- 1Faculty of Medicine, Doctoral School, “Ovidius” University, Constanţa, Romania
- 2General Hospital “Căi Ferate,” Galaţi, Romania
- 3Arestetic Clinic, Galaţi, Romania
- 4Faculty of Kinetotherapy, University “Dunărea de Jos,” Galaţi, Romania
- 5Department of Plastic Surgery and Reconstructive Microsurgery, Carol Davila University of Medicine and Pharmacy, Bucharest, Romania
- 6Clinical Department of Plastic Surgery and Reconstructive Microsurgery, “Prof. Dr. Agrippa Ionescu” Emergency Clinical Hospital, Bucharest, Romania
- 7Department of Dental Medicine, Faculty of Medicine and Pharmacy, “Dunărea de Jos” University, Galaţi, Romania
- 8Medical Assistance Service of the Municipality of Galaţi, Galaţi, Romania
- 9Leventer Medical Center, Bucharest, Romania
- 10“Elisabeta Doamna” Psychiatry Hospital, Galaţi, Romania
- 11Clinical Medical Department, Faculty of Medicine and Pharmacy, “Dunărea de Jos” University, Galaţi, Romania
- 12Dermatology Department, “Sfanta Cuvioasa Parascheva” Clinical Hospital of Infectious Diseases, Galaţi, Romania
- 13N. Paulescu National Institute of Diabetes, Bucharest, Romania
- 14Multidisciplinary Integrated Center of Dermatological Interface Research MIC-DIR (Centrul Integrat Multidisciplinar de Cercetare de Interfata Dermatologica - CIM-CID), “Dunărea de Jos” University, Galaţi, Romania
Abrikossoff tumors, also known as granular cell tumors (GCT), originate from Schwann cells. The most common location is in the oral cavity, followed by the skin, but they can also be found in the breast, digestive tract, tracheobronchial tree, or central nervous system. They can affect both sexes at any age, with a higher incidence between 30 and 50 years and a slight predisposition for female sex. They are usually solitary tumors but may also be multifocal. Most of the time, they are benign, with malignancy being exceptional in <2% of cases. Clinically, they appear as solid, well-defined, painless tumors, located subcutaneously with dimensions that can reach up to 10 cm. The definitive diagnosis is based on the immunohistochemical examination, and the treatment for benign tumors consists of surgical excision. Chemotherapy or radiotherapy may be required for malignant lesions, but the treatment regimens and their benefits remain unclear. This manuscript presents the case of a 12-year-old girl with a benign GCT, located in the skin on the mandibular line.
1. Introduction
Abrikossoff tumors, also known as granular cell tumors, are rare and often benign soft tissue tumors of Schwann cell origin (1). Although a granular cell tumor (GCT) usually develops in the skin or oral mucosa, it has been described as seen in many other organs (2). GCT typically presents as a solitary tumor, although it may sometimes be multiple, and in recent years, there have been reports of cases associated with Noonan syndrome and neurofibromatosis (3). It has also been described in association with other diseases. The vast majority of cases are reported in the skin and subcutaneous tissue. However, 2% of Abrikossoff tumors can be malignant (4). There is no predisposition for a certain sex although they can be found at all ages, and they predominate between the fourth and sixth decades of life (1, 5).
2. Case report
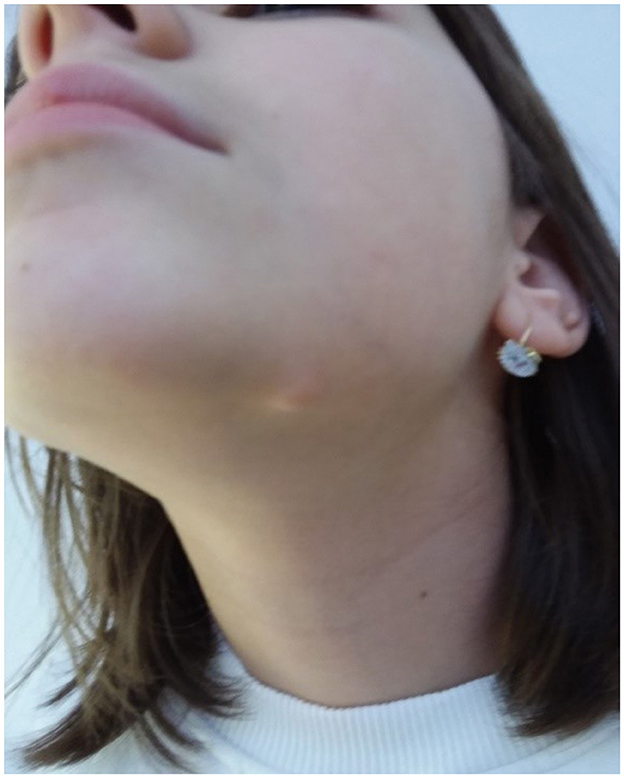
A 12-year-old female patient presented with a 12 mm mass in the area of the left mandible. Examination showed a 15 mm round, well-defined, non-tender, skin-colored nodular lesion, with a slightly depressed center (Figure 1).
An excision biopsy under local anesthesia was carried out and atypical-appearing, partially fatty tissue was removed, which was sent for histopathology. An Abrikossoff tumor was diagnosed, and a wider excision was carried out. Immunohistochemistry of the first piece of the tissue showed a tumor containing residual granular Abrikossoff cells surrounding the dermal fibrous scar tissue. The tumor appeared to have been completely excised, with the exception of a tumor fascicle extending perineurally, involving the deep margins of the resected area. Immediately adjacent to and beneath, this is an area of tumor proliferation comprised of cubes, sheets, and arches of large cells with small, round nuclei, central nuclei with an abundant eosinophillic, granular cytoplasm (Figures 2, 3).
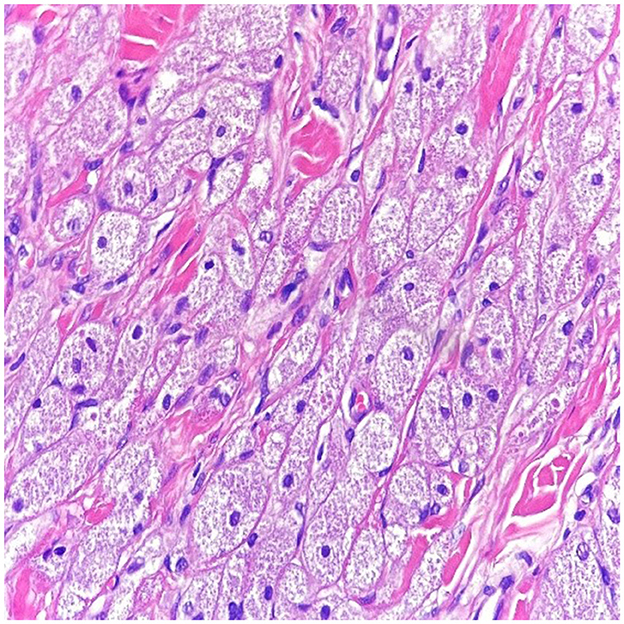
Figure 2. In high power magnification (10 x magnification-H & E stain), the cells have abundant eosinophilic granular cytoplasm with a small nucleus within a collagenous stroma.
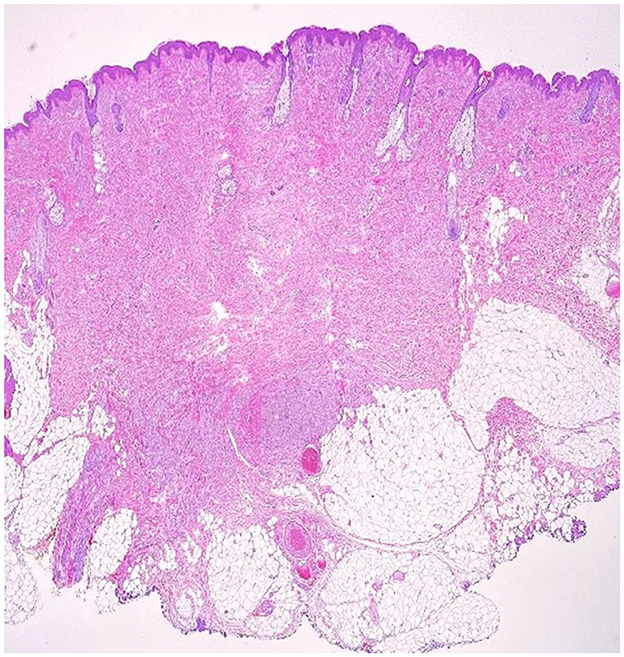
Figure 3. Cutaneous fragment with central fibrosis in the dermis, aside from this fibrous tissue, there is a non-encapsulated tumor proliferation with irregular borders and perineural extension (10 × magnification–H & E stain).
S100 protein was diffusely expressed by the entire tumor and was not expressed at the level of the resection limit, demonstrating the complete excision of the tumor mass, with the exception of an area of tumor cells extending around a nerve strand that reaches to the deeper margins of the resected lesion (Figures 2–4 correspond to the microscopic aspect of the tumor).
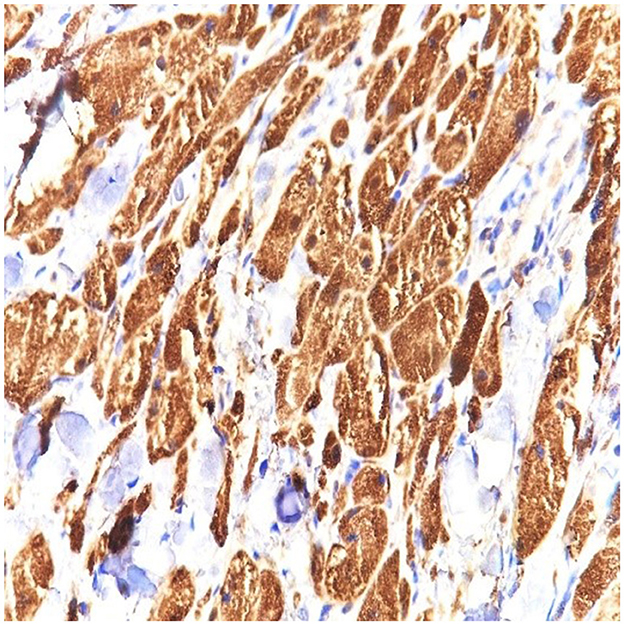
Figure 4. S100 stain in granular cell tumor strongly stains the cytoplasm and the nuclei of the granular cells. Strong and diffuse S100 expression is characteristic of this tumor (40 × magnification).
The cytoplasms of the tumor cells stain positively for CD68 in a granular manner.
Staining for tyrosinase was negative in the tumor but positive in the melanocytes, which were of normal number at the dermoepidermal junction. PRAME (Preferentially Expressed Antigen in Melanoma) was not expressed by tumor cells. The postoperative course was without complications.
3. Discussion
The etiology of these tumors is not known; initially, they were thought to be derived from myoblasts, and this fact is supported by the usual infiltration in the muscle fibers and by the damage of the tongue, a muscular organ (6). They were subsequently considered as developmental cells, histiocytes, neuroendocrine cells, fibroblasts, or undifferentiated mesenchymal cells (1). Fisher and Wechsler first showed by electron microscopy that these tumors are derived from nerve cells (7). In 1990, Mozur, Schultz, and Myers, using specific antibodies, also confirmed that GCT was derived from nerve cells (8), and with the advent of immunohistochemistry, it was established that GCT was derived from Schwann cells (9). GCTs are not common in children, they appear more between 30 and 50 years of age, and the distribution by sex is not clear in some studies predominates in women (9, 10), and in other studies, it predominates in men (11–13).
In terms of location, in order of frequency, they can be found at the level of the tongue (the most common) followed by the torso and limbs (5). Most of the time the tumor is solitary, but there can be multiple foci, including in the internal organs, with the percentage of multiple localizations reaching, according to some studies, up to 30% of cases (4). Multiple localizations in adults have been associated with some diseases such as neurofibromatosis and Hodgkin's lymphoma (4), and in children, they have been associated with cryptorchidism, pulmonary stenosis, congenital heart disease, or Noonan syndrome (14). Noonan syndrome is part of a group of diseases called RASopathies, characterized by genetic mutations in RAS proteins, that play an important role in cell differentiation and development, comprising Noonan syndrome and neurofibromatosis, Legius syndrome, and Costello syndrome and Leopard (PTPN1 gene mutation) (5, 15). Extracutaneous localization may affect the mammary gland, mediastinum, thyroid, larynx and trachea, lungs, ovary, testicle, heart, digestive tract, urinary tract, and rarely the central nervous system, but the central nervous system has the most severe clinical manifestations (1). At the breast level, GCT represents < 0.1% of all tumors and < 6% of all GCT tumors, and the preferred location being the superior-internal quadrant (16).
Clinically, it manifests as a round, painless, skin-colored tumor, located subcutaneously, with slow growth, with somewhat unclear margins, measuring between 5 mm and 10 cm in diameter, sometimes with a warty appearance due to epidermal hyperplasia (5, 6, 17). As the tumor affects the innervation of the skin, sometimes skin contractions occur. Malignancy is very rare, < 2% of cases, and is considered clinically as malignant only when they metastasize and when the size of the tumor exceeds 4 cm (18, 19). Malignant tumors have an accelerated growth rate and can cause metastasis to the lungs, bones, and brain, and metastases can be detected by positron emission tomography and F-18 fluorodeoxyglucose (16, 18). The differential diagnosis comprises lipoma, dermatofibroma, fibro-histiocytoma, pilar cyst, basal cell carcinoma, squamous cell carcinoma, pilomatrixoma, and other types of lesions (20). When they appear on the breast, they may look like breast cancer, but the therapeutic behavior and prognosis are completely different, and sometimes they can be concomitant with invasive ductal carcinoma either in the same breast or in the opposite breast (14, 21).
What is important to follow is the potential conversion from benign to malignant, which is a necessary clinical and histopathological correlation because some very precise criteria of malignancy are missing only on a histological basis. Other diagnostic methods, which may permit a more accurate assessment of the degree of malignancy as well as prognosis in such cases may exist (22).
Histologically, GCT is an unencapsulated tumor consisting of large polyhedral cells with small hyperchromatic central nuclei and a cytoplasm with abundant eosinophilic granules due to the accumulation of secondary lysosomes in the cytoplasm, often extending to the superficial hypodermis. Tumor cells often look like large eosinophilic granules surrounded by a transparent halo known as Milian's pustulo-ovoid bodies, the number of which increases with the age of the tumor (11). Occasionally, binuclear cells, stripped nuclei, dirty nuclei, and intranuclear inclusions may also be observed. The overlying epithelium is often characterized by prominent pseudoepitheliomatous hyperplasia that can be confused with squamous cell carcinoma if the biopsy is taken superficially. Pseudoepitheliomatous hyperplasia can be considered not as a tumor extension but rather as a reaction-type change in the underlying tumor (21).
Immunohistochemistry is positive for S100 protein, CD68 antigen (KP-1), and (neuron-specific enolase) (NSE). Some tumors may be S100-negative and are known as non-neural GCTs; these tumors have recently been reported to overexpress ALK and cyclin D1 and are probably different entities (22).
Fanburg and Smith proposed a classification to evaluate the malignancy of these tumors. Thus, he proposed the following parameters of analysis (23):
• Necrosis
• Spindling
• Vesicular nuclei with large nucleoli
• >2 mitoses/10 high-power fields at × 200 magnification
• High nuclear-to-cytoplasmic ratio
• Pleomorphism
Tumors that did not meet any of these criteria were considered benign, and those that met one or two criteria were considered atypical, and those with three or more were classified as malignant.
Since we are discussing about a pediatric case, the first differential diagnosis would be a spitzoid melanocytic tumor; therefore, tyrosinase and PRAME were performed. In our case, we used tyrosinase, which is positive in melanocytic proliferations and negative in Abrikossoff tumor.
From a histopathologic point of view, we can consider also other differential diagnoses, such as congenital granular cell epulis, cutaneous non-neural granular cell tumor, hibernoma, malignant melanoma, and granular cell dermatofibroma.
Other innovative imaging techniques can be used for diagnosis, such as optical coherence tomography (OCT). Optical coherence tomography (OCT) is an emerging imaging technique that is capable of acquiring high-resolution cross-sectional images of a tissue, being similar to ultrasound; however, infrared waves are used instead of sound waves (24).
The OCT images of the GCT reveal verrucous epidermal hyperplasia, seen as hyperreflective, uneven surface of the tissue. The dermo-epidermal junction is obscured in the OCT images of GCT, while it is discernible in the adjacent healthy skin. Blood vessels are visible in the dermis of the healthy skin but not in the images of GCT (25). Other emerging imaging techniques, such as reflective confocal microscopy, photoacoustic imaging, may also be combined with OCT to improve the diagnosis of GCT (26).
The simple excision of the tumor is the treatment of choice, followed by histopathological examination and possibly immunohistochemical examination. Only if the resection edges are positive is secondary recovery recommended. In malignant tumors, surgical treatment can be supplemented with chemotherapy or radiation therapy, the benefits of which remain unclear (27). The recommended monitoring is done for 10 years as the data from the literature show a local recurrence rate of up to 8% for situations with surgical negative margins and nearly 20% for those with positive surgical margins (28).
Abrikossoff tumor is a rare entity; thus, our case will contribute to growing the body of evidence on its presentation and potential therapy, as well as pave the way for further research. We did not report possible correlations with the COVID “era” nor did we find associations with SARS-CoV 2 infection, as in other cases (29).
4. Conclusion
GCT are a rare entity. They are usually benign, occasionally malignant. The diagnosis is based on the S100 immunohistochemical examination. The treatment of choice was simple surgical excision. In the case of benign tumors, the evolution and prognosis are favorable. Clinicians should be aware of its existence and bear it in mind as a possible differential diagnosis.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The study was conducted in accordance with the Declaration of Helsinki and it was conducted in a private clinic of plastic and aesthetics surgery procedures. It was approved by the Institutional Review Board of the Arestetic Clinic from Galaţi, Romania (63/04.07.2022). Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin. Written informed consent was obtained from the individual's next of kin for the publication of any potentially identifiable images or data included in this article.
Author contributions
VA, RJ, and AT: conceptualization. TT, FB, and RT: software. VA, FB, and AT: validation. VA: methodology, formal analysis, data curation, and writing—original draft preparation. VA, LM, and MM: resources. LN, LM, and MM: writing—review and editing. FB, LM, and MM: visualization. TT: supervision. All authors have read and agreed to the final version of the manuscript.
Funding
The APC was supported by Dunarea de Jos University of Galaţi.
Acknowledgments
The authors wish to acknowledge that the present study was academically supported by the Dunarea de Jos University of Galaţi, Romania, through the research center Multidisciplinary Integrated Center of Dermatological Interface Research MIC-DIR (Centrul Integrat Multidisciplinar de Cercetare de Interfata Dermatologica - CIM-CID).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Pushpa G, Karve PP, Subashini K, Narasimhan MN, Ahmad PB. Abrikossoff's tumor: an unusual presentation. Indian J Dermatol. (2013) 58:407. doi: 10.4103/0019-5154.117335
2. Calonje E, Tsai KE. Soft-tissue tumours and tumour-like condi-tions. Chapter 137 In:Griffiths C, Barker J, Bleiker T, Chalmers R, Creamer D, , editors. Rook's Textbook of Dermatology 9th ed. London: Wiley (2016).
3. Muscardin LM, Paradisi M, Provini A, Cota C, Marzetti G. Multiple cutaneous granular cell tumors, joint hypermobility and mild facial dysmorphism in a child. Int J Dermatol. (2006) 45:847–50. doi: 10.1111/j.1365-4632.2004.02476.x
4. Weedon D. Neural and neuroendocrine tumors. Chapter 37. In:Weedon's skin pathology. 3rd, ed. Philadelphia: Churchill-Livingstone (2010). p. 878–80.
5. Marcoval J, Bauer-Alonso A, Llobera-Ris C, Moreno-Vilchez C, Penín RM, Bermejo j, et al. Tumor de células granularesEstudio clínico de 81 pacientes. Actas Dermosifiliogr. (2021) 112:441–6. doi: 10.1016/j.ad.2020.11.012
6. Gayen T, Das A, Shome K, Bandyopadhyay D, Das D, Saha A. Granular cell tumor: an uncommon benign neoplasm. Indian JDermatol. (2015) 60:322. doi: 10.4103/0019-5154.156453
7. Fisher ER, Wechsler H. Granular cell myoblastoma-a misnomer. Electron microscopic and histochemical evidence concerning its Schwann cell derivation and nature (granular cell schwannoma). Cancer. (1962) 15:936–54. doi: 10.1002/1097-0142(196209/10)15:5<936::aid-cncr2820150509>3.0.co;2-f
8. Mozur MT, Schultz JJ, Myers JL. Granular cell tumor:immunohistochemical analysis of 21 benign tumors and one malignant tumor. Arch Pathol Lab Med. (1990) 114:692–6.
9. Aoyama K, Kamio T, Hirano A, Seshimo A, Kameoka S. Granular cell tumors: a report of six cases. World J Surg Oncol. (2012) 10:204. doi: 10.1186/1477-7819-10-204
10. Mobarki M, Dumollard JM, Dal Col P, Camy F. Peoc'h M, Karpathiou G. Granular cell tumor a study of 42 casesand systemic review of the literature. Pathol Res Pract. (2020) 216:152865. doi: 10.1016/j.prp.2020.152865
11. Torrijos-Aguilar A, Alegre-de Miquel V, Pitarch-Bort G, Mercader-García P, Fortea-Baixauli JM. Cutaneous granular cell tumor: a clinical and pathologic analysis of 34 cases. Actas Dermosifiliogr. (2009) 100:126–32. doi: 10.1016/S1578-2190(09)70028-9
12. Bamps S, Oyen T, Legius E, Vandenoord J, Stas M. Multiple granular cell tumors in a child with Noonan syndrome. Eur J PediatrSurg. (2013) 23:257–9. doi: 10.1055/s-0032-1322537
13. Schrader KA, Nelson TN, De Luca A, Huntsman DG, McGillivray BC. Multiple granular cell tumors are an associated feature of LEOPARD syndrome caused by mutation in PTPN11. Clin Genet. (2009) 75:185–9. doi: 10.1111/j.1399-0004.2008.01100.x
14. Pérez-González YC, Pagura L, Llamas-Velasco M, Cortes-LambeaL, Kutzner H, Requena L. Primary cutaneous malignant granular cell tumor: an immunohistochemical study and review of the literature. Am J Dermatopathol. (2015) 37:334–40. doi: 10.1097/DAD.0000000000000106
15. Mirza FN, Tuggle CT, Zogg CK, Mirza HN, Narayan D. Epidemiology of malignant cutaneous granular cell tumors: a united states population-based cohort analysis using the surveillance, epidemiology, and end results (SEER) database. J Am Acad Dermatol. (2018) 78:490–7.e1. doi: 10.1016/j.jaad.2017.09.062
16. Yu-Fong Chang J, Hwang MJ, Sun A, Chiang CP. Granular cell tumor: case report. J Dent Sci. (2021) 16:1018–9. doi: 10.1016/j.jds.2021.02.002
17. Salaouatchi MT, De Breucker S, Rouvière H, Lesage V, Rocq LJA, Vandergheyns F, et al. A rare case of a metastatic malignant abrikossoff tumor. Case Rep Oncol. (2021) 14:1868–75. doi: 10.1159/000520385
18. Tatu AL. Umbilicated blue black lesion on the lateral thorax. J Cutan Med Surg. (2017) 21:252. doi: 10.1177/1203475417694859
19. Marinescu SA, Tatu AL, Mihai IR, Giuglea C. Correlations between clinics, dermoscopy and histopathology in a female with two dermatofibromas - a case report. Rom J Morphol Embryol. (2016) 57:323–6. Available online at: https://rjme.ro/RJME/resources/files/570116323326.pdf
20. Becelli R, Perugini M, Gasparini G, Cassoni A, Fabiani F. Abrikossoff's tumor. J Craniofac Surg. (2001) 12:78–81. doi: 10.1097/00001665-200101000-00013
21. Olivier L, Naraynsingh V, Hassranah D, Cassim C. Abrikossoff tumor clinically mimicking carcinoma in accessory axillary breast tissue. Cureus. (2022) 14:e21733. doi: 10.7759/cureus.21733
22. Torre-Castro J, Moya-Martínez C, Núñez-Hipólito L, Mendoza-Cembranos MD, Eraña-Tomás I, Jo-Velasco M, et al. Three additional cases of non-neural granular cell tumor with novel immunohistochemical findings. J Cutan Pathol. (2020) 47:1026–32. doi: 10.1111/cup.13801
23. Fanburg-Smith JC1, Meis-Kindblom JM, Fante R, Kindblom LG. Malignant granular cell tumor of soft tissue: diagnostic criteria and clinicopathologic correlation. Am J Surg Pathol. (1998) 22:779–94. doi: 10.1097/00000478-199807000-00001
24. Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W, et al. Optical coherence tomography. Science. (1991) 254:1178–81. doi: 10.1126/science.1957169
25. Tes D, Aber A, Zafar M, Horton L, Fotouhi A, Xu Q, et al. Granular cell tumor imaging using optical coherence tomography. Biomed Eng Comput Biol. (2018) 9:1179597218790250. doi: 10.1177/1179597218790250
26. Broggi G, Verzì AE, Caltabiano R, Micali G, Lacarrubba F. Correlation between in vivo reflectance confocal microscopy and horizontal histopathology in skin cancer: a review. Front Oncol. (2021) 11:653140. doi: 10.3389/fonc.2021.653140
27. Bur D, Ibraheim MK, Freemyer B, Koshelev M. Granular cell tumor: importance of histopathology in determining malignant potential. Dermatol Online J. (2021) 27:10. doi: 10.5070/D327553617
28. Brown AC, Audisio RA, Regitnig P. Granular cell tumour of the breast. Surg Oncol. (2011) 20:97–105. doi: 10.1016/j.suronc.2009.12.001
Keywords: tumor, skin cancer, Abrikossoff, granular cell tumor, facial skin, Schwann cells, oral tumors
Citation: Ardeleanu V, Jecan RC, Moroianu M, Teodoreanu RN, Tebeica T, Moroianu LA, Bujoreanu FC, Nwabudike LC and Tatu AL (2023) Case report: Abrikossoff's tumor of the facial skin. Front. Med. 10:1149735. doi: 10.3389/fmed.2023.1149735
Received: 22 January 2023; Accepted: 28 April 2023;
Published: 31 May 2023.
Edited by:
Laura Atzori, University of Cagliari, ItalyReviewed by:
Giorgio Filosa, Retired, Jesi, ItalyLeena Dennis Joseph, Sri Ramachandra University, India
Copyright © 2023 Ardeleanu, Jecan, Moroianu, Teodoreanu, Tebeica, Moroianu, Bujoreanu, Nwabudike and Tatu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lavinia Alexandra Moroianu, bGF2aW5pYS5tb3JvaWFudUB5YWhvby5jb20=; Florin Ciprian Bujoreanu, ZmxvcmluLmJ1am9yZWFudUBnbWFpbC5jb20=
†These authors have contributed equally to this work
 Valeriu Ardeleanu1,2,3,4†
Valeriu Ardeleanu1,2,3,4† Lavinia Alexandra Moroianu
Lavinia Alexandra Moroianu Florin Ciprian Bujoreanu
Florin Ciprian Bujoreanu Lawrence Chukwudi Nwabudike
Lawrence Chukwudi Nwabudike Alin Laurentiu Tatu
Alin Laurentiu Tatu