing a metastatic testicular extranodal NK/T-cell lymphoma: a case report and a review of literature. Urol Case Rep. (2020) 33:101357. doi: 10.1016/j.eucr.2020.101357
25. Güler G, Altinok G, Uner AH, Sungur A. CD56+ lymphoma presenting as a testicular tumor. Leuk Lymphoma. (1999) 36:207–11. doi: 10.3109/10428199909145967
26. Chen A, Mokrane F-Z, Schwartz LH, Morschhauser F, Stamatoullas A, Schiano de Colella J-M, et al. Early 18F-FDG PET/CT response predicts survival in relapsed or refractory hodgkin lymphoma treated with nivolumab. J Nucl Med. (2020) 61:649–54. doi: 10.2967/jnumed.119.232827
27. Cottereau A-S, Nioche C, Dirand A-S, Clerc J, Morschhauser F, Casasnovas O, et al. 18F-FDG PET dissemination features in diffuse large B-cell lymphoma are predictive of outcome. J Nucl Med. (2020) 61:40–5. doi: 10.2967/jnumed.119.229450
28. Fujiwara H, Maeda Y, Nawa Y, Yamakura M, Ennishi D, Miyazaki Y, et al. The utility of positron emission tomography/computed tomography in the staging of extranodal natural killer/T-cell lymphoma. Eur J Haematol. (2011) 87:123–9. doi: 10.1111/j.1600-0609.2011.01645.x
29. Liu C, Zhang Y, Zhang Y, Wang M, Liu R, Liu X, Hu S. Diagnostic value of 18F-FDG PET/CT for cutaneous extranodal natural killer/T-cell lymphoma, nasal type. Nucl Med Commun. (2016) 37:446–52. doi: 10.1097/MNM.0000000000000463
30. Huang M, Wang R, Shen G, Tian R. Nasal-type NK/T-cell lymphoma involvement of the vocal cord on FDG PET/CT. Clin Nucl Med. (2022) 47:914–5. doi: 10.1097/RLU.0000000000004276
31. Wang H, Shen G, Jiang C, Li L, Cui F, Tian R. Prognostic value of baseline, interim and end-of-treatment 18F-FDG PET/CT parameters in extranodal natural killer/T-cell lymphoma: a meta-analysis. PLoS ONE. (2018) 13:e0194435. doi: 10.1371/journal.pone.0194435
32. Paes FM, Kalkanis DG, Sideras PA, Serafini AN. FDG PET/CT of extranodal involvement in non-Hodgkin lymphoma and Hodgkin disease. Radiographics. (2010) 30:269–91. doi: 10.1148/rg.301095088
33. Lim CH, Yoon SE, Kim SJ, Cho J, Ko YH, Lee K-H, et al. Metabolic activity of extranodal NK/T cell lymphoma on 18F-FDG PET/CT according to immune subtyping. Sci Rep. (2021) 11:5879. doi: 10.1038/s41598-021-85332-0
34. Xu P, Guo R, You J, Cheng S, Li J, Zhong H, et al. Dynamic evaluation of the prognostic value of 18F-FDG PET/CT in extranodal NK/T-cell lymphoma, nasal type. Ann Hematol. (2021) 100:1039–47. doi: 10.1007/s00277-021-04466-3
35. Sin KM, Ho SKD, Wong BYK, Gill H, Khong P-L, Lee EYP. Beyond the lymph nodes: FDG-PET/CT in primary extranodal lymphoma. Clin Imaging. (2017) 42:25–33. doi: 10.1016/j.clinimag.2016.11.006
36. Wu H-B, Wang Q-S, Wang M-F, Li H-S, Zhou W-L, Ye X-H, et al. Utility of 18F-FDG PET/CT for staging NK/T-cell lymphomas. Nucl Med Commun. (2010) 31:195–200. doi: 10.1097/MNM.0b013e32833310fa
37. Yang C, Wu W, Zhou H, Zhao S, Tian R, Xiang M, et al. 18F-FDG PET/CT plays a limited role in replacing bone marrow biopsy for newly diagnosed advanced-stage patients with extranodal natural killer/T-cell lymphoma. Front Oncol. (2022) 12:894804. doi: 10.3389/fonc.2022.894804
38. Zhou X, Lu K, Geng L, Li X, Jiang Y, Wang X. Utility of PET/CT in the diagnosis and staging of extranodal natural killer/T-cell lymphoma: a systematic review and meta-analysis. Medicine. (2014) 93:e258. doi: 10.1097/MD.0000000000000258
39. Kim SJ, Choi JY, Hyun SH, Ki C-S, Oh D, Ahn YC, et al. Risk stratification on the basis of Deauville score on PET-CT and the presence of Epstein-Barr virus DNA after completion of primary treatment for extranodal natural killer/T-cell lymphoma, nasal type: a multicentre, retrospective analysis. Lancet Haematol. (2015) 2:e66–74. doi: 10.1016/S2352-3026(15)00002-2
40. Bai B, Huang H-Q, Cai Q-C, Fan W, Wang X-X, Zhang X, et al. Predictive value of pretreatment positron emission tomography/computed tomography in patients with newly diagnosed extranodal natural killer/T-cell lymphoma. Med Oncol. (2013) 30:339. doi: 10.1007/s12032-012-0339-0
41. Pak K, Kim BS, Kim K, Kim IJ, Jun S, Jeong YJ, et al. Prognostic significance of standardized uptake value on F18-FDG PET/CT in patients with extranodal nasal type NK/T cell lymphoma: a multicenter, retrospective analysis. Am J Otolaryngol. (2018) 39:1–5. doi: 10.1016/j.amjoto.2017.10.009
42. Lee J, Suh C, Park YH, Ko YH, Bang SM, Lee JH, et al. Extranodal natural killer T-cell lymphoma, nasal-type: a prognostic model from a retrospective multicenter study. J Clin Oncol. (2006) 24:612–8. doi: 10.1200/JCO.2005.04.1384
43. International Non-Hodgkin's Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin's lymphoma. N Engl J Med. (1993) 329:987–94. doi: 10.1056/NEJM199309303291402
44. Suzuki R, Suzumiya J, Yamaguchi M, Nakamura S, Kameoka J, Kojima H, et al. Prognostic factors for mature natural killer (NK) cell neoplasms: aggressive NK cell leukemia and extranodal NK cell lymphoma, nasal type. Ann Oncol. (2010) 21:1032–40. doi: 10.1093/annonc/mdp418
45. Huang J-J, Zhu Y-J, Xia Y, Zhao W, Lin T-Y, Jiang W-Q, et al. A novel prognostic model for extranodal natural killer/T-cell lymphoma. Med Oncol. (2012) 29:2183–90. doi: 10.1007/s12032-011-0030-x
46. Li H, Shao G, Zhang Y, Chen X, Du C, Wang K, et al. Nomograms based on SUVmax of 18F-FDG PET/CT and clinical parameters for predicting progression-free and overall survival in patients with newly diagnosed extranodal natural killer/T-cell lymphoma. Cancer Imaging. (2021) 21:9. doi: 10.1186/s40644-020-00379-y
47. Guo R, Hu X, Song H, Xu P, Xu H, Rominger A, et al. Weakly supervised deep learning for determining the prognostic value of 18F-FDG PET/CT in extranodal natural killer/T cell lymphoma, nasal type. Eur J Nucl Med Mol Imaging. (2021) 48:3151–61. doi: 10.1007/s00259-021-05232-3
48. Wang H, Zhao S, Li L, Tian R. Development and validation of an 18F-FDG PET radiomic model for prognosis prediction in patients with nasal-type extranodal natural killer/T cell lymphoma. Eur Radiol. (2020) 30:5578–87. doi: 10.1007/s00330-020-06943-1
49. Au W-Y, Ma S-Y, Chim C-S, Choy C, Loong F, Lie AKW, et al. Clinicopathologic features and treatment outcome of mature T-cell and natural killer-cell lymphomas diagnosed according to the World Health Organization classification scheme: a single center experience of 10 years. Ann Oncol. (2005) 16:206–14. doi: 10.1093/annonc/mdi037
50. Yang Y, Zhang Y-J, Zhu Y, Cao J-Z, Yuan Z-Y, Xu L-M, et al. Prognostic nomogram for overall survival in previously untreated patients with extranodal NK/T-cell lymphoma, nasal-type: a multicenter study. Leukemia. (2015) 29:1571–7. doi: 10.1038/leu.2015.44
51. Mao S, Diao C, Cao L. Primary small intestinal extranodal NK/T cell lymphoma, nasal type with kidney involvement: a rare case report and literature review. Diagn Pathol. (2022) 17:75. doi: 10.1186/s13000-022-01254-z
52. Kobayashi T, Hangaishi A, Yamamoto G, Shinohara A, Morikawa T, Takazawa Y, et al. Successful treatment of secondary NK/T-cell lymphoma of the testis. Ann Hematol. (2013) 92:997–8. doi: 10.1007/s00277-012-1657-1
53. Drénou B, Lamy T, Amiot L, Fardel O, Caulet-Maugendre S, Sasportes M, et al. CD3- CD56+ non-Hodgkin's lymphomas with an aggressive behavior related to multidrug resistance. Blood. (1997) 89:2966–74. doi: 10.1182/blood.V89.8.2966
54. Yamaguchi M, Suzuki R, Oguchi M. Advances in the treatment of extranodal NK/T-cell lymphoma, nasal type. Blood. (2018) 131:2528–40. doi: 10.1182/blood-2017-12-791418
55. Ahn HK, Suh C, Chuang SS, Suzumiya J, Ko YH, Kim SJ, et al. Extranodal natural killer/T-cell lymphoma from skin or soft tissue: suggestion of treatment from multinational retrospective analysis. Ann Oncol. (2012) 23:2703–7. doi: 10.1093/annonc/mds096
56. Hu S, Zhou D, Zhang W. The optimal timing of radiotherapy in the combined modality therapy for limited-stage extranodal NK/T cell lymphoma (ENKTL): a systematic review and meta-analysis. Ann Hematol. (2018) 97:2279–87. doi: 10.1007/s00277-018-3479-2
57. Lee J, Au W-Y, Park MJ, Suzumiya J, Nakamura S, Kameoka J-I, et al. Autologous hematopoietic stem cell transplantation in extranodal natural killer/T cell lymphoma: a multinational, multicenter, matched controlled study. Biol Blood Marrow Transplant. (2008) 14:1356–64. doi: 10.1016/j.bbmt.2008.09.014
58. Tse E, Zhao W-L, Xiong J, Kwong Y-L. How we treat NK/T-cell lymphomas. J Hematol Oncol. (2022) 15:74. doi: 10.1186/s13045-022-01293-5
59. de Mel S, Hue SS-S, Jeyasekharan AD, Chng W-J, Ng S-B. Molecular pathogenic pathways in extranodal NK/T cell lymphoma. J Hematol Oncol. (2019) 12:33. doi: 10.1186/s13045-019-0716-7
Keywords: natural killer/T-cell lymphoma, testis, computed tomography, 18F-FDG PET/CT, case report, magnetic resonance imaging
Citation: Huang W, Liu X, Li L, Zhang Y, Gao Y, Gao J and Kang L (2023) Multimodality imaging evaluation of primary testicular extranodal natural killer/T-cell lymphoma: two case reports. Front. Med. 10:1183564. doi: 10.3389/fmed.2023.1183564
Received: 10 March 2023; Accepted: 11 May 2023;
Published: 30 May 2023.
Edited by:
Egesta Lopci, University of Milan, ItalyReviewed by:
Isinsu Kuzu, Ankara University, TürkiyeAleksandr Shulyak, National Academy of Medical Sciences of Ukraine, Ukraine
Xin Wang, Shandong Provincial Hospital, China
Copyright © 2023 Huang, Liu, Li, Zhang, Gao, Gao and Kang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lei Kang, a2FuZ2xlaUBiam11LmVkdS5jbg==
 Wenpeng Huang
Wenpeng Huang Xiaonan Liu2
Xiaonan Liu2 Liming Li
Liming Li Yongbai Zhang
Yongbai Zhang Yuan Gao
Yuan Gao Jianbo Gao
Jianbo Gao Lei Kang
Lei Kang