- 1Graduate School, Guizhou University of Traditional Chinese Medicine, Guiyang, China
- 2Department of Rheumatology and Immunology, Second Affiliated Hospital of Guizhou University of Chinese Medicine, Guiyang, China
Introduction: Acute generalized exanthematous pustulosis (AGEP) is a rare condition characterized by superficial pustules following drug ingestion or infection. Currently, there is no clear link between rheumatism and AGEP. It has been described that hydroxychloroquine (HCQ) is a rare cause of acute generalized epidermal necrolysis (AGEP). Presently, there are limited studies on HCQ-induced AGEP. We aimed to explore the clinical features and associated gene expression of AGEP induced after HCQ treatment exposure in rheumatology patients.
Methods: We assessed patients with HCQ-induced AGEP diagnosed at the Second Affiliated Hospital of Guizhou University of Chinese Medicine between January 1, 2017, and May 1, 2022. We also reviewed similar cases reported in specific databases.
Results: The study included five females (mean age, 40.2 years), and the mean time from initiation of HCQ treatment to symptom onset was 12.2 d. All patients received steroids and allergy medications after HCQ discontinuation, and the rash completely resolved within an average of 25.2 d. We performed whole exome sequencing and Sanger validation in our patient sample. CARD14 gene mutations were detected in three patients. Additionally, seven mutation sites were detected.
Discussion: HCQ-induced AGEP may have a longer latency period and regression time than AGEP induced by other drugs. Our patients all experienced CARD14 gene mutations. AGEP often resolves with topical therapy and drug discontinuation, although some cases require systemic steroid therapy. In the future, patients with rheumatism should pay attention to the effectiveness of HCQ during treatment and be aware of the associated skin toxicity.
1. Introduction
A rheumatic disease is an inflammatory disorder that affects the skin, mucous membranes, bone, joints, and surrounding soft tissues including muscles, synovium, bursa, and tendons (1). The most common symptom of acute generalized exanthematous pustulosis is the presence of numerous non-follicular, sterile pustules on top of an edematous background (2). It is usually accompanied by fever and peripheral blood leukocytosis (3). About 90% of AGEP cases are caused by drugs such as antibiotics, antifungals, diltiazem, and antimalarial agents (4, 5). Approximately 20% of patients with AGEP develop systemic involvement; mostly impaired liver, kidney, lung function and, in severe cases, multiple organ failure, diffuse intravascular coagulation, and death (6, 7). Hydroxychloroquine (HCQ) sulfate regulates immune function and can be used clinically as initial treatment for malaria, rheumatoid arthritis (RA), and systemic lupus erythematosus (SLE) (8, 9). With the widespread use of HCQ, an increasing number of adverse events are being reported in clinical practice, among which AGEP is rare. Owing to its low incidence, we have limited information on AGEP. Previous research has demonstrated that some patients with AGEP carry mutations in the IL36RN and CARD14 genes, suggesting that AGEP may have a genetic basis (10). In addition, there is evidence that AGEP is linked to mutations in the CARD14 gene, and the predicted p. (Arg430Trp) variant of heterozygous c.1288C c.1288C > T transition (11). We conducted a literature search and found only a few reported cases of Chinese clusters (12, 13). Therefore, the study aimed to describe the clinical and genetic characteristics of Chinese patients with HCQ-induced AGEP.
2. Materials and methods
We observed five patients of HCQ-induced AGEP at the Second Affiliated Hospital of Guizhou University of Chinese Medicine between January 1, 2017, and May 1, 2022. The patients were diagnosed with HCQ-induced AGEP based on EuroSCAR scores validated by (5); five patients scored 5–12 points, indicating a definite or probable diagnosis of AGEP (5). We collected the following information from the medical records of these patients: demographic characteristics, clinical features, onset date, time of symptom resolution, and laboratory test results. In addition, we used next-generation whole exome sequencing to evaluate the mutated gene, and performed Sanger verification on the mutant gene; IL36RN and CARD14 mutation sites were reported. Our gene sequencing experiments were all entrusted to the Beijing Luhe Huada Gene Technology Co., Ltd., Wuhan, China, and the specific process can be found on the company’s website.1 Furthermore, we reviewed similar cases reported in the Wanfang Data, PubMed, Embase, China National Knowledge Infrastructure (CNKI), Web of Science, and other databases from their inception to May 2022. Search terms included “acute generalized exanthematous pustulosis,” “AGEP,” “HCQ,” “hydroxychloroquine,” and “side effects.” The present study was approved by the Institutional Review Committee of the Second Affiliated Hospital of Guizhou University of Chinese Medicine and conducted in accordance with the Declaration of Helsinki. Throughout the study, all participants provided their informed consent.
3. Results
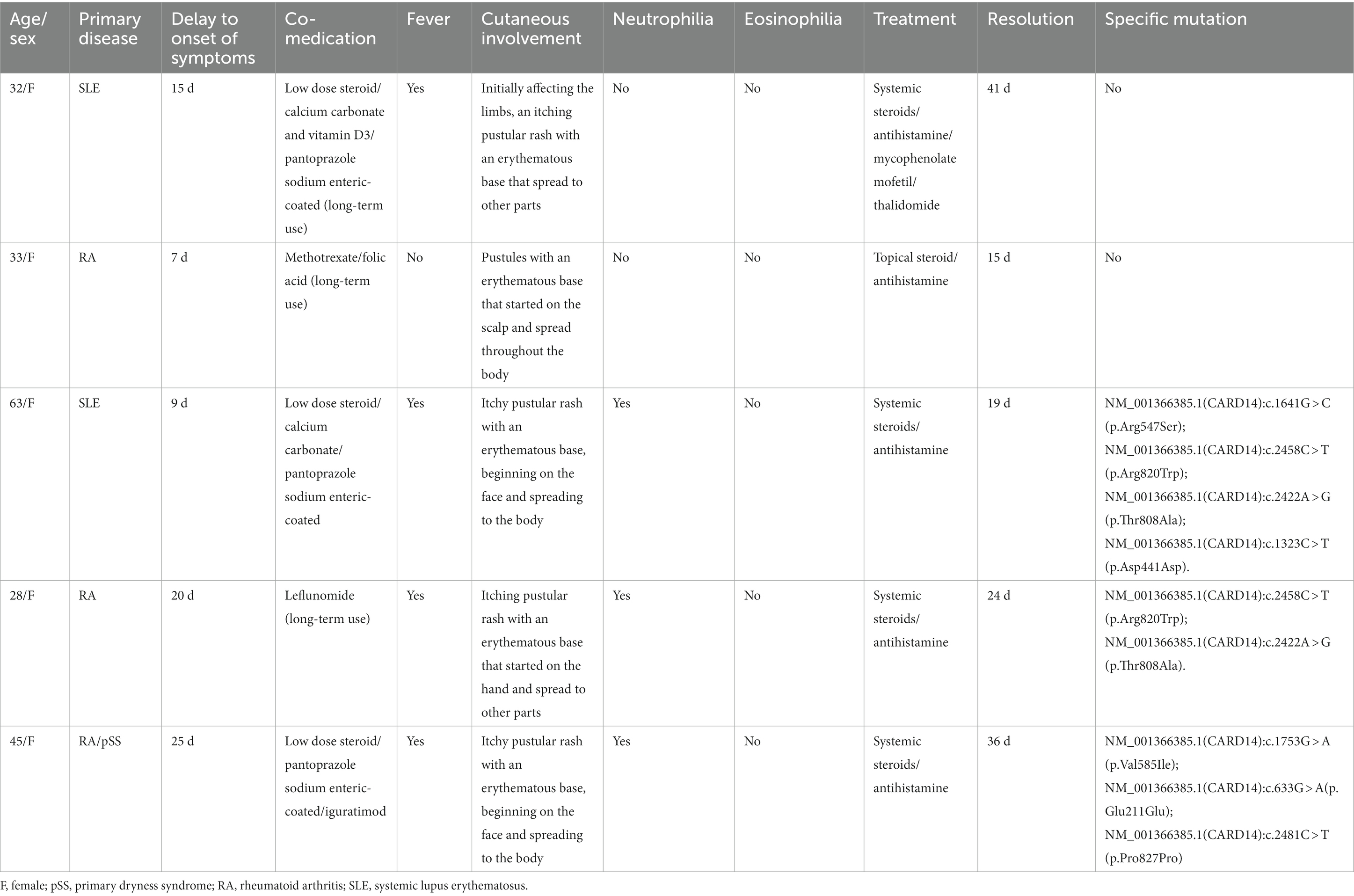
The average age at diagnosis was 40.2 years (range, 28–63 years) for all five participants. Patient history included two cases of RA, one case of RA with Sjogren’s syndrome, and two cases of SLE. None of the five patients had any skin reactions or a personal or family history of psoriasis. The details of each case are presented in Table 1.
Our five cases were accustomed to hospital treatment because of a poor response to the long-term use of disease-modifying antirheumatic drugs (DMARDs), such as methotrexate for rheumatism, and skin allergic reactions caused by adjusting to the use of HCQ. The dosage of HCQ among the five patients was the same: two tablets, administered twice daily at the same time. The average delay in the appearance of a rash in all five cases was 12.2 d after admission (range: 7–25 d). In all patients, the skin eruptions were non-follicular and pinhead-sized, resulting from sudden, acute onset. The rash was initially seen on the cheeks and face in two patients, on a limb joint in one patient, on the scalp in one patient, and on the hands in one patient. In all cases, a rapid spread of the rash throughout the body was observed, with facial involvement (Figures 1A–F). After the rash appeared, all patients immediately stopped HCQ treatment. No patients developed mucosal involvement or lymphatic system disease. Over the course of symptom duration, four patients developed fever and all patients had pruritis.
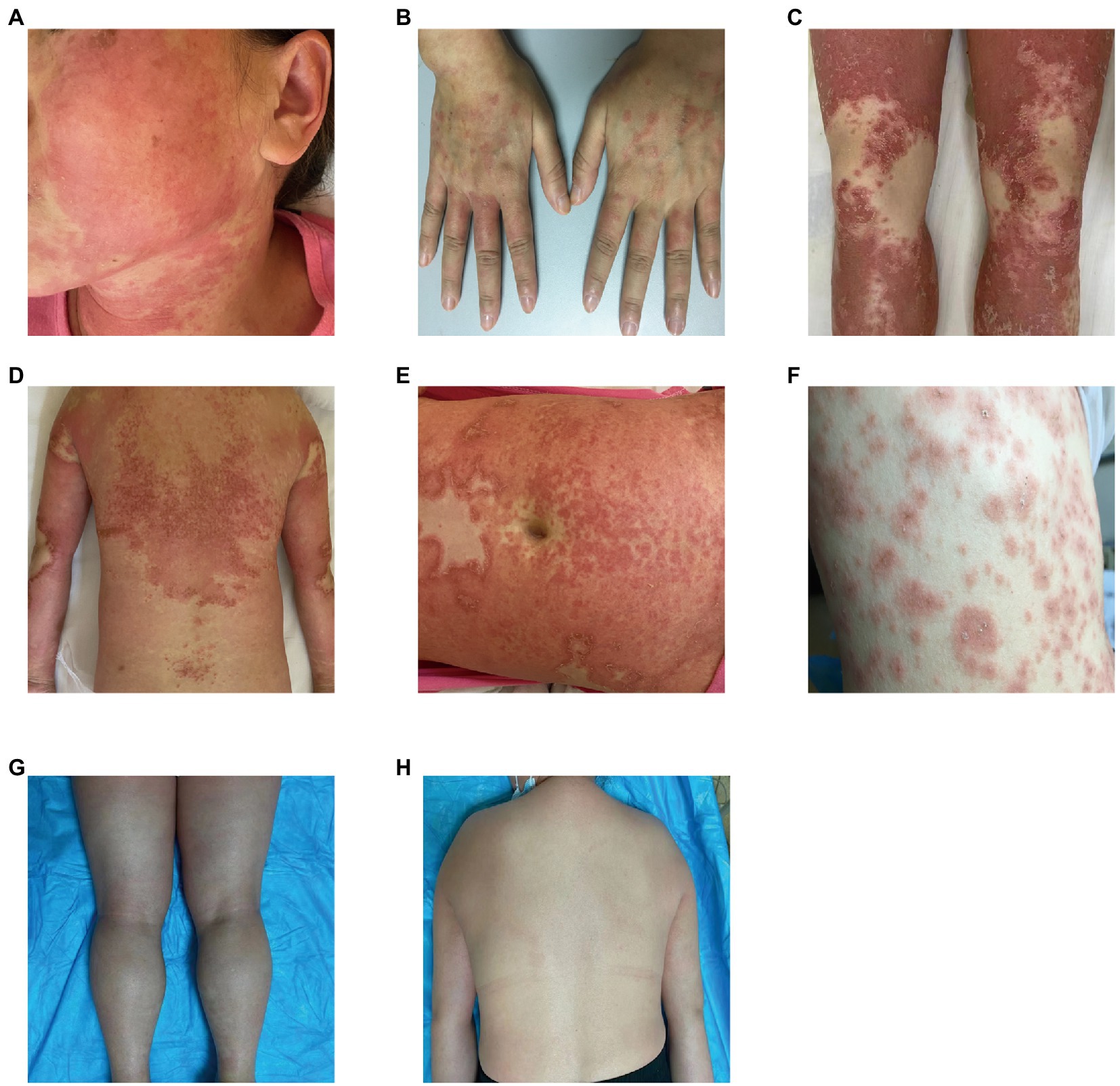
Figure 1. Rash before and after treatment. (A) Rash on the face; (B) rash on the hands; (C) rash on the legs; (D) rash on the back; (E) rash on the abdomen; (F) rash on the chest; (G) improvement in the rash on the legs; and (H) improvement in the rash on the back.
Laboratory results showed that the percentage of neutrophils increased to 81.3% (normal, 40–75%) in three patients, and the percentage of eosinophils decreased to 0.3% (normal, 0.4–8.0%) in two patients. C-reactive protein levels increased to 11.21 mg/L (the norm, 0–10 mg/L) in one patient, and the erythrocyte sedimentation rate increased to 59 mm/L (normal, 0–20 mm/L) in one patient. Several routine laboratory tests were also performed, including renal and hepatic function and serologic tests to determine influenza, hepatitis B, enteroviruses, and mycoplasmas, which were unremarkable. The bacteriological and mycological findings of pustules were negative.
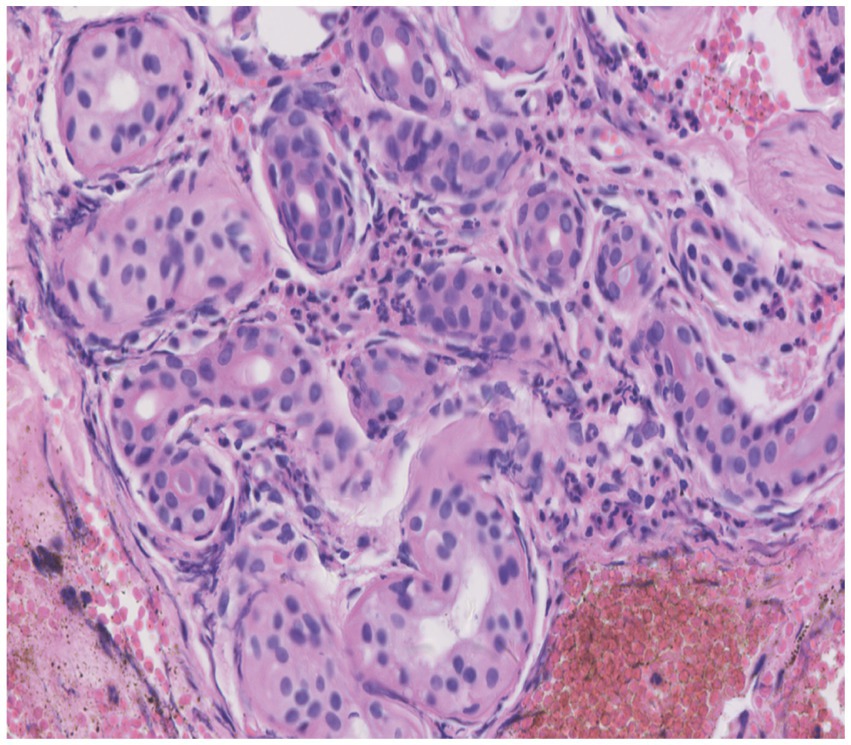
Skin tissue biopsy showed histopathological changes in the skin, where the epidermis showed mild vacuolar degeneration and varying numbers of neutrophil infiltrates just beneath the surface of the deep dermis—primarily the superficial dermis—as well as cavernous pustules under the epidermis; an example of these changes is presented in Figure 2. We performed whole exome sequencing and Sanger validation on all five patients and identified CARD14 gene mutations in three patients. Seven mutation sites were detected: c.1641G > C (p.Arg547Ser) heterozygous mutation, c.2422A > G (p.Thr808Ala) heterozygous/homozygous mutation, c.2458C > T (p.Arg820Trp) heterozygous mutation, c.1323C > T (p.Asp441Asp) heterozygous mutation, c.633G > A (p.Glu211Glu) heterozygous mutation, c.1753G > A (p.Val585Ile) heterozygous mutation, and c.2481C > T (p.Pro827Pro) heterozygous mutation (Figure 3). No mutations were identified in the IL-36RN gene.
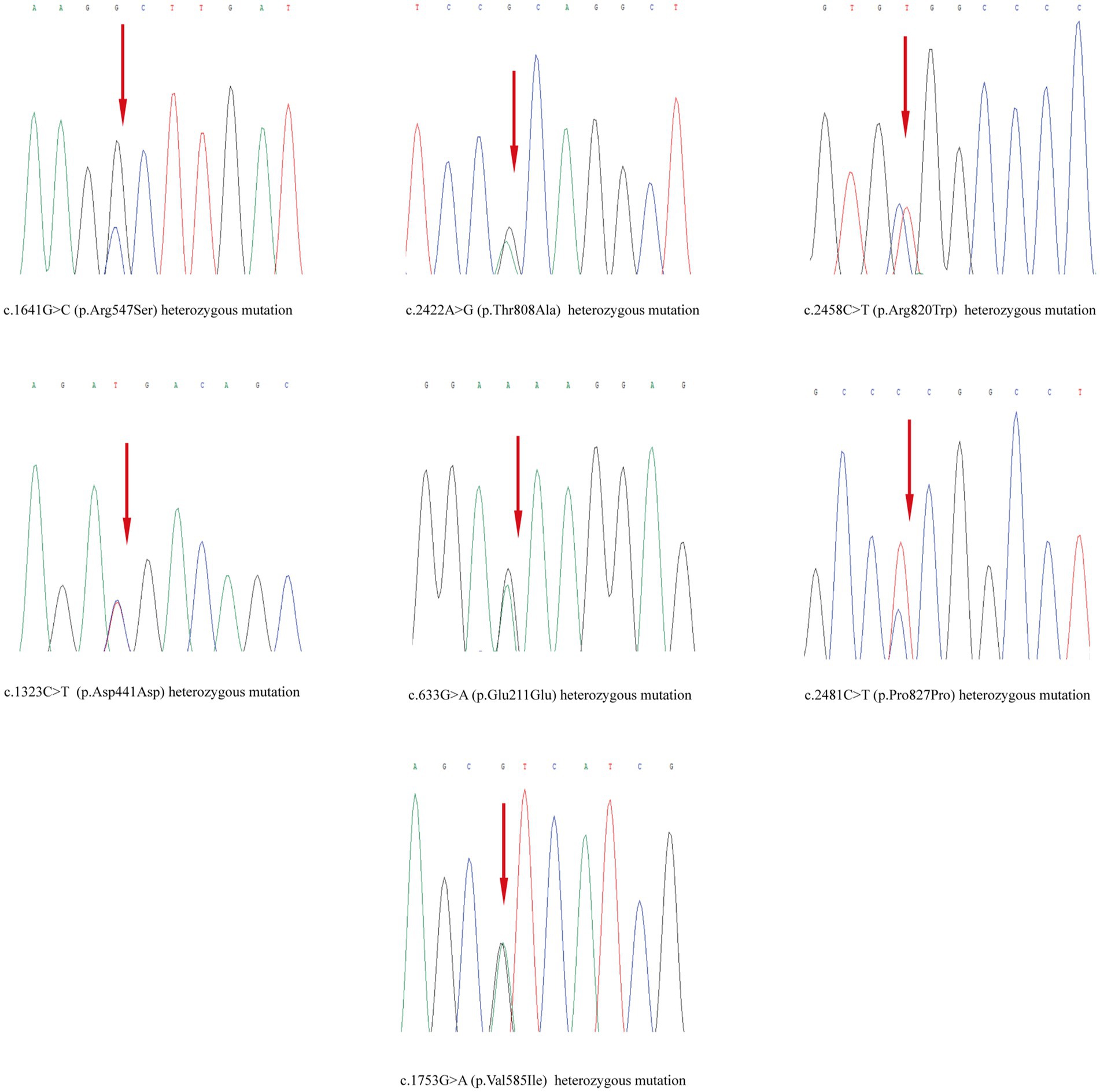
Figure 2. Histopathological examination of a skin biopsy from the left chest showed mild vacuolar degeneration of epidermal basal cells, varying numbers of neutrophil infiltrates in the superficial-deep dermis (mainly in the superficial dermis), and epidermal cavernous pustule formation (hematoxylin and eosin staining; original magnification, ×400).
One patient was treated with topical steroids plus antihistamines, three patients received systemic steroids plus antihistamines, and one patient was treated with steroids plus antihistamines, mycophenolate mofetil, and thalidomide. All patients achieved good results after treatment, the rash completely resolved within 15–45 d after HCQ discontinuation, and diffuse superficial desquamation was observed during treatment. All patients were followed-up for 6 months to 1 year, without rash recurrence (Figures 1G,H).
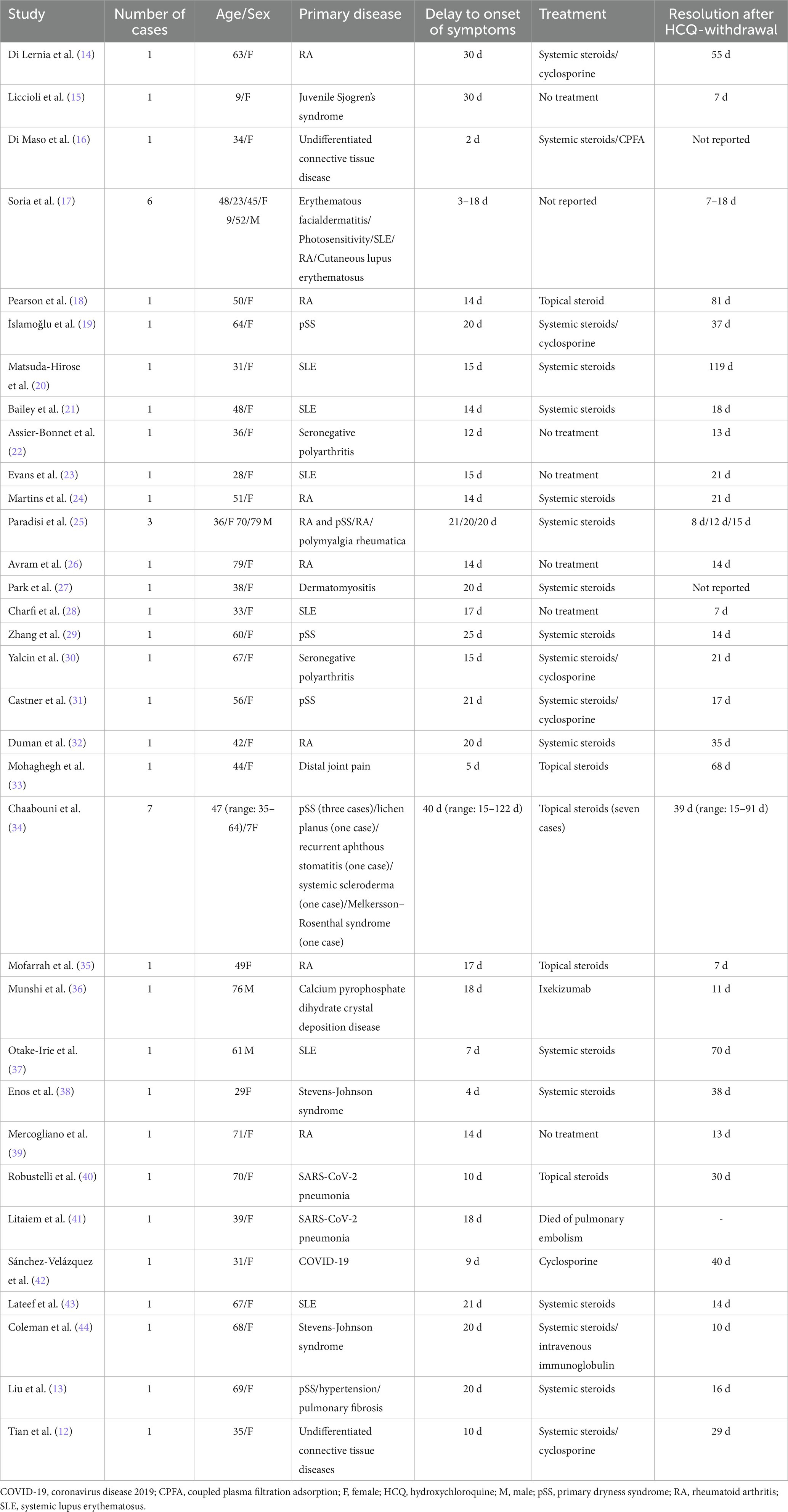
From our literature search strategy, 221 relevant articles were retrieved. After screening, 33 valid articles were obtained, including 46 similar cases. Table 2 shows the clinical features of these cases. The cases we reported were similar to those reported in the literature. These similarities concerned the greater proportion of females, average age of onset and mean delay to diagnosis, treatment with topical or systemic steroids, and complete rash resolution within 7–119 d.
4. Discussion
AGEP is an acute-onset non-follicular aseptic impetigo that typically presents with sudden high fever and a rash characterized by a dense distribution of needle tip to soybean-sized pustules based on diffuse flushing, usually without mucosal and general organ damage (29). The incidence of AGEP is approximately 1–5/1,000,000/year. Approximately 90% of patients are on medication prior to disease onset, mainly in the form of antibiotics, with β-lactam medications being the most common (45). In a small number of patients, AGEP is caused by an infection or other factors (46) including other drugs that rheumatological patients may take to fight biologics-related infections, e.g., doxycycline or new anticoagulants (dabigatran) (47, 48). The specific pathogenesis of AGEP remains incompletely understood (49). HCQ has immunosuppressive, anti-inflammatory, and possibly antiviral properties, and common adverse effects include headache, various types of rashes, pruritus, and gastrointestinal dysfunctions, such as nausea, vomiting, and diarrhea (50). Several dermatological side-effects have been associated with the use of HCQ, including dryness, pigmentation, itching, alopecia, urticaria, measles, contact dermatitis, and worsening psoriasis, of which AGEP is one of the more serious but rare types (51). With the wide application of HCQ in clinical practice, HCQ-induced AGEP should be seriously considered.
The incubation time of AGEP is generally 3–30 d, and the median time of occurrence is 15 d after starting drug treatment. The time from drug exposure to the appearance of a rash depends on the causative drug; primitive mycin and amoxicillin can cause AGEP rash onset 1 d after the first dose, whereas other drugs trigger a reaction only after 1–2 weeks (52). For instance, carbamazepine-induced AGEP may take 5–35 d to manifest symptoms (53); when diltiazem induces AGEP, symptom onset occurs within 1–3 weeks (54). Previous cases of HCQ-induced AGEP ranged from 2 to 30 d between the initiation of HCQ treatment and rash onset (15, 52). Rash onset in the cases we reported ranged from 7 to 25 d, which is similar to results reported in the literature. In addition, studies have found that blood drug levels peak after 5–6 weeks of HCQ trial treatment, which may be the reason for the slow appearance of AGEP symptoms (55). This indicates that HCQ-related AGEP may be associated with its metabolic properties.
In the cases we studied, there were no mutations in the IL-36RN gene; however, we identified novel mutations in the CARD14 gene in three cases at seven mutation sites. Of the three patients, one had SLE, one had RA, and one had RA with Sjogren’s syndrome. None of the three patients was in an active disease state, and there were no obvious symptoms of joint pain, swelling, skin allergies, or mouth ulcers. To date, CARD14 gene mutations have been associated with various diseases that produce widespread pustular rashes, such as generalized pustular psoriasis (GPP), psoriatic arthritis, and pityriasis rosea (10, 56). In addition, mutations in other CARD genes are known to cause pustular skin disorders, similar to Burau syndrome (CARD15/NOD2 mutations) (57). Owing to the limited number of reported cases, further research is needed to determine whether CARD14 mutations form the molecular basis of AGEP.
The typical manifestations of AGEP include tens to hundreds of non-follicular pinhead-sized sterile pustules that appear rapidly as edematous erythema, generally originating on the face or intertriginous areas of the body, and rapidly spreading to the trunk and limbs. Histopathology on sub-corneal pustules located in the superficial layer of the epidermis reveals the presence of immune cells—mainly neutrophils and occasionally eosinophils—and an almost unchanged epidermis below the pustules, showing only infiltrated neutrophils and mild intercellular edema (2). In the five patients we reported, the rash initially appeared on the cheeks and facial area, joints of the extremities, scalp, and hands. Afterward, the rash spread rapidly to other parts of the body. All cases had facial involvement, which is consistent with the clinical presentation of AGEP. HCQ-related AGEP can be clearly distinguished from GPP—which recurs often—and there may be typical psoriasis lesions before the appearance of pustules; these appear on the original plaque or rash and can expand and fuse to form “pus lakes.” In addition, some patients with GPP have a family history, and the histopathological manifestations are psoriasis-like hyperplasia of the epidermis with keratosis, Kogoj micro-abscesses in the upper part of the spinous layer, and superficial dermal telangiectasia and tortuous vessels (58). In this sample, there was no family history of psoriasis, the rash appeared after HCQ treatment, and pustule formation was observed on histopathology, which is not the case in patients with GPP. Therefore, patients with suspected HCQ-induced AGEP may benefit from histopathology and skin biopsy.
AGEP is self-limiting and has a short disease course, and lesions generally disappear within 15 d of stopping the causative drug. However, HCQ-induced AGEP is prolonged and recurrent, ranging from 7–81 d (18, 37). As HCQ has a longer half-life, HCQ-associated AGEP lesions may last for longer, i.e., 1–2 months (19). All five patients in our sample were discharged within 15–45 d, similar to the results reported in the literature. The first principles and priorities of AGEP treatment are cessation of exposure to suspected triggers and symptomatic supportive care, avoiding antibiotics as much as possible, except in cases where co-infection is suspected; in such cases, antibiotics should be cautiously used (59). The use of topical emollients may aid the treatment of mild symptoms, whereas patients with severe symptoms require systemic steroids to relieve itching, inhibit telangiectasia and inflammation, and shorten the course of the disease; for recalcitrant disease, systemic therapy is recommended with dapsone and cyclosporine (60). Our patients received topical or systemic steroids and antihistamines, and the most severely ill patient also received mycophenolate and thalidomide, which rarely present with serious complications and sequelae. The mortality rate of AGEP is less than 5% and is often due to multi-organ dysfunction. Patients at high mortality risk typically have comorbidities or extensive skin lesions with mucosal involvement (4). The condition rarely recurs after treatment, but exposure to the same trigger may lead to recurrence. None of the patients we reported on had any recurrence after a 1–2 year-follow-up period.
Herein, we reported five cases of HCQ-induced AGEP. The need to distinguish HCQ-induced AGEP from GPP is of clinical concern. Compared to AGEP induced by other drugs, the latency and regression time of AGEP caused by HCQ are longer. AGEP often resolves with topical therapy and with drug discontinuation, although systemic steroid therapy may be necessary in some cases. In addition, a new CARD14 mutation was identified in our study and was validated using Sanger sequencing.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Medical Ethics Committee of the Second Affiliated Hospital of Guizhou University of Chinese Medicine. The patients/participants provided their written informed consent to participate in this study.
Author contributions
W-kM and X-mYa: conceptualization. C-mC: methodology. Y-zY: software. W-kM, X-mYa, and FL: validation. HX: formal analysis. X-mYu: investigation. FL: data curation. FL: writing—first draft preparation. W-kM and X-mYa: writing—review and editing. All authors contributed to the article and approved the submitted version.
Funding
The study was funded by the Guizhou Province Science and Technology Plan Project, Grant/Award Number: Oiankehe Platform Talent [2020] 2202, [2016] 5650; Guizhou Province Science and Technology Plan Project, Grant/Award Number: Guizhou Qiankehe Support [2021] General 006.
Acknowledgments
We thank all the researchers and participants who contributed to this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
1. Wesemael, TV, Huizinga, T, and Toes, R. From Phenotypeto pathophysiology—placing rheumatic diseases in an immunological perspective. Rheumatol DJTL. (2022) 4:e166–7. doi: 10.1016/S2665-9913(21)00369-6
2. Hadavand, MA, Kaffenberger, B, Cartron, AM, and Trinidad, JCL. Clinical presentation and Management of Atypical and Recalcitrant Acute Generalized Exanthematous Pustulosis. J Am Acad Dermatol. (2022) 87:632–9. doi: 10.1016/j.jaad.2020.09.024
3. Duong, TA, Valeyrie-Allanore, L, Wolkenstein, P, and Chosidow, O. Severe cutaneous adverse reactions to drugs. Lancet (London, England). (2017) 390:1996–2011. doi: 10.1016/s0140-6736(16)30378-6
4. Szatkowski, J, and Schwartz, RA. Acute generalized Exanthematous Pustulosis (Agep): a review and update. J Am Acad Dermatol. (2015) 73:843–8. doi: 10.1016/j.jaad.2015.07.017
5. Sidoroff, A, Dunant, A, Viboud, C, Halevy, S, Bavinck, JN, Naldi, L, et al. Risk factors for acute generalized Exanthematous Pustulosis (Agep)-results of a multinational case-control study (Euroscar). Br J Dermatol. (2007) 157:989–96. doi: 10.1111/j.1365-2133.2007.08156.x
6. Creadore, A, Desai, S, Alloo, A, Dewan, AK, Bakhtiar, M, Cruz-Diaz, C, et al. Clinical characteristics, disease course, and outcomes of patients with acute generalized Exanthematous Pustulosis in the us. JAMA Dermatol. (2022) 158:176–83. doi: 10.1001/jamadermatol.2021.5390
7. Hotz, C, Valeyrie-Allanore, L, Haddad, C, Bouvresse, S, Ortonne, N, Duong, TA, et al. Systemic involvement of acute generalized Exanthematous Pustulosis: a retrospective study on 58 patients. Br J Dermatol. (2013) 169:1223–32. doi: 10.1111/bjd.12502
8. Schrezenmeier, E, and Dörner, T. Mechanisms of action of Hydroxychloroquine and Chloroquine: implications for rheumatology. Nat Rev Rheumatol. (2020) 16:155–66. doi: 10.1038/s41584-020-0372-x
9. Wang, M, Cao, R, Zhang, L, Yang, X, Liu, J, Xu, M, et al. Remdesivir and Chloroquine effectively inhibit the recently emerged novel coronavirus (2019-Ncov) in vitro. Cell Res. (2020) 30:269–71. doi: 10.1038/s41422-020-0282-0
10. Podlipnik, S, Castellanos-Moreira, R, Florez-Enrich, H, Arostegui, JI, and Mascaró, JM Jr. Acute generalized Exanthematous Pustulosis and polyarthritis associated with a novel Card14 mutation. Australas J Dermatol. (2018) 59:e70–3. doi: 10.1111/ajd.12669
11. Navarini, AA, Simpson, MA, Borradori, L, Yawalkar, N, and Schlapbach, C. Homozygous missense mutation in Il36rn in generalized Pustular Dermatosis with intraoral involvement compatible with both Agep and generalized Pustular psoriasis. JAMA Dermatol. (2015) 151:452–3. doi: 10.1001/jamadermatol.2014.3848
12. Tian, Q, Xu, B, and Zhou, X. A case of hydroxychloroquine causing refractory acute generalized exanthematous impetigo. Chinese. J Dermatol. (2018) 51:64–5. doi: 10.3760/cma.j.issn.0412-4030.2018.01.021
13. Liu, JJ, Yang, JJ, Li, H, and Huang, S. Pharmacological analysis of acute generalized exanthematous impetigo caused by hydroxychloroquine sulfate in patients with Sjogren's syndrome. Primary Med Forum. (2019) 23:5140–1. doi: 10.19435/j.1672-1721.2019.35.083
14. Di Lernia, V, Grenzi, L, Guareschi, E, and Ricci, C. Rapid clearing of acute generalized Exanthematous Pustulosis after Administration of Ciclosporin. Clin Exp Dermatol. (2009) 34:e757–9. doi: 10.1111/j.1365-2230.2009.03480.x
15. Liccioli, G, Marrani, E, Giani, T, Simonini, G, Barni, S, and Mori, F. The first pediatric case of acute generalized Exanthematous Pustulosis caused by Hydroxychloroquine. Pharmacology. (2019) 104:57–9. doi: 10.1159/000500406
16. Di Maso, V, Cozzi, M, Gerini, U, Bedina, E, Olivo, E, Bianco, F, et al. Coupled plasma filtration adsorption for treatment of capillary leak syndrome superimposed to acute generalized Exanthematous Pustolosis: a case report. Blood Purif. (2020) 49:372–8. doi: 10.1159/000503770
17. Soria, A, Barbaud, A, Assier, H, Avenel-Audran, M, Tétart, F, Raison-Peyron, N, et al. Cutaneous adverse drug reactions with Antimalarials and Allergological skin tests. Dermatol (Basel, Switzerland). (2015) 231:353–9. doi: 10.1159/000438787
18. Pearson, KC, Morrell, DS, Runge, SR, and Jolly, P. Prolonged Pustular eruption from Hydroxychloroquine: an unusual case of acute generalized Exanthematous Pustulosis. Cutis. (2016) 97:212–6.
19. İslamoğlu, ZGK, and Karabağli, P. A case of recalcitrant acute generalized Exanthematous Pustulosis with Sjogren's syndrome: successfully treated with low-dose cyclosporine. Clin Case Rep. (2019) 7:1721–4. doi: 10.1002/ccr3.2352
20. Matsuda-Hirose, H, Sho, Y, Yamate, T, Nakamura, Y, Saito, K, Takeo, N, et al. Acute generalized Exanthematous Pustulosis induced by Hydroxychloroquine successfully treated with Etretinate. J Dermatol. (2020) 47:e53–4. doi: 10.1111/1346-8138.15185
21. Bailey, K, McKee, D, Wismer, J, and Shear, N. Acute generalized Exanthematous Pustulosis induced by Hydroxychloroquine: first case report in Canada and review of the literature. J Cutan Med Surg. (2013) 17:414–8. doi: 10.2310/7750.2013.12105
22. Assier-Bonnet, H, Saada, V, Bernier, M, Clerici, T, and Saïag, P. Acute generalized Exanthematous Pustulosis induced by Hydroxychloroquine. Dermatol (Basel, Switzerland). (1996) 193:70–1. doi: 10.1159/000246211
23. Evans, CC, and Bergstresser, PR. Acute generalized Exanthematous Pustulosis precipitated by Hydroxychloroquine. J Am Acad Dermatol. (2004) 50:650–1. doi: 10.1016/s0190-9622(03)02733-6
24. Martins, A, Lopes, LC, Paiva Lopes, MJ, and Rodrigues, JC. Acute generalized Exanthematous Pustulosis induced by Hydroxychloroquine. Eur J Dermatol: EJD. (2006) 16:317–8.
25. Paradisi, A, Bugatti, L, Sisto, T, Filosa, G, Amerio, PL, and Capizzi, R. Acute generalized Exanthematous Pustulosis induced by Hydroxychloroquine: three cases and a review of the literature. Clin Ther. (2008) 30:930–40. doi: 10.1016/j.clinthera.2008.05.014
26. Avram, MM, and Hoang, M. Case Records of the Massachusetts General Hospital. Case 20-2009. A 79-year-old woman with a blistering cutaneous eruption. N Engl J Med. (2009) 360:2771–7. doi: 10.1056/NEJMcpc0900639
27. Park, JJ, Yun, SJ, Lee, JB, Kim, SJ, Won, YH, and Lee, SC. A case of Hydroxychloroquine induced acute generalized Exanthematous Pustulosis confirmed by accidental Oral provocation. Ann Dermatol. (2010) 22:102–5. doi: 10.5021/ad.2010.22.1.102
28. Charfi, O, Kastalli, S, Sahnoun, R, and Lakhoua, G. Hydroxychloroquine-induced acute generalized Exanthematous Pustulosis with positive patch-testing. Indian J Pharm. (2015) 47:693–4. doi: 10.4103/0253-7613.169589
29. Zhang, Z, and Liu, X. Images in clinical medicine. Acute generalized Exanthematous Pustulosis. N Engl J Med. (2015) 372:161. doi: 10.1056/NEJMicm1401196
30. Yalçın, B, Çakmak, S, and Yıldırım, B. Successful treatment of Hydroxychloroquine-induced recalcitrant acute generalized Exanthematous Pustulosis with cyclosporine: case report and literature review. Ann Dermatol. (2015) 27:431–4. doi: 10.5021/ad.2015.27.4.431
31. Castner, NB, Harris, JC, and Motaparthi, K. Cyclosporine for corticosteroid-refractory acute generalized Exanthematous Pustulosis due to Hydroxychloroquine. Dermatol Ther. (2018) 31:e12660. doi: 10.1111/dth.12660
32. Duman, H, Topal, IO, Kocaturk, E, Cure, K, and Mansuroglu, I. Acute generalized Exanthematous Pustulosis induced by Hydroxychloroquine: a case with atypical clinical presentation. An Bras Dermatol. (2017) 92:404–6. doi: 10.1590/abd1806-4841.20175561
33. Mohaghegh, F, Jelvan, M, and Rajabi, P. A case of prolonged generalized Exanthematous Pustulosis caused by Hydroxychloroquine-literature review. Clin Case Rep. (2018) 6:2391–5. doi: 10.1002/ccr3.1811
34. Chaabouni, R, Bahloul, E, Ennouri, M, Atheymen, R, Sellami, K, Marrakchi, S, et al. Hydroxychloroquine-induced acute generalized Exanthematous Pustulosis: a series of seven patients and review of the literature. Int J Dermatol. (2021) 60:742–8. doi: 10.1111/ijd.15419
35. Mofarrah, R, Mofarrah, R, Oshriehye, M, Ghobadi Aski, S, Nazemi, N, and Nooshiravanpoor, P. The necessity of patch testing in determining the causative drug of Agep. J Cosmet Dermatol. (2021) 20:2156–9. doi: 10.1111/jocd.13841
36. Munshi, M, Junge, A, Gadaldi, K, Yawalkar, N, and Heidemeyer, K. Ixekizumab for treatment of refractory acute generalized Exanthematous Pustulosis caused by Hydroxychloroquine. JAAD Case Rep. (2020) 6:634–6. doi: 10.1016/j.jdcr.2020.05.014
37. Otake-Irie, H, Nakajima, S, Okamoto, N, Toichi, E, Nomura, T, and Kabashima, K. Prolonged acute generalized Exanthematous Pustulosis and atypical target-like lesions induced by Hydroxychloroquine. J Dermatol. (2020) 47:e387–8. doi: 10.1111/1346-8138.15537
38. Enos, T, Jeong, HS, Vandergriff, T, Jacobe, HT, and Chong, BF. Acute generalized Exanthematous Pustulosis induced by empiric Hydroxychloroquine for presumed Covid-19. Dermatol Ther. (2020) 33:e13834. doi: 10.1111/dth.13834
39. Mercogliano, C, Khan, M, Lin, C, Mohanty, E, and Zimmerman, R. Agep overlap induced by Hydroxychloroquine: a case report and literature review. J Commun Hosp Int Med Perspect. (2018) 8:360–2. doi: 10.1080/20009666.2018.1547089
40. Robustelli Test, E, Vezzoli, P, Carugno, A, Raponi, F, Gianatti, A, Rongioletti, F, et al. Acute generalized Exanthematous Pustulosis with erythema Multiforme-like lesions induced by Hydroxychloroquine in a woman with coronavirus disease 2019 (Covid-19). J Eur Acad Dermatol Venereol: JEADV. (2020) 34:e457–9. doi: 10.1111/jdv.16613
41. Litaiem, N, Hajlaoui, K, Karray, M, Slouma, M, and Zeglaoui, F. Acute generalized Exanthematous Pustulosis after Covid-19 treatment with Hydroxychloroquine. Dermatol Ther. (2020) 33:e13565. doi: 10.1111/dth.13565
42. Sánchez-Velázquez, A, Arroyo-Andrés, J, Falkenhain-López, D, Peralto, JLR, Romero, PLO, Díaz, RR, et al. Hydroxychloroquine-induced acute generalized Exanthematous Pustulosis: an adverse reaction to keep in mind during Covid-19 pandemic. J der Deutschen Dermatologischen Gesellschaft = J German Soc Dermatol: JDDG. (2021) 19:896–8. doi: 10.1111/ddg.14354
43. Lateef, A, Tan, KB, and Lau, TC. Acute generalized Exanthematous Pustulosis and toxic epidermal Necrolysis induced by Hydroxychloroquine. Clin Rheumatol. (2009) 28:1449–52. doi: 10.1007/s10067-009-1262-4
44. Coleman, I, Ruiz, G, Brahmbhatt, S, and Ackerman, L. Acute generalized Exanthematous Pustulosis and Stevens-Johnson syndrome overlap due to Hydroxychloroquine: a case report. J Med Case Rep. (2020) 14:210. doi: 10.1186/s13256-020-02504-8
45. Mashiah, J, and Brenner, S. A systemic reaction to patch testing for the evaluation of acute generalized Exanthematous Pustulosis. Arch Dermatol. (2003) 139:1181–3. doi: 10.1001/archderm.139.9.1181
46. Davidovici, BB, Pavel, D, Cagnano, E, Rozenman, D, and Halevy, S. Acute generalized Exanthematous Pustulosis following a spider bite: report of 3 cases. J Am Acad Dermatol. (2006) 55:525–9. doi: 10.1016/j.jaad.2006.05.010
47. Trüeb, RM, and Burg, G. Acute generalized Exanthematous Pustulosis due to doxycycline. Dermatol (Basel, Switzerland). (1993) 186:75–8. doi: 10.1159/000247308
48. Schrom, K, Pacifico, A, Conic, RRZ, Pigatto, PDM, Malagoli, P, Morrone, A, et al. Dabigatran-associated acute generalized Exanthematous Pustulosis (Agep) in a psoriatic patient undergoing Ixekizumab and its Pathogenetic mechanism. Dermatol Ther. (2019) 32:e13018. doi: 10.1111/dth.13018
49. Nakamizo, S, Kobayashi, S, Usui, T, Miyachi, Y, and Kabashima, K. Clopidogrel-induced acute generalized Exanthematous Pustulosis with elevated Th17 cytokine levels as determined by a drug lymphocyte stimulation Test. Br J Dermatol. (2010) 162:1402–3. doi: 10.1111/j.1365-2133.2010.09705.x
50. Aviña-Zubieta, JA, Galindo-Rodriguez, G, Newman, S, Suarez-Almazor, ME, and Russell, AS. Long-term effectiveness of antimalarial drugs in rheumatic diseases. Ann Rheum Dis. (1998) 57:582–7. doi: 10.1136/ard.57.10.582
51. Nirk, EL, Reggiori, F, and Mauthe, M. Hydroxychloroquine in rheumatic autoimmune disorders and beyond. EMBO Mol Med. (2020) 12:e12476. doi: 10.15252/emmm.202012476
52. Bahloul, E, Jallouli, M, Garbaa, S, Marzouk, S, Masmoudi, A, Turki, H, et al. Hydroxychloroquine-induced hyperpigmentation in systemic diseases: prevalence, clinical features and risk factors: a cross-sectional study of 41 cases. Lupus. (2017) 26:1304–8. doi: 10.1177/0961203317700486
53. Son, CH, Lee, CU, Roh, MS, Lee, SK, Kim, KH, and Yang, DK. Acute generalized Exanthematous Pustulosis as a manifestation of carbamazepine hypersensitivity syndrome. J Investig Allergol Clin Immunol. (2008) 18:461–4.
54. Fernández-Ruiz, M, López-Medrano, F, García-Ruiz, F, and Rodríguez-Peralto, JL. Diltiazem-induced acute generalized Exanthemic Pustulosis: a case and review of the literature. Actas Dermo-Sifiliograficas. (2009) 100:725–7. doi: 10.1016/S0001-7310(09)72291-4
55. Munster, T, Gibbs, JP, Shen, D, Baethge, BA, Botstein, GR, Caldwell, J, et al. Hydroxychloroquine concentration-response relationships in patients with rheumatoid arthritis. Arthritis Rheum. (2002) 46:1460–9. doi: 10.1002/art.10307
56. Armstrong, AW, and Read, C. Pathophysiology, clinical presentation, and treatment of psoriasis: a review. JAMA. (2020) 323:1945–60. doi: 10.1001/jama.2020.4006
57. Fuchs-Telem, D, Sarig, O, van Steensel, MA, Isakov, O, Israeli, S, Nousbeck, J, et al. Familial Pityriasis Rubra Pilaris is caused by mutations in Card14. Am J Hum Genet. (2012) 91:163–70. doi: 10.1016/j.ajhg.2012.05.010
58. Hoegler, KM, John, AM, Handler, MZ, and Schwartz, RA. Generalized Pustular psoriasis: a review and update on treatment. J Eur Acad Dermatol Venereol: JEADV. (2018) 32:1645–51. doi: 10.1111/jdv.14949
59. Hoetzenecker, W, Nägeli, M, Mehra, ET, Jensen, AN, Saulite, I, Schmid-Grendelmeier, P, et al. Adverse cutaneous drug eruptions: current understanding. Semin Immunopathol. (2016) 38:75–86. doi: 10.1007/s00281-015-0540-2
Keywords: acute generalized exanthematous pustulosis, hydroxychloroquine, CARD14, gene mutation, gene sequencing
Citation: Luo F, Yuan X-m, Xiong H, Yang Y-z, Chen C-m, Ma W-k and Yao X-m (2023) Clinical features of acute generalized exanthematous pustulosis caused by hydroxychloroquine in rheumatology patients and exploration of CARD14 gene mutations. Front. Med. 10:1161837. doi: 10.3389/fmed.2023.1161837
Edited by:
Miao Pan, Children’s National Hospital, United StatesReviewed by:
Xiang Dong Jian, Qilu Hospital, Shandong University, ChinaSavas Yayli, Koç University, Türkiye
Giovanni Damiani, University of Milan, Italy
Copyright © 2023 Luo, Yuan, Xiong, Yang, Chen, Ma and Yao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xue-ming Yao, eXhtaW5nMTlAZm94bWFpbC5jb20=
 Feng Luo
Feng Luo Xue-mei Yuan1
Xue-mei Yuan1 Wu-kai Ma
Wu-kai Ma