- 1Cardiology Department, Emergency Institute for Cardiovascular Diseases and Transplantation Târgu Mureș, Târgu Mureș, Romania
- 2Physiology Department, University of Medicine, Pharmacy, Science and Technology “George Emil Palade” of Târgu Mureș, Târgu Mureș, Romania
Radiological and interventional cardiology procedures are in continuous expansion, leading to an important increase in the incidence of contrast-associated acute kidney injury (CA-AKI). Although numerous methods of CA-AKI prevention have been studied, at present, there is no consensus on the definition of this entity or on its prevention. In this paper, we aim to provide a critical analysis of the existing data on the epidemiology, pathophysiology, and clinical significance of CA-AKI. Existing and emergent approaches for CA-AKI prevention are also discussed, with a focus on parenteral fluid administration and on the most recent clinical and experimental data. We also emphasize a number of questions that remain to be answered, and we identify hotspots for future research.
1 Introduction
For decades, physicians have been concerned about the increasing use of contrast agents and their risk of inducing acute kidney injury (1). Despite substantial research, there are still many unknown aspects related to contrast-associated acute kidney injury (CA-AKI). Consensus appears to exist among physicians that contrast-induced complications are not clinically significant, and some authors question the very existence of this entity (2). Meanwhile, numerous clinical studies support a deleterious effect of contrast agents on kidney function, and animal studies clearly showed harmful effects (3–7).
The use of less nephrotoxic substances has reduced the incidence of CA-AKI (8). However, at this point, there is no consensus on the exact incidence of CA-AKI in the era of modern contrast agents, nor on the medium- and long-term impact of CA-AKI. Also, a wide array of strategies with prophylactic and/or therapeutic potential have been evaluated, but there is no consensus on the most effective strategy. Data regarding preventive measures such as fluid administration, pharmacological strategies, use of different contrast agents, and renal replacement therapies are conflicting. Isotonic saline administration seems to be, however, the most effective way to prevent CA-AKI (9–11). In addition, although many factors have been studied, with the exception of basal renal function, there is no consensus on the factors that increase the risk of CA-AKI.
In this paper, we aim to provide a critical analysis of the existing data on the epidemiology, pathophysiology, and clinical significance of CA-AKI. Existing and emergent approaches for CA-AKI prevention are also discussed, with a focus on parenteral hydration and on the most recent clinical and experimental data in this field.
2 Epidemiology of contrast-associated acute kidney injury in the era of modern iodinated contrast media
In a recent study including more than 7,000 patients, CA-AKI occurred in 6.5% of patients receiving intra-arterial administration of contrast media (12). In other studies, intra-arterial contrast administration led to CA-AKI in more than 13% of patients, whereas the risk of CA-AKI following intravenous contrast administration appeared to be negligeable (13). Development of CA-AKI after cardiac and radiologic procedures is associated with a significant increase in morbidity, mortality, and costs, and with prolonged hospitalization (14). At present, CA-AKI is defined as an impairment of renal function after parenteral administration of contrast media in the absence of other identifiable causes, characterized by an increase in serum creatinine (SCr) of at least 0.5 mg/dL (≥44 μmol/L) or ≥ 25% above baseline within the first 48 h after contrast administration (14). This is also the definition most frequently used in clinical trials. The European Society of Urogenital Radiology defines CA-AKI based on an increase in SCr by ≥0.5 mg/dL or ≥ 25% within the first 3 days after intravascular administration of contrast medium, without an alternative etiology (15).
The incidence of CA-AKI is increasing mainly due to the increase in the number of interventional procedures. In the United States only, over one million cardiac catheterization procedures are performed annually (16). The continuous development of interventional cardiology has also led to increasingly complex procedures that require administration of considerably larger amounts of contrast material (17). According to the study by Jennings et al., the crude rate of angiography and percutaneous coronary interventions (PCI) performed in Ireland increased by 47.8 and 35.9%, respectively, from 2004 to 2011, with rates of 6,689 and 1,825 per million individuals in 2011 (17). Among those cases, the reported incidence of CA-AKI ranged from 0 to >50% (18–21). Similar increases in the number and complexity of interventional cardiology procedures were also seen in more recent studies performed in Spain and Switzerland (22, 23).
A recent review and meta-analysis pointed out a higher incidence of CA-AKI after intra-arterial than after intravenous contrast medium administration, but the overall incidence of CA-AKI seemed to be decreasing over time, together with the need for dialysis. The incidence of CA-AKI was 9% and that of kidney failure requiring dialysis was 0.5% after intra-arterial administration of contrast medium (24). According to Chalikias et al. (25), the incidence of CA-AKI after PCI ranges from 2 to 20%, depending on baseline kidney function.
In addition to the increasing number of angiographies and computed tomography (CT) examinations, causing an increasingly number of patients to be exposed to contrast media, the population exposed to these procedures is also older and sicker, and the volume of contrast media administered is also higher in these patients (26–31).
The incidence of CA-AKI also seems to be influenced by several other major factors, including the type, volume, and route (arterial vs. venous) of contrast media administered. While there is agreement that intra-arterial contrast agent administration can cause acute kidney injury and that the incidence of CA-AKI is significantly higher after intra-arterial than after intravenous contrast administration, retrospective studies suggest that intravenous contrast material administration for CT examinations may associate a similar risk of CA-AKI to non-contrast CT (32, 33). Meanwhile, according to a systematic review and meta-analysis published in 2016, CA-AKI risk did not differ among types of low-osmolar contrast media and there was also no difference between the route of administration of the contrast media (34).
3 Brief overview of the pathophysiology of contrast-associated acute kidney injury
The exact mechanisms of CA-AKI remain to date unknown. Several causes have been described and there are two major theories regarding the mechanism of CA-AKI: direct cytotoxic effects of contrast media and renal vasoconstriction resulting in medullary hypoxemia (Figure 1) (21). Contrast agents have been shown to exhibit direct toxic effects on renal tubular cells, caused by oxygen free radicals release, altered mitochondrial function, and apoptosis, all culminating in renal ischemia (35, 36). Contrast agents exert their toxic effects mainly on renal tubular epithelial cells, causing vacuolization and osmotic nephrosis, and on endothelial cells. Although the exact mechanisms responsible for contrast media-induced cytotoxicity remain elusive, these agents have been shown to stimulate cellular pathways involved in apoptosis by activating caspase-3, caspase-9, and the bcl2 pathway (37). In addition, administration of contrast media has been linked to redistribution of cell membrane proteins and disruption of intercellular junctions, DNA damage, mitochondrial dysfunction, and a significant reduction in cell proliferation, leading to alterations in cell polarity, loss of cytoskeletal integrity, displacement of membrane proteins and cellular adhesion molecules, and necrosis of tubular epithelial cells (38–42).
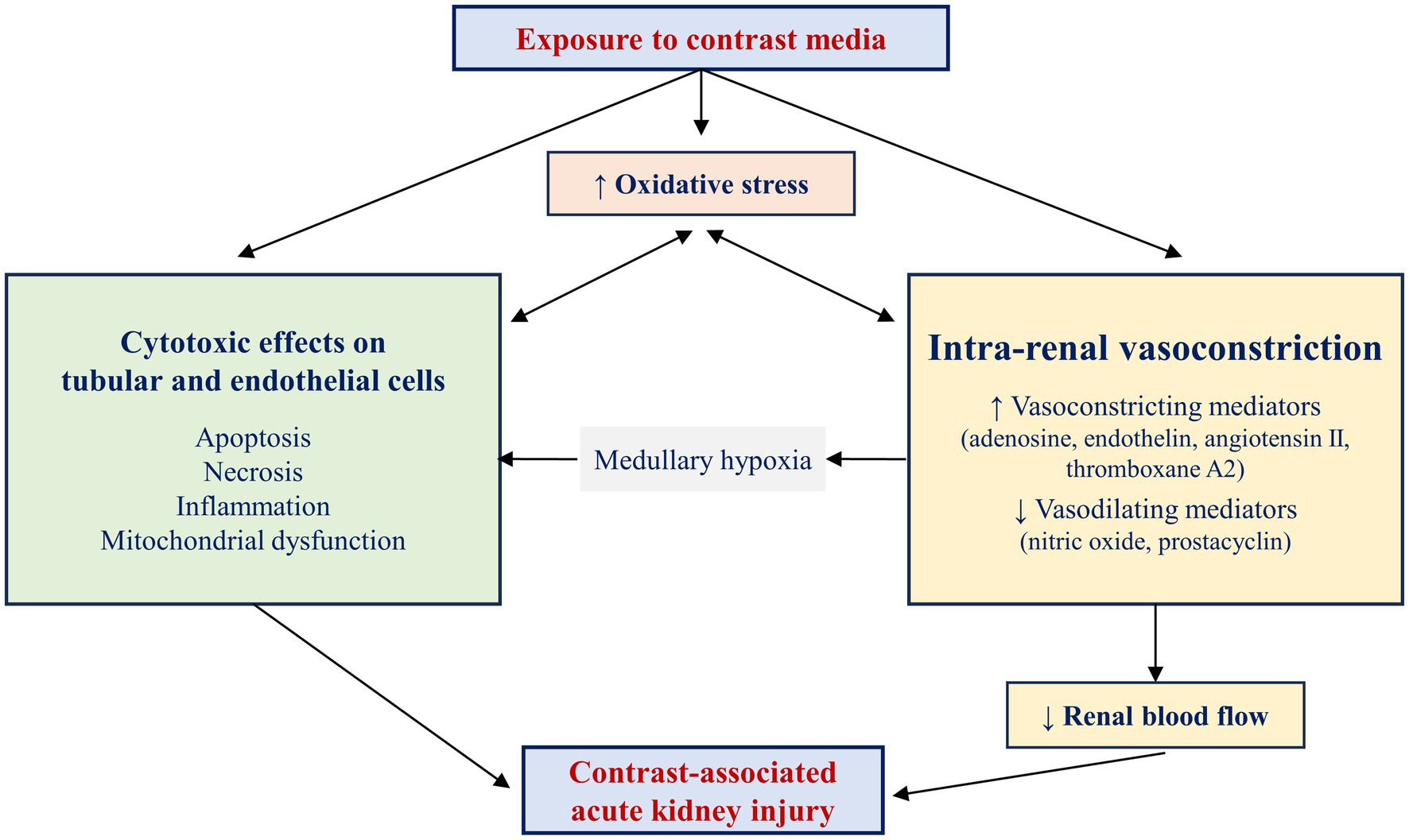
Figure 1. Pathophysiology of contrast-associated acute kidney injury. Two main mechanisms contribute to the pathogenesis of contrast-associated acute kidney injury: direct cytotoxic effects on tubular and endothelial cells and intrarenal vasoconstriction, favored by an imbalance between vasoconstricting and vasodilating mediators. The medullary hypoxia resulting from renal vasoconstriction further alters the structure and function of renal tubular cells. The increased production of free oxygen species and the consequent increase in oxidative stress aggravate renal vasoconstriction and exert toxic effects on renal tubular cells. In parallel, renal ischemia and local cytotoxic effects aggravate oxidative stress, creating a vicious cycle. The coexistence and mutual stimulation of these mechanisms eventually result in contrast-associated acute kidney injury.
The toxicity of contrast media on the tubular and endothelial cells is further accentuated by oxidative stress and hypoxia (43, 44). Increased adenosine and endothelin release in response to contrast agent administration leads to renal vasoconstriction, whereas reduced nitric oxide and prostaglandin synthesis decreases the vasodilation of the renal vascular bed (36). Together, these mechanisms are responsible for ischemia in the deeper portion of the outer medulla, an area with increased oxygen demand. Many of the deleterious effects of contrast agents on the kidneys are mediated by increased production of oxygen free radicals and oxidative stress. Medullary hypoxia and adenosine catabolism increase the production of oxygen free radicals, which not only scavenge nitric oxide, but also increase the synthesis of vasoconstricting substances such as endothelin, adenosine, angiotensin II, and thromboxane A2. Constriction of glomerular and peritubular capillaries and of vasa recta further leads to alterations in renal perfusion and blood flow autoregulation, and to distal ischemia (45, 46). At its turn, ischemia then increases the formation of oxygen free radicals, thus leading to a vicious circle in which ischemia promotes oxidative stress and oxidative stress aggravates the preexisting ischemia. Experiments performed on isolated mouse kidney vessels showed that decreased nitric oxide availability and increased superoxide production induced by contrast media resulted in more pronounced vasoconstriction in the afferent than in the efferent arterioles, providing a pathophysiological explanation for the reduction in glomerular filtration following administration of contrast media (47). In addition, increased oxidants levels alter mitochondrial and nuclear DNA, lead to damage in membrane lipids and cellular proteins, and activate c-Jun N-terminal, ERK, and p38-MAPK kinases, promote apoptosis and necrosis via activation of caspase-3 and caspase-9 (48, 49).
4 Clinical significance of contrast-associated acute kidney injury
4.1 Predictors of contrast-associated acute kidney injury
While in the general population the incidence of CA-AKI has been estimated to be <2%, in patients at high risk (patients with diabetes mellitus [DM], congestive heart failure, chronic renal impairment, and older age) the incidence is estimated to be >20% and up to 30% (50). In 2008, Mehran et al. (14) proposed a risk score for prediction of CA-AKI after PCI. In this score, points are given for the presence of hypotension, use of intra-aortic balloon pump, symptoms of congestive heart failure, age, anemia, DM, volume of contrast media administered, and the estimated glomerular filtration rate (eGFR). According to the Mehran risk score, a score < 6 points indicates a risk for CA-AKI of 7.5%, a risk score between 6 points and 10 points indicates a risk for CA-AKI of 14%, a risk score between 11 points and 16 points indicates a risk for CA-AKI of 26%, and a risk score > 16 points indicates a risk for CA-AKI of 57% following intra-arterial administration of contrast media (14).
Many risk factors have been reported to increase the risk of CA-AKI, but few of them have been identified as independent predictors of CA-AKI. Barett et al. analyzed a series of clinical trials using multivariate analysis and identified baseline renal disease, heart failure, DM, and the dose of contrast media administered as predictors of increased risk of CA-AKI, with pre-existing renal disease being the most significant predictor of CA-AKI (51).
Data regarding the role of DM as a risk factor for CA-AKI remain controversial. In Mehran’s risk score, DM is included as an independent predictor of CA-AKI. However, few articles have evaluated risk score models among patients with DM undergoing PCI. Hyperglycemia is associated with an increase in oxygen-derived free radicals, which will lead to vasoconstriction, one of the most important mechanisms involved in the pathophysiology of CA-AKI (52–54). Yao and co-workers proposed a simple clinical identification tool for predicting contrast-induced nephropathy in patients with DM undergoing PCI. The score includes four predictive factors and demonstrated good discrimination and predictive ability for CA-AKI and clinical outcomes after PCI (52). The study population was divided into groups according to the risk score: low (5.9% risk of CA-AKI), moderate (32.9% risk of CA-AKI), and high (60% risk of CA-AKI) risk score (52). Oxidative stress and inflammation have also been incriminated in CA-AKI occurrence in patients with acute ST-segment elevation myocardial infarction (36). Malnutrition and baseline inflammatory status have also been recently proposed as risk factors for CA-AKI in patients undergoing PCI (55, 56).
4.2 Impact of contrast media on the risk of contrast-associated acute kidney injury
At present, contrast media are commonly divided into high-osmolar (osmolality in the range of 1,000 to 2,000 mOsm/kg), low-osmolar (osmolality in the range of 500 to 1,000 mOsm/kg), and iso-osmolar (osmolality in the range of 290 to 300 mOsm/kg) contrast media. The introduction of low- and iso-osmolar contrast media has determined a reduction in the incidence of CA-AKI following intra-arterial contrast administration, particularly in high-risk patients (57).
In 1989, Schwab and co-workers published the results of a randomized controlled trial comparing a high-osmolar (diatrizoate) with a low-osmolar (iopamidol) contrast agent in 443 patients undergoing cardiac catheterization. They were unable to demonstrate a difference in the incidence of nephrotoxicity between the two groups (8, 58). Two years later, Taliercio and co-workers demonstrated, however, that the use of low-osmolar contrast medium (iopamidol) was less nephrotoxic than that of high-osmolar contrast medium (diatrizoate) in high-risk patients undergoing cardiac angiography. These findings were strengthened by the prospective randomized trial by Rudnick et al., which demonstrated that in patients with DM alone or combined with preexisting renal insufficiency undergoing cardiac angiography, the risk of CA-AKI was significantly lower when low- and iso-osmolar contrast media were used (20). In the Nephrotoxicity in High-Risk Patients Study of Iso-Osmolar and Low-Osmolar Non-Ionic Contrast Media (NEPHRIC) study by Aspelin et al. the incidence of CA-AKI was much lower when iodixanol was used rather than a low-osmolar non-ionic medium in patients with DM and in patients with preexisting renal insufficiency undergoing angiography (8). In patients undergoing invasive cardiac procedures, isoosmolal and low-osmolality agents have shown a significant reduction in the risk of CA-AKI compared with high-osmolality agents (57). In that study, iodixanol (an iso-osmolar agent) was shown to have the lowest risk for CA-AKI in patients at high risk (patients with chronic kidney disease and diabetes) (57). Iodixanol was shown to be superior to low-osmolality agents in this subset of patients and in those with renal dialysis and is thus recommended by The National Kidney Foundation Kidney Disease Outcome Quality Initiative Guidelines (57).
The risk of CA-AKI also increases as contrast volume increases. Thus, the recommended contrast doses in patients with chronic kidney disease are <30 mL for diagnostic catheterization and < 100 mL if a PCI is planned (57). According to the European Society of Cardiology, there are three simple ways of calculating maximum contrast volume to reduce the risk of CA-AKI. For example, < 100 mL should be used if significant chronic kidney disease is present and PCI is planned, or the volume of contrast should be adjusted according to SCr and body weight (5 x kg/SCr) or by multiplying 4 times the eGFR (calculated using the Cockroft-Gault or the Modification in Diet in Renal Disease equations) (57). According to recent data, use of systems specifically designed to reduce the volume of contrast media administered could further reduce risk of CA-AKI (59).
4.3 Clinical impact of contrast-associated acute kidney injury
4.3.1 Short term implications of contrast-associated acute kidney injury
Contrast-associated acute kidney injury is associated with prolonged hospitalization and increased hospital-related costs, higher morbidity, and increased short-term mortality (60–66), although emergent hemodialysis is rarely needed following administration of iodinated contrast media (67). There are consistent data regarding the prolongation of hospitalization in patients with CA-AKI, proving that the higher the creatinine values, the higher the number of days of hospitalization, which will increase at its turn the socio-economic burden for the medical systems (68). According to Rihal et al., patients who developed CA-AKI after coronary angiography and PCI also had markedly higher incidence of in-hospital mortality than those who did not develop CA-AKI (22% vs. 1.4%; p < 0.0001) (68). Another retrospective trial demonstrated that even a small (0.25 mg/dL to 0.5 mg/dL) increase in SCr was associated with an increase of in-hospital mortality (69). Meanwhile, in a secondary analysis of the PRESERVE cohort, underlying chronic kidney disease was associated with cardiovascular events by day 90 following angiography, but this was not the case for CA-AKI, most of which was stage 1 (70).
4.3.2 Long-term implication of contrast-associated acute kidney injury
In addition to short-term complications, CA-AKI is also associated with long-term mortality. Altered hemodynamics is associated with increased risk of CA-AKI and higher mortality rates. However, according to Sun et al., in a group of 696 patients with acute myocardial infarction and left ventricular ejection fraction >40%, CA-AKI was an independent predictor of long-term mortality, regardless of hemodynamic abnormalities (71).
5 Prophylactic strategies for contrast-associated acute kidney injury
5.1 Periprocedural fluid administration
Many randomized clinical trials have studied different pharmacological agents to prevent CA-AKI. However, according to an update published in the e-journal of the European Society of Cardiology Council for Cardiology Practice, intravenous fluid volume loading with isotonic saline (normal saline 0.9%) is considered the single most important measure to prevent the occurrence of CA-AKI (57). Volume resuscitation with isotonic saline is particularly important in hypovolemic patients. Questions remain, however, regarding the most adequate fluid and the optimal route of fluid administration – oral or intravenous (72). Trivedi and co-workers evaluated 53 patients undergoing cardiac catheterization. The patients were randomized into two groups: one group, consisting of 27 patients, received intravenous normal saline for 24-h (1 mL/kg/h) beginning 12 h prior to catheterization, and the other group, consisting of 26 patients, was allowed unrestricted oral fluids. Ten out of the 53 subjects developed acute renal insufficiency. The incidence of acute renal insufficiency was significantly lower in the group with saline administration (1 out of 27) as compared to the group allowed unrestricted oral fluids (9 out of 26; p = 0.005 between the two groups; relative risk: 0.11; 95% confidence interval 0.015 to 0.790) (73).
A randomized comparison of two fluid regimens (isotonic [0.9%] vs. hypotonic [0.45%] saline) performed in 1,620 patients undergoing coronary angioplasty was published by Mueller and co-workers, demonstrating that isotonic saline administration was superior to hypotonic hydration in the prevention of contrast media-associated nephropathy. In the group with isotonic saline administration, 5 of 685 patients developed CA-AKI (0.7%; 95% confidence interval 0.1–1.4%) vs. 14 of 698 patients in the hypotonic hydration group (2%; 95% confidence interval 1.0–3.1%; p = 0.04 between groups) (74). In the POSEIDON trial, left ventricular end-diastolic pressure-guided volume expansion with isotonic saline was more efficient than the standard (1.5 mL/kg/h) saline administration protocol in preventing CA-AKI in patients undergoing cardiac catheterization (75). Forced diuresis with dopamine, furosemide, or mannitol have shown no benefit in the prevention of CA-AKI, and their use has even been described to be harmful in different studies (76).
Current guidelines recommend prophylactic isotonic saline administration pre- and post-arterial administration of iodinated contrast media for prevention of CA-AKI in high-risk patients, based on expert consensus. The recommended protocol is 1 mL/kg/h 12 h before and continued for 24 h after the procedure or 0.5 mL/kg/h if the left ventricular ejection fraction is less than or equal to 35% and in heart failure patients with New York Heart Association class >2 according to the ESC/EACTS Guidelines on Myocardial Revascularization published in 2018 (53).
The A Maastricht Contrast-Induced Nephropathy Guideline (AMACING) trial demonstrated that no isotonic saline administration was non-inferior to prophylactic saline administration among patients with chronic kidney disease (eGFR 45–59 mL/min/1.73m2) and diabetes or at least two other risk factors (anemia, cardiovascular disease, ongoing nonsteroidal anti-inflammatory drugs or diuretics usage, age > 75 years), or multiple myeloma or lymphoplasmocytic lymphoma with small chain proteinuria receiving intravascular (intra-arterial or intravenous) contrast. A total of 660 patients were enrolled in that study, with a follow-up period of 35 days; 43% of patients were older than 75 years, 41% were females, 32% were diabetic, and all patients received the same low-osmolar contrast medium (iopromide). In patients with prophylactic isotonic saline administration, the incidence of symptomatic heart failure episodes was significantly higher than in the group with no saline administration. There was no difference between the two groups in the incidence of renal failure and all-cause mortality. However, due to its inclusion and exclusion criteria, the findings can only be applied to hemodynamically stable outpatients, with eGFR >30 mL/min/1.73m2, who received a relatively small amount of contrast media (< 100 mL) (77).
In 2017, Giacoppo and co-workers published the results of a meta-analysis that evaluated data from 124 trials and 28,240 patients investigating different strategies used to prevent CA-AKI. According to their results, fluid administration alone was the least effective preventive strategy for CA-AKI, and in patients with DM none of the strategies used to prevent CA-AKI was effective. Compared to saline administration alone, six other strategies were more effective in reducing the incidence of CA-AKI in that study: statins, N-acetylcysteine, xanthines, sodium bicarbonate, ischemic preconditioning, and the combination of N-acetylcysteine plus sodium bicarbonate (78).
The duration of peri-procedural fluid administration also remains a matter of debate. Previously, patients undergoing coronary angiography were admitted for overnight intravenous fluid administration, and a 12-h isotonic saline administration protocol was applied before and after the procedure. Accumulating data indicate, however, similar results for CA-AKI prevention with 1-h prior and 6-h post-procedural fluid administration (79, 80). The Optimal timing of hydration to erase contrast-associated nephropathy (OTHER CAN) study also failed to show a significant difference in CA-AKI incidence in patients with moderate renal impairment when comparing overnight i.v. to bolus fluid administration (p = 0.13) (81). Administration of 250 mL of sodium bicarbonate before contrast administration was also compared with 2,000 mL normal saline administration pre- and post-coronary intervention and was found to be non-inferior (82). In a more recent study, simplified hydration with normal saline from 1 h before to 4 h after coronary angiography at a rate of 3 mL/kg/h was non-inferior to standard hydration with normal saline 12 h before and 12 h after coronary angiography at a rate of 1 mL/kg/h in preventing CA-AKI (83).
5.2 Pharmacological agents for prevention of contrast-associated acute kidney injury
Studies on the benefit of natriuretic peptides, aminophylline, theophylline, statins, and ascorbic acid on CA-AKI prevention have yielded mixed results (Table 1). Many other pharmacological agents have also been studied in order to reduce the risk of CA-AKI. Until now, there are inconsistent data about angiotensin converting enzyme inhibitors / angiotensin II receptor blockers, calcium channel blockers, theophylline, ascorbic acid, and statins (84–88). Dopamine, fenoldopam, and atrial natriuretic peptide have shown no benefit, and forced diuresis with mannitol or furosemide is not indicated and may even be dangerous (88). Prostaglandin E1, also known as alprostadil, has been proposed as an effective preventative measure for CA-AKI. A meta-analysis including 19 randomized controlled trials has recently been published, showing that alprostadil might be associated with a reduction in the incidence of CA-AKI. Of the 19 trials, 13 reported the use of prostaglandin E1 to prevent CA-AKI in patients undergoing interventions for coronary heart disease. Even though the results of this meta-analysis showed that the use of prostaglandin E1 might be associated with a significant reduction in CA-AKI, the results require further verification (89).
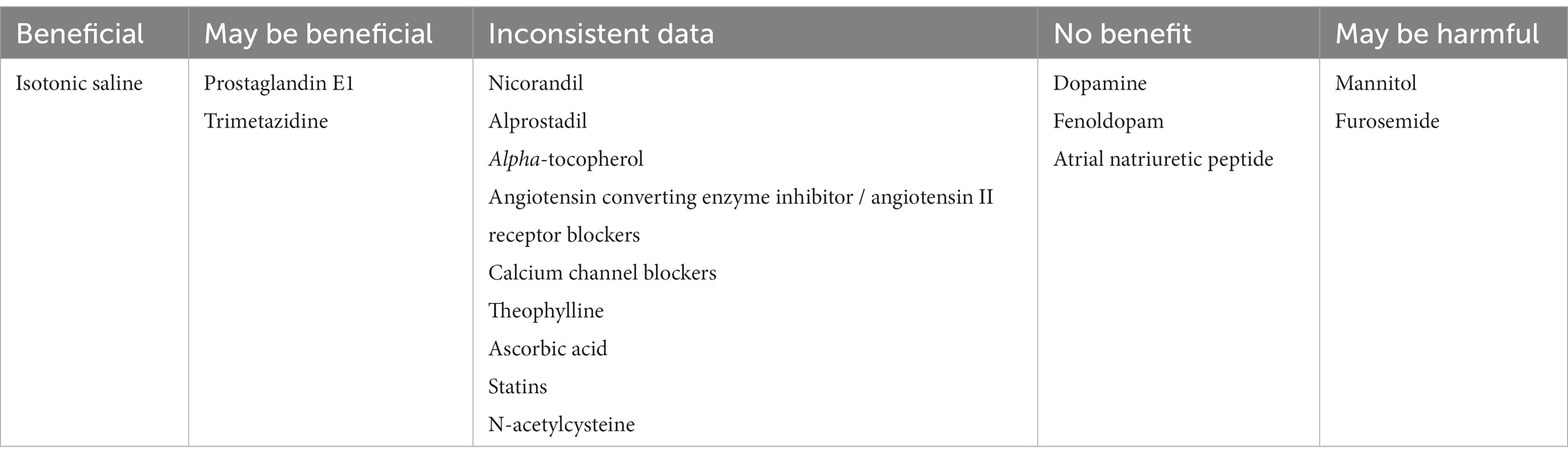
Table 1. Impact of pharmacologic strategies on the occurrence of contrast-associated acute kidney injury.
N-acetylcysteine has been widely used for the prevention of CA-AKI in high-risk patients, mainly because of its favorable side effect profile, low costs, and positive results from some randomized clinical studies. Data are available at present from over 40 clinical trials using N-acetylcysteine for prevention of CA-AKI in high-risk patients, and even though the studies with negative results outnumber those with positive results with a 2:1 ratio, the benefit in the positive studies was significant. The reason for these contradictory results is not yet known but might be related to the use of different types and volumes of contrast agents, different invasive procedures, and different dosages, timings, and routes of N-acetylcysteine administration, or simply to the fact that N-acetylcysteine is not effective in this setting. The first article about the use of N-acetylcysteine to prevent CA-AKI was published by Tepel and co-workers back in 2000. The study included 83 patients undergoing CT with a low-osmolality contrast agent (iopromide). Patients were randomly assigned in two groups: a study group, consisting of 41 patients, who received acetylcysteine (600 mg twice daily) and saline solution (0.45%) intravenously before and after contrast administration, and a control group, consisting of 42 patients, who received placebo and saline. While in the study group only 1 (2%) out of the 41 patients developed CA-AKI, in the control group 9 (21%) out of the 42 patients developed CA-AKI (p = 0.01 between groups) (90). In contrast to those results, another randomized controlled trial of N-acetylcysteine to prevent CA-AKI in patients undergoing cardiac angiography published by Durham and co-workers was unable to prove an additional benefit of acetylcysteine along with fluid administration. Seventy-nine patients were enrolled in that study, and there was no significant difference between groups at baseline in any measured parameter. There was no difference between groups in the mean duration of angiography, mean volume of contrast, or mean total intravenous saline administered, and there was also no significant difference between groups in the incidence of CA-AKI. Contrast-associated acute kidney injury occurred in 9 out of 41 patients (22%) in the control group and in 10 out of 38 patients (26.3%) in the group receiving acetylcysteine, with a non-significant statistical difference between the two groups (91). The main differences between Tepel’s and Durham’s trials were the dosage and timing of acetylcysteine administration, the route of administration being the same in both studies. While in Tepel’s trial the patients received acetylcysteine 600 mg orally on the day of administration of the contrast agent and on the day before, on top of intravenous saline administration (90), in Durham’s study the patients received N-acetylcysteine 1,200 mg orally 1 hour prior to and 3 hours following cardiac catheterization (91). Since the elimination half-time of acetylcysteine is 2.1 h and oral administration leads to peak serum levels in approximately 1 hour, the administration in Durham’s trial appears to be more rational from the standpoint of acetylcysteine pharmacokinetics (91). In 2004, Briguori and co-workers emphasized for the first time the potential importance of acetylcysteine dosage. Their study indicated that double dose (i.e., 1,200 mg, orally, twice daily) of N-acetylcysteine seems to be more effective than the standard dose (i.e., 600 mg, orally, twice daily) in preventing CA-AKI, especially in patients receiving high volumes of intra-arterial low-osmolality contrast agents (92). According to the American Society of Nephrology, until a well-powered definitive study will be performed, the use of acetylcysteine is probably reasonable because the drug is safe, well tolerated by patients, and inexpensive. The dose recommended is the dose used by Tepel (i.e., 600 mg twice daily, on the day before and the day of exposure to contrast) (93). An update about CA-AKI from the e-journal of the European Society of Cardiology Council for Cardiology Practice states that the use of acetylcysteine is not contraindicated but does not advocate for its use either (57).
A meta-analysis of 124 trials about preventive strategies for CA-AKI in patients undergoing percutaneous coronary procedures which included N-acetylcysteine, statins, saline, natriuretic peptides, peripheral ischemic preconditioning, ascorbic acid, dopaminergic agents, N-acetylcysteine + sodium bicarbonate, and sodium bicarbonate alone, was published in May 2017. From all the evaluated measures, statin administration was associated with marked and consistent reduction in CA-AKI compared with saline, while the other nine preventive strategies failed to demonstrate any reduction in the incidence of CA-AKI (78). Nicorandil, alprostadil, and alpha-tocopherol were also associated with a significant reduction of CA-AKI according to some authors, while others failed to demonstrate the same beneficial effects (94–96). Association of trimetazidine 35 mg twice daily with saline administration 12 h before and after coronary angiography was also shown to reduce the incidence of CA-AKI in some studies (97).
5.3 Avoiding potentially nephrotoxic agents
The American College of Radiology Guideline recommended until 2013 temporary discontinuation of metformin in patients undergoing procedures with intravenous administration of contrast media. The American College of Radiology Guideline from 2016 separates these patients into two groups: the group with eGFR ≥30 mL/min/1.73m2, in which there is no need to discontinue metformin either prior to or following the administration of contrast media, and the group with eGFR <30 mL/min/1.73m2, in which metformin should be temporarily discontinued at the time of or prior to the procedure, withheld for 48 h subsequent to the procedure, and reinstituted only after renal function has been re-evaluated and found to be normal (98).
According to a meta-analysis performed by Wang et al., (99) the use of renin-angiotensin-aldosterone system blockers, including angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers, could be associated with an increased risk of developing CA-AKI. However, larger clinical trials, with more strict inclusion criteria, are needed in order to clarify this hypothesis. Other pharmacological agents, such as certain antibiotics, anticonvulsant, antineoplastic, nonsteroidal anti-inflammatory, hypouricemic, and immunosuppressive agents can also cause acute tubular necrosis, leading to nephrotoxicity and renal impairment, and should thus be avoided in these patients (100).
6 Gaps in knowledge and future research
Despite the accumulating data regarding CA-AKI, there are still many unknown aspects related to this condition, particularly in patients with vascular disease, in whom chronic ischemia and inflammation (101) could further contribute to alterations in kidney function. Future studies will have to provide new information regarding the optimal methods of prevention and treatment of this condition following intra-arterial administration of contrast media, which is more and more often encountered in clinical practice. Studies will also have to clarify the long-term impact of CA-AKI on kidney function, to avoid further complications (102). Prophylactic strategies are insufficiently effective, and this is largely due to the fact that the pathophysiology of CA-AKI is not yet sufficiently understood. Further studies will thus have to elucidate CA-AKI pathophysiology and to identify the optimal prophylactic strategies.
The definition of CA-AKI is currently based only on SCr values, but there is no unanimously accepted definition for CA-AKI, and SCr might be too coarse to identify subtle, but potentially relevant changes in renal function following administration of contrast media. Finer markers, such as neutrophil gelatinase-associated lipocalin (NGAL), cystatin-C, interleukin-18, or beta-2 microglobulin may be needed to allow earlier and more accurate detection of CA-AKI (103).
Although CA-AKI is considered largely regressive, this conclusion is based only on creatinine monitoring, which is, as mentioned above, a very rough marker. The pathophysiology of CA-AKI, which involves tubular necrosis, suggests that it is very unlikely that contrast agents will have absolutely no impact on the kidney in the long term, at least in certain high-risk patients. Studies using more sensitive markers will have to evaluate this aspect. If a degree of renal damage, even subclinical, persists, this could be relevant if the patient is subsequently exposed to nephrotoxic agents or repeated administration of contrast media. Indeed, in a recent study, we showed, using repeated NGAL evaluation, that acute renal injury was much more common than reflected by SCr, affecting almost 18% of patients undergoing angioplasty procedures. Moreover, our data indicated that unlike CA-AKI, which was regressive at the 1-month follow-up, subclinical kidney injury was still present after 1 month in more than half of patients in whom the kidneys were initially affected by the contrast media (104).
7 Conclusion
Contrast-associated acute kidney injury is relatively frequent after intra-arterial administration of contrast agents and has a clinical impact in the short, medium, and long term. Despite the numerous studies carried out, there is still no consensus regarding the predictors and the most effective prophylactic measures for CA-AKI. Overall, data from prospective clinical trials indicate that most prophylactic measures provide negligible impact on CA-AKI. The only exceptions are periprocedural isotonic saline administration and usage of a small volume of contrast media, which have proven their effectiveness in CA-AKI prophylaxis in several large clinical trials. Further studies will have to confirm or deny the protective role of other agents, such as N-acetylcysteine, nicorandil, alprostadil, statins, ascorbic acid, trimetazidine, and others. For intravenous administration of iodinated contrast media, reviewing patients’ medications, optimizing cardiac output, and reducing the doses of contrast agent are efficient means to prevent post-procedural increase in plasma creatinine.
Author contributions
CS and RP drafted the manuscript. AS supervised the work. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
CA-AKI, contrast-associated acute kidney injury; CT, computed tomography; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; NGAL, neutrophil gelatinase-associated lipocalin; PCI, percutaneous coronary interventions; SCr, serum creatinine.
References
1. Vandenberghe, W, and Hoste, E. Contrast-associated acute kidney injury: does it really exist, and if so, what to do about it? F1000 Faculty Rev. (2019) 8:753. doi: 10.12688/f1000research.16347.1
2. McDonald, JS, McDonald, RJ, Carter, RE, Katzberg, RW, Kallmes, DF, and Williamson, EE. Risk of intravenous contrast material-mediated acute kidney injury: a propensity score-matched study stratified by baseline-estimated glomerular filtration rate. Radiology. (2014) 271:65–73. doi: 10.1148/radiol.13130775
3. Bakris, GL, Lass, N, Gaber, AO, Jones, JD, and Burnett, JC Jr. Radiocontrast medium-induced declines in renal function: a role for oxygen free radicals. Am J Phys. (1990) 258:F115–20. doi: 10.1152/ajprenal.1990.258.1.F115
4. Weisbord, SD, and du Cheryon, D. Contrast-associated acute kidney injury is a myth: no. Intensive Care Med. (2018) 44:107–9. doi: 10.1007/s00134-017-5015-6
5. Rudnick, MR, Leonberg-Yoo, AK, Litt, HI, Cohen, RM, Hilton, S, and Reese, PP. The controversy of contrast-induced nephropathy with intravenous contrast: what is the risk? Am J Kidney Dis. (2020) 75:105–13. doi: 10.1053/j.ajkd.2019.05.022
6. Zhang, F, Lu, Z, and Wang, F. Advances in the pathogenesis and prevention of contrast-induced nephropathy. Life Sci. (2020) 259:118379. doi: 10.1016/j.lfs.2020.118379
7. Liss, P, Nygren, A, Revsbech, NP, and Ulfendahl, HR. Measurements of oxygen tension in the rat kidney after contrast media using an oxygen microelectrode with a guard cathode. Adv Exp Med Biol. (1997) 411:569–76. doi: 10.1007/978-1-4615-5865-1_70
8. Aspelin, P, Aubry, P, Fransson, SG, Strasser, R, Willenbrock, R, and Berg, KJ. Nephrotoxicity in high-risk patients study of Iso-Osmolar and low-Osmolar non-ionic contrast media study investigators. Nephrotoxic effects in high-risk patients undergoing angiography. N Engl J Med. (2003) 348:491–9. doi: 10.1056/NEJMoa021833
9. Nijssen, EC, Nelemans, PJ, Rennenberg, RJ, van Ommen, V, and Wildberger, JE. Prophylactic intravenous hydration to protect renal function from intravascular iodinated contrast material (AMACING): long-term results of a prospective, randomised, controlled trial. EClinicalMedicine. (2018) 4-5:109–16. doi: 10.1016/j.eclinm.2018.10.007
10. Au, TH, Bruckner, A, Mohiuddin, SM, and Hilleman, DE. The prevention of contrast-induced nephropathy. Ann Pharmacother. (2014) 48:1332–42. doi: 10.1177/1060028014541996
11. Cardoso, PP. Contrast-induced nephropathy: can we better predict and prevent it? Rev Port Cardiol (Engl Ed). (2021) 40:499–500. doi: 10.1016/j.repce.2021.07.019
12. Mohebi, R, Karimi Galougahi, K, Garcia, JJ, Horst, J, Ben-Yehuda, O, Radhakrishnan, J, et al. Long-term clinical impact of contrast-associated acute kidney injury following PCI: an ADAPT-DES substudy. JACC Cardiovasc Interv. (2022) 15:753–66. doi: 10.1016/j.jcin.2021.11.026
13. Schönenberger, E, Martus, P, Bosserdt, M, Zimmermann, E, Tauber, R, Laule, M, et al. Kidney injury after intravenous versus intra-arterial contrast agent in patients suspected of having coronary artery disease: a randomized trial. Radiology. (2019) 292:664–72. doi: 10.1148/radiol.2019182220
14. Caixeta, A, Nikolsky, E, and Mehran, R. Contrast-induced nephropathy: prevention and management of high-risk patients. Indian Heart J. (2008) 60:524–31.
15. Thomsen, HS. Guidelines for contrast media from the European Society of Urogenital Radiology. AJR Am J Roentgenol. (2003) 181:1463–71. doi: 10.2214/ajr.181.6.1811463
16. Slicker, K, Lane, WG, Oyetayo, OO, Copeland, LA, Stock, EM, Michel, JB, et al. Daily cardiac catheterization procedural volume and complications at an academic medical center. Cardiovasc Diagn Ther. (2016) 6:446–52. doi: 10.21037/cdt.2016.05.02
17. Jennings, S, Bennett, K, Shelley, E, Kearney, P, Daly, K, and Fennell, W. Trends in percutaneous coronary intervention and angiography in Ireland, 2004-2011: implications for Ireland and Europe. Int J Cardiol Heart Vessel. (2014) 4:35–9. doi: 10.1016/j.ijchv.2014.08.001
18. Manske, CL, Sprafka, JM, Strony, JT, and Wang, Y. Contrast nephropathy in azotemic diabetic patients undergoing coronary angiography. Am J Med. (1990) 89:615–20. doi: 10.1016/0002-9343(90)90180-l
19. Morcos, SK, Thomsen, HS, and Webb, JA. Contrast-media-induced nephrotoxicity: a consensus report. Contrast media safety committee, European Society of Urogenital Radiology (ESUR). Eur Radiol. (1999) 9:1602–13. doi: 10.1007/s003300050894
20. Rudnick, MR, Goldfarb, S, Wexler, L, Ludbrook, PA, Murphy, MJ, Halpern, EF, et al. Nephrotoxicity of ionic and nonionic contrast media in 1196 patients: a randomized trial. Iohexol Cooperative Study Kidney Int. (1995) 47:254–61. doi: 10.1038/ki.1995.32
21. Rundback, JH, Nahl, D, and Yoo, V. Contrast-induced nephropathy. J Vasc Surg. (2011) 54:575–9. doi: 10.1016/j.jvs.2011.04.047
22. Serrador Frutos, AM, Jiménez-Quevedo, P, Pérez de Prado, A, and Pan Álvarez-Ossorio, M. Spanish cardiac catheterization and coronary intervention registry. 26th official report of the Spanish Society of Cardiology Working Group on cardiac catheterization and interventional cardiology (1990-2016). Rev Esp Cardiol (Engl Ed). (2017) 70:1110–20. doi: 10.1016/j.rec.2017.10.017
23. Wagener, M, Boeddinghaus, J, Gaemperli, O, Räber, L, Nietlispach, F, Meier, P, et al. Trends in coronary and structural heart interventions in Switzerland over the last 16 years and impact of COVID-19: insights from the National Swiss PCI survey. J Clin Med. (2022) 11:7459. doi: 10.3390/jcm11247459
24. Wu, MY, Lo, WC, Wu, YC, Lin, TC, Lin, CH, Wu, MS, et al. The incidence of contrast-induced nephropathy and the need of Dialysis in patients receiving angiography: a systematic review and Meta-analysis. Front Med (Lausanne). (2022) 9:862534. doi: 10.3389/fmed.2022.862534
25. Chalikias, G, Drosos, I, and Tziakas, DN. Contrast-induced acute kidney injury: an update. Cardiovasc Drugs Ther. (2016) 30:215–28. doi: 10.1007/s10557-015-6635-0
26. Toms, AP, Cash, CJ, Linton, SJ, and Dixon, AK. Requests for body computed tomography: increasing workload, increasing indications and increasing age. Eur Radiol. (2001) 11:2633–7. doi: 10.1007/s003300101052
27. Murphy, SW, Barrett, BJ, and Parfrey, PS. Contrast nephropathy. J Am Soc Nephrol. (2000) 11:177–82. doi: 10.1681/ASN.V111177
28. Fishbane, S, Durham, JH, Marzo, K, and Rudnick, M. N-acetylcysteine in the prevention of radiocontrast-induced nephropathy. J Am Soc Nephrol. (2004) 15:251–60. doi: 10.1097/01.asn.0000107562.68920.92
29. Gleeson, TG, and Bulugahapitiya, S. Contrast-induced nephropathy. AJR Am J Roentgenol. (2004) 183:1673–89. doi: 10.2214/ajr.183.6.01831673
30. Maeder, M, Klein, M, Fehr, T, and Rickli, H. Contrast nephropathy: review focusing on prevention. J Am Coll Cardiol. (2004) 44:1763–71. doi: 10.1016/j.jacc.2004.06.075
31. Goldenberg, I, and Matetzky, S. Nephropathy induced by contrast media: pathogenesis, risk factors and preventive strategies. CMAJ. (2005) 172:1461–71. doi: 10.1503/cmaj.1040847
32. Aksoy, F, Aydın Baş, H, Bağcı, A, and Basri, SH. Predictive value of oxidant and antioxidant status for contrast-induced nephropathy after percutaneous coronary intervention for ST-segment elevation myocardial infarction. Rev Port Cardiol (Engl Ed). (2021) 40:489–97. doi: 10.1016/j.repce.2021.07.018
33. Ribeiro, AL, Sousa, FB, Juchem, BC, Zimerman, A, Bernardi, G, Vivan, MA, et al. Incidence of contrast-associated acute kidney injury: a prospective cohort. J Bras Nefrol. (2023) 25:S0101–S28002023005031501. doi: 10.1590/2175-8239-jbn-2023-0019en
34. Eng, J, Wilson, RF, Subramaniam, RM, Zhang, A, Suarez-Cuervo, C, Turban, S, et al. Comparative effect of contrast media type on the incidence of contrast-induced nephropathy: a systematic review and Meta-analysis. Ann Intern Med. (2016) 164:417–24. doi: 10.7326/M15-1402
35. McCullough, PA, Choi, JP, Feghali, GA, Schussler, JM, Stoler, RM, Vallabahn, RC, et al. Contrast-induced acute kidney injury. J Am Coll Cardiol. (2016) 68:1465–73. doi: 10.1016/j.jacc.2016.05.099
36. Persson, PB, Hansell, P, and Liss, P. Pathophysiology of contrast medium-induced nephropathy. Kidney Int. (2005) 68:14–22. doi: 10.1111/j.1523-1755.2005.00377.x
37. Romano, G, Briguori, C, Quintavalle, C, Zanca, C, Rivera, NV, Colombo, A, et al. Contrast agents and renal cell apoptosis. Eur Heart J. (2008) 29:2569–76. doi: 10.1093/eurheartj/ehn197
38. Haller, C, and Hizoh, I. The cytotoxicity of iodinated radiocontrast agents on renal cells in vitro. Investig Radiol. (2004) 39:149–54. doi: 10.1097/01.rli.0000113776.87762.49
39. Sendeski, MM. Pathophysiology of renal tissue damage by iodinated contrast media. Clin Exp Pharmacol Physiol. (2011) 38:292–9. doi: 10.1111/j.1440-1681.2011.05503.x
40. Ward, DB, Brown, KC, and Valentovic, MA. Radiocontrast agent diatrizoic acid induces mitophagy and oxidative stress via calcium dysregulation. Int J Mol Sci. (2019) 20:4074. doi: 10.3390/ijms20174074
41. Chevalier, RL. The proximal tubule is the primary target of injury and progression of kidney disease: role of the glomerulotubular junction. Am J Physiol Renal Physiol. (2016) 311:F145–61. doi: 10.1152/ajprenal.00164.2016
42. Cheng, AS, and Li, X. The potential biotherapeutic targets of contrast-induced acute kidney injury. Int J Mol Sci. (2023) 24:8254. doi: 10.3390/ijms24098254
43. Vlachopanos, G, Schizas, D, Hasemaki, N, and Georgalis, A. Pathophysiology of contrast-induced acute kidney injury (CIAKI). Curr Pharm Des. (2019) 25:4642–7. doi: 10.2174/1381612825666191210152944
44. Bansal, S, and Patel, RN. Pathophysiology of contrast-induced acute kidney injury. Interv Cardiol Clin. (2020) 9:293–8. doi: 10.1016/j.iccl.2020.03.001
45. Sendeski, MM, Persson, AB, Liu, ZZ, Busch, JF, Weikert, S, Persson, PB, et al. Iodinated contrast media cause endothelial damage leading to vasoconstriction of human and rat vasa recta. Am J Physiol Renal Physiol. (2012) 303:F1592–8. doi: 10.1152/ajprenal.00471.2012
46. Basile, DP, Anderson, MD, and Sutton, TA. Pathophysiology of acute kidney injury. Compr Physiol. (2012) 2:1303–53. doi: 10.1002/cphy.c110041
47. Liu, ZZ, Viegas, VU, Perlewitz, A, Lai, EY, Persson, PB, Patzak, A, et al. Iodinated contrast media differentially affect afferent and efferent arteriolar tone and reactivity in mice: a possible explanation for reduced glomerular filtration rate. Radiology. (2012) 265:762–71. doi: 10.1148/radiol.12120044
48. Sies, H, Berndt, C, and Jones, DP. Oxidative stress. Annu Rev Biochem. (2017) 86:715–48. doi: 10.1146/annurev-biochem-061516-045037
49. Briguori, C, Donnarumma, E, Quintavalle, C, Fiore, D, and Condorelli, G. Contrast-induced acute kidney injury: potential new strategies. Curr Opin Nephrol Hypertens. (2015) 24:145–53. doi: 10.1097/MNH.0000000000000106
50. McCullough, PA, Wolyn, R, Rocher, LL, Levin, RN, and O'Neill, WW. Acute renal failure after coronary intervention: incidence, risk factors, and relationship to mortality. Am J Med. (1997) 103:368–75. doi: 10.1016/s0002-9343(97)00150-2
51. Barrett, BJ. Contrast-induced nephropathy: we need all the data to discern the truth. Am J Kidney Dis. (2009) 54:587–9. doi: 10.1053/j.ajkd.2009.06.011
52. Yao, ZF, Shen, H, Tang, MN, Yan, Y, and Ge, JB. A novel risk assessment model of contrast-induced nephropathy after percutaneous coronary intervention in patients with diabetes. Basic Clin Pharmacol Toxicol. (2021) 128:305–14. doi: 10.1111/bcpt.13501
53. Neumann, FJ, Sousa-Uva, M, Ahlsson, A, Alfonso, F, Banning, AP, Benedetto, U, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. (2019) 40:87–165. doi: 10.1093/eurheartj/ehy394
54. Mehran, R, Aymong, ED, Nikolsky, E, Lasic, Z, Iakovou, I, Fahy, M, et al. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol. (2004) 44:1393–9. doi: 10.1016/j.jacc.2004.06.068
55. Chen, L, Zhang, S, Luo, M, He, C, You, Z, Zhang, L, et al. Assessing the predictive value of different nutritional indexes for contrast-associated acute kidney injury in patients undergoing percutaneous coronary intervention. Circ J. (2023). doi: 10.1253/circj.CJ-23-0479
56. Zeng, JL, Xiang, YF, Zhang, LW, Chen, LC, Chen, JH, Liang, WJ, et al. Predictive value of systemic inflammation score for contrast-associated acute kidney injury and adverse outcomes among patients undergoing elective percutaneous coronary intervention. J Inflamm Res. (2023) 16:2845–54. doi: 10.2147/JIR.S419831
57. Moreno Arribas, J, and Alegria-Barrero, E. How to prevent contrast-induced nephropathy in patients undergoing invasive cardiac procedures. Cardiol Res Pract. (2009) 7:25.
58. Schwab, SJ, Hlatky, MA, Pieper, KS, Davidson, CJ, Morris, KG, Skelton, TN, et al. Contrast nephrotoxicity: a randomized controlled trial of a nonionic and an ionic radiographic contrast agent. N Engl J Med. (1989) 320:149–53. doi: 10.1056/NEJM198901193200304
59. Paolucci, L, De Micco, F, Bezzeccheri, A, Scarpelli, M, Esposito, G, Airoldi, F, et al. Contrast media volume reduction with the DyeVertTM system to prevent acute kidney injury in stable patients undergoing coronary procedures. Catheter Cardiovasc Interv. (2023) 102:655–62. doi: 10.1002/ccd.30809
60. Levy, EM, Viscoli, CM, and Horwitz, RI. The effect of acute renal failure on mortality. A cohort analysis. JAMA. (1996) 275:1489–94. doi: 10.1001/jama.275.19.1489
61. Kusirisin, P, Chattipakorn, SC, and Chattipakorn, N. Contrast-induced nephropathy and oxidative stress: mechanistic insights for better interventional approaches. J Transl Med. (2020) 18:400. doi: 10.1186/s12967-020-02574-8
62. Güzel, T, Aktan, A, Demir, M, Özbek, M, and Aslan, B. Relationship between contrast-induced nephropathy and long-term mortality after percutaneous coronary intervention in patients with chronic coronary total occlusion. Rev Assoc Med Bras (1992). (2022) 68:1078–83. doi: 10.1590/1806-9282.20220283
63. Aksu, U, Gulcu, O, Aksakal, E, Kalkan, K, and Topcu, S. Long-term mortality and contrast-induced nephropathy. Angiology. (2019) 70:783. doi: 10.1177/0003319718823628
64. Adolph, E, Holdt-Lehmann, B, Chatterjee, T, Paschka, S, Prott, A, Schneider, H, et al. Renal insufficiency following radiocontrast exposure trial (REINFORCE): a randomized comparison of sodium bicarbonate versus sodium chloride hydration for the prevention of contrast-induced nephropathy. Coron Artery Dis. (2008) 19:413–9. doi: 10.1097/MCA.0b013e3283021ac6
65. Bartholomew, BA, Harjai, KJ, Dukkipati, S, Boura, JA, Yerkey, MW, Glazier, S, et al. Impact of nephropathy after percutaneous coronary intervention and a method for risk stratification. Am J Cardiol. (2004) 93:1515–9. doi: 10.1016/j.amjcard.2004.03.008
66. Subramanian, S, Tumlin, J, Bapat, B, and Zyczynski, T. Economic burden of contrast-induced nephropathy: implications for prevention strategies. J Med Econ. (2007) 10:119–34. doi: 10.3111/200710119134
67. Tsai, TT, Patel, UD, Chang, TI, Kennedy, KF, Masoudi, FA, Matheny, ME, et al. Contemporary incidence, predictors, and outcomes of acute kidney injury in patients undergoing percutaneous coronary interventions: insights from the NCDR Cath-PCI registry. J Am Coll Cardiol Interv. (2014) 7:1–9. doi: 10.1016/j.jcin.2013.06.016
68. Rihal, CS, Textor, SC, Grill, DE, Berger, PB, Ting, HH, Best, PJ, et al. Incidence and prognostic importance of acute renal failure after percutaneous coronary intervention. Circulation. (2002) 105:2259–64. doi: 10.1161/01.cir.0000016043.87291.33
69. Weisbord, SD, Chen, H, Stone, RA, Kip, KE, Fine, MJ, Saul, MI, et al. Associations of increases in serum creatinine with mortality and length of hospital stay after coronary angiography. J Am Soc Nephrol. (2006) 17:2871–7. doi: 10.1681/ASN.2006030301
70. Murugan, R, Boudreaux-Kelly, MY, Kellum, JA, Palevsky, PM, and Weisbord, S. Contrast-associated acute kidney injury and cardiovascular events: a secondary analysis of the PRESERVE cohort. Clin Kidney J. (2023) 16:2626–38. doi: 10.1093/ckj/sfad214
71. Sun, G, Chen, P, Wang, K, Li, H, Chen, S, Liu, J, et al. Contrast-induced nephropathy and long-term mortality after percutaneous coronary intervention in patients with acute myocardial infarction. Angiology. (2019) 70:621–6. doi: 10.1177/0003319718803677
72. Hossain, MA, Costanzo, E, Cosentino, J, Patel, C, Qaisar, H, Singh, V, et al. Contrast-induced nephropathy: pathophysiology, risk factors, and prevention. Saudi J Kidney Dis Transpl. (2018) 29:1–9. doi: 10.4103/1319-2442.225199
73. Trivedi, HS, Moore, H, Nasr, S, Aggarwal, K, Agrawal, A, Goel, P, et al. A randomized prospective trial to assess the role of saline hydration on the development of contrast nephrotoxicity. Nephron Clin Pract. (2003) 93:C29–34. doi: 10.1159/000066641
74. Mueller, C, Buerkle, G, Buettner, HJ, Petersen, J, Perruchoud, AP, Eriksson, U, et al. Prevention of contrast media-associated nephropathy: randomized comparison of 2 hydration regimens in 1620 patients undergoing coronary angioplasty. Arch Intern Med. (2002) 162:329–36. doi: 10.1001/archinte.162.3.329
75. Brar, SS, Aharonian, V, Mansukhani, P, Moore, N, Shen, AY, Jorgensen, M, et al. Haemodynamic-guided fluid administration for the prevention of contrast-induced acute kidney injury: the POSEIDON randomised controlled trial. Lancet. (2014) 383:1814–23. doi: 10.1016/S0140-6736(14)60689-9
76. Sudarsky, D, and Nikolsky, E. Contrast-induced nephropathy in interventional cardiology. Int J Nephrol Renovasc Dis. (2011) 4:85–99. doi: 10.2147/IJNRD.S21393
77. Nijssen, EC, Rennenberg, RJ, Nelemans, PJ, Essers, BA, Janssen, MM, Vermeeren, MA, et al. Prophylactic hydration to protect renal function from intravascular iodinated contrast material in patients at high risk of contrast-induced nephropathy (AMACING): a prospective, randomised, phase 3, controlled, open-label, non-inferiority trial. Lancet. (2017) 389:1312–22. doi: 10.1016/S0140-6736(17)30057-0
78. Giacoppo, D, Gargiulo, G, Buccheri, S, Aruta, P, Byrne, RA, Cassese, S, et al. Preventive strategies for contrast-induced acute kidney injury in patients undergoing percutaneous coronary procedures: evidence from a hierarchical Bayesian network Meta-analysis of 124 trials and 28 240 patients. Circ Cardiovasc Interv. (2017) 10:e004383. doi: 10.1161/CIRCINTERVENTIONS.116.004383
79. Taylor, AJ, Hotchkiss, D, Morse, RW, and McCabe, J. PREPARED: preparation for angiography in renal dysfunction: a randomized trial of inpatient vs outpatient hydration protocols for cardiac catheterization in mild-to-moderate renal dysfunction. Chest. (1998) 114:1570–4. doi: 10.1378/chest.114.6.1570
80. Torigoe, K, Tamura, A, Watanabe, T, and Kadota, J. 20-hour preprocedural hydration is not superior to 5-hour preprocedural hydration in the prevention of contrast-induced increases in serum creatinine and cystatin C. Int J Cardiol. (2013) 167:2200–3. doi: 10.1016/j.ijcard.2012.05.122
81. Krasuski, RA, Beard, BM, Geoghagan, JD, Thompson, CM, and Guidera, SA. Optimal timing of hydration to erase contrast-associated nephropathy: the OTHER CAN study. J Invasive Cardiol. (2003) 15:699–702.
82. Khwaja, A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. (2012) 120:c179–84. doi: 10.1159/000339789
83. Liu, Y, Tan, N, Huo, Y, Chen, SQ, Liu, J, Wang, Y, et al. Simplified rapid hydration prevents contrast-associated acute kidney injury among CKD patients undergoing coronary angiography. JACC Cardiovasc Interv. (2023) 16:1503–13. doi: 10.1016/j.jcin.2023.03.025
84. Ix, JH, McCulloch, CE, and Chertow, GM. Theophylline for the prevention of radiocontrast nephropathy: a meta-analysis. Nephrol Dial Transplant. (2004) 19:2747–53. doi: 10.1093/ndt/gfh468
85. Bagshaw, SM, and Ghali, WA. Theophylline for prevention of contrast-induced nephropathy: a systematic review and meta-analysis. Arch Intern Med. (2005) 165:1087–93. doi: 10.1001/archinte.165.10.1087
86. Neumayer, HH, Junge, W, Küfner, A, and Wenning, A. Prevention of radiocontrast-media-induced nephrotoxicity by the calcium channel blocker nitrendipine: a prospective randomised clinical trial. Nephrol Dial Transplant. (1989) 4:1030–6.
87. Carraro, M, Mancini, W, Artero, M, Stacul, F, Grotto, M, Cova, M, et al. Dose effect of nitrendipine on urinary enzymes and microproteins following non-ionic radiocontrast administration. Nephrol Dial Transplant. (1996) 11:444–8. doi: 10.1093/oxfordjournals.ndt.a027309
88. Naeem, M, McEnteggart, GE, Murphy, TP, Prince, E, Ahn, S, and Soares, G. Fenoldopam for the prevention of contrast-induced nephropathy (CIN)-do we need more trials? A meta-analysis Clin Imaging. (2015) 39:759–64. doi: 10.1016/j.clinimag.2015.02.003
89. Zhang, JZ, Kang, XJ, Gao, Y, Zheng, YY, Wu, TT, Li, L, et al. Efficacy of alprostadil for preventing of contrast-induced nephropathy: a meta-analysis. Sci Rep. (2017) 7:1045. doi: 10.1038/s41598-017-01160-1
90. Tepel, M, van der Giet, M, Schwarzfeld, C, Laufer, U, Liermann, D, and Zidek, W. Prevention of radiographic-contrast-agent-induced reductions in renal function by acetylcysteine. N Engl J Med. (2000) 343:180–4. doi: 10.1056/NEJM200007203430304
91. Durham, JD, Caputo, C, Dokko, J, Zaharakis, T, Pahlavan, M, Keltz, J, et al. A randomized controlled trial of N-acetylcysteine to prevent contrast nephropathy in cardiac angiography. Kidney Int. (2002) 62:2202–7. doi: 10.1046/j.1523-1755.2002.00673.x
92. Briguori, C, Colombo, A, Violante, A, Balestrieri, P, Manganelli, F, Paolo Elia, P, et al. Standard vs double dose of N-acetylcysteine to prevent contrast agent associated nephrotoxicity. Eur Heart J. (2004) 25:206–11. doi: 10.1016/j.ehj.2003.11.016
93. Fishbane, S. N-acetylcysteine in the prevention of contrast-induced nephropathy. Clin J Am Soc Nephrol. (2008) 3:281–7. doi: 10.2215/CJN.02590607
94. Ma, X, Li, X, Jiao, Z, and Zhang, Y. Nicorandil for the prevention of contrast-induced nephropathy: a meta-analysis of randomized controlled trials. Cardiovasc Ther. (2018) 36:2. doi: 10.1111/1755-5922.12316
95. Xie, J, Jiang, M, Lin, Y, Deng, H, and Li, L. Effect of Alprostadil on the prevention of contrast-induced nephropathy: a Meta-analysis of 36 randomized controlled trials. Angiology. (2019) 70:594–612. doi: 10.1177/0003319719825597
96. Monami, M, Cignarelli, A, Pinto, S, D'Onofrio, L, Milluzzo, A, Miccoli, R, et al. Alpha-tocopherol and contrast-induced nephropathy: a meta-analysis of randomized controlled trials. Int J Vitam Nutr Res. (2021) 91:188–96. doi: 10.1024/0300-9831/a000573
97. Ibrahim, TA, El-Mawardy, RH, El-Serafy, AS, and El-Fekky, EM. Trimetazidine in the prevention of contrast-induced nephropathy in chronic kidney disease. Cardiovasc Revasc Med. (2017) 18:315–9. doi: 10.1016/j.carrev.2017.02.006
98. Shah, AD, McHargue, C, Yee, J, and Rushakoff, RJ. Intravenous contrast in patients with diabetes on metformin: new common sense guidelines. Endocr Pract. (2016) 22:502–5. doi: 10.4158/EP151123.CO
99. Wang, W, Qu, W, Sun, D, and Liu, X. Meta-analysis of effect of renin-angiotensin-aldosterone system blockers on contrast-induced nephropathy. J Renin-Angiotensin-Aldosterone Syst. (2020) 21:147032032091958. doi: 10.1177/1470320320919587
100. Walker, EM Jr, Fazekas-May, MA, and Bowen, WR. Nephrotoxic and ototoxic agents. Clin Lab Med. (1990) 10:323–54. doi: 10.1016/S0272-2712(18)30572-9
101. Serban, RC, Balan, AI, Perian, M, Pintilie, I, Somkereki, C, Huţanu, A, et al. Atrial electrical remodeling induced by chronic ischemia and inflammation in patients with stable coronary artery disease. Chin J Physiol. (2019) 62:11–6. doi: 10.4103/CJP.CJP_2_19
102. Șerban, RC, Șuș, I, Lakatos, EK, Demjen, Z, Ceamburu, A, Fișcă, PC, et al. Chronic kidney disease predicts atrial fibrillation in patients with ST-segment elevation myocardial infarction treated by primary percutaneous coronary intervention. Acta Cardiol. (2019) 74:472–9. doi: 10.1080/00015385.2018.1521558
103. Banda, J, Duarte, R, Dix-Peek, T, Dickens, C, Manga, P, and Naicker, S. Biomarkers for diagnosis and prediction of outcomes in contrast-induced nephropathy. Int J Nephrol. (2020) 2020:8568139–11. doi: 10.1155/2020/8568139
Keywords: contrast-associated acute kidney injury, percutaneous coronary intervention, periprocedural fluid administration, prevention, risk prediction
Citation: Somkereki C, Palfi R and Scridon A (2024) Prevention of contrast-associated acute kidney injury in an era of increasingly complex interventional procedures. Front. Med. 10:1180861. doi: 10.3389/fmed.2023.1180861
Edited by:
Elham Ahmadian, Tabriz University of Medical Sciences, IranReviewed by:
Yiwei Liu, Shanghai Children’s Medical Center, ChinaTakahide Kodama, Toranomon Hospital, Japan
Copyright © 2024 Somkereki, Palfi and Scridon. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alina Scridon, YWxpbmFzY3JpZG9uQGdtYWlsLmNvbQ==
 Cristina Somkereki
Cristina Somkereki Renata Palfi1
Renata Palfi1 Alina Scridon
Alina Scridon