- Division of Gastroenterology and Hepatology, Department of Internal Medicine, Chonnam National University Medical School, Gwangju, Republic of Korea
Background: Tailored therapy has been widely used for patients with Helicobacter pylori (H. pylori) infection in South Korea. Herein, we evaluated the treatment outcomes of tailored clarithromycin-based triple therapy (TT) in patients infected with H. pylori.
Methods: We enrolled 460 patients without A2142G and A2143G point mutations by dual priming oligonucleotide-based polymerase chain reaction who had taken TT and undergone the urease breath test to evaluate eradication in clinical practice. Eradication rates according to the treatment duration and dose of clarithromycin were analyzed.
Results: Among 460 patients (164 women, median age 63.0 years), 250 patients underwent TT with full-dose clarithromycin (TT-full CLA), and 216 patients underwent TT with half-dose clarithromycin (TT-half CLA). The eradication rates were 88.0% (220/250) in patients with TT-full CLA and 85.2% (179/210) in patients with TT-half CLA. In 250 patients with TT-full CLA, the eradication rates were 86.8% (33/38) in patients with 7-day TT-full CLA and 88.2% (187/212) in patients with 10-day or 14-day TT-full CLA (P = 0.788). In 210 patients with TT-half CLA, the eradication rates were 84.2% (139/165) in those with a 7-day TT-half CLA and 88.9% (40/45) in those with a 10-day or 14-day TT-half CLA (P = 0.436).
Conclusion: For patients with H. pylori infection without A2142G and A2143G point mutations by DPO-PCR in clinical practice, treatment extension above 7-day TT with full CLA did not improve the eradication rates. Future studies on the treatment outcomes of TT-half CLA considering effectiveness and compliance are warranted.
1. Introduction
Helicobacter pylori (H. pylori) is associated with variable gastric diseases including peptic ulcer disease, gastric adenocarcinoma, gastric preneoplastic diseases, and gastric mucosa-associated lymphoid tissue lymphoma (1, 2). H. pylori eradication has been recommended for managing these potentially curable or related diseases (1, 3). However, the eradication success rate has decreased with the increase in H. pylori antibiotic resistance (4). Recent international guidelines such as the Maastricht V/Florence Consensus or Italian guidelines stated that other treatment regimens, rather than clarithromycin-based triple therapy (TT), should be considered in regions with high clarithromycin resistance in the absence of H. pylori susceptibility testing (5, 6). According to a recent nationwide survey conducted in South Korea, the H. pylori seroprevalence rate in 2015 and 2016 was 51.0% (7). Moreover, antibiotic resistance mapping of H. pylori in South Korea reported a clarithromycin resistance rate of 17.8% (4). Even though South Korea is now an area with high clarithromycin resistance (4), the Korean guidelines still state that clarithromycin-based triple therapy should be used as the first-line regimen for H. pylori eradication, which is specified to be used for 14 days (3). It also recommends performing a clarithromycin resistance test when 7-day clarithromycin-based TT is considered a first-line treatment (3).
There is an established association between clarithromycin resistance and various point mutations in the peptidyl transferase region domain V of the 23S rRNA gene (8). Recently, several molecular assays for detecting clarithromycin resistance in H. pylori based on the detection of mutations in the 23S rRNA genes have been suggested (1, 9, 10). Among molecular assays, a commercial dual-priming oligonucleotide (DPO) primer has been developed to detect single-nucleotide polymorphisms using a one-step PCR assay by Chun et al. (11). This test can be performed by gastric biopsy, even processed by the rapid urease test, and identify H. pylori with sensitivity, specificity, and concordance rates of 97.7, 83, and 90%, respectively, compared with bacterial cultures or the rapid urease tests (12–15). It can also verify the presence of mutations, especially A2142G and A2143G point mutations of the 23S rRNA gene related to clarithromycin resistance. There are several reports regarding H. pylori eradication rates of tailored TT in patients with clarithromycin-susceptible H. pylori determined by DPO-PCR. The overall eradication rate was 87–90% (16–19), which was close to those in TT without clarithromycin resistance determined by the culture method (20, 21). As bacterial H. pylori cultures and antimicrobial resistance tests are time-consuming, expensive, and difficult to perform, they cannot be employed easily in clinical practice. Conversely, molecular assays including DPO-PCR can be relatively easily accessible in clinical practice.
Even though tailored therapies based on the antimicrobial susceptibility test have improved eradication rates, eradication failure has still been reported in 10–15% of patients (16–19). Therefore, this study aimed to examine the efficacy of tailored TT considering treatment duration and the dosage of clarithromycin in patients infected with H. pylori without the A2143G and A2142G point mutations of the 23S rRNA gene in clinical practice.
2. Methods
This was a retrospective observational study. We identified 622 patients older than 18 years of age and younger than 80 years of age, in whom H. pylori infection was confirmed by a DPO-based multiplex PCR during esophagogastroduodenoscopy (EGD) and had undergone urease breath test for eradication verification after taking more than 80% of the prescribed medication in the Chonnam National University Hospital between Jan 2019 and July 2021. Among 622 patients, 151 patients with the A2142G and/or A2143G point mutations of the 23S rRNA gene were excluded from the study, as they received other treatment regimens other than TT. We also excluded 11 patients who did not take TT. Finally, 460 patients were included in this study. We extracted the data of demographics or clinical characteristics and eradication success rates according to the eradication regimens from the medical chart review. The Institutional Review Board of Chonnam National University Hospital approved this study (IRB Number: CNUH-2022-351). We followed the ethical principles of the Declaration of Helsinki.
2.1. Clarithromycin resistance test by PCR
We obtained two gastric biopsy specimens from the antrum and body of patients during EGD, for which a DPO-based multiplex PCR test (Seegen Inc., Seoul, Korea) was performed to diagnose H. pylori infection by detecting the deoxyribonucleic acid (DNA) extraction. The amplified DNA products were identified using an ultraviolet transilluminator in electrophoresis. A single 621-bp DNA product was considered a wild-type H. pylori. The presence of the A2142G and 2143G mutations resulted in DNA bands at 475-bp and 19-bp, respectively.
2.2. Eradication regimens and confirmation of H. pylori eradication
In this study, all patients were treated with acid suppressants [proton pump inhibitor (PPI)- or potassium-competitive acid blocker (P-CAB)]-based triple therapy, which consisted of 1000 mg of amoxicillin twice a day, 500 mg or 250 mg of clarithromycin twice a day, and acid suppressants [PPIs (rabeprazole 20 mg, pantoprazole 40 mg, oresomeprazole 20 mg twice a day), or P-CAB (50 mg of tegoprazan twice a day)] for 7, 10, or 14 days, respectively.
At least 4 weeks after treatment completion, the patients visited the hospital to take a standard 13C-urea breath test (UBiTKit, Otsuka Pharmaceutical Co. Ltd., Tokyo, Japan) to find out whether H. pylori eradication was successful or not. Eradication was defined as a negative result obtained in the test.
2.3. Statistical analysis
Continuous variables are expressed as the median and interquartile range (IQR), and categorical variables are expressed as numbers with percentages (%). Eradication rates according to the treatment duration (7 vs. 10 or 14 days) and dose of clarithromycin [full CLA (500 mg of clarithromycin twice a day) vs. half CLA (250 mg of clarithromycin twice a day)] were analyzed using Fisher's exact t-test. Two-tailed P-values of <0.05 were considered statistically significant. All statistical analyses were conducted using SPSS 27.0 for Windows (SPSS Inc., Chicago, IL, United States).
3. Results
3.1. Baseline demographic and clinical characteristics
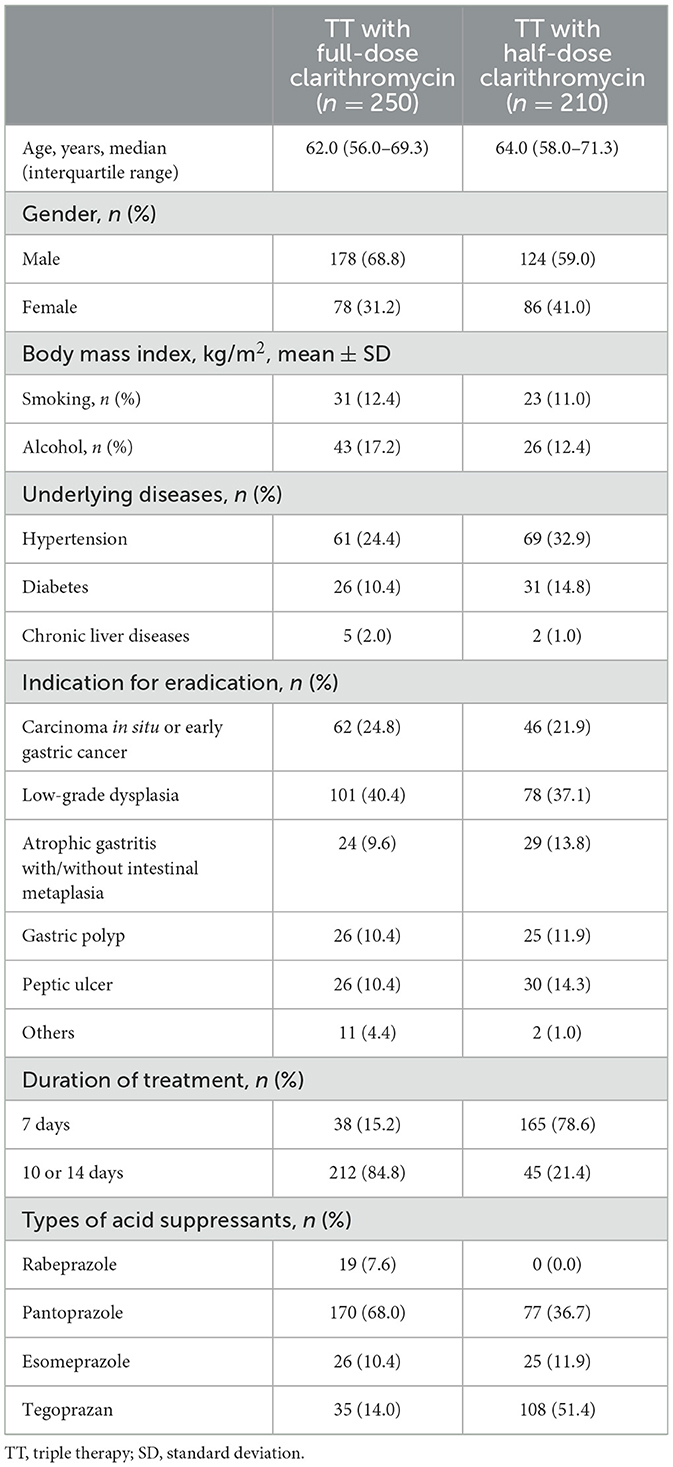
We enrolled 460 patients without the A2142G and A2143G point mutations of 23S rRNA certified by the DPO-PCR test. Table 1 shows the baseline demographic and clinical characteristics of the enrolled patients. The median age (IQR) was 63.0 (57.0–70.0) years, and there were 164 (35.7%) women. There were 250 patients who had taken TT with full CLA (TT-full CLA) and 210 who had taken TT with half CLA (TT-half CLA). The most common indication for H. pylori eradication was gastric low-grade dysplasia (179, 38.9%), followed by carcinoma in situ or early gastric cancer (108, 23.5%). The overall eradication rates were 86.7% (399/460).
3.2. Eradication rates of H. pylori in patients who had taken TT with full-dose clarithromycin
Among the 250 patients who had received TT-full CLA, 212 patients received 10-day or 14-day TT-full CLA, and 38 patients received 7-day TT-full CLA. The overall eradication rates were 88.0% (220/250). There was no significant difference in the eradication rates of H. pylori in terms of treatment duration; 86.8% (33/38) in the 7-day TT-full CLA, and 88.2% (187/212) in the 10-day or 14-day TT full-CLA (P = 0.788).
3.3. Eradication rates of H. pylori in patients who had taken TT with half-dose clarithromycin
There were 210 patients who had received TT with half-dose clarithromycin (TT-half CLA). The overall eradication rates were 85.2% (179/210). Among them, 165 patients received TT 7-day TT-half CLA. The eradication rates of H. pylori were 84.2% (139/165) in the 7-day TT-half CLA and 88.9% (40/45) in the 10-day or 14-day TT-half CLA (P = 0.635).
3.4. Comparison of the eradication rates of H. pylori between patients with full-dose clarithromycin and those with half-dose clarithromycin
We compared the eradication rates among four groups (7-day TT-full CLA, 10-day or 14-day TT-full CLA, 7-day TT-half CLA, and 10-day or 14-day TT-half CLA). The eradication rates were not significantly different among the four groups (P = 0.806, Figure 1). We also performed the subgroup analysis according to the duration of TT. In 7-day TT, there was no significant difference in the eradication rates between TT-full CLA and TT-half CLA (P = 0.806). Moreover, in 10-day or 14-day TT, there was no significant difference in the eradication rates between TT-full CLA and TT-half CLA (P > 0.999).
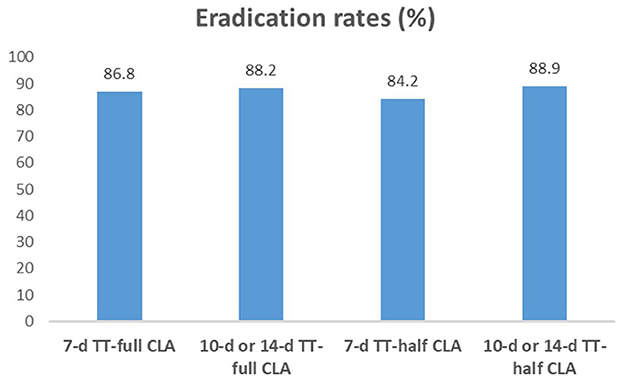
Figure 1. H. pylori eradication rates in each group. The eradication rates were not significantly different among the four groups (P > 0.05). In 7-day TT, there was no significant difference in the eradication rates between TT-full CLA (86.8%) and TT-half CLA (84.2%, P = 0.806). Moreover, in 10-day or 14-day TT, there was no significant difference in the eradication rates between TT-full CLA (88.2%) and TT-half CLA (88.9%, P > 0.999).
4. Discussion
The efficacy of clarithromycin-containing regimens for H. pylori eradication has decreased due to increased antibiotic resistance (22–24). There are several alternative regimens to increase the efficacy of H. pylori eradication through the extension of the treatment duration and adjusting the antibiotic type and dose. These therapeutic regimens include bismuth quadruple therapy, sequential therapy, hybrid therapy, concomitant therapy, vonoprazan-containing triple therapies, or adjunctive therapies with probiotics (3, 5, 6, 25, 26). However, the use of these new regimens remains limited by the lack of efficacy improvement and gradually increasing adverse events. Another method of increasing the efficacy of H. pylori eradication is the antimicrobial susceptibility test-guided tailored treatment (19, 26). This study showed that the eradication rate of tailored TT-full CLA was almost 90%. There is no benefit in the extension of treatment duration of tailored TT-full CLA, and tailored TT-half CLA may be considered.
Clarithromycin resistance can be assessed by phenotype detection using a culture with the agar dilution method or the E-test and by genotypic methods using PCR. Even though culture methods can provide an overall evaluation of the clarithromycin resistance of H. pylori, regardless of the intrinsic mechanism involved, they are still time-consuming, and the cultivation of H. pylori is difficult even in expert hands (27). There are several molecular assays for detecting clarithromycin resistance in H. pylori based on the detection of mutation in the 23S rRNA gene (1, 8–10, 26). PCR-based techniques for H. pylori showed high sensitivity and specificity and provided the mutation types associated with antibiotic resistance. Several detection methods for point mutations at the 23S rRNA of H. pylori include PCR followed by restriction fragment length polymorphism analysis (10), quantitative PCR assay, DPO-based multiplex PCR, fluorescent in situ hybridization (28), or a peptide nucleotide acid probe-based qPCR test (9). Recently, multiplex real-time PCR has been used for one-step detection of the mutations of multiple sites to predict the resistance of clarithromycin and levofloxacin (29). In this study, a molecular method using DPO-PCR was used to identify key mutations (A2143G, A2142G) responsible for clarithromycin resistance. DPO-PCR had a sensitivity of 87.5% and a specificity of 91.3% on the basis of the C13-urea breath test (15). H. pylori without a point mutation certified by DPO-PCR has a low tendency for clarithromycin resistance. Although some guidelines recommend the 14-day treatment durations for H. pylori eradication (5, 25, 30), recent several studies showed acceptable eradication rates of ~90% with 7-day TT in patients with clarithromycin-susceptible H. pylori (31–33). A recent meta-analysis suggested that susceptibility-guided therapy was more effective than empirical therapy for treatment-naive patients, especially in areas where the clarithromycin resistance rate is >20% (34). In the present study, the eradication rates with 7-day TT-full CLA and 10-day or 14-day TT-full CLA against H. pylori without A2142G and A2143G point mutations were 86.8 and 88.2%, respectively, which were similar to the findings of previous studies (31–33). Therefore, TT beyond 7 days needs to be discussed considering various situations.
A previous meta-analysis demonstrated that half-dose clarithromycin-based therapy is equally effective as full-dose clarithromycin-based therapy for H. pylori eradication (35). However, clarithromycin resistance was not considered in that study. The dose of clarithromycin does not affect the therapeutic outcomes in the treatment of clarithromycin-resistant H. pylori. Therefore, to evaluate the pure effect of clarithromycin dosage (full-dose or half-dose) for H. pylori eradication, it is necessary to analyze the eradication rate in patients with clarithromycin-sensitive H. pylori. Clinicians need to choose antibiotic doses in terms of efficacy, antibiotics-related adverse events, and compliance. A large-scale observational study demonstrated that the adverse events increased with higher doses of clarithromycin (36). Therefore, half-dose clarithromycin may increase compliance by decreasing adverse events. In the present study, we compared the eradication rates of TT-half CLA against H. pylori without A2142G and A2143G point mutations. The eradication rate of 7-day TT-half CLA was 84.2%, which was regarded as unacceptable, while the eradication rates increased up to ~90% when the treatment duration was extended. Therefore, 7-day TT-half CLA may be insufficient even for clarithromycin-sensitive H. pylori eradication, especially in areas with high clarithromycin resistance. However, if eradication rates can be increased through more potent acid inhibition or treatment duration extension, it may be one of the treatment options with the benefit of decreasing adverse events and increasing compliance.
This study has several limitations. First, there is a lack of information about patients' compliance with regimens and adverse events, as this study was retrospective. However, we enrolled patients who took >80% of the prescribed medication, and there were no reported severe adverse events during the observational period of H. pylori eradication from the medical chart review. Second, there are the inherent risks of selection bias and information bias because this is a single-center retrospective study. Third, several types of acid suppressants (PPIs and P-CAB) were used for the eradication regimen. The metabolism of PPI is affected by the enzymatic activity, cytochrome P450 enzymes, and CYP2C19 with genetic differences. Conversely, P-CAB is not dependent on the CYP2C19 genotype and provides the long-standing inhibition of gastric acid secretion. While vonoprazan showed higher eradication rates compared with PPI (37, 38), tegoprazan used in this study showed similar eradication rates, compared with PPI (39). Moreover, there was no significant difference between PPIs and P-CAB for clarithromycin-susceptible H. pylori eradication (40). Therefore, the impact of the use of tegoprazan on the eradication rates may be insignificant in this study. Nonetheless, this is the first study showing the efficacy of TT-half CLA in patients with clarithromycin-susceptible H. pylori. In future, a randomized controlled study with a multicenter and large sample size needs to be performed to solve these problems.
In conclusion, in patients with H. pylori infection without A2142G and A2143G point mutations of the 23S rRNA, the extension of treatment for more than 7 day TT-full CLA did not improve the eradication rates. Future studies on the treatment outcomes of TT-half CLA need to be performed considering effectiveness and compliance.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Institutional Review Board of Chonnam National University Hospital (IRB Number: CNUH-2022-351). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
SHC and MSP contributed to the data collection and drafting of the manuscript. DK, H-SY, and H-SK participated in the review and editing of the draft. S-YP contributed to the study conceptualization, design, writing the original draft, and critical revision of the draft. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by the Chonnam National University Hospital Biomedical Research Institute (Grant Number: BCRI 23033) and the National Research Foundation of Korea (NRF) grant funded by the Korean government (Grant Number: 2021R1F1A1059503).
Acknowledgments
We would like to thank Ban-Seok Kim for data collection.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Tshibangu-Kabamba E, Yamaoka Y. Helicobacter pylori infection and antibiotic resistance - from biology to clinical implications. Nat Rev Gastroenterol Hepatol. (2021) 18:613–29. doi: 10.1038/s41575-021-00449-x
2. Guevara B, Cogdill AG. Helicobacter pylori: a review of current diagnostic and management strategies. Dig Dis Sci. (2020) 65:1917–31. doi: 10.1007/s10620-020-06193-7
3. Jung HK, Kang SJ, Lee YC, Yang HJ, Park SY, Shin CM, et al. Evidence-based guidelines for the treatment of helicobacter pylori infection in Korea 2020. Gut Liver. (2021) 15:168–95. doi: 10.5009/gnl20288
4. Lee JH, Ahn JY, Choi KD, Jung HY, Kim JM, Baik GH, et al. Nationwide antibiotic resistance mapping of Helicobacter pylori in Korea: a prospective multicenter study. Helicobacter. (2019) 24:e12592. doi: 10.1111/hel.12592
5. Malfertheiner P, Megraud F, O'Morain CA, Gisbert JP, Kuipers EJ, Axon AT, et al. Management of Helicobacter pylori infection-the maastricht V/florence consensus report. Gut. (2017) 66:6–30. doi: 10.1136/gutjnl-2016-312288
6. Romano M, Gravina AG, Eusebi LH, Pellegrino R, Palladino G, Frazzoni L, et al. Management of Helicobacter pylori infection: guidelines of the Italian Society of Gastroenterology (SIGE) and the Italian Society of Digestive Endoscopy (SIED). Dig Liver Dis. (2022) 54:1153–61. doi: 10.1016/j.dld.2022.06.019
7. Lee JH, Choi KD, Jung HY, Baik GH, Park JK, Kim SS, et al. Seroprevalence of Helicobacter pylori in Korea: a multicenter, nationwide study conducted in 2015 and 2016. Helicobacter. (2018) 23:e12463. doi: 10.1111/hel.12463
8. Owen RJ. Molecular testing for antibiotic resistance in Helicobacter pylori. Gut. (2002) 50:285–9. doi: 10.1136/gut.50.3.285
9. Nahm JH, Kim WK, Kwon Y, Kim H. Detection of Helicobacter pylori with clarithromycin resistance-associated mutations using peptide nucleic acid probe-based melting point analysis. Helicobacter. (2019) 24:e12634. doi: 10.1111/hel.12634
10. Agudo S, Pérez-Pérez G, Alarcón T, López-Brea M. Rapid detection of clarithromycin resistant Helicobacter pylori strains in Spanish patients by polymerase chain reaction-restriction fragment length polymorphism. Rev Esp Quimioter. (2011) 24:32–6.
11. Chun JY, Kim KJ, Hwang IT, Kim YJ, Lee DH, Lee IK, et al. Dual priming oligonucleotide system for the multiplex detection of respiratory viruses and SNP genotyping of CYP2C19 gene. Nucleic Acids Res. (2007) 35:e40. doi: 10.1093/nar/gkm051
12. Woo HY, Park DI, Park H, Kim MK, Kim DH, Kim IS, et al. Dual-priming oligonucleotide-based multiplex PCR for the detection of Helicobacter pylori and determination of clarithromycin resistance with gastric biopsy specimens. Helicobacter. (2009) 14:22–8. doi: 10.1111/j.1523-5378.2009.00654.x
13. Kim SY, Park JM, Lim CH, Lee HA, Shin GY, Choe Y, et al. Types of 23S ribosomal RNA point mutations and therapeutic outcomes for Helicobacter pylori. Gut Liver. (2021) 15:528–36. doi: 10.5009/gnl20225
14. Lehours P, Siffré E, Mégraud F. DPO multiplex PCR as an alternative to culture and susceptibility testing to detect Helicobacter pylori and its resistance to clarithromycin. BMC Gastroenterol. (2011) 11:112. doi: 10.1186/1471-230X-11-112
15. Lee HJ, Kim JI, Cheung DY, Kim TH, Jun EJ, Oh JH, et al. Eradication of Helicobacter pylori according to 23S ribosomal RNA point mutations associated with clarithromycin resistance. J Infect Dis. (2013) 208:1123–30. doi: 10.1093/infdis/jit287
16. Lopez-Gongora S, Puig I, Calvet X, Villoria A, Baylina M, Munoz N, et al. Systematic review and meta-analysis: susceptibility-guided versus empirical antibiotic treatment for Helicobacter pylori infection. J Antimicrob Chemother. (2015) 70:2447–55. doi: 10.1093/jac/dkv155
17. Kim JL, Cho SJ, Chung SJ, Lee A, Choi J, Chung H, et al. Empiric versus clarithromycin resistance-guided therapy for Helicobacter pylori based on polymerase chain reaction results in patients with gastric neoplasms or gastric mucosa-associated lymphoid tissue lymphoma: a randomized controlled trial. Clin Transl Gastroenterol. (2020) 11:e00194. doi: 10.14309/ctg.0000000000000194
18. Suzuki S, Kusano C, Horii T, Ichijima R, Ikehara H. The ideal Helicobacter pylori treatment for the present and the future. Digestion. (2022) 103:62–8. doi: 10.1159/000519413
19. Kang S, Kim Y, Ahn JY, Jung HY, Kim N, Na HK, et al. Role of antimicrobial susceptibility testing before first-line treatment containing clarithromycin for Helicobacter pylori eradication in the clinical setting. Antibiotics. (2021) 10:214. doi: 10.3390/antibiotics10020214
20. Kwon YH, Kim N, Lee JY, Choi YJ, Yoon K, Nam RH, et al. Comparison of the efficacy of culture-based tailored therapy for Helicobacter pylori eradication with that of the traditional second-line rescue therapy in Korean patients: a prospective single tertiary center study. Scand J Gastroenterol. (2016) 51:270–6. doi: 10.3109/00365521.2015.1095352
21. Lee JW, Kim N, Nam RH, Lee SM, Kwon YH, Sohn SD, et al. Favorable outcomes of culture-based Helicobacter pylori eradication therapy in a region with high antimicrobial resistance. Helicobacter. (2019) 24:e12561. doi: 10.1111/hel.12561
22. Savoldi A, Carrara E, Graham DY, Conti M, Tacconelli E. Prevalence of antibiotic resistance in Helicobacter pylori: a systematic review and meta-analysis in World Health Organization regions. Gastroenterology. (2018) 155:1372–82.e17. doi: 10.1053/j.gastro.2018.07.007
23. Graham DY, Fischbach L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut. (2010) 59:1143–53. doi: 10.1136/gut.2009.192757
24. Thung I, Aramin H, Vavinskaya V, Gupta S, Park JY, Crowe SE, et al. Review article: the global emergence of Helicobacter pylori antibiotic resistance. Aliment Pharmacol Ther. (2016) 43:514–33. doi: 10.1111/apt.13497
25. Fallone CA, Chiba N, van Zanten SV, Fischbach L, Gisbert JP, Hunt RH, et al. The toronto consensus for the treatment of Helicobacter pylori infection in adults. Gastroenterology. (2016) 151:51–69.e14. doi: 10.1053/j.gastro.2016.04.006
26. Hu Y, Zhu Y, Lu NH. Novel and effective therapeutic regimens for Helicobacter pylori in an era of increasing antibiotic resistance. Front Cell Infect Microbiol. (2017) 7:168. doi: 10.3389/fcimb.2017.00168
27. Giorgio F, Principi M, De Francesco V, Zullo A, Losurdo G, Di Leo A, et al. Primary clarithromycin resistance to Helicobacter pylori: is this the main reason for triple therapy failure? World J Gastrointest Pathophysiol. (2013) 4:43–6. doi: 10.4291/wjgp.v4.i3.43
28. Demiray-Gürbüz E, Yilmaz Ö, Olivares AZ, Gönen C, Sarioglu S, Soytürk M, et al. Rapid identification of Helicobacter pylori and assessment of clarithromycin susceptibility from clinical specimens using FISH. J Pathol Clin Res. (2016) 3:29–37. doi: 10.1002/cjp2.57
29. Zhao Y, Li Y, Luan Z, Ma C, Yang L, Zhang W, et al. Establishment of a TaqMan-MGB probe multiplex real-time PCR system for one-step levofloxacin and clarithromycin resistant Helicobacter pylori detection. J Microbiol Methods. (2022) 192:106393. doi: 10.1016/j.mimet.2021.106393
30. Graham DY, Moss SF. Antimicrobial susceptibility testing for Helicobacter pylori is now widely available: when, how, why. Am J Gastroenterol. (2022) 117:524–8. doi: 10.14309/ajg.0000000000001659
31. Cho JH, Jeon SR, Kim HG, Jin SY, Park S. Cost-effectiveness of a tailored Helicobacter pylori eradication strategy based on the presence of a 23S ribosomal RNA point mutation that causes clarithromycin resistance in Korean patients. J Gastroenterol Hepatol. (2019) 34:700–6. doi: 10.1111/jgh.14383
32. Gweon TG, Kim JS, Kim BW. An economic modeling study of Helicobacter pylori eradication: comparison of dual priming oligonucleotide-based multiplex polymerase chain reaction and empirical treatment. Gut Liver. (2018) 12:648–54. doi: 10.5009/gnl18079
33. Lee JW, Kim SJ, Choi CW, Kim HJ, Kang DH, Kim HW, et al. Seven-day triple therapy is sufficient to eradicate infection caused by Helicobacter pylori without 23S rRNA point mutation. Medicine. (2021) 100:e26133. doi: 10.1097/MD.0000000000026133
34. Gingold-Belfer R, Niv Y, Schmilovitz-Weiss H, Levi Z, Boltin D. Susceptibility-guided versus empirical treatment for Helicobacter pylori infection: a systematic review and meta-analysis. J Gastroenterol Hepatol. (2021) 36:2649–58. doi: 10.1111/jgh.15575
35. Harb AH, Chalhoub JM, Abou Mrad R, Sharara AI. Systematic review and meta-analysis: full- vs. half-dose anti-microbials in clarithromycin-based regimens for Helicobacter pylori eradication. Aliment Pharmacol Ther. (2015) 42:131–41. doi: 10.1111/apt.13259
36. Fujioka T, Aoyama N, Sakai K, Miwa Y, Kudo M, Kawashima J, et al. A large-scale nationwide multicenter prospective observational study of triple therapy using rabeprazole, amoxicillin, and clarithromycin for Helicobacter pylori eradication in Japan. J Gastroenterol. (2012) 47:276–83. doi: 10.1007/s00535-011-0487-6
37. Chey WD, Mégraud F, Laine L, López LJ, Hunt BJ, Howden CW. Vonoprazan triple and dual therapy for Helicobacter pylori infection in the United States and Europe: randomized clinical trial. Gastroenterology. (2022) 163:608–19. doi: 10.1053/j.gastro.2022.05.055
38. Sugimoto M, Yamaoka Y. Role of vonoprazan in Helicobacter pylori eradication therapy in Japan. Front Pharmacol. (2018) 9:1560. doi: 10.3389/fphar.2018.01560
39. Choi YJ, Lee YC, Kim JM, Kim JI, Moon JS, Lim YJ, et al. Triple therapy-based on tegoprazan, a new potassium-competitive acid blocker, for first-line treatment of Helicobacter pylori infection: a randomized, double-blind, phase III, clinical trial. Gut Liver. (2022) 16:535–46. doi: 10.5009/gnl220055
Keywords: Helicobacter pylori, eradication, clarithromycin, resistance, point mutation
Citation: Cho SH, Park MS, Park S-Y, Kim DH, You H-S and Kim H-S (2023) Effectiveness of 7-day triple therapy with half-dose clarithromycin for the eradication of Helicobacter pylori without the A2143G and A2142G point mutations of the 23S rRNA gene in a high clarithromycin resistance area. Front. Med. 10:1150396. doi: 10.3389/fmed.2023.1150396
Received: 24 January 2023; Accepted: 27 February 2023;
Published: 22 March 2023.
Edited by:
Antonietta G. Gravina, University of Campania Luigi Vanvitelli, ItalyReviewed by:
Amin Talebi Bezmin Abadi, Tarbiat Modares University, IranJu Yup Lee, Keimyung University, Republic of Korea
Raffaele Pellegrino, University of Campania Luigi Vanvitelli, Italy
Copyright © 2023 Cho, Park, Park, Kim, You and Kim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Seon-Young Park, ZHJwc3lAbmF2ZXIuY29t
†These authors have contributed equally to this work and share first authorship
 Seong Hyun Cho†
Seong Hyun Cho† Seon-Young Park
Seon-Young Park Hye-Su You
Hye-Su You